Abstract
Purpose:
Current first-line treatment options in patients with metastatic urothelial carcinoma (mUC) unfit to receive cisplatin-containing chemotherapy include PD-1/L1 inhibitors and carboplatin-containing chemotherapy. However, the optimal sequencing of these therapies remains unclear.
Material and methods:
We conducted a multicenter retrospective analysis. Consecutive cisplatin-ineligible patients with mUC treated with first-line carboplatin-containing chemotherapy followed sequentially by second-line PD-1/L1 inhibitor, or the reverse order, were included. Patient demographics, objective response, time to treatment failure for first-line (TTF1) and second-line (TTF2) therapy, interval between end of first-line and initiation of second-line treatment (Interval1L-2L), and overall survival (OS) were collected. Multivariate analysis was conducted to examine the association of sequencing on OS.
Results:
In this multicenter retrospective study, we identified 146 cisplatin-ineligible patients with mUC treated with first-line (1L) PD-1/L1 inhibitor therapy followed by second-line (2L) carboplatin-containing chemotherapy (Group 1, n=43) or the reverse sequence (Group 2, n=103). In the overall cohort, median age was 72, 76% were men, and 18% had liver metastasis. In both groups, objective response rates were higher with carboplatin-containing chemotherapy (45.6% 1L, 44.2% 2L) compared to PD-1/L1 inhibitors (9.3% 1L, 21.3% 2L). On multivariate analysis, treatment sequence was not associated with overall survival (HR 1.05, p=0.85). Site of metastasis was the only factor significantly associated with overall survival (p=0.002).
Conclusions:
In this biomarker-unselected cohort of cisplatin-ineligible patients with mUC, PD-1/L1 inhibitor followed by carboplatin-containing chemotherapy and the reverse sequence had comparable OS.
Keywords: Immune checkpoint inhibitors, carboplatin chemotherapy, treatment sequencing, urothelial carcinoma, bladder cancer
Introduction
Metastatic urothelial carcinoma (mUC) is generally considered incurable. Cisplatin-containing chemotherapy remains the standard of care for cisplatin-eligible patients.1 However, many patients are unfit to receive cisplatin due to poor performance status (PS), renal dysfunction and/or other comorbidities.2
For patients who are unfit to receive cisplatin-containing chemotherapy, first-line treatment had traditionally been carboplatin-containing chemotherapy.3 PD-1/L1 inhibitors were approved initially as monotherapy for patients with mUC who experienced progression after platinum-containing chemotherapy.4–8 Thereafter, the therapeutic armamentarium for cisplatin-ineligible mUC in the first line setting expanded to include PD-1/L1 inhibitors.9, 10 Pembrolizumab and atezolizumab were FDA-approved as first-line therapies for cisplatin-ineligible patients based on rapid and durable responses in single arm phase II trials.
Subsequently, the front-line approval of pembrolizumab and atezolizumab monotherapy were modified to restrict their use for patients with high tumor tissue PD-L1 expression or those who are unfit also for carboplatin in this first line setting. This is due to an excess of early deaths in the PD-L1 low group noted after treatment with PD-1/L1 inhibitors alone compared to cisplatin- or carboplatin-based chemotherapy in two ongoing randomized phase III trials, KEYNOTE-361 and IMvigor130. For patients whose tumor PD-L1 expression status is unknown or high, the optimal treatment sequence of carboplatin-containing chemotherapy and PD-1/L1 inhibitors is unknown.
Materials and Methods
Study design
To examine whether there is an optimal treatment sequence of carboplatin-containing chemotherapy and PD-1/L1 inhibitors in mUC, we conducted a multicenter retrospective analysis including cisplatin-ineligible patients with mUC who had received both a PD-1/L1 inhibitor and carboplatin-based chemotherapy in sequence. Deidentified data were collected including patient demographics, outcomes and treatment durations. The impact of sequencing on overall survival (OS) was evaluated while accounting for known prognostic factors.
Participants
Consecutive cisplatin-ineligible patients with mUC treated with first-line carboplatin-containing chemotherapy followed sequentially by second-line PD-1/L1 inhibitor, or the reverse order, were included. Patients who had received cisplatin-containing perioperative chemotherapy were allowed if the interval between chemotherapy completion and initiation of first-line therapy for metastatic disease exceeded one year. Patient demographics, clinical variables, and outcomes – including investigator-assessed best objective response, time to treatment failure for first-line (TTF1) and second-line (TTF2) therapy, interval between end of first-line treatment and initiation of second-line treatment (Interval1L-2L), and overall survival (OS) – were collected.
Statistical analysis
The primary objective was the association between treatment sequence and OS. Multivariate analysis was conducted to examine the association of the following factors with OS from initiation of second-line treatment in the two groups: TTF1 plus Interval1L-2L, hemoglobin, ECOG-PS, and sites of metastasis at start of 1L treatment.
Results
Patient characteristics
Overall, 146 cisplatin-ineligible patients with mUC who received first-line PD-1/L1 monotherapy followed by second-line carboplatin-containing chemotherapy (Group 1, n=43) or the reverse sequence (Group 2, n=103) were included from 10 international tertiary centers. The median age was 72, 76% were men, 78% had ECOG-PS of 0–1, 18% patients had liver metastases, and 6% had received prior peri-operative cisplatin-based chemotherapy. Pre-1L treatment characteristics were balanced between groups with the exception for higher proportion of men in Group 1 (Supplemental Table 1).
Association of sequencing of therapy with outcomes
Median follow-up was 50 weeks (range: 9–148). On multivariate analysis, treatment sequence was not associated with OS (HR 1.05, p=0.85). Site of metastasis was the only factor significantly associated with OS (p=0.002), with liver metastasis conferring shorter OS (Table 1). In both groups, the objective response rate (ORR) was higher with carboplatin-containing chemotherapy compared to PD-1/L1 inhibitors. Specifically, in Group 1, ORR for first-line PD-1/PD-L1 inhibitor was 9.3% and ORR for second-line carboplatin-containing chemotherapy was 44.2%. In Group 2, ORR for first-line carboplatin-containing chemotherapy was 45.6% and ORR for second-line PD-1/PD-L1 inhibitor was 21.3% (Supplemental Table 2).
Table 1:
Multivariate analysis between overall survival and treatment sequence.
| HR (95% CI) | P-value | |
|---|---|---|
| Treatment sequence 1 | ||
| PD-1/L1 → Carbo | 1.05 (0.62–1.77) | 0.85 |
| Carbo → PD-1/L1 (Ref) | - | |
|
| ||
| TTF1 + Interval between 1L and 2L | 1.27 (0.77–2.11) | 0.35 |
|
| ||
| Hemoglobin | ||
| <10 | 1.33 (0.74–2.40) | 0.34 |
| ≥10 (Ref) | - | |
|
| ||
| ECOG performance status | ||
| 0–1 (Ref) | - | 0.83 |
| 2–3 | 1.07 (0.60–1.90) | |
|
| ||
| Site(s) of metastasis | ||
| LN only (Ref) | - | 0.002 |
| Non-liver | 1.49 (0.84–2.63) | |
| Liver | 3.23 (1.69–6.19) | |
On univariate analysis, treatment sequence was insignificant for OS: HR 1.16 (0.72–1.87), p=0.53.
Abbreviations: 1L, first-line treatment; 2L, second-line treatment; Carbo, carboplatin-containing chemotherapy; HR, hazard ratio; LN, lymph node; Ref, reference; TTF, time to treatment failure.
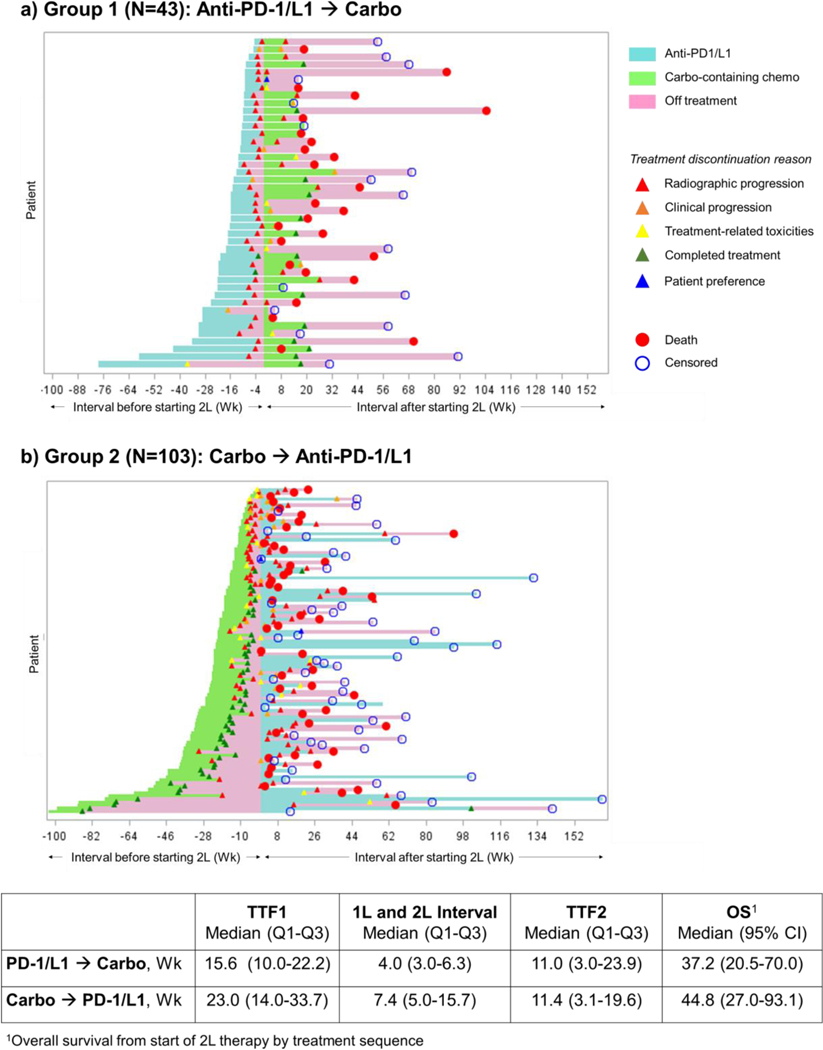
The median TTF1, Interval1L-2L, and TTF2 were 15.6 weeks (IQR 10.0–22.2), 4.0 weeks (IQR 3.0–6.3), and 11.0 weeks (IQR 3.0–23.9) in Group 1, and 23.0 weeks (IQR 14.0–33.7), 7.4 weeks (IQR 5.0–15.7), and 11.4 weeks (IQR 3.1–19.6) in Group 2, respectively (Figure 1). Most patients discontinued PD-1/PD-L1 inhibitor treatment for radiographic or clinical progression (93% in Group 1, 64% in Group 2). The most common reasons for discontinuing carboplatin-containing chemotherapy were progressive disease (47% in Group 1 and 41% in Group 2) and completion of planned chemotherapy (33% in Group 1, 49% in Group 2) (Supplemental Table 3).
Figure 1:
Swimmer’s plot by treatment sequence.
Abbreviations: 1L, first-line treatment; 2L, second-line treatment; Carbo, carboplatin-containing chemotherapy; OS, overall survival.
Discussion
Immune checkpoint inhibitors (ICI) that target negative regulators of the immune system, particularly the PD-1/L1 axis, have changed the paradigm of treatment for metastatic urothelial carcinoma. Pembrolizumab demonstrated OS benefit in the post-platinum setting compared to investigator’s choice of chemotherapy with objective response rate (ORR) of 21%, and received FDA approval in May 2017 for this indication. Between May 2016 and May 2017, four additional PD-1/L1 inhibitors – atezolizumab, nivolumab, durvalumab and avelumab – were approved in patients whose disease progressed during or after platinum-containing chemotherapy. In April-May 2017, pembrolizumab and atezolizumab were granted accelerated approval for the first-line treatment of cisplatin-ineligible locally advanced or metastatic UC based on single-arm phase II studies demonstrating ORR of 20%−25% and favorable toxicity profile, broadening the role of ICI.
With these rapid FDA approvals and significant enthusiasm around ICIs, many patients with advanced UC who are unfit to receive cisplatin were favored to receive an PD-1/L1 inhibitor instead of conventional carboplatin-containing chemotherapy, with the goal of achieving durable responses while experiencing fewer side effects. However, emerging data suggest that carboplatin-containing chemotherapy remains an important treatment modality for cisplatin-ineligible patients. In June 2018, due to an excess of early deaths in the PD-L1 low group with PD-1/L1 inhibitors compared to platinum-containing chemotherapy in the phase III IMvigor130 and KEYNOTE-361 studies, the FDA revised the first-line indications for pembrolizumab and atezolizumab to patients who are cisplatin-ineligible and who have high expression of PD-L1, or patients who are ineligible for any platinum-containing chemotherapy. Interim analysis of IMvigor130 showed that atezolizumab plus platinum/gemcitabine chemotherapy significantly and modestly improved progression-free survival compared to placebo plus platinum/gemcitabine chemotherapy; however, mature OS data are awaited.11 The combination of PD1/L1 inhibitors with platinum-based chemotherapy or CTLA-4 immune checkpoint inhibitors have all not been clearly successful in IMvigor130 and other recently reported phase III trials, KEYNOTE-361 and DANUBE.11–13 Indeed, first-line PD1/L1 inhibitor monotherapy has still not been demonstrated to improve OS compared to platinum-based chemotherapy in phase III trials in patients who were unselected or harbored tumors with high PD-L1 expression (IMvigor130, KEYNOTE-361, DANUBE). More recently, following initial signals of benefit for switch maintenance pembrolizumab, the phase III JAVELIN Bladder 100 trial demonstrated improved OS for switch maintenance avelumab plus best supportive care (BSC) versus BSC alone in patients with mUC regardless of PD-L1 status who had stable or responding disease after 4–6 cycles of platinum-based first-line chemotherapy.14, 15 These results led to the FDA approval of avelumab maintenance treatment on June 30, 2020 for patients with locally advanced (unresectable) or metastatic UC who have not progressed after 4–6 cycles of first-line platinum-containing chemotherapy. Approximately 50% of patients in Group 2 in our study discontinued 1L chemotherapy for reasons other than progression, and may have qualified for switch maintenance avelumab similar to patients in the JAVELIN Bladder-100. However, our study was conducted when post-platinum PD1/L1 inhibitors were approved to treat progressive post-platinum disease and the interval between the end of first-line chemotherapy and initiation of second-line immunotherapy in this group is often long (median 10 weeks, up to 87 weeks). This is distinct from the maintenance immunotherapy strategy in the JAVELIN Bladder-100 trial, where treatment was initiated within 10 weeks after completion of chemotherapy.
Our study examined whether there is an optimal treatment sequence of carboplatin-containing chemotherapy and PD-1/L1 inhibitors in patients with mUC as first and second line treatment, by examining the conditional survival of patients who have received both classes of agents. Cisplatin-eligible patients were excluded since there is currently no approved indication to use PD1/L1 inhibitors in patients with mUC who are fit to receive cisplatin-containing chemotherapy. We examined OS from the landmark of initiation of second-line therapy, given that inclusion for the study was conditional on receiving both lines of therapy.
While no significant difference in OS based on treatment sequencing was observed on multivariate analysis, insights can be glimpsed from these results. In an era where there is at times untampered enthusiasm for immunotherapy, this real world dataset can be sobering. The ORR of PD-1/L1 inhibitors ranged from 9–20%, and ORR from traditional carboplatin-based chemotherapy is 2.5 times higher. Consistent with our results, there is retrospective data showing notable ORR to 2L platinum-containing chemotherapy after 1L anti-PD1/L1 immunotherapy.17 The ORR observed with first-line PD-1/L1 inhibitors (9.3%) was lower than anticipated in previously reported phase II trials (23–29%) 9, 10. This may reflect our real-world patient population as well as patient selection criteria with the requirement to have received both PD1/L1 inhibitor and carboplatin-based chemotherapy. Patients who enjoyed durable remissions with first-line PD1/L1 inhibitors may be poorly represented in our population since they may not have required second-line treatment during the period when data were collected for this study. In addition, approximately 50% of patients in the real world have been reported to not receive second-line therapy, partly due to poor ECOG-PS and comorbidities.16
Our study is limited by its retrospective nature, moderate sample size, several selection and confounding biases, lack of central imaging review, and lack of biomarker analysis. Tumor PD-L1 status was unavailable for the majority of patients, as patients in this study were treated before the regulatory revision of first-line labels for pembrolizumab and atezolizumab in June 2018. Despite these limitations, this study contributes meaningful clinical data, and results can be useful to guide clinical decision making. Carboplatin-containing chemotherapy should remain a mainstay of care, particularly when higher response rates are desired, for example in patients with significant disease burden or symptomatic disease.
The therapeutic landscape for the treatment of locally advanced (unresectable) and metastatic UC is likely to be a highly dynamic arena. An ongoing phase III trial (EV-302) is evaluating the role of enfortumab vedotin combined with pembrolizumab as first-line therapy with or without platinum compared to platinum-containing chemotherapy. Another important question in the field is whether erdafitinib, an FGFR1–4 inhibitor, should be sequenced before or after a PD-1/L1 inhibitor in patients with locally advanced (unresectable) or metastatic UC with progression after platinum-based chemotherapy. In an open-label phase II study of erdafitinib in patients with locally advanced (unresectable) or metastatic UC, only 1 of 22 patients responded to prior ICI before receiving erdafitinib20. Other retrospective data showed similar ORR of approximately 25% to nivolumab or atezolizumab regardless of activating FGFR3 alteration status.18–20
Conclusion
In conclusion, in this biomarker-unselected cohort, cisplatin-ineligible patients with mUC treated sequentially with PD-1/L1 inhibitor and carboplatin-containing chemotherapy had comparable OS, regardless of treatment sequence. Carboplatin-based chemotherapy was associated with high ORR when given as 1L or 2L treatment (approximately 45%), while ORR for PD-1/L1 inhibitor was higher when given as 2L vs 1L treatment (approximately 20% vs 10%). The sequence of carboplatin-containing chemotherapy followed by PD-1/PD-L1 inhibitors also had a longer off treatment period before initiation of second-line therapy. These data lend support to the use of carboplatin-containing chemotherapy in the first-line setting in a number of patients. With the recent approval of avelumab maintenance treatment based on JAVELIN Bladder 100 trial results, there’s even stronger rationale to use carboplatin-containing chemotherapy upfront in cisplatin-ineligible patients regardless of PD-L1 status. The role of first-line pembrolizumab or atezolizumab monotherapy in unselected patients with unknown PD-L1 status is limited to those who are ineligible for any platinum-containing therapy. In cisplatin-ineligible patients, first-line PD-1/L1 inhibitor alone is a reasonable option if the patient’s tumor highly expresses PD-L1 based on the companion assay, with the caveat that phase III trials have not demonstrated significantly improved overall survival compared to chemotherapy. Indeed, one benefit of the JAVELIN Bladder-100 paradigm may be the sequenced delivery of both platinum-based chemotherapy and avelumab in sequence without interruption of therapy. Emerging and mature data from recently reported Phase III clinical trials, including IMvigor130, KEYNOTE-361, CHECKMATE-901, DANUBE, NILE and EV-302, will further refine the role of chemotherapy and immunotherapy for patients with advanced urothelial cancer. Precision medicine employing known clinical factors, genomic (e.g. tumor mutation burden), transcriptomic (e.g. gene expression subtype) and protein expression data (e.g. PD-L1 expression) will hopefully play a major role in the optimal sequencing of platinum-based chemotherapy and PD-1/L1 inhibitors.
Supplementary Material
Key of definitions for abbreviations
- 1L
First-line
- 2L
Second-line
- BSC
Best supportive care
- ECOG-PS
Eastern Cooperative Oncology Group Performance status
- FDA
Food and Drug Administration
- HR
Hazard ratio
- ICI
Immune checkpoint inhibitors
- IQR
Interquartile range
- mUC
Metastatic urothelial carcinoma
- ORR
Objective response rate
- OS
Overall survival
- PD-1
Programmed cell death protein 1
- PD-L1
Programmed death-ligand 1
- TTF
Time to treatment failure
References
- 1.von der Maase H, Sengelov L, Roberts JT et al. : Long-term survival results of a randomized trial comparing gemcitabine plus cisplatin, with methotrexate, vinblastine, doxorubicin, plus cisplatin in patients with bladder cancer. J Clin Oncol, 23: 4602, 2005 [DOI] [PubMed] [Google Scholar]
- 2.Galsky MD, Hahn NM, Rosenberg J. et al. : Treatment of patients with metastatic urothelial cancer “unfit” for Cisplatin-based chemotherapy. J Clin Oncol, 29: 2432, 2011 [DOI] [PubMed] [Google Scholar]
- 3.De Santis M, Bellmunt J, Mead G. et al. : Randomized phase II/III trial assessing gemcitabine/carboplatin and methotrexate/carboplatin/vinblastine in patients with advanced urothelial cancer who are unfit for cisplatin-based chemotherapy: EORTC study 30986. J Clin Oncol, 30: 191, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bellmunt J, de Wit R, Vaughn DJ et al. : Pembrolizumab as Second-Line Therapy for Advanced Urothelial Carcinoma. New England Journal of Medicine, 376: 1015, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Rosenberg JE, Hoffman-Censits J, Powles T. et al. : Atezolizumab in patients with locally advanced and metastatic urothelial carcinoma who have progressed following treatment with platinum-based chemotherapy: a single-arm, multicentre, phase 2 trial. Lancet, 387: 1909, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Powles T, O’Donnell PH, Massard C. et al. : Efficacy and Safety of Durvalumab in Locally Advanced or Metastatic Urothelial Carcinoma: Updated Results From a Phase 1/2 Open-label Study. JAMA Oncol: e172411, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Patel MR, Ellerton J, Infante JR et al. : Avelumab in metastatic urothelial carcinoma after platinum failure (JAVELIN Solid Tumor): pooled results from two expansion cohorts of an open-label, phase 1 trial. Lancet Oncol, 19: 51, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Sharma P, Retz M, Siefker-Radtke A. et al. : Nivolumab in metastatic urothelial carcinoma after platinum therapy (CheckMate 275): a multicentre, single-arm, phase 2 trial. Lancet Oncol, 2017 [DOI] [PubMed] [Google Scholar]
- 9.Balar AV, Castellano D, O’Donnell PH et al. : First-line pembrolizumab in cisplatin-ineligible patients with locally advanced and unresectable or metastatic urothelial cancer (KEYNOTE-052): a multicentre, single-arm, phase 2 study. Lancet Oncol, 2017 [DOI] [PubMed] [Google Scholar]
- 10.Balar AV, Galsky MD, Rosenberg JE et al. : Atezolizumab as first-line treatment in cisplatin-ineligible patients with locally advanced and metastatic urothelial carcinoma: a single-arm, multicentre, phase 2 trial. Lancet, 389: 67, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Galsky MD, Arija JAA, Bamias A. et al. : Atezolizumab with or without chemotherapy in metastatic urothelial cancer (IMvigor130): a multicentre, randomised, placebo-controlled phase 3 trial. Lancet, 395: 1547, 2020 [DOI] [PubMed] [Google Scholar]
- 12. https://www.astrazeneca.com/media-centre/press-releases/2020/update-on-phase-iii-danube-trial-for-imfinzi-and-tremelimumab-in-unresectable-stage-iv-bladder-cancer-06032020.html.
- 13. https://investors.merck.com/news/press-release-details/2020/Merck-Provides-Update-on-Phase-3-KEYNOTE-361-Trial-Evaluating-KEYTRUDA-pembrolizumab-as-Monotherapy-and-in-Combination-with-Chemotherapy-in-Patients-with-Advanced-or-Metastatic-Urothelial-Carcinoma/default.aspx.
- 14.Powles T PS, Voog E, et al. : Maintenance avelumab + best supportive care (BSC) versus BSC alone after platinum-based first-line (1L) chemotherapy in advanced urothelial carcinoma (UC): JAVELIN Bladder 100 phase III interim analysis. 10.1200/JCO.2020.38.18_suppl.LBA1 [DOI] [Google Scholar]
- 15.Galsky MD, Mortazavi A, Milowsky MI et al. : Randomized Double-Blind Phase II Study of Maintenance Pembrolizumab Versus Placebo After First-Line Chemotherapy in Patients With Metastatic Urothelial Cancer. J Clin Oncol, 38: 1797, 2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Bamias A, Tzannis K, Harshman LC et al. : Impact of contemporary patterns of chemotherapy utilization on survival in patients with advanced cancer of the urinary tract: a Retrospective International Study of Invasive/Advanced Cancer of the Urothelium (RISC). Ann Oncol, 29: 361, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Feld E, Harton J, Meropol NJ et al. : Effectiveness of First-line Immune Checkpoint Blockade Versus Carboplatin-based Chemotherapy for Metastatic Urothelial Cancer. Eur Urol, 76: 524, 2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Wang L, Gong Y, Saci A. et al. : Fibroblast Growth Factor Receptor 3 Alterations and Response to PD-1/PD-L1 Blockade in Patients with Metastatic Urothelial Cancer. Eur Urol, 2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Sharma P, Retz M, Siefker-Radtke A. et al. : Nivolumab in metastatic urothelial carcinoma after platinum therapy (CheckMate 275): a multicentre, single-arm, phase 2 trial. Lancet Oncol, 18: 312, 2017 [DOI] [PubMed] [Google Scholar]
- 20.Loriot Y, Necchi A, Park SH et al. : Erdafitinib in Locally Advanced or Metastatic Urothelial Carcinoma. N Engl J Med, 381: 338, 2019 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.