Abstract
Background:
Accurate tuberculosis infection (TBI) tests are critical for pregnant women, especially those with HIV, who have a high risk of TB disease.
Methods:
We enrolled interferon gamma release assay (IGRA)+ pregnant women with and without HIV in a longitudinal study, followed up at delivery and 6 months postpartum. Tuberculin skin test (TST) and IGRA were compared by HIV status at each timepoint.
Results:
Of 165 enrolled IGRA+ pregnant women: 35 (21%) had HIV and were on antiretroviral therapy with median CD4 of 476 (IQR 399–586). Compared to antepartum, significantly fewer women remained IGRA+ at delivery [HIV+ n=21/35 (62%, p=0.009); HIV− n=100/130 (77%, p=0.002)] and postpartum [HIV+ n=30/35 (87%, p=0.03); HIV− n=116/130 (89%, p=0.01)]. IGRA/TST discordance was high in pregnant women (HIV+: 51%; HIV−: 25%). Median IFN-γ was lowest for all women at delivery; significantly lower in women with HIV at all timepoints compared to women without HIV. TB incidence was 50/ 1000 person-years and 18/1000 person-years among women with and without HIV respectively.
Conclusions:
Pregnancy affects TBI test results and reduces IFN-γ response to M. tuberculosis stimulation. Despite adequate CD4 counts, women with HIV express less IFN-γ than women without HIV, which may explain the high TB incidence in postpartum women with HIV.
Keywords: Tubercular Infection (TBI); Interferon Gamma Release Assay (IGRA); Tuberculin Skin Test (TST); pregnancy, Human Immune Deficiency Virus (HIV); Interferon Gamma (IFN-γ)
INTRODUCTION
Tuberculosis (TB) disease is a leading cause of maternal mortality worldwide, especially among women living with HIV (WHO, 2015). Globally, more than 200,000 pregnant women develop TB annually, over 30% of those cases occur in Southeast Asia(Chopra et al., 2017, Gupta et al., 2007, Sugarman et al., 2014). TB incidence is highest within 90 days postpartum and leads to poor maternal and infant outcomes, including maternal death, preterm birth, and low birth weight (Getahun et al., 2012, Mathad and Gupta, 2012).
Before the combined antiretroviral therapy (cART) era, risk of TB disease was almost 10-fold greater in pregnant women with HIV to those without (Pillay et al., 2001); there are few data on TB incidence in the cART era. Data from non-pregnant populations, however, suggests that people living with well-controlled HIV still have higher risk of TB disease than people without HIV(WHO, 2019). This is of particular concern in pregnant women with HIV since pregnancy may independently increase the risk of TB disease(Zenner et al., 2012).
During a normal pregnancy, cytokines produced at the feto-maternal interface suppress production of inflammatory cytokines, such as interferon gamma (IFN-γ), which are essential in preventing the progression of TB infection (TBI) to disease(Chaouat et al., 1996). This suppression increases as pregnancy progresses and returns to baseline by 3–6 months postpartum, but may take longer in women with HIV(Chaouat et al., 1996, Mathad et al., 2016, Mathad and Gupta, 2012, Zenner et al., 2012). The effect of these immune changes on TB incidence is unknown. Cross-sectional studies of pregnant women with HIV report significant discordance between the tuberculin skin test (TST) and interferon gamma release assay (IGRA). Our previous study in HIV-infected pregnant women shows that women with IGRA+/TST− results have a lower expression of IFN-γ and IL-2 compared to women with IGRA+/TST+ results, suggesting that the discordant phenotype has a biologic cause (LaCourse et al., 2017, Theron et al., 2020). None of these studies have longitudinally compared the immune response to M. tuberculosis (MTB) antigens in women with versus without HIV.
Our objective was to investigate how stages of pregnancy and HIV impact responses to TBI tests in a longitudinal cohort of pregnant women with HIV and without HIV. The goals of this work were to compare the performance of TBI tests over time in pregnancy, and examine the agreement of TBI tests in pregnant women with and without HIV. We also examined the effect of HIV on M. tuberculosis (MTB)-specific immune responses to gain further insight into the pathophysiology of TB in pregnant women and TB progression.
METHODS
Between June 2016 and December 2019, we conducted an observational prospective cohort study, PRegnancy Associated Changes In Tuberculosis Immunology (PRACHITi; R01HD081929) in women with and without HIV at Sassoon Government Hospital, a large urban hospital in Pune, India.
Study population
Pregnant women over 18 years of age who presented to the antenatal clinic with gestational age between 13–34 weeks were screened for the study. We excluded women with a history of TB disease in the last two years, those currently on immunosuppressants or antibiotics of 15 days duration, or those with haemoglobin <7.5 gms (to mitigate adverse effects of additional blood draw for the study) (Fig 1). We initially excluded women receiving isoniazid preventive therapy (IPT) to better assess progression from infection to disease, but in 2017 the Indian national guidelines changed to recommend IPT for all with HIV, including pregnant women. Hence, the protocol was amended to include individuals on IPT in the study from 2017 onwards.
Figure 1. PRegnancy Associated CHanges in Tuberculosis Immunology (PRACHITI) subanalysis of IGRA+ subjects study design.
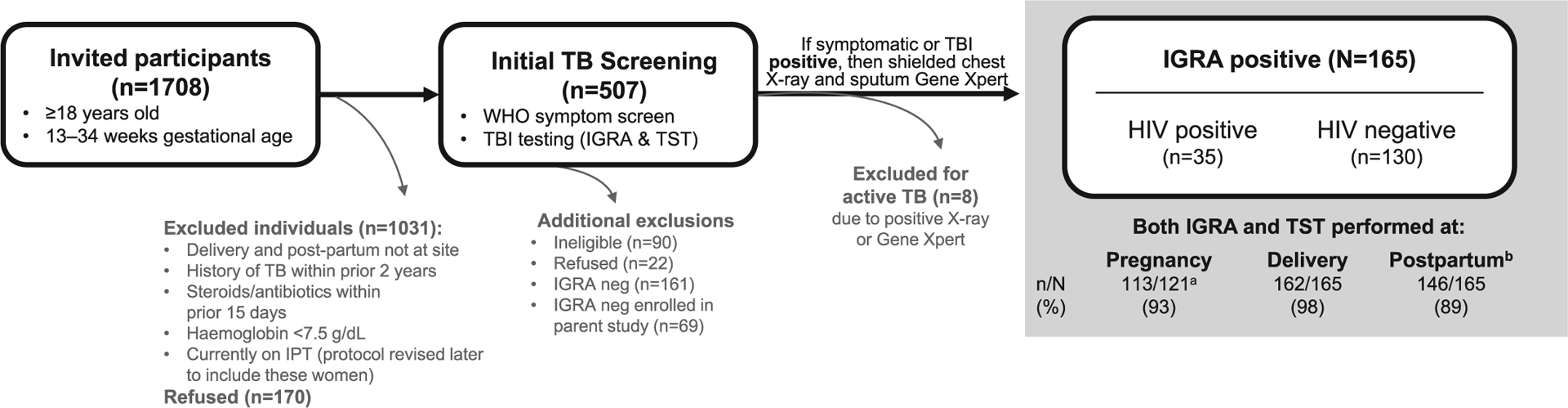
Individuals who were symptom screen and TBI negative [IGRA negative, n=69 (HIV+ n=44; HIV− n=25)] were not included in the present analysis to understand the impact of HIV and pregnancy stage on quantitative and qualitative changes in IGRA results. a 121 subjects expected for third trimester follow-up. b Assessments made at six months postpartum.
Screening, Enrollment and Follow up
We assessed TBI using the IGRA (QuantiFERON-TB Gold In-Tube (QGIT)) test and the TST. For this analysis, we included only women with a positive IGRA at study entry to understand the impact of pregnancy stage on IGRA results. If a woman had a positive World Health Organization (WHO) TB symptom screen(WHO, 2011) or a positive TBI test at study screening or at any time during the study, TB disease was ruled out through sputum Cepheid GeneXpert MTB RIF assay, followed by culture if MTB was detected, shielded chest radiography, and abdominal sonography (USG) as applicable. Women diagnosed with TB disease were excluded and referred for treatment. Women without TB disease were enrolled and followed until 1 year postpartum, with WHO symptom screen at each visit, repeat TBI testing done within 5 days of delivery and at 6 months postpartum. These timepoints were chosen based on known differences in test performance from our previous studies(Mathad et al., 2016, Mathad et al., 2014).
Phlebotomy for QGIT was performed prior to Mantoux TST placement (SPAN diagnostics); 3 mL for QGIT or 4 mL for QGIT-Plus (QGIT-Plus was used after QGIT production was stopped in 2017). Samples were processed and interpreted as per package insert (Quantiferon plus 2019, Quantiferon2018). The quantitative IFN-γ response was measured from the supernatant of the TB antigen tubes. For consistency in interpretation of results, IFN-γ response to TB antigen 1 tube minus NIL in QGIT and QGIT-plus was used in this analysis. TST was placed in the forearm (5 tuberculin units of purified protein derivative) and read by a study nurse at 48–72 hours. A positive TST was defined as induration ≥5 mm for women with HIV and ≥10 mm for those without HIV. Agreement between the TBI tests was defined as IGRA+/TST+ or IGRA−/TST−.
All participants provided written, informed consent prior to enrollment. The study was approved by the Institutional Review Board of Byramjee Jejeebhoy Government Medical College, Johns Hopkins University, Weill Cornell Medicine, and by the Health Ministry Screening Committee of the Indian Council Medical Research, India.
Statistical Analysis
Primary outcomes were longitudinal changes in TBI results and in IFN-γ response to M. tuberculosis (MTB) antigen by IGRA at pregnancy, delivery, and postpartum in IGRA+ pregnant women with and without HIV. TB disease progression was a secondary outcome. Sociodemographic/clinical characteristics and primary outcomes were summarized as proportions and medians with interquartile range (IQR) and compared by HIV status using Fisher’s exact test and Wilcoxon rank sum test, as appropriate. To assess the effect of covariates on longitudinal changes in linear IFN-γ response to MTB, a univariable and multivariable mixed effects model was used, in which the individuals were treated as random effects. IFN-γ response had constant correlation structure over time with increase in lag time hence exchangeable correlation was used in the mixed effects models. Variables that were significantly different (p<0.05) between women with and without HIV in the univariable analysis were included in the multivariable model. We also included variables that could be clinically relevant for the immune response to MTB (e.g. stage of pregnancy, TST, HIV status). With a sample size of at least 32 HIV infected and 60 HIV uninfected women, we had 90% power to detect a difference in IFN γ levels between women with and without HIV with a 5% significance level. Stata version 14.2 (StataCorp. 2015. Stata Statistical Software: Release 14.2 College Station, TX: StataCorp LP) was used for analysis.
RESULTS
Baseline demographics and clinical characteristics
For the parent PRACHITI study, we enrolled 234 HIV infected and uninfected pregnant women, of which 165 were IGRA+ (35 with HIV and 130 without HIV) and were included in this analysis (Table 1, Figure 1). Median age at enrolment was 23 years (IQR: 21 – 27) and median gestational age was 22 weeks (IQR: 18 – 27). The majority were unemployed from an urban area and 33% had a daily household income of less than $5 USD (~350 INR). More than 75% of participants were BCG vaccinated as determined by the scar, over 10 years ago as reported by the women; see Table 1 for additional demographic details. At enrolment, five women (3 women with HIV and 2 without HIV) had TB exposure within their household in the previous 2 years. All women with HIV were on antiretroviral therapy (ART) with a median CD4 count of 476 cells/mm3 (IQR 399 – 586) and viral load of <40 copies/ml (IQR 0–109).
Table. 1.
Socio demographic and clinical profile longitudinal cohort of initially IGRA+ pregnant women
| Characteristics | Overall n=165 | H1V+ n=35 | H1V−n=130 |
|---|---|---|---|
| Sociodemographic features | |||
| Age in years, Median (1QR) | 23 (21 – 27) | 26 (22 – 30) | 22 (20 – 26) * |
| Urban residence, n (%) | 155 (93%) | 28 (80%) | 127 (98%) * |
| Household income: $1–5/day, n (%) | 55 (33%) | 17 (49%) | 38 (29%) * |
| Unemployed (Housewife), n (%) | 140 (85%) | 26 (74%) | 114 (88%) |
| Socioeconomic Status (modified Kuppuswamy scale) | |||
| Lower | 100 (61%) | 26 (74%) | 74 (57%) |
| Middle/Upper | 65 (39%) | 9 (26%) | 56 (43%) |
| One room tenement, n (%) | 63 (38%) | 19 (54%) | 44 (34%) * |
| Live with >3 family members, n (%) | 80 (49%) | 11 (31%) | 69 (53%) * |
| Education ≤ to 4th grade, n (%) | 40 (24%) | 14 (40%) | 26 (20%) * |
| Clinical features and relevant laboratory results | |||
| Gestational age at entry, Median (1QR) | 22 (18 – 27) | 23 (18 – 29) | 21 (18 – 26) |
| Gestational age at delivery | |||
| Preterm | 15 (9%) | 5 (14%) | 10 (8%) |
| Term | 147 (91%) | 30 (86%) | 117 (92%) |
| BCG vaccinated | 127 (77%) | 27 (77.1%) | 100 (76.9%) |
| BCG given | |||
| > 10 yrs ago | 120 (72.7%) | 25 (71.4%) | 95 (73.1%) |
| Don’t Know | 45 (27.3%) | 10 (28.6%) | 35 (26.9%) |
| Past H/O TB-previous 2 years | 5 (3.0%) | 3 (8.6%) | 2 (1.5%) |
| Mid-upper arm circumference | |||
| Normal ≥ 22 – 27 cms | 93 (56%) | 23 (66%) | 70 (54%) |
| Underweight < 22 cms | 41 (25%) | 6 (17%) | 35 (27%) |
| Overweight > 27 – 31 cms | 26 (16%) | 5 (14%) | 21 (16%) |
| Obese ≥ 31 cms | 5 (3%) | 1 (3%) | 4 (3%) |
| Anemia | |||
| Hemoglobin < 9 gm/dL | 17 (10%) | 6 (17%) | 11 (8%) |
| Tuberculin skin test | |||
| Positive | 115 (69.7%) | 17 (48.6%) | 98 (75.4%)* |
| ≥5mm &<10mm | 1 (0.9%) | 0 | 1 (1.0%) |
| > 10mm | 114 (99.1%) | 17 (100%) | 97 (99%) |
| CD4/mm3, Median (1QR)a | - | 476 (399 – 586) | NA |
| Median H1V viral load a copies/mL (1QR) | - | < 40 (0 – 109) | NA |
| H1V viral load > 400 copies | - | 4 (11%) | NA |
| On ART at enrollment a | - | 35 (100%) | NA |
All data presented as number (%) unless otherwise indicated.
Significant at <0.05
Abbreviations: ART, antiretroviral treatment; HIV, human immunodeficiency virus; IQR, interquartile range
Data is presented for women with HIV (n=35)
Overall retention rate of the parent study was over 90%. Of the 165 women included in this analysis, 162 (98%) returned for delivery and 146 (89%) returned for 6 months postpartum visits (Figure 1).
Longitudinal TST and IGRA results differed by HIV status
By design, all women included in this analysis had a positive IGRA test at study entry. Regardless of HIV status, all women had a significant decrease in IGRA positivity at delivery (HIV+: 100% to 62%, p<0.01; HIV−: 100% to 77%, p<0.01) (Figure 2). Between delivery and 6 months postpartum, IGRA positivity increased significantly in both women with and without HIV (HIV+: 62% to 87%, p<0.05; HIV−: 77% to 89%, p<0.05) (Figure 2). The change in IGRA positivity was greater in women with HIV than those without, though this comparison did not reach statistical significance between pregnancy and delivery (Δ: −38% in HIV+ vs. −23% in HIV−, p=0.07) or between delivery and 6 months postpartum (Δ: +25% in HIV+ vs 13% in HIV−, p=0.7).
Figure 2. Performance of IGRA and TST is inconsistent during peripartum.
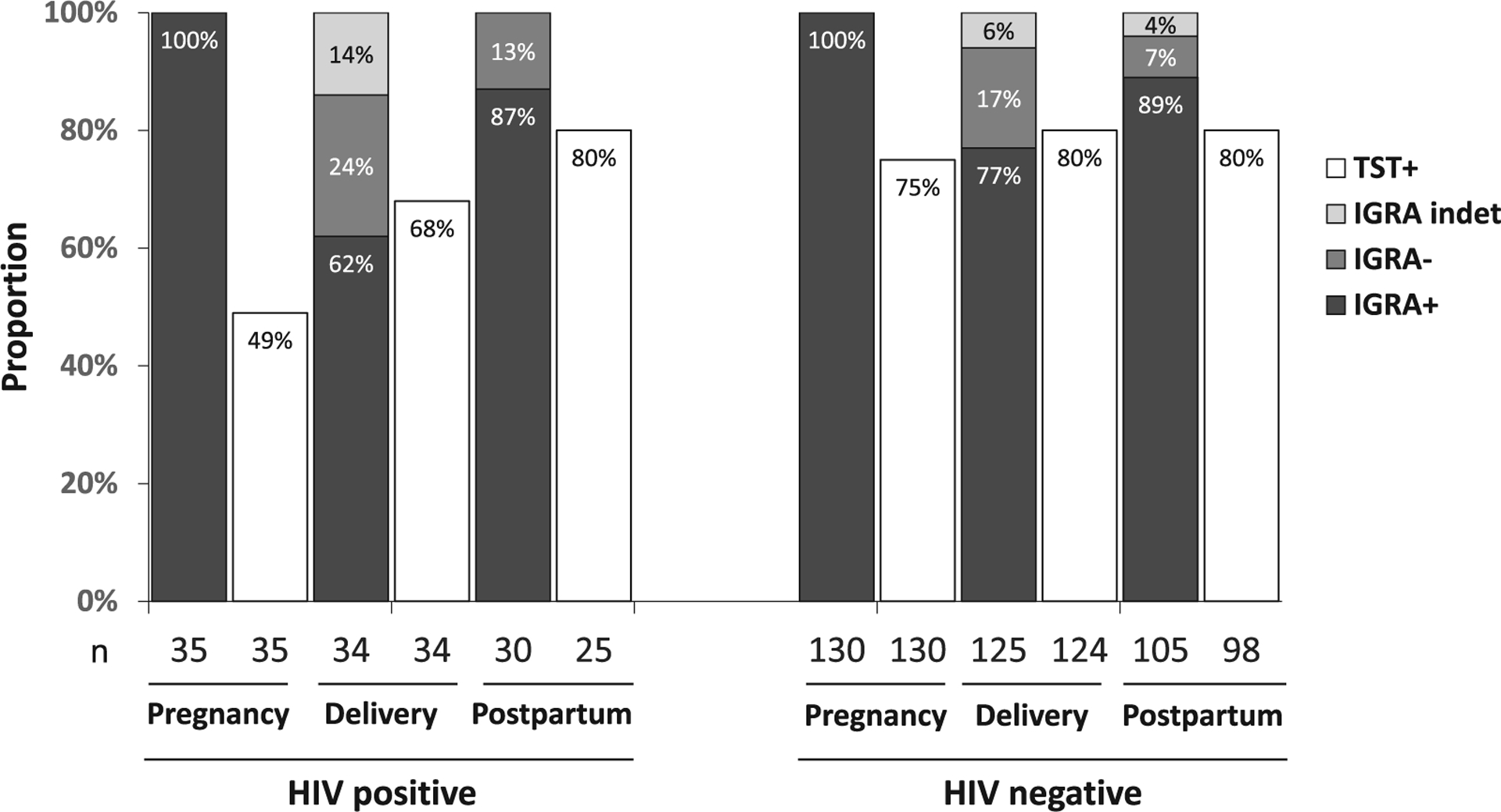
Qualitative performance of an interferon gamma release assay (IGRA) and tuberculin skin test (TST) at each pregnancy stage in a longitudinal cohort of initially IGRA+ pregnant women with and without HIV in Pune, India (n=165)., IGRA performance declined at delivery, characterized by lowest IGRA positivity (proportion ≥0.35IU/mL) and highest rate of indeterminant results (nil >8.0IU/mL or mitogen <0.50IU/mL); IGRA positivity did not differ significantly according to HIV status, although women without HIV tended to remain IGRA+ at delivery (p=0.07. IGRA+/TST+ concordance was particularly low among women living with HIV compared to those without HIV (p=0.003); TST positivity improved at delivery and 6 months postpartum with no significant differences by HIV status.
For TST, 17 of 35 (49%) pregnant women with HIV and 98 of 130 (75%) without HIV had a positive test (p=0.004) (Figure 2) with a median size of 16mm in HIV infected and 19mm in HIV uninfected pregnant women (Table 1& S Table.1). There was a numerical increase in TST positivity from pregnancy to delivery in women with HIV (49% vs. 68% p=0.11) and without HIV (75% vs 80% p=0.14). While TST positivity numerically increased between delivery and postpartum in women with HIV (68% vs 80% p=0.3), it did not change in HIV-uninfected (80% vs 80% p=0.9). For all time points, median TST induration was ≥15mm regardless of HIV status. (S Table.1)
Low concordance of IGRA and TST
TST positivity was lower than IGRA positivity at pregnancy, delivery and postpartum in all women, regardless of HIV status. (Figure 2) Compared to women without HIV, women with HIV had lower IGRA+/TST+ concordance at pregnancy (49% HIV+ vs. 75% HIV−; p=0.003) (Table 2). Women without HIV had >70% IGRA/TST agreement at all timepoints. In women with HIV, IGRA/TST agreement was 49% during pregnancy, 68% at delivery with improvement to 77% at 6 months postpartum (Table 2).
Table 2.
Agreement among interferon gamma release assay (IGRA) and tuberculin skin test (TST) results by pregnancy stage in a cohort of initially IGRA+ women with and without HIV in Pune, India
| Pregnancy Stage | Test Discordancea | Test Concordance | Agreementb | |
|---|---|---|---|---|
| IGRA+/TST+ | IGRA−/TST− | |||
| H1V-positive | ||||
| Pregnancy | 51.4 | 48.6 | NA | 48.6 |
| Delivery | 17.6 | 52.9 | 14.7 | 67.6 |
| Postpartum | 23.1 | 73.1 | 3.85 | 76.9 |
| H1V-negative | ||||
| Pregnancy | 24.6 | 75.4 | NA | 75.4 |
| Delivery | 16.1 | 67.2 | 9.6 | 77.4 |
| Postpartum | 24.2 | 70.7 | 2.02 | 72.7 |
All data presented as % unless otherwise indicated.
Abbreviations: CI, confidence interval; IGRA, interferon gamma release assay; NA, not applicable; TST, tuberculin skin test; HIV, human immunodeficiency virus
Defined as IGRA+/TST− or IGRA−/TST+.
Defined as IGRA+/TST+ or IGRA−/TST−.
Quantitative IFN-γ response to MTB antigen varies with stage of pregnancy and HIV status
Women with HIV also had a lower IFN-γ response at all timepoints compared to women without HIV; the difference was significant during pregnancy (1.5 IU/mL [IQR 0.8–5.2] vs 4.6 IU/mL [IQR 3.7–5.8], p=0.002) and at delivery (0.5 IU/mL [IQR 0.4–1.9] vs 2.0 IU/mL [IQR 0.3–3.4], p=0.007), but did not reach statistical significance at 6 months postpartum (1.8 IU/mL[IQR 0.9–6.1] vs 4.8 IU/mL [IQR 3.9–8.2], p=0.11) (Figure 3). A multivariable mixed effect model with random effects identified the following as contributing factors for longitudinal change in quantitative IFN-γ response: being pregnant compared to delivery (mean deviation [MD] slope 1.35 [95% CI: 0.58, 2.12], p=0.001), postpartum compared to delivery (MD slope 1.31 [95% CI: 0.5, 2.11] p=0.001), and having HIV (MD slope −1.55 [95% CI: −2.86, −0.23] p=0.005) (S Table 2).
Figure 3. IFN-γ response decreased from pregnancy to delivery and increased from delivery to 6 months postpartum regardless of HIV status.
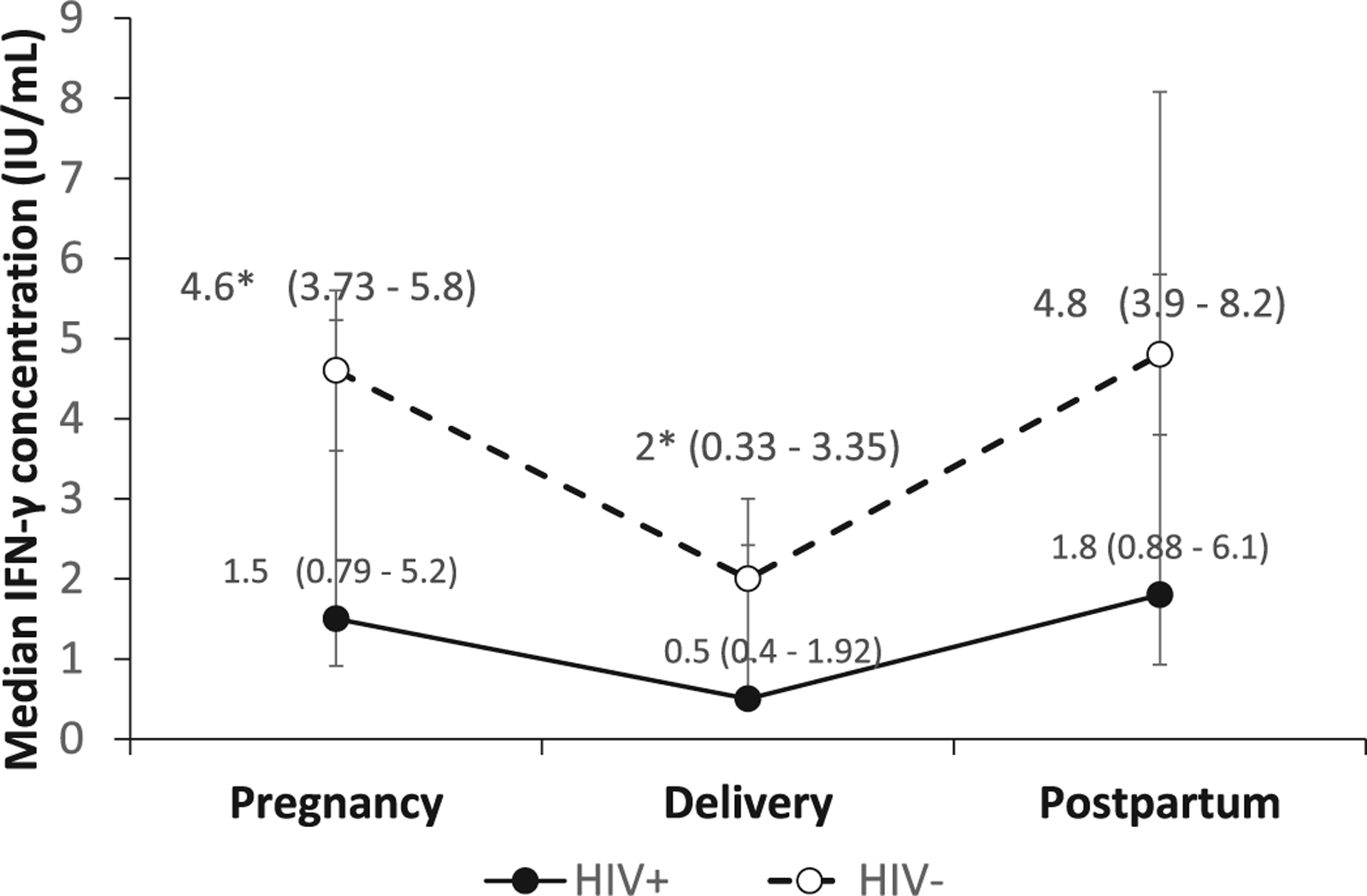
Quantitative IFN-γ response (median IFN-γ concentration measured in TB antigen 1 tube using the QuantiFERON-TB Gold In-Tube and QuantiFERON-TB Gold plus assay) to Mycobacterium tuberculosis antigen stimulation at each pregnancy stage in a longitudinal cohort of initially IGRA+ pregnant women with and without HIV in Pune, India. Response decreased from pregnancy to delivery (p<0.01) and increased from delivery to 6 months postpartum (p<0.01), regardless of HIV status. Women living with HIV had a lower IFN-γ response than women without HIV at all timepoints; the difference was significant during pregnancy and delivery, as indicated by the asterisk (p<0.01).
Error bar represents 95% confidence interval.
TB progression was higher in women with versus without HIV
During the course of the study, nine enrolled women (5 HIV+, 4 HIV−) progressed from TBI to TB disease. One case (HIV+) was diagnosed during the third trimester of pregnancy, and eight cases (4 HIV+, 4 HIV−) were diagnosed during postpartum (S Table 3). As such, the overall TB incidence was 28 per 1000 person-years (95% CI 13 – 53). Stratified by HIV, the TB incidence was 50 per 1000 person-years among women with HIV and 18 per 1000 person-years among women without HIV. Of the 50 (18 HIV+; 32 HIV−) IGRA+/TST− pregnant women at enrolment, 44% (8 HIV+; 14 HIV−) converted to a positive TST (IGRA+/TST+) at delivery (S Table 4). Of the 8 women with HIV who converted to IGRA+/TST+ concordance at delivery, 3 (37%) progressed to TB disease prior to 6 months postpartum. None of the 14 HIV uninfected women with conversion to IGRA+/TST+ at delivery progressed to TB disease (S Table 4). IPT was initiated in the national program for women with HIV in 2017, but only three of our enrolled 35 women with HIV (two during pregnancy and one at postpartum) were given IPT. Two of these three women did not complete the treatment, one of which developed TB disease.
DISCUSSION
We found that the performance of both the IGRA and TST are affected by stages of pregnancy, with a nadir of positive results at delivery. The TST returned fewer positive results than the IGRA, while the IGRA had significant qualitative and quantitative changes throughout the peripartum period. Despite having CD4+ counts over 400 cells/mm3, IGRA+ women with HIV consistently had a lower quantitative response to MTB antigens at every timepoint than IGRA+ women without HIV. TB progression was higher among women with HIV than in women without HIV. The majority of incident TB cases were diagnosed within 6 months postpartum.
There are few data on the impact of HIV on TB-specific immune function during pregnancy. To our knowledge, ours is the first study to compare the performance of these tests over time in women with and without HIV. We found that more TBI was diagnosed by IGRA than TST which is consistent with other studies from high incidence settings in pregnant women with HIV and high risk non-pregnant population without HIV (LaCourse et al., 2017, Mathad et al., 2014, Pai et al., 2005, Zwerling et al., 2012).
Though fewer TBI cases were identified by TST, the test is believed to be less informative in BCG-vaccinated populations and where exposure to non-tuberculous mycobacteria (NTM) is high (Hoft et al., 2000, Pai et al., 2014). More than 75% of our pregnant women aged ~23 years in the study were BCG-vaccinated, presumably with a single dose in their infancy according to Indian immunization policy. Yet, there was still 70% agreement between tests. Since our women were BCG vaccinated >10 years ago and > 99% had a TST reaction of >10mm (Table 1 and S Table 1), it is highly likely that TST reaction in our study women signifies true TB infection as shown in the literature (Farhat et al., 2006, Sharma et al., 2017). The high discordance of TBI tests in our study may be related to the immune suppression of pregnancy as has been reported in studies of pregnant women in other high prevalence settings(LaCourse et al., 2017, Worjoloh et al., 2011). Lower IFN-γ and IL2 may prevent delayed hypersensitivity reaction to TST but does not affect the ELISA measurement of IFN-γ in IGRA, making IGRA a more sensitive test compared to TST (Mathad et al., 2016, Mathad et al., 2014).
Our finding that women with HIV continued to express lower IFN-γ, despite being on cART and with adequate CD4 counts, is consistent with emerging data in non-pregnant populations with TBI (Gupta et al., 2013). Evidence indicates that people with HIV are at greater risk of developing TB disease even before profound CD4 loss occurs (Day et al., 2017, Riou et al., 2016). More recent studies in ART-naïve adults with HIV demonstrate that proliferation of MTB-specific CD4 cells, especially those that express IFN-γ, is impaired in those with TBI and HIV compared to those with TBI and without HIV (Day et al., 2017, Riou et al., 2016). Another study found that both cART-experienced (CD4>300) and cART-naïve HIV infected individuals had markedly reduced levels of IFN-γ-secreting-CD4 T-cells compared to healthy controls(Sutherland et al., 2006). Because IFN-γ is the main cytokine required to maintain immune control over MTB infection,(Cavalcanti et al., 2012) the possible defect in functional capacity of CD4+ T cells in HIV infected people may explain why pregnant women with HIV in our study remained at higher risk of developing TB disease than women without HIV.
Most women who developed TB disease did so within 6 months postpartum. After delivery, most postpartum women stay home, and are less exposed to TB, suggesting that these cases represent progression from TBI to TB disease rather than new infection. We hypothesize that the lower IFN-γ levels in pregnancy may allow TB progression during pregnancy, while also masking clinical symptoms. Once the immune system recovers in the early postpartum period, this incipient disease becomes overt. Our hypothesis is supported by our own data showing increased IFN-γ levels in the postpartum period as well as a large epidemiological study, which also shows a 2-fold increased TB incidence during the early postpartum period(Gupta, 2007, Zenner, 2012).
Our data and others suggest that pregnant women should be considered a high-risk group for TB progression and, therefore, TB preventive therapy. We found an overall TB incidence of 28 per 1000 person-years, which is 24.6 times higher than the TB incidence of 1.14 per 1000 person years reported from the general population in the same state in India(TB Facts Org, 2018).Furthermore, pregnant women with HIV had an even higher TB incidence, 2.8 times higher than that of women without HIV. This suggests that the combination of pregnancy and HIV, despite being well controlled, still results in a disproportionately high risk of TB disease.
However, administration of IPT during pregnancy remains controversial. At the time of this study, IPT was just being introduced for pregnant women with HIV in India and only 3 women with HIV in our study had ever taken IPT. Though the WHO has recommended IPT for all HIV infected individuals including pregnant women since 2011(WHO, 2011), few TB-endemic countries implement this recommendation for fear of toxicity. A recent study found that pregnant women with HIV who took IPT during pregnancy had an increased risk of adverse pregnancy outcomes compared to women administered IPT postpartum(Gupta et al., 2019, Gupta et al., 2020). Because other studies report no increased risk of adverse outcomes with antepartum IPT use, there is confusion over the optimal timing of IPT in pregnant and postpartum women(Hamada et al., 2020, Salazar-Austin et al., 2020). Rifampicin preventive therapy (RPT) is a safe and effective alternative for non-pregnant people (Campbell et al., 2020, Flynn et al., 2020, Menzies et al., 2018, Sterling et al., 2020). While WHO acknowledges the safety of rifampicin in pregnancy, it still recommends IPT for TB prevention in pregnancy until safety data are available for other preventive therapies such as rifapentine containing regimens (WHO, 2020).
Our study and others suggest that a positive IGRA in pregnancy, especially in women with HIV, is associated with higher risk of TB disease postpartum(Jonnalagadda et al., 2010). Notably, the majority of women with HIV who developed TB disease in our study had IGRA+/TST− discordance during pregnancy and converted to IGRA+/TST+ at delivery. Since our study pregnant women were all IGRA positive, the TST conversion at delivery is less likely to be a new exposure but rather a change in the immune response to TST. Exploration of the predictive value of this phenotype in larger studies of pregnant women in TB endemic countries may be warranted to identify women at the highest risk of developing TB disease, in whom the benefit of TB preventive therapy (TPT) would clearly outweigh any potential risk (Schnittman et al., 2021).
Our longitudinal study design had several strengths, including an excellent retention rate (>90%), which enabled us to track changes in IGRA and TST at pregnancy, delivery and postpartum. Our rigorous approach of screening all pregnant and postpartum women with the WHO-recommended TB symptoms screen also allowed us to actively identify incident cases of TB in this population. We also had limitations: by including only IGRA-positive women in our analysis we were unable to comment on conversions from negative to positive results from pregnancy to delivery or postpartum and progression to TB disease in IGRA negative women. In previous studies by our group and others, the risk of postpartum TB was highest in IGRA+ women(Jonnalagadda et al., 2010, Jonnalagadda et al., 2013, Mathad et al., 2016). Because we performed serial TSTs, the increase in TST positivity from pregnancy to delivery, especially in women with HIV, could reflect a boosting effect from the second TST placement. Boosting is usually maximal within 1 to 5 weeks from the initial test, but can be seen for over one year(Hecker et al., 1997, Menzies, 1999, Nayak and Acharjya, 2012). Initiation of IPT for women with HIV during the study could have influenced the TBI test results as well as IFN-γ response to TB antigen at various time points. Very few women with HIV were initiated on IPT during the study period and repeat analyses excluding those on IPT showed no significant changes in longitudinal IGRA or TST positivity and median IFN-γ response. Importantly, by including women with and without HIV, we were able to assess relative changes in both populations and assess the impact of HIV on the performance of IGRA and TST during the peripartum period.
CONCLUSIONS
Systematically screening pregnant women with TBI for TB disease would identify an important pool of women at high-risk of TB progression (WHO, 2018). Our study showed that the immune changes of pregnancy and the postpartum period affect the performance of the TST and IGRA leading to missed diagnosis. Importantly, our study also documented that IGRA+ women with well-controlled HIV still have decreased expression of IFN-γ in response to MTB antigens compared to IGRA+ women without HIV.
Immunological changes place pregnant and early postpartum women at high risk of developing TB disease and they should be prioritized for TPT. Identifying pregnant women that have the highest risk of developing TB disease remains a challenge, and is further complicated by the potential risks of taking IPT in pregnancy. Healthcare providers should utilize systematic screening for TB symptoms combined with microbiologic and radiologic testing, as indicated, to identify and treat pregnant women with TB disease early, improving the survival of herself and her family.
Supplementary Material
Acknowledgements
We acknowledge the clinical support provided by the Department of Obstetrics & Gynecology of B J Government Medical College, Pune India for our study mothers. We would like to acknowledge the study team of clinicians, counsellors, research nurses, lab and data management staff for their contribution to the quality of the collected study data and for the excellent retention rate. We acknowledge the editorial and data preparation help provided by Katherine N McIntire MD for the manuscript. Additional editorial support was provided by Meredith Whitaker, PhD of Alphabet Health (New York, NY), according to Good Publication Practice guidelines (https://www.ismpp.org/gpp3) with generous support from Jessica and Natan Bibliowicz. MA, VK, AG, NG and JSM are supported by the Eunice Shriver National Institutes of Child Health and Development of the National Institutes of Health under award number R01HD081929 and by the Ujala Foundation (Newtown Square, PA). JSM and AG are also supported by the National Institute of Allergy and Infectious Diseases of the National Institutes of Health under award number K23AI129854 and UM1AI069465, respectively. AG receives support from the Gilead Foundation, Makhija Foundation, and the Wyncote Foundation. JSM received support from the Kellen Foundation. We also recognize the support of Persistent (Pune, India) that supported our electronic data management system for the study. The funding source is not involved in the study design; in the collection, analysis and interpretation of data; in the writing of the report; and in the decision to submit the article for publication. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health or the other listed foundations.
Funding source
Eunice Shriver National Institutes of Child Health and Development of the National Institutes of Health under award number R01HD081929. In addition the principal investigators of the study are supported through National Institute of Allergy and Infectious Diseases of the National Institutes of Health under award number K23AI129854 and UM1AI069465.
Footnotes
Ethical Approval statement
The study was approved by the Institutional Review Board of Byramjee Jejeebhoy Government Medical College, Johns Hopkins University, Weill Cornell Medicine, and by the Health Ministry Screening Committee of the Indian Council Medical Research, India. All participants provided written, informed consent prior to enrollment.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Supplementary materials
Supplementary material associated with this article can be found, in the online version, at doi:10.1016/j.ijid.2021.09.010.
References
- Campbell JR, Trajman A, Cook VJ, Johnston JC, Adjobimey M, Ruslami R, et al. Adverse events in adults with latent tuberculosis infection receiving daily rifampicin or isoniazid: post-hoc safety analysis of two randomised controlled trials. Lancet Infect Dis 2020;20(3):318–29. doi: 10.1016/s1473-3099(19)30575-4. [DOI] [PubMed] [Google Scholar]
- Cavalcanti YV, Brelaz MC, Neves JK, Ferraz JC, Pereira VR. Role of TNF-Alpha, IFN-Gamma, and IL-10 in the Development of Pulmonary Tuberculosis. Pulm Med 2012;2012. doi: 10.1155/2012/745483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chaouat G, Menu E, Smedt Dd, Khrihnan L, Hui L, Meliani AA, et al. The emerging role of IL-10 in pregnancy. Am J Reprod Immunol 1996;35(4):325–9. doi: 10.1111/j.1600-0897.1996.tb00488.x. [DOI] [PubMed] [Google Scholar]
- Chopra S, Siwatch S, Aggarwal N, Sikka P, Suri V. Pregnancy outcomes in women with tuberculosis: a 10-year experience from an Indian tertiary care hospital. Trop Doct 2017;47(2):104–9. doi: 10.1177/0049475516665765. [DOI] [PubMed] [Google Scholar]
- Day CL, Abrahams DA, Harris LD, van Rooyen M, Stone L, de Kock M, et al. HIV-1 Infection Is Associated with Depletion and Functional Impairment of. J Immunol 2017;199(6):2069–80. doi: 10.4049/jimmunol.1700558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farhat M, Greenaway C, Pai M, Menzies D. False-positive tuberculin skin tests: what is the absolute effect of BCG and non-tuberculous mycobacteria? Int J Tuberc Lung Dis 2006;10(11):1192–204. [PubMed] [Google Scholar]
- Flynn AG, Aiona K, Haas MK, Reves R, Belknap R. Clinical Characteristics of Active Tuberculosis Diagnosed After Starting Treatment for Latent Tuberculosis Infection. Clin Infect Dis 2020;71(5):1320–3. doi: 10.1093/cid/ciz1157. [DOI] [PubMed] [Google Scholar]
- Getahun H, Sculier D, Sismanidis C, Grzemska M, Raviglione M. Prevention, diagnosis, and treatment of tuberculosis in children and mothers: evidence for action for maternal, neonatal, and child health services. J Infect Dis 2012;205(Suppl 2):S216–27. doi: 10.1093/infdis/jis009. [DOI] [PubMed] [Google Scholar]
- Gupta A, et al. Postpartum tuberculosis incidence and mortality among HIV-infected women and their infants in Pune, India, 2002–2005. Clin Infect Dis 2007;45(2):241–9. doi: 10.1086/518974. [DOI] [PubMed] [Google Scholar]
- Gupta A, Montepiedra G, Aaron L, Theron G, McCarthy K, Bradford S, et al. Isoniazid Preventive Therapy in HIV-Infected Pregnant and Postpartum Women. N Engl J Med 2019;381(14):1333–46. doi: 10.1056/NEJMoa1813060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gupta A, Montepiedra G, Theron G. HIV, Pregnancy, and Isoniazid Preventive Therapy. Reply. N Engl J Med 2020;382(12):1184–5. doi: 10.1056/NEJMc1916664. [DOI] [PubMed] [Google Scholar]
- Gupta RK, Lawn SD, Bekker LG, Caldwell J, Kaplan R, Wood R. Impact of human immunodeficiency virus and CD4 count on tuberculosis diagnosis: analysis of city-wide data from Cape Town, South Africa. Int J Tuberc Lung Dis 2013;17(8):1014–22. doi: 10.5588/ijtld.13.0032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamada Y, Figueroa C, Martín-Sánchez M, Falzon D, Kanchar A. The safety of isoniazid tuberculosis preventive treatment in pregnant and postpartum women: systematic review and meta-analysis. Eur Respir J 2020;55(3). doi: 10.1183/13993003.01967-2019. [DOI] [PubMed] [Google Scholar]
- Hecker MT, Johnson JL, Whalen CC, Nyole S, Mugerwa RD, Ellner JJ. Two-step tuberculin skin testing in HIV-infected persons in Uganda. Am J Respir Crit Care Med 1997;155(1):81–6. doi: 10.1164/ajrccm.155.1.9001293. [DOI] [PubMed] [Google Scholar]
- Hoft DF, Brown RM, Belshe RB. Mucosal bacille calmette-Guérin vaccination of humans inhibits delayed-type hypersensitivity to purified protein derivative but induces mycobacteria-specific interferon-gamma responses. Clin Infect Dis 2000;30(Suppl 3):S217–22. doi: 10.1086/313864. [DOI] [PubMed] [Google Scholar]
- Jonnalagadda S, Lohman Payne B, Brown E, Wamalwa D, Maleche Obimbo E, Majiwa M, et al. Latent tuberculosis detection by interferon γ release assay during pregnancy predicts active tuberculosis and mortality in human immunodeficiency virus type 1-infected women and their children. J Infect Dis 2010;202(12):1826–35. doi: 10.1086/657411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jonnalagadda SR, Brown E, Lohman-Payne B, Wamalwa D, Farquhar C, John-Stewart GC. Predictive value of interferon-gamma release assays for postpartum active tuberculosis in HIV-1-infected women. Int J Tuberc Lung Dis 2013;17(12):1552–7. doi: 10.5588/ijtld.13.0239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LaCourse SM, Cranmer LM, Matemo D, Kinuthia J, Richardson BA, Horne DJ, et al. Effect of Pregnancy on Interferon Gamma Release Assay and Tuberculin Skin Test Detection of Latent TB Infection Among HIV-Infected Women in a High Burden Setting. J Acquir Immune Defic Syndr 2017;75(1):128–36. doi: 10.1097/qai.0000000000001298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mathad JS, Bhosale R, Balasubramanian U, Kanade S, Mave V, Suryavanshi N, et al. Quantitative IFN-γ and IL-2 Response Associated with Latent Tuberculosis Test Discordance in HIV-infected Pregnant Women. Am J Respir Crit Care Med 2016;193(12):1421–8. doi: 10.1164/rccm.201508-1595OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mathad JS, Bhosale R, Sangar V, Mave V, Gupte N, Kanade S, et al. Pregnancy differentially impacts performance of latent tuberculosis diagnostics in a high-burden setting. PLoS One 2014;9(3):e92308. doi: 10.1371/journal.pone.0092308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mathad JS, Gupta A. Tuberculosis in pregnant and postpartum women: epidemiology, management, and research gaps. Clin Infect Dis 2012;55(11):1532–49. doi: 10.1093/cid/cis732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Menzies D Interpretation of repeated tuberculin tests. Boosting, conversion, and reversion. Am J Respir Crit Care Med 1999;159(1):15–21. doi: 10.1164/ajrccm.159.1.9801120. [DOI] [PubMed] [Google Scholar]
- Menzies D, Adjobimey M, Ruslami R, Trajman A, Sow O, Kim H, et al. Four Months of Rifampin or Nine Months of Isoniazid for Latent Tuberculosis in Adults. N Engl J Med 2018;379(5):440–53. doi: 10.1056/NEJMoa1714283. [DOI] [PubMed] [Google Scholar]
- Nayak S, Acharjya B. Mantoux test and its interpretation. Indian Dermatol Online J 2012;3(1):2–6. doi: 10.4103/2229-5178.93479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pai M, Denkinger CM, Kik SV, Rangaka MX, Zwerling A, Oxlade O, et al. Gamma interferon release assays for detection of Mycobacterium tuberculosis infection. Clin Microbiol Rev 2014;27(1):3–20. doi: 10.1128/cmr.00034-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pai M, Gokhale K, Joshi R, Dogra S, Kalantri S, Mendiratta DK, et al. Mycobacterium tuberculosis infection in health care workers in rural India: comparison of a whole-blood interferon gamma assay with tuberculin skin testing. JAMA 2005;293(22):2746–55. doi: 10.1001/jama.293.22.2746. [DOI] [PubMed] [Google Scholar]
- Pillay T, Khan M, Moodley J, Adhikari M, Padayatchi N, Naicker V, et al. The increasing burden of tuberculosis in pregnant women, newborns and infants under 6 months of age in Durban, KwaZulu-Natal. S Afr Med J 2001;91(11):983–7. [PubMed] [Google Scholar]
- QuantiFERON®-TB Gold Plus. (QFT®-Plus) ELISA Package Insert 04/2019. 2019
- QuantiFERON®-TB Gold (QFT®) Elisa package insert 2 Plate kit ELISA 06/2018. 2018
- Riou C, Strickland N, Soares AP, Corleis B, Kwon DS, Wherry EJ, et al. HIV Skews the Lineage-Defining Transcriptional Profile of Mycobacterium tuberculosis-Specific CD4+ T Cells. J Immunol 2016;196(7):3006–18. doi: 10.4049/jimmunol.1502094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salazar-Austin N, Cohn S, Lala S, Waja Z, Dooley KE, Hoffmann CJ, et al. Isoniazid Preventive Therapy and Pregnancy Outcomes in Women Living With Human Immunodeficiency Virus in the Tshepiso Cohort. Clin Infect Dis 2020;71(6):1419–26. doi: 10.1093/cid/ciz1024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schnittman SR, Byakwaga H, Buom Y, Kabakyenga J, Matthews LT, Burdo TH, et al. Changes in Immune Activation during Pregnancy and the Postpartum Period in Treated HIV Infection. Open Forum Infect Dis 2021. ofab245. doi: 10.1093/ofid/ofab245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharma SK, Vashishtha R, Chauhan LS, Sreenivas V, Seth D. Comparison of TST and IGRA in Diagnosis of Latent Tuberculosis Infection in a High TB-Burden Setting. PLoS One 2017;12(1). doi: 10.1371/journal.pone.0169539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sterling TR, Njie G, Zenner D, Cohn DL, Reves R, Ahmed A, et al. Guidelines for the Treatment of Latent Tuberculosis Infection: Recommendations from the National Tuberculosis Controllers Association and CDC, 2020. MMWR Recomm Rep 2020;69(1):1–11. doi: 10.15585/mmwr.rr6901a1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sugarman J, Colvin C, Moran AC, Oxlade O. Tuberculosis in pregnancy: an estimate of the global burden of disease. Lancet Glob Health 2014;2(12):e710–16. doi: 10.1016/S2214-109X(14)70330-4. [DOI] [PubMed] [Google Scholar]
- Sutherland R, Yang H, Scriba TJ, Ondondo B, Robinson N, Conlon C, et al. Impaired IFN-gamma-secreting capacity in mycobacterial antigen-specific CD4 T cells during chronic HIV-1 infection despite long-term HAART. AIDS 2006;20(6):821–9. doi: 10.1097/01.aids.0000218545.31716.a4. [DOI] [PubMed] [Google Scholar]
- TB Statistics India. TBFACTSorg 2018. (Accessed 8 Apr 2020)
- Theron G, Montepiedra G, Aaron L, McCarthy K, Nahida C, Jean-Philippe P, et al. Individual and Composite Adverse Pregnancy Outcomes in a Randomized Trial on Isoniazid Preventative Therapy Among Women Living With HIV. Clin Infect Dis 2020. doi: 10.1093/cid/ciaa1482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WHO. Guidelines for intensified tuberculosis case-finding and isoniazid preventive therapy for people living with HIV in resource-constrained settings. World Health Organization; 2011. http://apps.who.int/iris/bitstream/handle/10665/44472/9789241500708_eng.pdf?sequence=1(accessed 20 Feb 2021). [Google Scholar]
- WHO. Global tuberculosis report https://www.who.int/tb/publications/global_report/gtbr15_main_text.pdf: World Health Organization; 2015. (accessed 22 Feb 2020) [Google Scholar]
- WHO. Global tuberculosis report https://apps.who.int/iris/handle/10665/274453: WHO; 2018. (Accessed 8 Apr 2020) [Google Scholar]
- WHO. HIV-Associated Tuberculosis. https://www.who.int/tb/areas-of-work/tb-hiv/tbhiv_factsheet_2016_web.pdf: World Health Organization; 2019. (Accessed 10 Feb 2021) [Google Scholar]
- WHO. WHO consolidated guidelines on tuberculosis: tuberculosis preventive treatment: Module 1: prevention. https://www.who.int/publications/i/item/9789240001503: WHO; 2020. [PubMed] [Google Scholar]
- Worjoloh A, Kato-Maeda M, Osmond D, Freyre R, Aziz N, Cohan D. Interferon gamma release assay compared with the tuberculin skin test for latent tuberculosis detection in pregnancy. Obstet Gynecol 2011;118(6):1363–70. doi: 10.1097/AOG.0b013e31823834a9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zenner D, et al. Risk of tuberculosis in pregnancy: a national, primary care-based cohort and self-controlled case series study. Am J Respir Crit Care Med 2012;185(7):779–84. doi: 10.1164/rccm.201106-1083OC. [DOI] [PubMed] [Google Scholar]
- Zwerling A, van den Hof S, Scholten J, Cobelens F, Menzies D, Pai M. Interferon-gamma release assays for tuberculosis screening of healthcare workers: a systematic review. Thorax 2012;67(1):62–70. doi: 10.1136/thx.2010.143180. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


