Summary
Background
Due to the critical role of folates in neurodevelopment, it is important to understand potential interactions between anti-HIV drugs used during pregnancy, and folate delivery pathways in the placenta. This study investigates the effect of dolutegravir (DTG) exposure on the functional expression of the reduced folate carrier (RFC), proton-coupled folate transporter (PCFT), and folate receptor-α (FRα) in the placenta.
Methods
Human placental cell lines, human placental explants, and a pregnant mouse model treated with clinically relevant concentrations of DTG were used. Gene and protein expression were assessed by qPCR, immunoblot and immunohistochemical assays. Folate transport function was measured by applying radioisotope-based transport assays.
Findings
In placental cells, clinically relevant DTG exposure for 3h or 6h was associated with a modest but significant reduction in the expression of RFC and PCFT both at the mRNA and protein levels, as well as decreased uptake of RFC and PCFT substrates [3H]-methotrexate and [3H]-folic acid, respectively. In pregnant mice, DTG administration was associated with an increase in both placental RFC and PCFT mRNA expression, accompanied by a decrease in placental FRα mRNA under folate-deficient dietary conditions.
Interpretation
These findings demonstrate a potential interaction between DTG and folate transport pathways in the placenta, particularly in vivo, under folate deficient conditions, potentially impacting folate delivery to the foetus in the context of DTG-based ART during pregnancy.
Funding
Funded by Ontario HIV Treatment Network, grant #506657; and Eunice Kennedy Shriver National Institute of Child Health & Human Development of the National Institutes of Health, award #R01HD104553.
Keywords: Dolutegravir, folate transport, folate deficiency, placenta, antiretroviral therapy, neural tube defects
RESEARCH IN CONTEXT.
Evidence before this study
Dolutegravir (DTG) is increasingly used amongst pregnant women living with HIV. A 2018 report from the Tsepamo surveillance study initially identified a 0·94% risk of neural tube defects (NTDs) amongst women taking DTG-based antiretroviral therapy (ART) from conception, compared to 0·12% amongst women taking non-DTG ART from conception. Follow-up reports from the Tsepamo study and others have identified a small or no increased risk of NTDs associated with DTG from conception, however, potential interactions of DTG with developmental pathways in early gestation remain unclear. Due to the established link between NTDs and folate dysregulation in pregnancy, a few studies have investigated interactions between DTG and placental folate transport, though findings are limited. One study identified DTG as a non-competitive inhibitor of folate-receptor mediated folate uptake at the placenta, associated with developmental toxicity in zebrafish. A second study suggested that DTG does not inhibit placental folate uptake pathways at clinically relevant concentrations in cell line models.
Added value of this study
This study uses three models of the placenta, i.e., an in vitro cell culture model, an ex vivo human placental explant model, and an in vivo animal model, to investigate interactions between DTG and expression of placental folate transport mechanisms. In each model, we characterized the expression of key folate transport pathways at the mRNA and protein level, establishing a framework for understanding and further investigating ART interactions with folate uptake at the placenta. Furthermore, we observed modest but significant alterations in folate transport pathways, including in vitro functional impairment of placental folate uptake, and a corresponding altered mRNA expression of folate transporters in the in vitro, ex vivo and in vivo models, at clinically relevant concentrations of DTG, particularly under folate deficient conditions.
Implications of all the available evidence
In agreement with previous studies, these findings further suggest an interaction between DTG and folate uptake at the placenta, through altered functional expression of folate transporters and the folate receptor. Decreased functional expression could have implications for folate access during early neurodevelopment, and could ultimately exacerbate fetal folate deficiency and associated developmental defects in settings where folate supplementation is uncommon. However, relatively modest changes in transporter and receptor expression observed in our models, together with the low risk of DTG-associated NTDs identified in surveillance studies, suggest limited clinical implications of such an interaction in the context of folate supplementation.
Alt-text: Unlabelled box
Introduction
Folates are a family of biomolecules derived from vitamin B9, obtained in the diet through vegetables, legumes and fortified grains.1 Due to their role in nucleic acid synthesis and repair, amino acid synthesis, and gene regulation, folates are particularly important during times of rapid tissue growth and development, including during pregnancy.2,3 Due to the demand of the developing embryo, folate requirements during pregnancy are considerably greater than in non-pregnant adults, at 400 µg/day.2,4 Folate deficiency in pregnancy is linked to adverse outcomes including preterm births, low birthweight, and neurogenic defects such as neural-tube defects (NTDs).5,6 Folic acid (FA) fortification of wheat flour and other grains, as well as folate supplementation during pregnancy, greatly improves infant health outcomes.2,5
Many adverse pregnancy outcomes linked to maternal folate deficiency are also associated with drugs which impair transplacental folate uptake, particularly antifolate drugs used in chemotherapy.7,8 In order to ensure safe use of novel drugs in pregnant populations, it is essential to understand potential interactions with folate delivery pathways. Transplacental folate delivery involves the co-ordinated activity of three specific folate transport mechanisms: the folate receptor isoforms α (FOLR1/FRα) and β (FOLR2/FRβ), the reduced folate carrier (RFC), and the proton coupled folate transporter (PCFT).9, 10, 11 FRα is primarily expressed on the apical side of the placental villi, in the syncytiotrophoblast, and is thought to act as the primary mechanism of placental folate uptake via receptor-mediated endocytosis.9,10 FRα is also expressed in the neural folds and neural-tube of the developing embryo, and is necessary for neural-tube development.12 PCFT, a proton/folate co-transporter, has a low affinity for folates in the placental environment, and plays a greater role in dietary folate uptake in the low pH environment of the gastrointestinal tract.9,13 At the placenta, similar to FRα, PCFT is localized at the apical membrane of the syncytiotrophoblast, is internalized in cytoplasmic vesicles during FRα -mediated folate uptake, and is thought to export folate into the cytoplasm following endosome acidification.14 Finally, RFC is a bidirectional transporter expressed at both the apical and basolateral membranes, which further contributes to folate transport across the placenta, particularly across the basolateral membrane of the syncytiotrophoblast.9,11 Interference in folate transport and metabolism by fetotoxic drugs has long been reported, particularly involving antiepileptics such as valproic acid and carbamazepine and antifolates such as methotrexate (MTX).15, 16, 17
Antiretroviral therapy (ART) during pregnancy has been effective in reducing rates of vertical HIV transmission, with dolutegravir (DTG) quickly becoming a preferred component of contemporary ART regimens due to its low cost, high tolerability and barrier to resistance.18 In May 2018, the Tsepamo study in Botswana reported cases of NTDs among 426 pregnant women who had been taking DTG-based ART from conception at a rate of 0·94% compared to 0·12% amongst infants born to women taking non-DTG ART from the time of conception.19 This early signal prompted concern regarding the safety of DTG use during pregnancy, and led the World Health Organization to temporarily suspend recommendation of DTG in this population. Reassuring follow-up findings from the Tsepamo study and others demonstrated lower rates of NTDs, though in some cases still higher than rates in populations exposed to non-DTG based ART;20, 21, 22, 23 however as DTG use continues to rise among pregnant women, and women of childbearing age living with HIV, it remains critical to better understand the implications of in utero DTG exposure on fetal development. Recent reports have identified potential interactions between DTG and folate uptake pathways, though the implications of these findings remain unclear, and findings in in vivo models are limited.24, 25, 26
In light of findings from the Tsepamo study, and due to the established link between impaired folate delivery and NTDs, we hypothesized that DTG exposure affects the functional expression of folate transporters and receptors in the placenta. Using in vitro placental cell culture models, in vivo pregnant murine models, and ex vivo human models of the antiretroviral drug-exposed placenta, this study aimed to characterize the impact of DTG exposure on the functional expression of placental FRα, PCFT and RFC, in order to better understand potential effects of DTG exposure on folate delivery during pregnancy.
Methods
Study approval
The study protocol was approved by the Mount Sinai Hospital Research Ethics Board, Sinai Health System REB# 02-0061A and University Health Network Research Ethics Board REB# 10-0277-A. All subjects donating placental tissues gave written informed consent in accordance with the Declaration of Helsinki. Tissues were obtained through the BioBank program at the Research Centre for Women's and Infants’ Health at Mount Sinai Hospital. All research using human tissues was performed in a biosafety level II certified laboratory by qualified staff trained in biological and chemical safety protocols, and in accordance with Health Canada guidelines and regulations. All animal experiments were approved by the University Health Network Animal Use Committee (protocol #2575.25) and performed according to the policies and guidelines of the Canadian Council on Animal Care.
Human placental tissue isolation
Placentae were collected from patients undergoing elective surgical termination of pregnancy and from healthy deliveries at term (first trimester (5-10 weeks), second trimester (12-20 weeks), and term (38-40 weeks)). Placental tissue specimens were processed within 2h of collection. Tissues were dissected to 1cm3 blocks, washed free of blood and fixed overnight in 4% PFA, followed by three washes in phosphate buffered saline (PBS). Tissues were then processed through a series of increasing ethanol solutions (50-100%), cleared in xylene and embedded for paraffin immunohistochemistry (IHC). Sections were freshly cut before the IHC protocol at 5 µm, placed on Superfrost microscope slides, and dried overnight.
For ex vivo treatment with DTG, placental tissue specimens were placed into 1% PBS without Ca2+ or Mg2+ and dissected into villous clusters of about 15-30 mg. Villous explants were then cultured in 12-well plates in DMEM/F12 media (containing antibiotic (Normocin; InvivoGen) and 1xITS-A (insulin, transferrin and selenium-A; Invitrogen)), in the presence of 2000 ng/mL DTG, 4000 ng/mL DTG or DMSO (control) for 3h or 6h, in a humidified incubator at 37°C, 5% CO2, and 95% air atmosphere, and subsequently processed for RNA isolation.
Mouse treatment and tissue collection
Mouse treatment and tissue collection was performed as previously established.27 C57BL/6J mice bred in-house (original breeders from Jackson Laboratory RRID:IMSR JAX:000664), were maintained under a 12-h light/dark cycle, with ad libitum access to food and water. Mice in the folate sufficient groups were fed a standard folate-sufficient laboratory diet containing 7 mg/kg of folate (Teklad LM-485 Mouse/Rat Sterilizable Diet #7012). Relatively high levels of folate in the laboratory diet yield adequate folate doses (2–3 mg/kg) following diet sterilization. Mice in the folate deficient groups were placed on a folic acid-deficient laboratory diet which provides 0·1-0·2 mg/kg folic acid (Envigo TD. 160606), for 2 weeks prior to mating. Female mice (7-12 weeks of age) were mated with males at a ratio of 2:1 or 3:1. Presence of vaginal plug was denoted as gestational day (GD) 0·5. Plugged females were randomly assigned to a treatment arm and housed in cages with 5 dams/cage. Pregnancy was confirmed by weight gain on GD 9·5 (>1·5 g).
DTG and tenofovir/emtricitabine (TDF/FTC) were purchased as prescription drugs. Pills were crushed, suspended in distilled water, and administered once daily by oral gavage (100 µL/mouse). Drug suspensions were prepared fresh each morning. Drug dosing was selected to yield maternal plasma levels equivalent to those reported in pregnant women, as determined in pilot studies. The 1x-DTG+TDF/FTC group received 2·5 mg/kg DTG + 50 mg/kg TDF + 33·3 mg/kg FTC, yielding DTG peak plasma concentrations of ∼3000 ng/ml, and DTG Ctrough concentrations ∼ 150 ng/mL. These values are within the therapeutic range.28 The 5x-DTG+TDF/FTC group received 12·5 mg/kg DTG + 50 mg/kg TDF + 33·3 mg/kg FTC, yielding DTG peak plasma concentrations of ∼12,000 ng/ml, and DTG Ctrough concentrations ∼ 250 ng/mL. Control mice received 100 µL/mouse of distilled water once daily. On the day of plug detection (GD 0·5), plugged mice that were on the folic acid sufficient diet or folic acid deficient diet were randomly allocated to control (water), 1x-DTG, 5x-DTG, 1x-DTG+TDF/FTC or 5x-DTG+TDF/FTC groups. Dams exposed to control, 1x-DTG or 5x-DTG (without TDF/FTC) were sacrificed on GD 10·5, just after the time of neural-tube closure.29 Dams exposed to control, 1x-DTG+TDF/FTC or 5x-DTG+TDF/FTC were sacrificed on GD 15·5, representing later stages of neurogenesis and time of accelerated fetal growth.
The weight of each dam was recorded before euthanasia by CO2 inhalation. Post euthanasia, cervical dislocation was performed. The uterus was surgically removed and the number and location of embryos and resorptions was recorded. The placentae from dams sacrificed on GD 10·5 and GD 15·5 were cleaned by teasing out the embryo and the yolk sac attached, frozen in liquid nitrogen and stored at -80°C.
Human placental cell culture
The immortalized human first trimester trophoblast cell line, HTR-8/SVneo, and two choriocarcinoma cell lines, JAR and BeWo, were used in this study. The HTR-8/SVneo cell line (ATCC Cat# CRL-3271, RRID:CVCL_7162) was a kind gift from Dr. Isabella Caniggia (Mount Sinai Hospital, Toronto, Canada), while JAR and BeWo cells were purchased from the American Type Culture Collection (ATCC Cat# HTB-144, RRID:CVCL_0360 and ATCC Cat# CCL-98, RRID:CVCL_0044). BeWo and JAR cells were validated by the ATCC. The HTR-8/SVneo cell line was authenticated by IDEXX BioResearch in December 2017. After receiving HTR-8 SV neo cells from our collaborator Dr. Isabella Caniggia's laboratory, we have further verified it as a trophoblast cell line that expresses both CK8 and HLAG and does not express CD90 a fibroblast marker by IHC and western. HTR-8/SVneo cells (passage 11-18) and JAR cells (passage 2-10) were maintained in RPMI-1640 media supplemented with 10% FBS and 1% penicillin/streptomycin. BeWo cells were maintained in DMEM supplemented with 10% FBS and 1% penicillin/streptomycin. The cell lines were kept in a humidified incubator at 37°C, 5% CO2, and 95% air atmosphere. Cells were subcultured with 0·25% trypsin-EDTA when they reached 90-100% confluence, and the culture media was changed every two to three days. Cells exposed to DTG treatment were first seeded into 6-well plates at an initial density of 50,000 cells/cm2 and grown to confluence for three days. For functional studies, cells were plated into 24-well plates at a density of 50,000 cells/cm2 and grown to confluence for three days before performing the transport assay.
DTG treatment of placental cell lines
Confluent HTR-8/SVneo and JAR cell monolayers grown on six-well plates were treated with either DMSO (vehicle control) or DTG (500-4000 ng/mL) for a period of 3h or 6h at 37°C. Ex vivo human placental explants were treated for 3h or 6h with either DMSO (vehicle control) or DTG (2000 ng/mL or 4000 ng/mL). The range of drug concentrations was based on reported human pharmacokinetic data and the human therapeutic concentrations from the literature-based summary.28 At the desired time interval, treated cells and tissues were collected using TRIzol for gene expression analysis, or radioimmunoprecipitation assay buffer for protein expression analysis.
Immunohistochemical analysis
IHC analysis of placental protein localization was performed on 5µm sections using the streptavidin peroxidase method as described previously.30 Antigen retrieval was performed by microwaving the slides in 10mM sodium citrate (pH 6). Slides were blocked using serum free protein blocking solution (Dako) for 1 hr at room temperature, followed by overnight incubation at 4°C in primary antibody for FRα (1:50, Abcam, Cat# ab221543), RFC (1:1000, Sigma-Aldrich Cat# AV44167, RRID:AB_1848995) or PCFT (1:100, Sigma Aldrich, Cat# SAB2108339). Subsequent incubations with anti-rabbit (1:1000, Abcam, Cat# ab64256) biotinylated secondary antibody and streptavidin-horseradish peroxidase (HRP)-conjugated tertiary reagent (Invitrogen) were carried out for 1 hr each at room temperature. Incubation with or rabbit IgG (Abcam, Cat# ab6702) in place of primary antibody served as a negative control. Specific signals were detected using a Liquid DAB+ (3,3-diaminobenzidine) Chromogen System (Dako). To ensure fair comparison across gestation the same development time was applied to all sections. Slides were counterstained using Gill's No.1 haematoxylin (Sigma-Aldrich), dehydrated in ascending series of ethanol, cleared in xylene, and mounted with Cytoseal (Thermo Fisher Scientific). Photographs were taken with an Olympus DP72 camera mounted on a BX61 Olympus microscope using the CellSens Standard program.
Gene expression analysis
The mRNA expression of folate transporters/receptor was assessed using quantitative real-time PCR (qPCR). Total RNA was isolated from HTR-8/SVneo, JAR and BeWo cells or human placental tissues using TRIzol (Invitrogen). RNA concentrations (absorbance at 260 nm) and purity (absorbance ratio 260/280) were measured using Nanodrop One Spectrophotometer (Thermo Scientific). Isolated RNA (2μg) was treated with DNase I to remove contaminating DNA and reverse transcribed to cDNA using a high-capacity reverse transcription cDNA kit (Applied Biosystems) following the manufacturer's instructions. Specific human/mouse TaqMan primers for FOLR1/Folr1 (FRα/Frα; Hs01124179_91/Mm00433355_m1), SLC19A1/Slc19a1 (RFC/Rfc; Hs00953344_m1/Mm00446220_m1) and SLC46A1/Slc46a1 (PCFT/Pcft; Hs00560565_m1/ Mm00546630_m1) obtained from Life Technologies were used with TaqMan qPCR chemistry. All assays were performed in triplicates with the housekeeping gene for human/mice HPRT1/Hprt1 (Hs00168719_m1/Mm03024075 m1) as an internal control. For each gene, the critical threshold cycle (CT) was normalized to HPRT1/Hprt1 using the comparative CT method. The difference in CT values (ΔCT) between the gene of interest and the control housekeeping gene was then normalized to the corresponding ΔCT of the vehicle control (ΔΔCT) and expressed as 2−ΔΔCT to represent the relative difference in mRNA expression for each gene.
Immunoblot analysis
Protein expression was measured by immunoblot as previously described,31 with some modifications. Briefly, cells or placental tissues lysates were prepared in a modified radioimmunoprecipitation assay buffer (50 mM Tris pH 7·5, 150 mM NaCl, 1 mM EGTA, 1 mM sodium o-vanadate, 0·25% (v/v) sodium deoxycholic acid, 0·1% (v/v) sodium dodecyl sulphate (SDS), 1% (v/v) NP-40, 200μM PMSF, 0·1% (v/v) protease inhibitor). Total protein concentrations were quantified using Bradford's protein assay (Bio-Rad Laboratories) with BSA as a standard. Total protein (50 μg) was mixed with 1x Laemmli buffer and 10% β-mercaptoethanol and separated on 10% SDS-polyacrylamide gel, and electro-transferred onto a polyvinylidene fluoride membrane overnight at 4°C. The non-specific sites were blocked by incubating the blots with 5% skim milk prepared in tris-buffered saline solution containing 0·1% Tween 20 for 1·5h at room temperature. The blots were further incubated overnight at 4°C with primary rabbit monoclonal anti- FRα antibody (1:500 Abcam Cat# ab221543), rabbit polyclonal anti-SLC19A1 (RFC) antibody (1:250, Sigma-Aldrich Cat# AV44167, RRID:AB_1848995), rabbit polyclonal anti-SLC46A1 (PCFT) antibody (1:250, Sigma Aldrich Cat# SAB2108339) or mouse monoclonal anti-β-actin antibody (1:1000, Santa Cruz Biotechnology, sc-517582). The blots were then incubated with corresponding horseradish peroxidase-conjugated anti-rabbit (1:5000) or anti-mouse (1:5000) secondary antibody for 1·5h. Protein bands were detected using enhanced chemiluminescence SuperSignal West Pico System (Thermo Fisher Scientific) and imaged using the ChemiDoc MP Imaging System (Bio-Rad).
Transport assay
Functional assays with [3H]-FA (a PCFT and FRα substrate) and [3H]-MTX (an RFC substrate) (Moravek Biochemicals, MT783 and MT701) were performed using the published protocol of our laboratory with minor modifications.32 All transport assays were performed using transport buffer (Hank's balanced salt solution supplemented with 0·01% BSA and 25 mM HEPES (pH 7·4) or 25 mM MES (pH 5·5)). HTR-8/SVneo and JAR cells were allowed to grow confluent on 24-well plates, and pre-incubated in the transport buffer at 37°C for 15 minutes. Transport was initiated by adding 0·5 mL of transport buffer (pH 7·4 or 5·5) containing 50 nM [3H]-FA or [3H]-MTX at 37°C. The specific RFC/PCFT inhibitor pemetrexed (PMX) was added to both pre-incubation and radioactive transport buffer to examine the effect of inhibitors. At the desired time interval, the radioactive medium was aspirated, and each well was washed twice with ice-cold PBS and solubilized in 1 mL of 1% Triton X-100 at 37oC for 40 minutes. Cells were collected and mixed with 3 mL of PicoFluor 40 scintillation fluid (PerkinElmer Life and Analytical Sciences), and radioactivity was measured by the Beckman Coulter LS6500 Scintillation counter. When analysing data, correction for non-specific binding and variable quench time was performed by estimating the retention of the radiolabelled compound in the cells after a zero time of exposure. This time was determined by aspirating radioactive buffer immediately after its addition into the well, followed by two washes of ice-cold PBS and collection of cells for liquid scintillation counting. Cellular uptake of radiolabelled FA or MTX was normalized to total protein content per well, which was quantified by the Bio-Rad DC Protein Assay kit with BSA as standard.
Data analysis
All in vitro experiments were repeated at least three times, using cells from different passages. Experiments with human tissues were repeated with tissues from at least 6 donors. Animal experiments were repeated with at least 15 placentae from 2-21 dams per treatment. Each data point from a single experiment represents triplicates trials. Analysis of in vivo animal data and ex vivo human data was performed by experimenters blinded to group assignment. For animal experiments, mRNA expression was assessed using a mixed effects model, which included treatment as a fixed effect, and litter as a random effect. Results are generally presented as mean ± SD. Scatter plots are presented with the median. All statistical analyses and data obtained from functional studies with HTR-8/SVneo were fitted into the appropriate models by non-linear least-squares analysis using Prism 7 software (GraphPad Software Inc., San Diego, CA, USA). Statistical significance between two groups was analysed by a Mann-Whitney U test. Multiple group comparisons for unpaired data were analysed using a one-way analysis of variance (ANOVA) test or a Kruskal-Wallis test where appropriate, both with Bonferroni's post hoc test. Multiple group comparisons for paired data were analysed using Freidman's Test, with Dunn's post hoc test. p < 0·05 was considered statistically significant.
Role of the funding source
The funders approved the study design. The funders played no role in experimental work, data collection, analysis, interpretation, or drafting of the manuscript.
RESULTS
Expression of folate transporters and receptor in human placental cell lines
We first aimed to characterize the expression of FRα, RFC, and PCFT in three unique human in vitro models of the placenta: the third trimester choriocarcinoma cell line, BeWo; the first trimester choriocarcinoma cell line, JAR; and the immortalized first trimester trophoblast cell line, HTR-8/SVneo. mRNA expression of FOLR1 (FRα), SLC19A1 (RFC), and SLC46A1 (PCFT), was robust in all three cell lines, with the exception of FOLR1, which displayed relatively low expression in the HTR-8/SVneo cell line (Fig. 1a). At the protein level, applying immunoblot analysis, the expression of RFC and PCFT was readily detectable in the HTR-8/SVneo, JAR and BeWo cell lines, while the FRα protein expression was much lower compared to RFC and PCFT in the HTR-8/SVneo cell line, which corresponds to the low level of FOLR1 mRNA expression detected in this cell line (Fig. 1b, c).
Figure 1.
In vitro expression of folate transporters and Folate receptor-α in human placental cell lines. a) Relative mRNA expression of SLC19A1, SLC46A1 and FOLR1 was determined in HTR-8/SVneo, JAR and BeWo cells by qPCR, normalized to the housekeeping gene HPRT1 (n=3). b) Protein expression of RFC, PCFT and FRα, in JAR, HTR-8/SVneo and BeWo cell lines. Expression was quantified relative to β-actin, by densitometric analysis of immunoblots (n=3). (c) Representative immunoblot demonstrating expression of RFC, PCFT, FRα and β-actin, in JAR, HTR-8/SVneo and BeWo cell lines. Results are presented as mean relative expression ± SD.
Folate transporters and receptor localization in human placental tissue
In addition to our in vitro assessment of protein expression in human placental cell lines by qPCR, we assessed the gestational age-dependent expression and localization of FRα, RFC, and PCFT by IHC in human placental tissue sections. Tissue sections from the first trimester (5 weeks, 7-8 weeks, 10-12 weeks), early second trimester (17-20 weeks), and term (38-40 weeks), were immuno-stained and visualized using light microscopy. We identified robust staining of FRα in early first trimester placental tissue (Fig. 2), localized to the syncytiotrophoblast, which comprises the outer layer of the placental villi.9 FRα staining gradually decreased in intensity as the first trimester progressed to the second trimester. At term FRα staining was observed in the syncytiotrophoblast of the smaller intermediate and terminal villi (Fig. 2, upper panel). Staining for PCFT and RFC was weaker and localized to the syncytiotrophoblast, some weak staining of the cytoplasm of the mesenchymal stromal cells within the villous core was observed at 10-12 weeks of gestation (Fig. 2 middle and bottom panels). No staining was identified in the negative control (rabbit IgG), confirming that the signals we observed from FRα, RFC, and PCFT antibodies were specific.
Figure 2.
Folate transporter and receptor localization in human placental tissue sections. Immunohistochemical localization of folate receptor alpha (FRα, top), proton-coupled folate transporter (PCFT, middle) and reduced folate carrier (RFC, bottom), in 5 week, 7-8 week, 10-12 week, 17 to 20 week and term38, 39, 40 week human placenta. Tissues were immunostained and visualized using confocal microscopy operated with ZEN software using a 20x objective lens. (Scale bar, 100 µM). Staining was not present in appropriate negative controls (rabbit IgG, inset).
Effect of DTG exposure on folate transporters and receptor expression in human placental explants
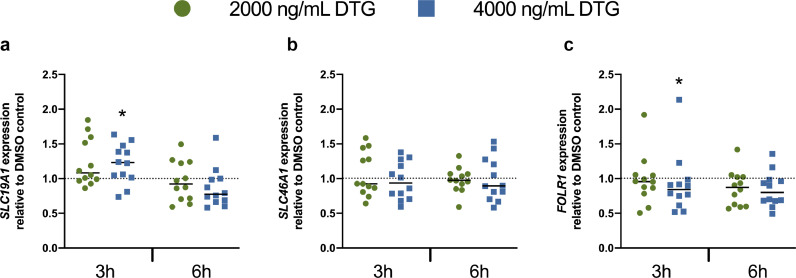
We next investigated the effect of DTG treatment on the mRNA expression of FRα, RFC, and PCFT in human first trimester placental explants. Explants were collected from human placenta between 6 and 12 weeks’ gestation and treated ex vivo with clinically relevant DTG concentrations of 2000 ng/mL DTG or 4000 ng/mL of DTG for 3h or 6h. Despite considerable inter-individual variability in expression, we identified a modest upregulation of SLC19A1, and a modest downregulation of FOLR1 following 3h exposure to the 4000 ng/mL dose of DTG (Figure 3a, c). No changes were identified at 2000 ng/mL DTG exposure. Furthermore no interaction between DTG exposure and expression was identified following the longer, 6h DTG treatment (figure 3a, b, c).
Figure 3.
Effect of DTG exposure on folate transporter and receptor mRNA expression in human first trimester placental explants. Relative mRNA expression of a) SLC19A1, b) SLC46A1 and c) FOLR1 was determined in first trimester human placental explants from healthy donors, by qPCR (n=6). Placental explants were treated ex vivo with 2000 ng/mL DTG, 4000 ng/mL DTG, or vehicle control (DMSO) for 3h or 6h. For each donor, expression levels in the DTG-treated placental explants was normalized to those in the vehicle control. Each point represents relative expression in DTG-treated explants, normalized to expression in the corresponding vehicle-treated explants for a given donor. Solid line indicates mean relative mRNA expression. Vehicle control is indicated by the dotted line. Expression of SLC19A1 and FOLR1 following 3h DTG treatment at 4000 ng/mL was significantly greater than in the vehicle treated control. *, p<0•05 (Friedman test with Dunn's post hoc test).
Effect of in vitro DTG exposure on folate transporters and receptor expression in placental cell lines
In order to investigate the effect of DTG treatment on placental FRα, RFC, and PCFT mRNA expression, HTR-8/SVneo cells (Fig. 4) were treated for 3h or 6h with 500 ng/mL DTG, 2000 ng/mL DTG, 4000 ng/mL DTG or vehicle control (DMSO), representing clinically relevant plasma concentrations.33 Transporter and receptor expression was assessed at the mRNA level by qPCR. We identified a significant downregulation in mRNA expression of SLC19A1 in HTR-8/SVneo cells treated with 2000 ng/mL or 4000 ng/mL DTG for 3h, with no effect following 6h of treatment (Fig. 4a). Furthermore, we identified a trend of dose-dependent downregulation in SLC46A1 mRNA expression in cells exposed to DTG for 6h, with a significant reduction in SLC46A1 expression in cells exposed to the highest dose of DTG (4000 ng/mL) (Fig. 4b).
Figure 4.
Effect of in vitro DTG exposure on transporter and receptor expression in HTR-8/SVneo cells. HTR-8/SVneo cells were treated for 3h or 6h with DMSO, 500 ng/mL DTG, 2000 ng/mL DTG or 4000 ng/mL DTG. a) SLC19A1 and b) SLC46A1 expression was determined by qPCR, normalized to the housekeeping gene HPRT1 and to the vehicle-treated control (DMSO). Protein expression of c) RFC and d) PCFT in DTG treated HTR-8/SVneo cells was quantified relative to β-actin, by densitometric analysis of immunoblots. Representative immunoblot for e) RFC and f) PCFT in 6h DTG-treated HTR-8/SVneo cells. Results are presented as mean relative mRNA expression ± SD, from n=3 independent experiments. *, p<0•05 (one-way ANOVA with Bonferroni's post-hoc test).
Transporter protein expression was investigated by immunoblot analysis. We identified a dose-dependent downregulation in expression of both RFC and PCFT at the protein level, in HTR-8/SVneo cells exposed to DTG for 6h, with significant downregulation reached in cells exposed to the highest dose of DTG (4000 ng/mL) (Fig. 4c, d).
Effect of in vitro DTG exposure on transporter and receptor function in placental cell lines
We investigated the ability of DTG exposure to affect the function of folate transport mechanisms in the first trimester HTR-8/SVneo and JAR cell lines. RFC does not transport FA, but is a transporter of its reduced forms including 5-methytetrahydrofolate and related antifolate drugs such as MTX.34 To assess the effect of DTG exposure on RFC function, cells were exposed to MTX at pH 7·4, following DTG treatment. Under these conditions, 6h DTG treatment was associated with 30-40% reduction in [3H]-MTX uptake in the HTR-8/SVneo cell line. [3H]-MTX uptake was similarly reduced in JAR cells following a 3h DTG treatment (Fig. 5a, b).
Figure 5.
Effect of in vitro DTG exposure on transporter and receptor function in placental cell lines. To assess the effect of DTG exposure on transporter and receptor function, HTR-8/SVneo and JAR cells were treated with respective transporters/receptor substrates ([3H-MTX or [3H]-FA) and/or inhibitors (PMX or excess substrate concentration), following a 3h or 6h DTG exposure at clinically relevant concentrations. a) In HTR-8/SVneo cells and b) JAR cells, cellular uptake of [3H]-methotrexate (50 nM) was measured over 1 minute at pH 7·4, 37°C to assess the activity of RFC. c) In HTR-8/SVneo cells and e) JAR cells, cellular uptake of [3H]-folic acid (50 nM) was measured over 1 minute at pH 5·5, 37°C to assess the activity of PCFT. e) In JAR cells only, cellular uptake of [3H]-folic acid was measured over 1h at pH 7·4, 37°C to assess the function of FRα. Results are presented as mean uptake, relative to vehicle control ± SD, for n=3 independent experiments. *, p<0·05; **, p<0·01; ***, p<0·001 (one-way ANOVA, with Bonferroni's post-hoc test).
To assess the function of PCFT, transport assays were conducted with the PCFT substrate [3H]-FA at pH 5·5, conditions which favour PCFT activity over that of FRα (i.e., PCFT is a proton-coupled co-transporter). Following 3h and 6h DTG treatment of HTR-8/SVneo cells, uptake of [3H]-FA was reduced 35-45%, with a less pronounced effect in JAR cells (Fig. 5c, d). As expected, the uptake of [3H]-FA was significantly inhibited by the PCFT inhibitor, PMX, indicating specific PCFT-mediated transport.
To investigate the functional activity of FRα, we assessed the uptake of [3H]-FA in JAR cells at pH 7·4, optimal pH conditions favouring the activity of FRα over PCFT. Following 3h and 6h exposures to DTG, uptake of [3H]-FA was significantly reduced by 20% (Fig. 5e).
Expression of folate transporters and receptor in DTG-treated mouse placenta in vivo
In order to investigate the effect of chronic DTG-exposure on placental expression of FRα, RFC, and PCFT, we used a mouse model previously validated to represent clinically relevant DTG exposures in pregnancy.28 Pregnant mice on a folate sufficient diet were treated once daily for 10·5 days with DTG at 1x (2·5mg/kg/day) or 5x (12·5 mg/kg/day) doses; or once daily for 15·5 days with DTG + TDF/FTC at either 1x (2·5 mg/kg/day DTG + 50 mg/kg/day TDF + 33·3 mg/kg/day FTC) or 5x (12·5 mg/kg/day DTG + 50 mg/kg/day TDF + 33·3 mg/kg/day) doses. These doses were previously demonstrated to achieve clinically relevant maternal plasma concentrations.28 Mice placentae collected at GD 15·5 were treated with DTG + TDF/FTC. However, to mimic the in vitro experiments, placentae collected at GD 10·5 mice were treated with DTG only. Placental mRNA expression was assessed by qPCR, with expression normalized to the housekeeping gene Hprt1, as well as to the vehicle-treated control. In this cohort, DTG exposure at the 1x-DTG dose was associated with a significant but modest upregulation in the placental expression of Folr1 at GD 10·5, as well as a modest upregulation in placental expression of Slc19a1 at GD 15·5 (Figure 6a, b). DTG exposure was not associated with significantly altered expression of Slc19a1 or Slc46a1 at GD 10·5, nor Slc46a1 or Folr1 at GD 15·5.
Figure 6.
Expression of folate transporters and receptor in DTG-treated folate-sufficient mouse placenta. Effect of DTG treatment on Slc19a1 (left), Slc46a1 (middle) and Folr1 (right) placental mRNA expression in a) GD 10·5 (n=15 for vehicle, n=16 for 1x-DTG treated, and n=15 for 5x-DTG treated groups), and b) GD 15·5 mouse placenta (n=40 for vehicle, n=33 for 1x-DTG+TDF/FTC treated, and n=40 for 5x-DTG+TDF/FTC treated groups). Vehicle = water; 1x-DTG = 2·5 mg/kg; 5x-DTG = 12·5 mg/kg); E/T = emtricitabine (33·3 mg/kg) + tenofovir disoproxil fumarate (50 mg/kg). Results presented as median expression normalized to the housekeeping gene Hprt1, and plotted relative to vehicle control. *, p<0·05; **, p<0·01; ****, p<0·0001 (mixed effects model to account for litter effects).
Risks of neurodevelopmental defects, including drug-associated NTDs are more pronounced in regions where folate supplementation in pregnancy is not widespread. To simulate these conditions, we further investigated mRNA expression of Folr1, Slc19a1, and Slc46a1 in mice fed on a folate-deficient diet. In this cohort, chronic exposure to the 5x dose of DTG was associated with a significant upregulation of Slc19a1expression in GD 10·5 mouse placenta, and Slc19a1 as well as Slc46a1 expression in 15·5 mouse placenta. 5x-DTG exposure was further associated with a significant downregulation in placental Folr1 expression at GD 10·5 but not GD 15·5 (Fig. 7a, b).
Figure 7.
Expression of folate transporters and receptor in DTG-treated folate-deficient mouse placenta. Effect of DTG treatment on Slc19a1 (left), Slc46a1 (middle) and Folr1 (right) placental mRNA expression in a) GD 10·5 (n=15 for vehicle, 16 for 1x-DTG treated, and 18 for 5x-DTG treated groups), and b) GD 15·5 mouse placenta (n=34 for vehicle, 40 for 1x-DTG + E/T treated, and 32 for 5x-DTG + E/T treated groups) under folate-deficient conditions. Vehicle = water; 1x-DTG = 2·5 mg/kg; 5x-DTG = 12·5 mg/kg); E/T = emtricitabine (33·3 mg/kg) + tenofovir disoproxil fumarate (50 mg/kg). Results presented as median expression normalized to the housekeeping gene Hprt1, and plotted relative to vehicle control. *, p<0·05; **, p<0·01 (mixed effects model to account for litter effects).
Discussion
In this study we have characterized the expression of FRα, RFC, and PCFT, in three unique models of folate transport at the placenta. In each model, we further assessed the effect of DTG exposure on the expression and/or function of folate transport pathways. Findings from each model were distinct, but collectively suggest a potential interaction between DTG and placental folate delivery, not through direct inhibition, but through altered gene expression.
Beginning with the 2018 Tsepamo report of NTDs amongst pregnant women taking DTG from the time of conception, recent clinical and preclinical reports have identified mixed findings regarding folate delivery and toxicity amongst women taking DTG, with several studies identifying little or no risk.19, 20, 21, 22,24,26,27,35 In contrast, Cabrera et. al. identified developmental toxicity amongst zebrafish embryos exposed to DTG.24 Using an ART mouse model comparable to the one described in our study, Mohan et al. similarly identified an increased risk of fetal defects amongst pregnant mice exposed to DTG from conception, including lower fetal and placental weights, vascular defects, and 0·47% rate of NTDs, similar to the 0·3% rate identified in the Tsepamo study.27 Interestingly, Mohan et al. also identified a significant increase in fetal total folate levels in pregnant mice exposed to 5x (12·5 mg/kg/day) DTG on a folate sufficient diet compared to control and 1x (2·5mg/kg/day) DTG, suggesting a potential fetal protective mechanism following DTG exposure.
Recent in vitro findings, regarding an interaction between DTG and folate transport pathways are also mixed, due in part to differences in the models used. Using competitive binding studies in HeLa and HTR-8/SVneo cells, Cabrera et al. identified DTG as a partial antagonist of FRα, with an IC50 value of 4·4 µM, within reported therapeutic plasma concentrations of 3-10 µM 24. In contrast, Zamek-Gliszczynski et al. investigated FRα mediated uptake of FA in an overexpressing renal epithelial cell line (MDCKII), and identified a 36% ± 5·7% inhibition of FRα -mediated FA uptake by DTG at 57·3 ± 4·2 µM, concentrations well above the therapeutic range, as well as above the cut-off for clinical relevance described in the study.25 Zamek-Gliszczynski et al. also investigated transport of MTX and FA by RFC and PCFT respectively, and concluded that DTG as well as bictegravir, cabotegravir, and elvitegravir, would not inhibit folate transport pathways under clinical conditions.
Our findings are in part consistent with the conclusions of Zamek-Gliszczynski et al. in suggesting that impaired folate delivery at therapeutic DTG concentrations may be primarily caused not by direct inhibition but instead through altered transporter expression and/or regulation. The study design of both Zamek-Gliszczynski et al., involving overexpression of FRα, PCFT, and RFC in MDCKII cells, and/or relatively short incubation times (30 minutes), are sufficient for intended investigation of direct inhibition, but are not reflective of clinically relevant chronic exposure which may impact gene expression. In the HTR-8/SVneo and JAR cell lines, which were treated with DTG for 3h and 6h, we observed a downregulation in RFC and PCFT expression. As expected, the downregulation in RFC mRNA expression in HTR-8/SVneo following 3h DTG treatment was accompanied by both reduced protein expression and RFC transport of [3H]-MTX at 6h of treatment, suggesting a direct link between altered expression and function. Similar findings were observed at 6h in the expression of PCFT, but not for FRα due to low expression in HTR-8/SVneo cells.
In the in vivo mouse model of placental folate delivery, we observed modest changes in the expression of the folate transporters and receptors following administration of clinically relevant doses of DTG throughout pregnancy. Amongst the models investigated in this study, chronic DTG exposure and folate deficient diet applied in the animal model is most reflective of the conditions experienced in clinical settings such as Botswana where the risk of NTDs was first identified. Notably, in the folate deficient condition, the administration of DTG resulted in a modest upregulation of placental RFC and PCFT mRNA at both GD 10·5 and GD 15·5 time points. Importantly, this upregulation of RFC at the mRNA level was also identified in our ex vivo treatment of human placental explants treated with 4000 ng/mL DTG. Transporter upregulation and increased folate uptake in response to folate deficiency, has been previously reported as a compensatory mechanism.13,36,37 Our findings suggest that in vivo DTG further stimulates RFC and PCFT upregulation in response to folate deficiency. RFC, which is essential for folate transport across the basolateral membrane of the syncytiotrophoblast, is regulated in part by the activity of transcription factors including Sp1, upstream stimulatory factor 1, vitamin D receptor and nuclear respiratory factor 1.38, 39, 40, 41 Transcription factors which respond to xenobiotic exposures, most notably the aryl hydrogen receptor (AhR), have also been implicated in the regulation of solute carrier (SLC) transporters including RFC.39 Halwachs et al., for example demonstrated the AhR-mediated downregulation of RFC following exposure to an AhR agonist.42 Whether DTG interacts with these or other pathways remains to be explored.
In contrast, we report a modest reduction in the expression of Folr1 amongst GD 10·5 folate deficient placentae exposed to DTG. This finding was also consistent with a downregulation of FOLR1 identified in our ex vivo treatments of human placental explants. Regulation of human FOLR1 is governed by transcription factors including the glucocorticoid and oestrogen receptors, as well as the hepatocyte nuclear factor 4-alpha, and Sp1.43,44 Interactions with these pathways could impact the expression of FRα. For example exposure to 17β-oestradiol and xenobiotic oestrogen receptor modulators such as tamoxifen, has been associated with a downregulation in FORL1 expression in cervical and ovarian carcinoma cells.45 As FRα constitutes the primary mechanism of placental folate uptake during this sensitive stage of neurogenesis, reduction in FRα expression could significantly limit folate accessibility in an already folate deficient environment, potentially even in light of parallel increases in RFC and PCFT expression.
Differences in findings amongst the three models investigated in the present study highlight the difficulties in the establishment of a clinically relevant model of placental folate transport. The in vitro models allowed for a detailed investigation of gene expression and folate uptake, however low expression of FOLR1 in HTR-8/SVneo limited its use in studies of FRα function, while in contrast, the characteristics of the choriocarcinoma cell lines JAR and BeWo, limit their translatability. Furthermore, findings in ex vivo treated human placental explants, while most relevant to human pregnancy, displayed high variability in transporter expression, and are relatively limited in assessing folate transport function. Of the models presented in this work, the in vivo murine model is perhaps most reflective of the chronic DTG exposure, as well as folate deficiency, experienced by many pregnant women living with HIV, taking DTG. However, differences in placental anatomy between human and mouse somewhat limit translatability, particularly as similar findings were not consistently observed in our ex vivo human model. Ultimately, application of these models together provides a holistic perspective of folate delivery mechanisms in placenta. Our findings in all three models suggest a potential interaction between DTG and placental folate delivery, which may have bearing on the early neurodevelopment in the context of ART.
Declaration of interests
LS received personal support for participating in a ViiV organized Think Tank. The authors have no further competing interests related to this study.
Acknowledgments
Contributors
JCG, MTH, and WD performed the immunoblot, qPCR, and transport assays, and analysed the data. HM performed the breeding, animal treatments and tissue collection with help from JCG. CD performed the IHC assay and data analysis. JCG, MTH and RB verified the underlying data. RB and LS conceived the study and directed the research. JCG, MTH, and RB drafted the manuscript. All authors have read and approved the final version of the manuscript.
Acknowledgments
The HTR-8/SVneo cell line was a kind gift from Dr. Isabella Caniggia, to whom we are grateful. We would also like to thank Ms. Mona Adib for her technical contributions to this study. This work was supported by an Ontario HIV Treatment Network grant #506657, awarded to RB and LS; and by the Eunice Kennedy Shriver National Institute Of Child Health & Human Development of the National Institutes of Health under Award Number R01HD104553 awarded to LS. HM was funded by a CTN/CIHR Postdoctoral Fellowship. JCG was funded by a CIHR Doctoral Award. The funders played no role in the experimental work, data collection, analysis, or interpretation of the data, or drafting of the manuscript. The corresponding author had full access to all the data in the study and had final responsibility for the decision to submit for publication.
Data sharing
All relevant data have been presented in the manuscript. All requests for or questions about the data can be initiated by contacting r.bendayan@utoronto.ca.
Footnotes
Supplementary material associated with this article can be found in the online version at doi:10.1016/j.ebiom.2021.103771.
Appendix. Supplementary materials
References
- 1.Ducker G.S., Rabinowitz J.D. One-Carbon Metabolism in Health and Disease. Cell Metabolism. 2017 Jan 10;25(1):27–42. doi: 10.1016/j.cmet.2016.08.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Tamura T., Picciano M.F. Folate and human reproduction. Am J Clin Nutr. 2006 May;83(5):993–1016. doi: 10.1093/ajcn/83.5.993. [DOI] [PubMed] [Google Scholar]
- 3.Tibbetts A.S., Appling D.R. Compartmentalization of Mammalian folate-mediated one-carbon metabolism. Annu Rev Nutr. 2010 Aug 21;30:57–81. doi: 10.1146/annurev.nutr.012809.104810. [DOI] [PubMed] [Google Scholar]
- 4.Institute of Medicine (US) Standing Committee on the Scientific Evaluation of Dietary Reference Intakes and its Panel on Folate . National Academies Press (US); Washington (DC): 1998. Other B Vitamins, and Choline. Dietary Reference Intakes for Thiamin, Riboflavin, Niacin, Vitamin B6, Folate, Vitamin B12, Pantothenic Acid, Biotin, and Choline [Internet]http://www.ncbi.nlm.nih.gov/books/NBK114310/ [cited 2021 Mar 20]. (The National Academies Collection: Reports funded by National Institutes of Health). Available from: [PubMed] [Google Scholar]
- 5.Fekete K., Berti C., Cetin I., Hermoso M., Koletzko B.V., Decsi T. Perinatal folate supply: relevance in health outcome parameters. Matern Child Nutr. 2010 Sep 21;6(Suppl 2):23–38. doi: 10.1111/j.1740-8709.2010.00261.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Steele J.W., Kim S.-E., Finnell R.H. One-carbon metabolism and folate transporter genes: Do they factor prominently in the genetic etiology of neural tube defects? Biochimie. 2020 Jun;173:27–32. doi: 10.1016/j.biochi.2020.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Matok I., Gorodischer R., Koren G., Landau D., Wiznitzer A., Levy A. Exposure to folic acid antagonists during the first trimester of pregnancy and the risk of major malformations. Br J Clin Pharmacol. 2009 Dec;68(6):956–962. doi: 10.1111/j.1365-2125.2009.03544.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Allen LH. Causes of vitamin B12 and folate deficiency. Food Nutr Bull. 2008 Jun;29(2 Suppl):S20–S34. doi: 10.1177/15648265080292S105. [DOI] [PubMed] [Google Scholar]
- 9.Zhao R., Diop-Bove N., Visentin M., Goldman I.D. Mechanisms of Membrane Transport of Folates into Cells and Across Epithelia. Annu Rev Nutr. 2011 Aug:31. doi: 10.1146/annurev-nutr-072610-145133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Yasuda S., Hasui S., Yamamoto C., Yoshioka C., Kobayashi M., Itagaki S., et al. Placental folate transport during pregnancy. Biosci Biotechnol Biochem. 2008 Sep;72(9):2277–2284. doi: 10.1271/bbb.80112. [DOI] [PubMed] [Google Scholar]
- 11.Solanky N., Requena Jimenez A., D'Souza S.W., Sibley C.P., Glazier J.D. Expression of folate transporters in human placenta and implications for homocysteine metabolism. Placenta. 2010 Feb;31(2):134–143. doi: 10.1016/j.placenta.2009.11.017. [DOI] [PubMed] [Google Scholar]
- 12.Saitsu H., Ishibashi M., Nakano H., Shiota K. Spatial and temporal expression of folate-binding protein 1 (Fbp1) is closely associated with anterior neural tube closure in mice. Dev Dyn. 2003 Jan;226(1):112–117. doi: 10.1002/dvdy.10203. [DOI] [PubMed] [Google Scholar]
- 13.Qiu A., Jansen M., Sakaris A., Min S.H., Chattopadhyay S., Tsai E., et al. Identification of an intestinal folate transporter and the molecular basis for hereditary folate malabsorption. Cell. 2006 Dec 1;127(5):917–928. doi: 10.1016/j.cell.2006.09.041. [DOI] [PubMed] [Google Scholar]
- 14.Zhao R., Min S.H., Wang Y., Campanella E., Low P.S., Goldman I.D. A Role for the Proton-coupled Folate Transporter (PCFT-SLC46A1) in Folate Receptor-mediated Endocytosis. J Biol Chem. 2009 Feb 13;284(7):4267–4274. doi: 10.1074/jbc.M807665200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Speidel B.D., Mea.dow., Sr. Maternal epilepsy and abnormalities of the fetus and newborn. The Lancet. 1972 Oct 21;300(7782):839–843. doi: 10.1016/s0140-6736(72)92209-x. [DOI] [PubMed] [Google Scholar]
- 16.Fathe K., Palacios A., Finnell R.H. Novel Mechanism for Valproate-Induced Teratogenicity. Birth Defects Res A Clin Mol Teratol. 2014 Aug;100(8):592–597. doi: 10.1002/bdra.23277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Keating E., Gonçalves P., Campos I., Costa F., Martel F. Folic acid uptake by the human syncytiotrophoblast: Interference by pharmacotherapy, drugs of abuse and pathological conditions. Reprod Toxicol. 2009 Dec;28(4):511–520. doi: 10.1016/j.reprotox.2009.07.001. [DOI] [PubMed] [Google Scholar]
- 18.Scarsi K.K., Havens J.P., Podany A.T., Avedissian S.N., Fletcher C.V. HIV-1 Integrase Inhibitors: a Comparative Review of Efficacy and Safety. Drugs. 2020 Nov;80(16):1649–1676. doi: 10.1007/s40265-020-01379-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Zash R., Makhema J., Shapiro R.L. Neural-tube defects with dolutegravir treatment from the time of conception. New Engl J Med. 2018;379(10):979–981. doi: 10.1056/NEJMc1807653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Zash R., Holmes L., Diseko M., Jacobson D.L., Brummel S., Mayondi G., et al. Neural-Tube Defects and Antiretroviral Treatment Regimens in Botswana. New Engl J Med. 2019 Aug;381(9):827–840. doi: 10.1056/NEJMoa1905230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Chandiwana N.C., Chersich M., Venter W.D.F., Akpomiemie G., Hill A., Simmons B., et al. Unexpected interactions between dolutegravir and folate: randomized trial evidence from South Africa. AIDS. 2021 Feb 2;35(2):205–211. doi: 10.1097/QAD.0000000000002741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Pereira G.F.M., Kim A., Jalil E.M., Fernandes Fonseca F., Shepherd B.E., Veloso V.G., et al. Dolutegravir and pregnancy outcomes in women on antiretroviral therapy in Brazil: a retrospective national cohort study. Lancet HIV. 2021 Jan;8(1):e33–e41. doi: 10.1016/S2352-3018(20)30268-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Sibiude J., Le Chenadec J., Mandelbrot L., Dollfus C., Matheron S., Lelong N., et al. Risk of birth defects and perinatal outcomes in HIV-infected women exposed to integrase strand inhibitors during pregnancy. AIDS. 2021 Feb 2;35(2):219–226. doi: 10.1097/QAD.0000000000002719. [DOI] [PubMed] [Google Scholar]
- 24.Cabrera R.M., Souder J.P., Steele J.W., Yeo L., Tukeman G., Gorelick D.A., et al. The antagonism of folate receptor by dolutegravir: Developmental toxicity reduction by supplemental folic acid. AIDS. 2019 Nov;33(13):1967–1976. doi: 10.1097/QAD.0000000000002289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Zamek-Gliszczynski M.J., Zhang X., Mudunuru J., Du Y., Chen J.-L., Taskar K.S., et al. Clinical Extrapolation of the Effects of Dolutegravir and Other HIV Integrase Inhibitors on Folate Transport Pathways. Drug Metab Dispos. 2019 Aug;47(8):890–898. doi: 10.1124/dmd.119.087635. [DOI] [PubMed] [Google Scholar]
- 26.Stanislaus D.J., Posobiec L.M., Laffan S.B., Solomon H.M., Ziejewski M.K. Romach EH. Absence of developmental and reproductive toxicity in animals exposed to dolutegravir. Birth Defects Res. 2020 Feb 1;112(3):245–261. doi: 10.1002/bdr2.1635. [DOI] [PubMed] [Google Scholar]
- 27.Mohan H., Lenis M.G., Laurette E.Y., Tejada O., Sanghvi T., Leung K.-Y., et al. Dolutegravir in pregnant mice is associated with increased rates of fetal defects at therapeutic but not at supratherapeutic levels. EBioMedicine. 2021 Jan;63 doi: 10.1016/j.ebiom.2020.103167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kala S., Watson B., Zhang J.G., Papp E., Guzman Lenis M., Dennehy M., et al. Improving the clinical relevance of a mouse pregnancy model of antiretroviral toxicity; a pharmacokinetic dosing-optimization study of current HIV antiretroviral regimens. Antivir Res. 2018;159(May):45–54. doi: 10.1016/j.antiviral.2018.09.008. [DOI] [PubMed] [Google Scholar]
- 29.Greene N.D.E., Copp A.J. Neural Tube Defects. Annu Rev Neurosci. 2014;37:221–242. doi: 10.1146/annurev-neuro-062012-170354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Hazan A.D., Smith S.D., Jones R.L., Whittle W., Lye S.J., Dunk C.E. Vascular-leukocyte interactions: mechanisms of human decidual spiral artery remodeling in vitro. Am J Pathol. 2010 Aug;177(2):1017–1030. doi: 10.2353/ajpath.2010.091105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Hoque M.T., Shah A., More V., Miller D.S., Bendayan R. In vivo and ex vivo regulation of breast cancer resistant protein (Bcrp) by peroxisome proliferator-activated receptor alpha (Pparα) at the blood-brain barrier. J Neurochem. 2015 Dec;135(6):1113–1122. doi: 10.1111/jnc.13389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kis O., Zastre J.A., Ramaswamy M., Bendayan R. pH dependence of organic anion-transporting polypeptide 2B1 in Caco-2 cells: potential role in antiretroviral drug oral bioavailability and drug-drug interactions. J Pharmacol Exp Ther. 2010 Sep 1;334(3):1009–1022. doi: 10.1124/jpet.110.166314. [DOI] [PubMed] [Google Scholar]
- 33.Castagna A., Maggiolo F., Penco G., Wright D., Mills A., Grossberg R., et al. Dolutegravir in antiretroviral-experienced patients with raltegravir- and/or elvitegravir-resistant HIV-1: 24-week results of the phase III VIKING-3 study. J Infect Dis. 2014 Aug 1;210(3):354–362. doi: 10.1093/infdis/jiu051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Matherly L.H., Hou Z. Structure and Function of the Reduced Folate Carrier: a Paradigm of A Major Facilitator Superfamily Mammalian Nutrient Transporter. Vitam Horm. 2008:79. doi: 10.1016/S0083-6729(08)00405-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.drew Hill.An Aa, P Cl.ayden, Thorne C., Christie R., Zash R. Safety and pharmacokinetics of dolutegravir in HIV-positive pregnant women: a systematic review. J Virus Erad. 2018 Apr 1;4(2):66–71. doi: 10.1016/S2055-6640(20)30247-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Liu M., Ge Y., Cabelof D.C., Aboukameel A., Heydari A.R., Mohammad R., et al. Structure and regulation of the murine reduced folate carrier gene: identification of four noncoding exons and promoters and regulation by dietary folates. J Biol Chem. 2005 Feb 18;280(7):5588–5597. doi: 10.1074/jbc.M412662200. [DOI] [PubMed] [Google Scholar]
- 37.Said H.M., Chatterjee N., Haq R.U., Subramanian V.S., Ortiz A., Matherly L.H., et al. Adaptive regulation of intestinal folate uptake: effect of dietary folate deficiency. Am J Physiol Cell Physiol. 2000 Dec;279(6):C1889–C1895. doi: 10.1152/ajpcell.2000.279.6.C1889. [DOI] [PubMed] [Google Scholar]
- 38.Liu M., Whetstine J.R., Payton S.G., Ge Y., Flatley R.M., Matherly L.H. Roles of USF, Ikaros and Sp proteins in the transcriptional regulation of the human reduced folate carrier B promoter. Biochem J. 2004 Oct 15;383(Pt 2):249–257. doi: 10.1042/BJ20040414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Alam C., Hoque M.T., Sangha V., Bendayan R. Nuclear respiratory factor 1 (NRF-1) upregulates the expression and function of reduced folate carrier (RFC) at the blood-brain barrier. FASEB J. 2020 Aug;34(8):10516–10530. doi: 10.1096/fj.202000239RR. [DOI] [PubMed] [Google Scholar]
- 40.Alam C., Hoque M.T., Finnell R.H., Goldman I.D., Bendayan R. Regulation of Reduced Folate Carrier (RFC) by Vitamin D Receptor at the Blood-Brain Barrier. Mol Pharm. 2017 Nov 6;14(11):3848–3858. doi: 10.1021/acs.molpharmaceut.7b00572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Alam C., Aufreiter S., Georgiou C.J., Hoque M.T., Finnell R.H., O'Connor D.L., et al. Upregulation of reduced folate carrier by vitamin D enhances brain folate uptake in mice lacking folate receptor alpha. Proc Natl Acad Sci U S A. 2019 Aug 27;116(35):17531–17540. doi: 10.1073/pnas.1907077116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Halwachs S., Lakoma C., Gebhardt R., Schäfer I., Seibel P., Honscha W. Dioxin mediates downregulation of the reduced folate carrier transport activity via the arylhydrocarbon receptor signalling pathway. Toxicol Appl Pharmacol. 2010 Jul;246(1–2):100–106. doi: 10.1016/j.taap.2010.04.020. [DOI] [PubMed] [Google Scholar]
- 43.Saikawa Y., Price K., Hance K.W., Chen T.Y., Elwood P.C. Structural and functional analysis of the human KB cell folate receptor gene P4 promoter: cooperation of three clustered Sp1-binding sites with initiator region for basal promoter activity. Biochemistry. 1995 Aug 8;34(31):9951–9961. doi: 10.1021/bi00031a018. [DOI] [PubMed] [Google Scholar]
- 44.Salbaum J.M., Finnell R.H., Kappen C. Regulation of Folate Receptor 1 Gene Expression in the Visceral Endoderm. Birth Defects Res A Clin Mol Teratol. 2009 Apr;85(4):303–313. doi: 10.1002/bdra.20537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Kelley K.M.M., Rowan B.G., Ratnam M. Modulation of the folate receptor alpha gene by the estrogen receptor: mechanism and implications in tumor targeting. Cancer Res. 2003 Jun 1;63(11):2820–2828. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.