Abstract
Severe trauma is a leading cause of mortality. Its pathophysiology, progression, and outcome are complex and heterogenous. In this issue of Cell Reports Medicine, Wu et al. from the PAMPer study provide new insights into the potential underlying biology based on multi-omics analysis.1
Severe trauma is a leading cause of mortality. Its pathophysiology, progression, and outcome are complex and heterogenous. In this issue of Cell Reports Medicine, Wu et al. from the PAMPer study provide new insights into the potential underlying biology based on multi-omics analysis.1
Main text
Unintended injuries represent a leading cause of premature death worldwide. In 2020, critical injuries took nearly 200,000 lives in the United States, only behind the heart disease, cancer, and COVID-19.2 Patients with critical injuries, or severe trauma, have a high mortality rate, and over 50% of them die at the scene of injury or before hospitalization. After hospitalization, the majority of early deaths occurring within the first 2 days can be attributed to traumatic brain injury (TBI) and hemorrhagic shock, while the later deaths were largely due to sepsis and multiple organ failure.3 Over the last two decades, the mortality rate has significantly improved in the specialized trauma centers.4 However, the prehospital mortality rate, in other words deaths in minutes to a few hours after injury, has not been improved.5 Although the coagulopathy and hemorrhagic shock are more approachable to treatment, damage to the central nervous system or TBI still remains a major challenge in patients with severe trauma.6
The Prehospital Air Medical Plasma (PAMPer) trial was designed to test the efficacy and safety of prehospital plasma resuscitation in critically injured patients at risk for hemorrhagic shock, through a multicenter phase 3 trial at the University of Pittsburgh Medical Center.7 During the air medical transport to nine level-I trauma centers, patients were randomized to receive a standard-care or allogeneic thawed plasma resuscitation. Clinical data from 501 patients showed a significant improvement in mortality at 30 days in the plasma resuscitation group.7 To further understand the complex human response to severe trauma and the potential underlying biological mechanism(s) of plasma-based resuscitation in reducing mortality, the PAMPer study investigators have been analyzing the patient clinical data in relation to serum biomarkers including endotheliopathy biomarkers, cytokines, and lipidomics.7,8
In the this issue of Cell Reports Medicine, Wu et al. reported the metabolomic and proteomic datasets and completed a 6-layer multi-omic map for the PAMPer cohort.1 Their metabolomic and proteomic analysis at admission, 24 h, and 72 h after hospitalization revealed an abrupt and massive release of cellular constituents into the circulation. The metabolomic analysis revealed seven metabolite modules that evolved differently between the resolving and non-resolving (severe) conditions across the three time points. These metabolite modules were further validated in the TD-2 cohort of 472 blunt trauma survivors, indicating that the metabolic shift in severe trauma is closely related to clinical outcome. The proteomics analysis in 151 PAMPer patients for 1305 proteins in plasma revealed that the majority of proteins elevated in the serum at hospitalization, particularly in the non-resolving cases, reflect a massive release of cellular constituents caused by injury.
With the integrated 6-layer multi-omic array from 88 patients, the authors pooled 200 variables highly correlating with injury severity, lactic acidosis, and prothrombin time based on the international normalized ratio (IRN). They further identified two clusters. The first cluster consisting of inflammatory cytokines, endothelial and tissue injury markers, cellular components and stress response, energy substrates, and metabolic intermediates was named “Systemic Storm.” The second cluster enriched in lipids, lipid transport proteins, coagulation factors and enzymes was named “Massive Consumption.” These two patterns clearly depicted the injury-induced immediate changes in circulating proteins and metabolites, as well as endangering coagulopathy,1 providing a critical insight to the hyperacute phase of the injury.
Importantly, a multi-layer analysis in 173 patients at hospitalization identified two endotypes: (1) one mainly representing high levels of endothelial injury markers that were associated with a lower 30-day survival rate, and (2) the other representing lower levels of endothelial injury markers and associated with a significantly higher 30-day survival rate with ∼25% net difference. Interestingly, these endotypes particularly influenced differential outcomes in two subgroups of trauma patients that received prehospital plasma resuscitation. Only patients with TBI and high levels of endothelial injury markers responded well to plasma administration, with approximately 70% reduction in mortality, whereas patients without brain injury and lower levels of endothelial injury markers had a marginal response to plasma administration. These data are very exciting and indicate that prehospital plasma resuscitation could be a promising intervention for patients with severe TBI (Figure 1).8
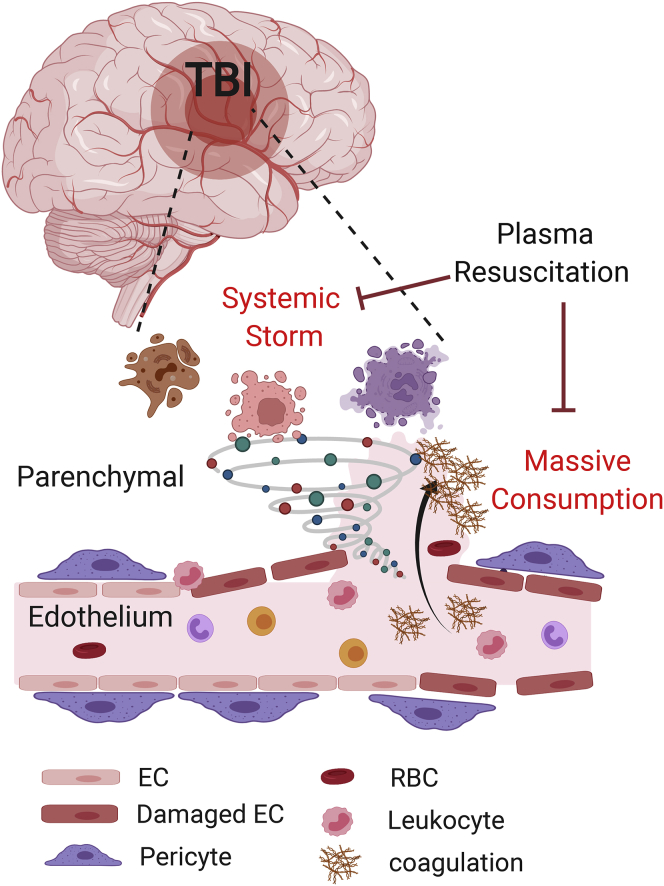
Figure 1.
Prehospital plasma resuscitation improves outcome in traumatic brain injury (TBI) patients
Critical trauma leads to a “Systemic Storm” caused by release of several cellular constituents including cytokines and metabolites into the circulation, and a “Massive Consumption” pattern associated with an increase in lipids, coagulation factors, and enzymes in the circulation. Prehospital plasma resuscitation in the PAMPer trial attenuated both the Systemic Storm and Massive Consumption patterns and improved survival in TBI patients with endothelial injuries. EC, endothelial cell; RBC, red blood cell.
The current findings have provided the needed empirical data on the benefit of plasma-based prehospital intervention for trauma. For the development of point-of-care diagnostics to identify the right endotype subgroup, the authors tested an array of serum biomarkers and found that the combination of a brain injury-specific marker UCHL1 (Ubiquitin C-terminal hydrolase L1), an endothelial injury marker sydencan 1, and inflammatory cytokines IL17A and interferon-inducible protein 10 (IP-10) offer the best predictive value based on 173 studied patients. Therefore, this biomarker panel may provide a practical tool to stratify trauma patients for effective early treatment.
Overall, the PAMPer study has provided an important evaluation of prehospital plasma resuscitation in the critically injured and demonstrated its efficacy in reducing mortality in TBI patients. This method provides a simple, practical, and likely effective approach that can be easily integrated into the existing prehospital trauma care systems throughout the world.9 The current study provided not only the two biological signatures, Systemic Storm and Massive Consumption, underlying the early pathophysiological changes upon critical injuries, but also identified a vascular endotype contributing to the outcomes of complex injuries. The selective responsiveness of TBI patients with endothelial injury to plasma administration is consistent with the notion that the blood brain barrier breakdown is likely a key component of its pathogenesis and that preserving the neurovascular function is critical.10 While the overall survival in hospitalized trauma patients has been improving, the total death toll is still rising in the US every year, which calls for more effective prevention of injuries and violence.
Acknowledgments
The work of B.V.Z. is supported by NIH grants R01AG023084, R01NS090904, R01NS034467, R01AG039452, 1R01NS100459, 5P01AG052350, and 5P50AG005142; Alzheimer’s Association strategic 509279 grant; Cure Alzheimer’s Fund; and the Foundation Leducq Transatlantic Network of Excellence grant 16 CVD 05. The work of Z.Z. is supported by NIH grants R01AG061288, R01NS110687, R03AG063287, R21AG066090 and RF1NS122060; BrightFocus Foundation grant A2019218S; and US Department of Defense grant W81XWH2010424.
Declaration of interests
The authors declare no competing interests.
Contributor Information
Zhen Zhao, Email: zzhao@usc.edu.
Berislav V. Zlokovic, Email: zlokovic@usc.edu.
References
- 1.Wu J., Vodovotz Y., Abdelhamid S., Guyete F.X., Yaffe M.B., Gruen D.S., Cyr A., Okonkwo D.O., Kar U.K., Krishnamoorthi N., et al. Multi-omic analysis in injured humans: Patterns align with outcomes and treatment responses. Cell Rep. Med. 2021;2 doi: 10.1016/j.xcrm.2021.100478. 100478-1–100478-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ahmad F.B., Anderson R.N. The Leading Causes of Death in the US for 2020. JAMA. 2021;325:1829–1830. doi: 10.1001/jama.2021.5469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Baker C.C., Oppenheimer L., Stephens B., Lewis F.R., Trunkey D.D. Epidemiology of trauma deaths. Am. J. Surg. 1980;140:144–150. doi: 10.1016/0002-9610(80)90431-6. [DOI] [PubMed] [Google Scholar]
- 4.Oyeniyi B.T., Fox E.E., Scerbo M., Tomasek J.S., Wade C.E., Holcomb J.B. Trends in 1029 trauma deaths at a level 1 trauma center: Impact of a bleeding control bundle of care. Injury. 2017;48:5–12. doi: 10.1016/j.injury.2016.10.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Sobrino J., Shafi S. Timing and causes of death after injuries. Proc. Bayl. Univ. Med. Cent. 2013;26:120–123. doi: 10.1080/08998280.2013.11928934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Brohi K., Gruen R.L., Holcomb J.B. Why are bleeding trauma patients still dying? Intensive Care Med. 2019;45:709–711. doi: 10.1007/s00134-019-05560-x. [DOI] [PubMed] [Google Scholar]
- 7.Sperry J.L., Guyette F.X., Brown J.B., Yazer M.H., Triulzi D.J., Early-Young B.J., Adams P.W., Daley B.J., Miller R.S., Harbrecht B.G., et al. PAMPer Study Group Prehospital Plasma during Air Medical Transport in Trauma Patients at Risk for Hemorrhagic Shock. N. Engl. J. Med. 2018;379:315–326. doi: 10.1056/NEJMoa1802345. [DOI] [PubMed] [Google Scholar]
- 8.Gruen D.S., Guyette F.X., Brown J.B., Okonkwo D.O., Puccio A.M., Campwala I.K., Tessmer M.T., Daley B.J., Miller R.S., Harbrecht B.G., et al. Association of Prehospital Plasma With Survival in Patients With Traumatic Brain Injury: A Secondary Analysis of the PAMPer Cluster Randomized Clinical Trial. JAMA Netw. Open. 2020;3:e2016869. doi: 10.1001/jamanetworkopen.2020.16869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.World Health Organization . 2005. Prehospital trauma care systems.https://www.who.int/publications/i/item/prehospital-trauma-care-systems [Google Scholar]
- 10.Sweeney M.D., Zhao Z., Montagne A., Nelson A.R., Zlokovic B.V. Blood-Brain Barrier: From Physiology to Disease and Back. Physiol. Rev. 2019;99:21–78. doi: 10.1152/physrev.00050.2017. [DOI] [PMC free article] [PubMed] [Google Scholar]