Abstract
Introduction and importance
Primary adrenal leiomyosarcoma (PAL) is an extremely rare neoplasm that usually arises from the smooth muscle cells of the adrenal or adjacent vascular structures. The tumor is asymptomatic until it grows up and develops a mass effect in the retroperitoneal region. Although there are about 50 reported valid cases, surgical intervention is mandatory in the majority of patients.
Case presentation
Herein, we report the case of a 32-year-old healthy woman with a chief complaint of vague abdominal pain. After initial clinical and radiological examinations, we found a large retroperitoneal mass located around the right adrenal gland. Due to the patient's pain, a laparotomy was performed, and a large mass was resected with free margins. Immunohistochemical examination was positive for vimentin, smooth muscle actin (SMA), and desmin. Therefore, the diagnosis of PAL was confirmed.
Conclusion
Although PAL is an uncommon malignancy, its diagnostic and therapeutic approaches are almost straightforward. A computed tomography scan can show the characteristics of the tumor and direct the management. Surgical resection is the mainstay of treatment, and the effects of adjuvant therapies have not been apparent yet.
Keywords: Adrenal gland, Adrenal gland neoplasms, Leiomyosarcoma, Adrenalectomy, Case report
Highlights
-
•
A 32-year-old woman presented to us complaining of vague abdominal pain without any specific signs and symptoms.
-
•
After imaging studies, a large retroperitoneal mass was found around the right adrenal gland.
-
•
The patient was operated with a midline laparotomy and the mass was resected with free margins.
-
•
Immunohistochemical examination was positive for pathological markers of leiomyosarcoma.
-
•
The surgery was performed successfully, and the patient was discharged in a good condition.
1. Introduction
Primary adrenal leiomyosarcoma (PAL) is a soft tissue neoplasm with a smooth muscle origin. The PAL possibly originates from the smooth muscles of the adrenal vascular tissue. The leiomyosarcoma of the adrenal gland is an extremely rare malignancy, with about 50 cases currently reported in the English literature. This entity does not have any specific clinical manifestations and serum biomarkers. In addition, the radiologic features of adrenal leiomyosarcoma have not been described well. Therefore, the tumor predominantly presents with compressive effects on adjacent structures [1]. Herein, we report the case of a 32-year-old female that was approached as an adrenal incidentaloma, subsequently diagnosed as the adrenal leiomyosarcoma by pathological examination. This work has been reported in line with the SCARE criteria [2].
2. Case presentation
A 32-year-old woman presented to our surgical clinic with a complaint of vague pain in the right flank that had started three months ago. Over the three months, the pain became deteriorated, necessitating medical treatment. The patient underwent abdominal ultrasonography four months earlier for a routine checkup. That study revealed a suprarenal mass incidentally found in the right upper quadrant of the abdomen. Because the patient was asymptomatic at the time of the ultrasound, she was advised to be closely observed with no need for surgical removal or biopsy. She had no other symptoms, including weight loss, fever or night chilling. She had a history of laparoscopic cholecystectomy due to symptomatic biliary stone five years ago. Her medical and drug history was unremarkable.
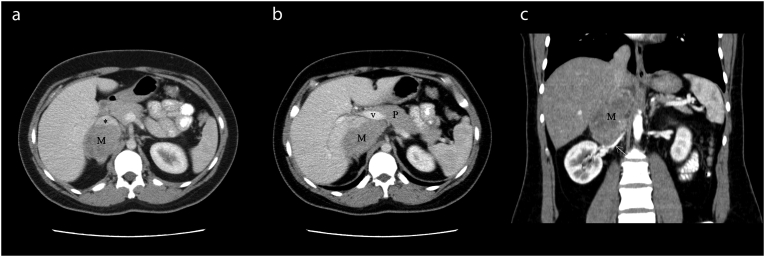
On physical examination, her vital signs were normal. Abdominal palpitation revealed a flank discomfort without any tenderness or lymphadenopathy. The patient underwent an abdominopelvic computed tomography (CT) scan with intravenous (IV) contrast. The CT scan showed an enhancing soft tissue mass with the size of 106 × 53 × 61 mm located in the right adrenal region with compression effect over the posterior aspect of the inferior vena cava (IVC), with forwarding displacement of the vein (Fig. 1A). Anteromedial extension close to the posterior aspect of the pancreatic neck and posterior wall of the portal vein was noted without any evidence of invasion (Fig. 1B). The mass was in close contact with the liver and right crus of the diaphragm, superiorly and the right renal artery and vein, inferiorly (Fig. 1C). No regional lymphadenopathy was identified.
Fig. 1.
Axial images of abdominal CT scan (A) demonstrate the mass (M) in the right adrenal region, which has compressed and anteriorly displaced the IVC(*).
A more superior axial section (B) shows the anteromedial extension of the tumor to reach the posterior aspect of the portal vein (v) close to the pancreatic neck(P). In coronal reconstruction image (C) right renal artery (arrow) is depicted along the inferior-medial margin of the mass.
All the laboratory tests, including complete blood count, renal and hepatic functional tests, electrolytes level, urine analysis, 24-hour urine collection for metanephrine and normetanephrine, and 17-ketosteroid, plasma metanephrine level, and overnight 1-mg dexamethasone suppression test, were within normal limits.
The patient underwent right open radical adrenalectomy with the probable diagnosis of right adrenal carcinoma based on radiologic findings. With the help of the midline incision, the right adrenal mass was measured about 10 × 5 × 5 cm with no apparent invasion to the adjacent structures. No ascites or liver metastasis was inspected. The tumor was dissected from the IVC and portal vein, and resected with all peri-tumoral fat and para-aortic lymphatic tissue from the diaphragm to the level of the right renal vein with no additional damage to the adjacent structures. There were no complications in the postoperative period, and the patient was discharged three days after the surgery.
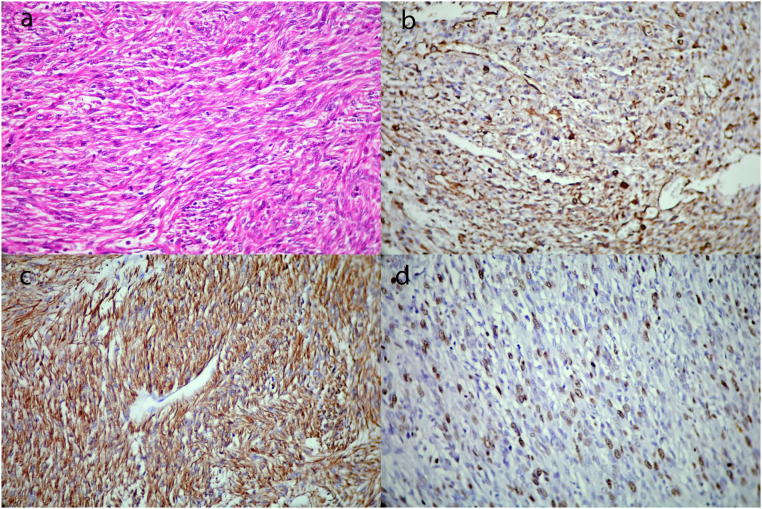
Gross pathological examination revealed multiple irregular tan-brown soft to elastic tissue fragments with vague nodular cut surfaces and small orange areas at the periphery of one fragment, totally measured 10 × 8 × 3.5 cm. The microscopic examination showed infiltrative adrenal neoplasm characterized by intersecting fascicles of spindle cells with elongate blunt-ended nuclei, eosinophilic cytoplasms, and moderate pleomorphism (Fig. 2A). Frequent mitotic figures (7 mitoses/10HPF) and multiple foci of necrosis (<50%) were present. On immunohistochemistry study, the tumor cells were positive for vimentin, SMA, and desmin; however, no immunoreactivity for cytokeratin, S100, SOX10, Melan-A, CD117, and P53 was detected. The ki-67 proliferative index was up to 30% (Fig. 2B, C, and D). Based on the aforementioned findings, the diagnosis of conventional leiomyosarcoma was confirmed.
Fig. 2.
Fascicles of mitotically active intersecting spindle cells with moderate pleomorphism (A ×400, H&E) that show diffuse immunoreactivity for vimentin(B) and desmin(C) with a high Ki-67 proliferative index(D).
After two months, we visited the patients in the clinic. She didn't complain of pain or any other symptoms. Imaging studies showed no local recurrence or distant metastasis.
3. Discussion
Adrenal leiomyomas and leiomyosarcomas are two rare smooth muscle tumors for which there are no known risk factors or predisposing conditions. However, about 50% of reported leiomyomas were associated with the human immunodeficiency virus. According to a systematic review conducted in five online databases (March 2020), about 45 valid cases of leiomyosarcoma have been reported so far [1]. Also, we searched the same databases with the same search strategy again to find more relevant cases. Finally, we assume that there are five more reported cases of PAL until now (November 2021).
Although leiomyosarcomas with other origins are well-known in the literature, PAL is more uncommon. Its diagnosis, management, and prognosis are not known well.
Similar to other adrenal tumors, they usually present as retroperitoneal masses [1]. Given that no specific clinical manifestation has been described to date, unspecific symptoms, such as abdominal/flank pain or discomfort, weight loss, and anorexia, have been reported [3]. In general, tissue enlargement is the most important cause of developing these symptoms due to the lack of tumor functionality. Of the 50 valid reported cases, almost all have developed symptoms due to the mass effect of the enlarged tumor. However, some patients presented with fever at the time of diagnosis [1], [4].
As the tumor grows silently, the diagnosis depends on imaging studies in the majority of cases. Ultrasound has remained the mainstay of abdominal imaging for years; nevertheless, in these cases, it cannot show specific features other than the size of the tumor. Therefore, a CT with IV contrast is widely used to determine the accurate tumor size, location, adjacent organ invasion, portal vein involvement, and distant metastasis [5].
In a CT scan, heterogeneous or lobulated well-defined mass, with or without necrosis, can be observed. Furthermore, leiomyosarcoma can invade the IVC and adjacent organs [6]. Although in the majority of cases, the IVC is just displaced due to mass effect, thrombus might be formed inside the cava [7]. In addition, imaging solely cannot show portal or IVC invasion. In the present case, the CT just showed the displacement of the IVC; nonetheless, we encountered IVC invasion during the operation.
Similar to any other symptomatic retroperitoneal masses, surgery is a necessary step in the management of PAL. Surgery removes the primary source of discomfort and helps the clinician decide better for additional postoperative therapies. Except for the cases with distant metastasis or severe vascular invasion, almost every PAL needs to be resected [8]. Both laparotomy and minimally invasive approaches can be used, and one method cannot be recommended due to the scarcity of data. Theoretically, it seems that tumors with smaller sizes and without any adjacent organ or vascular invasion can be resected via a minimally invasive approach. Some studies reported the successful resection of PAL with the aid of laparoscopy [9]. Although laparoscopy is used successfully, robotic adrenalectomy in the context of PAL has not been performed yet. Considering the potential advantages of robotic surgery, such as shorter operative time and hospital stay, it can be recommended in the future [10].
Considering the rarity of PAL, genetic and molecular patterns are less described. On the other hand, some associated hub-genes have been found in the molecular pathogenetic of different types of leiomyosarcomas. Five hub-genes such as KDR, CCL21, SELP, DPT, and DCN have been introduced for uterine leiomyosarcoma [11]. Also, chromosomal mutations, homologous-recombination DNA-repair deficiency and microsatellite instability (MSI) mutation pathways have been found in uterine leiomyosarcomas [12]. Because identifying these genes is essential in the targeted-gene therapy of sarcoma, knowing these genes will help clinicians improve outcomes. Different genetic mutations and associated expressed proteins have been described in the literature so far. SMA is the most positive biomarker in the cases. Also, desmin, vimentin (c-20), S100, CD34, CD47, CD68, CD117 and CK Mak6 are positive in some patients [1]. Although, immunotherapy for PDL-1(CD274) is used for uterine sarcomas, no available report is found around the web about the benefits of this treatment for PAL [13]. In general, it seems that known genetic mutations for other types of leiomyosarcomas are not specifically associated with PAL.
There is not a universal consensus about the use of chemotherapy, radiotherapy, or combination therapy. Several methods and regimens have been described; however, a safe conclusion could not be drawn using the available data [1], [14]. On the other hand, the effect of additional therapies on short-term or long-term follow-ups is not apparent. According to the rarity of PAL, almost all available information is recorded in case reports. Therefore, we cannot recommend or declare specific methods for the diagnosis, management, and follow-up of the disease. Other diagnostic tools, such as positron emission tomography (PET)/CT, and robotic surgery might be used in the future.
4. Conclusion
In conclusion, PAL is a rare soft tissue neoplasm that usually arises from smooth muscles of the adrenal gland or IVC. Unspecific clinical signs and symptoms, such as abdominal/flank pain or weight loss, might exist. Although ultrasound is not helpful, IV contrast CT can show different tumor features, including size, location, or distant metastasis. Except for the presence of substantial unresectable masses or distant metastasis, the surgical removal of the tumor with free margins is mandatory. No specific additional therapy, such as chemotherapy or radiation therapy, is generally recommended, and long-term follow-up varies in each case.
Ethical approval
This article does not contain any studies with human participants or animals performed by any of the authors.
Sources of funding
The authors didn't receive any financial support for this report.
CRediT authorship contribution statement
BO: Study design and The Chief Surgeon. AZ and SM: Case presentation and data gathering. HB: Images gathering, interpretation, and preparation. EJ: Pathology examination consultation. ME: Writing and reviewing the manuscript.
All authors read and approved the final manuscript.
Guarantor
Dr. Manoochehr Ebrahimian.
Registration of research studies
Not applicable.
Consent
Written informed consent was obtained from the patient for publication of this case report and accompanying images. A copy of the written consent is available for review by the Editor-in-Chief of this journal on request.
Provenance and peer review
Not commissioned, externally peer-reviewed.
Declaration of competing interest
The authors declared no potential conflict of interests with respect to the research, authorship, and/or publication of this article.
Acknowledgments
None.
References
- 1.Sakellariou M., Dellaportas D., Peppa M., Schizas D., Pikoulis E., Nastos K. Review of the literature on leiomyoma and leiomyosarcoma of the adrenal gland: a systematic analysis of case reports. In Vivo. 2020;34(5):2233–2248. doi: 10.21873/invivo.12034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Agha R.A., Franchi T., Sohrabi C., Mathew G., Kerwan A., Thoma A., et al. The SCARE 2020 guideline: updating consensus surgical CAse REport (SCARE) guidelines. Int. J. Surg. 2020;84:226–230. doi: 10.1016/j.ijsu.2020.10.034. [DOI] [PubMed] [Google Scholar]
- 3.Linos D., Kiriakopoulos A.C., Tsakayannis D.E., Theodoridou M., Chrousos G. Laparoscopic excision of bilateral primary adrenal leiomyosarcomas in a 14-year-old girl with acquired immunodeficiency syndrome (AIDS) Surgery. 2004;136(5):1098–1100. doi: 10.1016/j.surg.2003.07.007. [DOI] [PubMed] [Google Scholar]
- 4.Thamboo T.P., Liew L.C., Raju G.C. Adrenal leiomyosarcoma: a case report and literature review. Pathology. 2003;35(1):47–49. [PubMed] [Google Scholar]
- 5.Wang Y., Teng Y., Na S., Yuan Y. Pleomorphic leiomyosarcoma of the adrenal gland in a young woman: a case report and review of the literature. OncoTargets Ther. 2020;13:4705. doi: 10.2147/OTT.S254162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Atilgan A.O., Akcay E.Y., Ozdemir B. 2019. Leiomyosarcoma of the inferior vena cava and renal vein: a case report. [Google Scholar]
- 7.Öztürk H. Vena cava ınvasion by adrenal leiomyosarcoma. Rare Tumors. 2014;6(2):55–56. doi: 10.4081/rt.2014.5275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Stoner P., Schlachterman A., Ghayee H., Lu L., Gupte A. First reported case of endoscopic ultrasound-guided core biopsy yielding diagnosis of primary adrenal leiomyosarcoma: 2098. Off. J. Am. Coll. Gastroenterol. 2017;112 doi: 10.1155/2018/8196051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Quildrian S., Califano I., Carrizo F., Daffinoti A., Calónico N. Primary adrenal leiomyosarcoma treated by laparoscopic adrenalectomy. 2015;62(9):472–473. doi: 10.1016/j.endonu.2015.05.008. [DOI] [PubMed] [Google Scholar]
- 10.Brandao L.F., Autorino R., Laydner H., Haber G.P., Ouzaid I., De Sio M., et al. Robotic versus laparoscopic adrenalectomy: a systematic review and meta-analysis. Eur. Urol. 2014;65(6):1154–1161. doi: 10.1016/j.eururo.2013.09.021. [DOI] [PubMed] [Google Scholar]
- 11.Shen X., Yang Z., Feng S., Li Y. Identification of uterine leiomyosarcoma-associated hub genes and immune cell infiltration pattern using weighted co-expression network analysis and CIBERSORT algorithm. World J. Surg. Oncol. 2021;19(1):223. doi: 10.1186/s12957-021-02333-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Choi J., Manzano A., Dong W., Bellone S., Bonazzoli E., Zammataro L., et al. Integrated mutational landscape analysis of uterine leiomyosarcomas. Proc. Natl. Acad. Sci. U. S. A. 2021;118(15) doi: 10.1073/pnas.2025182118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Budczies J., Mechtersheimer G., Denkert C., Klauschen F., Mughal S.S., Chudasama P., et al. PD-L1 (CD274) copy number gain, expression, and immune cell infiltration as candidate predictors for response to immune checkpoint inhibitors in soft-tissue sarcoma. Oncoimmunology. 2017;6(3) doi: 10.1080/2162402X.2017.1279777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bhalla A., Sandhu F., Sieber S. Primary adrenal leiomyosarcoma: a case report and review of the literature. Conn. Med. 2014;78(7) [PubMed] [Google Scholar]