Abstract
Background
Despite the use of modern drug-eluting stents (DES), in-stent restenosis (ISR) may still occur in as many as 2–10% of percutaneous coronary interventions (PCI) in certain lesion/patient subsets. ISR causes increased morbidity after stent implantation; acute myocardial infarction is a frequent correlate to a clinical ISR, arising in 5–10% of cases. Compared to de novo stenosis, patients with ISR also present more frequently with symptoms of unstable angina pectoris (45% versus 61%). In this article, we discuss the risk factors for ISR and the corresponding diagnostic measures and effective treatment strategies.
Methods
This review is based on pertinent publications retrieved by a selective search in PubMed, with special attention to current international guidelines and specialist society recommendations.
Results
The type of implanted stent, the presence of diabetes mellitus, previous bypass surgery, and small vessel caliber are predictors for ISR. In their guidelines, the European specialist societies (ESC/EACTS) recommend repeated PCI with DES implantation or drug-coated balloon (DCB) angioplasty as the methods of choice for the treatment of ISR. This approach is supported by evidence from meta-analyses. The RIBS-IV trial showed that revascularization treatment of the target lesion is needed less often after everolimus-eluting stent (EES) implantation than after DCB dilatation (11 [7.1%] versus 24 [15.6%]; p = 0.015; hazard ratio: 0.43; 95% confidence interval: [0.21; 0.87]).
Conclusion
Because the pathogenesis of ISR is multifactorial, differentiated risk stratification is necessary. The identification of patient-, stent-, and lesion-related predictors is particularly important, as the most effective way to combat ISR is to prevent it.
cme plus
This article has been certified by the North Rhine Academy for Continuing Medical Education. Participation in the CME certification program is possible only over the internet: cme.aerzteblatt.de. The deadline for submission is 23 September 2022.
In-stent restenosis (ISR) is characterized by progressive narrowing of a coronary lesion previously treated with a stent (1). ISR is a result of stent-induced mechanical injury to the arterial wall of the treated segment (2) (figure). Angiographic ISR refers to a significant narrowing of the coronary lumen in the area of previously stented lesion (3). Restenosis detected using coronary angiography is commonly defined as a binary event with re-narrowing of ≥ 50% of the vessel diameter (4). The term “binary” indicates that patients are allocated to one of two groups: either the group with narrowing ≥ 50% or the group with narrowing <50%.
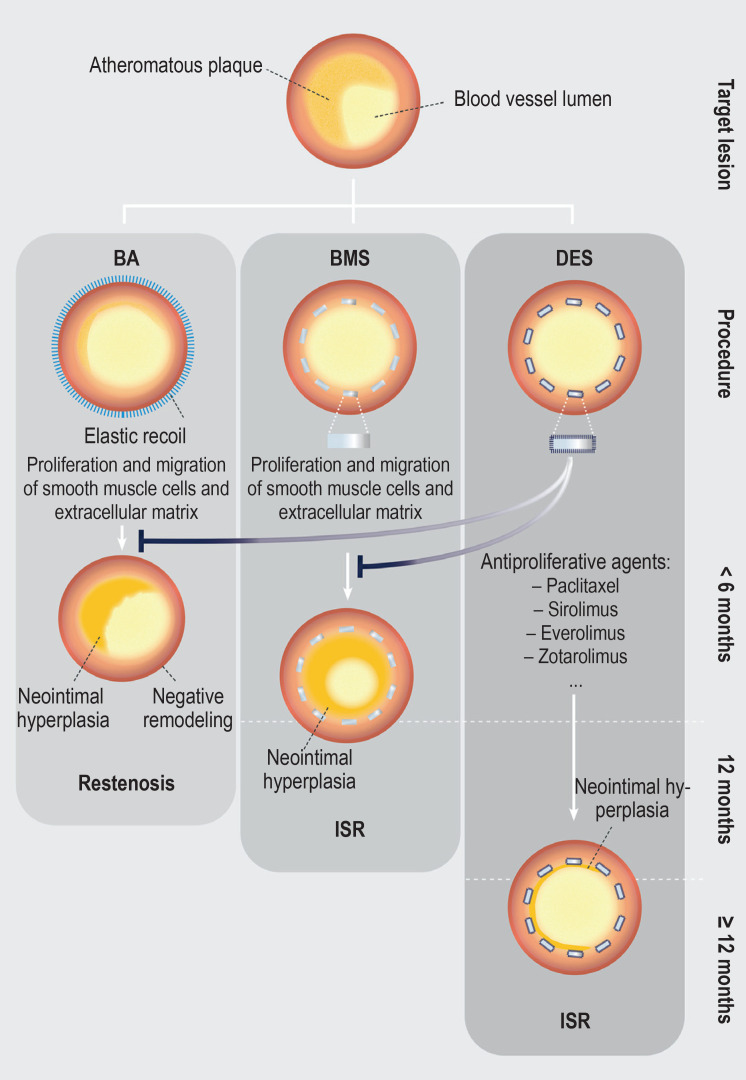
Figure.
Development of coronary in-stent restenoses after percutaneous coronary intervention (adapted from [40])
Left: Coronary restenosis after standard balloon angioplasty is primarily caused by elastic recoil of the vessel wall. In addition, the injury to the coronary artery activates the proliferation and migration of smooth muscle cells as well as the creation of an extracellular matrix. The formation of neointimal hyperplasia can ultimately lead to the development of restenosis.
Center: As it causes a higher degree of vascular injury, the implantation of a bare metal stent (BMS) increases the extent of neointimal hyperplasia and consequently the risk of developing in-stent restenosis (ISR).
Right: Drug-eluting stents (DES) release antiproliferative agents and thus reduce the extent of neointimal hyperplasia along with the risk of developing ISR.
Clinically, ISR is characterized by delayed recurrence of signs and symptoms of cardiac ischemia after initially successful angioplasty (3). In-stent restenosis affects the quality of life of a patient and also is of prognostic significance. Among patients diagnosed with ISR at a 6-month routine follow-up examination after initially successful placement of a bare metal stent (BMS), Schühlen et al. (5) demonstrated a case fatality rate of 8.8% (versus 6.0% in patients without ISR; p = 0.02). Magalhaes et al. (6) analyzed the clinical presentation of 909 patients after percutaneous coronary intervention (PCI).They found that acute myocardial infarction was a common correlate of clinically apparent in-stent restenosis after deployment of a BMS, first-generation drug-eluting stent (DES) and second-generation DES (10.6%, 10.1% and 5.2%, respectively; p = 0,273). Furthermore, in a retrospective analysis (7), patients with ISR after DES implantation more commonly presented with signs and symptoms of unstable angina pectoris compared to patients with de-novo stenosis (61% versus 45%) and the rate of major adverse cardiovascular events was 17% (versus 10%; hazard ratio [HR]: 1.98; 95% confidence intervals: [1.58; 2.46]).
Yet, despite the continuous advancement and optimization of stent technology, the prevalence of ISR has remained almost unchanged. And although patients have been treated with new drug-eluting stents with modified design, absorbable polymers and new antiproliferative agents, approximately 10% of all patients treated with PCI are still likely to develop ISR (8). Given the high prevalence of ISR, the potentially fatal adverse events associated with ISR, and the more than 300 000 PCIs performed in Germany each year, it is crucial to identify risk factors for ISR and to understand the pathophysiological causes of ISR (e1). These will be discussed in the context of established diagnostic methods and effective treatment strategies.
Methods
This review is based on pertinent publications retrieved from a selective literature search in the PubMed database; current international guidelines and expert recommendations were also taken into account. The search criteria used are listed in the Box.
BOX. Literature search.
The literature search for this review was performed in the PubMed database. The search criteria listed below were used in the basic search. The author performed an initial screening based on the manuscript title. Further pertinent articles were derived from the references of the articles identified as relevant. The search was extended according to the specific subtopics (“IVUS“/“OCT“/“angiography“/“treatment“) at the respective locations.
Date: 1 January 1990 to 31 January 2021
Language: English
Terms: (restenosis[tiab]) AND coronar*[tiab]) AND stent[tiab]) OR (“coronary artery disease” [tiab] OR CAD[tiab] OR “coronary heart disease” [tiab] OR CHD[tiab] OR “acute myocardial infarction”[tiab] OR AMI[tiab] OR “acute coronary syndrome”[tiab] OR ACS[tiab] OR NSTEMI[tiab] OR STEMI[tiab] OR “unstable angina”[tiab]) AND (“coronary intervention”[tiab] OR PCI[tiab] OR “coronary stenting”[tiab] OR “coronary artery stent”[tiab] OR “drug-eluting stent”[tiab] OR DES[tiab] OR “drug eluting stent” [tiab] OR bare-metal stent[tiab] OR BMS[tiab] OR “bare metal stent” [tiab]) AND (restenosis[tiab])
Pathogenesis
Percutaneous coronary intervention to treat atherosclerotic plaques may result in a barotrauma with injury to the endothelium and the vessel wall (9). Restenosis after standard balloon angioplasty is primarily caused by elastic recoil of the vessel wall (4, 10) (figure). In addition, the vascular injury triggers an inflammatory response, characterized by an increase in circulating biomarkers, such as fibrinogen, amyloid A and C-reactive protein (4, 10, 11). Moreover, the injury to the coronary artery activates the proliferation and migration of smooth muscle cells (12). The creation of an extracellular matrix ultimately leads to the formation of neointimal tissue, which, in case of excessive hyperplasia, can cause restenosis (9, 12).
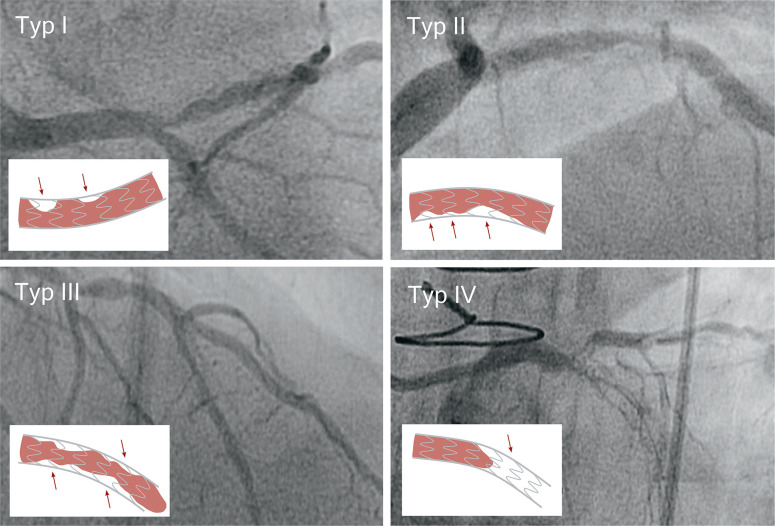
While BMS implantation leads to a reduction in elastic recoil forces, the extent of neointimal hyperplasia is increased by the more severe vascular injury (4). In addition, by reducing blood flow, the stent struts trigger activation of blood coagulation which, in turn, leads to the formation of fibrin clots. The presence of clots then promotes the migration of macrophages into the injured vessel wall (9, 10). DES release antiproliferative agents and thereby reduce both recoil forces and neointima formation (12). Mehran et al. (13) proposed an angiographic classification of ISR based on the local distribution of intimal hyperplasia in relation to the implanted stent (Figure 1).
Figure 1:
Angiographic classification of in-stent restenosis (ISR) (adapted from [13])
Type I: Focal ISR with lesions ≤ 10 mm in length. Lesions can be located between neighboring stents, at the proximal or distal end of the stent, within the stent or at any combination of the above locations.
Type II: Diffuse ISR with lesions >10 mm in length. The lesions are limited to the stent, without extending beyond its ends.
Type III: Diffuse, proliferative ISR. The lesions are >10 mm in length and extend beyond the edges of the stent.
Type IV: ISR with total occlusion. Lesions with TIMI-0 flow*.
* TIMI-0 flow, no coronary perfusion; TIMI-1 flow, significantly slowed coronary blood flow; the categories range from TIMI-0 to TIMI-3.
Background
In 1987, Sigwart and Puel were the first to implant a self-expanding BMS (e2). This stent implantation resulted in an improved initial treatment outcome; however, two major limitations emerged in later work. Serruys et al. (e3) observed stent thrombosis events in 24% of patients; 78% of these were documented within 14 days after implantation. In 33% of patients (32% of stents), ISR events were observed during follow-up. New insights into acute vascular injury, BMS and ISR as the result of a strong healing reaction led to the development of drug-eluting stents; their use was associated with a significant reduction in ISR events (e4). DES locally release controlled amounts of antiproliferative agents which are distributed in the vessel wall and exert immunosuppressive (inhibitors of the mechanistic target of rapamycin [mTOR]) or antiproliferative effects (paclitaxel, a taxane, mitosis inhibitor) on smooth muscle cells (12).
However, these anti-restenotic effects come at the cost of delayed arterial healing and are associated with an increased incidence of stent thrombosis (14). The underlying cause is an adverse paradoxical effect: The released drug inhibits the physiological tissue repair reaction and consequently fibrin removal. This, in turn, results in the activation of the prothrombotic cascade and is associated with recruitment of inflammatory cells as well as excessive production of chemokines and growth factors (11). The use of modified DES reduced the risk of ISR and the need for reintervention on the target vessel; as a result, the clinical outcome improved significantly (e5, e6).
Over time, differences in the development of ISR are noted. While neointima formation peaks 3 to 6 months after BMS implantation, a right shift of the time curve occurs after DES implantation; consequently, years after the intervention patients are still at risk of neointima formation (4, 15). In keeping with this, Räber et al. (16) demonstrated a continuous increase in lumen loss after implantation of first-generation drug-eluting stents. This increase in lumen loss together with the persistent risk of very late stent thrombosis (annual rate of 0.65% [0.40; 0.90]) indicates that the injured vessel wall may not have healed completely even five years after implantation of a first-generation DES.
Risk factors for developing ISR
Risk factors for developing ISR can be divided into patient-related risk factors and risk factors related to the design of the coronary stent. In addition, characteristics of the lesion treated, including vascular properties, and the outcome of stent implantation contribute to the development of ISR (table). In a retrospective study (17) on patients after BMS implantation, ISR events were observed in 30% of lesions; after second-generation DES implantation (e.g. polymer-based everolimus-eluting stents), this proportion dropped significantly to 12%. The use of first-generation DES compared to BMS (OR 0.35 [0.31; 0.39]) and the use of second-generation DES compared to first-generation DES (OR 0.67 [0.58; 0.77]) are independent predictors of lower ISR rates in the common follow-up period of 6 to 8 months after PCI.
Table. Risk factors for the development of in-stent restenosis.
| Patient-related risk factors | Stent-related risk factors | Lesion-related risk factors | Implantation-related risk factors |
| Diabetes mellitus (4, 17, 21) | BMS (versus DES) (4, 17) | Small target vessel (4, 17, 18) | Inadequate stent expansion (22– 25) – Stent malapposition to the vessel wall (24) – Reduced minimum lumen area in the stent (24) – Non-symmetric stent expansion (24) |
| Arterial hypertension (21) | First-generation DES (versus newer generations) (4, 17, 18) | Ostial lesion (21) | Final diameter stenosis (18) |
| Previous bypass surgery (17, 18) | Thick stent struts (4, 20) | Longer stented lesion (4, 17) | |
| Heart failure (21) | Complex morphology of the lesion (4, 17) | ||
| Length of the stenosis (>20mm) (21, 26) |
BMS, bare metal stent; DES, drug-eluting stent
Kastrati et al. (18) confirmed the type of stent used as a strong predictor of ISR. Late healing together with local inflammatory reactions and hypersensitivity reactions to the drug and the polymer are new causes of ISR—despite continuous advancements in the stent platform (8, 19). Comparing two types of stents, Pache et al. (20) highlighted the importance of also taking into account the structural design of the stent. Thinner stent struts are associated with fewer angiographic and clinical restenosis events.
With regard to patient-related factors, diabetes mellitus (OR 1.32 [1.19; 1.46]) and previous bypass surgery, regardless of location, (OR 1.38 [1.20; 1.58]) were identified as independent predictors of ISR (17). Angiographic (lesion-related) risk factors include length of the stented lesion (OR 1.27 [1.21; 1.33] for each increase by 10 mm), complex lesions (OR 1.35 [1.21; 1.51]) and small vessel sizes (OR 1.59 [1.52; 1.68] for each decrease of 0.5 mm). According to Elezi et al. (e7), however, patients with smaller vessels are more likely to suffer from conditions such as diabetes mellitus or multivessel coronary artery disease which are per se associated with poorer outcomes after stent implantation. A subanalysis of the PRESTO study (21) found increased ISR rates in patients with arterial hypertension or congestive heart failure.
The analysis of lesion-related characteristics showed statistically significant associations between increased ISR rates and lesion length of more than 20 millimeter and ostial lesions. Final diameter stenosis was also identified as an independent risk factor; each diameter increase of 5% was associated with an OR of 1.30 [1.15; 1.47] (18). Incomplete expansion of the stent (stent underexpansion) after implantation is also regarded as a meaningful predictor (22– 24). Hong et al. (25) analyzed 21 cases of DES restenosis and showed that in 43% (9 cases) ISR was caused by stent underexpansion. (table)
Intracoronary imaging
Intracoronary imaging techniques, such as intravascular ultrasound (IVUS) and optical coherence tomography (OCT), provide insight into the pathophysiology underlying the development of restenosis (27) (Figure 2). IVUS is based on an ultrasound examination of the coronary arteries and allows the assessment of the extent and distribution of intimal tissue (13, 28). De Jaegere et al. (24) demonstrated in patients who underwent IVUS-guided stent deployment that, despite acceptable angiographic outcomes, the majority of stents was not fully expanded. Insufficient stretching of the stent, known to be a strong predictor of ISR, can be detected with IVUS and subsequently corrected under IVUS guidance (22). OCT uses infrared light and has an about ten times higher axial resolution compared to IVUS (e6). OCT has been shown to be superior to IVUS in the detection of malapposition of stent struts (lack of contact between stent strut and arterial wall), stent edge-related dissections and stent fractures (22). Furthermore, it can more reliably detect the presence of neoatherosclerosis (27). In a meta-analysis, Nerlekar et al. (29) showed that IVUS-guided DES implantation reduced the rate of severe cardiovascular events (OR 0.73 [0.64; 0.85], p<0.001). Furthermore, the use of OCT is associated with improved prognosis. For example, data of the Pan-London PCI cohort (30) showed a statistically significant, non-adjusted difference in mortality between patients with OCT-guided PCI and patients with IVUS-guided PCI and patients with angiography-guided PCI (7.7% versus 12.2% versus 15.7%, p<0.0001).
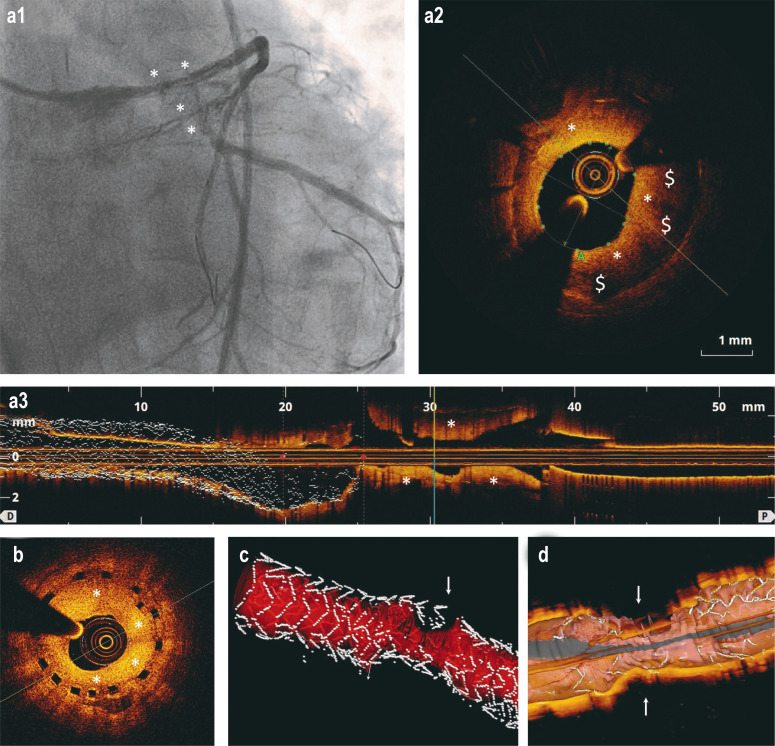
Figure 2:
In-stent restenosis after DES implantation and implantation of bioresorbable coronary stents (scaffolds): selected coronary angiography and optical coherence tomography (OCT) findings
a) The images show an in-stent restenosis (*) (a1: coronary angiography; a2: cross-section in OCT; a3: longitudinal section in OCT) in the area of the left anterior descending artery (LAD) at three months after implantation of a DES in the main stem of the left coronary artery. The area of the lumen is 3.11mm2 (normal >8 mm2). The dark areas ($) are peri-strut low intensity areas (PSLIA) and suggestive of immature, rapidly growing neointima.
b) OCT in a 67-year-old man who presented without symptoms at the 6-month follow-up after implantation of a bioresorbable scaffold. The image shows a high-grade in-stent restenosis (*) in the circumflex artery (Cx).
c–d) 3D reconstructions of in-stent restenoses (arrows) in OCT. In Figure c, the blood vessel lumen is marked in red.
DES, drug-eluting stent
Management of in-stent restenosis
The current guidelines of the European Society of Cardiology/European Association for Cardio-Thoracic Surgery (ESC/EACTS) (27) recommend a myocardial revascularization procedure for patients who develop restenosis with associated angina symptoms or coronary ischemia (27). In this situation, repeat PCI is considered the method of choice. A class 1A recommendation is made for both DES implantation and drug-coated balloon (DCB) angioplasty. Data of randomized clinical trials and network meta-analyses provided the supportive evidence and demonstrated the benefits of these first-line treatment strategies (31– 34).
DCBs are coated with lipophilic agents, such as the cytostatic drug paclitaxel, and, as mitosis inhibitors, inhibit neointimal proliferation. Concerns about suspected excess mortality after treatment with Paclitaxel-coated DCBs in peripheral interventions led to the increased use of Limus-based DCBs (8, 35). Smaller pilot studies using sirolimus-coated DCBs have shown positive initial results (8). Cutting balloons and scoring balloons are additional treatment options capable of reducing the tendency to develop restenosis (8, 36). Vascular brachytherapy (VBT) is a complex treatment modality which is now only used by few centers. The released radioactive beta radiation can reduce the proliferative reaction of the artery, resulting in lower ISR recurrence rates (8, 36).
The use of drug-based treatment regimens alone is not an effective strategy to reduce ISR recurrence rates; moreover, it is associated with the occurrence of adverse events (8). While the use of abciximab was considered as effective in early studies, this benefit was not confirmed by later research findings (36, 37). Oral application of sirolimus was once discussed as a treatment approach, but corresponding long-term data are lacking. In addition, the higher incidence of adverse drug reactions is another argument against the use of this treatment regime (36, 38).
Management of ISR by implanting a further DES or using DCB is superior to balloon angioplasty alone (27). Siontis et al. (31) confirmed this finding in a meta-analysis, comparing various strategies for managing ISR against each other. In the management of percentage diameter stenosis, PCI with implantation of an everolimus-eluting stent was more effective than DCB angioplasty, with a difference of -9.0% [-15.8; -2.2]. The difference was -9.4% [-17.4; 1.4] versus sirolimus-eluting stents (SES), versus paclitaxel-eluting stents (PES) -10.2% [-18.4; 2.0], versus vascular brachytherapy (VBT) -19.2% [-28.2; -10.4], versus bare metal stents -23.4% [-36.2; -10.8], and versus balloon angioplasty -24.2% [-32.2; -16.4].
However, ESC/EACTS point out that, with regard to stent-in-stent treatment, the superiority of the anti-restenotic effectiveness of new-generation DES has to be weighed during the long-term follow-up against the disadvantage of having to implant further metal layers (27). Inferences about clinical events should be made with caution, since none of the in-stent restenosis studies conducted had adequate statistical power for clinical endpoints (27, 39).
The results of the RIBS-IV study (32) show a significant reduction in the need for repeat revascularization of the target lesion (11 [7.1%] versus 24 [15.6%]; p = 0.015; HR: 0.43 [0.21; 0.87]) after everolimus-eluting stent (EES) implantation compared to DCB dilation. On average, 11.8 patients need to be treated with EES (instead of DCB) for one extra patient not to reach the study outcome of revascularization of the target lesion at three years after EES-ISR treatment (32, 33). For BMS-ISR, the number needed to treat at one year after the intervention was 19.2 (34).
Clinical implications
Routine angiographic follow-up after coronary stent implantation is obsolete. Patients should only be followed up with angiography if they are symptomatic or have proven ischemia. For patients with ISR plus signs of myocardial ischemia, ESC/EACTS recommend repeat revascularization (27). In this recommendation, coronary intervention with DES implantation and DCB angioplasty are regarded as equivalent treatment options. In addition, the guidelines (27) recommend the use of intracoronary imaging to optimize patient outcome and to inform the creation of individual treatment plans. With regard to the mechanisms underlying stent failure, optical coherence tomography can be recommended as the method of choice (22). To assess lesions of the left main stem coronary artery, which have a high prevalence of ISR, preference should be given to intravascular ultrasound (22). The use of imaging technologies during the intervention can significantly reduce the risk of ISR. For example, severely calcified lesions need to be adequately prepared to prevent insufficient expansion of the stent to be implanted (8). However, in patients with complex findings and recurrent diffuse in-stent restenosis, a surgical procedure should be considered. The decision to perform coronary artery bypass surgery usually requires the involvement of a multidisciplinary heart team.
Clinical evaluation of new catheter-based techniques to treat in-stent restenosis is currently underway. One of these new treatment modalities is coronary lithotripsy which can break down calcified lesions using shock waves. This technique is currently being assessed in clinical trials on patients with ISR and underexpanded stents (8). In addition, next-generation sirolimus-coated DCB technology should be evaluated for the treatment of DES-ISR in randomized trials. Further intensive research is needed to identify the patient population which could benefit from new treatment techniques. Additionally, their long-term benefits must be evaluated and then compared to proven treatment strategies. Finally, the most effective approach to manage in-stent restenosis is to prevent it from occurring in the first place. For this, the use of imaging modalities as well as the further development of implantation techniques and stent technologies are essential.
Questions on the article in issue 38/2021: Coronary In-stent Restenosis— Predictors and Treatment.
The submission deadline is 23 September 2022. Only one answer is possible per question. Please select the answer that is most appropriate.
Question 1
At what degree of narrowing of the vessel diameter in coronary angiography is the term binary restenosis used?
≥ 15%
≥ 20%
≥ 30%
≥ 35%
≥ 50%
Question 2
In approximately what percentage of percutaneous coronary interventions (PCIs) occurrence of in-stent restenosis can be expected despite the use of current treatment standards?
5%
10%
20%
40%
80%
Question 3
What does the abbreviation BMS stand for in the context of coronary stents?
Balloon and metal stent
Biological matrix stent
Bone matrix stent
Bare metal stent
Biological material stent
Question 4
In the angiographic classification, a diffuse in-stent restenosis with lesions limited to the stent and measuring >10 mm in length is classified as what type?
Type 0
Type I
Type II
Type III
Type IV
Question 5
What imaging techniques are abbreviated as OCT and IVUS?
Optical coherence tomography and intravascular ultrasound
Optical computed tomography and intravascular ultrasound
Operative coherence tomography and invasive vascular ultrasound
Optical coherence tomography and intraoperative visualization by ultrasound
Operative computer technology and intravascular ultrasound
Question 6
According to study results, which metabolic disease is a risk factor for in-stent restenosis?
Hyperthyroidism
Lactose intolerance
Amyloidosis
Wilson‘s disease
Diabetes mellitus
Question 7
Based on the Pan-London PCI cohort data, various intervention techniques were evaluated. For which technique was the lowest case fatality reported?
OCT-guided PCI
IVUS-guided PCI
Angiography-guided PCI
PCI without imaging
CT-guided PCI
Question 8
Which recommendation is made in the article for routine angiographic follow-up after coronary stent implantation?
It should be routinely performed in all patients.
It should only be performed in symptomatic patients or patients with proven ischemia.
It should only be performed in patients with a risk factor for ISR.
It should only be performed in patients with at least two risk factors for ISR.
It should only be performed in patients aged 70 years and older
Question 9
What effect is attributed to active substances released from drug-eluting stents?
Reduction of the extent of neointimal hyperplasia
Prevention of neointimal hyperplasia formation
Shortening of arterial healing time
Faster reaching of the peak of neointima formation
Reduction of the risk of late stent thrombosis
Question 10
What does the term malapposition denote in the context of implanted stents?
Missing information about the position of the stent
The stent was implanted in the wrong section of the blood vessel.
The stent location cannot be determined using standard imaging modalities.
Missing contact between stent struts and blood vessel wall
The removal of the stent because of wrong positioning
Acknowledgments
Translated from the original German by Ralf Thoene, MD.
Footnotes
Conflict of interest
Prof. Gori received consultant and lecture fees from Abbott Vascular.
The remaining authors declare no conflict of interest.
References
- 1.Lee MS, Banka G. In-stent restenosis. Interv Cardiol Clin. 2016;5:211–220. doi: 10.1016/j.iccl.2015.12.006. [DOI] [PubMed] [Google Scholar]
- 2.Stone GW, Ellis SG, Cox DA, et al. One-year clinical results with the slow-release, polymer-based, paclitaxel-eluting TAXUS stent: the TAXUS-IV trial. Circulation. 2004;109:1942–1947. doi: 10.1161/01.CIR.0000127110.49192.72. [DOI] [PubMed] [Google Scholar]
- 3.Kuntz RE, Baim DS. Defining coronary restenosis. Newer clinical and angiographic paradigms. Circulation. 1993;88:1310–1323. doi: 10.1161/01.cir.88.3.1310. [DOI] [PubMed] [Google Scholar]
- 4.Byrne RA, Joner M, Kastrati A. Stent thrombosis and restenosis: what have we learned and where are we going? The Andreas Gruntzig Lecture ESC 2014. Eur Heart J. 2015;36:3320–3331. doi: 10.1093/eurheartj/ehv511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Schühlen H, Kastrati A, Mehilli J, et al. Restenosis detected by routine angiographic follow-up and late mortality after coronary stent placement. Am Heart J. 2004;147:317–322. doi: 10.1016/j.ahj.2003.10.002. [DOI] [PubMed] [Google Scholar]
- 6.Magalhaes MA, Minha S, Chen F, et al. Clinical presentation and outcomes of coronary in-stent restenosis across 3-stent generations. Circ Cardiovasc Interv. 2014;7:768–776. doi: 10.1161/CIRCINTERVENTIONS.114.001341. [DOI] [PubMed] [Google Scholar]
- 7.Buchanan KD, Torguson R, Rogers T, et al. In-stent restenosis of drug-eluting stents compared with a matched group of patients with de novo coronary artery stenosis. Am J Cardiol. 2018;121:1512–1518. doi: 10.1016/j.amjcard.2018.02.033. [DOI] [PubMed] [Google Scholar]
- 8.Shlofmitz E, Iantorno M, Waksman R. Restenosis of drug-eluting stents: a new classification system based on disease mechanism to guide treatment and state-of-the-art review. Circ Cardiovasc Interv. 2019;12 doi: 10.1161/CIRCINTERVENTIONS.118.007023. e007023. [DOI] [PubMed] [Google Scholar]
- 9.Fanelli C, Aronoff R. Restenosis following coronary angioplasty. Am Heart J. 1990;119:357–368. doi: 10.1016/s0002-8703(05)80028-6. [DOI] [PubMed] [Google Scholar]
- 10.Gori T. Vascular wall reactions to coronary stents-clinical implications for stent failure. Life (Basel) 2021;11 doi: 10.3390/life11010063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gori T. Endothelial function: a short guide for the interventional cardiologist. Int J Mol Sci. 2018;19 doi: 10.3390/ijms19123838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Stefanini GG, Holmes DR. Jr. Drug-eluting coronary-artery stents. N Engl J Med. 2013;368:254–265. doi: 10.1056/NEJMra1210816. [DOI] [PubMed] [Google Scholar]
- 13.Mehran R, Dangas G, Abizaid AS, et al. Angiographic patterns of in-stent restenosis: classification and implications for long-term outcome. Circulation. 1999;100:1872–1878. doi: 10.1161/01.cir.100.18.1872. [DOI] [PubMed] [Google Scholar]
- 14.Piccolo R, Stefanini GG, Franzone A, et al. Safety and efficacy of resolute zotarolimus-eluting stents compared with everolimus-eluting stents: a meta-analysis. Circ Cardiovasc Interv. 2015 doi: 10.1161/CIRCINTERVENTIONS.114.002223. [DOI] [PubMed] [Google Scholar]
- 15.Kastrati A, Schomig A, Dietz R, Neumann FJ, Richardt G. Time course of restenosis during the first year after emergency coronary stenting. Circulation. 1993;87:1498–1505. doi: 10.1161/01.cir.87.5.1498. [DOI] [PubMed] [Google Scholar]
- 16.Raber L, Wohlwend L, Wigger M, et al. Five-year clinical and angiographic outcomes of a randomized comparison of sirolimus-eluting and paclitaxel-eluting stents: results of the Sirolimus-Eluting Versus Paclitaxel-Eluting Stents for Coronary Revascularization LATE trial. Circulation. 2011;123:2819–2828. doi: 10.1161/CIRCULATIONAHA.110.004762. [DOI] [PubMed] [Google Scholar]
- 17.Cassese S, Byrne RA, Tada T, et al. Incidence and predictors of restenosis after coronary stenting in 10 004 patients with surveillance angiography. Heart. 2014;100:153–159. doi: 10.1136/heartjnl-2013-304933. [DOI] [PubMed] [Google Scholar]
- 18.Kastrati A, Dibra A, Mehilli J, et al. Predictive factors of restenosis after coronary implantation of sirolimus- or paclitaxel-eluting stents. Circulation. 2006;113:2293–2300. doi: 10.1161/CIRCULATIONAHA.105.601823. [DOI] [PubMed] [Google Scholar]
- 19.Lee SY, Hong MK, Jang Y. Formation and transformation of neointima after drug-eluting stent implantation: insights from optical coherence tomographic studies. Korean Circ J. 2017;47:823–832. doi: 10.4070/kcj.2017.0157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Pache J, Kastrati A, Mehilli J, et al. Intracoronary stenting and angiographic results: strut thickness effect on restenosis outcome (ISAR-STEREO-2) trial. J Am Coll Cardiol. 2003;41:1283–1288. doi: 10.1016/s0735-1097(03)00119-0. [DOI] [PubMed] [Google Scholar]
- 21.Singh M, Gersh BJ, McClelland RL, et al. Clinical and angiographic predictors of restenosis after percutaneous coronary intervention: insights from the Prevention of Restenosis With Tranilast and Its Outcomes (PRESTO) trial. Circulation. 2004;109:2727–2731. doi: 10.1161/01.CIR.0000131898.18849.65. [DOI] [PubMed] [Google Scholar]
- 22.Raber L, Mintz GS, Koskinas KC, et al. Clinical use of intracoronary imaging. Part 1: guidance and optimization of coronary interventions. An expert consensus document of the European Association of Percutaneous Cardiovascular Interventions. Eur Heart J. 2018;39:3281–3300. doi: 10.1093/eurheartj/ehy285. [DOI] [PubMed] [Google Scholar]
- 23.Borovac JA, D‘Amario D, Vergallo R, et al. Neoatherosclerosis after drug-eluting stent implantation: a novel clinical and therapeutic challenge. Eur Heart J Cardiovasc Pharmacother. 2019;5:105–116. doi: 10.1093/ehjcvp/pvy036. [DOI] [PubMed] [Google Scholar]
- 24.de Jaegere P, Mudra H, Figulla H, et al. Intravascular ultrasound-guided optimized stent deployment. Immediate and 6 months clinical and angiographic results from the Multicenter Ultrasound Stenting in Coronaries Study (MUSIC Study) Eur Heart J. 1998;19:1214–1223. doi: 10.1053/euhj.1998.1012. [DOI] [PubMed] [Google Scholar]
- 25.Hong MK, Mintz GS, Lee CW, et al. Intravascular ultrasound predictors of angiographic restenosis after sirolimus-eluting stent implantation. Eur Heart J. 2006;27:1305–1310. doi: 10.1093/eurheartj/ehi882. [DOI] [PubMed] [Google Scholar]
- 26.Bourassa MG, Lesperance J, Eastwood C, et al. Clinical, physiologic, anatomic and procedural factors predictive of restenosis after percutaneous transluminal coronary angioplasty. J Am Coll Cardiol. 1991;18:368–376. doi: 10.1016/0735-1097(91)90588-z. [DOI] [PubMed] [Google Scholar]
- 27.Neumann FJ, Sousa-Uva M, Ahlsson A, et al. 2018 ESC/EACTS Guidelines on myocardial revascularization. Eur Heart J. 2019;40:87–165. doi: 10.1093/eurheartj/ehy855. [DOI] [PubMed] [Google Scholar]
- 28.Maehara A, Matsumura M, Ali ZA, Mintz GS, Stone GW. IVUS-guided versus OCT-guided coronary stent implantation: a critical appraisal. JACC Cardiovasc Imaging. 2017;10:1487–1503. doi: 10.1016/j.jcmg.2017.09.008. [DOI] [PubMed] [Google Scholar]
- 29.Nerlekar N, Cheshire CJ, Verma KP, et al. Intravascular ultrasound guidance improves clinical outcomes during implantation of both first- and second-generation drug-eluting stents: a meta-analysis. EuroIntervention. 2017;12:1632–1642. doi: 10.4244/EIJ-D-16-00769. [DOI] [PubMed] [Google Scholar]
- 30.Jones DA, Rathod KS, Koganti S, et al. Angiography alone versus angiography plus optical coherence tomography to guide percutaneous coronary intervention: outcomes from the Pan-London PCI Cohort. JACC Cardiovasc Interv. 2018;11:1313–1321. doi: 10.1016/j.jcin.2018.01.274. [DOI] [PubMed] [Google Scholar]
- 31.Siontis GC, Stefanini GG, Mavridis D, et al. Percutaneous coronary interventional strategies for treatment of in-stent restenosis: a network meta-analysis. Lancet. 2015;386:655–664. doi: 10.1016/S0140-6736(15)60657-2. [DOI] [PubMed] [Google Scholar]
- 32.Alfonso F, Perez-Vizcayno MJ, Cuesta J, et al. 3-year clinical follow-up of the RIBS IV clinical trial: a prospective randomized study of drug-eluting balloons versus everolimus-eluting stents in patients with in-stent restenosis in coronary arteries previously treated with drug-eluting stents. JACC Cardiovasc Interv. 2018;11:981–991. doi: 10.1016/j.jcin.2018.02.037. [DOI] [PubMed] [Google Scholar]
- 33.Alfonso F, Pérez-Vizcayno MJ, Cárdenas A, et al. A prospective randomized trial of drug-eluting balloons versus everolimus-eluting stents in patients with in-stent restenosis of drug-eluting stents: the RIBS IV randomized clinical trial. J Am Coll Cardiol. 2015;66:23–33. doi: 10.1016/j.jacc.2015.04.063. [DOI] [PubMed] [Google Scholar]
- 34.Alfonso F, Pérez-Vizcayno MJ, Cárdenas A, et al. A randomized comparison of drug-eluting balloon versus everolimus-eluting stent in patients with bare-metal stent-in-stent restenosis: the RIBS V clinical trial (Restenosis Intra-stent of Bare Metal Stents: paclitaxel-eluting balloon vs. everolimus-eluting stent). J Am Coll Cardiol. 2014;63:1378–1386. doi: 10.1016/j.jacc.2013.12.006. [DOI] [PubMed] [Google Scholar]
- 35.Katsanos K, Spiliopoulos S, Kitrou P, Krokidis M, Karnabatidis D. Risk of death following application of paclitaxel-coated balloons and stents in the femoropopliteal artery of the leg: a systematic review and meta-analysis of randomized controlled trials. J Am Heart Assoc. 2018;7 doi: 10.1161/JAHA.118.011245. e011245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Alfonso F, Byrne RA, Rivero F, Kastrati A. Current treatment of in-stent restenosis. J Am Coll Cardiol. 2014;63:2659–2673. doi: 10.1016/j.jacc.2014.02.545. [DOI] [PubMed] [Google Scholar]
- 37.Moustapha A, Assali AR, Sdringola S, et al. Abciximab administration and clinical outcomes after percutaneous intervention for in-stent restenosis. Catheter Cardiovasc Interv. 2002;56:184–187. doi: 10.1002/ccd.10166. [DOI] [PubMed] [Google Scholar]
- 38.Kufner S, Hausleiter J, Ndrepepa G, et al. Long-term risk of adverse outcomes and new malignancies in patients treated with oral sirolimus for prevention of restenosis. JACC Cardiovasc Interv. 2009;2:1142–1148. doi: 10.1016/j.jcin.2009.08.015. [DOI] [PubMed] [Google Scholar]
- 39.Xu B, Qian J, Ge J, et al. Two-year results and subgroup analyses of the PEPCAD China in-stent restenosis trial: a prospective, multicenter, randomized trial for the treatment of drug-eluting stent in-stent restenosis. Catheter Cardiovasc Interv. 2016;87(1):624–629. doi: 10.1002/ccd.26401. [DOI] [PubMed] [Google Scholar]
- 40.Torrado J, Buckley L, Durán A, et al. Restenosis, stent thrombosis, and bleeding complications: navigating between Scylla and Charybdis. J Am Coll Cardiol. 2018;71:1676–1695. doi: 10.1016/j.jacc.2018.02.023. [DOI] [PubMed] [Google Scholar]
- E1.MedMarket Diligence, LLC. Global dynamics of surgical and interventional cardiovascular procedures, 2015-2022. (Report #C500) MedMarket Diligence. 2016 [Google Scholar]
- E2.Sigwart U, Puel J, Mirkovitch V, Joffre F, Kappenberger L. Intravascular stents to prevent occlusion and restenosis after transluminal angioplasty. N Engl J Med. 1987;316:701–706. doi: 10.1056/NEJM198703193161201. [DOI] [PubMed] [Google Scholar]
- E3.Serruys PW, Strauss BH, Beatt KJ, et al. Angiographic follow-up after placement of a self-expanding coronary-artery stent. N Engl J Med. 1991;324:13–17. doi: 10.1056/NEJM199101033240103. [DOI] [PubMed] [Google Scholar]
- E4.Stefanini GG, Taniwaki M, Windecker S. Coronary stents: novel developments. Heart. 2014;100:1051–1061. doi: 10.1136/heartjnl-2012-303522. [DOI] [PubMed] [Google Scholar]
- E5.Stettler C, Wandel S, Allemann S, et al. Outcomes associated with drug-eluting and bare-metal stents: a collaborative network meta-analysis. Lancet. 2007;370:937–948. doi: 10.1016/S0140-6736(07)61444-5. [DOI] [PubMed] [Google Scholar]
- E6.Ullrich H, Münzel T, Gori T. Coronary stent thrombosis—predictors and prevention. Dtsch Arztebl Int. 2020;117:320–326. doi: 10.3238/arztebl.2020.0320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- E7.Elezi S, Dibra A, Mehilli J, et al. Vessel size and outcome after coronary drug-eluting stent placement: results from a large cohort of patients treated with sirolimus- or paclitaxel-eluting stents. J Am Coll Cardiol. 2006;48:1304–1309. doi: 10.1016/j.jacc.2006.05.068. [DOI] [PubMed] [Google Scholar]