Abstract
Traumatic neuroma is not a true neoplasm but a reparative proliferation of axons, Schwann cells, and fibroblasts at the proximal end of transected or injured nerves resulting from trauma or surgery. Breast traumatic neuroma after breast surgery, with or without clinical symptoms, has rarely been reported. Once found, it should be differentiated from tumor recurrence, and tissue confirmation is necessary, although it is small in size and demonstrates a benign appearance in imaging studies. Herein, we present two cases of traumatic neuroma at the mastectomy site. They were incidentally encountered during ultrasound evaluation of mastectomy beds given concerns for potential recurrence or malignancy, and pathologic confirmation by ultrasound-guided core needle biopsy was sufficient for the diagnosis.
Keywords: Traumatic neuroma, Mastectomy, Ultrasound Imaging
Introduction
Traumatic neuroma (TN) is an uncommon benign lesion that develops at the proximal end of a severed nerve after trauma or surgery as a reparative process consisting of hyperplastic proliferation of neural fibers and connective tissue. It occurs most frequently in the lower extremities after amputation, followed by occurrence in the head and neck [1]. TN in patients who have undergone breast cancer surgery, including breast conservation or mastectomy, rarely occurs and has an incidence rate of 0.09% [2]. To date, imaging findings of breast neuromas have been infrequently reported but have included reports of sporadic cases [2], [3], [4], [5], [6], [7], [8]. Breast TN can appear as a painful or palpable symptomatic small nodule and can be incidentally observed during a routine follow-up examination of the breast after surgery. Once found, it presents a diagnostic challenge because TN should be distinguished from breast cancer recurrence. Conducive ultrasound (US) features of breast TN, such as an oval shape, circumscribed margin, tail sign, restricted blood flow, and benign or soft elasticity, have been introduced; however, pathologic confirmation is needed due to concerns regarding the possibility of local cancer recurrence. Due to its rarity and benignity, imaging findings other than US have been limited. Herein, we present two new cases of TN at the mastectomy site along with imaging findings of US, including elastography, computed tomography (CT), and positron emission tomography-computed tomography (PET-CT).
Case report
Case 1
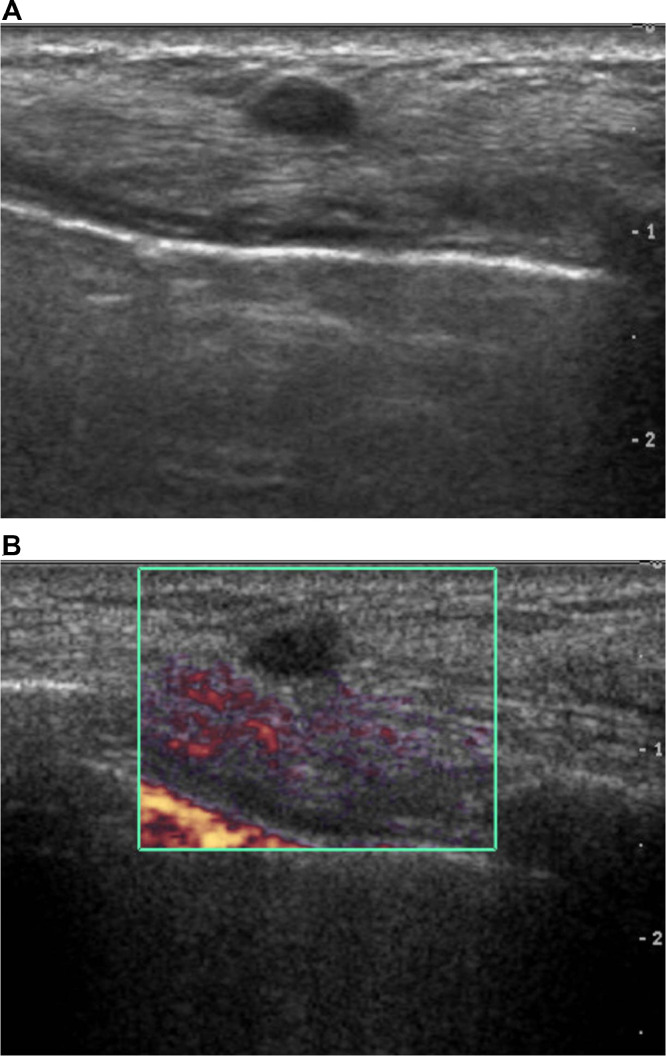
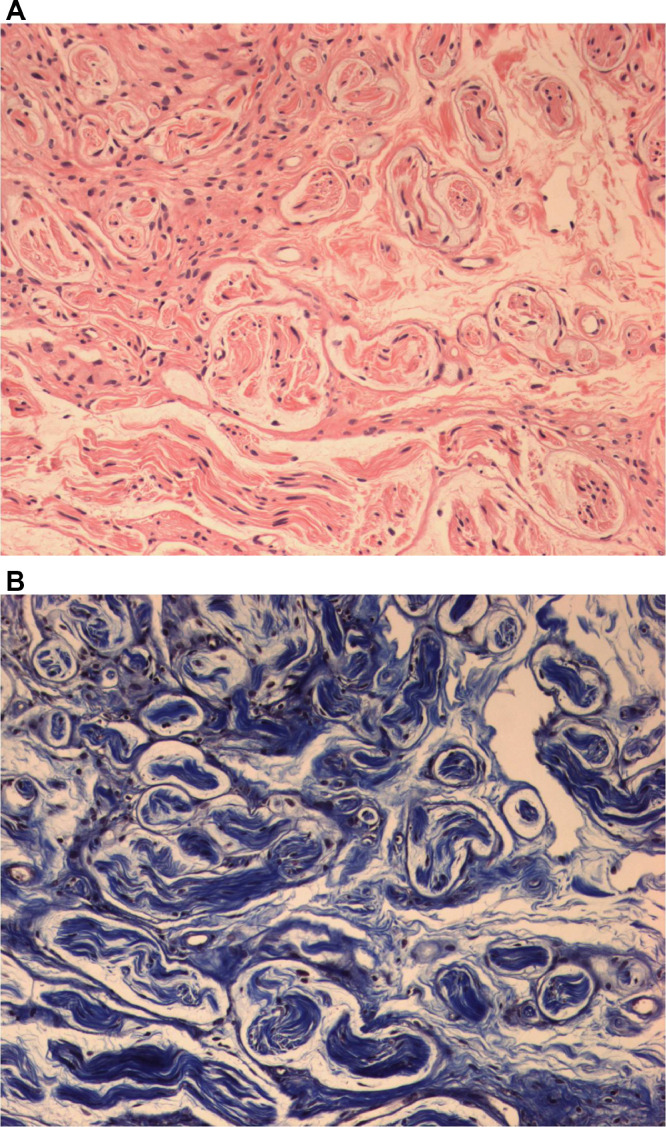
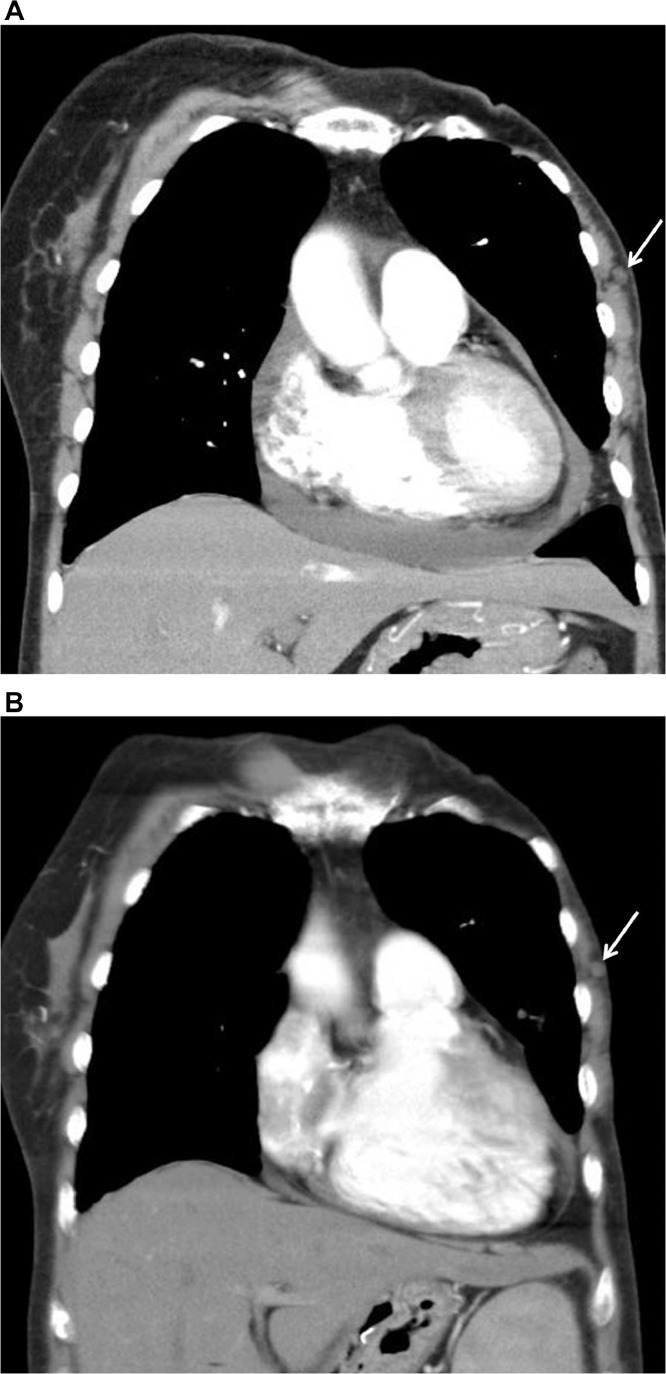
A 74-year-old woman presented with dyspnea. Chest CT showed pericardial effusion and focal pulmonary thromboembolism in the right lobar artery. The patient's past medical history included a left modified radical mastectomy for breast cancer about 20 years prior to treatment at our institution. The record for histologic type and stage of the previous lesion was unavailable because the patient had undergone breast surgery at another institution. Due to the possibility of malignant effusion, the attending physician requested a whole-breast US evaluation of the right breast and the left mastectomy bed to evaluate the possible cause. Gray scale US revealed an oval-shaped hypoechoic 0.7 cm sized nodule with a partially indistinct margin in the 3 o'clock direction of the left chest wall (Fig. 1A). There was no evidence of vascularity within the lesion in the Power Doppler study (Fig. 1B). However, because of the patient's medical history of previous breast cancer and sonographic features, the lesion was classified as category 4 according to the Breast Imaging Reporting and Data System and thereby required a biopsy. US-guided core needle biopsy was performed using a 14-gauge needle. On histological examination, the biopsied tissue depicted a tangled mass of axonal bundles in the connective tissue stroma, which was consistent with traumatic neuroma (Fig. 2A and B). The patient began treatment with warfarin, and the symptoms resolved. On a thorough review of a previous chest CT scan, a small nodule showing a density similar to that of the adjacent muscle was correlated with the traumatic neuroma, and it showed no interval change on the chest CT after one year of evaluating recurrent pulmonary thromboembolism (Fig. 3A and B).
Fig. 1.
A & B. Gray scale ultrasound of Case 1 shows an oval-shaped, indistinct, hypoechoic mass in the deep subcutaneous fat layer of the mastectomy site (A). On Power Doppler, no intralesional blood flow can be observed (B).
Fig. 2.
A & B. Histopathologic examination by core needle biopsy of Case 1 reveals mixed axons, Schwann cells, perineurial fibroblasts, and thick collagen (hematoxylin and eosin stain, original magnification × 200) (A). Trichrome stain reveals perifascicular collagen bundles (× 200) (B).
Fig. 3.
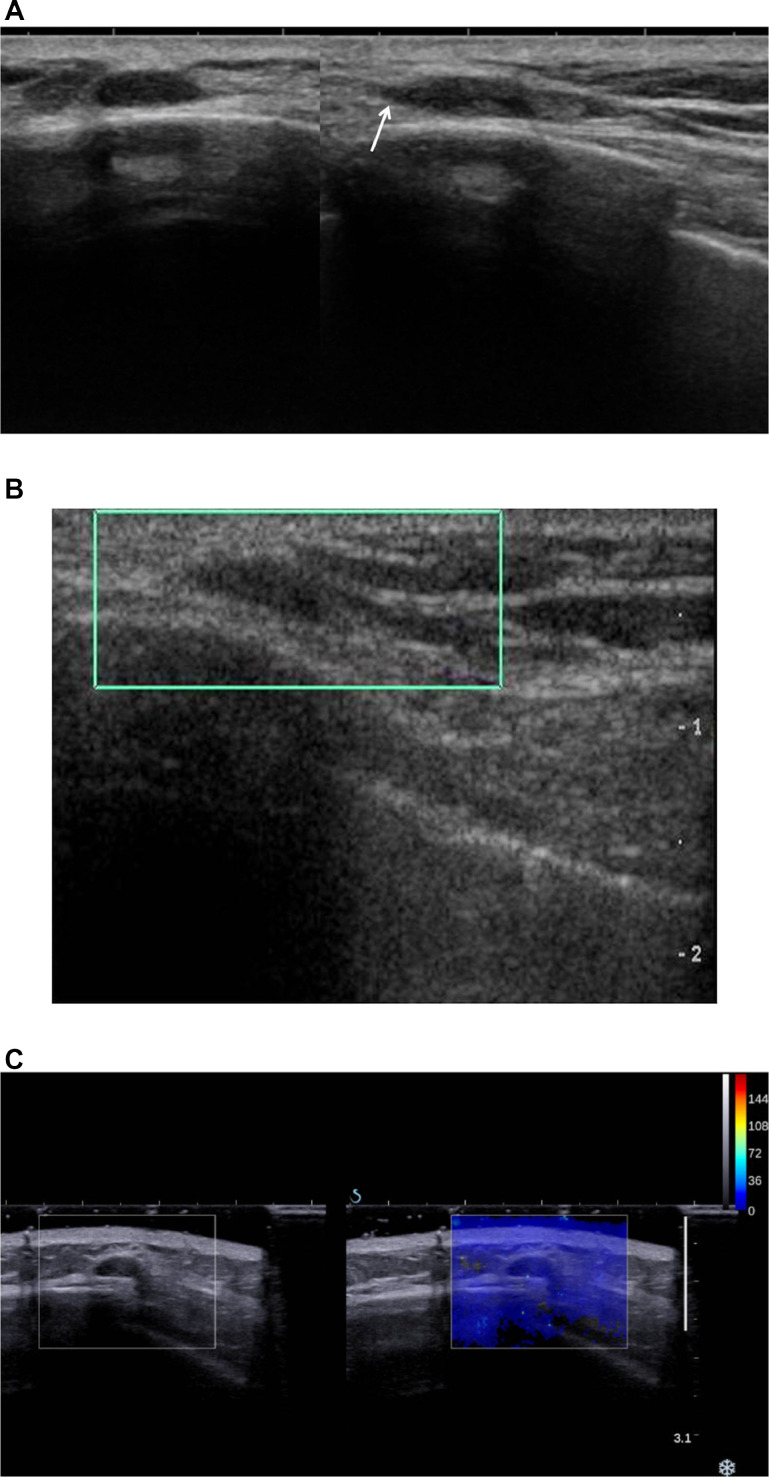
A & B. Coronal reconstructed image of chest computed tomography (CT) for dyspnea shows pericardial effusion and a small, isodense nodule in the left chest wall (arrow) (A). Chest CT performed after one year due to recurrent pulmonary thromboembolism shows the stability of the small nodule (arrow) (B).
Case 2
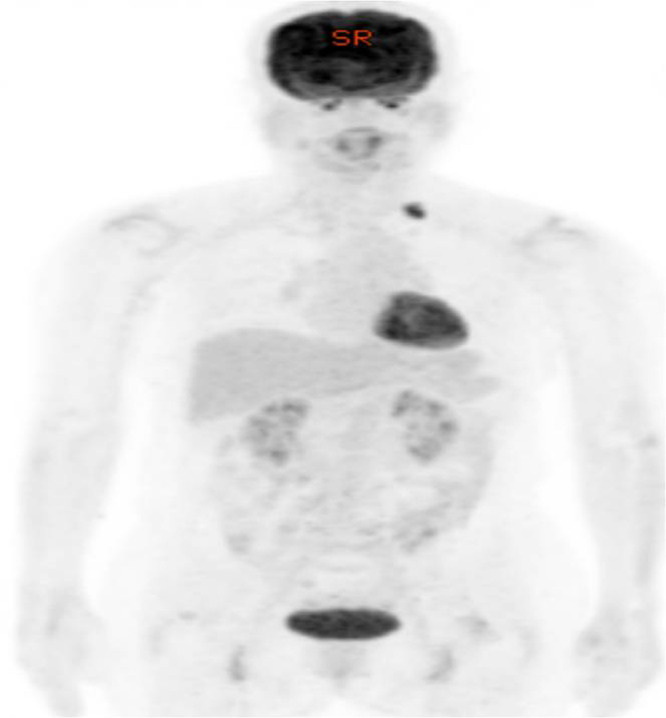
A 62-year-old woman was referred for US evaluation of the whole right breast, the left mastectomy bed, and the left supraclavicular fossa because of an abnormal uptake on the left supraclavicular fossa on F18-fluorodeoxyglucose PET-CT during recurrence and metastatic workup (Fig. 4). The patient underwent a left modified radical mastectomy for breast cancer (T1cN0M0) six years prior to treatment at our institution, followed by hormone therapy for five years. At the time of the present admission, the patient was asymptomatic, and the patient's physical examination results were normal. Gray scale US showed an oval, circumscribed, hypoechoic mass with fusiform tapering of one end, corresponding to the tail sign in the 2 o'clock direction of the left chest wall measuring 0.5 × 0.4 cm in diameter (Fig. 5A). There was no increased intralesional blood flow on color Doppler study (Fig. 5B), and the nodule was soft on elasticity assessment by shear wave elastography (Fig. 5C). Sonographic assessment revealed a Breast Imaging Reporting and Data System category 3 lesion; however, the possibility of breast cancer recurrence could not be excluded. For pathologic confirmation, US-guided core biopsy using a 14-gauge needle was performed on the chest wall lesion, and fine needle aspiration cytology using a 23-gauge needle was performed on an enlarged lymph node in the left supraclavicular area. On histologic examination, the biopsied tissue revealed traumatic neuroma, and the result of fine needle aspiration was reactive, with no evidence of malignancy (not shown). Since the patient wanted to remove the lymph node, excision was performed, and the final result was tuberculosis (not shown).
Fig. 4.
Torso view of positron emission tomography-computed tomography (PET-CT) of Case 2 shows abnormal metabolic uptake on PET-CT in the left supraclavicular fossa.
Fig. 5.
A, B & C. Transverse (left) and longitudianl (right) view of gray scale ultrasound shows an oval-shaped, circumscribed, hypoechoic mass with tail sign (arrow) in the deep subcutaneous fat layer of the mastectomy site (A). On color Doppler, no intralesional blood flow can be observed (B). On the shear wave elastographic evaluation, the nodule shows soft elasticity (C).
Discussion
TN is not a true neoplasm but reactive hyperplasia after nerve injury, consisting of multiple interlacing fascicles of nerve fibers in the condensed fibrotic tissue of the nerve sheath. Foltan et al. suggested that TN may arise due to simultaneous nerve fiber regeneration and excessive fibrous tissue proliferation, resulting in contraction of neural fibers within the fibrous scar and leading to a chronic defensive proliferation of nerve fibers and fibrous scar tissue [1].
TNs are classified into two types as follows: spindle neuromas that result from the chronic stimulation of a non-severed nerve and terminal neuromas that occur in a transected nerve [2]. Traumatic neuromas in the mastectomy site belong to the terminal neuroma group. While TNs at other sites are usually accompanied by symptoms, such as pain or palpable nodules, those associated with mastectomy were asymptomatic in half of all cases reported in the literature by imaging findings [3]. Both cases in this study were also incidentally identified on US examination. TNs at the mastectomy site are usually located in the pectoralis muscle layer or subcutaneous fat layer of the lateral aspect of the chest wall, near the surgical scar. This is because they are related to postsurgical damage of branches of the long thoracic nerve and lateral cutaneous and anterior cutaneous branches of the intercostal nerve [2], [3], [4].
According to several previous studies on breast TNs, they usually show a benign appearance in imaging studies [2], [3], [4], [5], [6], [7], [8]. In the previously reported cases observed on mammography, TN was observed as a small, oval, circumscribed, isodense mass on the mastectomy site or small architectural distortion in breast conservation surgery [6,8]. US mostly shows an oval shape, circumscribed margin with parallel orientation, no vascularity, benign or soft elasticity, and tail sign (fusiform tapering of one end of the mass or focal thickening of the nerve) [2], [3], [4], [5], [6], [7]. When the TN is visible on magnetic resonance imaging, isointense signal on T1-weighted images and high signal on T2-weighted images with benign type 1 enhancement curve have been noted [2]. Only one CT finding was reported by Baltalarli et al., wherein the lesion was triangular in shape based on the pectoralis muscle with isoattenuation to the muscle [7]. In our Case 1, TN was noted in the subcutaneous fat layer as a small isoattenuated nodule to the adjacent chest wall muscle through retrospective imaging review and was stable at follow-up. Thus, TN in both cases presented in this study showed all the typical US findings, isoattenuation to the adjacent muscle on CT, and no fluorodeoxyglucose uptake on PET-CT.
The most important differential diagnosis in cases of TN is tumor recurrence. According to previous literature, tumor recurrence tends to be close to the skin surface or subcutaneous fat layer, showing an irregular, not circumscribed, nonparallel, hypoechoic mass with increased tumor vascularity on US, while TN seems to be more deeply located adjacent to or within the muscle layer, oval in shape, circumscribed, parallel-oriented, and hypoechoic with poor vascularity [2,4,9]. If the tail sign is present, it is more useful for differentiation. However, a few previous cases of TN were depicted in the subcutaneous fat layer [3,7], and the present cases were also observed in the subcutaneous fat layer, which was closer to the muscle than the skin. Despite these differential imaging findings, pathological confirmation is necessary for patients with a personal history of breast cancer or post status of breast cancer surgery. US-guided core needle biopsy is safe and sufficiently reveals benignity, and a quick diagnosis may reduce overwhelming management or anxiety. However, patients who have undergone breast surgery, such as mastectomy with or without reconstruction, may experience difficulty in the biopsy procedure because the lesions are close to the skin, pectoralis muscle, or implant capsule. In this case, the biopsy may be performed by approaching from a slightly farther position to sufficiently ensure setting the needle parallel to the chest wall or using the hydrodissection technique of instilling a local anesthetic or normal saline around the lesion to displace anteriorly or posteriorly and create a safe zone for needle passage [10].
In conclusion, a TN can occur at the mastectomy site as a small nodule with or without symptoms. Its clinical scenario and imaging findings with US-guided core biopsy can establish a safe and quick diagnosis and help dictate appropriate management in patients who have undergone prior breast cancer surgery.
Patient consent
Written informed consent was not necessary because no patient data has been included in the manuscript.
Footnotes
Acknowledgments: There is no funding source
Competing interests: The authors have declared that no competing interests exist.
References
- 1.Foltán R, Klíma K, Špačková J, Šedý J. Mechanism of traumatic neuroma development. Med Hypotheses. 2008;71(4):572–576. doi: 10.1016/j.mehy.2008.05.010. [DOI] [PubMed] [Google Scholar]
- 2.AlSharif S, Ferré R, Omeroglu A, El Khoury ME, Mesurolle B. Imaging features associated with posttraumatic breast neuromas. A.J.R. Am J Roentgenol. 2016;206(3):660–665. doi: 10.2214/AJR.14.14035. [DOI] [PubMed] [Google Scholar]
- 3.Chen W, Zhang H, Huang J, Li Y, Zhang Z, Peng Y. Traumatic neuroma in mastectomy scar: Two case reports and review of the literature. Medicine. 2019;98(15) doi: 10.1097/MD.0000000000015142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Sung HS, Kim YS. Ultrasonographic features of traumatic neuromas in breast cancer patients after mastectomy. Ultrasonography. 2017;36(1):33–38. doi: 10.14366/usg.16029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kim EY, Kang DK, Kim TH, Kim KS, Yim H. Traumatic neuroma in a breast cancer patient after modified radical mastectomy: a case report. J Korean Soc Radiol. 2011;64(5):515–518. doi: 10.3348/jksr.2011.64.5.515. [DOI] [Google Scholar]
- 6.Fitzpatrick KA, Borders MH, MacKerricher WS, Bracamonte ER. Traumatic neuroma of the breast after mastectomy. Appl Radiol. 2017;46:26–28. [Google Scholar]
- 7.Baltalarlı B, Demirkan N, Yağcı B. Traumatic neuroma: Unusual benign lesion occurring in the mastectomy scar. Clin Oncol (R Coll Radiol) 2004;16(7):503–504. doi: 10.1016/j.clon.2004.07.007. [DOI] [PubMed] [Google Scholar]
- 8.Salemis NS. Traumatic neuroma as a rare cause of intractable neuropathic breast pain following cancer surgery: Management and review of the literature. Intractable Rare Dis Res. 2018;7(3):185–190. doi: 10.5582/irdr.2018.01041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Al-Khalili R, Alzeer A, Nguyen GK, Crane EP, Song JH, Jeon JL, et al. Palpable lumps after mastectomy: Radiologic-pathologic review of benign and malignant masses. RadioGraphics. 2021;41(4) doi: 10.1148/rg.2021200161. 967–89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bhatt AA, Whaley DH, Lee CU. Ultrasound-guided breast biopsies: basic and new techniques. J Ultrasound Med. 2021;40(7):1427–1443. doi: 10.1002/jum.15517. [DOI] [PMC free article] [PubMed] [Google Scholar]