Abstract
Objectives:
To better understand the role of nutrition in older adults (aged 50 years or older) with bipolar disorders (OABD), we conducted a systematic review of the literature and appraise existing evidence.
Methods:
Following PRISMA guidelines, we searched databases including Medline/PubMed, PsychINFO, EMBASE, CINAHL, Scopus, Web of Science, Cochrane Register, FDA website, and clinical trial registries through 2019 for eligible reports. The search string combined MeSH terms for bipolar disorder, nutrition and older adults. This was supplemented by snowball searching of references in relevant studies and authors were contacted to request their work where necessary. All included studies were rated with the National Institutes of Health Study Quality Assessment Tools based on study designs.
Results:
Of 2280 papers screened, ten studies including eight observational and two interventional studies. The topic foci of the papers examined several nutrients, (including vitamin B12, vitamin D, coenzyme Q10, homocysteine, and folate), nutritional deficiencies and biochemical correlates. The prevalence rates of deficiencies varied with specific nutrients (3.7% to 71.6% for Vitamin B12 and 34.6% for Vitamin D), and between inpatient versus outpatient populations. While nutritional interventions appeared to be associated with improvement in both affective and cognitive outcomes, the sample sizes of OABD varied and were generally small.
Conclusion:
While there is evidence for the benefits of nutritional interventions on affective, cognitive and overall outcome in OABD, the quality of the evidence is limited. Our findings underscore the need for high quality studies to inform evidence-based guidelines for nutritional assessment and supplemention in OABD.
Keywords: Bipolar disorder, Depression, Geriatrics, Nutrition, Older adults, Mania
1. INTRODUCTION
Recent international collaborative research indicates that suboptimal diet contributes significantly to the global burden of diseases with 11 million deaths and loss of 255 million disability-adjusted life-years attributable to dietary risk factors in 2017 (1–3). Despite the evidence linking poor nutrition to disease, nutrition and nutritional supplements are often overlooked in the assessment and treatment of patients generally, and in individuals with bipolar disorder (BD) in particular.
Older adults with bipolar disorder (OABD) may be especially prone to nutritional deficiencies due to various age-related factors that can diminish nutrient intake and absorption. Reduced dietary intake of nutrients may occur due to decreased sensory function, decreased appetite and dysphagia with advanced age (4). Causes of decreased nutrient absorption that are more common in older adults include: age related changes in the gut or atrophic gastritis; peptic ulcer disease with Helicobacter pylori infection; gastric and intestinal resections and taking medications that can interfere with nutrient absorption. Many of these interfering medications are commonly prescribed and include inhibitors of gastric acid secretion such as proton pump inhibitors and histamine-2 receptor antagonists; anti-epileptics, metformin, methotrexate, triamterene and trimethoprim (5, 6). Importantly, nutritional status, particularly vitamin levels, can influence cognitive function and affective symptoms in older adults with mood disorders, including BD (7–10).
In addition to the lack of awareness of diet and nutritional deficiencies in the care of OABD, clinicians are often unaware of the increasing use of nutritional supplements by their patients. Herbal and nutritional compounds are used widely by older adults and have been reported to be taken by nearly one in three older adults with bipolar disorder or major depression (11). Most individuals who use these supplements do not mention this to their clinicians, putting them at risk for potential drug-supplement interactions with adverse health effects.
Given the clinical importance of nutritional deficiencies and nutritional supplement use, a working group within the International Society for Bipolar Disorders (ISBD) (12) OABD taskforce undertook a systematic review with the following objectives in mind: 1) to synthesize literature evidence on nutritional deficiencies and supplements in OABD; 2) to review the quality of research evidence on nutritional deficiencies and supplements in relation to the epidemiology, pathophysiology, clinical treatment, outcome and wellbeing of OABD, and 3) to make recommendations regarding further research that will promote evidence-based assessment and management of OABD.
2. METHODS
2.1. Eligibility criteria
We followed the preferred reporting items for systematic reviews and meta-analyses (PRISMA) guidelines in conducting the literature search in this review (13, 14). All literature for all years through 2019 was searched. The age cut-off for OABD for eligible studies in this review is 50 years and older based on the knowledge that around 50 years adults already have decreased absorption of nutrients, which is a primary age-related cause of nutritional deficiency (15, 16). Screening for nutrient deficiencies has been recommended in people aged 50 years and older in the United States and elsewhere in the world (15–18). We included all study designs including retrospective, observational, cross-sectional, and intervention trial but excluded case reports. Other inclusion criteria were availability of study full-text in the English language,19 and a clearly described representation of OABD among the study participants.
2.2. Information sources
We searched databases including Medline/PubMed, PsychINFO, EMBASE, Web of Science, Cochrane Register, the Food and Drug Administration (FDA) website, and international clinical trial registries for all years through 2019 for eligible reports. The bibliographies of included studies and relevant reviews were snow-ball searched for additional studies. Study authors were also contacted to request their work where necessary.
2.3. Search strategy
We used a search strategy addressing the following key terms in various permutations: nutrition, older adults, and bipolar disorder combined with OR and AND. Search strings included “diet* OR Diet, Food, and Nutrition OR food OR nutrition* OR “nutritional status” OR “nutritional physiological phenomenon” OR micronutrient* vitamin* OR multivitamin* OR vitamin B OR folate OR B6 OR B12 OR niacin OR vitamin C OR vitamin D OR vitamin E OR calcium OR chromium OR iron OR magnesium OR zinc OR artemisin OR caffeine OR dehydroepiandrosterone OR DHEA OR echinacea OR fish oil OR GABA OR gamma-amino butyric acid OR garlic OR ginkgo OR glucosamine OR karaela OR melatonin OR methylsulfonylmethane OR primrose oil OR probiotics OR S-adenosylmethionine OR St. Johns wort OR tryptophan OR tyrosine OR valerian AND “old age” OR aged OR elder* OR geriatric* OR geriatric psychiatry OR older OR aging OR ageing AND “bipolar disorder” OR manic depressi* OR bipolar depression. Search strings were performed in titles, abstracts, major topic/subject headings, and MeSH headings.
2.4. Study selection
Study selection, review of included studies and data extraction were conducted by Andrew T. Olagunju (ATO), Julie A. Morgan (JAM), Awais Aftab (AA), and supervised by William T. Regenold (WTR). Titles and abstracts were screened independently by at least two authors to shortlist studies for further review. The full texts of the shortlisted studies were reviewed by at least two authors independently according to the inclusion criteria. Inconsistencies about inclusion or exclusion of studies were resolved by discussion between authors or in consultation with WTR or other members of the task force to reach consensus.
2.5. Quality/bias assessment
As studies with different designs and representation of older adults were eligible, the Study Quality Assessment Tools of the National Institutes of Health (NIH) for the assessment of Observational Cohort and Cross-Sectional Studies, Case-Control, and Controlled Intervention Studies were used to assess the quality of included studies.20 We rated individual studies on a range of 12–14 items depending on their designs to produce a comprehensive overview of the risk of bias in individual studies and highlighted relevant overall quality limitations.
2.6. Data collection process
Data items collected from eligible reports included study characteristics, nutrients, main findings and implications for OABD (Table 1). In total, we screened 2280 titles and abstracts to produce a shortlist of 92 potential reports for full-text review. Of these 92 reports, 10 studies were selected for inclusion in the final review. (See Figure 1)
Table 1:
Studies included in the review examining nutrition in older adults with bipolar disorder
| Study | Design | Sample, n, age, females | Nutrients | Main findings |
|---|---|---|---|---|
|
Bell et al., 1990 USA |
R | N=102 (BD=22), age = 60–100 years, females=84 (82%) | Folate, Vitamin B12 | Folate levels correlated negatively with age at onset of psychiatric illness and length of hospitalization. Biochemically interrelated vitamins such as B12 and folate may exert both a separate and a concomitant influence on affect and cognition. |
|
Bell et al., 1990 USA |
R | N=60 (BD=8), mean age= 74.5 (SD= 5.9) years, females=45(75%) |
Folate, Vitamin B12 | There were strong positive correlations for B12 and cognitive measures within the psychotic depression, but not in other diagnostic categories including BD. |
|
Forester et al., 2012 USA |
RCT |
N=10, (BD-All), age range ≥55 years, females=7(70%) | Coenzyme Q10 | Reduction in depression symptom severity during treatment with high-dose CoEnzyme Q10 for OABD |
|
Forester et al. 2015 USA |
RCT | N=19, (BPD=All), age range 56–78 years, gender= females, 9(47.4%) | Coenzyme Q10 | CoQ10 showed antidepressant effects in OABD suggesting that mitochondrial enhancing therapies may play a role in the treatment of affective illness. Specific symptom domains such as lassitude, apparent sadness and poor concentration may preferentially respond to such energy enhancing therapies. |
|
Gronli et al., 2014 Norway |
C | N=95 (BD= 10), age >64 years, females=63.2% | Vitamin D | Compared with controls, Vitamin D deficiency was associated with patient status, but did not differ significantly among across different psychiatric diagnoses |
| Isaac et al., 2015 India |
R | N=60 (BD=3), age > 64 years, females=26(43.3%) | Vitamin B12 | This study suggested Vitamin B12 deficiency could be associated with memory loss in diverse neuropsychiatric populations including OABD. |
|
Keaton et al., 2009 USA |
C | N=100 (BD=50), age ≥50 years (mean age 68.7 ±9.74 years), females=70(70.0%) | Herbal and Nutritional compounds | The use of herbal and nutritional compounds (HNC) was more common in elders with bipolar disorder compared to those with major depression. Most individuals did not discuss HNC use with their physicians. |
|
Lachner et al., 2014 USA |
R | N=374 (BD=50), age>50 years, females=194(51.9%) | Vitamin B12 | No difference in Vitamin B12 level comparing cognitive disorders vs. noncognitive disorders, in older adult psychiatric inpatients. |
|
Lapid et al., 2013 USA |
R | N=171(BD=11), age≥65 years, females=86 (61%) | Vitamin D |
Hypovitaminosis D was common among psychogeriatric patients but there was no association between Vitamin D level with diagnosis and global cognitive function. |
|
Scott et al., 2004 USA |
R | N=34 (BD=16%), mean age=75 years, female=15(44.1%) | Folate Homocysteine Vitamin B12 | Low folate concentration was associated with decreased hippocampal and amygdala volume as well as with brain white matter disease. Elevated homocysteine level was also associated with brain white matter disease. |
BD- bipolar disorders, BCAA- branched-chain amino acid, C-cross-sectional, CC-case control, MTHFR- Methylene tetrahydrofolate reductase, N-total number of sample, NR-not reported, OABD- Older adults with bipolar disorders, R-retrospective chart review, RCT-randomized controlled clinical trial, YMRS- Young Mania Rating Scale
Figure 1.
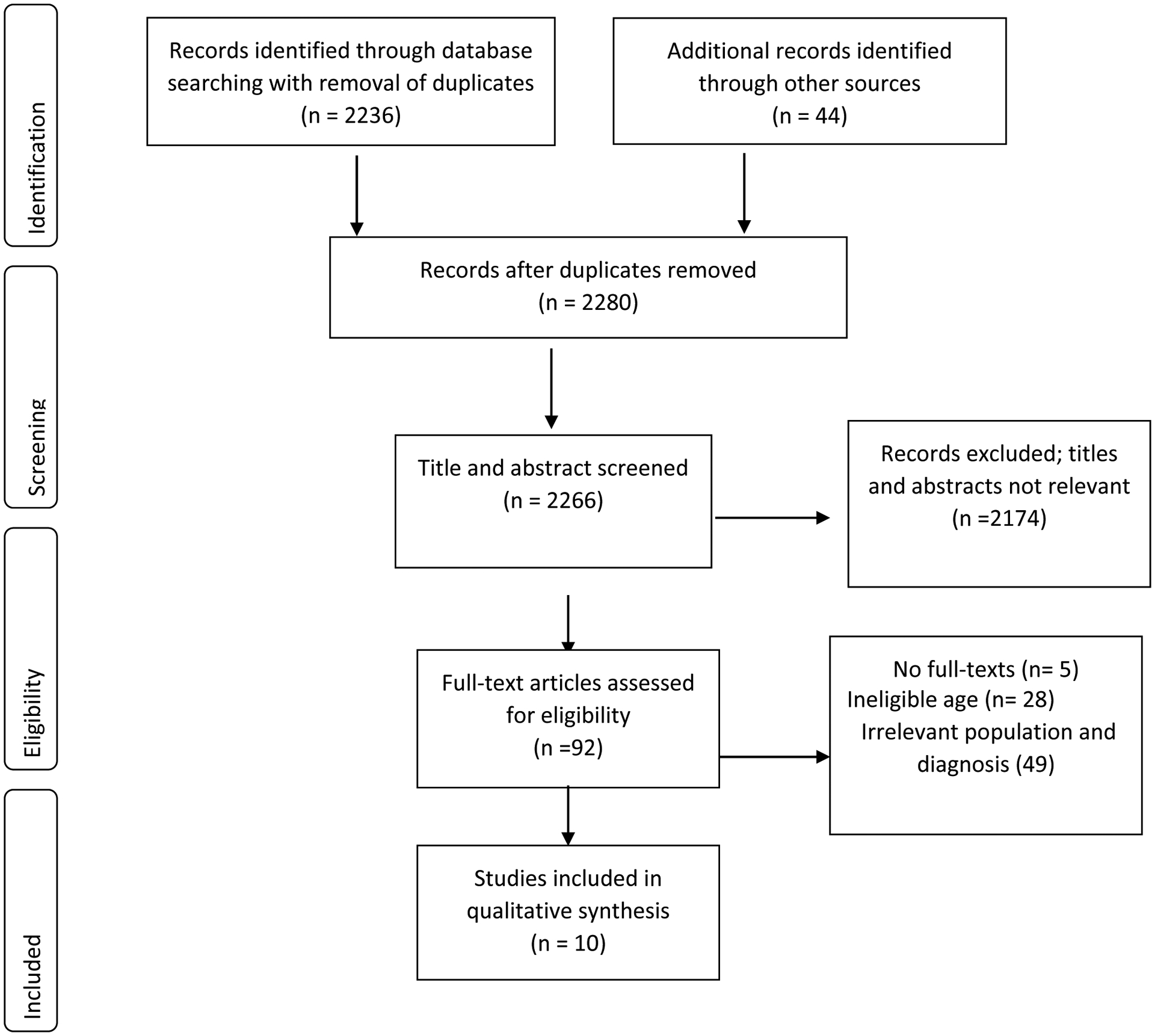
Flow of studies through the systematic review
3.0. RESULTS
3.1. Study characteristics
Ten studies were included in the review (5,7,8,10,11,21–24, 25). The publication dates of these reports spanned three decades from 1990 to 2015. Of the ten studies, six were retrospective chart reviews, two were cross-sectional studies, and the remaining two were clinical trials. Considering the study participants, in two studies all participants were adults diagnosed with BD with a representation of OABD, (21, 22) while in the remaining studies (n=8), participants with BD represented a subset of the total sample. In general, the topic foci in the included studies addressed different aspects of nutrition and nutritional deficiencies, including biochemical, epidemiological and clinical factors, and nutritional supplements in OABD. All were able to specify the sample sizes of OABD in the included studies aged 50 years or older, ranging from 3–50. Follow-up was limited as the majority of the studies reported cross-sectional observations. (See table 1)
3.2. Biochemical, clinical and epidemiological findings on nutrients
Table 2 presents findings on specific nutrients and nutritional supplements covered in the ten studies included in this review. In total, four classes of nutrients including vitamins, minerals, and dietary herbs and nutritional supplements are covered in the studies and described below.
Table 2:
Quality assessment with NIH scale-tool based on study design
| Study | Study Quality Assessment Tools criteria | |||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| SD | 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 | 11 | 12 | 13 | 14 | OR | |
| Bell et al., 1990 | OCCSS | Yes | Yes | NA | Yes | NA | NA | Yes | NA | Yes | NA | Yes | NA | NA | Yes | Fair |
| Bell et al., 1990 | OCCSS | Yes | Yes | Yes | Yes | NA | NA | Yes | NA | Yes | NA | Yes | NA | NA | Yes | Fair |
| Forester et al., 2012 | CIS | No | NA | No | No | No | Yes | No | No | Yes | Yes | Yes | NR | NA | No | Good |
| Forester et al. 2015 | CIS | No | NA | No | No | No | Yes | No | NA | Yes | Yes | Yes | NR | NA | No | Good |
| Gronli et al., 2014 | CCS | Yes | Yes | No | No | Yes | Yes | Yes | No | Yes | NA | No | Yes | - | - | Fair |
| Isaac et al., 2015 | OCCSS | Yes | Yes | Yes | Yes | No | NA | NA | NA | NA | No | Yes | NA | NA | Yes | Fair |
| Keaton et al., 2009 | OCCSS | Yes | Yes | Yes | Yes | No | NA | NA | NA | NA | No | Yes | NA | NA | Yes | Fair |
| Lachner et al., 2014 | OCCSS | Yes | Yes | Yes | Yes | No | NA | NA | NA | NA | No | Yes | NA | NA | Yes | Fair |
| Lapid et al., 2013 | OCCSS | Yes | Yes | Yes | Yes | No | NA | NA | NA | NA | No | Yes | NA | NA | Yes | Fair |
| Scott et al., 2004 | OCCSS | Yes | Yes | Yes | Yes | No | NA | NA | NA | Yes | No | Yes | No | NA | Yes | Fair |
1–14 are quality assessment items/criteria; CCS- case-controlled studies; CD- cannot determine; CIS- controlled intervention studies, NIH- National Institute of Health; NA- not applicable; NR-not reported; OCCSS- observational cohort and cross-sectional studies; OR-overall rating; SD-study design.
3.2.1. Vitamins
Five retrospective chart reviews (5, 7, 8, 10, 23) focused on vitamin B12 and/or folate. Importantly, several reports looked at vitamin B12 deficiency in mixed populations of older adults including patients with BD. The prevalence rates of Vitamin B12 deficiency ranged from 3.7%8 to 71.6%.10 Lower levels of vitamin B12 correlated strongly with memory loss and poor cognitive performance in psychotic depression (8). Approximately one quarter of geriatric clinic outpatients with B12 deficiency had neuropsychiatric symptoms with behavioural disturbance, memory loss and sensorimotor disorders being most common (10). Folate deficiency was reported to be 1.3% in acute geropsychiatric inpatients (7). One study concluded that the biochemically interrelated vitamins, B12 and folate, may exert both separate and concomitant influences on affect and cognition and that poorer vitamin status may contribute to certain geropsychiatric disorders that have later life onset and lack a familial predisposition (7).
Another study investigated the relationships between Vitamin B12 and folate levels as well as homocysteine (HCY) levels on brain volume changes and brain white matter disease in geriatric patients with psychiatric disorders (23). These authors found that, low serum concentrations of folate, but not of B12, were associated with magnetic resonance imaging (MRI) evidence of brain white-matter disease and smaller hippocampus and amygdala brain-volume measures. Elevated HCY, which can result from folate deficiency, was also associated with MRI evidence of brain white matter disease (23). Finally, one study found that among older adult psychiatric inpatients, B12 serum levels and percentages of probable and possible B12 deficiency did not differ significantly between cognitive disorder patients and non-cognitive disorder patients, including OABD, suggesting that it was reasonable to routinely screen older adult psychiatric inpatients for B12 deficiency whether or not cognitive disorder symptoms were present (5). One retrospective chart review study (24) and cross-sectional study(25) addressed vitamin D in OABD. Vitamin D deficiency prevalence rates ranged from 3.7 % to 34.6%. Vitamin D insufficiency (less severe and defined as levels <30 ng/mL) was reported in approximately 75% of psychiatric inpatients in two cross sectional assessments (25). However, no associations were found between vitamin d levels and a screen of global cognitive function or psychiatric diagnoses (24, 25).
3.2.2. Dietary, Herbs and Nutritional Supplements
In one cross sectional study of OABD and older adults with major depression (11), herbs and nutraceutical (HNC) products were ingested by 30% of individuals. About 40% incorrectly believed that HNC products were FDA-regulated at that time. The majority (64%) had not discussed the use of HNC with treating physicians and some preferred to take HNC compared to physician-prescribed psychotropic medications (14–20%). The use of HNC was more common in those with BD (44%) than those with unipolar major depression (16%).
Two open label trials (21, 22) reported significant reduction in the severity of depressive symptoms with high-dose CoEnzyme Q10 in OABD (21). These findings could support a role of therapy targeted at mitochondrial function in affective disorders (22).
3.2.3. Study Quality Assessment
Quality assessment of all included studies (n = 10) was rated fair (n=8) and good (n=2) with risk of bias items included in the tool. The limited representation of OABD represented a risk of bias. Notwithstanding the limitations in the quality of the studies, the reported outcomes on nutritional deficiencies and supplements in OABD were considered the best available evidence for the recommendations made. (See table 2)
4. DISCUSSION
4.1. Clinical implications
Most of the studies reviewed—seven of ten studies (70%) focused on three vitamins—vitamin B12, vitamin D and folate and the consequences of their deficiencies. These studies support a relationship between vitamin B12 deficiency and both affective symptoms and cognitive impairment in OABD (7, 8). They also provide evidence for associations between folate levels and hippocampus and amygdala volumes and brain MRI white matter hyper- intensities (WMHs) in older adult psychiatric patients (23). Vitamin D deficiency was reported to be common in older adult psychiatric patients. Generally, vitamin deficiencies did not differ across psychiatric disorders, indicating that screening for these deficiencies in older adults should not be limited to particular diagnostic groups such as cognitive disorders. Evidence for a therapeutic role for vitamin supplementation was limited. There is some evidence for a benefit from folate supplementation for OABD taking lithium.
Tissue levels of homocysteine can be elevated due to vitamin B12 or folate deficiencies. Four studies examined the relationships among vitamin B12, folate and homocysteine levels and their associations with psychiatric symptoms or evidence of brain disease (23). These studies support an inverse relationship between B12 and folate blood levels on one hand and homocysteine blood levels on the other in individuals with bipolar depression. They also suggest that HCY blood levels are elevated in individuals with BD and support an association between elevated levels and impairment in executive function and in the extent of brain WMHs. These studies provide evidence for measuring HCY blood levels along with vitamin B12 and folate levels in OABD.
Studies of diet and nutritional supplements revealed that supplement use is very common among OABD (11). Furthermore, supplement use was typically not discussed with health providers (11).
Clinical trials of adjunctive nutritional supplements found preliminary evidence for a benefit from Coenzyme Q10 on depressive symptoms in OABD (21, 22). The positive results in these supplement studies require replication in a larger group of OABD prior to their consideration for routine clinical use in OABD.
4.2. Study limitations
There are several study limitations of this review. The included studies were not homogenous nor entirely focused on OABD. Of the ten articles included in the review, only two reported study results exclusively from a sample or population of OABD. By expanding our inclusion criteria to capture studies with some significant number of OABD participants, we were able to report a more comprehensive review of studies. The majority of these studies were retrospective chart reviews and cross-sectional studies, while the number of RCTs was limited. Consequently, inferences on causal relationships between the nutrients studied and the pathophysiology or treatment of OABD is limited. Furthermore, none of the included studies (n=10) looked at the potential contributions of gender and the type of bipolar disorder (I and II) to their study findings despite the clinical importance of these two factors (26).
4.3. Conclusions
Scientific literature on nutrition and nutritional supplements in OABD exists but is limited in both quantity and quality. However, we can draw several conclusions from this review. First, because older adults are prone to nutritional deficits and suffer from several vitamin deficiencies that influence cognition and affective symptoms, there should be consideration given to better research to develop clinical guidelines for routine screening for deficiencies of vitamin B12, folate and vitamin D in OABD. Second, screening for elevated blood levels of HCY that result from vitamin deficiencies should also be considered. Notably there is need for well-designed and powered clinical trials to examine the effects of nutritional interventions including dietary questionnaires, vitamin deficiency screening, HCY blood level screening, and adjunctive nutrient supplementation in OABD.
Highlights.
Nutrition is critical to physical health and general well-being of older adults with bipolar disoder (OABD), however nutritional deficiencies is common albeit varies for specific nutrients.
While there is evidence for the benefits of nutritional interventions on affective, cognitive and overall outcome in OABD, the quality of the evidence is limited.
Findings in this review underscore the need for high quality studies for development of evidence-based guideline for assessing and supplementing nutrition in OABD.
ACKNOWLEDGEMENTS
The authors thank the International Society for Bipolar Disorders executives, and staff for their support for this task force. Our gratitude also goes to Ms Maureen Bell of the University of Adelaide library for her support and advice.
DISCLOSURE INFORMATION
Financial Disclosure information for Martha Sajatovic MD are stated below:
Research grants within past 3 years: Otsuka, Alkermes, Janssen, International Society for Bipolar Disorders, Reuter Foundation, Woodruff Foundation, Reinberger Foundation, National Institutes of Health (NIH), Centers for Disease Control and Prevention (CDC)
Consultant: Alkermes, Bracket, Otsuka, Janssen, Neurocrine, Health Analytics
Royalties: Springer Press, Johns Hopkins University Press, Oxford Press, UpToDate
CME activities: American Physician’s Institute, MCM Education, CMEology, Potomac Center for Medical Education, Global Medical Education, Creative Educational Concepts
Financial Disclosure information: Dr. Gatchel reports other relevant financial activities from Merck, grants from Alzheimer’s Association, grants from BrightFocus Foundation, outside the submitted work.
Footnotes
CONFLICTS OF INTEREST
The authors declare no conflicts of interest to the content of the manuscript.
REFERENCES
- 1.GBD 2017 Diet Collaborators. Health effects of dietary risks in 195 countries, 1990–2017: a systematic analysis for the Global Burden of Disease Study 2017. Lancet (London, England). 2019;393(10184):1958–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.GBD 2016 Risk Factors Collaborators. Global, regional, and national comparative risk assessment of 84 behavioural, environmental and occupational, and metabolic risks or clusters of risks, 1990–2016: a systematic analysis for the Global Burden of Disease Study 2016. Lancet (London, England). 2017;390(10100):1345–422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.GBD 2017 Risk Factor Collaborators. Global, regional, and national comparative risk assessment of 84 behavioural, environmental and occupational, and metabolic risks or clusters of risks for 195 countries and territories, 1990–2017: a systematic analysis for the Global Burden of Disease Study 2017. Lancet (London, England). 2018;392(10159):1923–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Schiffman SS. Intensification of sensory properties of foods for the elderly. J Nutr. 2000;130(4S Suppl):927S–30S. [DOI] [PubMed] [Google Scholar]
- 5.Lachner C, Martin C, John D, Nekkalapu S, Sasan A, Steinle N, et al. Older adult psychiatric inpatients with non-cognitive disorders should be screened for vitamin B12 deficiency. Journal of Nutrition, Health and Aging. 2014;18(2):209–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Langan RC, Goodbred AJ. Vitamin B12 Deficiency: Recognition and Management. Am Fam Physician. 2017;96(6):384–9. [PubMed] [Google Scholar]
- 7.Bell IR, Edman JS, Marby DW, Satlin A, Dreier T, Liptzin B, et al. Vitamin B12 and folate status in acute geropsychiatric inpatients: affective and cognitive characteristics of a vitamin nondeficient population. Biol Psychiatry. 1990;27(2):125–37. [DOI] [PubMed] [Google Scholar]
- 8.Bell IR, Edman JS, Miller J, Hebben N, Linn RT, Ray D, et al. Relationship of normal serum vitamin B12 and folate levels to cognitive test performance in subtypes of geriatric major depression. J Geriatr Psychiatry Neurol. 1990;3(2):98–105. [DOI] [PubMed] [Google Scholar]
- 9.Coppen A, Chaudhry S, Swade C. Folic acid enhances lithium prophylaxis. J Affect Disord. 1986;10(1):9–13. [DOI] [PubMed] [Google Scholar]
- 10.Issac TG, Soundarya S, Christopher R, Chandra SR. Vitamin B12 deficiency: an important reversible co-morbidity in neuropsychiatric manifestations. Indian journal of psychological medicine. 2015;37(1):26–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Keaton D, Lamkin N, Cassidy KA, Meyer WJ, Ignacio RV, Aulakh L, et al. Utilization of herbal and nutritional compounds among older adults with bipolar disorder and with major depression. Int J Geriatr Psychiatry. 2009;24(10):1087–93. [DOI] [PubMed] [Google Scholar]
- 12.(ISBD) ISoBD. https://www.isbd.org/active-task-forces. 2019.
- 13.Liberati A, Altman DG, Tetzlaff J, Mulrow C, Gotzsche PC, Ioannidis JP, et al. The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate health care interventions: explanation and elaboration. Annals of internal medicine. 2009;151(4):W65–94. [DOI] [PubMed] [Google Scholar]
- 14.Moher D, Liberati A, Tetzlaff J, Altman DG. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. Annals of internal medicine. 2009;151(4):264–9, w64. [DOI] [PubMed] [Google Scholar]
- 15.Hughes CF, Ward M, Hoey L, McNulty H. Vitamin B12 and ageing: current issues and interaction with folate. Ann Clin Biochem. 2013. July;50(Pt 4):315–29. [DOI] [PubMed] [Google Scholar]
- 16.Langan RC, Goodbred AJ. Vitamin B12 Deficiency: Recognition and Management. Am Fam Physician. 2017. September 15;96(6):384–389. [PubMed] [Google Scholar]
- 17.Government of South Wales, Australia: A Practical Guide to Effective Engagement with Older People. Retrieved on 27/01/2020 from: https://www.sahealth.sa.gov.au/wps/wcm/connect/efc56a004efc69f1b7ccf79ea2e2f365/Better+Together+-+A+Practical+Guide+to+Effective+Engagement+with+Older+People.pdf?MOD=AJPERES&CACHEID=ROOTWORKSPACE-efc56a004efc69f1b7ccf79ea2e2f365-mMADUnH
- 18.Institute of Medicine Panel on folate, other B vitamins and choline. Dietary Reference Intake: Thiamin, Riboflavin, Niacin, Vitamin B6, Vitamin B12, Pantothenic Acid, Biotin and Choline Washington DC: National Academy Press, 1998, pp. 306–56. [PubMed] [Google Scholar]
- 19.Bauer M, Glenn T, Conell J, Rasgon N, Marsh W, Sagduyu K, et al. Common use of dietary supplements for bipolar disorder: a naturalistic, self-reported study. Int J Bipolar Disord. 2015;3(1):29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.NIH National Heart LaBIS, quality assessment tools [cited 2017 M. Available from: https://wwwnhlbinihgov/health-pro/guidelines/in-develop/cardiovascular-riskreduction/tools. 2017
- 21.Forester BP, Harper DG, Georgakas J, Ravichandran C, Madurai N, Cohen BM. Antidepressant effects of open label treatment with coenzyme Q10 in geriatric bipolar depression. Journal of clinical psychopharmacology. 2015;35(3):338–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Forester BP, Zuo CS, Ravichandran C, Harper DG, Du F, Kim S, et al. Coenzyme Q10 effects on creatine kinase activity and mood in geriatric bipolar depression. J Geriatr Psychiatry Neurol. 2012;25(1):43–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Scott TM, Tucker KL, Bhadelia A, Benjamin B, Patz S, Bhadelia R, et al. Homocysteine and B vitamins relate to brain volume and white-matter changes in geriatric patients with psychiatric disorders. Am J Geriatr Psychiatry. 2004;12(6):631–8. [DOI] [PubMed] [Google Scholar]
- 24.Lapid MI, Drake MT, Geske JR, Mundis CB, Hegard TL, Kung S, et al. Hypovitaminosis D in psychogeriatric inpatients. J Nutr Health Aging. 2013;17(3):231–4. [DOI] [PubMed] [Google Scholar]
- 25.Gronli O, Kvamme JM, Jorde R, Wynn R. Vitamin D deficiency is common in psychogeriatric patients, independent of diagnosis. BMC Psychiatry. 2014;14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Müller JK, Leweke FM. Bipolar disorder: clinical overview. Med Monatsschr Pharm. 2016. September;39(9):363–9. [PubMed] [Google Scholar]


