Abstract
Corticosteroid insensitivity in asthma limits the ability to effectively manage severe asthma, which is characterized by persistent airway inflammation, airway hyperresponsiveness (AHR), and airflow obstruction despite corticosteroid treatment. Recent reports indicate that corticosteroid insensitivity is associated with increased interferon-γ (IFN-γ) levels and T-helper (Th) 1 lymphocyte infiltration in severe asthma. Signal transducer and activator of transcription 1 (STAT1) activation by IFN-γ is a key signaling pathway in Th1 inflammation; however, its role in the context of severe allergic airway inflammation and corticosteroid sensitivity remains unclear. In this study, we challenged wild-type (WT) and Stat1−/− mice with mixed allergens (MA) augmented with c-di-GMP [bis-(3′-5′)-cyclic dimeric guanosine monophosphate], an inducer of Th1 cell infiltration with increased eosinophils, neutrophils, Th1, Th2, and Th17 cells. Compared with WT mice, Stat1−/− had reduced neutrophils, Th1, and Th17 cell infiltration. To evaluate corticosteroid sensitivity, mice were treated with either vehicle, 1 or 3 mg/kg fluticasone propionate (FP). Corticosteroids significantly reduced eosinophil infiltration and cytokine levels in both c-di-GMP + MA-challenged WT and Stat1−/− mice. However, histological and functional analyses show that corticosteroids did not reduce airway inflammation, epithelial mucous cell abundance, airway smooth muscle mass, and AHR in c-di-GMP + MA-challenged WT or Stat1−/− mice. Collectively, our data suggest that increased Th1 inflammation is associated with a decrease in corticosteroid sensitivity. However, increased airway pathology and AHR persist in the absence of STAT1 indicate corticosteroid insensitivity in structural airway cells is a STAT1 independent process.
Keywords: asthma, corticosteroid insensitivity, STAT1, Th1 inflammation
INTRODUCTION
Corticosteroids have broad anti-inflammatory effects that contribute to the effective management of asthma symptoms and exacerbations. In contrast to the corticosteroid-sensitive mild asthma, people with severe asthma exhibit greater airway inflammation, remodeling, airway hyperresponsiveness (AHR), and more frequent exacerbations despite treatment with higher doses of corticosteroids (1, 2). Although the mechanisms that modulate corticosteroid sensitivity and asthma severity remain poorly understood, adaptive immune pathways are thought to play a key role (3). T-helper (Th) 2 inflammation has a central role in many of the pathophysiological and structural features of asthma; however, recent studies suggest that a more complex inflammatory milieu may contribute to corticosteroid insensitivity in severe asthma (4). Studies aimed to define phenotypes and endotypes in severe asthma have identified associations between activation of additional non-Th2 pathways, such as Th1 inflammation and corticosteroid insensitivity (3, 5, 6).
Th1 inflammation can be activated upon viral or bacterial infection and is characterized by increased Th1 lymphocyte infiltration and interferon-γ (IFN-γ) levels (7). Studies in children and adults with severe asthma have identified IFN-γ-producing CD4+ T lymphocytes in bronchoalveolar lavage samples (8, 9). Although the source that leads to increased Th1 inflammation remains unclear, studies show that patients with severe asthma have increased colonization of pathogenic bacteria in their airways (10). These observations are supported by studies showing enhanced allergic airway inflammation and corticosteroid insensitivity in mice with Th1 inflammation driven by adoptive transfer of IFN-γ-producing Th1 lymphocytes, administration of lipopolysaccharide or the bacterial second messenger, c-di-GMP [bis-(3′-5′)-cyclic dimeric guanosine monophosphate] (8, 11–13). Furthermore, genetic and pharmacological disruption of IFN-γ signaling has been shown to reduce AHR and airway remodeling in mice (8, 11), implicating Th1 inflammation in functional and structural changes in severe asthma. Although Th1 inflammation has been associated with corticosteroid insensitivity in asthma, it remains unclear how Th1-related signaling pathways affect corticosteroid sensitivity.
Signal transducer and activation of transcription factor (STAT1) is a transcription factor that is activated by IFN-γ and mediates proinflammatory responses in immune and airway structural cells (14). Given the critical role for STAT1 in Th1 lymphocyte differentiation (7), Stat1−/− mice exhibit reduced Th1 responses and IFN-γ production (15, 16). Previous studies demonstrated that STAT1 phosphorylation induced by IFN-γ remained enhanced in the presence of corticosteroid treatment in vitro (6, 17). Given the potential role for Th1 inflammation in severe asthma, it is important to understand the role of STAT1 in the context of corticosteroid sensitivity.
In this study, we examined corticosteroid sensitivity in wild-type (WT) and Stat1−/− mice using a chronic mixed allergen (MA) model augmented with c-di-GMP. We hypothesized that ablation of STAT1 would disrupt Th1 inflammation and increase corticosteroid sensitivity in chronic severe allergic airway inflammation. However, our data demonstrate reduced corticosteroid sensitivity in both WT and Stat1−/− mice. Examination of immunological, functional, and structural aspects of chronic allergic airway inflammation shows that persistent airway remodeling remains increased in Stat1−/− mice despite treatment with corticosteroids. Our studies demonstrate that corticosteroid insensitivity remains in the absence of STAT1 signaling during severe allergic airway inflammation.
MATERIALS AND METHODS
Mouse Allergen Sensitization and Challenge
Mouse studies were approved by the Nationwide Children’s Hospital Institutional Animal Care and Use Committee. Wild-type C57BL/6J (Stock No. 000664) and Stat1−/− (Stock No. 012606) mice were procured from Jackson Laboratory (Bar Harbor, ME). Mice were maintained in Research Building III at the Abigail Wexner Research Institute and provided food and water ad libitum. Adult male and female mice were mated to generate pup litters that were randomly assigned a treatment group. Newborn male and female mice were intranasally (in) sensitized and challenged with PBS or mixed allergens (MA), which consists of 10 µg Alternaria alternata (Stallergenes Greer, Lenoir, NC), 10 µg Aspergillus fumigatus (Stallergenes Greer), 10 µg Dermatophagoides pteronyssinus (Stallergenes Greer), and 10 µg ovalbumin (OVA) plus 0.5 µg c-di-GMP (GMP; InvivoGen), three times per week for 7 wk. During the last 2 wk, mice were intraperitoneally (ip) injected with vehicle (PBS containing 0.015% DMSO) or 1 mg/kg or 3 mg/kg FP (FP; Cat. No. 20703; Cayman Chemical, Ann Arbor, Michigan) on the same day as MA challenge. Necropsy was performed ∼24 h after last allergen challenge (Fig. 1A). Mice were euthanized by intraperitoneal injection with 90–120 mg/kg ketamine and 5 mg/kg xylazine.
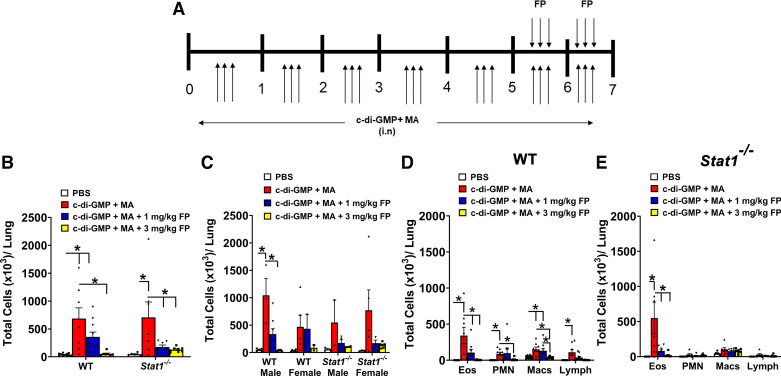
Figure 1.
Corticosteroids reduce total airway immune cell and eosinophilic infiltration. A: mice were challenged intranasally (in) with c-di-GMP and mixed allergen (MA) 3 times a week for 7 wk. In addition, mice were treated with either vehicle or 1 or 3 mg/kg fluticasone propionate (FP) 3 times a week during weeks 6 and 7. B: immune cell infiltration in bronchoalveolar lavage (BAL) is increased in both c-di-GMP + MA-challenged wild-type (WT) and Stat1−/− mice. Treatment with 1 mg/kg FP reduced total BAL cells in c-di-GMP + MA-challenged Stat1−/− mice. n = 6–11 mice per group. C: comparison of BAL immune cell infiltration between male and female WT and Stat1−/− mice. Treatment with 3 mg/kg FP reduced total BAL cells in allergen-challenged male and female mice. n = 2–8 male and female mice per group. D: differential cell counts show that treatment with 1 and 3 mg/kg FP significantly reduces BAL eosinophil, neutrophil, and macrophage infiltration in WT mice. E: treatment with 1 and 3 mg/kg FP significantly reduces eosinophilic infiltration in c-di-GMP + MA-challenged Stat1−/− mice. Male and female mice were analyzed separately to assess sex-related differences. Data are presented as means ± SE, n = 6–11 mice per group, P < 0.05. *Significant difference.
Bronchoalveolar Lavage Harvesting
Bronchoalveolar lavage fluid (BAL) was harvested as previously described (18). Briefly, whole lungs were lavaged with PBS three times. Collected BALF was centrifuged at 300 g for 5 min. Supernatant was collected and stored at −80°C for further analyses. Cellular pellets were resuspended in PBS for total and differential cell count analysis. Total counts were performed using a hemocytometer. Cytospins were generated on microscope slides and differentially stained using a modified Wright-Giemsa Stain (Newcomer Supply, 9112B, Middleton, WI). Cells were imaged on a bright-field microscope and counted by a blinded investigator until a total of 200 cells had been counted.
Lung Function
Once anesthetized, the trachea was exposed and cannulated with a 19-gauge blunt tip cannula. The mouse was placed on a 37°C heating pad and attached to Y-tubing on the Flexivent (SCIREQ, Montreal, Quebec, Canada). A series of measurements including Snapshot (resistance, compliance, elastance) maneuvers were performed following nebulization with PBS, 6.25, 12.5, 25, and 50 mg/mL methacholine (Millipore-Sigma, St. Louis, MO). Airway hyperresponsiveness is reported as total resistance in response to methacholine.
Cytokine Analyses
Lung tissue was homogenized in RIPA buffer, processed, and protein concentration measured using Bradford assay. Each well in custom Meso scale U-Plex Multiplex ELISA plates (Meso Scale Discovery, Rockville, MD) was coated with antibodies against IL-4, IL-17A, and IFN-γ. Analytes were measured in whole lung homogenates following manufacturer instructions. IL-13 levels in BAL were measured using ELISA (Cat. No. M1300CB, R&D Systems, Minneapolis, MN) according to the manufacturer’s instructions.
Western Blotting
Samples were quantified by BCA assay (Pierce) and 25 µg of protein was analyzed per sample. Samples were separated on a 14% SDS-PAGE gel and transferred to nitrocellulose membrane (Bio-Rad). Membranes were blocked for 1 h at room temperature in 1% milk/TBS-T and then incubated in primary antibody overnight at 4°C. Primary antibodies were purchased from Cell Signaling (Danvers, MA): monoclonal rabbit anti-Stat1 antibody at 1:1,000 dilution, Cat. No. 14994S; polyclonal rabbit antiphospho-Stat1 (Ser727) antibody at 1:500 dilution, Cat. No. 9177S; monoclonal rabbit anti-Stat6 at 1:500, Cat. No. 5397S; and monoclonal rabbit anti-phospho-Stat6 (Tyr641) at 1:500, Cat. No. 56554S. The following day, samples were washed in Tris-buffered saline-Tween 20 (TBST), incubated with secondary antibodies (donkey anti-rabbit 800CW or donkey anti-mouse 680RD, 1:20,000, LI-COR) for 1 h at room temperature, washed with TBST, and imaged on an Odyssey Clx (LI-COR). Protein quantification was performed using ImageStudio Software (LI-COR).
Histopathological Analyses
Left lung lobes were inflated with 10% neutral-buffered formalin at 25 cm H2O. Lungs were processed, paraffin embedded, cut into 6 µm sections, and stained with hematoxylin and eosin (H&E, pathological assessment). H&E slides were analyzed and scored for inflammation (0–4 scale) and bronchial-associated lymphoid tissues (BALTs) were quantified by a blinded investigator. Inflammation scores are based on the degree of immune cell infiltration/aggregation around peribronchiolar and perivascular spaces. To quantify BALTs, compact lymphoid nodules were counted in proximal and distal regions. To quantify abundance of mucous cells in the airway epithelium, left lung lobe sections were stained with Alcian Blue-Periodic Acid Schiff (AB-PAS) and photomicrographs of four different airways were taken at ×100 using an Olympus BX-40 light microscope and digital camera (Olympus, Center Valley, PA). PAS-positive and -negative cells were quantified by a blinded investigator using ImageJ (NIH) and reported as a percentage of total airway epithelial cells counted.
Immunohistochemical Analyses for α-Smooth Muscle Actin
Formalin-fixed, paraffin embedded left lung lobe sections (6 µm) were used for immunohistochemical staining for α-smooth muscle actin (α-SMA). Sections were deparaffinized with xylene (2 × 5 min each) and rehydrated with graded ethanol (100%, 90%, 70%, and tap water). Antigen retrieval was performed with 10 mM sodium citrate at 100°C for 1 h. The slides were blocked for 1 h in TBS containing 4% goat serum and 0.04% Triton X-100. After washing, slides were incubated overnight in monoclonal mouse anti-α-SMA at 1:100 dilution (Millipore Sigma, St. Louis, MO, Cat. No. A2547). After incubation, slides were washed and incubated with anti-mouse-FITC secondary antibody (Millipore Sigma, Cat. No. F0257) at 1:1,000 dilution. Negative control slides followed the same protocol but without addition of a primary antibody. Slides were counterstained with DAPI, and images taken at ×100 using Lionheart (Biotek, Winooski, VT). Smooth muscle actin area was analyzed using ImageJ (NIH). Data were normalized to length of airway basement membrane to report as airway smooth muscle (ASM) mass per µm basement membrane.
Gene Expression Analyses
RNA (300–1000 ng) from profiled samples was cDNA transcribed using Oligo dT12–18 primers and Superscript IV (Thermo Fisher Scientific Applied Biosystems). Quantitative real-time PCR with TaqMan using Muc5ac (Mm0127618_m1), Muc5b (Mm00466391_m1), and GAPDH (Mm99999915_g1) primer sets was performed (Life Technologies) according to the manufacturer’s instructions. Samples were run on a QuantStudio 3 96-well Real-Time PCR system (Thermo Fisher Scientific Applied Biosystems). Results were analyzed using the comparative Ct method for TaqMan assays.
Flow Cytometry
Lungs were perfused with 5 mL of PBS through right ventricle of the heart, and then placed in C-tubes (Miltenyi Biotec, Gladbach, Germany) and digested for 30 min in 432 U/mL Collagenase Type IV (Worthington Biochemical, Lakewood, NJ, Cat. No. LS004188) and 64 U/mL DNAase I (Millipore Sigma, Cat. No. DN25-100MG) in DMEM medium. Lung tissue was homogenized using Gentlemacs octo dissociator (Miltenyi Biotec), strained through 70 μm nylon cell strainer, centrifuged at 300 g for 10 min, and resuspended in 10 mL of DMEM medium. Cells were counted using a hemocytometer. For Th stimulation, two million cells were incubated in DMEM medium containing leukocyte activation cocktail with Golgi plug (BD Biosciences, Cat. No. 550583), protein transported inhibitor containing Monensin (BD Biosciences, Cat. No. 554724), and 10% FBS for 37°C for 4 h. Cells were collected and permeabilized/fixed using fixation/permeabilization buffer and 1× permeabilization/wash buffer (BD Biosciences, Cat. No. 554714) and resuspended in 200 μL of PBS. Single cell suspensions were used in flow cytometry analysis.
Single cell suspensions were stained with various fluorochrome conjugated anti-mouse monoclonal antibodies for cellular phenotyping as follows: FITC-conjugated anti-CD45 (30-F11), BV421-conjugated anti-CD3 (17A2), PE-conjugated anti-CD4 (A161A1), Alexa 700-conjugated anti-IFNγ (XMG1.2), APC-conjugated anti-IL-4 (11B11), and APC-conjugated anti-IL-17A (TC11-18H10.1). To control for intracellular cytokine staining, APC (RTK2071) and Alexa fluor 700 (RTK2071) IgG1 isotypes were used. All antibodies were purchased from BioLegend. Data were acquired using a BD LSRII flow cytometer (BD Biosciences, San Diego, CA) and analyzed using FlowJo software (v. 10.7.1).
Statistical Analyses
Data were analyzed by performing two-way ANOVA with Bonferroni post hoc analysis for multiple comparisons. Data were analyzed and graphed using GraphPad Prism 9 Software (GraphPad, La Jolla, CA). Values are presented a means ± standard error and significant differences indicated by P < 0.05.
RESULTS
Corticosteroids Reduce Total Airway Immune Cell and Eosinophil Infiltration
Wild-type (WT) and Stat1−/− mice challenged with c-di-GMP + MA exhibited increased total BAL immune cells compared with PBS-challenged WT mice (Fig. 1B). BAL total cell counts were increased in both male and female mice (Fig. 1C). Differential immune cell analyses showed that c-di-GMP + MA-challenged WT mice exhibited significantly increased eosinophil, neutrophil, macrophage, and lymphocyte numbers in BAL (Fig. 1D). In contrast, neutrophils, macrophages, and lymphocytes were not increased in c-di-GMP + MA-challenged Stat1−/− mice (Fig. 1E). Treatment with 1 mg/kg fluticasone propionate (FP) significantly reduced total BAL immune cells in c-di-GMP + MA-challenged Stat1−/− mice, but not WT mice (Fig. 1B), whereas treatment with the higher dose of FP, 3 mg/kg, led to reduced total BAL and eosinophil cell numbers in c-di-GMP + MA-challenged WT and Stat1−/− mice (Fig. 1, B, D, and E). In addition to eosinophils, neutrophil and macrophage cell numbers were significantly reduced in c-di-GMP + MA-challenged WT mice treated with 3 mg/kg FP (Fig. 1, D and E). Effects of FP were comparable in male and female mice (Fig. 1C).
Genetic Deletion of STAT1 Reduces Th1 and Th17 Lymphocyte Populations and Interferon-γ Expression
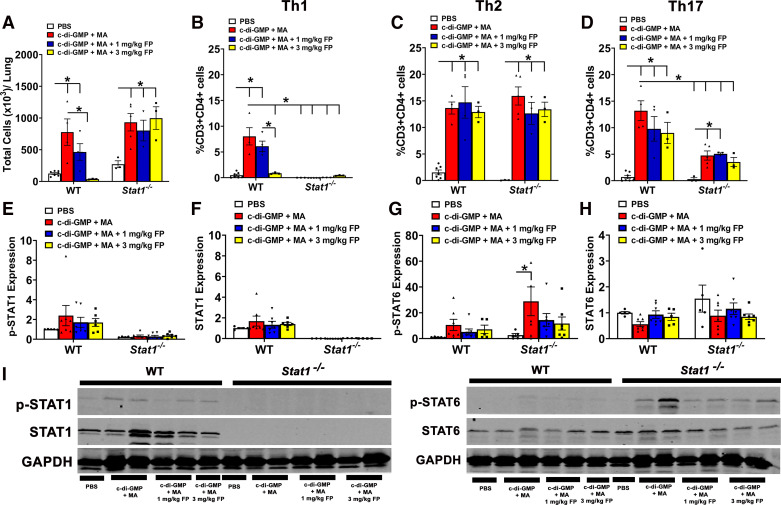
Recent studies have shown evidence of Th1 inflammation in lung-derived samples collected from patients with severe asthma (8). To assess T helper (Th) lymphocyte populations and their respective cytokines, we performed flow cytometric analyses and ELISA on whole lung homogenates, respectively. Total CD3+CD4+ T cell numbers were significantly increased in c-di-GMP + MA-challenged WT and Stat1−/− (Fig. 2A). Treatment with 3 mg/kg FP significantly reduced total CD3+CD4+ T cell numbers in WT mice; however, treatment with 1 and 3 mg/kg FP did not reduce total CD3+CD4+ T cell numbers in Stat1−/− mice (Fig. 2A). For Th1 inflammation, we analyzed for IFN-γ producing CD3+CD4+ T cells and IFN-γ levels. C-di-GMP + MA-challenged WT mice exhibited significantly increased Th1 cells (Fig. 2B) and IFN-γ levels (Table 1). Treatment with 3 mg/kg fluticasone propionate (FP) significantly reduced Th1 populations and IFN-γ levels in c-di-GMP + MA-challenged WT mice (Fig. 2B and Table 1). Increased Th1 populations and IFN-γ levels were not observed in c-di-GMP + MA-challenged Stat1−/− mice (Fig. 2B and Table 1). In addition, we did not observe significant changes in STAT1 phosphorylation (Fig. 2, E and I) or total STAT1 expression (Fig. 2, F and I) in the c-di-GMP + MA-challenged WT mice. Importantly, STAT1 expression was not detected in Stat1−/− mice.
Figure 2.
Genetic deletion of signal transducer and activator of transcription 1 (STAT1) reduces T-helper (Th) 1 lymphocyte populations. A: total CD3+CD4+ lymphocytes in wild-type (WT) mice, but not Stat1−/− mice, are reduced by fluticasone propionate (FP). B: Th1 lymphocytes are increased in c-di-GMP + MA-challenged WT mice but reduced in Stat1−/− mice. In WT mice, treatment with 3 mg/kg FP reduced this increase in Th1 cells. Th2 (C) and Th17 (D) lymphocytes are significantly increased in c-di-GMP + MA-challenged WT and Stat1−/− mice and not reduced with treatment with corticosteroids. C-di-GMP + MA-challenged Stat1−/− mice significantly reduced Th17 lymphocytes compared with WT counterparts. Stat1 phosphorylation (Ser727; E) and total protein levels (F) remain unchanged in c-di-GMP + MA WT mice, but were absent in Stat1−/− mice. G: Stat6 phosphorylation (Tyr641) is increased in GMP + MA-challenged Stat1−/− mice compared with PBS controls, whereas H: total Stat6 was unchanged in both WT and Stat1−/− mice. I: representative Western blots of phosphorylated (p)-STAT1, total STAT1 protein, p-STAT6, total STAT6 protein, and GAPDH. Data are presented as means ± SE, n = 3–7 mice per group, P < 0.05. *Significant difference. MA, mixed allergen.
Table 1.
Proinflammatory cytokine levels in chronic model
| Genotype | Cytokine | PBS | c-di-GMP + MA | c-di-GMP + MA + 1 mg/kg FP | c-di-GMP + MA + 3 mg/kg FP |
|---|---|---|---|---|---|
| Wild type | IFN-γ | 0.51 ± 0.04 | 2.48 ± 0.47* | 1.46 ± 0.66 | 0.633 ± 0.24$ |
| IL-4 | 0.21 ± 0.10 | 77.53 ± 17.96* | 8.35 ± 3.19$ | 6.28 ± 1.74$ | |
| IL-13 | 2.67 ± 0.77 | 286.78 ± 91.56* | 20.17 ± 10.83 | 7.64 ± 1.93$ | |
| IL-17A | 0.05 ± 0.01 | 226.50 ± 65.91* | 74.07 ± 48.28$ | 19.59 ± 10.11$ | |
| Stat1−/− | IFN-γ | 0.01 ± 0.02 | 0.86 ± 0.22 | 0.53 ± 0.26% | 0.19 ± 0.12% |
| IL-4 | 0.23 ± 0.170 | 153.80 ± 63.67* | 46.84 ± 21.80$ | 29.97 ± 9.08$ | |
| IL-13 | 2.43 ± 0.86 | 427.42 ± 136.93* | 71.71 ± 31.14$ | 42.67 ± 10.04$ | |
| IL-17A | 0.038 ± 0.03 | 189.08 ± 86.25 | 41.55 ± 25.37 | 14.37 ± 4.98 |
Data are presented as means ± SE, n = 5–10 mice per group, P < 0.05. FP, fluticasone propionate; MA, mixed allergen. *Significant difference from PBS wild-type (WT) mice, $significant effect of FP, and %significant difference from c-di-GMP + MA WT mice.
To assess Th2 inflammation, we measured Th2 lymphocytes (CD3+CD4+IL-4+) and IL-4 and IL-13 expression. Th2 lymphocytes were increased in c-di-GMP + MA-challenged WT and Stat1−/− mice and were not reduced with 1 or 3 mg/kg FP treatment (Fig. 2C). WT and Stat1−/− mice exhibited significant increases in both IL-4 and IL-13 levels (Table 1), whereas treatment with 1 mg/kg FP significantly reduced IL-4 levels in both WT and Stat1−/− mice challenged with c-di-GMP (Table 1). IL-13 levels were significantly reduced in c-di-GMP + MA WT and Stat1−/− mice with 3 mg/kg FP (Table 1). Since STAT6 is important for IL-4 and IL-13-mediated signaling (19), we measured expression of STAT6 phosphorylation (Tyr641) in lung homogenates. We found that STAT6 phosphorylation was significantly increased in GMP + MA-challenged Stat1−/− mice (Fig. 2, G and I), whereas total Stat6 levels remained unchanged (Fig. 2, H and I).
In regard to Th17 inflammation, Th17 (CD3+CD4+IL-17A+) cells and IL-17A levels were significantly higher in c-di-GMP + MA-challenged WT and Stat1−/− mice (Fig. 2D and Table 1). Treatment with 3 mg/kg FP significantly reduced IL-17A expression levels; however, Th17 lymphocytes remained increased. There was also a significant reduction of Th17 cell populations in c-di-GMP + MA-challenged Stat1−/− mice compared with their WT counterparts (Fig. 2D).
Genetic Deletion of STAT1 Does Not Improve Airway Pathology or AHR in Combination with Corticosteroids
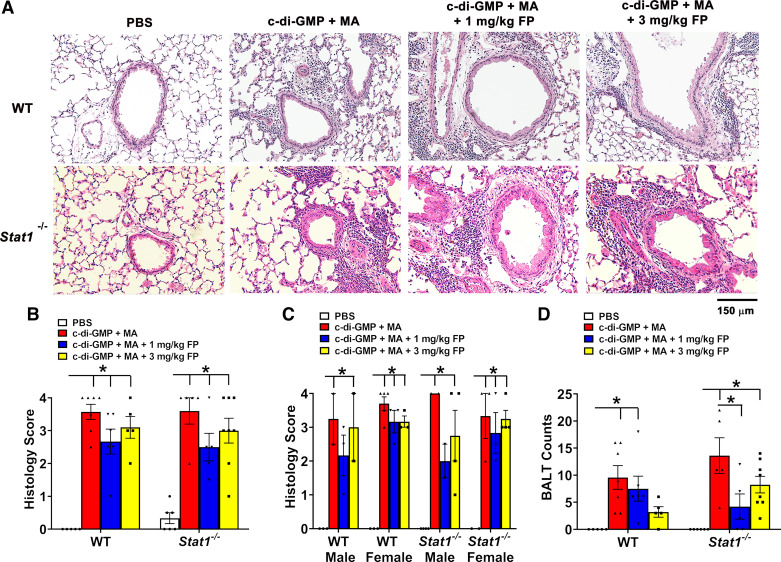
To histologically assess airway inflammation, H&E-stained lung sections were scored for immune cell infiltration in peribronchiolar and perivascular spaces, and bronchial-associated lymphoid tissue (BALTs) nodules quantified. Compared with PBS challenge, c-di-GMP + MA challenge significantly increased airway inflammation in WT and Stat1−/− mice (Fig. 3, A and B). These effects were not significantly reduced by treatment with 1 or 3 mg/kg FP (Fig. 3B). To analyze for sex-related differences in airway inflammation, male and female WT and Stat1−/− mice were analyzed separately. C-di-GMP + MA-challenged male and female mice exhibited a significantly higher degree of airway inflammation regardless of genotype. Treatment with 1 and 3 mg/kg FP did not significantly reduce airway inflammation in both male and females regardless of genotype (Fig. 3C). In regard to BALTs, c-di-GMP + MA-challenged WT and Stat1−/− mice exhibited significantly higher number of BALTs in analyzed lung sections compared with PBS-challenge controls (Fig. 3D). A significant reduction in BALT counts was only observed in c-di-GMP + MA-challenged Stat1−/− mice treated with 1 mg/kg FP (Fig. 3C).
Figure 3.
Ablation of signal transducer and activator of transcription 1 (STAT1) does not reduce allergic airway inflammation. A: representative photomicrographs of left lung lobe sections stained with hematoxylin and eosin (H&E). B: c-di-GMP + MA-challenged wild-type (WT) and Stat1−/− mice exhibited increased inflammation (increased histology score) in the peribronchiolar and perivascular spaces that was not reduced with corticosteroid treatment. C: comparison of airway inflammation between male and female WT and Stat1−/− mice. n = 2–5 male and female mice per group. D: number of bronchial-associated lymphoid tissues (BALTs) per lung section in GMP + MA-challenged WT and Stat1−/− mice were increased compared with PBS controls. Male and female mice were analyzed separately to assess sex-related differences. Data are presented as means ± SE, n = 4–8 mice per group, P < 0.05. *Significant difference. FP, fluticasone propionate; MA, mixed allergen.
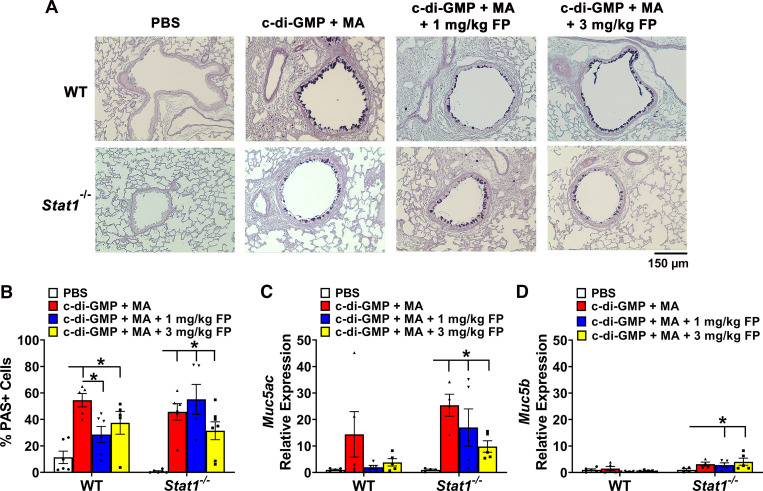
To examine the abundance of mucus-producing cells in the airway epithelium, lung sections were stained with AB-PAS and mucous cells counted. Cyclic-di-GMP + MA-challenged WT and Stat1−/−mice exhibited significant increases in mucous cells compared with PBS-challenged mice (Fig. 4, A and B). Treatment with 1 mg/kg FP significantly reduced the abundance of mucous cells in c-di-GMP + MA-challenged WT mice (Fig. 4B). Mucous cell abundance remained increased in WT and Stat1−/− mice despite treatment with 3 mg/kg FP (Fig. 4B). To determine mucin gene expression, we analyzed whole lung homogenates for expression of secreted mucin-associated genes, Muc5ac and Muc5b (Fig. 4, C and D). There were no significant differences in c-di-GMP + MA-challenged WT mice in Muc5ac and Muc5b mRNA expression. Conversely, c-di-GMP + MA-challenged Stat1−/− mice exhibited significantly higher Muc5ac expression that was not reduced with either FP treatment, whereas c-di-GMP + MA Stat1−/− mice also had significantly higher Muc5b expression compared with PBS-challenged Stat1−/− mice (Fig. 4, C and D).
Figure 4.
Ablation of signal transducer and activator of transcription 1 (STAT1) does not reduce presence of mucous cells in airway epithelium. A: representative photomicrographs of lung sections stained with Alcian Blue-Periodic Acid Schiff (AB-PAS). B: allergen-challenged wild-type (WT) and Stat1−/− mice exhibit increased mucous cell abundance. Muc5ac (C) and Muc5b (D) mRNA expression is increased in allergen-challenged Stat1−/− mice. Data are presented as means ± SE, n = 5–9 mice per group, P < 0.05. *Significant difference. FP, fluticasone propionate.
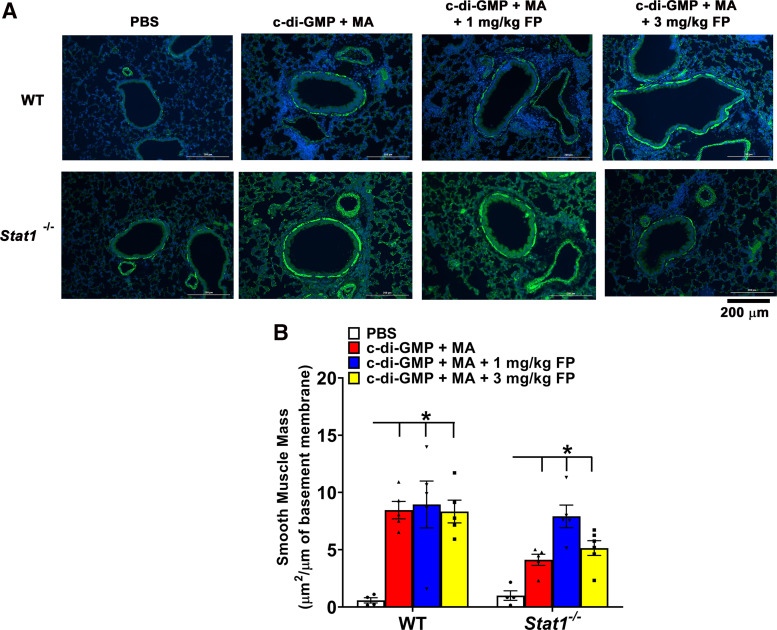
To assess airway remodeling, we measured airway smooth muscle (ASM) mass in lung sections stained for α-smooth muscle actin. C-di-GMP + MA-challenged WT and Stat1−/− mice exhibited greater ASM mass around the airways compared with PBS-challenged mice. The increases in ASM were not reduced in WT and Stat1−/− mice treated with 1 or 3 mg/kg FP (Fig. 5).
Figure 5.
Increased airway smooth muscle (ASM) mass persists in severe allergic airway inflammation. A: representative photomicrographs of lung sections immunohistochemically stained for α-smooth muscle actin (α-SMA) and DAPI. B: quantification of α-SMA around airways shows increased ASM mass in c-di-GMP + MA-challenged wild-type (WT) and Stat1−/− mice. These effects were not reduced by treatment with 1 or 3 mg/kg fluticasone propionate (FP). Data are presented as means ± SE, n = 4–6 mice per group, P < 0.05. *Significant difference. MA, mixed allergen.
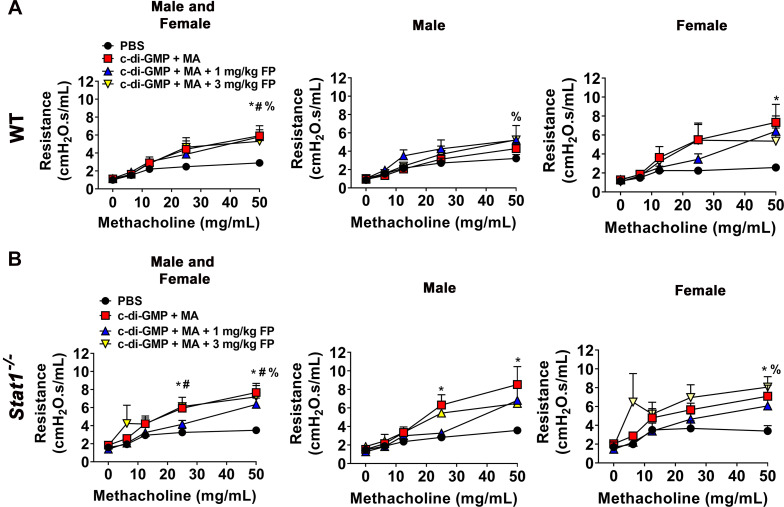
Airway hyperresponsiveness (AHR) was measured using forced oscillation maneuvers following administration of methacholine (0–50 mg/mL). C-di-GMP + MA-challenged WT and Stat1−/− mice showed significantly increased AHR compared with PBS-challenged mice. Treatment with 1 mg or 3 mg/kg fluticasone propionate did not significantly reduce AHR in WT and Stat1−/− mice (Fig. 6). To analyze for sex-related differences, we next analyzed AHR for males and females separately. C-di-GMP + MA-challenged WT and Stat1−/− female mice and Stat1−/− male mice exhibited significantly higher AHR compared with PBS-challenged mice. C-di-GMP + MA-challenged male WT and Stat1−/− female treated with 3 mg/kg FP also exhibited significantly higher AHR compared with PBS-controls (Fig. 6).
Figure 6.
Airway hyperresponsiveness (AHR) in chronic severe allergic inflammation. AHR is significantly increased in mixed allergen (MA) + GMP-challenged wild-type (WT) (A) and Stat1−/− (B) mice and is reduced by with the treatment with 1 or 3 mg/kg fluticasone propionate (FP). Male and female mice were analyzed separately to assess sex-related differences. Data are presented as means ± SE, n = 8–13 mice per group and n = 4–7 male and female mice per group, P < 0.05. *, #, %Significant difference from PBS.
DISCUSSION
People with severe asthma have a complex inflammatory environment that is thought to contribute to corticosteroid insensitivity (4). Studies suggest that Th1 inflammation promotes corticosteroid insensitivity (4, 8, 17); however, its role remains unclear. Consistent with previous studies, we show that enhanced Th1 inflammation was associated by corticosteroid insensitivity in mice chronically challenged with allergens and a bacterial second messenger (5, 8). C-di-GMP + MA-challenged WT mice exhibited mixed granulocytic cell infiltration with eosinophils and neutrophils, persistent airway inflammation, and dysfunction in airway epithelium and smooth muscle. In addition, Th1, Th2, and Th17 cell populations, as indicated by their respective effector cytokines, were present in our severe allergic airway inflammation model (Table 2). To understand the role of Th1-related pathways in severe allergic airway inflammation, we assessed corticosteroid sensitivity in Stat1−/− mice that exhibit dysfunctional Th1 signaling (20). Although we hypothesized that Stat1−/− mice would have improved corticosteroid sensitivity, we found that Stat1−/− mice remained largely insensitive to corticosteroids. Our data suggest that loss of the Th1-signaling pathway STAT1 does not affect corticosteroid sensitivity and altered airway pathology, inflammation, airway smooth muscle mass, and mucous cell abundance persisted (Table 2).
Table 2.
Summary of allergen responses and corticosteroid sensitivity in wild-type and Stat1−/− mice
| Endpoint | Effect of c-di-GMP + MA |
Corticosteroid Sensitivity |
||
|---|---|---|---|---|
| Wild Type | Stat1−/− | Wild Type | Stat1−/− | |
| Granulocytic infiltration | Eosinophil and neutrophil | Eosinophil | Moderate | Moderate |
| T Lymphocyte populations | Th1, Th2, Th17 | Th2, Th17lo | Moderate | Low |
| Lung inflammation | High | High | Low | Low |
| Mucous cell abundance | High | High | Low | Low |
| Airway smooth muscle mass and airway hyperresponsiveness | High | High | Low | Low |
MA, mixed allergen; Th, T-helper.
Although BAL immune cell infiltration displayed moderate corticosteroid sensitivity in WT and Stat1−/− mice, histological analyses showed persistent airway inflammation in both genotypes. Treatment with 1 and 3 mg/kg fluticasone propionate significantly reduced total BAL cell number in WT and Stat1−/− mice. These decreases were largely attributed to reduced eosinophil infiltration, which is consistent with previous studies in OVA (21) and house dust mite (8) models. In our model, WT but not Stat1−/− mice had neutrophil infiltration when challenged with c-di-GMP+ MA. Although neutrophils are associated with corticosteroid insensitivity in severe asthma, their infiltration is commonly associated with increased Th17 and not Th1 inflammation (13, 22, 23). Given the role of Th17 inflammation in neutrophil recruitment and lung infiltration (22, 24), reductions in neutrophils in Stat1−/− mice could be attributed to reduced Th17 lymphocytes and IL-17A expression. STAT1 signaling has been shown to regulate neutrophil infiltration chemoattracts (25, 26), suggesting Th1-associated signaling pathways can contribute to Th17 inflammation and neutrophil recruitment in allergic airway inflammation.
Recent studies led us to hypothesize that disruption of a key Th1 signaling pathway, such as STAT1, would lead to enhanced corticosteroid sensitivity and alleviate severe allergic airway inflammation (5, 6, 8). Ifnγ−/− mice challenged with house dust mite (HDM) and c-di-GMP exhibited reduced AHR even in the absence of corticosteroids, implicating Th1 inflammation in increased AHR (8). In addition, these mice showed no significant increase in expression of Th2 cytokines, IL-4, IL-5, and IL-13 (8). In contrast, our studies show that disrupting STAT1 signaling reduced IFN-γ expression and Th1 inflammation and did not lead to reduction in airway inflammation, AHR, or corticosteroid insensitivity. We found evidence that T lymphocyte infiltration and Th2 inflammation maybe enhanced in Stat1−/− mice. We observed increased CD3+CD4+ total cell numbers, Th2 lymphocytes, IL-4 and IL-13 levels, and STAT6 phosphorylation in allergen-challenged Stat1−/− mice. IL-4 and IL-13 are known to have profound effects on airway inflammation, AHR, remodeling, and mucous cell metaplasia, effects that involve STAT6 (19, 27–29). Although we observed that corticosteroids significantly reduced IL-4 and IL-13 expression levels in Stat1−/− mice, their levels did not return to baseline. IL-4 and IL-13 at relatively low levels have been shown to induce mucus production, AHR, and remodeling (27–30). We speculate that loss of Th1 inflammation within a complex inflammatory environment could allow the effects of Th2 inflammation to continue in the presence of corticosteroids.
Diminished lung function and airway remodeling contribute to airway narrowing and stiffening in severe asthmatics (2, 31). AHR and ASM mass, both key features of asthma, were corticosteroid insensitive in both WT and Stat1−/− mice. These key indicators of airway structure and function are commonly found to persist despite corticosteroid treatments in severe asthmatics (32, 33). Although it remains unclear why ASM are insensitive to corticosteroids in our models, the continued presence of one or more Th1, Th2, and/or Th17 effector cytokines during airway inflammation may play a role (34). Many of these cytokines are known to increase ASM inflammatory mediator production, hypercontractility, proliferation, and extracellular matrix deposition (30, 34–38). Although corticosteroids are able to reduce the effects of individual cytokines in vitro, the complex inflammatory environment and/or duration of allergen exposure may allow ASM dysfunction to persist. Despite the loss of the Th1 inflammation in Stat1−/− mice, we observed that AHR and ASM mass remained increased, which is a common feature in patients with severe asthma (2, 4, 33). The severity of airway inflammation was also exemplified by the presence of lymphoid cell aggregations or BALTs in allergen-challenged WT and Stat1−/− mice. The role of BALTs in asthma is not well understood. However, they are thought to play an important role in localized immune responses in the lung (39, 40) and have been observed in allergen-challenged mice (41–43) and patients with severe asthma (44).
Increased abundance of mucous cells in the airway epithelium is another important pathological feature in severe asthma that also adversely affects airway function by contributing to cough, wheeze, and airway obstruction (45, 46). Previous studies show that the airway epithelium in patients with severe asthma has higher abundance of mucous cells than healthy individuals (27, 46–48). We found significant increases in the abundance of mucous cells in the airways of chronically challenged WT and Stat1−/− mice. Treatment with 1 mg/kg FP was able to reduce mucous cell abundance in WT mice; however, mucous cell abundance remained increased in Stat1−/− mice. This observation is consistent with studies demonstrating the inability of corticosteroids to reduce mucous cell abundance in severe allergic airway inflammation (49, 50), making mucous cell metaplasia another important pathologic feature in severe asthma. Increases in mucin gene (Muc5ac and Muc5b) expression and mucus production are associated with increased Th2-associated inflammation (27). The observed increases in mucin gene expression in allergen-challenged Stat1−/− mice suggest persistent Th2-associated responses. Collectively, these data show that structural and functional aspects in our model remain largely insensitive to corticosteroids in WT and Stat1−/− mice.
Our findings in Stat1−/− mice are in contrast to previous studies in mouse models of severe allergic airway inflammation. In a c-di-GMP + house dust mite (GMP + HDM) model, Ifnγ−/− and Irf5−/− mice exhibited evidence of increased Th2 inflammation with greater eosinophil infiltration than allergen-challenged WT mice (5, 8). However, AHR was significantly reduced in Ifnγ−/− and Irf5−/− mice, whereas we found that airway inflammation and AHR remained increased in Stat1−/− mice. Although the differences from our study are not entirely clear, our sensitization/challenge strategy, use of fungal allergens (Aspergillus fumigatus, Alternaria alternata), and persistent airway remodeling could be contributing factors in the lack of reduced airway inflammation and corticosteroid sensitivity in Stat1−/− mice.
Although Th1 and Th17 inflammation are reduced in Stat1−/− mice, persistent airway inflammation and corticosteroid insensitivity may involve enhanced Th2 inflammation. Negative regulation of Th2 inflammatory responses by Th1 inflammation is an established and important immune regulatory mechanism (51, 52). This concept is highlighted in studies reporting a role for IFN-γ and STAT1 in suppressing Th2 inflammation. These effects have been attributed to interference with GATA3 expression, Stat6 phosphorylation, and Th2 lymphocyte differentiation (16, 53–55). In the lung, Stat1−/− mice develop enhanced Th2 inflammation in mouse models of fungal (56) and viral infections (57, 58). In addition, respiratory syncytial virus (RSV) infection has been shown to induce higher levels of IL-13 in the lungs of Stat1−/− mice compared with their WT counterparts, which lead to increases in mucous cell abundance (57, 58). The antagonistic role for Th1 inflammation in Th2-mediated responses is further demonstrated in recent studies showing that STAT1 is important for limiting ILC2 development and differentiation into effector cells that promote Th2 inflammation (59, 60). ILC2s have an important role in the initiation and maintenance of Th2 inflammation by secreting Th2 cytokines in allergic asthma (61). Persistence of Th2 inflammation may also affect other cells types, such as macrophages and airway structural cells, further contributing to corticosteroid insensitivity in the absence of STAT1.
Sex and circulating sex hormone levels are important factors when considering asthma prevalence. In children, males are much more likely to have asthma than females and to greater severity. As age progresses, females are found to have an increased asthma prevalence compared with males (62). We analyzed our BAL, H&E staining, and AHR to determine potential differences in allergen responses and corticosteroid sensitivity in male and female mice. We did not detect significant differences between males and females in regard to airway inflammation and AHR in WT and Stat1−/− mice. Despite these observations, little is known about sex differences, corticosteroid sensitivity, and asthma severity. Given the intersections between sex hormone and glucocorticoid signaling in the lung (63), sex is likely an important factor when considering corticosteroid sensitivity. Thus, future studies designed to determine the contribution of sex differences to corticosteroid insensitivity and severe asthma are needed.
Although we found that loss of STAT1 leads to enhancement of STAT6 phosphorylation, we did not examine activation of other pathways associated with Th1- and Th2-mediated signaling in asthma including IRF1, IRF5, and IRS2 (5, 64, 65). Additional limitations to our study include the semiquantitative histological methods used to sample and analyze lung inflammation and ASM mass. Our sampling captured only a portion of airway and lung sections which may not fully represent the effect of allergens and corticosteroids on lung pathology. Cytokine levels, BAL immune cell infiltration, and AHR were not entirely consistent with our histological analyses. Stereological techniques would likely provide more unbiased and comprehensive analysis of lung inflammation and airway remodeling (66). In addition, our understanding of potential sex differences in our model is limited due to relatively low numbers of male and female mice. However, our study does raise intriguing questions about differential corticosteroid sensitivity in males and females with asthma.
In conclusion, our data show that STAT1 is important for Th1 inflammation in severe allergic airway inflammation but not corticosteroid insensitivity. We observed persistent asthmatic features in allergen-challenged Stat1−/− mice treated with corticosteroids that may involve persistent airway structural alterations such as immune cell aggregation, presence of mucous cells in airway epithelium, and increased smooth muscle mass. These findings may also provide a potential avenue to explore corticosteroid insensitivity in the context of Th2hi severe asthma, which lacks Th1 and Th17 (67). Together, these data provide insight into the role of STAT1 and Th1 inflammation in chronic allergen exposure and has implications for understanding corticosteroid insensitivity in allergic asthma.
GRANTS
We acknowledge the funding support from National Institutes of Health R00 HL131682 ( to R. D. Britt and D. Jackson), R01 HL155095 (to R. D. Britt), R01 AI121405 (to M. Guerau-de-Arellano), R03 AI151769 (to M. Guerau-de-Arellano), and startup funds from the Abigail Wexner Research Institute at Nationwide Children’s Hospital.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
B.W.L., M.H.G., and R.D.B.J. conceived and designed research; B.W.L., D.J., S.A.A., J.W., M.G., S.G., E.C., A.V.B., and R.D.B.J. performed experiments; B.W.L., D.J., S.A.A., J.W., M.G., S.G., E.C., A.V.B., M.G.-d.-A., and R.D.B.J. analyzed data; B.W.L., M.G.-d.-A., M.H.G., and R.D.B.J. interpreted results of experiments; B.W.L. and R.D.B.J. prepared figures; B.W.L., M.G.-d.-A., M.H.G., and R.D.B.J. drafted manuscript; B.W.L., D.J., S.A.A., J.W., M.G., S.G., E.C., A.V.B., M.G.-d.-A., M.H.G., and R.D.B.J. edited and revised manuscript; B.W.L., D.J., S. A.A., J.W., M.G., S.G., E.C., A.V.B., M.G.-d.-A., M.H.G., and R.D.B.J. approved final version of manuscript.
REFERENCES
- 1.Hansbro PM, Kim RY, Starkey MR, Donovan C, Dua K, Mayall JR, Liu G, Hansbro NG, Simpson JL, Wood LG, Hirota JA, Knight DA, Foster PS, Horvat JC. Mechanisms and treatments for severe, steroid-resistant allergic airway disease and asthma. Immunol Rev 278: 41–62, 2017. doi: 10.1111/imr.12543. [DOI] [PubMed] [Google Scholar]
- 2.Saglani S. Childhood severe asthma: new insights on remodelling and biomarkers. Paediatr Respir Rev 24: 11–13, 2017. doi: 10.1016/j.prrv.2017.06.001. [DOI] [PubMed] [Google Scholar]
- 3.Ray A, Camiolo M, Fitzpatrick A, Gauthier M, Wenzel SE. Are we meeting the promise of endotypes and precision medicine in asthma? Physiol Rev 100: 983–1017, 2020. doi: 10.1152/physrev.00023.2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ray A, Raundhal M, Oriss TB, Ray P, Wenzel SE. Current concepts of severe asthma. J Clin Invest 126: 2394–2403, 2016. doi: 10.1172/JCI84144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Oriss TB, Raundhal M, Morse C, Huff RE, Das S, Hannum R, Gauthier MC, Scholl KL, Chakraborty K, Nouraie SM, Wenzel SE, Ray P, Ray A. IRF5 distinguishes severe asthma in humans and drives Th1 phenotype and airway hyperreactivity in mice. JCI Insight 2: e91019, 2017. doi: 10.1172/jci.insight.91019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Gauthier M, Chakraborty K, Oriss TB, Raundhal M, Das S, Chen J, Huff R, Sinha A, Fajt M, Ray P, Wenzel SE, Ray A. Severe asthma in humans and mouse model suggests a CXCL10 signature underlies corticosteroid-resistant Th1 bias. JCI Insight 2: e94580, 2017. doi: 10.1172/jci.insight.94580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Annunziato F, Cosmi L, Liotta F, Maggi E, Romagnani S. Human Th1 dichotomy: origin, phenotype and biologic activities. Immunology 144: 343–351, 2015. doi: 10.1111/imm.12399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Raundhal M, Morse C, Khare A, Oriss TB, Milosevic J, Trudeau J, Huff R, Pilewski J, Holguin F, Kolls J, Wenzel S, Ray P, Ray A. High IFN-γ and low SLPI mark severe asthma in mice and humans. J Clin Invest 125: 3037–3050, 2015. doi: 10.1172/JCI80911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Wisniewski JA, Muehling LM, Eccles JD, Capaldo BJ, Agrawal R, Shirley DA, Patrie JT, Workman LJ, Schuyler AJ, Lawrence MG, Teague WG, Woodfolk JA. TH1 signatures are present in the lower airways of children with severe asthma, regardless of allergic status. J Allergy Clin Immunol 141: 2048–2060.e13, 2018. doi: 10.1016/j.jaci.2017.08.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Zhang Q, Illing R, Hui CK, Downey K, Carr D, Stearn M, Alshafi K, Menzies-Gow A, Zhong N, Fan Chung K. Bacteria in sputum of stable severe asthma and increased airway wall thickness. Respir Res 13: 35, 2012. doi: 10.1186/1465-9921-13-35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kobayashi M, Ashino S, Shiohama Y, Wakita D, Kitamura H, Nishimura T. IFN-γ elevates airway hyper-responsiveness via up-regulation of neurokinin A/neurokinin-2 receptor signaling in a severe asthma model. Eur J Immunol 42: 393–402, 2012. doi: 10.1002/eji.201141845. [DOI] [PubMed] [Google Scholar]
- 12.Hadjigol S, Netto KG, Maltby S, Tay HL, Nguyen TH, Hansbro NG, Eyers F, Hansbro PM, Yang M, Foster PS. Lipopolysaccharide induces steroid-resistant exacerbations in a mouse model of allergic airway disease collectively through IL-13 and pulmonary macrophage activation. Clin Exp Allergy 50: 82–94, 2020. doi: 10.1111/cea.13505. [DOI] [PubMed] [Google Scholar]
- 13.Cui J, Pazdziorko S, Miyashiro JS, Thakker P, Pelker JW, Declercq C, Jiao A, Gunn J, Mason L, Leonard JP, Williams CM, Marusic S. TH1-mediated airway hyperresponsiveness independent of neutrophilic inflammation. J Allergy Clin Immunol 115: 309–315, 2005. doi: 10.1016/j.jaci.2004.10.046. [DOI] [PubMed] [Google Scholar]
- 14.Hu X, Ivashkiv LB. Cross-regulation of signaling pathways by interferon-γ: implications for immune responses and autoimmune diseases. Immunity 31: 539–550, 2009. doi: 10.1016/j.immuni.2009.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Sugawara I, Yamada H, Mizuno S. STAT1 knockout mice are highly susceptible to pulmonary mycobacterial infection. Tohoku J Exp Med 202: 41–50, 2004. doi: 10.1620/tjem.202.41. [DOI] [PubMed] [Google Scholar]
- 16.Thio CL, Lai AC, Chi PY, Webster G, Chang YJ. Toll-like receptor 9-dependent interferon production prevents group 2 innate lymphoid cell-driven airway hyperreactivity. J Allergy Clin Immunol 144: 682–697.9, 2019. doi: 10.1016/j.jaci.2019.03.008. [DOI] [PubMed] [Google Scholar]
- 17.Britt RD Jr, Thompson MA, Sasse S, Pabelick CM, Gerber AN, Prakash YS. Th1 cytokines TNF-α and IFN-γ promote corticosteroid resistance in developing human airway smooth muscle. Am J Physiol Lung Cell Mol Physiol 316: L71–L81, 2019. doi: 10.1152/ajplung.00547.2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Livraghi-Butrico A, Kelly EJ, Klem ER, Dang H, Wolfgang MC, Boucher RC, Randell SH, O'Neal WK. Mucus clearance, MyD88-dependent and MyD88-independent immunity modulate lung susceptibility to spontaneous bacterial infection and inflammation. Mucosal Immunol 5: 397–408, 2012. doi: 10.1038/mi.2012.17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Akiho H, Blennerhassett P, Deng Y, Collins SM. Role of IL-4, IL-13, and STAT6 in inflammation-induced hypercontractility of murine smooth muscle cells. Am J Physiol Gastrointest Liver Physiol 282: G226–G232, 2002. doi: 10.1152/ajpgi.2002.282.2.G226. [DOI] [PubMed] [Google Scholar]
- 20.Szabo SJ, Sullivan BM, Peng SL, Glimcher LH. Molecular mechanisms regulating Th1 immune responses. Annu Rev Immunol 21: 713–758, 2003. doi: 10.1146/annurev.immunol.21.120601.140942. [DOI] [PubMed] [Google Scholar]
- 21.Birrell MA, Battram CH, Woodman P, McCluskie K, Belvisi MG. Dissociation by steroids of eosinophilic inflammation from airway hyperresponsiveness in murine airways. Respir Res 4: 3, 2003. doi: 10.1186/rr197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Tesmer LA, Lundy SK, Sarkar S, Fox DA. Th17 cells in human disease. Immunol Rev 223: 87–113, 2008. doi: 10.1111/j.1600-065X.2008.00628.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ray A, Kolls JK. Neutrophilic inflammation in asthma and association with disease severity. Trends Immunol 38: 942–954, 2017. doi: 10.1016/j.it.2017.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.McKinley L, Alcorn JF, Peterson A, Dupont RB, Kapadia S, Logar A, Henry A, Irvin CG, Piganelli JD, Ray A, Kolls JK. TH17 cells mediate steroid-resistant airway inflammation and airway hyperresponsiveness in mice. J Immunol 181: 4089–4097, 2008. doi: 10.4049/jimmunol.181.6.4089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Duke KS, Taylor-Just AJ, Ihrie MD, Shipkowski KA, Thompson EA, Dandley EC, Parsons GN, Bonner JC. STAT1-dependent and -independent pulmonary allergic and fibrogenic responses in mice after exposure to tangled versus rod-like multi-walled carbon nanotubes. Part Fibre Toxicol 14: 26, 2017. doi: 10.1186/s12989-017-0207-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Fulkerson PC, Zimmermann N, Hassman LM, Finkelman FD, Rothenberg ME. Pulmonary chemokine expression is coordinately regulated by STAT1, STAT6, and IFN-γ. J Immunol 173: 7565–7574, 2004. doi: 10.4049/jimmunol.173.12.7565. [DOI] [PubMed] [Google Scholar]
- 27.Curran DR, Cohn L. Advances in mucous cell metaplasia: a plug for mucus as a therapeutic focus in chronic airway disease. Am J Respir Cell Mol Biol 42: 268–275, 2010. doi: 10.1165/rcmb.2009-0151TR. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Gour N, Wills-Karp M. IL-4 and IL-13 signaling in allergic airway disease. Cytokine 75: 68–78, 2015. doi: 10.1016/j.cyto.2015.05.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Zhu Z, Homer RJ, Wang Z, Chen Q, Geba GP, Wang J, Zhang Y, Elias JA. Pulmonary expression of interleukin-13 causes inflammation, mucus hypersecretion, subepithelial fibrosis, physiologic abnormalities, and eotaxin production. J Clin Invest 103: 779–788, 1999. doi: 10.1172/JCI5909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Manson ML, Safholm J, James A, Johnsson AK, Bergman P, Al-Ameri M, Orre AC, Karrman-Mardh C, Dahlen SE, Adner M. IL-13 and IL-4, but not IL-5 nor IL-17A, induce hyperresponsiveness in isolated human small airways. J Allergy Clin Immunol 145: 808–817.e2, 2020. doi: 10.1016/j.jaci.2019.10.037. [DOI] [PubMed] [Google Scholar]
- 31.Benayoun L, Druilhe A, Dombret MC, Aubier M, Pretolani M. Airway structural alterations selectively associated with severe asthma. Am J Respir Crit Care Med 167: 1360–1368, 2003. doi: 10.1164/rccm.200209-1030OC. [DOI] [PubMed] [Google Scholar]
- 32.Berry M, Morgan A, Shaw DE, Parker D, Green R, Brightling C, Bradding P, Wardlaw AJ, Pavord ID. Pathological features and inhaled corticosteroid response of eosinophilic and non-eosinophilic asthma. Thorax 62: 1043–1049, 2007. doi: 10.1136/thx.2006.073429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Bossley CJ, Saglani S, Kavanagh C, Payne DN, Wilson N, Tsartsali L, Rosenthal M, Balfour-Lynn IM, Nicholson AG, Bush A. Corticosteroid responsiveness and clinical characteristics in childhood difficult asthma. Eur Respir J 34: 1052–1059, 2009. doi: 10.1183/09031936.00186508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Prakash YS. Emerging concepts in smooth muscle contributions to airway structure and function: implications for health and disease. Am J Physiol Lung Cell Mol Physiol 311: L1113–L1140, 2016. doi: 10.1152/ajplung.00370.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Perkins C, Yanase N, Smulian G, Gildea L, Orekov T, Potter C, Brombacher F, Aronow B, Wills-Karp M, Finkelman FD. Selective stimulation of IL-4 receptor on smooth muscle induces airway hyperresponsiveness in mice. J Exp Med 208: 853–867, 2011. doi: 10.1084/jem.20100023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Chang Y, Al-Alwan L, Risse PA, Halayko AJ, Martin JG, Baglole CJ, Eidelman DH, Hamid Q. Th17-associated cytokines promote human airway smooth muscle cell proliferation. FASEB J 26: 5152–5160, 2012. doi: 10.1096/fj.12-208033. [DOI] [PubMed] [Google Scholar]
- 37.Risse PA, Jo T, Suarez F, Hirota N, Tolloczko B, Ferraro P, Grutter P, Martin JG. Interleukin-13 inhibits proliferation and enhances contractility of human airway smooth muscle cells without change in contractile phenotype. Am J Physiol Lung Cell Mol Physiol 300: L958–L966, 2011. doi: 10.1152/ajplung.00247.2010. [DOI] [PubMed] [Google Scholar]
- 38.Kudo M, Melton AC, Chen C, Engler MB, Huang KE, Ren X, Wang Y, Bernstein X, Li JT, Atabai K, Huang X, Sheppard D. IL-17A produced by αβ T cells drives airway hyper-responsiveness in mice and enhances mouse and human airway smooth muscle contraction. Nat Med 18: 547–554, 2012. doi: 10.1038/nm.2684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Hirahara K, Shinoda K, Morimoto Y, Kiuchi M, Aoki A, Kumagai J, Kokubo K, Nakayama T. Immune cell-epithelial/mesenchymal interaction contributing to allergic airway inflammation associated pathology. Front Immunol 10: 570, 2019. doi: 10.3389/fimmu.2019.00570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Elliot JG, Jensen CM, Mutavdzic S, Lamb JP, Carroll NG, James AL. Aggregations of lymphoid cells in the airways of nonsmokers, smokers, and subjects with asthma. Am J Respir Crit Care Med 169: 712–718, 2004. doi: 10.1164/rccm.200308-1167OC. [DOI] [PubMed] [Google Scholar]
- 41.Vroman H, Bergen IM, Li BW, van Hulst JA, Lukkes M, van Uden D, Hendriks RW, Kool M. Development of eosinophilic inflammation is independent of B-T cell interaction in a chronic house dust mite-driven asthma model. Clin Exp Allergy 47: 551–564, 2017. doi: 10.1111/cea.12834. [DOI] [PubMed] [Google Scholar]
- 42.Ramelli SC, Comer BS, McLendon JM, Sandy LL, Ferretti AP, Barrington R, Sparks J, Matar M, Fewell J, Gerthoffer WT. Nanoparticle delivery of anti-inflammatory LNA oligonucleotides prevents airway inflammation in a HDM model of asthma. Mol Ther Nucleic Acids 19: 1000–1014, 2020. doi: 10.1016/j.omtn.2019.12.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Guest IC, Sell S. Bronchial lesions of mouse model of asthma are preceded by immune complex vasculitis and induced bronchial associated lymphoid tissue (iBALT). Lab Invest 95: 886–902, 2015. doi: 10.1038/labinvest.2015.72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Hwang JY, Randall TD, Silva-Sanchez A. Inducible bronchus-associated lymphoid tissue: taming inflammation in the lung. Front Immunol 7: 258, 2016. doi: 10.3389/fimmu.2016.00258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Bergeron C, Tulic MK, Hamid Q. Airway remodelling in asthma: from benchside to clinical practice. Can Respir J 17: e85–e93, 2010. doi: 10.1155/2010/318029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Ordonez CL, Khashayar R, Wong HH, Ferrando R, Wu R, Hyde DM, Hotchkiss JA, Zhang Y, Novikov A, Dolganov G, Fahy JV. Mild and moderate asthma is associated with airway goblet cell hyperplasia and abnormalities in mucin gene expression. Am J Respir Crit Care Med 163: 517–523, 2001. doi: 10.1164/ajrccm.163.2.2004039. [DOI] [PubMed] [Google Scholar]
- 47.Kuyper LM, Pare PD, Hogg JC, Lambert RK, Ionescu D, Woods R, Bai TR. Characterization of airway plugging in fatal asthma. Am J Med 115: 6–11, 2003. doi: 10.1016/S0002-9343(03)00241-9. [DOI] [PubMed] [Google Scholar]
- 48.Aikawa T, Shimura S, Sasaki H, Ebina M, Takishima T. Marked goblet cell hyperplasia with mucus accumulation in the airways of patients who died of severe acute asthma attack. Chest 101: 916–921, 1992. doi: 10.1378/chest.101.4.916. [DOI] [PubMed] [Google Scholar]
- 49.Singh VP, Mabalirajan U, Pratap K, Bahal D, Maheswari D, Gheware A, Bajaj A, Panda L, Jaiswal A, Ram A, Agrawal A. House dust mite allergen causes certain features of steroid resistant asthma in high fat fed obese mice. Int Immunopharmacol 55: 20–27, 2018. doi: 10.1016/j.intimp.2017.11.014. [DOI] [PubMed] [Google Scholar]
- 50.Panda L, Gheware A, Rehman R, Yadav MK, Jayaraj BS, Madhunapantula SV, Mahesh PA, Ghosh B, Agrawal A, Mabalirajan U. Linoleic acid metabolite leads to steroid resistant asthma features partially through NF-κB. Sci Rep 7: 9565, 2017. doi: 10.1038/s41598-017-09869-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Finotto S, Neurath MF, Glickman JN, Qin S, Lehr HA, Green FH, Ackerman K, Haley K, Galle PR, Szabo SJ, Drazen JM, De Sanctis GT, Glimcher LH. Development of spontaneous airway changes consistent with human asthma in mice lacking T-bet. Science 295: 336–338, 2002. doi: 10.1126/science.1065544. [DOI] [PubMed] [Google Scholar]
- 52.Glanville N, Peel TJ, Schroder A, Aniscenko J, Walton RP, Finotto S, Johnston SL. Tbet deficiency causes T helper cell dependent airways eosinophilia and mucus hypersecretion in response to rhinovirus infection. PLoS Pathog 12: e1005913, 2016. doi: 10.1371/journal.ppat.1005913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Heller NM, Matsukura S, Georas SN, Boothby MR, Rothman PB, Stellato C, Schleimer RP. Interferon-γ inhibits STAT6 signal transduction and gene expression in human airway epithelial cells. Am J Respir Cell Mol Biol 31: 573–582, 2004. doi: 10.1165/rcmb.2004-0195OC. [DOI] [PubMed] [Google Scholar]
- 54.Jenner RG, Townsend MJ, Jackson I, Sun K, Bouwman RD, Young RA, Glimcher LH, Lord GM. The transcription factors T-bet and GATA-3 control alternative pathways of T-cell differentiation through a shared set of target genes. Proc Natl Acad Sci USA 106: 17876–17881, 2009. doi: 10.1073/pnas.0909357106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Cohn L, Homer RJ, Niu N, Bottomly K. T helper 1 cells and interferon γ regulate allergic airway inflammation and mucus production. J Exp Med 190: 1309–1318, 1999. doi: 10.1084/jem.190.9.1309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Leopold Wager CM, Hole CR, Wozniak KL, Olszewski MA, Wormley FL Jr.. STAT1 signaling is essential for protection against Cryptococcus neoformans infection in mice. J Immunol 193: 4060–4071, 2014. doi: 10.4049/jimmunol.1400318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Newcomb DC, Boswell MG, Huckabee MM, Goleniewska K, Dulek DE, Reiss S, Lukacs NW, Kolls JK, Peebles RS Jr.. IL-13 regulates Th17 secretion of IL-17A in an IL-10-dependent manner. J Immunol 188: 1027–1035, 2012. doi: 10.4049/jimmunol.1102216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Stier MT, Goleniewska K, Cephus JY, Newcomb DC, Sherrill TP, Boyd KL, Bloodworth MH, Moore ML, Chen K, Kolls JK, Peebles RS Jr.. STAT1 represses cytokine-producing group 2 and group 3 innate lymphoid cells during viral infection. J Immunol 199: 510–519, 2017. doi: 10.4049/jimmunol.1601984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Duerr CU, McCarthy CD, Mindt BC, Rubio M, Meli AP, Pothlichet J, Eva MM, Gauchat JF, Qureshi ST, Mazer BD, Mossman KL, Malo D, Gamero AM, Vidal SM, King IL, Sarfati M, Fritz JH. Type I interferon restricts type 2 immunopathology through the regulation of group 2 innate lymphoid cells. Nat Immunol 17: 65–75, 2016. doi: 10.1038/ni.3308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Moro K, Kabata H, Tanabe M, Koga S, Takeno N, Mochizuki M, Fukunaga K, Asano K, Betsuyaku T, Koyasu S. Interferon and IL-27 antagonize the function of group 2 innate lymphoid cells and type 2 innate immune responses. Nat Immunol 17: 76–86, 2016. doi: 10.1038/ni.3309. [DOI] [PubMed] [Google Scholar]
- 61.Halim TY, Steer CA, Matha L, Gold MJ, Martinez-Gonzalez I, McNagny KM, McKenzie AN, Takei F. Group 2 innate lymphoid cells are critical for the initiation of adaptive T helper 2 cell-mediated allergic lung inflammation. Immunity 40: 425–435, 2014. doi: 10.1016/j.immuni.2014.01.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Fuseini H, Newcomb DC. Mechanisms driving gender differences in asthma. Curr Allergy Asthma Rep 17: 19, 2017. doi: 10.1007/s11882-017-0686-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Luchak A, Solomon LA, Kanagalingam T, Vijeyakumaran M, Rowe BH, Cameron L. Comparative efficacy of glucocorticoid receptor agonists on Th2 cell function and attenuation by progesterone. BMC Immunol 21: 54, 2020. doi: 10.1186/s12865-020-00383-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Maier E, Duschl A, Horejs-Hoeck J. STAT6-dependent and -independent mechanisms in Th2 polarization. Eur J Immunol 42: 2827–2833, 2012. doi: 10.1002/eji.201242433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Junttila IS. Tuning the cytokine responses: an update on interleukin (IL)-4 and IL-13 receptor complexes. Front Immunol 9: 888, 2018. doi: 10.3389/fimmu.2018.00888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Schneider JP, Ochs M. Stereology of the lung. Methods Cell Biol 113: 257–294, 2013. doi: 10.1016/B978-0-12-407239-8.00012-4. [DOI] [PubMed] [Google Scholar]
- 67.Peters MC, Kerr S, Dunican EM, Woodruff PG, Fajt ML, Levy BD, Israel E, Phillips BR, Mauger DT, Comhair SA, Erzurum SC, Johansson MW, Jarjour NN, Coverstone AM, Castro M, Hastie AT, Bleecker ER, Wenzel SE, Fahy JV; National Heart, Lung and Blood Institute Severe Asthma Research Program 3. Refractory airway type 2 inflammation in a large subgroup of asthmatic patients treated with inhaled corticosteroids. J Allergy Clin Immunol 143: 104–113.e14, 2019. doi: 10.1016/j.jaci.2017.12.1009. [DOI] [PMC free article] [PubMed] [Google Scholar]