Abstract
Infective endocarditis (IE) is a life-threatening infection, which has had a notable increase in incidence in recent years. Although Staphylococci or Streptococci are the most common culprit organisms, rarer organisms have also been implicated and associated with a more aggressive disease course. We present a case of Enterobacter cloacae IE affecting the aortic valve in an 82-year-old male with an implantable permanent pacemaker. Our case demonstrates that a prolonged-course of beta-lactam therapy may be an effective, non-invasive management option for IE caused by multidrug-resistant gram-negative organisms.
Keywords: Device-associated endocarditis, Multi-drug resistance, Aortic valve endocarditis, Beta-lactamases producing organism
Introduction
The pathophysiology behind IE requires an inciting endocardial injury that is typically sustained from regurgitant jet blood flow. After endocardial injury has occurred, platelets and fibrin accumulate at narrow orifices of cardiac valves separating high pressure blood flow from low pressure flow. These sterile vegetations are then superinfected by circulating microorganisms due to transient bacteremia. This results in activation of the extrinsic coagulation pathway and deposition of fibronectin, making it difficult to eradicate the infection. It often takes several months for organisms to undergo phagocytosis or lysis [1].
Implantable cardiac devices (ICD), valvular heart disease and the presence of prosthetic valves predispose patients to the development of IE. However, frequently overlooked risk factors typically associated with multi-drug resistant (MDR) IE also includes healthcare contact, nursing home residence and chronic kidney disease and are often associated with a high risk of mortality.
Most cases of IE are caused by gram-positive organisms, though Gram-negative HACEK organisms can also be causative. However, IE caused by most non-HACEK gram negative organisms, with exception to E. Cloacae, is an infrequent occurrence due to the inability of these organisms to attach to the endocardium. In fact, in a prospective cohort study of 2761 patients with confirmed IE, only 49 cases were due to non-HACEK Gram-negative organisms and of these cases, only two reported cases were caused by Enterobacter spp [2], [7].
In the past, bacteremia due to E. Cloacae was typically associated with urosepsis, biliary disease, or malignancy, but in recent years, healthcare exposure and the presence of ICDs have also become significant risk factors. Despite the various clinical benefits of the newer generation of ICDs, the exponential increase of ICD placement and the invasive nature of the procedure introduces an increased risk for a disease process that carries fatal complications.
Given the fatal complications associated with IE, such as heart failure, heart block, perivalvular abscesses, and multiorgan failure, correctly diagnosing this disease process is crucial. Therefore, there should be a high index of suspicion for IE when a patient with a predisposing risk factor presents with fever and bacteremia. While IE can be treated with long-term antibiotics, there are specific indications for surgical intervention. IE associated with organisms that are typically MDR such as E. Cloacae is an immediate surgical indication. However, in this case report, we propose an alternative noninvasive management approach with long-term beta-lactam monotherapy.
Case presentation
An 82-year-old male presented to the hospital from his nursing home due to 7-day history of altered mental status and generalized weakness. He was diagnosed with coronary artery disease and underwent coronary artery bypass graft in 1990. He was later noted to have Mobitz type II atrioventricular block and underwent a pacemaker implantation in 2003. In addition, his medical history was relevant for heart failure with preserved ejection fraction, recurrent urinary tract infections, chronic kidney disease, and insulin-dependent diabetes mellitus type 2.
On admission, the patient was febrile at 39.8 C but vitals and physical exam were otherwise unremarkable. Initial laboratory findings revealed an elevated serum lactic acid level of 3.3 mmol/L and leukocyte count of 15,700/mm3 with neutrophilic predominance. Serum creatinine was also elevated at 1.48 mg/dL. Urinalysis was positive for leukocyte esterase and nitrates which suggested possible urosepsis so the patient was treated with intravenous (IV) piperacillin-tazobactam for broad spectrum coverage. On day 2 of hospitalization, urine culture grew Enterobacter cloacae complex.
Despite treatment, the patient’s condition continued to deteriorate. Repeated runs of non-sustained ventricular tachycardia were noted on telemetry. On physical examination, the patient had jugular venous distension and the presence of a new grade IV/VI systolic murmur heard at the left upper sternal border in addition to his persistent fever. Furthermore, he was noted to have elevated serum troponin and NT pro-BNP levels. Two sets of blood cultures were now positive for E. Cloacae further with resistance to piperacillin-tazobactam. As such, on day three, piperacillin-tazobactam was discontinued and ertapenem therapy was initiated.
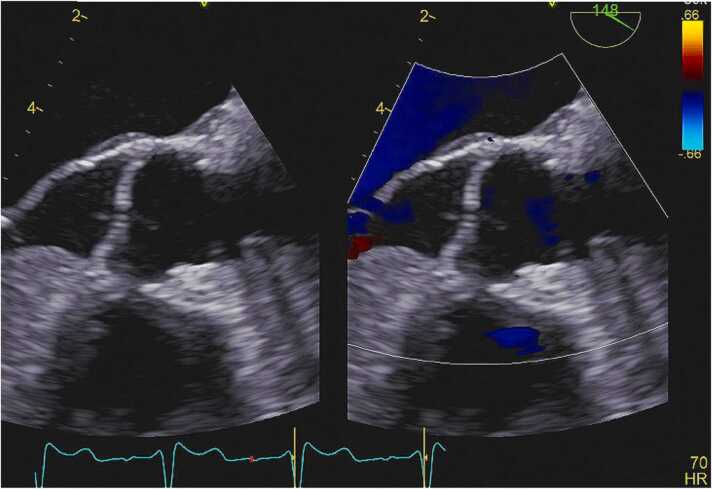
Given the patient’s fever, bacteremia, and presence of a new regurgitant murmur, the patient met 2 major and 1 minor duke’s criteria suggesting definite infective endocarditis. The diagnosis was confirmed after a transesophageal echocardiogram revealed a large independently mobile vegetation attached to the aortic valve. After multidisciplinary discussion, unfortunately the patient was deemed a poor candidate for cardiothoracic intervention given his advanced age and multiple comorbidities (Fig. 1).
Fig. 1.
Transesophageal echocardiogram revealing an independently mobile echodensity attached to the ventricular surface of the right coronary cusp of the aortic valve.
Over the course of his hospitalization, the patient’s clinical condition improved. The patient completed a total of 12 weeks of antibiotic therapy with ertapenem. During his outpatient follow-up, his repeat echocardiogram no longer revealed findings concerning for vegetations or significant valvular destruction and remains this way four months following his hospitalization. At present, approximately one year following his diagnosis of IE, the patient continues to have urological complications however, he has not had further cardiac complications. As per our literature review, this is one of the few case reports demonstrating effective management of IE caused by E. Cloacae with intravenous antibiotics alone.
Discussion
Although E. Cloacae is not the usual suspect associated with infective endocarditis, it has a high mortality rate which emphasizes the importance for clinicians to recognize how this causative organism affects our decision for management. E. Cloacae is a biofilm forming organism which results in the secretion of extracellular polymeric substances that support the colonization of bacterial organisms. Moreover, E. Cloacae contains a chromosome-encoded AmpC β-lactam genes which permit these organisms to intrinsically produce AmpC β-lactamase conferring resistance to most antibiotics including penicillins and cephalosporins [3].
The American Association for Thoracic Surgery Consensus Guidelines outline that mobile vegetations greater than 10 mm in length and symptoms of heart failure as seen in our patient, recommend urgent surgical intervention. Surgical removal of the vegetation with aortic valve replacement is typically performed in conjunction with prolonged antibiotic therapy [4]. Furthermore, the European Heart Rhythm Association consensus recommends lead extraction for patients with bacteremia with or without systemic symptoms and ICD related infective endocarditis [5]. Moreover, pacemaker lead cultures are collected to direct antimicrobial therapy and leads are reinserted after 72 h if blood cultures remain negative [6].
However, our patient had multiple comorbid conditions making this a high-risk procedure that he would not have tolerated. After weighing the risks and benefits of reducing bacterial load with surgical intervention, it was decided to manage the patient with a non-invasive approach. Our case report reveals an effective alternative therapy with prolonged beta-lactam therapy for high-risk patients.
Conclusion
In conclusion, we presented a rare case of infective endocarditis caused by E. Cloacae. In this scenario, it was successfully treated with beta-lactam monotherapy without cardiothoracic surgery or lead extraction. Ertapenem was chosen based on culture sensitivity and given that it is a broad-spectrum antibiotic with particularly favorable coverage for extended spectrum β-lactamase (ESBL)-producing GNB organisms such as the one implicated in this case. Although E. cloacae IE has been a rare occurrence thus far, we may see an increase in incidence in the foreseeable future. This case emphasizes the need to weigh risks against benefits of therapy recommended by current societal guidelines to ensure that the best treatment options is being offered in a unique clinical scenario. In our case, prolonged therapy with intravenous beta-lactams was an effective management approach in this clinical setting.
Declaration of interest and ethical statement
All authors meet the ICMJE authorship criteria. No competing interests between authors or declarations to disclose. Written informed consent was obtained from the patient for publication of this case report and accompanying images. A copy of the written consent is available for review by the Editor-in-Chief of this journal on request.
Intellectual property rights assignment or licence statement
I, Dilanthy Annappah, the Author have the right to grant and do grant on behalf of all authors, to ID Cases in accordance with the relevant stated licence terms for US Federal Government Employees to permit this Work, if accepted, to be published in ID Cases.
References
- 1.Werdan K., Dietz S., Löffler B., Niemann S., Bushnaq H., Silber R.E., et al. Mechanisms of infective endocarditis: pathogen-host interaction and risk states. Nat Rev Cardiol. 2014;11(1):35–50. doi: 10.1038/nrcardio.2013.174. Epub 2013 Nov 19. [DOI] [PubMed] [Google Scholar]
- 2.Baddour L.M., Wilson W.R., Bayer A.S., Fowler VG Jr, Tleyjeh I.M., Rybak M.J., et al. Infective endocarditis in adults: diagnosis, antimicrobial therapy, and management of complications: a scientific statement for healthcare professionals from the American Heart Association. Endorsed by: Infectious Disease Society of America. Circulation. 2015;132:1435–1486. doi: 10.1161/CIR.0000000000000296. [DOI] [PubMed] [Google Scholar]
- 3.Yoshino Y., Okugawa S., Kimura S., Makita E., Seo K., Koga I., et al. Infective endocarditis due to enterobacter cloacae resistant to third- and fourth-generation cephalosporins. J Microbiol Immunol Infect. 2015;48(2):226–228. doi: 10.1016/j.jmii.2012.07.015. [DOI] [PubMed] [Google Scholar]
- 4.AATS Surgical Treatment of Infective Endocarditis Consensus Guidelines Writing Committee C., Pettersson G.B., Coselli J.S., Writing C., Pettersson G.B., Coselli J.S., et al. 2016 American Association for Thoracic Surgery (AATS) consensus guidelines: surgical treatment of infective endocarditis. J Thorac Cardiovasc Surg. 2017;153:1241–1258. doi: 10.1016/j.jtcvs.2016.09.093. e29. [DOI] [PubMed] [Google Scholar]
- 5.Bongiorni M.G., Burri H., Deharo J.C., Starck C., Kennergren C., Saghy L., et al. 2018 Ehra expert consensus statement on lead extraction: recommendations on definitions, endpoints, research trial design, and data collection requirements for clinical scientific studies and registries: endorsed by APHRS/Hrs/LAHRS. EP Eur. 2018;20(7) doi: 10.1093/europace/euy050. 1217–1217. [DOI] [PubMed] [Google Scholar]
- 6.Kusumoto F.M., Schoenfeld M.H., Wilkoff B.L., Berul C.I., Birgersdotter-Green U.M., Carrillo R., et al. 2017 HRS expert consensus statement on cardiovascular implantable electronic device lead management and extraction. Heart Rhythm. 2017;14:503–551. doi: 10.1016/j.hrthm.2017.09.001. [DOI] [PubMed] [Google Scholar]
- 7.Morpeth S. Non-HACEK gram-negative Bacillus endocarditis. Ann Intern Med. 2007;147:829–835. doi: 10.7326/0003-4819-147-12-200712180-00002. [DOI] [PubMed] [Google Scholar]