Abstract
The ability of the c-Myc oncoprotein to potentiate apoptosis has been well documented; however, the mechanism of action remains ill defined. We have previously identified spatially distinct apoptotic pathways within the same cell that are differentially inhibited by Bcl-2 targeted to either the mitochondria (Bcl-acta) or the endoplasmic reticulum (Bcl-cb5). We show here that in Rat1 cells expressing an exogenous c-myc allele, distinct apoptotic pathways can be inhibited by Bcl-2 or Bcl-acta yet be distinguished by their sensitivity to Bcl-cb5 as either susceptible (serum withdrawal, taxol, and ceramide) or refractory (etoposide and doxorubicin). Myc expression and apoptosis were universally associated with Bcl-acta and not Bcl-cb5, suggesting that Myc acts downstream at a point common to these distinct apoptotic signaling cascades. Analysis of Rat1 c-myc null cells shows these same death stimuli induce apoptosis with characteristic features of nuclear condensation, membrane blebbing, poly (ADP-ribose) polymerase cleavage, and DNA fragmentation; however, this Myc-independent apoptosis is not inhibited by Bcl-2. During apoptosis, Bax translocation to the mitochondria occurs in the presence or absence of Myc expression. Moreover, Bax mRNA and protein expression remain unchanged in the presence or absence of Myc. However, in the absence of Myc, Bax is not activated and cytochrome c is not released into the cytoplasm. Reintroduction of Myc into the c-myc null cells restores Bax activation, cytochrome c release, and inhibition of apoptosis by Bcl-2. These results demonstrate a role for Myc in the regulation of Bax activation during apoptosis. Moreover, apoptosis that can be triggered in the absence of Myc provides evidence that signaling pathways exist which circumvent Bax activation and cytochrome c release to trigger caspase activation. Thus, Myc increases the cellular competence to die by enhancing disparate apoptotic signals at a common mitochondrial amplification step involving Bax activation and cytochrome c release.
Among the known proto-oncogenes, the cellular myc gene (c-myc) is one of those most frequently implicated in carcinogenesis. Deregulated expression of the structurally unaltered Myc protein is sufficient to drive continuous cell proliferation and programmed cell death in response to growth-promoting and growth-inhibitory signals, respectively. As a regulator of gene transcription, Myc is thought to drive such disparate activities by controlling distinct subsets of target genes. The ability of Myc to trigger apoptosis is key to the control of tumor development. Apoptosis is thought to function as an intrinsic safety mechanism to limit the life of a cell that acquires deregulated c-myc expression, thus preventing further transformation. However, loss of this function through any additional mutation that prevents Myc from triggering apoptosis will promote survival and strongly cooperate with Myc to allow the continued proliferation, mutation, and carcinogenic evolution of the affected clone (4, 5, 12, 18, 19, 25, 27, 37, 47, 49).
The apoptotic process can be divided into three interdependent phases: induction, decision, and execution. For simplicity, inducers have been categorized as those that trigger apoptosis through death receptor activation (e.g., CD95 and tumor necrosis factor receptor) and those that stimulate apoptosis by a non-death receptor mechanisms (e.g., chemotherapeutic drugs, metabolic inhibitors, and withdrawal of survival factors). The decision phase is largely regulated by the Bcl-2 family of apoptotic regulators, including both pro- and antiapoptotic members. These molecules integrate a wide variety of signaling cascades and, through protein-protein interaction and subcellular localization, determine whether the balance of signals dictates that cell death will proceed or terminate. A hallmark of the execution phase is the activation of caspases and their substrates that essentially dissolve the normal structure and function of a cell while compartmentalizing the remains for noninflammatory clearance and engulfment. Another feature that is sufficient, but not essential, for cell death is the collapse of mitochondrial membrane integrity. The release of mitochondrial constituents such as cytochrome c stimulates a potent execution program involving formation of the apoptosome, activation of downstream caspases, and the subsequent demise of the cell (21, 24, 35, 43).
The capacity of Myc to drive apoptosis was first established under growth-limiting conditions where its deregulated expression was uncoupled from growth factor controls (5, 18). Following growth and survival factor withdrawal, sustained c-myc expression triggered cells to undergo apoptosis. In addition, an essential role for Myc in T-cell receptor activation-induced apoptosis has been demonstrated in studies using antisense oligonucleotides (52). Myc is also a critical component of the death signal initiated by tumor necrosis factor and enhances the magnitude of response to stimulation of the CD95/Fas death receptor (15, 28, 29, 34). In addition to triggering apoptosis during growth factor deprivation, Myc has also been shown to enhance apoptosis induced by hypoxia, glucose deprivation, heat shock, chemotoxins, DNA damage, and cancer therapeutics (3, 26, 53, 56). Thus, Myc activation can sensitize cells to a wide variety of mechanistically distinct antiproliferative stimuli.
The role of Myc in potentiating apoptosis has led to several models for the mechanism of action (10, 17, 47, 55). One well-supported model suggests that Myc acts at a common node in the regulatory and effector machinery of apoptosis. In this dual-signal model, Myc alone is not sufficient to induce apoptosis but rather sensitizes cells to apoptotic stimuli that are insufficient or only weakly elicit the full death response. This model is also in agreement with results showing that Myc is essential for the induction of apoptosis by sublethal doses of cytotoxic agents or a block to cell proliferation but is not required in cases where the apoptotic stimulus is independently potent and sufficient to trigger apoptosis. It has been reported that Myc can induce the release of cytochrome c from the mitochondria (8, 30). However, neither the mechanism by which Myc elicits this response nor the precise step in the pathway activated by Myc has been identified. Thus, Myc is able to act as a sensitizer to numerous disparate triggers of apoptosis, but the mechanism remains unclear.
One of the most potent Myc-cooperating oncoproteins is Bcl-2, which functions as a global inhibitor of apoptosis, likely through multiple mechanisms (2, 24, 48). Endogenous Bcl-2 is expressed at both the outer mitochondrial membrane and the endoplasmic reticulum (ER) which is contiguous with the nuclear membrane. We have previously shown that apoptosis induced by serum withdrawal in the presence of ectopically expressed Myc can be suppressed equally well by Bcl-2 located at either the mitochondria or ER. By contrast, apoptosis induced by the apoptotic agonist etoposide is inhibited by Bcl-2 targeted specifically to the mitochondria but not the ER. These results show that by assessing the differential protection by organelle-targeted Bcl-2, we can distinguish spatially distinct apoptotic pathways triggered within the same cell. Moreover, these observations raised an important question as to whether the additional ER apoptotic pathway is Myc specific, or whether it is part of the overall apoptotic pathway initiated by serum withdrawal. To investigate whether Myc drives an ER-specific apoptotic pathway, we have examined apoptosis triggered by a variety of stimuli in the presence and absence of Myc, using organelle-specific Bcl-2.
Previous work aimed at addressing the role of Myc in apoptosis has been confounded by the lack of a cell system in which to study apoptosis in the complete absence of Myc. To date, most of the work done in this area has made use of an inducible chimeric MycER protein in the presence of endogenous Myc (references 10, 19, and 47 references therein). Here, we report the first use of c-myc null cells to determine the rate-limiting step controlled by Myc in apoptosis.
MATERIALS AND METHODS
Reagents.
Etoposide, cisplatin, doxorubicin, and taxol (paclitaxol) were purchased from Sigma. C2-ceramide was purchased from Calbiochem Pharmaceuticals.
Cell culture and cell lines.
TGR-1 and HO15.19 Rat1 fibroblasts have been previously described (38). HOMyc3 cells were kindly provided by M. Cole and have also been previously described (7). The targeted Bcl-2 mutants were constructed as described in reference (58). cDNAs encoding the different Bcl-2 constructs were subcloned into the retroviral vector pMNiresGFP, which was used to transfect the retroviral packaging cell line Phoenix-Eco. Viral supernatants were used to infect HO15.19 and HOMyc3 cells in the presence of Polybrene (8 μg/ml; Sigma) for 18 h. Cells successfully infected were selected by fluorescence-activated cells sorting to collect cells expressing the green fluorescent protein from the internal ribosomal entry sequence within the provirus of infected cells. A minimum of 250,000 cells were collected to constitute final pools of cells expressing the selected constructs. All cell lines were maintained in Dulbecco's H21 medium supplemented with 10% calf serum.
Cell growth and death assays.
DNA fragmentation was detected by cell death detection enzyme-linked immunosorbent assay (ELISA) (Roche Biochemicals), which was performed according to the manufacturer's instructions. Briefly, cells were seeded as subconfluent monolayers onto 60-mm-diameter tissue culture dishes and allowed to settle overnight. The following day, medium was replaced with fresh medium containing apoptotic agonist or vehicle control, and cells were further incubated at 37°C and 5% CO2 until time of assay. Cells were collected, and identical aliquots were further analyzed: one aliquot for cell death detection by ELISA, and a second aliquot that was used to derive cell counts. ELISA readings were normalized according to the cell number tested, and at least 10,000 cells were assayed from duplicate samples in each case. All experiments were done in duplicate and were repeated independently at least twice. Statistical analysis was conducted using the f test to determine whether the data curves for the Bcl-2-expressing cells differed significantly from those derived for control cells. Data curves for cells expressing the mutant Bcl-2 proteins were also compared to those for cells expressing the wild-type version of the protein. Curves were considered significantly different only when P were less than 0.05.
To measure the growth rates of HOMyc3 and HO15.19 cells expressing either empty vector or Bcl-2, 150,000 (HOMyc3) or 53,000 (HO15.19) cells were seeded per well onto six-well dishes. Adherent and nonadherent cells were then collected at the indicated time points and analyzed for cell viability by trypan blue exclusion. Duplicate samples were each counted three times independently to derive standard deviations for each time point.
Immunoblotting.
Subconfluent, growing cells were lysed in sample buffer (10% glycerol, 1% sodium dodecyl sulfate, 30 mM Tris-HCl [pH 6.8]), separated by sodium dodecyl sulfate-polyacrylamide gel electrophoresis, and then transferred to nitrocellulose membranes for subsequent immunodetection. Affinity-purified antibodies to rat Bcl-2, human Bcl-2 (Stan), and Bax (αIDI) (Exalpha Biologicals) were used at dilutions of 1:1,000, 1:10,000, and 1:5,000, respectively, anti-β-actin antibodies (Sigma) were used at a dilution of 1:10,000, affinity-purified anti-cytochrome c antibodies (Exalpha Biologicals) were used at a dilution of 1:5,000, and anti-poly (ADP-ribose) polymerase (PARP) antibodies (BioMol) were used at a dilution of 1:10,000. Blots were developed after incubation with peroxidase-conjugated secondary antibodies (Amersham), using the Renaissance enhanced chemiluminescence detection system (Mandel).
Cell fractionation.
Cells were seeded as subconfluent monolayers onto 100-mm-diameter tissue culture dishes and were treated as above for the ELISA cell death assay. At the indicated time points, cells were harvested using a rubber policeman and then pelleted by centrifugation in a clinical centrifuge for 3 min at 4°C. The cell pellet was washed twice with the cell buffer (250 mM sucrose, 20 mM HEPES [pH 7.5], 2 mM MgCl2, 1 mM sodium EDTA, 1 mM phenylmethylsulfonyl fluoride, 1 mM dithiothreitol, cocktail of protease inhibitors). The final pellet was resuspended in an equal volume of buffer suspension and held at 150 lb/in2 for 15 min on ice in a 45-ml nitrogen bomb (Parr Instruments), and cells were disrupted by releasing pressure. The nuclei and cell debris in the expelled lysate were removed by centrifugation at 500 × g for 2 min at 4°C. The resulting supernatant, termed whole-cell lysate, was then split into a cytosolic (S100) and pellet (P100) fraction by centrifugation at 100,000 × g for 1 h in Beckman TLA100 rotor at 4°C. Protein samples were then snap-frozen in liquid nitrogen and stored at −80°C. Densitometry was performed by phosphorimager analysis using Image Quant software.
Immunofluorescence.
Cells were seeded as subconfluent monolayers onto glass coverslips and treated as above for the ELISA cell death assay. Cells were fixed with 4% paraformaldehyde, permeabilized with 0.2% 3-[(3-cholamidopropyl)-dimethylammonio]-1-propanesulfonate (CHAPS) in phosphate-buffered saline, and processed conventionally for immunofluorescence. Cells were costained with the conformation-specific Bax antibody 6A7 (1:50 dilution; Exalpha Biologicals) and affinity-purified cytochrome c antibodies (1:750 dilution; Exalpha Biologicals). Cells stained with the 6A7 antibody were counted by two independent observers, using identical stored images of fields of cells generated with Zeiss LSM Image Browser software on the Carl Zeiss LSCM 510 system. The agreement between the two observers for the different conditions varied from 0 to 4.8% (median, 0.9%). Mitotracker staining was performed as described elsewhere (4a). Briefly, after exposure to drug, cells were treated with 150 nM Mitoracker (Molecular Probes). After incubation for 15 min in the dark, fresh medium was added and the cells were incubated for an additional 15 min in the dark. These samples were then processed for immunofluorescence of activated Bax, using 6A7 antibody as described above. For competition experiments, purified 6A7 antibody was incubated in 3% BSA bovine serum albumin–phosphate-buffered saline containing either 5 μg of purified Bax glutathione S-transferase (GST), 5 μg of GST–Bcl-2, or an equal volume of buffer at room temperature for 1 h prior to staining. This antibody was then used for immunofluorescence as described above.
Bax-GST is a carboxyl-terminal fusion of GST to amino acids 1 to 230 of Bax (plasmid pMAC1045). Gst-Bcl-2 is an amino-terminal fusion of GST to amino acids 1 to 187 of Bcl-2 (plasmid pMAC482). Both proteins were expressed in bacteria under the control of tac promoter and purified on glutathione-Sepharose 4B (Amersham Pharmacia Biotech) according to the manufacturer's protocol.
RESULTS
To compare signaling events and apoptotic pathways induced by different antiproliferative agents in the presence or absence of Myc, we used HO15.19 and HOMyc3 cell lines. HO15.19 cells are derived from an immortalized Rat1 fibroblast cell line, TGR-1, in which both alleles of the c-myc gene have been knocked out by homologous recombination (38); HOMyc3 cells are a c-myc null cell line in which an exogenous c-myc allele was stably introduced by retroviral infection (7). Retrovirus-containing human Bcl-2 constructs in which the C-terminal insertion sequence of the native protein has been replaced with the C-terminal insertion sequences of proteins expressed at either the mitochondria or the ER (as described previously [58]) were used to infect the TGR-1, HO15.19, and HOMyc3 cells. Selection of these infected cells resulted in the generation of pooled cell populations expressing empty vector, wild-type Bcl-2, Bcl-acta (targeted to the mitochondria), or Bcl-cb5 (targeted to the ER). These cells permit the evaluation of signaling events and apoptotic pathways induced by different agents in the presence or absence of Myc in the same genetic background.
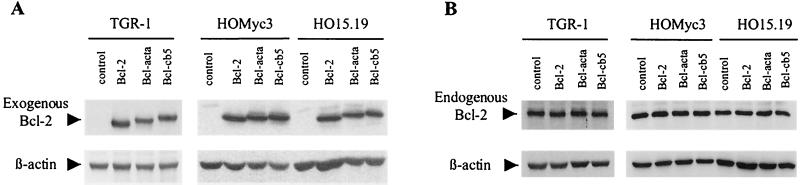
To verify and compare levels of Bcl-2 protein in the stably infected TGR-1, HO15.19, and HOMyc3 cell lines, protein extracts from subconfluently growing cells of each population were isolated for immunoblot analysis using an antibody specific to the exogenous human Bcl-2 protein (Fig. 1A) and endogenous rat Bcl-2 protein (Fig. 1B) as well as β-actin as a loading control. Immunoblot analysis showed that levels of relative ectopic Bcl-2 protein in each cell line were comparable and that the levels of the endogenous protein were not significantly different in any of the derived cell lines. Immunofluorescence and confocal microscopy demonstrated that the different Bcl-2 variants were correctly targeted in these cells as previously described (58) (data not shown).
FIG. 1.
Expression of targeted Bcl-2 protein in c-myc null and c-myc-reconstituted cell lines. HO15.19 and HOMyc3 cells were stably infected with control retrovirus or retrovirus carrying Bcl-2, Bcl-acta, or Bcl-cb5. Protein extracts from pooled cell populations (10 μg/lane) were analyzed by immunoblotting for ectopic human Bcl-2 (A) and endogenous rat Bcl-2 (B) expression. Blots were also probed for β-actin as a control for equal protein loading of lanes.
Spatially distinct apoptotic pathways are triggered in the presence of ectopic Myc.
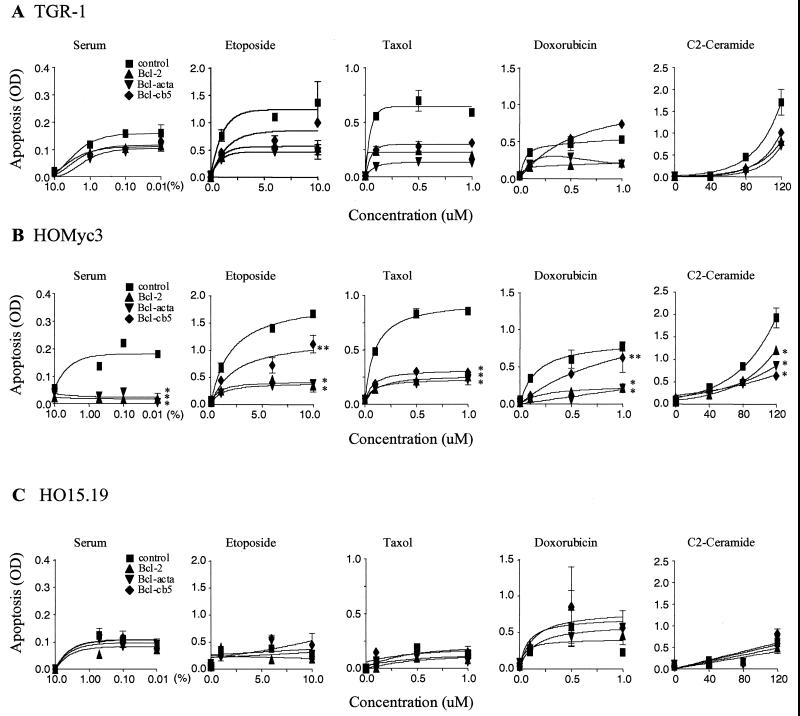
To screen a number of apoptotic agents for their antiproliferative effect on Rat1 fibroblast cells, we used the reduction of the tetrazolium salt 3-[4,5-dimethylthiazol-2-yl]-2,5-diphenyltetrazolium bromide (MTT assay). Five disparate apoptotic agonists (etoposide, taxol, ceramide, doxorubicin, and serum withdrawal) were chosen for further study, as they induced dose-dependent death responses that were inhibited by Bcl-2 in the Rat1 fibroblasts (data not shown). We then measured apoptosis using an ELISA for cellular DNA fragmentation in TGR-1, HO15.19, and HOMyc3 cell lines expressing the targeted Bcl-2 constructs after exposure to low, intermediate, or high doses of these stimuli.
After one cell cycle, both the TGR-1 and the c-myc-reconstituted cells (HOMyc3) underwent apoptosis in a dose-dependent manner that was inhibitable by Bcl-2 (Fig. 2A and B). Moreover, apoptosis was differentially inhibited by Bcl-2 targeted to specific subcellular locations. Consistent with our previous results, both Bcl-acta and Bcl-cb5, as well as Bcl-2, abrogated apoptosis induced upon serum withdrawal with equal efficacy. Interestingly, only Bcl-2 and Bcl-acta efficiently inhibited etoposide-induced apoptosis. Doxorubicin-induced apoptosis, like that induced by etoposide, was not inhibited by Bcl-cb5 yet was protected by Bcl-2 and Bcl-acta. By contrast, taxol- and ceramide-induced death was inhibited by Bcl-cb5 as well as Bcl-2 and Bcl-acta. As expected, HOMyc3 cells, which express an activated c-myc allele, showed higher levels of DNA fragmentation than the TGR-1 parental cells upon exposure to the same concentrations of apoptotic stimuli. However, the pattern of Bcl-2 protection did not alter despite higher levels of apoptosis in the presence of deregulated Myc expression. From these data, spatially distinct apoptotic pathways can be characterized as either Bcl-cb5 refractory or Bcl-cb5 inhibited, both of which can be potentiated by Myc. Moreover, Myc is not universally associated with an ER-specific apoptotic pathway (Fig. 2B). Interestingly, Bcl-acta was effective against all stimuli tested, suggesting a convergence point at the mitochondria for apoptotic signaling in the presence of Myc.
FIG. 2.
Distinct apoptotic pathways are triggered in the presence of Myc. TGR-1 (A), HOMyc3 (B), and HO15.19 (C) cells expressing empty vector control, Bcl-2, Bcl-acta, or Bcl-cb5 were exposed to a concentration range of serum, etoposide, taxol, doxorubin, and ceramide for one cell cycle (22 h for TGR-1 cells, 18 h for HOMyc3 cells, and 54 h for HO15.19 cells). Apoptosis was measured (optical density [OD]) by quantitation of DNA fragmentation. Statistical analysis of HOMyc3 data shows that the curves indicated by ∗ are significantly different from control curves (P < 0.001) and that curves indicated by ∗∗ are significantly different from Bcl-2 curves (P < 0.001). In the case of HO15.19 cells, statistical analysis shows that Bcl-2, Bcl-acta, and Bcl-cb5 curves are not significantly different from control curves in response to any of the above agonists (P > 0.05).
To determine whether Myc was critical to any one apoptotic pathway, and to further delineate the role of Myc in apoptosis induced by different agonists, we induced apoptosis in the c-myc null cells (HO15.19) expressing the targeted Bcl-2 constructs. HO15.19 cells were exposed to the same stimuli used above but for 54 h, to accommodate one cell cycle in these more slowly dividing cells (38). The levels of apoptosis observed in vector control cells or in cells expressing Bcl-2, Bcl-acta, or Bcl-cb5 in response to different agonists were indistinguishable and not statistically different (Fig. 2C). These results suggested that the extent of apoptosis induced by these stimuli after one cell cycle in HO15.19 cells may not have been sufficient to distinguish the protective effects of Bcl-2. To achieve levels of apoptosis which were comparable to the death seen in HOMyc3 cells, we assayed for apoptosis in HO15.19 cells over an extended time course.
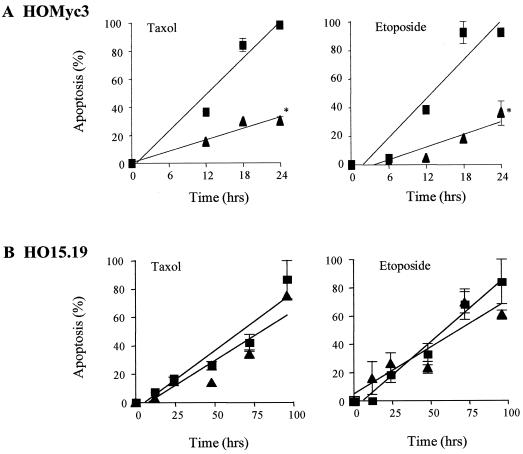
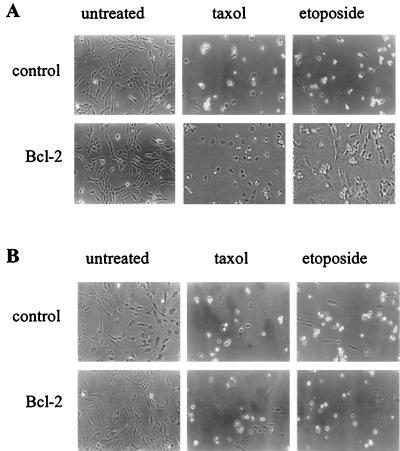
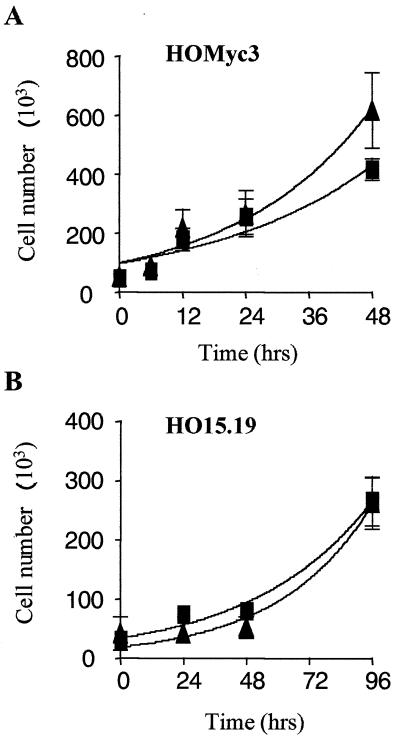
Time course experiments were performed using intermediate doses of either etoposide or taxol (Fig. 3), and apoptosis was further visualized and quantified by photomicrography (Fig. 4) and time-lapse cinematography as well as trypan blue exclusion (data not shown). Etoposide and taxol were used as examples of Bcl-cb5-refractory and -susceptible pathways, respectively. Upon exposure of HOMyc3 cells to either etoposide or taxol over an extended time course, maximum detectable apoptosis was achieved after 24 h. However, Bcl-2 was able to suppress apoptosis by approximately 50% (Fig. 3A). When HO15.19 cells were exposed to the same concentrations of taxol or etoposide, the levels of apoptosis at 96 h were equivalent to levels seen in the HOMyc3 control cells at 18 h (Fig. 3 and 4). However, statistical analysis of the kinetic curves showed no significant difference between the response of the HO15.19 control or HO15.19 Bcl-2 cells to either agonist (Fig. 3B). By contrast, concomitant analysis shows that ectopic Bcl-2 expression does not affect cell growth of either HO15.19 or HOMyc3 cells (Fig. 4 and 5). Thus, an indirect affect on cell growth could not account for the lack of Bcl-2 inhibition in HO15.19 cells induced to undergo apoptosis.
FIG. 3.
Bcl-2 can inhibit apoptosis in the presence, but not in the absence, of Myc expression. HOMyc3 (A) and HO15.19 (B) cells expressing either empty vector control (■) or Bcl-2 (▴) were exposed to 0.5 μM taxol or 6 μM etoposide. Cells were harvested at indicated time points and apoptosis was analyzed by DNA fragmentation. All samples were tested in duplicate, and each time course experiment was repeated at least twice with similar results. Statistical analysis shows that the curves indicated by ∗ are significantly different from control curves (P < 0.001).
FIG. 4.
Equivalent cell death occurs in c-myc null and c-myc-reconstituted cells exposed to etoposide or taxol, yet Bcl-2 confers survival only in the presence of Myc. (A) HOMyc3 cells expressing empty vector (control) or Bcl-2 were left untreated or exposed to 0.5 μM taxol or 6 μM etoposide and photographed at 18 h using in a phase-contrast microscope. (B) HO15.19 cells expressing empty vector (control) or Bcl-2 were treated with identical doses of taxol or etoposide and photographed under similar conditions at 96 h.
FIG. 5.
Ectopic Bcl-2 expression does not affect cell growth of either HO15.19 or HOMyc3 cells. HOMyc3 (A) and HO15.19 (B) cells expressing either empty vector control (■) or Bcl-2 (▴) were seeded subconfluently, and the total number of live cells was assessed at the indicated time points by trypan blue exclusion. Error bars indicate the standard deviation calculated for each time point (see Materials and Methods).
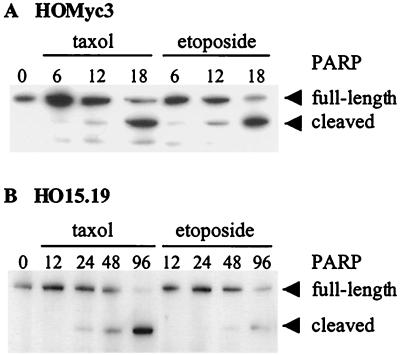
To confirm the apoptotic nature of the cell death occurring in the c-myc null and c-myc-reconstituted cells by an independent assay, we assayed for cleavage of the caspase substrate PARP in response to either taxol or etoposide. PARP cleavage was clearly observed at 12 h after exposure to either agonist in HOMyc3 cells and after 48 h exposure to taxol or etoposide in HO15.19 cells (Fig. 6). These results are consistent with time points at which DNA fragmentation was also observed in these cells by ELISA. As an additional measure of apoptosis, cells that had been exposed to an intermediate dose of each agonist were fixed and stained with 4′, 6′-diamidine-2-phenylindole dihydrochloride (DAPI). In all cases, the treated cells exhibited typical apoptotic morphologies: condensed chromatin, membrane blebbing, and nuclear fragmentation (data not shown). Therefore, in cells with ectopic Myc expression, mechanistically different apoptotic stimuli induce a rapid cell death by pathways that are spatially distinct and inhibited by Bcl-2. By contrast, in c-myc null cells, these agents elicit apoptosis, but cell death occurs more slowly and is not inhibitable by Bcl-2. As Bcl-2 is not effective, we cannot determine if these pathways remain spatially distinct in the absence of Myc.
FIG. 6.
PARP cleavage in response to etoposide or taxol in c-myc null and c-myc-reconstituted cells. HOMyc3 (A) and HO15.19 (B) cells were exposed to either 0.5 μM taxol or 6 μM etoposide, and protein was isolated at the indicated time points. PARP cleavage was detected by immunoblot analysis using a PARP-specific antibody. PARP cleavage is detected by the appearance of the smaller cleaved fragment and a relative decrease in full-length protein in cells undergoing apoptosis.
Myc is required for release of cytochrome c from mitochondria during apoptosis.
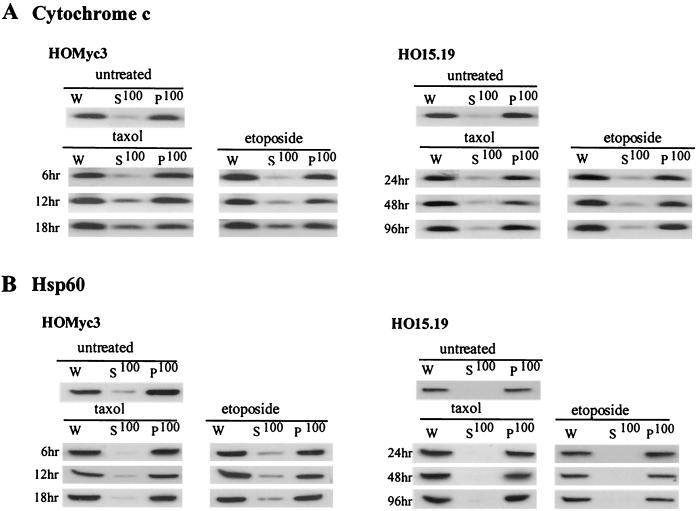
When Myc is expressed, Bcl-2 targeted specifically to the outer mitochondrial membrane is able to inhibit apoptosis induced by all agonists tested. The mitochondria are key regulators and amplifiers of the apoptotic response, and the release of cytochrome c from the mitochondria is a feature of apoptosis induced by many stimuli (22). Cytosolic cytochrome c plays a central role as a component of the apoptosome and forms a proapoptotic complex with Apaf-1 and caspase 9 to stimulate the effector caspase cascade. Moreover, in Rat1 fibroblasts with inducible ectopic expression of MycER, the release of cytochrome c is increased after exposure to several different apoptotic stimuli (30). To determine whether differences in this mediator of apoptosis were evident in HOMyc3 and HO15.19 cells, we investigated the release of cytochrome c by subcellular fractionation of cells disrupted by low-pressure nitrogen cavitation. This method offers benefits over other homogenization procedures, as cells lysed by cavitation in iso-osmotic buffer retain intact outer mitochondrial membranes (1). The cytosolic and membrane fractions (S100 and P100, respectively) from HOMyc3 and HO15.19 cells were analyzed by immunoblotting with affinity-purified antibodies to cytochrome c to assess the extent of cytochrome c release and with antibodies to Hsp60 as a mitochondrial marker and an indicator of the integrity of the inner mitochondrial membrane (Fig. 7). In untreated cells, cytochrome c was found in the membrane fraction, whereas after exposure of c-myc-reconstituted cells (HOMyc3) to either etoposide or taxol, cytochrome c was detected in the cytosolic fractions as early as 6 h after drug addition. However, in parallel experiments performed in the c-myc null cells (HO15.19), cytochrome c remained in the membrane fraction and was not detected in the cytosolic fractions even at later time points when PARP cleavage and DNA fragmentation were clearly detectable (Fig. 7A). Therefore, we have established that Myc expression is essential for the release of cytochrome c during apoptosis triggered by disparate apoptotic stimuli, consistent with previous reports that the induction of MycER enhances cytochrome c release by these agents (8, 30, 32).
FIG. 7.
Myc is essential for cytochrome c release. HOMyc3 and HO15.19 cells were exposed to either 0.5 μM taxol or 6 μM etoposide for the indicated times, and then cell lysates were prepared by nitrogen cavitation. W, whole-cell lysate after cavitation and removal of cell debris. Cytosolic proteins are in the supernatant fraction (S100), and membrane-associated proteins including mitochondrial proteins are in the pellet fraction (P100). Ten micrograms of whole-cell lysate and equivalent volumes of each fraction were analyzed by immunoblotting for cytochrome c (A) and Hsp60 (B).
Myc is not required for Bax translocation to membranes during apoptosis.
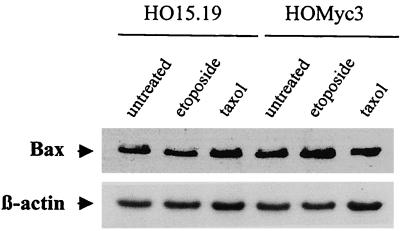
Although a role has been previously ascribed to Myc in the release of cytochrome c, no clues about mechanism have been forthcoming. Another critical feature of many apoptotic pathways previously linked to cytochrome c release is the translocation of Bax from the cytoplasm to the mitochondria, where it becomes inserted into the outer membrane (23, 33). It has been suggested that Myc may directly regulate Bax expression through binding to E-box elements in the promoter region of the Bax gene (40, 41). To address whether Myc was influencing mitochondrial events during apoptosis through a direct regulation of this proapoptotic molecule, total RNA was extracted from asynchronously growing cells, and the relative amount of Bax mRNA was determined. No significant differences in Bax transcript levels were detected in either the c-myc-null or c-myc-reconstituted cell lines compared to the parental TGR-1 cells (data not shown). Next, to examine whether Myc played a role in the up-regulation of Bax protein expression during apoptosis, the relative levels of Bax protein were compared in HO15.19 and HOMyc3 cells exposed to either etoposide or taxol. No differences in Bax protein expression were detected in either the presence or absence of Myc (Fig. 8). Together, these data suggest that Myc expression does not alter the expression profile of Bax during growth or apoptosis in these cells.
FIG. 8.
Myc expression does not alter the expression profile of Bax during growth or apoptosis. HOMyc3 and HO15.19 cells were exposed to either 6 μM etoposide or 0.5 μM taxol, and protein was isolated at 18 h (HOMyc3) or 96 h (HO15.19). Bax was detected by immunoblot analysis using a Bax-specific antibody. Blots were also probed for β-actin as a control for equal protein loading between lanes.
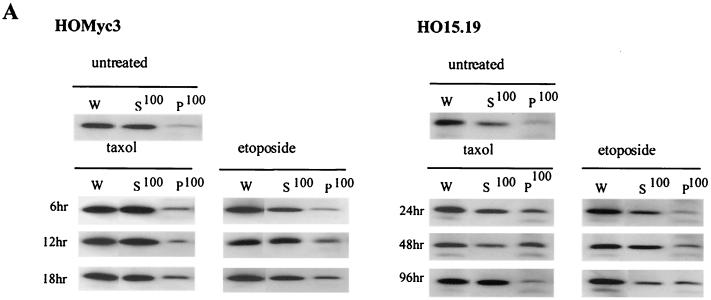
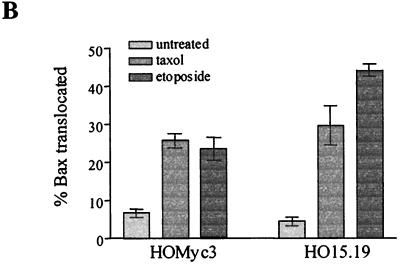
Translocation of Bax to the mitochondria and subsequent membrane insertion has been reported to induce cytochrome c release by the creation of a membrane pore or by altering the normal function of other preexisting mitochondrial pores (6, 16, 46). To determine whether the persistence of cytochrome c in mitochondria during apoptosis in c-myc null cells was due to a defect in Bax translocation, membrane and cytosolic protein fractions, as described for Fig. 7, were probed with a Bax-specific antibody (Fig. 9A). Although Bax was clearly in the cytosolic fractions of both untreated HO15.19 and HOMyc3 cells, after exposure to either etoposide or taxol, Bax was detectable in the membrane fractions of both cell types, indicating Bax translocation to membranes of both cell types in response to apoptotic agonists. The percentage of total Bax which relocalized to the membrane fraction during apoptosis was determined by densitometry of data from two independent experiments and shown to be on average 25.6 and 34.4% in HOMyc3 cells and 39.5 and 40.1% in HO15.19 cells in response to taxol and etoposide (Fig. 9B). Therefore, regardless of the Myc status in the cell, Bax is translocated to the mitochondria in response to different apoptotic agonists. Nevertheless, cytochrome c is released only in cells expressing exogenous Myc (Fig. 7A). This result suggests that Myc contributes to cytochrome c release downstream of Bax translocation.
FIG. 9.
Myc is not required for translocation of Bax to membranes. (A) HOMyc3 and HO15.19 cells were exposed to either 0.5 μM taxol or 6 μM etoposide for the indicated times, and then cell lysates were prepared by nitrogen cavitation as described for Fig. 7. Ten micrograms of whole-cell lysate (W) and equivalent volumes of each fraction were analyzed by immunoblotting for Bax. (B) Immunoblots as in panel A were analyzed by densitometry, and the percentage of total Bax localized to the P100 membrane fraction was calculated for HOMyc3 and HO15.19 cells either untreated or exposed to 0.5 μM taxol or 6 μM etoposide for 18 h (HOMyc3) or 96 h (HO15.19). Error bars indicated the range between two independent experiments.
Myc is required for activation of Bax.
Previous reports have suggested that translocation to the mitochondria and activation of Bax are regulated by a change in conformation that allows membrane integration via its insertion sequence (14, 23). The change in Bax conformation necessary for activation is detectable using the conformation-specific antibody 6A7 (42). Our observation in HO15.19 cells that cytochrome c release does not occur, despite translocation of Bax to mitochondria, demonstrates that translocation of Bax may not be synonymous with its activation. To determine if the latter process was regulated by Myc expression, we assessed Bax activation in HOMyc3 and HO15.19 cells after exposure to etoposide or taxol, using the 6A7 antibody, by confocal microscopy (Fig. 10). In addition, cells were costained with antibodies to cytochrome c to assess its release into the cytoplasm. To use the 6A7 monoclonal antibody to assess Bax conformation, it is necessary to permeabilize cells with CHAPS, as other detergents induce conformational changes in Bax that expose the 6A7 epitope. When cells are permeabilized with CHAPS, staining of cytochrome c in the cytosol is intense and clearly evident, while staining of cytochrome c in mitochondria is less intense. This is likely a consequence of inefficient permeabilization of mitochondria by CHAPS, as equivalent intensity of cytochrome c staining is evident in both the mitochondria and cytosol with other detergents (data not shown). Thus, permeabilization with CHAPS and subsequent staining allows for the simultaneous detection of Bax activation and cytochrome c release into the cytoplasm.
FIG. 10.
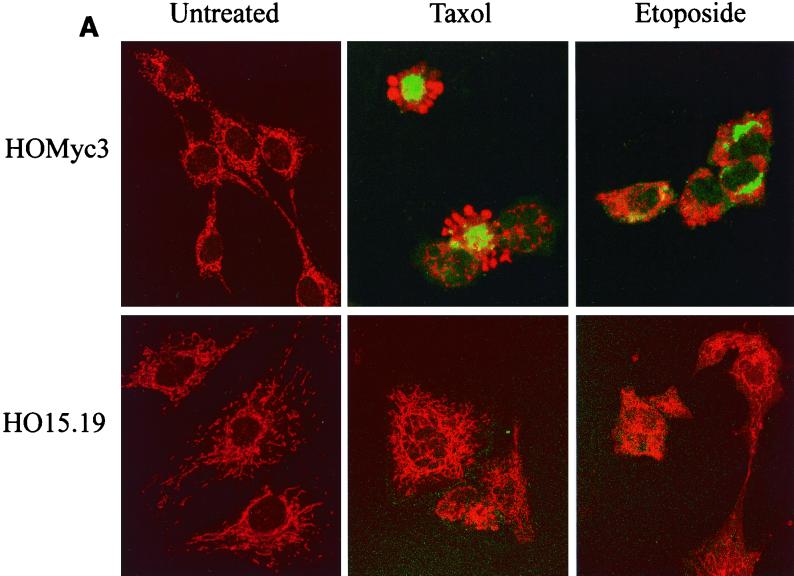
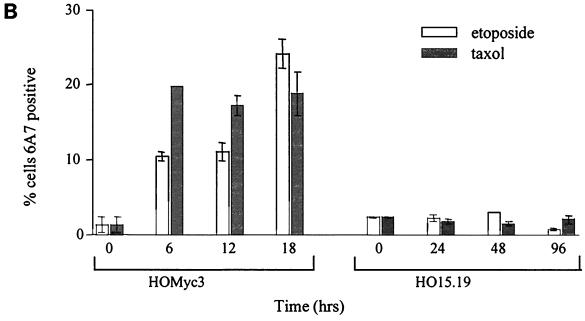
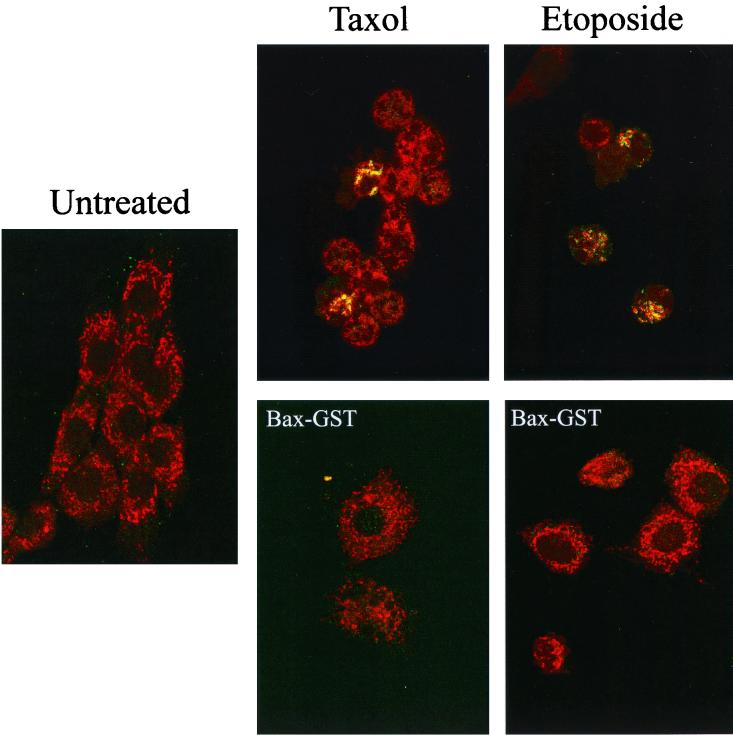
Bax is not activated in the absence of Myc. (A) HOMyc3 and HO15.19 cells were either left untreated or exposed to 0.5 μM taxol or 6 μM etoposide for 12 h (HOMyc3) or 48 h (HO15.19). The staining pattern of cytochrome c (red) and presence of activated Bax detected by the 6A7 antibody (green) were assessed by immunofluorescence and laser scanning confocal microscopy. All fields have been magnified similarly. Original confocal images can be viewed at http://www.science.mcmaster.ca/biochem/faculty/andrews/lab/index.html. (B) A minimum of three fields of 40 cells each were counted independently by two different observers for each of the indicated conditions and time points to determine the percentage of cells stained by 6A7. Error bars indicate the range between the two observers.
After exposure to either drug, a proportion of HOMyc3 cells were 6A7 positive, and only these cells showed evidence of cytochrome c release (Fig. 10A). The number of 6A7-positive cells was substantially greater in HOMyc3 cells exposed to either etoposide or taxol compared to untreated controls (Fig. 10B). At 18 h, 18 and 24% of HOMyc3 cells were 6A7 positive in response to taxol and etoposide, respectively. As this analysis can be conducted only on cells that are still adherent, and many of the HOMyc3 cells have detached from the dish at this time, these figures are relatively low estimates of proportion of cells with activated Bax. By contrast, we were we not able to detect 6A7-positive HO15.19 cells above background in taxol- or etoposide-treated cells, nor did we observe cytochrome c release in the HO15.19 cells, consistent with results obtained after cell fractionation and immunoblotting (Fig. 7A). Similar experiments using the 6A7 antibody in combination with Mitotracker, a mitochondrion-specific fluorescent dye, showed localization of activated Bax at the mitochondria (Fig. 11, top). The specificity of the 6A7-activated Bax interaction was confirmed by competition experiments with active-conformation Bax-GST fusion protein (Fig. 11, bottom). Pretreatment of 6A7 antibody with recombinant Bax-GST fusion protein was able to overide endogenous activated Bax-6A7 interactions. By contrast, recombinant GST–Bcl-2 was unable to bind and compete with 6A7 antibody for interaction with activated endogenous Bax protein (data not shown). We can conclude that 6A7 binds specifically to activated Bax in these cells. Taken together, these results show that Myc expression is necessary for the activation of Bax during apoptosis but is not required for the translocation of Bax to the mitochondria. Moreover, Bax translocation occurs prior to and is independent of the conformational change detected by the 6A7 antibody.
FIG. 11.
Activated Bax is localized at the mitochondria. HOMyc3 cells were either left untreated or exposed to 0.5 μM taxol or 6 μM etoposide for 18 h. The staining pattern of Mitotracker (red) and presence of activated Bax detected by the 6A7 antibody (green) were assessed by immunofluorescence and laser scanning confocal microscopy. All fields have been magnified similarly. Competition experiments were performed under similar conditions with the addition of 5 μg of recombinant Bax-GST fusion proteins in conjunction with 6A7 antibody (bottom) to verify the specificity of the activated Bax-6A7 interaction in these cells (see Materials and Methods).
DISCUSSION
First, our data demonstrate that spatially distinct apoptotic pathways triggered by a variety of stimuli can be inhibited by Bcl-2 and Bcl-acta and can be further characterized as susceptible or refractory to inhibition by Bcl-cb5. Apoptotic cascades stimulated by serum withdrawal, taxol, and C2-ceramide can be blocked by Bcl-cb5. By contrast, apoptosis in response to etoposide and doxorubicin stimulates a Bcl-cb5-refractory pathway. Interestingly, upon constitutive expression of Myc, apoptosis is potentiated, as expected, yet the pattern of Bcl-cb5 protection does not alter. As the activity of Bcl-cb5 does not partition with ectopic Myc expression in a global manner, Myc does not universally drive an apoptotic pathway that is associated with the ER. Consistent with these results, we did not see differences in ER-regulated pH or calcium concentrations in the presence or absence of Myc expression (S. Grinstein, K. Szaszi, E. L. Soucie, and L. Z. Penn, unpublished data). While the mechanisms of ER-regulated apoptosis and inhibition by Bcl-2 at this subcellular location are of interest, our data suggest that the role of Myc in apoptosis lies downstream at a position more central to both Bcl-cb5-sensitive and -refractory pathways.
Our analysis with the Bcl-2-targeted molecules clearly showed that apoptosis triggered in the presence of Myc expression by all agonists tested was universally inhibited by Bcl-acta, suggesting that Myc plays a role in the regulation of apoptosis at the level of the mitochondria. This is consistent with accumulating evidence suggesting that Myc action is focused at a common mitochondrial signaling element of many apoptotic pathways. Indeed, we show that Myc is essential for cytochrome c release from mitochondria in response to mechanistically distinct chemotherapeutic agents. These observations extend recent reports indicating that induction of MycER potentiates the release of cytochrome c following serum deprivation (8, 30, 32). Moreover, our analysis of the upstream processes that lead to cytochrome c release reveals that Bax translocation and activation are uncoupled, and the latter is dependent on Myc expression. To date, Bax translocation has been synonymous with activation; however, these events can be independently regulated, and Myc is required for Bax activation in conjunction with an apoptotic stimulus.
The lack of Bax activation in HO15.19 c-myc null cells likely accounts for the absence of cytochrome c release, as activated Bax has been shown to regulate this critical step in the control of apoptosis (14, 16, 31). Numerous mechanisms of Bax activation and cytochrome c release have been proposed, including Bax oligomerization, activation of an independent or preexisting mitochondrial pore, or a novel protein-protein interaction. Myc may directly activate Bax after both proteins translocate to the mitochondria in response to apoptotic stimuli. A similar mechanism has recently been described for the nuclear orphan receptor TR3/Nur77, another transcription factor that can induce apoptosis through the release of cytochrome c (36). Alternatively, Myc may indirectly control Bax activation and cytochrome c release, perhaps by regulating an upstream activator of Bax. Interestingly, during revision of this report, it was reported that apoptosis induced by serum withdrawal in the presence of deregulated Myc expression was deficient in Bax-null mouse embryo fibroblasts (40). This work further supports our data showing that Bax is critical for Myc potentiation of apoptosis. Further analysis of the c-myc null cell system is needed to identify the rate-limiting step controlled by Myc in the sensitization of cells to diverse apoptotic stimuli.
Analysis of this system shows that Bax expression is not directly regulated by Myc at the level of either mRNA or protein. Identification and verification of endogenous Myc target genes essential for apoptosis has been a slow and difficult process, due in part to the ubiquitous nature of Myc expression. A definitive all-or-none c-myc null cell system to address the identity, function, and regulation of Myc target genes has not been available. Both c-myc and N-myc knockout mice are embryonic lethal at approximately day 10, and mouse embryo fibroblasts null for myc expression cannot be derived (9, 13, 54). This major obstacle has been overcome through the development of the Rat1 somatic cell knockout system (38). This novel experimental tool has already had a significant impact on the field (7, 39, 44, 57). Comparison of c-myc null cells and null cells reconstituted with an exogenous allele of c-myc has been instrumental in distinguishing the putative Myc-regulated genes that are indeed dependent on Myc for regulation (7, 44). Moreover, it is thought that the Myc-regulated growth and death program can be uncoupled and that Myc regulates unique subsets of genes which in turn mediate these disparate activities (11, 20, 45). Clearly, this system will likely be instrumental in both identifying and verifying Myc-regulated genes critical for apoptosis.
The characteristics of apoptotic pathways that we have identified in HO15.19 and HOMyc3 cells are reminiscent of the two types of response that have been recently described for CD95 signaling (50, 51). In type I cells, where the components of the death-inducing signaling complex are not limiting, direct activation of downstream effector caspases by caspase 8-mediated cleavage is sufficient to induce apoptosis. This apoptosis is not blocked by Bcl-2. In type II cells, death receptor signaling to effector caspases through direct means is insufficient, and therefore a mitochondrial amplification loop that involves caspase 8 cleavage of Bid, Bax activation, and cytochrome c release is required. Our results parallel these observations; in the absence of Myc, cells undergo apoptosis by a type I-like, Bcl-2 noninhibited mechanism, whereas in the presence of Myc, a mitochondrion-dependent, Bcl-2-inhibited type II-like cell death is evident. Further investigation into the nature of this Myc-independent pathway is required, as it points to an alternative apoptotic pathway which is able to circumvent downstream signaling events at the mitochondria.
Our data further support the dual-signal model, as we show that Myc is essential but not sufficient for Bax activation, cytochrome c release, and apoptosis induction. Both Myc expression and a growth-inhibitory signal are required to trigger cell death. Our hypothesis is that Myc derepresses at least one level of apoptotic control, removing a rate-limiting event in the regulation of apoptosis and rendering cells more competent to die in response to a diverse set of stimuli. Our work shows that Myc likely regulates a common point of control, despite the diversity of signaling cascades with which it can collaborate to trigger apoptosis. We show that point of control to be at the level of Bax activation. This is consistent with previous reports showing Bcl-2 cooperates strongly with Myc in tumorigenesis, as it is a potent inhibitor of Myc-regulated apoptosis. Indeed, other inhibitors of Myc-stimulated apoptosis, such as insulin-like growth factor and Akt/protein kinase B, also function through a mechanism that targets Bcl-2 activity or cytochrome c release (20, 26, 30, 32). Together, these observations further support our data showing that Myc regulation of apoptosis is focused at the mitochondria and controls an apoptotic amplification step by regulating Bax activation.
ACKNOWLEDGMENTS
We thank members of the Penn lab for helpful discussions during preparation of the manuscript.
This work was supported by CIHR and NCIC grants to L.Z.P. as well as a CIHR grant to D.W.A. and B.L. E.S. is the recipient of a CIHR doctoral research award.
Footnotes
This paper is dedicated to the memory of Arnold Greenberg.
REFERENCES
- 1.Adachi S, Gottlieb R A, Babior B M. Lack of release of cytochrome C from mitochondria into cytosol early in the course of Fas-mediated apoptosis of Jurkat cells. J Biol Chem. 1998;273:19892–19894. doi: 10.1074/jbc.273.31.19892. [DOI] [PubMed] [Google Scholar]
- 2.Adams J M, Cory S. The Bcl-2 protein family: arbiters of cell survival. Science. 1998;281:1322–1326. doi: 10.1126/science.281.5381.1322. [DOI] [PubMed] [Google Scholar]
- 3.Alarcon R M, Rupnow B A, Graeber T G, Knox S J, Giaccia A J. Modulation of c-Myc activity and apoptosis in vivo. Cancer Res. 1996;56:4315–4319. [PubMed] [Google Scholar]
- 4.Amati B, Alevizopoulos K, Vlach J. Myc and the cell cycle. Front Biosci. 1998;3:D250–D268. doi: 10.2741/a239. [DOI] [PubMed] [Google Scholar]
- 4a.Annis, M. G., et al. Oncogene, in press.
- 5.Askew D S, Ashmun R A, Simmons B C, Cleveland J L. Constitutive c-myc expression in an IL-3-dependent myeloid cell line suppresses cell cycle arrest and accelerates apoptosis. Oncogene. 1991;6:1915–1922. [PubMed] [Google Scholar]
- 6.Brenner C, Cadiou H, Vieira H L, Zamzami N, Marzo I, Xie Z, Leber B, Andrews D, Duclohier H, Reed J C, Kroemer G. Bcl-2 and Bax regulate the channel activity of the mitochondrial adenine nucleotide translocator. Oncogene. 2000;19:329–336. doi: 10.1038/sj.onc.1203298. [DOI] [PubMed] [Google Scholar]
- 7.Bush A, Mateyak M, Dugan K, Obaya A, Adachi S, Sedivy J, Cole M. c-myc null cells misregulate cad and gadd45 but not other proposed c-Myc targets. Genes Dev. 1998;12:3797–3802. doi: 10.1101/gad.12.24.3797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Chang D W, Claassen G F, Hann S R, Cole M D. The c-Myc transactivation domain is a direct modulator of apoptotic versus proliferative signals. Mol Cell Biol. 2000;20:4309–4319. doi: 10.1128/mcb.20.12.4309-4319.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Charron J, Malynn B A, Fisher P, Stewart V, Jeannotte L, Goff S P, Robertson E J, Alt F W. Embryonic lethality in mice homozygous for a targeted disruption of the N-myc gene. Genes Dev. 1992;6:2248–2257. doi: 10.1101/gad.6.12a.2248. [DOI] [PubMed] [Google Scholar]
- 10.Cole M D, McMahon S B. The Myc oncoprotein: a critical evaluation of transactivation and target gene regulation. Oncogene. 1999;18:2916–2924. doi: 10.1038/sj.onc.1202748. [DOI] [PubMed] [Google Scholar]
- 11.Conzen S D, Gottlob K, Kandel E S, Khanduri P, Wagner A J, O'Leary M, Hay N. Induction of cell cycle progression and acceleration of apoptosis are two separable functions of c-Myc: transrepression correlates with acceleration of apoptosis. Mol Cell Biol. 2000;20:6008–6018. doi: 10.1128/mcb.20.16.6008-6018.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Dang C V, Resar L M, Emison E, Kim S, Li Q, Prescott J E, Wonsey D, Zeller K. Function of the c-Myc oncogenic transcription factor. Exp Cell Res. 1999;253:63–77. doi: 10.1006/excr.1999.4686. [DOI] [PubMed] [Google Scholar]
- 13.Davis A C, Wims M, Spotts G D, Hann S R, Bradley A. A null c-myc mutation causes lethality before 10.5 days of gestation in homozygotes and reduced fertility in heterozygous female mice. Genes Dev. 1993;7:671–682. doi: 10.1101/gad.7.4.671. [DOI] [PubMed] [Google Scholar]
- 14.Desagher S, Osen-Sand A, Nichols A, Eskes R, Montessuit S, Lauper S, Maundrell K, Antonsson B, Martinou J C. Bid-induced conformational change of Bax is responsible for mitochondrial cytochrome c release during apoptosis. J Cell Biol. 1999;144:891–901. doi: 10.1083/jcb.144.5.891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Dong J, Naito M, Tsuruo T. c-Myc plays a role in cellular susceptibility to death receptor- mediated and chemotherapy-induced apoptosis in human monocytic leukemia U937 cells. Oncogene. 1997;15:639–647. doi: 10.1038/sj.onc.1201237. [DOI] [PubMed] [Google Scholar]
- 16.Eskes R, Antonsson B, Osen-Sand A, Montessuit S, Richter C, Sadoul R, Mazzei G, Nichols A, Martinou J C. Bax-induced cytochrome C release from mitochondria is independent of the permeability transition pore but highly dependent on Mg2+ ions. J Cell Biol. 1998;143:217–224. doi: 10.1083/jcb.143.1.217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Evan G, Harrington E, Fanidi A, Land H, Amati B, Bennett M. Integrated control of cell proliferation and cell death by the c-myc oncogene. Philos Trans R Soc Lond B. 1994;345:269–275. doi: 10.1098/rstb.1994.0105. [DOI] [PubMed] [Google Scholar]
- 18.Evan G I, Wyllie A H, Gilbert C S, Littlewood T D, Land H, Brooks M, Waters C M, Penn L Z, Hancock D C. Induction of apoptosis in fibroblasts by c-myc protein. Cell. 1992;69:119–128. doi: 10.1016/0092-8674(92)90123-t. [DOI] [PubMed] [Google Scholar]
- 19.Facchini L M, Penn L Z. The molecular role of Myc in growth and transformation: recent discoveries lead to new insights. FASEB J. 1998;12:633–651. . (Review.) [PubMed] [Google Scholar]
- 20.Fanidi A, Harrington E A, Evan G I. Cooperative interaction between c-myc and bcl-2 proto-oncogenes. Nature. 1992;359:554–556. doi: 10.1038/359554a0. [DOI] [PubMed] [Google Scholar]
- 21.Green D R. Apoptotic pathways: paper wraps stone blunts scissors. Cell. 2000;102:1–4. doi: 10.1016/s0092-8674(00)00003-9. [DOI] [PubMed] [Google Scholar]
- 22.Green D R, Reed J C. Mitochondria and apoptosis. Science. 1998;281:1309–1312. doi: 10.1126/science.281.5381.1309. [DOI] [PubMed] [Google Scholar]
- 23.Gross A, Jockel J, Wei M C, Korsmeyer S J. Enforced dimerization of BAX results in its translocation, mitochondrial dysfunction and apoptosis. EMBO J. 1998;17:3878–3885. doi: 10.1093/emboj/17.14.3878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Gross A, McDonnell J M, Korsmeyer S J. BCL-2 family members and the mitochondria in apoptosis. Genes Dev. 1999;13:1899–1911. doi: 10.1101/gad.13.15.1899. [DOI] [PubMed] [Google Scholar]
- 25.Hanahan D, Weinberg R A. The hallmarks of cancer. Cell. 2000;100:57–70. doi: 10.1016/s0092-8674(00)81683-9. [DOI] [PubMed] [Google Scholar]
- 26.Harrington E A, Bennett M R, Fanidi A, Evan G I. c-Myc-induced apoptosis in fibroblasts is inhibited by specific cytokines. EMBO J. 1994;13:3286–3295. doi: 10.1002/j.1460-2075.1994.tb06630.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hueber A O, Evan G I. Traps to catch unwary oncogenes. Trends Genet. 1998;14:364–367. doi: 10.1016/s0168-9525(98)01520-0. [DOI] [PubMed] [Google Scholar]
- 28.Hueber A O, Zornig M, Lyon D, Suda T, Nagata S, Evan G I. Requirement for the CD95 receptor-ligand pathway in c-Myc-induced apoptosis. Science. 1997;278:1305–1309. doi: 10.1126/science.278.5341.1305. [DOI] [PubMed] [Google Scholar]
- 29.Janicke R U, Lee F H, Porter A G. Nuclear c-Myc plays an important role in the cytotoxicity of tumor necrosis factor alpha in tumor cells. Mol Cell Biol. 1994;14:5661–5670. doi: 10.1128/mcb.14.9.5661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Juin P, Hueber A O, Littlewood T, Evan G. c-Myc-induced sensitization to apoptosis is mediated through cytochrome c release. Genes Dev. 1999;13:1367–1381. doi: 10.1101/gad.13.11.1367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Jurgensmeier J M, Xie Z, Deveraux Q, Ellerby L, Bredesen D, Reed J C. Bax directly induces release of cytochrome c from isolated mitochondria. Proc Natl Acad Sci USA. 1998;95:4997–5002. doi: 10.1073/pnas.95.9.4997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kennedy S G, Wagner A J, Conzen S D, Jordan J, Bellacosa A, Tsichlis P N, Hay N. The PI 3-kinase/Akt signaling pathway delivers an anti-apoptotic signal. Genes Dev. 1997;11:701–713. doi: 10.1101/gad.11.6.701. [DOI] [PubMed] [Google Scholar]
- 33.Khaled A R, Kim K, Hofmeister R, Muegge K, Durum S K. Withdrawal of IL-7 induces Bax translocation from cytosol to mitochondria through a rise in intracellular pH. Proc Natl Acad Sci USA. 1999;96:14476–14481. doi: 10.1073/pnas.96.25.14476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Klefstrom J, Vastrik I, Saksela E, Valle J, Eilers M, Alitalo K. c-Myc induces cellular susceptibility to the cytotoxic action of TNF-alpha. EMBO J. 1994;13:5442–5450. doi: 10.1002/j.1460-2075.1994.tb06879.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kroemer G, Reed J C. Mitochondrial control of cell death. Nat Med. 2000;6:513–519. doi: 10.1038/74994. [DOI] [PubMed] [Google Scholar]
- 36.Li H, Kolluri S K, Gu J, Dawson M I, Cao X, Hobbs P D, Lin B, Chen G, Lu J, Lin F, Xie Z, Fontana J A, Reed J C, Zhang X. Cytochrome c release and apoptosis induced by mitochondrial targeting of nuclear orphan receptor TR3. Science. 2000;289:1159–1164. doi: 10.1126/science.289.5482.1159. [DOI] [PubMed] [Google Scholar]
- 37.Lowe S W, Lin A W. Apoptosis in cancer. Carcinogenesis. 2000;21:485–495. doi: 10.1093/carcin/21.3.485. [DOI] [PubMed] [Google Scholar]
- 38.Mateyak M K, Obaya A J, Adachi S, Sedivy J M. Phenotypes of c-Myc-deficient rat fibroblasts isolated by targeted homologous recombination. Cell Growth Differ. 1997;8:1039–1048. [PubMed] [Google Scholar]
- 39.Mateyak M K, Obaya A J, Sedivy J M. c-Myc regulates cyclin D-Cdk4 and -Cdk6 activity but affects cell cycle progression at multiple independent points. Mol Cell Biol. 1999;19:4672–4683. doi: 10.1128/mcb.19.7.4672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Mitchell K O, Ricci M S, Miyashita T, Dicker D T, Jin Z, Reed J C, El-Deiry W S. Bax is a transcriptional target and mediator of c-myc-induced apoptosis. Cancer Res. 2000;60:6318–6325. [PubMed] [Google Scholar]
- 41.Miyashita T, Reed J C. Tumor suppressor p53 is a direct transcriptional activator of the human bax gene. Cell. 1995;80:293–299. doi: 10.1016/0092-8674(95)90412-3. [DOI] [PubMed] [Google Scholar]
- 42.Nechushtan A, Smith C L, Hsu Y T, Youle R J. Conformation of the Bax C-terminus regulates subcellular location and cell death. EMBO J. 1999;18:2330–2341. doi: 10.1093/emboj/18.9.2330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Nicholson D W. Caspase structure, proteolytic substrates, and function during apoptotic cell death. Cell Death Differ. 1999;6:1028–1042. doi: 10.1038/sj.cdd.4400598. [DOI] [PubMed] [Google Scholar]
- 44.Oster S K, Marhin W W, Asker C, Facchini L M, Dion P A, Funa K, Post M, Sedivy J M, Penn L Z. Myc is an essential negative regulator of platelet-derived growth factor beta receptor expression. Mol Cell Biol. 2000;20:6768–6778. doi: 10.1128/mcb.20.18.6768-6778.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Packham G, Porter C W, Cleveland J L. c-Myc induces apoptosis and cell cycle progression by separable, yet overlapping, pathways. Oncogene. 1996;13:461–469. [PubMed] [Google Scholar]
- 46.Pastorino J G, Tafani M, Rothman R J, Marcineviciute A, Hoek J B, Farber J L. Functional consequences of the sustained or transient activation by Bax of the mitochondrial permeability transition pore. J Biol Chem. 1999;274:31734–31739. doi: 10.1074/jbc.274.44.31734. [DOI] [PubMed] [Google Scholar]
- 47.Prendergast G C. Mechanisms of apoptosis by c-Myc. Oncogene. 1999;18:2967–2987. doi: 10.1038/sj.onc.1202727. [DOI] [PubMed] [Google Scholar]
- 48.Reed J C. Bcl-2 family proteins. Oncogene. 1998;17:3225–3236. doi: 10.1038/sj.onc.1202591. [DOI] [PubMed] [Google Scholar]
- 49.Reed J C. Dysregulation of apoptosis in cancer. J Clin Oncol. 1999;17:2941–2953. doi: 10.1200/JCO.1999.17.9.2941. [DOI] [PubMed] [Google Scholar]
- 50.Scaffidi C, Fulda S, Srinivasan A, Friesen C, Li F, Tomaselli K J, Debatin K M, Krammer P H, Peter M E. Two CD95 (APO-1/Fas) signaling pathways. EMBO J. 1998;17:1675–1687. doi: 10.1093/emboj/17.6.1675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Scaffidi C, Schmitz I, Zha J, Korsmeyer S J, Krammer P H, Peter M E. Differential modulation of apoptosis sensitivity in CD95 type I and type II cells. J Biol Chem. 1999;274:22532–22538. doi: 10.1074/jbc.274.32.22532. [DOI] [PubMed] [Google Scholar]
- 52.Shi Y, Glynn J M, Guilbert L J, Cotter T G, Bissonnette R P, Green D R. Role for c-myc in activation-induced apoptotic cell death in T cell hybridomas. Science. 1992;257:212–214. doi: 10.1126/science.1378649. [DOI] [PubMed] [Google Scholar]
- 53.Shim H, Chun Y S, Lewis B C, Dang C V. A unique glucose-dependent apoptotic pathway induced by c-Myc. Proc Natl Acad Sci USA. 1998;95:1511–1516. doi: 10.1073/pnas.95.4.1511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Stanton B R, Perkins A S, Tessarollo L, Sassoon D A, Parada L F. Loss of N-myc function results in embryonic lethality and failure of the epithelial component of the embryo to develop. Genes Dev. 1992;6:2235–2247. doi: 10.1101/gad.6.12a.2235. [DOI] [PubMed] [Google Scholar]
- 55.Thompson E B. The many roles of c-Myc in apoptosis. Annu Rev Physiol. 1998;60:575–600. doi: 10.1146/annurev.physiol.60.1.575. [DOI] [PubMed] [Google Scholar]
- 56.Wagner A J, Kokontis J M, Hay N. Myc-mediated apoptosis requires wild-type p53 in a manner independent of cell cycle arrest and the ability of p53 to induce p21 waf1/cip1. Genes Dev. 1994;8:2817–2830. doi: 10.1101/gad.8.23.2817. [DOI] [PubMed] [Google Scholar]
- 57.Xiao Q, Claassen G, Shi J, Adachi S, Sedivy J, Hann S R. Transactivation-defective c-MycS retains the ability to regulate proliferation and apoptosis. Genes Dev. 1998;12:3803–3808. doi: 10.1101/gad.12.24.3803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Zhu W, Cowie A, Wasfy G W, Penn L Z, Leber B, Andrews D W. Bcl-2 mutants with restricted subcellular location reveal spatially distinct pathways for apoptosis in different cell types. EMBO J. 1996;15:4130–4141. [PMC free article] [PubMed] [Google Scholar]