Summary
Deep brain stimulation (DBS) entails neurosurgery to implant electrodes in specific brain structures to modulate the behavior of a particular neural circuit. DBS is best known for treating advanced Parkinson disease and can potentially be applicable to other motor and even cognitive dysfunctions. Here, we describe a detailed protocol allowing for electrode preparation, surgical procedures, stimulation delivery, and field potential recordings in both anesthetized and behaving mice, and the benefit evaluation of DBS at the fimbria-fornix by using a fear conditioning test.
For complete details on the use and execution of this protocol, please refer to Hao et al. (2015).
Subject areas: Model Organisms, Neuroscience, Cognitive Neuroscience, Behavior
Graphical abstract
Highlights
-
•
Construction of electrodes for in vivo stimulation or recording in freely moving mice
-
•
Stereotaxic implantation of electrodes under electrophysiological guidance
-
•
Field potential recording and chronic deep brain stimulation in freely moving mice
-
•
Fear conditioning training and memory test using ANY-maze system
Deep brain stimulation (DBS) entails neurosurgery to implant electrodes in specific brain structures to modulate the behavior of a particular neural circuit. DBS is best known for treating advanced Parkinson disease and can potentially be applicable to other motor and even cognitive dysfunctions. Here, we describe a detailed protocol allowing for electrode preparation, surgical procedures, stimulation delivery, and field potential recordings in both anesthetized and behaving mice, and the benefits of evaluating DBS at the fimbria-fornix by using a fear conditioning test.
Before you begin
To deliver DBS and evaluate its benefits in behaving animals, the following essential elements must be prepared (example products in materials and equipment).
-
1.
Dedicated space with a solder station, vise, and a dissection microscope for electrode construction.
-
2.
Materials for electrode construction (electrode wires, connectors, stainless steel tubing).
-
3.
A multimeter to check/verify the wire connections of the electrode assemblies.
-
4.
A procedure suite for mouse survival surgery to implant the electrodes into the mouse brain.
-
5.
An electrophysiology rig for field stimulation and recordings in anesthetized and freely moving mice, respectively.
-
6.
Dedicated space for daily DBS administration.
-
7.
A programmable stimulating device and stimulus isolators with constant current output.
-
8.
Dedicated space and equipment to evaluate the effect of DBS on learning and memory.
-
9.
A histological suite to verify the placement of DBS and recording electrodes in the brain.
Mouse colonies were bred and maintained under a 14 h light: 10 h dark cycle with standard mouse chow and water ad libitum in our on-site AALAS-accredited facility in the Jan and Dan Duncan Neurological Research Institute at Texas Children’s Hospital. (The choice of 14:10 as opposed to 12:12 was chosen by the facility to better mimic the extended period of daylight the animals would normally receive in the summertime.). Female heterozygous mice of Rett syndrome or wild type littermates were used in this study because they are more clinically relevant (Guy et al., 2007). Mice were group-housed before surgery, up to five per cage, and housed individually with nesting material in the cage after surgery to enable undisturbed recovery. All research and animal care procedures were approved by the Baylor College of Medicine Institutional Animal Care and Use Committee.
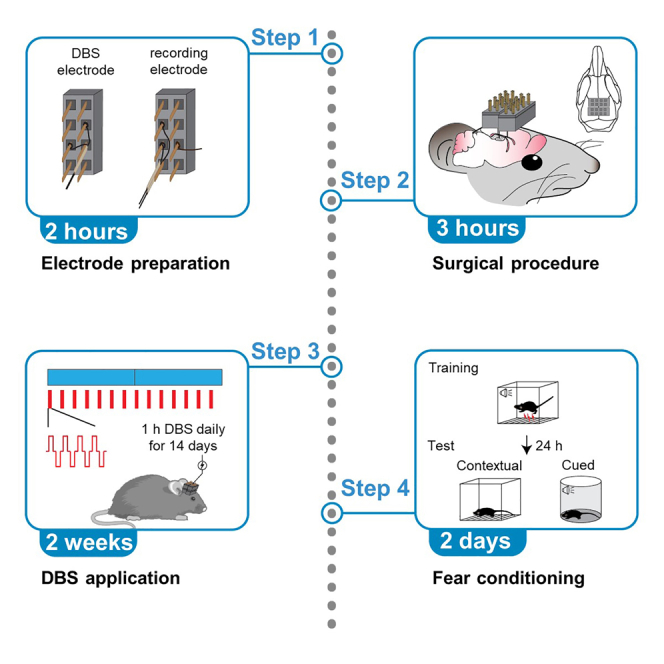
Construction of DBS electrode assembly
Timing: 25 min
The purpose of this step is to construct an electrode assembly for chronic DBS in freely moving mice.
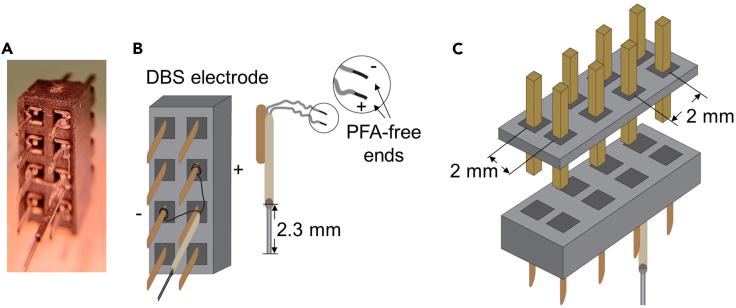
Note: The DBS electrode assembly consists of two wires attached to a female connector for unilateral DBS administration (Figure 1).
-
10.
Cut one piece of 26 Gauge stainless steel tubing (∼6 mm in length). Polish both ends on the diamond disc mounted on a rotary tool to fully open the ends and achieve a final tube length of ∼5 mm. Then flush the tube with Millipore water.
-
11.
Cut 2 pieces of perfluoroalkoxy alkane (PFA)-coated tungsten wire (50 μm bare diameter), each ∼20 mm long. Under a dissection microscope, insert both wires into the 26G tube. Then, bend each tungsten wire by ∼90° at the middle and remove 4–5 mm of the PFA coating from the ends not inserted through the tube.
-
12.
Vertically attach the tube with tungsten wires inside on the corresponding pin of the female connector by soldering them together (Figures 1A and 1B).
-
13.
Use a surgical tweezer to entwine the PFA-free portion of the tungsten wires around the base of the corresponding pins on the female connector and apply solder to strengthen the connection (Figures 1A and 1B).
Note: Solder flux may help with the soldering of stainless steel tube and tungsten wires. Avoid extra solder entering into the connector holes.
-
14.
Under a scaled dissection microscope, use surgical scissors to cut both coated tungsten wires that came out of the stainless steel tube so that the wires extend out of the tube by 2.3 mm (Figure 1B).
-
15.
Insert a matched double male connector for further connections (Figure 1C), and verify each wire connection with a multimeter to avoid shortcuts between the positive and negative poles.
Note: The free tips of the two tungsten wires should be as close as possible (no more than 0.15 mm apart).
Figure 1.
Wiring and layout of DBS electrode assembly
(A) Photograph of DBS electrode assembly.
(B and C) Diagram of the DBS electrode assembly. (B) Left, channel layout on a female connector (bottom view) and the wiring of each electrode wire. Right, finalized electrode wires extend the stainless steel tubing by 2.3 mm. The PFA-free ends are entwined around the base of the corresponding pins on the female connector. (C) Bottom, a female connector (top view) with wired electrodes. Top, the matched double male connector. PFA, perfluoroalkoxy alkane.
Construction of recording electrode assembly
Timing: 45 min
The purpose of this step is to construct an electrode assembly for field recordings in anesthetized and behaving mice.
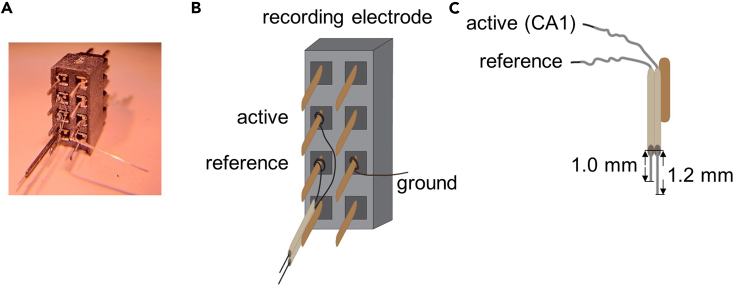
Note: The active probe of the recording electrode assembly is designed to target the CA1 region of the dorsal hippocampus (Figure 2). The reference probe is positioned in the ipsilateral corpus callosum.
-
16.
Cut two pieces of stainless steel tubing (30 Gauge), each ∼5 mm long. Polish both ends of each tube on the diamond disc mounted on a rotary tool to fully open the ends and to achieve a final tube length of ∼4 mm. Then flush the tubes with Millipore water. Dry the tubes and solder them together (side by side) to make a bundle.
Note: To prevent dust created during the polishing from blocking the tube ends, hold the tube with the tweezers and flick them occasionally to remove the dust. Also, be careful to avoid blocking tube holes by the fluid solder while soldering them together.
-
17.
Cut two pieces of PFA-coated tungsten wire, each ∼20 mm long. Under a dissection microscope, insert the wire into each tube. Then, bend each tungsten wire by ∼90° at the middle and remove 4–5 mm of PFA coating from the ends not inserted through the tubes.
-
18.
Vertically attach the tube bundle with tungsten wires inside on the corresponding pin of the female connector by soldering them briefly (Figures 2A and 2B).
-
19.
Use a surgical tweezer to entwine the PFA-free portion of the tungsten wires around the base of corresponding pins on the female connector (Figures 2A and 2B).
Note: Use solder flux to help with the soldering of the stainless steel tubes and the tungsten wires. Avoid extra solder entering into the connector holes.
-
20.
Under a scaled dissection microscope, use surgical scissors to cut both coated tungsten wires that came out of the stainless steel tubes so that one wire (active) extends past the tube tip by 1.2 mm and the other wire (reference) by 1.0 mm (Figure 2C).
-
21.
Use a multimeter to check/verify the proper connection of each wire.
-
22.
For the ground connection of the preamplifier (Figures 2A and 2B), cut a piece of PFA-coated silver wire (127 μm bare diameters) that is ∼20 mm long. Use a razor blade to remove the PFA coating by 3–4 mm at one end of the wire under a dissection microscope. Use a surgical tweezer to entwine the exposed end of silver wire around the base of the corresponding pin on the female connector. Solder each connection at the wired place of the connector.
Figure 2.
Wiring and layout of the recording electrode assembly
(A) Photograph of the recording electrode assembly.
(B and C) Diagram of the recording electrode assembly. (B) Channel layout on a female connector and the wiring of each electrode wire. (C) Finalized active electrode (targeting CA1 pyramidal cell layer) extends the stainless steel tube by 1.2 mm. The reference probe (aiming at the ipsilateral corpus callosum) is 0.2 mm shorter.
Build customized adaptor and wiring system for DBS application
Timing: 50 min
The purpose of this step is to prepare the cable that connects the implanted DBS head post to the stimulus isolator.
-
23.
Cut two pieces of thin wire (AWG 38) with ∼100 cm length and twist them together. Use a razor blade to remove the Teflon coating by 3–4 mm at both ends of each wire under a dissection microscope.
Note: Practice adjusting the cutting strength to avoid cutting through the wire while removing the coating. A blunt razor may work better than a new, sharp one.
-
24.
Connect the wires to the proper pins of the female connector by soldering. Make sure that the pinout matches the negative/positive poles of DBS electrode assembly. The other ends of the wires connect to the stimulus isolator through a commutator.
Note: A commutator allows uninterrupted electric connections while the tethered mice are moving inside the chamber during DBS.
-
25.
Use the 2-component special adhesive to glue the wire-pin connections and dry out.
-
26.
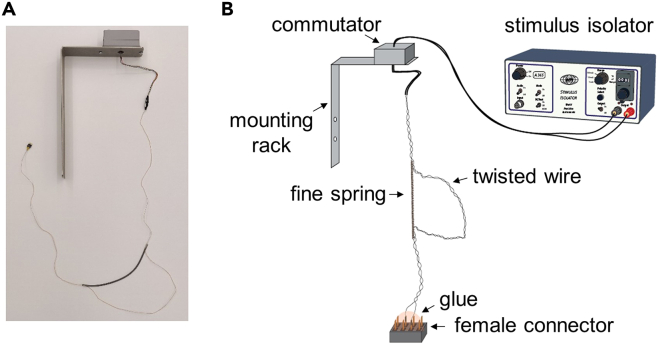
Attach a coil spring (18 cm in length, very fine) to the wire bundle to fold the middle 2/5 of the cable (Figure 3). The spring allows for some changes in wire length caused by mouse movement inside the DBS chamber.
Figure 3.
Wire system for DBS application in freely moving mice
(A) Photograph of the wire system.
(B) Diagram of the wire system. The wire connects the implanted DBS head post to the output of the stimulus isolator.
Key resources table
| REAGENT or RESOURCE | SOURCE | IDENTIFIER |
|---|---|---|
| Chemicals, peptides, and recombinant proteins | ||
| Oxygen | Airgas | UN1072 |
| Saline | Hospira, Inc. | NDC 0409-1966-02 |
| Meloxicam | Covetrus North America | NDC 11695-6936-1 |
| Isoflurane | Covetrus North America | NDC 11695-6777-2 |
| Artificial tears ointment | AKORN | NDC 17478-062-35 |
| Hair remover lotion | Church & Dwight Co., Inc | LL9023 |
| 70% ethanol | VWR | 89370-084 |
| Povidone-iodine | Purdue Products L.P. | NDC 67618-154-16 |
| Lidocaine hydrochloride | Sigma-Aldrich | 1366013 |
| Bupivacaine hydrochloride | Sigma-Aldrich | 1078507 |
| 8% hydrogen peroxide | The One Minute Miracle | #7722-84-1 |
| Dental cement (C&B Metabond) | Parkell | 375-0407 |
| 3M Vetbond tissue adhesive | Amazon | 1469SB |
| Acetic acid | Sigma-Aldrich | A6283 |
| 2-methylbutane | Millipore Sigma | Item # M32631 |
| Cresyl violet | Millipore Sigma | Item # C5042 |
| Tissue-Tek® O.C.T. compound | Sakura Finetek USA, Inc. | REF 4583 |
| Experimental models: organisms/strains | ||
| Mouse: female Mecp2+/- mice and wild type littermates (FVB.129 background), 1.5-2 months of age at surgery | (Guy et al., 2007) | N/A |
| Software and algorithms | ||
| Data acquisition software | Molecular Devices | pCLAMP 10 |
| ANY-maze behavioral tracking software | Stoelting Co. | Version 6.30 |
| Other | ||
| PFA-coated tungsten wire | A-M Systems | Cat. # 795500 |
| PFA-coated silver wire | A-M Systems | Cat. # 786000 |
| Stainless steel tubing, 30 Gauge | A-M Systems | Cat. # 832000 |
| Stainless steel tubing, 26 Gauge | A-M Systems | Cat. # 836000 |
| Sand paper | 3M | 400 Grit |
| Diamond disc (X926.7) | thin-flex | P71-0889 |
| Rotary tool kit | Avid Power | AEDG128 |
| Solder flux | MG Chemicals | FLUX H150 |
| Nylon surgical suture | AD Surgical | #S-N518R13 |
| Male connector | Harwin | M22-2580405 |
| Female connector | Harwin | M22-7140442 |
| Teflon-coated thin wire | Cooner Wire | CZ1187 |
| 2-component special adhesive | UHU®PLUS | 45700 |
| Coil spring | D.R. TEMPLEMAN CO. | 0.008" x 0.121"OD x 7" |
| House light | CEC Industries | #44 (6.3V, 1.575W ) |
| USB camera | Imaginesource | DMK 22AUC03 |
| Camera lens | FUJINON | YV5x2.7R4B-2 |
| Loudspeaker | BOSS AUDIO SYSTEMS | BRS40 |
| VWR micro slides | VWR International, LLC. | Cat. # 48311-703 |
| VWR micro cover glass | VWR International, LLC. | Cat. # 48393081 |
| Dissection microscope | A-M Systems | EMZ-5TR |
| Stereo microscope | ZEISS | Stemi 2000-C |
| Soldering station | Weller | Model WES51 |
| Multimeter | Fluke | Fluke 179 |
| 0.5 mL insulin syringes | ADW Diabetes | # SY8290328279 |
| Isoflurane vaporizer | Veterinary Anesthesia Systems, Inc. | Matrx VIP 3000 |
| Fiber optic illuminator | A-M Systems | 725910 |
| Small animal stereotaxic instrument | David Kopf | Model 962 |
| Glass bead sterilizer | Cellpoint Scientific, Inc. | 5-1450 |
| Homeothermic blanket system | Harvard Apparatus | 507220F |
| Animal fur trimmer | WAHL | #9861-900 |
| High speed drill | The Foredom Electric Company | Model 1474 |
| Heat therapy pump | Gaymar Industries, Inc. | Model # TP650 |
| Field potential recording chamber | Pinnacle Technology Inc. | 8228 |
| Faraday cage | Technical Manufacturing Corporation | No.81-344-04 |
| Stimulus isolator | World Precision Instruments | SYS-A365R |
| Stimulus isolator charger | World Precision Instruments | SYS-A362 |
| 4-Channel preamplifier | Pinnacle Technology Inc. | 8406-SE4 |
| 8-Channel mouse commutator | Pinnacle Technology Inc. | 8408 |
| Differential AC amplifier | A-M Systems | Model 1700 |
| Digitizer | Molecular Devices | 1440A |
| Programmable stimulator | A.M.P.I. | Master-8 |
| Batteries | Amazon | Size AA |
| Digital interface for ANY-maze | Stoelting | AMi-2 |
| Audio interface for ANY-maze | Stoelting | AMi-2 |
| Sound attenuating cubicle | Med Associates, Inc. | ENV-017M |
| Fear conditioning chamber | Med Associates, Inc. | ENV-307A |
| Shocker | Med Associates, Inc. | ENV-414S |
| Surgical tools: fine scissors, scalpel, fine forceps, coarse forceps, 0.7 mm burs | Fine Science Tools | https://www.finescience.com/en-US/ |
| Straight fissure crosscut bur | Dentalaire | https://www.dentalaireproducts.com/products/burs-and-diamond-discs/ |
| Cryostat microtome | Leica Biosystems | CM3050 S |
| Microscope | World Precision Instruments | W30S-LED |
Materials and equipment
Equipment and supplies for electrode construction and DBS wire system:
-
•
Dissection microscope (e.g., EMZ-5TR, A-M Systems).
-
•
Soldering station (e.g., MES51, Weller).
-
•
Solder flux (e.g., FLUX H150, MG Chemicals).
-
•
Rotary tool kit (e.g., AEDG128, Avid Power).
-
•
Diamond disc (e.g., P71-0889, thin-flex).
-
•
2-component special adhesive (Item # 45700, UHU®PLUS).
-
•
Stainless steel tubing, 26 Gauge (e.g., Cat. # 836000, A-M Systems).
-
•
Stainless steel tubing, 30 Gauge (e.g., Cat. # 832000, A-M Systems).
-
•
Sand paper (e.g., 400 Grit, 3M).
-
•
PFA-coated tungsten wire (e.g., Cat. # 795500, A-M Systems).
-
•
PFA-coated silver wire (e.g., Cat. # 786000, A-M Systems).
-
•
Teflon-coated thin wire (e.g., CZ1187, Cooner Wire).
-
•
Male connector (e.g., M22-2580405, Harwin).
-
•
Female connector (e.g., M22-7140442, Harwin).
-
•
Coil spring.
Setup and supplies for mouse surgery:
-
•
Local and general analgesics (dissolved in sterile saline). Local: e.g., 50/50 mix of 2% lidocaine & 0.5% bupivacaine diluted 1:20 in sterile saline, 0.04 mL/10 g body weight (6–8 h duration of action), injected subcutaneously around the site of implant. General: e.g., meloxicam, 5 mg/kg, subcutaneously (24 h duration of action).
-
•
Isoflurane anesthesia system (e.g., Matrx VIP 3000, Veterinary Anesthesia Systems, Inc.).
-
•
Oxygen supply (medical grade).
-
•
Small animal stereotaxic instrument (e.g., Model 962, David Kopf).
-
•
Stereo microscope (e.g., Stemi, 2000-C, ZEISS).
-
•
Fiber optic illuminator (e.g., Model 725910, A-M Systems).
-
•
Homeothermic blanket system (e.g., Model 507220F, Harvard Apparatus).
-
•
High speed drill (e.g., Model 1474, The Foredom Electric Company).
-
•
Glass bead sterilizer (e.g., Model 5-1450, Cellpoint Scientific, Inc.).
-
•
Heat therapy pump (e.g., TP650, Gaymar Industries, Inc.).
-
•
Animal fur trimmer (e.g., Item # 9861-900, WAHL).
-
•
Hair remover lotion (e.g., Item # LL9023, Church & Dwight Co., Inc.).
-
•
Surgical tools: fine scissors, scalpel, fine forceps, coarse forceps, etc. (e.g., from Fine Science Tools).
-
•
Straight fissure crosscut bur (e.g., Item #: 557, Dentalaire)
-
•
Isoflurane (e.g., NDC 11695-6777-2).
-
•
Dental cement (e.g., Item # 375-0407, Parkell).
-
•
Tissue adhesive (e.g., Item # 1469SB, 3M Vetbond).
-
•
Nylon surgical suture (e.g., Item # S-N518R13).
-
•
Dental drill bit with 0.7 mm tip.
-
•
Artificial tears ointment (e.g., NDC-17478-062-35, AKORN).
-
•
Sterile saline (e.g., NDC 0409-1966-02, Hospira, Inc.).
-
•
70% Ethanol.
-
•
Povidone-iodine.
-
•
8% Hydrogen peroxide.
-
•
0.5 mL syringe with 30-gauge needle.
Electrophysiology rig for field stimulation and recordings in anesthetized and freely moving mice:
-
•
Faraday cage (e.g., Model No. 81-344-04, Technical Manufacturing Corporation).
-
•
Stimulus isolator (e.g., SYS-A365R, World Precision Instruments).
-
•
Stimulus isolator charger (e.g., SYS-A362, World Precision Instruments).
-
•
Differential AC amplifier (e.g., Model 1700, A-M systems).
-
•
Digitizer (e.g., Model 1440A, Molecular Devices).
-
•
Data acquisition software (e.g., pClamp 10, Molecular Devices).
-
•
Preamplifier (e.g., Model 8406-SE4, Pinnacle Technology Inc.).
-
•
Commutator (e.g., Model 8408, Pinnacle Technology Inc.).
-
•
Recording chamber (e.g., Model 8228, Pinnacle Technology Inc.).
-
•
Multimeter (e.g., Model 179, Fluke).
-
•
±4.5 Volts power supply.
Setup for DBS:
-
•
Programmable stimulator (e.g., Master-8, A.M.P.I.).
-
•
Stimulus isolator (e.g., SYS-A365R, World Precision Instruments).
-
•
Stimulus isolator charger (e.g., SYS-A362, World Precision Instruments).
-
•
Commutator (e.g., Model 8408, Pinnacle Technology Inc.).
-
•
Plexiglas chamber (e.g., Model 8228, Pinnacle Technology Inc.).
Setup for fear conditioning paradigm:
-
•
Sound attenuating cubicle (e.g., ENV-017M, Med Associate, Inc.).
-
•
Fear conditioning chamber (e.g., ENV-307A, Med Associate, Inc.).
-
•
Shocker (e.g., ENV-414S, Med Associate, Inc.).
-
•
USB camera (e.g., DMK 22AUC03, The Imagingsource).
-
•
Camera lens (e.g., YV5x2.7R4B-2, FUJINON Corporation).
-
•
Loudspeaker (e.g., BRS40, BOSS AUDIO SYSTEMS).
-
•
House light (e.g., #44 (6.3V, 1.575W ), CEC Industries).
-
•
ANY-maze behavioral tracking software (Version 6.30, Stoelting Co.).
-
•
Digital interface for ANY-maze (Ami-2, Stoelting Co.).
-
•
Audio interface for ANY-maze (Ami-2, Stoelting Co.).
-
•
70% ethanol.
-
•
1% acetic acid.
Equipment and materials for histology
-
•
Cryostat microtome (e.g., CM3050 S, Leica).
-
•
Microscope (e.g., W30S-LED, World Precision Instruments).
-
•
2-methylbutane (e.g., Item # M32631, Millipore Sigma).
-
•
Cresyl violet (e.g., Item # C5042, Millipore Sigma).
-
•
Tissue-Tek® O.C.T. compound (e.g., REF 4583, Sakura Finetek USA, Inc.).
-
•
VWR micro slides (e.g., Cat. No. 48311-703, VWR International, LLC.).
-
•
VWR micro cover glass (e.g., Cat No. 48393081, VWR International, LLC.).
Alternatives: The specific products listed above are examples that we have used successfully, but they could be replaced by similar products.
Step-by-step method details
Surgical preparation
Timing: 30 min
-
1.Surgical instruments
-
a.Prepare scissors, blunt-end forceps, scalpel, spring scissors, spatula, burs, and needle holder.
-
b.Autoclave all these instruments.
-
a.
-
2.Drugs and chemicals
-
a.Anesthetic: isoflurane.
-
b.Analgesic: meloxicam (0.5 mg/mL), 50/50 mix of 2% lidocaine and 0.5% bupivacaine diluted 1:20 in sterile saline.
-
c.70% ethanol, betadine, 8% hydrogen peroxide.
-
a.
-
3.
Isoflurane anesthesia system, oxygen (medical grade), exhaust vacuum system of isoflurane.
-
4.
Stereotaxic apparatus, dipper, fur trimmer, hair remover lotion, heating blanket, eye ointment, drill, cotton swabs, dental acrylic, suture, tissue glue, disposable syringes, gown, gloves, masks.
Surgical implantation of the electrodes
Timing: 2 h
All the procedures should be performed under aseptic conditions that include appropriate personal protective equipment, autoclaved instruments, and a glass bead sterilizer.
-
5.
Set all autoclaved surgical instruments on a stainless steel tray.
-
6.
Inject mice at 6–8 weeks of age with meloxicam (5.0 mg/kg, S.C.) 30 min prior to administering anesthesia.
-
7.
Use an isoflurane vaporizer to anesthetize the mice with 2.5 % isoflurane in oxygen (1.0 L/min flow rate) for ∼5 min in the induction chamber. Fully anesthetized mice will not exhibit tail- or foot-pinch reflexes.
-
8.
Take the mice out from the induction chamber and lay them flat on a diaper. Carefully shave the region of interest and the surrounding area with a fur trimmer. Then evenly apply hair remover lotion on the shaved surgical area for ∼10 s to completely remove the fur from the skin.
-
9.
Put the mice back into the induction chamber until they lose pinch reflexes again. Then, quickly mount the anesthetized mice on the stereotaxic frame by locking the incisor and gently inserting the ear bar tips into the ear canals.
-
10.
Switch the isoflurane anesthesia to the nose connection of the frame. The mice should rest comfortably on a heating blanket. Reduce the isoflurane concentration to 1.0%–2.0% and maintain the anesthesia level throughout the procedure.
-
11.
Use a feedback-controlled heating blanket to maintain the mice’s body temperature at 36°C–37°C. Insert the temperature sensor probe with a lubricated tip part 7–8 mm into the rectum.
-
12.
Cover both the mice’s eyes with lubricant ophthalmic ointment to protect them from dryness and bright light during the surgery.
-
13.
Disinfect the skin of the surgical region with 70% ethanol followed by povidone-iodine for three runs.
-
14.
Inject a 50/50 mix of 2% lidocaine and 0.5% bupivacaine diluted 1:20 in sterile saline (0.04 mL/10 g body weight) subcutaneously along the incision line. Then wait for 2–3 min before making the incision.
-
15.
Gently hold the skin with blunt forceps and use a scalpel to make an incision along the midline between the eyes and the occiput (∼15 mm long) and pull the skin to the sides.
-
16.
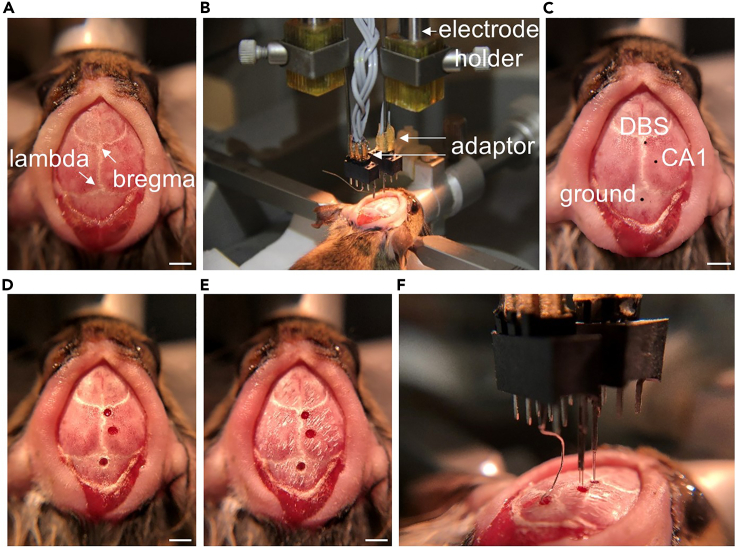
Use a scalpel blade together with spring scissors to clean the connecting tissues. Then, use a cotton swab soaked with 8% hydrogen peroxide followed with sterile saline to expose the skull surface. Let it dry so that the suture lines (bregma, lambda, etc.) on the skull are clearly visible (Figure 4A).
CRITICAL: Measure the height of the bregma and lambda and make sure that they are on the same vertical level, if necessary, by adjusting the head positioning. This is the premise of coordinate setting (Paxinos and Franklin, 2001).
-
17.Determine the coordinates for the targets of DBS and hippocampal recordings.
-
a.Attach the two electrode assemblies (DBS and recording electrodes) to the electrode holders mounted on the stereotaxic arms with custom-made adaptors (Figure 4B).
 CRITICAL: The adaptors for DBS and recording electrodes are specially made so that one side matches the designed pins of the electrodes and the other side connects to the output of the stimulus isolator and input of signal acquisition system, respectively.
CRITICAL: The adaptors for DBS and recording electrodes are specially made so that one side matches the designed pins of the electrodes and the other side connects to the output of the stimulus isolator and input of signal acquisition system, respectively. -
b.Determine the coordinates for each electrode assembly (DBS electrode: P0.2R0.24H2.3; recording electrode: P2.0R1.0H1.3 (the longer, active probe to the CA1); ground electrode: subdural space over the cerebellum) (Paxinos and Franklin, 2001) and mark each point on the skull with a black marker (Figure 4C).
-
c.Carefully use a surgical high-speed drill (0.7 mm tip diameter) to make the openings that allow electrode insertion at each mark on the skull (Figure 4D).
-
d.Then, make rough on the rest of the exposed skull surface for better cementing contact (Figure 4E). Gently touch the skull surface with the rolling edge of the straight fissure crosscut bur mounted to the surgical drill to make it rough evenly. Clean the skull with a cotton swab lightly soaked with 8% hydrogen peroxide and let it dry before electrode insertion.
-
a.
-
18.Evoked response-guided implantation of DBS and recording electrodes.
-
a.Connect the output of the stimulus isolator to the designed pins of the DBS electrode assembly. Then, connect the designed pins of the recording electrode assembly to the input of the Differential AC Amplifier (A-M Systems). The output of the amplifier goes to the inputs of the digitizer (DigiData 1440A, Molecular Devices) and the output of the digitizer goes to a USB port of the computer (Figure 5).
-
b.Use pClamp 10 software for data acquisition and experimental control. Use one of the digital outputs of the digitizer to trigger the stimulus isolator.
-
c.Set the stimulation as a single pulse: 0.1 ms pulse duration, 0.2 mA stimulus intensity, and repeat every 30 s.
-
d.Set the data acquisition at 10 kHz sampling rate, 100× amplification, 0.1 Hz–5 kHz filter band, and 50 ms sweep length.
-
e.Implant the ground wire to the subdural space over the cerebellum (Figure 4F).
-
f.Slowly lower both DBS electrode towards the fimbria-fornix and the recording electrode aimed at the CA1, to depths of 2.0 mm and 1.0 mm, respectively, as the pre-set depths.
-
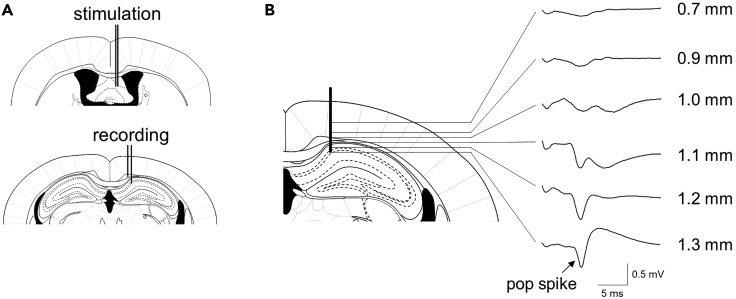
g.Start to record the evoked potentials induced by stimulation through the DBS electrode. Lower the DBS electrode and/or recording electrodes by 0.1 mm every 10 min and record the evoked responses for 3 min at each depth step. The depth profile of the evoked responses along the track of the recording electrode is shown in Figure 6. Once the DBS electrode reaches the fimbria-fornix and the recording electrode arrives at the pyramidal cell layer of the CA1 (e.g., the last depth of recording at 1.3 mm depth), stop lowering the electrodes and record the evoked response for 10 min.
-
h.Wait an additional 20 min before applying the cement.
-
a.
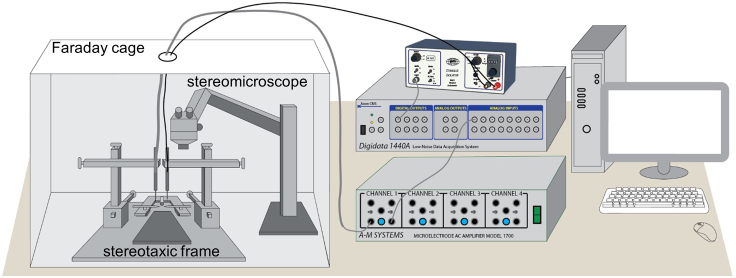
CRITICAL: 1) To reduce the interference of environmental electromagnetic noises, the mice should stay inside a Faraday cage that is properly grounded for the surgical procedure and signal recording (Figure 5); 2) It is important to slowly lower the electrode probes, increment by increment. This is to minimize the mechanical pressure of the electrodes on the brain tissue, which may cause the electrode to be placed too deep after the tissue bounces back; 3) it is possible to see tiny response or no response at all due to misplacement of the electrodes, especially the DBS electrode. If a second try of the DBS electrode insertion with 0.1–0.2 mm horizontal shift does not improve recording, terminate the attempt to implant the electrode and euthanize this animal; 4) during insertion of the electrodes, apply one or two small drops of sterilized saline at the skull openings to keep the exposed parts of the brain surface moist.
-
19.
Apply dental cement (C&B Metabond) sequentially at the skull openings with electrode wires, the rest of the skull surface, and the space between the skull and the bottom half of the female connectors. Make sure that all of the wires and wire connections at the connector pins are embedded into the cement.
-
20.
After the acrylic hardens (∼20 min), suture the incised skin at the rostral and the caudal. Attach the skin edges at both sides on the cement surface using tissue glue.
CRITICAL: Store the cement kit in 4°C to allow a longer operation window while the cement components are still cold during the procedure. Pay careful attention to avoid the fluid cement or the tissue glue entering the connector holes of the electrode assemblies. Failure to do so may not allow removing the adaptors from the electrode assemblies at the end of surgery or cause open electronic circuit(s) for DBS and/or field recordings afterwards.
-
21.
Withdraw the ear bars and loosen the nose clamp. Carefully detach the implanted head posts from the connected adaptors and insert the double male connectors into the connector holes of the electrode assemblies.
-
22.
Transfer the implanted mouse into a pre-warmed cage that is heated by a heat-therapy pump until the mouse completely wakes up from the anesthesia, usually within 30 min.
-
23.
For post-surgical care, singly house the animal and administer meloxicam (5.0 mg/kg, S.C.) daily for at least 3 days to reduce pain. Carefully observe the post-surgical recovery of the mice and provide necessary treatment, if there is minor dehydration or local infection, following approved procedures.
-
24.
Give the mice a 2-week recovery from the surgery before the next step.
Figure 4.
Surgical procedures for the implantation of DBS and recording electrodes
(A) The bregma and lambda are identified after the skull is cleaned and dried. Scale bars in (A), (C), (D), (E): 2 mm.
(B) Electrode assemblies mounted on the electrode holder of the stereotaxic arm through custom-made adaptors.
(C) Horizontal sites of the DBS, CA1, and ground are determined and marked on the skull.
(D) Small openings that allow electrode insertion are made with a high-speed drill.
(E) Rough skull surface for better cementing contact.
(F) Insertion of DBS, recording, and ground electrodes following coordination.
Figure 5.
Equipment setup for evoked response-guided implantation of DBS and recording electrodes
Surgical procedures are performed inside a Faraday cage. pClamp software is used to control the stimulation and data acquisition through the Digidata 1440A digitizer.
Figure 6.
Electrophysiological guidance of electrode implantation
(A) Schematic illustration of the targets for DBS probe (top) and the recording electrodes (bottom) in the brain.
(B) Depth profile of the evoked potentials induced by stimulation of the fimbria-fornix. Numbers on the right of each representative trace indicate the active recording electrode depth from the bregma after the DBS electrode reached the final depth.
Determine the DBS current intensity for each mouse
Timing: 30 min
The goal of this step is to optimize the current intensity of DBS for each behaving mouse (Hao et al., 2015; Shirvalkar et al., 2010; Suthana et al., 2012). Setting up the equipment for stimulation and recording is the same as for the surgical implantation except that the evoked potential signal is picked up through a preamplifier.
-
25.
Connect the preamplifier (± 4.5 V power supply) to the implanted recording electrode assembly and leave the animal in the recording chamber with corn bedding inside the Faraday cage. The output of the preamplifier is connected to the input of the differential AC amplifier via a commutator (Figure 7). Signals are amplified (100×, preamplifier and amplifier together), filtered (0.1 Hz–5 kHz bandpass), and digitized at 10 kHz. Connect the output of the stimulus isolator to the DBS electrode assembly through the commutator.
-
26.
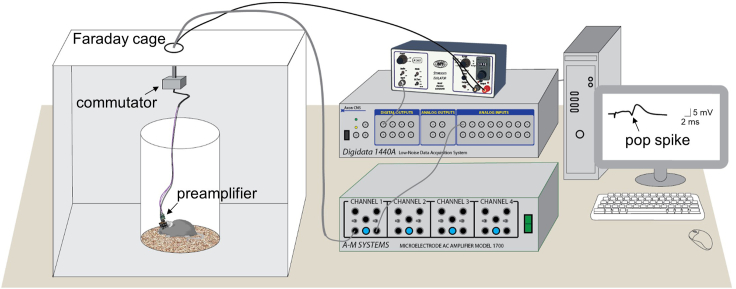
Scan the evoked responses with incremental stimulus intensity of a single pulse (60 μs duration) to determine the threshold for a population spike (pop spike, Figure 7). Take 80% of the threshold as the current intensity of DBS for this given mouse.
CRITICAL: The DBS current intensity is always below the threshold required to elicit an afterdischarge (e.g., a neuronal discharge that occurs after termination of the stimulus).
Figure 7.
Experimental setup for the recording of evoked potentials in the CA1 of freely moving mice
Both stimulation and recording signals pass through a commutator that counterbalances the animal movement inside the testing chamber. A representative threshold response of the pop spike is displayed.
Chronic DBS application in freely moving mice
Timing: 1.5 h
The goal of this step is to set up the system for chronic DBS in freely moving mice and administer the daily DBS.
-
27.Set up the DBS system (Figure 8).
-
a.Use a programmable stimulator (e.g., Master-8) and set the output as continuous train stimulation at 130 Hz, 60 μs pulse duration.
-
b.Connect the output of the programmable stimulator (e.g., Master-8) to the input of a stimulus isolator with constant current output (e.g., A365R). Set the isolator output as a bipolar rectangular pulse model, and the stimulus intensity to the given current value for each animal.
-
a.
-
28.
Connect the isolator output to the commutator and then to the implanted DBS electrode assembly through the custom-made adaptor and wire system (Figure 3).
-
29.
Leave the mouse in the Plexiglas chamber and deliver the chronic daily DBS (e.g., 1 h per day for 2 weeks in this study).
CRITICAL: 1) To ensure delivery of DBS, use a multimeter to check the connection between the stimulator and the DBS electrode assembly daily before DBS; 2) Habituate the mice in the DBS room for 30 min daily before connecting them to the DBS system; 3) Experimenter should be present during the DBS session to make sure the wire connections remain intact and monitor the mouse for any untoward events (e.g., you may discover an unexpected seizure tendency in a given mouse model and have to reduce or stop DBS).
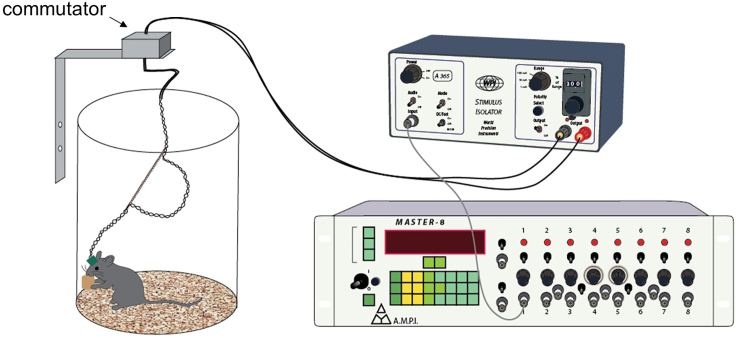
Figure 8.
System for chronic DBS delivery
A Master-8 stimulator generates the stimulus pattern that controls the output of a stimulus isolator. The constant current output pulses are delivered to the mouse through a commutator and the customized wiring system as shown in Figure 3.
Classic fear conditioning task
Timing: 30 min habituation + 10 min training + 20 min testing
The goal of this step is to evaluate the benefits of forniceal DBS on hippocampal learning and memory. ANY-maze software package provides experimental control, data acquisition and analysis.
-
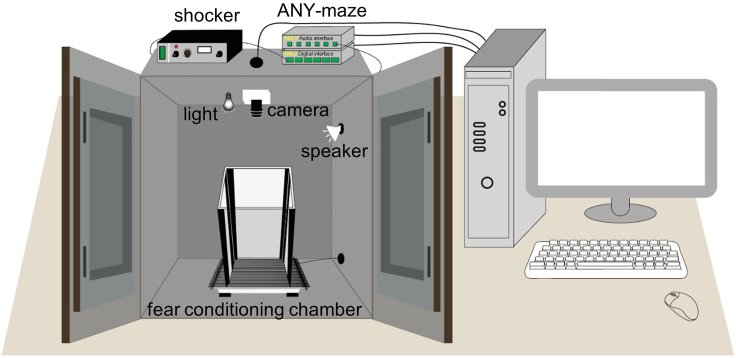
30.Set up the fear conditioning system (Figure 9).
-
a.Inside a sound attenuation cubicle, set up the fear conditioning chamber, an overhead USB camera, a loudspeaker, and a house light.
-
b.Connect the metal grating floor of the fear conditioning chamber to the electric shocker.
-
c.Install ANY-maze software on a PC computer.
-
d.Connect the USB camera to the computer.
-
e.Use AMi 2 audio and digital interfaces of ANY-maze to connect the loudspeaker and the shocker with the computer, respectively.
-
a.
-
31.
Set up the experimental parameters: sound, 5 KHz, 85 dB tone; foot shock, 0.7 mA current, 2 s.
-
32.
Three weeks after the 2-week DBS session, habituate the mice for 30 min before training or tests in the test room with 150 lux light intensity and 60 dB background white noise.
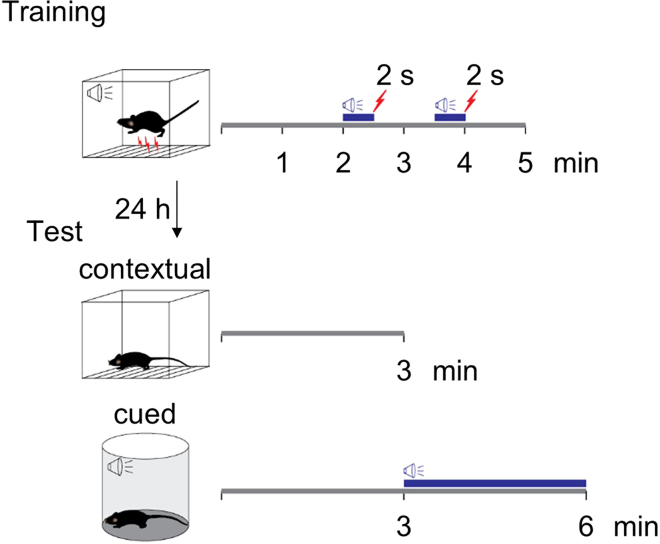
-
33.Fear conditioning training (Figure 10).
-
a.Clean the fear conditioning chamber with 70% ethanol.
-
b.Put a mouse into the fear conditioning chamber. After 2 min of exploration, deliver the first tone – foot shock pairing (30 s tone co-terminated with 0.7 mA, 2 s foot shock). Repeat this tone – foot shock pairing 1 min later. Bring the mouse to the home cage after an additional minute.
-
c.Clean the chamber with 70% ethanol for the next mouse.
-
a.
-
34.Retention test of fear memory 24 h after fear conditioning training.
-
a.For contextual fear memory, put the mice back into the training chamber once it has been cleaned with 70% ethanol. Record the mouse behavior for 3 min without tone or foot shock presentation for off-line analysis.
-
b.For the cued fear memory test, place the mice into a new environment that is different from the training chamber in shape, texture, smell (1% acetic acid), and house light intensity. After 3 min of exploration, the same tone for the training is presented for 3 min. Record the mouse behavior for a total of 6 min for off-line analysis.
-
a.
CRITICAL: Before using ANY-maze tracking system to score the freezing level of mice, it is crucial to manually calibrate the settings of freezing detection.
Figure 9.
Fear conditioning system
The fear conditioning chamber is set inside a sound attenuation cubicle mounted with a loudspeaker, an overhead camera, and a house light. ANY-maze is used to control the delivery of sound and foot shock, record the mouse behavior, and analyze the data.
Figure 10.
Fear conditioning protocol
For fear conditioning training, after 2 min of exploration in the conditioning chamber, mice receive two pairs of tone-foot shock presentations which are separated by 1 min. For contextual memory tests, the mice are scored for 3 min in the conditioning chamber without tone or foot shock. For cued memory tests, mice in a novel context receive 3 min of tone presentation after 3 min of exploration in the new chamber.
Histological verification of electrode placement
Timing: 2 h
The goal of this step is to histologically verify the tip sites of DBS and recording electrodes in the mouse brain.
-
35.
Euthanize the mice at the end of experiments. Then, apply anodal current (30 μA, 8 s) through one of the DBS electrodes and the recording electrode to the CA1 region.
-
36.
Dissect the mouse brain and freeze it in cold 2-methylbutane. Store the brain in −20°C for at least 24 h before sectioning.
-
37.
Cut the brain for 30 μm-thick sections using a cryostat microtome and mount the sections on the glass slides directly.
-
38.
Stain the sections with 0.4% cresyl violet for ∼1 min.
-
39.
Examine the sites of DBS and recording electrodes under a microscope (Figure 11).
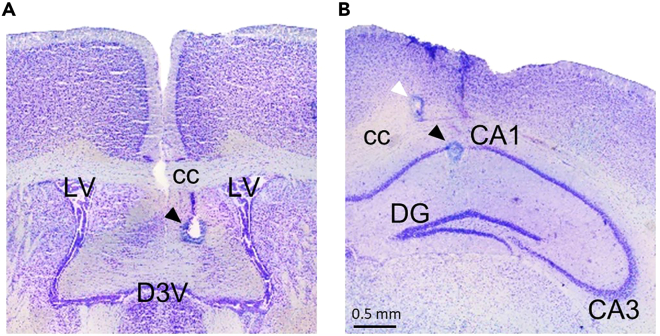
Figure 11.
Photomicrographs illustrating electrode placement
(A) DBS electrode in the fimbria-fornix (arrowhead).
(B) Active recording electrode in the CA1 (black arrowhead) and reference electrode in the corpus callosum (white arrowhead). cc, corpus callosum; LV, lateral ventricle; D3V, dorsal third ventricle; DG, dentate gyrus.
Expected outcomes
This protocol will help establish chronic DBS for days, weeks or even longer in awake, freely moving mice. There are several advantages to this protocol. First, the described electrode assemblies allow one to construct probes for intracranial field stimulation and/or recordings in general. Second, the stereotaxic surgical procedures may be used for the implantation of probes targeting different brain structures for chronic stimulation and/or recording in freely moving mice. Third, the custom-made E-Phys rig is useful for field recordings in both anesthetized and freely moving animals. Last but not least, DBS treatment targeting different brain regions may be used to assess its effects on cognition, motor, and psychiatric phenotypes of disease models (Lozano et al., 2019; Mankin and Fried, 2020). Our representative experiments in Rett syndrome mice showed that chronic DBS in the fimbria-fornix enhances hippocampal memory, synaptic plasticity, and neurogenesis (Hao et al., 2015). Forniceal DBS also rescues hippocampal circuit deficits and gene expression of Rett mice (Lu et al., 2016; Pohodich et al., 2018).
This DBS protocol may be extended to other mouse models of intellectual disability disorders (Hao et al., 2021). The technical details of electrode construction, stereotaxic surgery, and chronic DBS in this protocol will benefit the preclinical studies in mouse models of motor deficits, psychiatric disorder, and other neurological disorders (Edwards et al., 2017; Lozano et al., 2019).
Limitations
The DBS procedures are performed in freely moving mice in order to mimic its clinical application. It is therefore more complicated and technically challenging than delivering DBS in anesthetized mice. In particular, background and expertise in electrophysiology is required to set up the E-Phys rigs and recording evoked responses in the hippocampus. However, this E-Phys guidance improves the success rate of electrode targeting and, importantly, allows optimizing the DBS intensity (Hao et al., 2015; Hao et al., 2021; Shirvalkar et al., 2010; Suthana et al., 2012).
Experimenters may experience technical challenges in the construction of electrode assemblies initially. Be patient; developing skill in these procedures takes practice.
Troubleshooting
Problem 1
Difficulty in soldering the stainless steel tubes and the tungsten wires when constructing the electrode assemblies (step construction of DBS electrode assembly, 13; step construction of recording electrode assembly, 16, 18, and 22; step build customized adaptor and wiring system for DBS application, 24).
Potential solution
We recommend setting the soldering temperature to 450°C. Using solder flux helps. Entwining the tungsten wire on the connector pin for a few runs before soldering may increase the contact surface and reinforce the connection between the two parts.
Problem 2
Hot fluid solder enters into the tube or connector holes when soldering two stainless steel tubes together or soldering on the connector pins (step construction of DBS electrode assembly, 13; step construction of recording electrode assembly, 16, 18, and 22; step build customized adaptor and wiring system for DBS application, 24).
Potential solution
If this happens, the tubes or connectors should be discarded. Restart using a minimal amount of solder. Try a few times until success.
Problem 3
Shortcut between the two DBS electrode wires or between the active and reference wires of recording electrode, leading to crosstalk of the signals (step construction of DBS electrode assembly, 15; step construction of recording electrode assembly, 21; step build customized adaptor and wiring system for DBS application, 24).
Potential solution
Use the multimeter to check each electrode channel (from the matched male connector pins to the free end of the wires) before finalizing the electrode assembly. Make sure that each wire is connected and only connected to its designated connector pin.
Problem 4
Environmental noise during electrophysiological recordings (step surgical implantation of the electrodes, 18g; step determine the DBS current intensity for each mouse, 26).
Potential solution
First, ground the Faraday cage and other equipment properly. Second, during surgical procedures, if the fiber optic illuminator and the glass bead sterilizer need to be close to the mice inside the Faraday cage, turn them off and disconnect from the power, then place the power cables inside the Faraday cage. Third, make sure only the animal and the battery-powered preamplifier are inside the Faraday cage when recording the evoked potential in freely moving mice. Fourth, twist the active and reference cables may help reduce the noise. Fifth, use the notch filter of the amplifier if necessary.
Problem 5
Chronic DBS induces seizures in mice (step chronic DBS application in freely moving mice, 29).
Potential solution
Some mouse models might have an unsuspected tendency to develop seizures under stimulation. Closely watch the animals during DBS. Stop the DBS immediately if a mouse appears to show seizure-like behavior (e.g., chewing and facial twitching or myoclonic jerks (Li et al., 2014; Watanabe et al., 2011)). Wait for at least 30 min and try DBS with only 60% or less of the previous stimulus intensity.
Problem 6
Inaccurate automatic freezing scoring using ANY-maze (step classic fear conditioning task, 33 and 34).
Potential solution
This is rare but possible under some conditions. It is important to manually verify/correct the machine detection of freezing by at least two blinded, experienced experimenters. Adjust the parameter setting of freezing detection in the experimental protocol. In addition, make sure that the light intensity inside the sound attenuating box is even and consistent. Usually, reducing the light reflection of the conditioning/testing chambers by evenly sanding the inside surface helps the ANY-maze system reliably detect freezing.
Resource availability
Lead contact
Further information and requests for resources and technical comments should be directed to and will be fulfilled by the lead contact, Jianrong Tang (jtang1@bcm.edu).
Materials availability
This study did not generate new unique reagents.
Acknowledgements
We would like to thank Zhenyu Wu for help with the mouse colony. This work was supported in part by the National Institute of Neurological Disorders and Stroke (R01NS100738 to J.T.), the LouLou Foundation (to J.T.), the Eunice Kennedy Shriver National Institute of Child Health and Human Development (U54HD083092, P50HD103555 to Baylor College of Medicine Intellectual and Developmental Disabilities Research Center, Neuroconnectivity Core and Circuit Modulation Core), the In Vivo Neurophysiology Core of Jan and Dan Duncan Neurological Research Institute at Texas Children’s Hospital, the Chao Family Foundation, and the Cockrell Family Foundation. We thank Dr. Jin Xu and Vicky Brandt for reading the manuscript.
Author contributions
Conceptualization, J.T.; Methodology, J.T., Q.W., and B.T.; Investigation, Q.W. and B.T.; Writing, J.T. and Q.W. Funding Acquisition, J.T.; Resources, J.T.; Supervision, J.T. All authors discussed and approved the manuscript.
Declaration of interests
The authors declare no competing interests.
Data and code availability
This study did not generate code.
References
- Edwards C.A., Kouzani A., Lee K.H., Ross E.K. Neurostimulation devices for the treatment of neurologic disorders. Mayo Clin. Proc. 2017;92:1427–1444. doi: 10.1016/j.mayocp.2017.05.005. [DOI] [PubMed] [Google Scholar]
- Guy J., Gan J., Selfridge J., Cobb S., Bird A. Reversal of neurological defects in a mouse model of Rett syndrome. Science. 2007;315:1143–1147. doi: 10.1126/science.1138389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hao S., Tang B., Wu Z., Ure K., Sun Y., Tao H., Gao Y., Patel A.J., Curry D.J., Samaco R.C., et al. Forniceal deep brain stimulation rescues hippocampal memory in Rett syndrome mice. Nature. 2015;526:430–434. doi: 10.1038/nature15694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hao S., Wang Q., Tang B., Wu Z., Yang T., Tang J. CDKL5 deficiency augments inhibitory input into the dentate gyrus that can be reversed by deep brain stimulation. J. Neurosci. 2021 doi: 10.1523/JNEUROSCI.1010-21.2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li B., Wang L., Sun Z., Zhou Y., Shao D., Zhao J., Song Y., Lv J., Dong X., Liu C., et al. The anticonvulsant effects of SR 57227 on pentylenetetrazole-induced seizure in mice. PLoS ONE. 2014;9:e93158. doi: 10.1371/journal.pone.0093158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lozano A.M., Lipsman N., Bergman H., Brown P., Chabardes S., Chang J.W., Matthews K., McIntyre C.C., Schlaepfer T.E., Schulder M., et al. Deep brain stimulation: current challenges and future directions. Nat. Rev. Neurol. 2019;15:148–160. doi: 10.1038/s41582-018-0128-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu H., Ash R.T., He L., Kee S.E., Wang W., Yu D., Hao S., Meng X., Ure K., Ito-Ishida A., et al. Loss and gain of MeCP2 cause similar hippocampal circuit dysfunction that is rescued by deep brain stimulation in a rett syndrome mouse model. Neuron. 2016;91:739–747. doi: 10.1016/j.neuron.2016.07.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mankin E.A., Fried I. Modulation of human memory by deep brain stimulation of the entorhinal-hippocampal circuitry. Neuron. 2020;106:218–235. doi: 10.1016/j.neuron.2020.02.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paxinos G., Franklin K.B.J. Academic Press; 2001. The Mouse Brain in Stereotaxic Coordinates. [Google Scholar]
- Pohodich A.E., Yalamanchili H., Raman A.T., Wan Y.W., Gundry M., Hao S., Jin H., Tang J., Liu Z., Zoghbi H.Y. Forniceal deep brain stimulation induces gene expression and splicing changes that promote neurogenesis and plasticity. Elife. 2018;7 doi: 10.7554/eLife.34031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shirvalkar P.R., Rapp P.R., Shapiro M.L. Bidirectional changes to hippocampal theta-gamma comodulation predict memory for recent spatial episodes. Proc. Natl. Acad. Sci. U S A. 2010;107:7054–7059. doi: 10.1073/pnas.0911184107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suthana N., Haneef Z., Stern J., Mukamel R., Behnke E., Knowlton B., Fried I. Memory enhancement and deep-brain stimulation of the entorhinal area. N. Engl. J. Med. 2012;366:502–510. doi: 10.1056/NEJMoa1107212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watanabe Y., Kaida Y., Fukuhara S., Takechi K., Uehara T., Kamei C. Participation of metabotropic glutamate receptors in pentetrazol-induced kindled seizure. Epilepsia. 2011;52:140–150. doi: 10.1111/j.1528-1167.2010.02764.x. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
This study did not generate code.