Abstract
Objective
The objective was to investigate the association between cognitive impairment and healthcare resource utilization (HCRU) and quality of life (QoL) among patients with schizophrenia.
Methods
Data from the Adelphi Schizophrenia Disease Specific Programme™, a point-in-time survey of physicians and their patients, were collected in the United States between July–October 2019. Psychiatrists reported on patient cognitive function, HCRU, housing circumstances and employment status for their next 10 consulting adult patients with schizophrenia. Patients were classified as having no/mild or moderate/severe cognitive impairment and asked to complete a QoL questionnaire voluntarily. Multiple regression analysis estimated the association between severity of cognitive impairment and patient outcomes adjusting for patient demographics and clinical characteristics.
Results
Psychiatrists (n=124) reported on 651 and 484 patients with no/mild and moderate/severe cognitive impairment, respectively. Moderate/severe vs. no/mild cognitive impairment was associated with greater odds of hospitalization related to schizophrenia relapse within the last 12 months (adjusted odds ratio [aOR] [95% CI] = 2.23 [1.53–3.24]) and being unemployed due to disability (aOR = 2.39 [1.65–3.45]). Patients with moderate/severe vs. no/mild cognitive impairment had worse average QoL (EuroQoL 5-dimension [EQ-5D] Health Index: difference = −0.09 [−0.13 to −0.04]; EQ-5D Visual Analogue Scale: difference = −7.0 [−13.0 to −1.0]) and overall life satisfaction (Quality of Life Enjoyment and Satisfaction Questionnaire-Short Form: difference = −8.4 [−14.1 to −2.8]).
Conclusions
Moderate/severe cognitive impairment among patients with schizophrenia was associated with worse patient outcomes including greater risk of hospitalizations related to schizophrenia relapse. Treatment to improve cognitive function could benefit the large proportion of patients with schizophrenia who suffer from cognitive impairment.
Keywords: Schizophrenia, cognitive impairment, healthcare resource utilization, employment, quality of life
1. Introduction
Schizophrenia, a chronic psychiatric disorder, has a mean lifetime prevalence of 1.1% in the United States (US) (McGrath et al., 2008). A core feature of schizophrenia is cognitive impairment, which is characterized by substandard neurocognition (processing speed, attention, learning, memory, problem-solving and working memory) and/or social cognition (emotion processing, social perception, attributional bias and mentalizing) (Green et al., 2019). Up to 75% of patients with schizophrenia experience neurocognitive impairment (Silberstein and Harvey, 2019).
Levels of neurocognitive impairment among patients with schizophrenia range, on average, up to 2 standard deviations (SDs) below healthy individuals (Mesholam-Gately et al., 2009). Among patients with schizophrenia, level of cognitive impairment varies from near-normal to severe deficits (Green et al., 2020; Habtewold et al., 2020). Cognitive impairment is persistent throughout life stages and changes in schizophrenia symptoms (Keefe, 2014). Additionally, cognition in patients with schizophrenia may deteriorate over time (Parlar and Heinrichs, 2021).
Cognitive impairment is predictive of functional outcomes and disability among patients with schizophrenia (Green, 2016; Harvey et al., 2019) and has also been associated with worse community functioning (Green, 2016), lower patient quality of life (QoL) (DeRosse et al., 2018; Pascal de Raykeer et al., 2019) and greater healthcare resource utilization (HCRU) (Hori et al., 2020). Therefore, cognitive impairment likely contributes to the $281.6 billion annual economic burden of schizophrenia in the US (Murphy et al., 2021). However, more severe levels of cognitive impairment among patients with schizophrenia may be associated with worse outcomes and greater burden.
There is a lack of published data on the association between more severe levels of cognitive impairment compared to no or mild cognitive impairment and patient outcomes. The objective of this study was to evaluate the association of moderate/severe vs. no/mild cognitive impairment and HCRU, employment status, housing circumstances and QoL as well as to report real-world estimates of outcomes among patients with schizophrenia in the US.
2. Methods
2.1. Data source
This analysis was conducted using data from the Adelphi Schizophrenia Disease Specific Programme (DSP)™, a point-in-time survey collecting data from psychiatrists and their patients in the US between July and October 2019. The DSP uses a convenience sample of psychiatrists recruited by field-based interviewers. Physicians were eligible to participate in this survey if they were personally responsible for treatment decisions and management of patients with schizophrenia. The complete survey methods have been described previously (Anderson et al., 2008).
Participating psychiatrists provided information on their next 10 eligible consulting patients with schizophrenia (8 outpatients and 2 inpatients, when possible). Patients were eligible for the survey if they were adults (age ≥ 18 years old), had a physician-confirmed diagnosis of schizophrenia, visited their psychiatrist, and were not currently taking part in a clinical trial. Patients were excluded from the analysis if they were not currently receiving treatment for schizophrenia.
The psychiatrist-completed patient record forms included patient demographics, clinical characteristics, cognition, employment status, housing circumstances and HCRU due to schizophrenia relapses. The same patients were asked to voluntarily complete a patient self-completion questionnaire to assess their QoL and life satisfaction.
2.2. Cognitive impairment
Psychiatrists described their patients' current cognitive impairment as one of the following: not at all cognitively impaired, borderline, mild, moderate, marked, severe or among the most extreme cognitive impairment. For this analysis, not at all cognitively impaired, borderline or mild cognitive impairment were classified as no/mild; and moderate, marked, severe or among the most extreme were classified as moderate/severe. A binary split was chosen because patients with mild cognitive impairment were not expected to have the same outcomes as patients with moderate or greater cognitive impairment. Physician-reported cognition has previously been used to assess the correlation between cognitive impairment and HCRU and patient QoL (Tundia et al., 2012).
2.3. Patient outcomes and other variables
The psychiatrist reported on patient demographics including age, sex, race/ethnicity, the highest level of education, health insurance type and treatment setting, as well as clinical characteristics including Body Mass Index (BMI), time since diagnosis with schizophrenia, number of medications, smoking status, cardiometabolic comorbidities (diabetes, dyslipidemia, gastroesophageal reflux disease [GERD], hypertension and obesity) and psychiatric comorbidities (anxiety, alcohol use disorder, depression, insomnia and substance use disorder).
The primary outcome of interest was HCRU related to schizophrenia relapse, which was physician-reported based on patient records and history available to the physician. Hospitalizations, emergency room (ER) visits resulting in hospitalization, days hospitalized and healthcare practitioner (HCP) visits were reported for the previous 12 months and also since diagnosis for hospitalizations. Secondary physician-reported outcomes included employment status and housing circumstances. Employment status included full-time employment, part-time employment, student/homemaker/retired, unemployed due to disability, unemployed for other reason or sick leave. Housing circumstances included living alone, living with a partner/spouse, living with relatives/friends/others, living in supported housing or living in a homeless shelter.
Patient-reported QoL was measured via the EuroQoL 5-dimension (EQ-5D) Health Index (scale 0–1 with a higher score indicating a better health state) and EQ-5D Visual Analogue Scale (VAS; scale 0–100 with a higher score indicating a better health state) (Brooks, 1996; The EuroQol Group, 1990). Life satisfaction was measured with the Quality of Life Enjoyment and Satisfaction Questionnaire-Short Form (Q-LES-Q-SF; scale 0–100 with a higher score indicating greater life satisfaction) (Endicott et al., 1993).
2.4. Ethical considerations
Psychiatrists were compensated financially upon survey completion according to fair market research rates. Data collection was conducted in line with European Pharmaceutical Marketing Research Association guidelines (European Pharmaceutical Market Research Association (EphMRA), 2021). Ethics approval was obtained from the Western Institutional Review Board (AG8618). Each survey was performed in full accordance with the US Health Insurance Portability and Accountability Act 1996 (US Department of Health and Human Services, 2003), and Health Information Technology for Economic and Clinical Health Act legislation (ICC/ESOMAR, 2016).
2.5. Statistical analysis
Descriptive statistics included means and SDs for continuous variables, and proportions for categorical variables. Statistical difference by level of cognitive impairment was tested using the analysis of variance for continuous variables and chi-squared tests for categorical variables. Linear regressions were used for continuous QoL outcomes, logistic regressions for binary employment status and housing outcomes and negative binomial regressions for HCRU outcomes expressed in counts. All analyses controlled for age, sex, BMI, level of education, insurance type, and number of concomitant medications and pills. Regressions for HCRU and QoL also controlled for housing circumstances and employment status. Standard errors were clustered by physician. The goal of the adjusted analysis was to provide an estimate of real-world patient outcomes in clinical practice. Average margin estimates and 95% confidence intervals (CIs) were reported for adjusted means and proportions. Adjusted odds ratios (aOR) were reported for logistic regressions and adjusted incidence rate ratios (aIRRs) were reported for negative binomial regressions. All analyses were conducted using Stata v17 (StataCorp LLC, College Station, Texas, USA). Statistical significance was defined as p-value <0.05.
3. Results
3.1. Patient characteristics
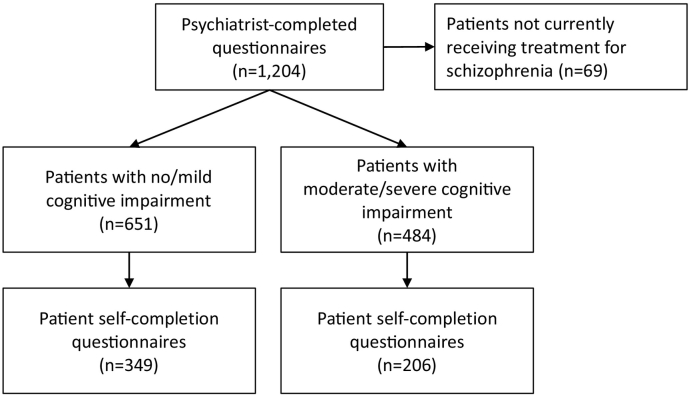
Psychiatrists (n = 124) reported on 651 patients with no/mild cognitive impairment and 484 patients with moderate/severe cognitive impairment currently receiving treatment for schizophrenia (Fig. 1). Of those patients, 349 (54%) and 206 (43%) patients with no/mild and moderate/severe cognitive impairment, respectively, completed patient self-completion questionnaires.
Fig. 1.
Flow diagram.
On average, patients were 40 years old and 8.2 years from their schizophrenia diagnosis (Table 1). A significantly greater proportion of patients with moderate/severe vs. no/mild cognitive impairment was male (64.9 vs. 53.9%, p < 0.05), did not complete high school (27.7 vs. 10.1%, p < 0.05), had Medicaid (47.1 vs. 33.7%) or Medicare (27.9 vs. 16.3%) health insurance (p < 0.05), and was treated in an inpatient setting (23.8 vs. 14.1%, p < 0.05).
Table 1.
Patient demographics and clinical characteristics.
| Total (n = 1135) | No/mild (n = 651) | Moderate/severe (n = 484) | p-valuea | |
|---|---|---|---|---|
| Ageb, mean years (SD) | 40.0 (15.8) | 38.8 (14.9) | 41.7 (16.9) | 0.002 |
| Maleb, n (%) | 665 (58.6) | 351 (53.9) | 314 (64.9) | <0.001 |
| BMIb, kg/m2, mean (SD) | 27.4 (4.9) | 27.1 (4.8) | 27.7 (5.1) | 0.027 |
| Ethnicityb, n (%) | 0.172 | |||
| White/Caucasian | 697 (61.4) | 408 (62.7) | 289 (59.7) | |
| African American | 267 (23.5) | 132 (20.3) | 135 (27.9) | |
| Hispanic/Latino | 73 (6.4) | 46 (7.1) | 27 (5.6) | |
| Other | 98 (8.6) | 65 (10.0) | 33 (6.8) | |
| Educationb, n (%) | <0.001 | |||
| Did not complete high school | 200 (17.6) | 66 (10.1) | 134 (27.7) | |
| High school diploma/GED | 572 (50.4) | 329 (50.5) | 243 (50.2) | |
| College degree, 2-year | 202 (17.8) | 143 (22.0) | 59 (12.2) | |
| College degree, 4-year | 95 (8.4) | 73 (11.2) | 22 (4.5) | |
| Graduate degree/trade school/certificate program | 53 (4.7) | 32 (4.9) | 21 (4.3) | |
| Other | 13 (1.1) | 8 (1.2) | 5 (1.0) | |
| Years since schizophrenia diagnosisc, mean (SD) | 8.2 (10.8) | 7.4 (10.3) | 9.9 (11.6) | 0.036 |
| Treatment settingb, d, n (%) | <0.001 | |||
| Inpatient | 207 (13.2) | 92 (14.1) | 115 (23.8) | |
| Outpatient | 928 (81.8) | 559 (85.9) | 369 (76.2) | |
| Any health insurancee, n (%) | 0.777 | |||
| Yes | 1016 (95.0) | 594 (95.2) | 422 (94.8) | |
| No | 53 (5.0) | 30 (4.8) | 23 (5.2) | |
| Among patients with health insurance, type of health insurancef, n (%) | <0.001 | |||
| Medicaid | 387 (39.3) | 193 (33.7) | 194 (47.1) | |
| Commercial | 267 (27.1) | 200 (35.0) | 67 (16.3) | |
| Medicare | 208 (21.1) | 93 (16.3) | 115 (27.9) | |
| Private (Health insurance exchange plan, Cobra) | 100 (10.2) | 77 (13.5) | 23 (5.6) | |
| Other (Tricare/Veteran's healthcare, other) | 22 (2.2) | 9 (1.6) | 13 (3.2) | |
| Smokingg, n (%) | <0.001 | |||
| Current smoker | 354 (33.5) | 159 (26.3) | 195 (43.1) | |
| Ex-smoker | 200 (18.9) | 121 (20.0) | 79 (17.5) | |
| Never smoked | 503 (47.6) | 325 (53.7) | 178 (39.4) | |
| Cardiometabolic comorbiditiesb, n (%) | ||||
| Hypertension | 258 (22.7) | 108 (16.6) | 150 (31.0) | <0.001 |
| Dyslipidemia | 198 (17.4) | 91 (14.0) | 107 (22.1) | <0.001 |
| Obesity | 192 (16.9) | 90 (13.8) | 102 (21.1) | 0.001 |
| GERD | 76 (6.7) | 44 (6.8) | 32 (6.6) | 1.000 |
| Diabetes without chronic complications | 75 (6.6) | 34 (5.2) | 41 (8.5) | 0.039 |
| Psychiatric comorbiditiesb, n (%) | ||||
| Anxiety | 309 (27.2) | 161 (24.7) | 148 (30.6) | 0.031 |
| Depression | 156 (13.7) | 78 (12.0) | 78 (16.1) | 0.055 |
| Stress | 146 (12.9) | 70 (10.8) | 76 (15.7) | 0.015 |
| Insomnia | 132 (11.6) | 57 (8.8) | 75 (15.5) | <0.001 |
| Substance use disorder | 69 (6.1) | 26 (4.0) | 43 (8.9) | 0.001 |
| Alcohol use disorder | 63 (5.6) | 24 (3.7) | 39 (8.1) | 0.002 |
| Number of medications not including schizophreniah, mean (SD) | 1.7 (2.1) | 1.4 (1.8) | 2.2 (2.4) | <0.001 |
Abbreviations: BMI, Body Mass Index; GED, General Education Development (high school equivalency diploma); GERD, gastroesophageal reflux disease; kg, kilogram; m, meter; n, number of patients; SD, standard deviation.
Notes.
P-values are reported for differences between the two groups.
Reported for full sample of 651 and 484 patients with no/mild and moderate/severe cognitive impairment, respectively.
Time since schizophrenia diagnosis reported for 358 and 194 patients with no/mild and moderate/severe cognitive impairment, respectively.
A quota of 8 outpatients and 2 inpatients was set for each physician to ensure an adequate number of inpatients for analysis.
Any health insurance reported for 624 and 445 patients with no/mild and moderate/severe cognitive impairment, respectively.
Type of health insurance reported for 572 and 412 patients with no/mild and moderate/severe cognitive impairment, respectively. A conflicting value of no health insurance reported for one patient with moderate/severe cognitive impairment was not included.
Smoking status reported for 605 and 452 patients with no/mild and moderate/severe cognitive impairment, respectively.
Number of medications reported for 545 and 356 patients with no/mild and moderate/severe cognitive impairment, respectively.
Comorbidities were more prevalent among patients with moderate/severe vs. no/mild cognitive impairment including cardiometabolic (hypertension: 31.0 vs. 16.6%; dyslipidemia: 22.1 vs. 14.0%; obesity: 21.1 vs. 13.8%; diabetes without chronic conditions: 8.5 vs. 5.2%; all p < 0.05) and psychiatric (anxiety: 30.6 vs. 24.7%; stress: 15.7 vs. 10.8%; insomnia: 15.5 vs. 8.8%; substance use disorder: 8.9 vs. 4.0%; alcohol use disorder: 8.1 vs. 3.7%; all p < 0.05) comorbidities.
3.2. Patient outcomes
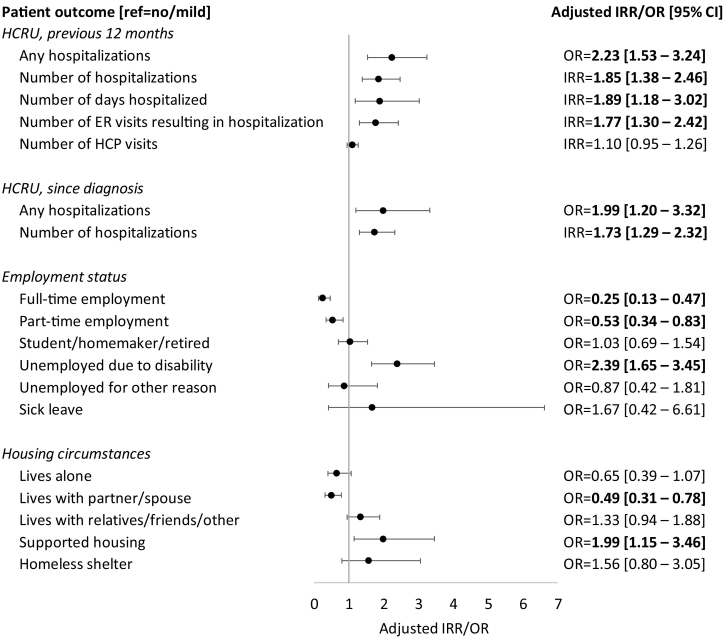
The association of moderate/severe cognitive impairment with HCRU, employment status and housing circumstances are shown in Fig. 2. The average margin estimates are shown in Table 2.
Fig. 2.
Adjusted outcomes related to severity of cognitive impairment
Abbreviations: CI, confidence interval; HCP, healthcare practitioner; HCRU, healthcare resource utilization; IRR, incidence rate ratio; OR, odds ratio; ref., reference.
Notes: Bold text indicates statistical significance of moderate/severe vs. no/mild cognitive impairment based on 95% CI. Employment status and housing circumstances outcomes adjusted for patient age, sex, BMI, level of education, insurance type, and number of concomitant medications and pills. HCRU outcomes additionally controlled for employment status and housing circumstances.
Table 2.
Adjusted physician-reported patient outcomes.
| No/mild (n = 651) | Moderate/severe (n = 484) | |
|---|---|---|
| HCRU related to schizophrenia relapse in the previous 12 months | ||
| Any hospitalizations | ||
| n | 499 | 322 |
| Proportion [95% CI] | 25.2% [18.3–32.0%] | 42.8% [34.4–51.2%] |
| Number of hospitalizations | ||
| n | 499 | 322 |
| Mean [95% CI] | 0.3 [0.2–0.4] | 0.6 [0.4–0.7] |
| Number of days hospitalized | ||
| n | 484 | 297 |
| Mean [95% CI] | 1.8 [1.0–2.6] | 3.4 [2.3–4.5] |
| Number of ER visits resulting in hospitalization | ||
| n | 495 | 309 |
| Mean [95% CI] | 0.2 [0.2–0.3] | 0.4 [0.3–0.5] |
| Number of HCP visits | ||
| n | 478 | 313 |
| Mean [95% CI] | 7.7 [6.8–8.6] | 8.4 [7.3–9.6] |
| HCRU related to schizophrenia relapse since diagnosis | ||
| Any hospitalizations | ||
| n | 380 | 249 |
| Proportion [95% CI] | 61.6% [52.6–70.6%] | 76.2% [68.4–83.9%] |
| Number of hospitalizations | ||
| n | 304 | 139 |
| Mean [95% CI] | 1.1 [0.8–1.3] | 1.8 [1.3–2.4] |
| Employment status | ||
| n | 520 | 338 |
| Full-time employment, proportion [95% CI] | 6.1% [3.0–9.1%] | 1.6% [0.4–2.8%] |
| Part-time employment, proportion [95% CI] | 22.3% [17.5–27.0%] | 13.2% [8.5–18.0%] |
| Student/homemaker/retired, proportion [95% CI] | 12.9% [9.4–16.5%] | 13.3% [9.0–17.5%] |
| Unemployed due to disability, proportion [95% CI] | 29.7% [24.3–35.1%] | 50.2% [41.7–58.7%] |
| Unemployed for other reason, proportion [95% CI] | 3.3% [1.7–4.9%] | 2.9% [0.7–5.1%] |
| Sick leave, proportion [95% CI] | 1.4% [0.1–2.7%] | 2.3% [0.3–4.4%] |
| Housing circumstances | ||
| n | 526 | 342 |
| Lives alone, proportion [95% CI] | 14.9% [10.8–19.1%] | 10.2% [6.0–14.4%] |
| Lives with partner/spouse, proportion [95% CI] | 21.4% [16.7–26.1%] | 11.7% [7.3–16.1%] |
| Lives with relatives/friends/other, proportion [95% CI] | 45.2% [39.6–50.8%] | 52.2% [45.2–59.2%] |
| Lives in supported housing, proportion [95% CI] | 4.5% [2.3–6.7%] | 8.5% [4.7–12.4%] |
| Lives in a homeless shelter, proportion [95% CI] | 2.5% [0.6–4.5%] | 3.9% [1.4–6.5%] |
Abbreviations: ER, emergency room; HCP, healthcare practitioner; HCRU, healthcare resource utilization; n, number of patients.
Notes: Average margin estimates are reported for means and proportions. Employment status and housing circumstances outcomes adjusted for patient age, sex, BMI, level of education, insurance type, and number of concomitant medications and pills. HCRU outcomes additionally controlled for employment status and housing circumstances.
After adjusting for patient demographic and clinical characteristics as well as employment status and housing circumstances, the number of hospitalizations related to schizophrenia relapse in the previous 12 months was significantly greater in patients with moderate/severe vs. no/mild cognitive impairment (average margin estimate [95% CI] = 0.6 vs. 0.3, aIRR [95% CI] = 1.85 [1.38–2.46], p < 0.05). Patients with moderate/severe vs. no/mild cognitive impairment had a greater number of days hospitalized (3.4 vs. 1.8, aOR = 1.89 [1.18–3.02], p < 0.05) and ER visits resulting in hospitalization (0.4 vs. 0.2, aOR = 1.77 [1.30–2.42], p < 0.05). The number of hospitalizations since diagnosis was also greater in patients with moderate/severe vs. no/mild cognitive impairment (1.8 vs. 1.1, aOR = 1.73 [1.29–2.32], p < 0.05).
After adjusting for patient demographic and clinical characteristics, half of patients with moderate/severe cognitive impairment were unemployed due to disability compared to less than one third of patients with no/mild cognitive impairment (50.2 vs. 29.7%, aOR = 2.39 [1.65–3.45], p < 0.05). The proportion of patients in full- or part-time employment was significantly lower in patients with moderate/severe vs. no/mild cognitive impairment (full-time: 1.6 vs. 6.1%, aOR = 0.25 [0.13–0.47], p < 0.05; part-time: 13.2 vs. 22.3%, aOR = 0.53 [0.34–0.83], p < 0.05). The proportion of patients living with a partner or spouse was significantly lower (11.7 vs. 21.4%, aOR = 0.49 [0.31–0.78], p < 0.05) and the proportion of patients living in supported housing was greater (8.5 vs. 4.5%, aOR = 1.99 [1.15–3.46], p < 0.05) in patients with moderate/severe vs. no/mild cognitive impairment.
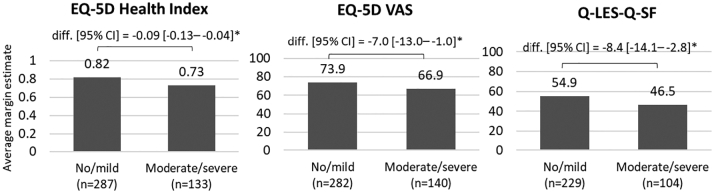
Fig. 3 shows the average margin estimate and difference in QoL scores in patients with no/mild and moderate/severe cognitive impairment.
Fig. 3.
Adjusted patient QoL outcomes
Abbreviations: BMI, Body Mass Index; CI, confidence interval; diff., difference; EQ-5D, EuroQoL 5-dimension; n, number of patients; Q-LES-Q-SF, Quality of Life Enjoyment and Satisfaction Questionnaire-Short Form; VAS, Visual Analogue Scale.
Notes: *Indicates significance vs. no/mild cognitive impairment based on 95% CI. Outcomes adjusted for patient age, sex, BMI, level of education, housing circumstances, employment status, insurance type, and number of concomitant medications and pills.
After adjusting for patient demographic, clinical characteristics, employment status and housing circumstances, QoL was significantly lower in patients with moderate/severe vs. no/mild cognitive impairment (EQ-5D Health Index: 0.73 vs. 0.82, difference = −0.09 [−0.13 to −0.04], p < 0.05; EQ-5D VAS: 66.9 vs. 73.9, difference = −7.0 [−13.0 to −1.0], p < 0.05). Patients with moderate/severe cognitive impairment also reported significantly lower average life satisfaction compared to patients with no/mild cognitive impairment (46.5 vs. 54.9, difference = −8.4 [−14.1 to −2.8], p < 0.05).
4. Discussion
Patients with moderate/severe cognitive impairment had a significantly greater disease burden compared to patients with no/mild cognitive impairment after controlling for patient demographics and clinical characteristics. The average number of hospitalizations, ER visits and days hospitalized in the previous 12 months were almost two times greater in patients with moderate/severe compared to no/mild cognitive impairment. Half of patients with moderate/severe cognitive impairment were unemployed due to disability compared to less than one third of those with no/mild cognitive impairment. The proportion of patients with moderate/severe cognitive impairment living in supported housing was almost twice that of no/mild cognitive impairment. Patients with moderate/severe cognitive impairment also reported worse QoL and life satisfaction.
The results of this US survey were consistent with the conclusions of an earlier DSP survey conducted in Europe (not peer-reviewed), which reported that severity of cognitive impairment predicted HCRU and health-related QoL (Tundia et al., 2012). Comparing patients with moderate/severe to no/mild cognitive impairment, we reported significant differences in HCRU, employment status and living circumstances as well as a clinically important difference in QoL (EQ-5D difference > 0.08) (Le et al., 2013). No other studies were found that reported real-world outcomes for patients with moderate/severe cognitive impairment.
Severe cognitive impairment likely includes impairments in multiple components of neurocognition and social cognition. HCRU has been shown to be associated with working memory, verbal memory, and executive function (Hori et al., 2020). Working memory and verbal memory have also been directly associated with employment and independent living, respectively (Pothier et al., 2019; Shamsi et al., 2011). Multiple aspects of cognition have been associated with QoL (Pascal de Raykeer et al., 2019). This supports the large differences in HCRU, employment, housing, and QoL in patients with moderate/severe vs. no/mild cognitive impairment reported in this survey.
The relationship between cognitive impairment and outcomes among patients with schizophrenia is complex and could be mediated by schizophrenia disease severity. An earlier analysis of this survey population reported significant associations between the level of schizophrenia disease severity and HCRU, employment status, housing circumstances and patient QoL (Kadakia et al., 2021). Future studies could further investigate the causality between schizophrenia disease severity and cognition.
Treatment for cognitive impairment associated with schizophrenia could decrease the clinical and economic burden of disease for patients with schizophrenia. Cognitive remediation, a behavioral training intervention, may improve cognition and functioning but is limited from the HCP perspective by training and staff requirements (Bowie, 2019; Bowie et al., 2020; Vita et al., 2021). No pharmacologic treatment has been indicated for the treatment of cognitive impairment associated with schizophrenia. Trial design modifications, such as screening trial participants for cognitive impairment and stratifying results based on level of cognitive impairment, have been recommended to increase the chance of finding effective treatment for cognitive impairment associated with schizophrenia (Cotter et al., 2019; Keefe et al., 2013; Zhu, 2020).
The DSP survey has several limitations. Firstly, severity of cognitive impairment was based on psychiatrists' judgements of their patients and not confirmed using a validated assessment of cognitive performance. Secondly, the survey was not based on a random sample of psychiatrists or patients. Psychiatrist participation was influenced by willingness to complete the survey and patients that visited the psychiatrist more frequently were more likely to be selected. Additionally, no procedure was in place to confirm the consecutive selection of patients. Therefore, the patients in this analysis may not reflect the general schizophrenia patient population. Thirdly, recall bias was possible for the physician-reported HCRU outcomes and patient-reported QoL outcomes. However, referring to medical records and the short recall period (7 days) should have limited the bias for physicians and patients, respectively. Fourthly, the participation rate was lower for patient self-completion questionnaires among patients with moderate/severe compared to no/mild schizophrenia (43% vs. 54%). If the most severe patients did not complete the questionnaire, QoL results may be a conservative estimate. Fifthly, the number of concomitant medications and pills, which were included as adjustment variables in the regression analysis, could not be converted to standardized units. Finally, causal relationships between cognitive impairment among patients with schizophrenia and patient outcomes could not be tested due to the point-in-time design of this survey.
5. Conclusions
This survey investigated the association between the severity of cognitive impairment and patient outcomes among patients with schizophrenia. Moderate/severe vs. no/mild cognitive impairment was associated with significantly greater HCRU due to relapse, unemployment due to disability, risk of living in supported housing and worse patient QoL. Treatment for cognitive impairment associated with schizophrenia could reduce the clinical and economic burden of schizophrenia.
Previous presentations
Earlier versions of this work were presented as posters at the 2021 American Psychiatric Association Annual Meeting (online, May 1-3, 2021), European College of Neuropsychopharmacology Congress (Lisbon, Portugal, October 2-5, 2021), Annual Neuroscience Education Institute Psychopharmacology Congress (Colorado Springs, CO, November 4-7, 2021) and US Psychiatric and Mental Health Congress (San Antonio, TX, October 29-November 1, 2021).
Availability of data and material
Data collection was undertaken by Adelphi Real World as part of an independent survey, entitled the Adelphi Schizophrenia Disease Specific Programme, subscribed to by multiple pharmaceutical companies of which one was Sunovion. Sunovion did not influence the original survey through either contribution to the design of questionnaires or data collection. All data that support the findings of this survey are the intellectual property of Adelphi Real World.
Declaration of funding
The survey data reported on in this manuscript are from the Adelphi Schizophrenia Disease Specific Programme to which Sunovion subscribed.
CRediT authorship contribution statement
All authors were directly involved in the analysis of the survey data, interpretation of results, drafting of the manuscript and providing final review.
Declaration of competing interest
A. Kadakia, Q. Fan, C. Dembek and G. R. Williams are employees of Sunovion. J. Shepherd, H. Bailey and C. Walker are employees of Adelphi Real World.
Acknowledgements
We thank Barbara Blaylock (Blaylock Health Economics LLC) for providing medical writing support.
References
- Anderson P., Benford M., Harris N., Karavali M., Piercy J. Real-world physician and patient behaviour across countries: disease-specific programmes - a means to understand. Curr. Med. Res. Opin. 2008;24(11):3063–3072. doi: 10.1185/03007990802457040. [DOI] [PubMed] [Google Scholar]
- Bowie C.R. Cognitive remediation for severe mental illness: state of the field and future directions. World Psychiatry. 2019;18(3):274–275. doi: 10.1002/wps.20660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bowie C.R., Bell M.D., Fiszdon J.M., et al. Cognitive remediation for schizophrenia: an expert working group white paper on core techniques. Schizophr. Res. 2020;215:49–53. doi: 10.1016/j.schres.2019.10.047. [DOI] [PubMed] [Google Scholar]
- Brooks R. EuroQol: the current state of play. Health Policy. 1996;37(1):53–72. doi: 10.1016/0168-8510(96)00822-6. [DOI] [PubMed] [Google Scholar]
- Cotter J., Barnett J.H., Granger K. The use of cognitive screening in pharmacotherapy trials for cognitive impairment associated with schizophrenia. Front. Psychiatry. 2019;10:648. doi: 10.3389/fpsyt.2019.00648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeRosse P., Nitzburg G.C., Blair M., Malhotra A.K. Dimensional symptom severity and global cognitive function predict subjective quality of life in patients with schizophrenia and healthy adults. Schizophr. Res. 2018;195:385–390. doi: 10.1016/j.schres.2017.10.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Endicott J., Nee J., Harrison W., Blumenthal R. Quality of life enjoyment and satisfaction questionnaire: a new measure. Psychopharmacol. Bull. 1993;29(2):321–326. [PubMed] [Google Scholar]
- European Pharmaceutical Market Research Association (EphMRA) EphMRA; Basel (CH): 2021. Code of conduct [internet]https://www.ephmra.org/standards/code-of-conduct/ Available from: [Google Scholar]
- Green M.F. Impact of cognitive and social cognitive impairment on functional outcomes in patients with schizophrenia. J. Clin. Psychiatry. 2016;77(Suppl. 2):8–11. doi: 10.4088/JCP.14074su1c.02. [DOI] [PubMed] [Google Scholar]
- Green M.F., Horan W.P., Lee J. Nonsocial and social cognition in schizophrenia: current evidence and future directions. World Psychiatry. 2019;18(2):146–161. doi: 10.1002/wps.20624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Green M.J., Girshkin L., Kremerskothen K., Watkeys O., Quidé Y. A systematic review of studies reporting data-driven cognitive subtypes across the psychosis spectrum. Neuropsychol. Rev. 2020;30(4):446–460. doi: 10.1007/s11065-019-09422-7. [DOI] [PubMed] [Google Scholar]
- Habtewold T.D., Rodijk L.H., Liemburg E.J., et al. A systematic review and narrative synthesis of data-driven studies in schizophrenia symptoms and cognitive deficits. Transl. Psychiatry. 2020;10(1):244. doi: 10.1038/s41398-020-00919-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harvey P.D., Strassnig M.T., Silberstein J. Prediction of disability in schizophrenia: symptoms, cognition, and self-assessment. J. Exp. Psychopathol. 2019;10(3) [Google Scholar]
- Hori H., Atake K., Katsuki A., Yoshimura R. Effects of the number of hospitalizations on cognitive function in Japanese patients with stable schizophrenia. CNS Spectr. 2020:1–6. doi: 10.1017/S1092852920001728. [online ahead of print] [DOI] [PubMed] [Google Scholar]
- ICC/ESOMAR International code on market, opinion and social research and data analytics [internet]. [place unknown]: ICC/ESOMAR. 2016. https://www.esomar.org/uploads/public/knowledge-and-standards/codes-and-guidelines/ICCESOMAR_Code_English_.pdf Available from:
- Kadakia A., Fan Q., Shepherd J., et al. Point-in-time survey of healthcare resource utilization, employment, quality of life and caregiver status by disease severity in patients with schizophrenia in the US. Curr Med Res Opin. 2021:1–10. doi: 10.1080/03007995.2021.2007690. [online ahead of print] [DOI] [PubMed] [Google Scholar]
- Keefe R.S. The longitudinal course of cognitive impairment in schizophrenia: an examination of data from premorbid through posttreatment phases of illness. J. Clin. Psychiatry. 2014;75(Suppl. 2):8–13. doi: 10.4088/JCP.13065su1.02. [DOI] [PubMed] [Google Scholar]
- Keefe R.S., Buchanan R.W., Marder S.R., et al. Clinical trials of potential cognitive-enhancing drugs in schizophrenia: what have we learned so far? Schizophr. Bull. 2013;39(2):417–435. doi: 10.1093/schbul/sbr153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Le Q.A., Doctor J.N., Zoellner L.A., Feeny N.C. Minimal clinically important differences for the EQ-5D and QWB-SA in post-traumatic stress disorder (PTSD): results from a doubly randomized preference trial (DRPT) Health Qual. Life Outcomes. 2013;11:59. doi: 10.1186/1477-7525-11-59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGrath J., Saha S., Chant D., Welham J. Schizophrenia: a concise overview of incidence, prevalence, and mortality. Epidemiol. Rev. 2008;30:67–76. doi: 10.1093/epirev/mxn001. [DOI] [PubMed] [Google Scholar]
- Mesholam-Gately R.I., Giuliano A.J., Goff K.P., Faraone S.V., Seidman L.J. Neurocognition in first-episode schizophrenia: a meta-analytic review. Neuropsychology. 2009;23(3):315–336. doi: 10.1037/a0014708. [DOI] [PubMed] [Google Scholar]
- Murphy T., Krasa H., Lavigne G., et al. Schizophrenia & Psychosis Action Alliance; Alexandria, VA: 2021. Societal costs of schizophrenia & related disorders. [online ahead of print] [Google Scholar]
- Parlar M.E., Heinrichs R.W. Cognitive decline and impairment in schizophrenia spectrum disorders reconsidered. Schizophr. Res. 2021;228:626–632. doi: 10.1016/j.schres.2020.11.020. [DOI] [PubMed] [Google Scholar]
- Pascal de Raykeer R., Hoertel N., Blanco C., et al. Effects of depression and cognitive impairment on quality of life in older adults with schizophrenia spectrum disorder: results from a multicenter study. J. Affect. Disord. 2019;256:164–175. doi: 10.1016/j.jad.2019.05.063. [DOI] [PubMed] [Google Scholar]
- Pothier W., Cellard C., Corbière M., et al. Determinants of occupational outcome in recent-onset psychosis: the role of cognition. Schizophr. Res. Cogn. 2019;18 doi: 10.1016/j.scog.2019.100158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shamsi S., Lau A., Lencz T., et al. Cognitive and symptomatic predictors of functional disability in schizophrenia. Schizophr. Res. 2011;126(1–3):257–264. doi: 10.1016/j.schres.2010.08.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silberstein J., Harvey P.D. Cognition, social cognition, and self-assessment in schizophrenia: prediction of different elements of everyday functional outcomes. CNS Spectr. 2019;24(1):88–93. doi: 10.1017/S1092852918001414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- The EuroQol Group EuroQol–a new facility for the measurement of health-related quality of life. Health Policy. 1990;16(3):199–208. doi: 10.1016/0168-8510(90)90421-9. [DOI] [PubMed] [Google Scholar]
- Tundia N.L., Bhor M., Duhig A.M., Hass S., Perry R., Milligan G. Impact of positive and negative symptoms and cognitive impairment on health outcomes and health care resource utilization in European patients with schizophrenia [poster abstract] Value Health. 2012;15(4):A92. [Google Scholar]
- US Department of Health and Human Services Summary of the Health Insurance Portability and Accountability Act (HIPAA) privacy rule [internet]. Washington (DC): US Department of Health and Human Services. 2003. http://www.hhs.gov/sites/default/files/privacysummary.pdf Available from:
- Vita A., Barlati S., Ceraso A., et al. Effectiveness, core elements, and moderators of response of cognitive remediation for schizophrenia: a systematic review and meta-analysis of randomized clinical trials. JAMA Psychiatry. 2021;78(8):848–858. doi: 10.1001/jamapsychiatry.2021.0620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu T. Challenges of psychiatry drug development and the role of human pharmacology models in early development-a drug developer's perspective. Front. Psychiatry. 2020;11 doi: 10.3389/fpsyt.2020.562660. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data collection was undertaken by Adelphi Real World as part of an independent survey, entitled the Adelphi Schizophrenia Disease Specific Programme, subscribed to by multiple pharmaceutical companies of which one was Sunovion. Sunovion did not influence the original survey through either contribution to the design of questionnaires or data collection. All data that support the findings of this survey are the intellectual property of Adelphi Real World.