Key Points
Question
For preterm infants with respiratory distress syndrome supported with continuous positive airway pressure (CPAP), does selective administration of surfactant via a thin catheter at a low oxygenation threshold improve survival without bronchopulmonary dysplasia compared with continuation of CPAP?
Findings
In this randomized clinical trial including 485 infants with a gestational age of 25 to 28 weeks and respiratory distress syndrome, minimally invasive surfactant therapy delivered via a thin catheter at a fraction of inspired oxygen threshold of 0.30 or greater within 6 hours of birth compared with sham (control) treatment resulted in attainment of the composite outcome of death or bronchopulmonary dysplasia in 43.6% vs 49.6%, respectively, of infants in the 2 groups. This difference was not statistically significant.
Meaning
Among preterm infants with respiratory distress syndrome, surfactant administration via a thin catheter did not result in a statistically significant reduction in the likelihood of the composite outcome of death or bronchopulmonary dysplasia.
Abstract
Importance
The benefits of surfactant administration via a thin catheter (minimally invasive surfactant therapy [MIST]) in preterm infants with respiratory distress syndrome are uncertain.
Objective
To examine the effect of selective application of MIST at a low fraction of inspired oxygen threshold on survival without bronchopulmonary dysplasia (BPD).
Design, Setting, and Participants
Randomized clinical trial including 485 preterm infants with a gestational age of 25 to 28 weeks who were supported with continuous positive airway pressure (CPAP) and required a fraction of inspired oxygen of 0.30 or greater within 6 hours of birth. The trial was conducted at 33 tertiary-level neonatal intensive care units around the world, with blinding of the clinicians and outcome assessors. Enrollment took place between December 16, 2011, and March 26, 2020; follow-up was completed on December 2, 2020.
Interventions
Infants were randomized to the MIST group (n = 241) and received exogenous surfactant (200 mg/kg of poractant alfa) via a thin catheter or to the control group (n = 244) and received a sham (control) treatment; CPAP was continued thereafter in both groups unless specified intubation criteria were met.
Main Outcomes and Measures
The primary outcome was the composite of death or physiological BPD assessed at 36 weeks’ postmenstrual age. The components of the primary outcome (death prior to 36 weeks’ postmenstrual age and BPD at 36 weeks’ postmenstrual age) also were considered separately.
Results
Among the 485 infants randomized (median gestational age, 27.3 weeks; 241 [49.7%] female), all completed follow-up. Death or BPD occurred in 105 infants (43.6%) in the MIST group and 121 (49.6%) in the control group (risk difference [RD], −6.3% [95% CI, −14.2% to 1.6%]; relative risk [RR], 0.87 [95% CI, 0.74 to 1.03]; P = .10). Incidence of death before 36 weeks’ postmenstrual age did not differ significantly between groups (24 [10.0%] in MIST vs 19 [7.8%] in control; RD, 2.1% [95% CI, −3.6% to 7.8%]; RR, 1.27 [95% CI, 0.63 to 2.57]; P = .51), but incidence of BPD in survivors to 36 weeks’ postmenstrual age was lower in the MIST group (81/217 [37.3%] vs 102/225 [45.3%] in the control group; RD, −7.8% [95% CI, −14.9% to −0.7%]; RR, 0.83 [95% CI, 0.70 to 0.98]; P = .03). Serious adverse events occurred in 10.3% of infants in the MIST group and 11.1% in the control group.
Conclusions and Relevance
Among preterm infants with respiratory distress syndrome supported with CPAP, minimally invasive surfactant therapy compared with sham (control) treatment did not significantly reduce the incidence of the composite outcome of death or bronchopulmonary dysplasia at 36 weeks’ postmenstrual age. However, given the statistical uncertainty reflected in the 95% CI, a clinically important effect cannot be excluded.
Trial Registration
anzctr.org.au Identifier: ACTRN12611000916943
This randomized clinical trial includes preterm infants with a gestational age of 25 to 28 weeks who were supported with continuous positive airway pressure and compares the effect of selective application of minimally invasive surfactant therapy at a low fraction of inspired oxygen threshold vs control treatment on survival without bronchopulmonary dysplasia.
Introduction
For the newly born preterm infant, published guidelines recommend continuous positive airway pressure (CPAP) for initial respiratory support rather than intubation and ventilation,1,2 with the expectation of equivalent or better outcomes, including a reduction in the risk of bronchopulmonary dysplasia, the chronic disease of the preterm lung.3 For the composite outcome of death or bronchopulmonary dysplasia, a meta-analysis of data from clinical trials4,5,6,7 in preterm infants with a gestational age of less than 30 weeks showed an association favoring initial CPAP with a relative risk (RR) of 0.91 (95% CI, 0.84-0.99).8 In these trials, exogenous surfactant, which is a proven therapy for respiratory distress syndrome (RDS),9 was only administered in the CPAP group if relatively high thresholds (0.40-0.60) for fraction of inspired oxygen (Fio2) were exceeded.
It remains unclear whether the benefit of initial CPAP on respiratory outcomes in these trials would have been greater if, in the CPAP group, surfactant had been selectively administered at a lower Fio2 threshold in infants with more prominent features of RDS. CPAP alone often fails to provide sufficient support in such cases,10,11 and in a large nonrandomized study12 this pathway of delayed intubation was associated with deleterious consequences, including an increased risk of death or bronchopulmonary dysplasia.
Without an endotracheal tube, preterm infants supported with CPAP lack the usual conduit for instillation of exogenous surfactant, thus invoking the dilemma of how to administer surfactant therapy to those with features of RDS.9 Among numerous less invasive alternatives, intratracheal administration of surfactant via a thin catheter has emerged as a practicable and propitious solution,13,14,15 but the benefits of this approach, including in relation to death or bronchopulmonary dysplasia, remain uncertain.
The OPTIMIST-A trial (one of cOllaborative Paired Trials Investigating Minimally Invasive Surfactant Therapy) tested the hypothesis that administration of surfactant via a thin catheter (minimally invasive surfactant therapy [MIST]) would reduce the incidence of the composite outcome of death or bronchopulmonary dysplasia or its components.
Methods
Study Design and Oversight
The trial was an investigator-initiated, international, multicenter randomized clinical trial of a blinded intervention conducted at 33 tertiary-level neonatal intensive care units in Australia, Canada, Israel, New Zealand, Qatar, Singapore, Slovenia, the Netherlands, Turkey, the UK, and the US. The human research ethics committees of all participating centers approved the trial protocol16 (Supplement 1). Prospective written parental consent was obtained prenatally or postnatally. An independent data and safety monitoring committee reviewed the interim analyses for safety and efficacy. The statistical analysis followed the statistical analysis plan (Supplement 2), in which the method of analysis outlined in the trial protocol was expanded upon, including nomination of the components of the primary outcome as end points, and identification of a group of key clinical and safety outcomes from among the secondary outcomes.
Participants
Infants were considered for inclusion if they (1) were within the gestational age range of 25 weeks 0 days and 28 weeks 6 days, (2) had been inborn at a study center and admitted to the neonatal intensive care unit, and (3) were supported with CPAP or noninvasive positive pressure ventilation for respiratory insufficiency without prior intubation. Infants were eligible if supported with a CPAP level of 5 cm H2O to 8 cm H2O and requiring Fio2 of 0.30 or greater within the first 6 hours of life. Prior administration of caffeine was expected as part of standard management,17 but not protocolized. Infants were ineligible if, in the judgment of treating clinicians, there was an imminent need for intubation, the cause of the respiratory insufficiency was not RDS, or a serious congenital anomaly was present.
Randomization and Masking
Randomization and the intervention were undertaken by a treatment team comprising staff not currently involved in the infant’s care, including at least 1 proceduralist trained in the technique of MIST and an assistant (neonatal nurse or respiratory therapist).
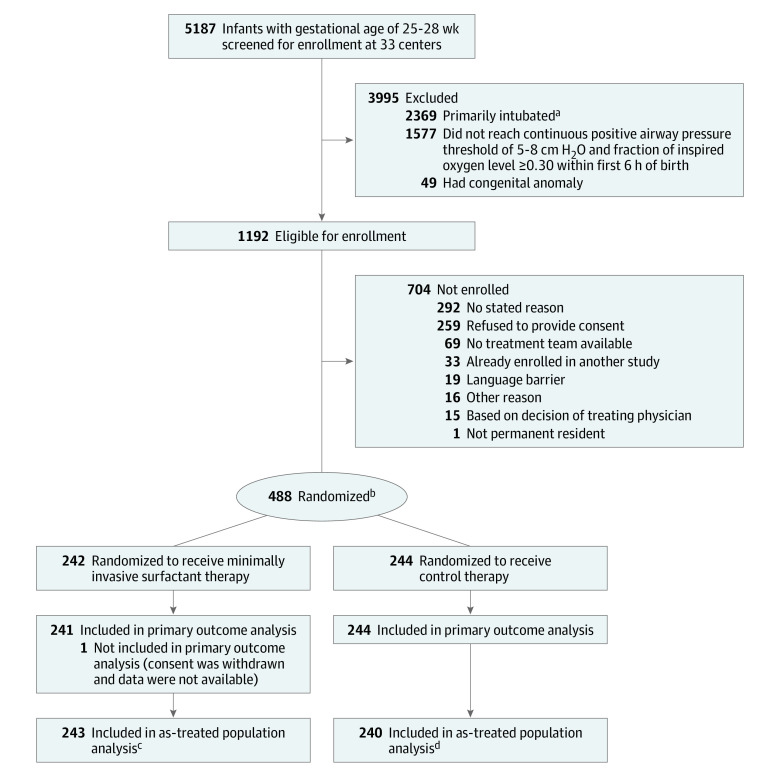
Infants were randomized 1:1 to the MIST group or the sham treatment (control) group via a computer-generated code linked to a corresponding opaque sealed envelope (Figure). The randomization sequence used permuted block sizes of 2, 4, or 6 with stratification by study center and gestational age. Multiple births were randomized independently.
Figure. Screening, Enrollment, Randomization, and Follow-up in the OPTIMIST-A Randomized Clinical Trial.
aIntubated in the delivery room or shortly after arrival in the neonatal intensive care unit; no continuous positive airway pressure administered.
bTwo infants were excluded after randomization (1 randomization failure and 1 ineligible because required fraction of inspired oxygen was 0.24).
cIncludes 6 infants who received active therapy in error (2 incorrect randomization envelope opened and 4 treatment allocation inadvertently not followed). Excludes 4 infants who received control therapy (2 were randomized to active therapy but required intubation immediately after randomization, 1 for whom surfactant instillation catheter was not available, and 1 inadvertently treated as control).
dIncludes 2 infants who received control therapy in error (randomized to active therapy but surfactant instillation catheter unavailable and treatment allocation inadvertently not followed). Excludes 6 infants who received active therapy in error (2 incorrect randomization envelope opened and 4 treatment allocation inadvertently not followed).
Clinicians and parents were blinded to the study intervention with screening of the infant’s bedspace from external view. Central physiological monitors were disconnected whenever possible and a study oximeter was used to display heart rate and oxygen saturation.
Interventions
The MIST intervention was administered using the Hobart method as described previously15,18 (Video; a longer version is also available at https://youtu.be/wAkNATfH9S0). Administration of a sedating premedication was not done; intraoral 25% sucrose, intravenous atropine, or both, were allowed. Surfactant was instilled using a 16-gauge vascular catheter (Angiocath, Becton Dickinson) or a proprietary catheter (LISAcath, Chiesi Farmaceutici), marked to indicate the correct insertion depth beyond the vocal cords (1.5 cm for gestational age <27 weeks; 2 cm otherwise). Via direct laryngoscopy, the catheter was inserted into the trachea (maximum of 3 attempts) and surfactant (200 mg/kg of poractant alfa; Chiesi Farmaceutici) was administered in 3 to 4 aliquots, with a 10-second pause between each. CPAP was applied throughout, aiming to optimize surfactant dispersion from the trachea promoted by spontaneous breathing. Infants with apnea initially received tactile stimulation. Positive pressure inflations by mask were used for refractory apnea, persistent hypoxemia, or bradycardia.
Video. Demonstration of the Hobart Method of Minimally Invasive Surfactant Therapy in a Resuscitation Manikin Equivalent to a 27-Week Gestation 1-kg Preterm Infant.
This video demonstrates surfactant instillation via a thin catheter (the Hobart method) in a preterm infant manikin. This is the technique of minimally invasive surfactant therapy (MIST) that was used as the active therapy in this randomized clinical trial. A longer version of the video is available at https://youtu.be/wAkNATfH9S0.
Infants in the control group received a sham intervention consisting only of transient repositioning. The interactions of the treatment team and the duration of the procedure mimicked that of the MIST procedure.
After the study intervention, infants were returned to their original position, standard monitoring was restored, and care resumed by treating clinicians. Three members of the staff, including the bedside nurse, completed a brief questionnaire that asked whether they could discern which intervention the infant had received.
Infants in both groups were intubated if requiring Fio2 of 0.45 or greater (or by clinician discretion when requiring Fio2 >0.40) or if there was severe or recurrent apnea or persistent respiratory acidosis. Once intubated, surfactant could be administered according to clinical judgement. Surfactant administration by thin catheter was not allowed in either group after the intervention. Other aspects of clinical management were not protocolized.
Outcomes
The primary outcome was the composite of death prior to 36 weeks’ postmenstrual age or physiological bronchopulmonary dysplasia19 assessed at 36 weeks’ postmenstrual age by blinded study personnel. Infants were assigned a diagnosis of bronchopulmonary dysplasia if at 36 weeks’ postmenstrual age they were: (1) supported with mechanical ventilation, CPAP, or high-flow nasal cannula therapy (at a rate of ≥2 L/min), or (2) receiving supplemental oxygen with actual or effective Fio2 of 0.30 or greater, or (3) receiving oxygen with Fio2 less than 0.30 and did not pass an air trial or no air trial was done per physician request.
The secondary outcomes included the components of the primary composite outcome and 6 key clinical and safety outcomes that were prespecified based on their importance in relation to early respiratory management, in-hospital care, and potential effect on long-term outcome. The outcomes were pneumothorax requiring drainage, need for intubation within 72 hours of birth, grade III or IV intraventricular hemorrhage,20 the composite of death during hospitalization or major morbidity, and each of these considered separately. Major morbidity was defined as any of the following: intraventricular hemorrhage grade III or IV; cystic periventricular leukomalacia; retinopathy of prematurity stage 3 or greater; or physiological bronchopulmonary dysplasia.21 There were 15 additional binary secondary outcomes and 7 continuous secondary outcomes.
Additional protocolized secondary outcomes not reported herein include length of stay in the intensive care unit (variably defined worldwide), hospital billings and calculated cost of hospitalization, and outcomes beyond the first hospitalization, including death or major disability at 2 years’ postmenstrual age and respiratory morbidity during the first 2 years. Exploratory procedural and safety outcomes related to the MIST intervention were ascertained as were adverse events. Further details appear in the statistical analysis plan (Supplement 2). All deaths and unexpected serious adverse events were reported to local ethics committees and the trial management center within 5 days and were reviewed by the data and safety monitoring committee.
Sample Size Calculation
The study aimed to recruit 606 infants, providing 90% power to detect an absolute risk reduction of 13% (RR reduction of 33%) in the incidence of death or bronchopulmonary dysplasia, which for the control group was projected to be 38% based on observational data.11
Statistical Analysis
For the primary outcome and its components, the RR (with 95% CI) comparing active treatment with control was estimated according to randomization group using a generalized linear model (GLM), adjusting for gestational age strata and incorporating a cluster-robust SE calculation to account for clustering by study site using Stata version 16 (StataCorp). The GLM used the modified Poisson approach of Zou22 with a logarithmic link function. An extended GLM additionally adjusted for covariates (birth weight <10th percentile, sex, mode of delivery, plurality, antenatal glucocorticoid exposure, and 5-minute Apgar score) known to influence the primary outcome.12
Sensitivity analyses for the primary outcome and its components were conducted for the as-treated and per-protocol populations. For other outcomes, the RRs or mean differences were estimated using GLMs. The median difference using quantile regression was estimated as appropriate. The treatment effect for dichotomous outcomes also was estimated post hoc as the risk difference (RD), using a GLM approach with Gaussian error distribution (to avert convergence difficulties with low prevalence outcomes) and linear link function. No adjustments for multiple comparisons were made so the findings for the analyses of the secondary outcomes should be interpreted as exploratory.
Preplanned exploratory subgroup analyses were performed for the primary and other prespecified outcomes by gestational age strata, Fio2 required at randomization (0.30-0.35 and >0.35), and geographic region, assessing any interaction between subgroup and treatment assignment in the GLM. The frequency of missing data was low and available case analysis was used. Two-tailed P values <.05 were labeled as significant.
Results
Infants were enrolled between December 16, 2011, and March 26, 2020. Four planned data and safety monitoring committee interim analyses of safety and efficacy (blinded to the study group) recommended that recruitment continue. Recruitment ceased on March 26, 2020, after research activities were put in abeyance indefinitely at most participating centers due to the COVID-19 pandemic. The trial steering committee, unaware of the interim study results, ceased enrollment at this juncture with 488 infants recruited. Patient follow-up (to hospital discharge) was completed on December 2, 2020, and 1 infant in the MIST group remained in the hospital.
Study Infants
A total of 5187 infants were screened at 33 participating centers, 488 were randomized, and data from 485 infants were included in the primary analysis (Figure). These infants had a median gestational age of 27.3 weeks (IQR, 26.4-28.1 weeks) and 241 of 485 infants (49.7%) were female. Baseline characteristics of the study infants overall were similar between the groups (Table 1); however, the frequency of male sex, incomplete or no steroid exposure, and multiple birth within the gestational age stratum of 25 to 26 weeks were each 12% to 14% higher in the MIST group (eTable 1 in Supplement 3).
Table 1. Baseline Characteristics.
| Minimally invasive surfactant therapy (n = 241)a |
Control treatment (n = 244)a |
|
|---|---|---|
| Demographic characteristics | ||
| Gestational age, median (IQR), wk | 27.3 (26.3-28.1) | 27.3 (26.4-28.0) |
| Birth weight | ||
| Mean (SD), g | 929 (221) | 928 (216) |
| <10th Percentile | 34 (14.1) | 34 (13.9) |
| Sex | ||
| Female | 116 (48.1) | 125 (51.2) |
| Male | 125 (51.9) | 119 (48.8) |
| Plurality and birth order | ||
| Singleton | 150 (62.2) | 169 (69.3) |
| First of multiples | 41 (17.0) | 32 (13.1) |
| Second or subsequent multiple | 50 (20.7) | 43 (17.6) |
| Peripartum details | ||
| Exposure to antenatal glucocorticoids, doses prior to delivery | ||
| ≥2 | 156 (64.7) | 175 (71.7) |
| 1 | 63 (26.1) | 50 (20.5) |
| 0 | 22 (9.1) | 19 (7.8) |
| Delivery mode | ||
| Vaginal | 45 (18.7) | 52 (21.3) |
| Cesarean with labor | 96 (39.8) | 82 (33.6) |
| Cesarean without labor | 100 (41.5) | 110 (45.1) |
| Apgar score at 5 min, median (IQR)b | 8 (7-9) | 8 (7-9) |
| Clinical state at randomization | ||
| Age, mean (SD), h | 2.9 (1.4) | 2.8 (1.5) |
| Continuous positive airway pressure level required at randomization, median (IQR), cm H2O | 7 (6-8) | 7 (6-8) |
| Fraction of inspired oxygen | ||
| Level required at randomization, median (IQR) | 0.35 (0.30-0.39) | 0.35 (0.30-0.40) |
| Level required ≤0.35 | 152 (63.1) | 150 (61.5) |
Data are expressed as No. (%) unless otherwise indicated.
Indicates success of transition at birth. The score range is 0 to 10. A score of 0 to 2 is given for each of the following: heart rate, respiratory effect, reflex irritability, muscle tone, and skin color. An Apgar score greater than 7 at 5 minutes after birth generally indicates a satisfactory transition for a preterm infant.
Primary Outcome and Components of the Primary Outcome
Death or bronchopulmonary dysplasia assessed at 36 weeks’ postmenstrual age occurred in 105 infants (43.6%) in the MIST group and in 121 infants (49.6%) in the control group (RD, −6.3% [95% CI, −14.2% to 1.6%]; RR, 0.87 [95% CI, 0.74 to 1.03]; P = .10) (Table 2). Death occurred prior to 36 weeks’ postmenstrual age in 24 infants (10.0%) in the MIST group and in 19 infants (7.8%) in the control group (RD, 2.1% [95% CI, −3.6% to 7.8%]; RR, 1.27 [95% CI, 0.63 to 2.57]; P = .51). The incidence of bronchopulmonary dysplasia in survivors to 36 weeks’ postmenstrual age was reduced in the MIST group (81/217 [37.3%] vs 102/225 [45.3%] in the control group; RD, −7.8% [95% CI, −14.9% to −0.7%]; RR, 0.83 [95% CI, 0.70 to 0.98]; P = .03). This pattern of findings for the primary outcome and components of the primary outcome was unchanged in the analysis using the extended GLM with additional covariates (eTable 2 in Supplement 3) and in the as-treated and per-protocol populations (eTable 3 in Supplement 3).
Table 2. Primary Outcome Analysis.
| No./total (%) | Risk difference, % (95% CI)a |
Relative risk (95% CI)a |
P value | ||
|---|---|---|---|---|---|
| Minimally invasive surfactant therapy | Control treatment | ||||
| Death or bronchopulmonary dysplasiab | 105/241 (43.6) | 121/244 (49.6) | −6.3 (−14.2 to 1.6) | 0.87 (0.74 to 1.03) | .10 |
| Death prior to 36 weeks’ postmenstrual age | 24/241 (10.0) | 19/244 (7.8) | 2.1 (−3.6 to 7.8) | 1.27 (0.63 to 2.57) | .51 |
| Bronchopulmonary dysplasia in survivors to 36 weeks’ postmenstrual ageb | 81/217 (37.3) | 102/225 (45.3) | −7.8 (−14.9 to −0.7) | 0.83 (0.70 to 0.98) | .03 |
Adjusted for gestational strata.
Bronchopulmonary dysplasia (physiological definition) was assessed at 36 weeks’ postmenstrual age and was defined as (1) requiring mechanical respiratory support (mechanical ventilation, continuous positive airway pressure, or high-flow nasal cannula therapy ≥2 L/min); (2) if not requiring mechanical respiratory support, requiring oxygen with actual or effective fraction of inspired oxygen (Fio2) of 0.30 or greater; (3) receiving oxygen with Fio2 less than 0.30 and failed air trial; or (4) receiving oxygen with Fio2 less than 0.30 and air trial not performed per physician request (n = 2 infants; 1 infant in each treatment group).
Secondary Outcomes
Among the 6 key clinical and safety outcomes, the need for intubation within 72 hours of birth was reduced in the MIST group (36.5% vs 72.1% in the control group; RD, −35.8% [95% CI, −47.2% to −24.4%]; RR, 0.50 [95% CI, 0.40 to 0.64]; P < .001), as was the incidence of pneumothorax requiring drainage (4.6% vs 10.2%; RD, −5.8% [95% CI, −10.2% to −1.4%]; RR, 0.44 [95% CI, 0.25 to 0.78]; P = .005) (Table 3). The cumulative proportion of infants requiring intubation in the 2 groups diverged during the first 24 hours (eFigure 1 in Supplement 3). The incidence of other key clinical and safety outcomes was not statistically significantly different between groups.
Table 3. Prespecified Key Clinical and Safety Outcomes.
| Outcomea | No./total (%) | Risk difference, % (95% CI)b |
Relative risk (95% CI)b |
P value | |
|---|---|---|---|---|---|
| Minimally invasive surfactant therapy | Control treatment | ||||
| Pneumothorax requiring drainage | 11/241 (4.6) | 25/244 (10.2) | −5.8 (−10.2 to −1.4) | 0.44 (0.25 to 0.78) | .005 |
| Need for intubation within 72 h of birth | 88/241 (36.5) | 176/244 (72.1) | −35.8 (−47.2 to −24.4) | 0.50 (0.40 to 0.64) | <.001 |
| Intraventricular hemorrhage grade III or IV | 18/241 (7.5) | 24/244 (9.8) | −2.4 (−6.3 to 1.5) | 0.75 (0.48 to 1.19) | .23 |
| Death or major morbidity during first hospitalizationc | 116/241 (48.1) | 136/244 (55.7) | −7.9 (−18.6 to 2.7) | 0.86 (0.70 to 1.05) | .14 |
| Death during first hospitalization | 28/241 (11.6) | 20/244 (8.2) | 3.3 (−2.2 to 8.9) | 1.41 (0.73 to 2.69) | .30 |
| Major morbidity during first hospitalization in survivorsc | 88/213 (41.3) | 116/224 (51.8) | −10.3 (−20.8 to 0.2) | 0.80 (0.64 to 1.00) | .05 |
The outcomes are listed in likely chronological order of appearance and were prespecified as key clinical and safety outcomes based on their primacy in relation to early respiratory management, in-hospital care, and potential effect on long-term outcome in the gestational age range in this study.
Adjusted for gestational strata.
Major morbidity includes intraventricular hemorrhage grade III or IV, cystic periventricular leukomalacia, retinopathy of prematurity greater than stage II (assessed throughout hospitalization), or physiological bronchopulmonary dysplasia at 36 weeks’ postmenstrual age.21
Significant differences favoring the MIST group were noted for 4 of 15 binary secondary outcomes (Table 4). Treatment with MIST was associated with a reduced requirement for intubation at any time (54.8% vs 81.1% in the control group; RD, −26.7% [95% CI, −39.8% to −13.5%]; RR, 0.67 [95% CI, 0.54 to 0.84]), incidence of patent ductus arteriosus requiring medical therapy (35.3% vs 45.5%; RD, −10.5% [95% CI, −20.2% to −0.9%]; RR, 0.77 [95% CI, 0.60 to 0.99]), and in need for oxygen therapy at home in survivors to hospital discharge (14.7% vs 21.9%; RD, −7.1% [95% CI, −11.6% to −2.5%]; RR, 0.68 [95% CI, 0.52 to 0.88]).
Table 4. Secondary Outcomes.
| No./total (%)a | Between-group difference, % (95% CI)b |
Relative risk (95% CI)b |
||
|---|---|---|---|---|
| Minimally invasive surfactant therapy | Control treatment | |||
| Respiratory binary outcomes | ||||
| Intubation at any time | 132/241 (54.8) | 198/244 (81.1) | −26.7 (−39.8 to −13.5) | 0.67 (0.54 to 0.84) |
| Requirement for surfactant therapy via endotracheal tube | 79/241 (32.8) | 167/244 (68.4) | −35.7 (−47.7 to −23.7) | 0.48 (0.37 to 0.62) |
| Pulmonary hemorrhage | 17/241 (7.1) | 15/244 (6.1) | 0.8 (−3.4 to 5.0) | 1.13 (0.60 to 2.14) |
| Oxygen therapy at day 28 (in survivors to day 28) | 172/221 (77.8) | 183/227 (80.6) | −2.7 (−10.3 to 4.9) | 0.97 (0.88 to 1.06) |
| Bronchopulmonary dysplasiac in survivors to 36 weeks’ postmenstrual age | 94/217 (43.3) | 107/225 (47.6) | −4.1 (−11.2 to 3.0) | 0.91 (0.78 to 1.07) |
| Mechanical respiratory support at 36 weeks’ postmenstrual age (in survivors) | 53/217 (24.4) | 67/225 (29.8) | −5.3 (−12.5 to 2.0) | 0.82 (0.64 to 1.06) |
| Oxygen therapy at home in survivors to hospital discharged | 31/211 (14.7) | 49/224 (21.9) | −7.1 (−11.6 to −2.5) | 0.68 (0.52 to 0.88) |
| Nonrespiratory binary outcomes | ||||
| Patent ductus arteriosus requiring medical therapy | 85/241 (35.3) | 111/244 (45.5) | −10.5 (−20.2 to −0.9) | 0.77 (0.60 to 0.99) |
| Late-onset sepsis | 55/241 (22.8) | 63/244 (25.8) | −3.2 (−13.7 to 7.3) | 0.88 (0.58 to 1.33) |
| Intraventricular hemorrhage any grade | 85/241 (35.3) | 91/244 (37.3) | −2.4 (−12.2 to 7.3) | 0.93 (0.72 to 1.22) |
| Cystic periventricular leukomalacia | 6/241 (2.5) | 16/244 (6.6) | −4.1 (−8.9 to 0.8) | 0.38 (0.14 to 1.02) |
| Necrotizing enterocolitis | ||||
| Modified Bell stage ≥2 | 12/241 (5.0) | 17/244 (7.0) | −2.0 (−6.5 to 2.5) | 0.72 (0.31 to 1.65) |
| Requiring surgery | 4/241 (1.7) | 5/244 (2.0) | −0.4 (−2.6 to 1.7) | 0.79 (0.26 to 2.45) |
| Spontaneous intestinal perforation | 4/241 (1.7) | 6/244 (2.5) | −0.9 (−3.7 to 2.0) | 0.65 (0.16 to 2.57) |
| Retinopathy of prematurity (≥stage 3) | 19/241 (7.9) | 24/244 (9.8) | −2.2 (−8.7 to 4.4) | 0.78 (0.38 to 1.63) |
| Continuous outcomes | ||||
| No. of surfactant doses, median (IQR)e | (n = 241) 1 (1 to 2) |
(n = 244) 1 (0 to 1) |
||
| Duration of mechanical ventilation via endotracheal tube, median (IQR), d | (n = 240) 1 (0 to 7) |
(n = 244) 4 (0 to 13) |
−1.96 (−3.19 to −0.73)f,g | |
| Duration of CPAP, median (IQR), dh | (n = 240) 17 (6 to 33) |
(n = 244) 22 (8 to 35) |
−4.62 (−8.41 to −0.84)f,g | |
| Duration of mechanical ventilation plus CPAP, median (IQR), d | (n = 240) 25 (8 to 45) |
(n = 244) 32 (12 to 48) |
−8.13 (−13.98 to −2.27)f,g | |
| Duration of all forms of mechanical respiratory support, median (IQR), d | (n = 240) 40 (14 to 60) |
(n = 244) 45 (25 to 64) |
−6.42 (−11.95 to −0.89)f,g | |
| Time required to regain birth weight, mean (SD), d | (n = 231) 10.1 (4.2) |
(n = 236) 10.6 (5.1) |
−0.56 (−1.21 to 0.08)i | |
| Duration of hospitalization, mean (SD), d | (n = 238) 80 (47) |
(n = 243) 84 (38) |
−3.62 (−11.44 to 4.19)i | |
Abbreviation: CPAP, continuous positive airway pressure.
Unless otherwise indicated. The protocolized secondary outcomes not reported are: length of stay in intensive care unit (variably defined worldwide); hospital billings and calculated hospitalization cost; and outcomes beyond first hospitalization (additional details appear in Supplement 1).
Adjusted for gestational strata.
Clinically defined as the requirement for oxygen, mechanical respiratory support, or both, at 36 weeks’ postmenstrual age. Mechanical respiratory support included mechanical ventilation, CPAP, or high-flow nasal cannula therapy (at rate of ≥2 L/min).
Data are missing for 2 infants in the minimally invasive surfactant therapy group.
The mean number of doses given per infant was 1.42 in the minimally invasive surfactant therapy group and 0.96 in the control group.
Data are severely skewed.
Between-group difference expressed as median difference (95% CI).
Includes noninvasive positive pressure ventilation.
Between-group difference expressed as mean difference (95% CI).
The requirement for surfactant therapy via endotracheal tube also was reduced after treatment with MIST (32.8% vs 68.4% in the control group); the mean total number of doses of surfactant administered was 1.42 in the MIST group and 0.96 in the control group (Table 4). The MIST group showed reductions in 4 of 6 continuous secondary outcomes quantifying duration of therapy: mechanical ventilation (1 day vs 4 days in the control group; median difference, −1.96 [95% CI, −3.19 to −0.73] days), CPAP (17 days vs 22 days; median difference, −4.62 [95% CI, −8.41 to −0.84] days), mechanical ventilation plus CPAP (25 days vs 32 days; median difference, −8.13 [95% CI, −13.98 to −2.27] days), and all forms of mechanical respiratory support (40 days vs 45 days; median difference, −6.42 [95% CI, −11.95 to −0.89 days]) (Table 4).
Subgroups
In an exploratory analysis by gestational age subgroups for the outcome of death prior to 36 weeks’ postmenstrual age, there was a statistically significant interaction in relation to the treatment effect (control group favored at lower gestational age and MIST group favored at higher gestational age; P = .01 for interaction), but not for the composite of death or bronchopulmonary dysplasia or for bronchopulmonary dysplasia in survivors (eTable 4 in Supplement 3). Evidence of interaction between gestational age stratum and group allocation also was seen in relation to the need for intubation within 72 hours of birth and death during hospitalization (eTable 5 in Supplement 3). The need for intubation within 72 hours of birth was 49% for the MIST group vs 72% for the control group within the gestational age stratum of 25 to 26 weeks and was 29% vs 72% within the gestational age stratum of 27 to 28 weeks (P = .02 for interaction).
For the secondary outcomes, the pattern of treatment effects did not appear to differ between gestational age strata (eTable 6 in Supplement 3). Subgroup analysis by Fio2 level required at randomization showed no statistically significant interaction between the subgroups (eTables 7-8 in Supplement 3). Incidence of the primary outcome and its components varied considerably in the control group between geographic regions (eFigure 2 in Supplement 3), but there was no statistically significant interaction in the effect of MIST across regions (P > .05 for interaction terms for the primary outcome and its components).
Exploratory Outcomes
The trachea was catheterized during the first attempt in 76% of the MIST interventions (eTable 9 in Supplement 3). Hypoxemia and bradycardia were common but transient, with positive pressure inflations applied in 14% of cases, and emergent intubation needed in 1 infant. The surfactant instillation procedure was found to be effective (defined in footnote for eTable 9 in Supplement 3) in 88% of cases, with a median absolute reduction of 0.10 in Fio2 level required at 4 hours after the procedure. Clinical staff correctly identified which intervention had been performed in 33% of cases, were incorrect in 11%, and were unsure in 56%.
Adverse Events
A total of 54 serious adverse events were reported (eTable 10 in Supplement 3), which were evenly distributed between treatment groups (10.3% of infants in the MIST group vs 11.1% in the control group) and were not considered to be related to participation in the study. Infants who died were receiving comparable levels of respiratory support before the intervention with those who survived for both study groups. Within the MIST group, the frequency of procedural complications and the disease trajectory during the 24 hours after the procedure appeared to be similar (eTable 11 in Supplement 3).
Discussion
In this multicenter, randomized clinical trial in preterm infants supported with CPAP and exhibiting features of RDS, administration of surfactant via a thin catheter at a low oxygenation threshold, compared with continuation of CPAP, did not significantly reduce the incidence of the composite outcome of death or bronchopulmonary dysplasia at 36 weeks’ postmenstrual age. A clinically important true treatment effect cannot be excluded, with the 95% CI including the possibility of an absolute reduction in death or bronchopulmonary dysplasia of 14%, which was higher than originally hypothesized.
Analysis of the components of the primary outcome showed an absolute increase in death of 2% prior to 36 weeks’ postmenstrual age favoring the control group (with an upper bound of 7.8%), which was not statistically significant. If representative of a real difference in mortality, this would clearly temper enthusiasm for MIST potentially arising from other findings of this trial. For both randomization groups, deaths were reported to be due to a multiplicity of causes and were distributed throughout the first months of life. Site investigators (who were blinded to treatment group) did not attribute any deaths to enrollment in the trial. In exploratory subgroup analysis for the outcome of death prior to 36 weeks’ postmenstrual age, there was a significant interaction suggesting a higher mortality risk in the gestational age stratum of 25 to 26 weeks associated with allocation to the MIST group. This finding may have been due in part to a chance imbalance in the risk profile for this subgroup, but behooves caution in application of MIST in preterm infants at the most immature gestational ages, particularly in regions in which mortality for such infants remains high.
There was an absolute decrease of 8% in bronchopulmonary dysplasia in survivors to 36 weeks’ postmenstrual age favoring the MIST group. An effect of MIST on the incidence of bronchopulmonary dysplasia has plausibility by virtue of the observed reduction in exposure to positive pressure ventilation by endotracheal tube, a known intermediary in the development of injury to the preterm lung.23,24 However, the pathogenesis of bronchopulmonary dysplasia is multifactorial,3 with many contributory factors not alterable by a single dose of surfactant on day 1.
One previous trial compared surfactant delivery via a thin catheter with continuation of CPAP in 220 preterm infants, noting a reduction in need for intubation within 72 hours of birth (RR, 0.61 [95% CI, 0.42-0.88]), but no clear effect on bronchopulmonary dysplasia at 36 weeks’ postmenstrual age (RR, 0.59 [95% CI, 0.26-1.36]).25 With a larger sample size, blinded intervention, and protocolized intubation thresholds, the present study provides more robust and definitive evidence regarding this mode of surfactant delivery.
This trial is, to our knowledge, the largest study to date to investigate administration of surfactant to preterm infants once an oxygen requirement of 30% has been surpassed. This is a lower threshold for selective surfactant therapy than used in previous large clinical trials in preterm infants with a gestational age of less than 30 weeks requiring CPAP, in which surfactant administration only occurred (after intubation) if the Fio2 requirement exceeded 0.40 to 0.60.4,5,6,7 The choice of an Fio2 entry threshold of 0.30 in the present study was based on data from a cohort of infants with gestational ages of 25 to 28 weeks who were receiving CPAP from the outset. Among infants for whom the Fio2 requirement exceeded 0.30 in early life, most had moderate or severe RDS radiologically, and there was a high rate of CPAP failure (odds ratio, 5.6 [95% CI, 1.7-18.0]).11 In the present study, exploratory subgroup analysis in infants with baseline Fio2 requirement in the range of 0.30 to 0.35 showed the pattern of treatment effects after MIST to be not discernibly different from those of infants with an Fio2 requirement greater than 0.35 at randomization, suggesting that an oxygen requirement of 30% (rather than higher) is an appropriate intervention threshold.
Given the design of the trial, the question of whether surfactant delivered via endotracheal tube at the same treatment threshold would have conferred the same benefits as MIST cannot be answered. However, a recent network meta-analysis of clinical trials including infants receiving noninvasive respiratory support identified surfactant delivery via a thin catheter, rather than by brief intubation, to be the strategy most strongly associated with a reduction in death or bronchopulmonary dysplasia.26 Furthermore, a Cochrane review of head-to-head trials of surfactant delivery via a thin catheter or endotracheal tube has provided strong evidence in favor of the thin catheter delivery method (for death or bronchopulmonary dysplasia: RR, 0.59 [95% CI, 0.48-0.73]).27
The Hobart method of MIST using a semirigid catheter appeared to be practicable, needing only 1 attempt in three-quarters of cases, in line with other studies.25,28,29 The proportion of infants experiencing transient bradycardia or hypoxemia was higher than reported previously, but in the case of hypoxemia the proportion was similar to standard intubation.30 The need for positive pressure ventilation by mask and for emergent intubation was low in the MIST group.
Limitations
This study has several limitations. First, after slow recruitment, enrollment ceased at the onset of the COVID-19 pandemic at 81% of the planned recruitment target, meaning that the study was underpowered for detection of a treatment effect of the magnitude originally hypothesized. The combination of the overall rate of commencement of CPAP (54%) at all study centers and the proportion of infants supported with CPAP that qualified for the trial (44%) imposed a barrier to recruitment. Lack of availability of treatment team personnel and loss of equipoise at some centers also played a role.
Second, data on numerous clinically relevant outcomes were collected and multiple statistical comparisons were performed, raising the possibility of type I error. A cautious interpretation of the findings is warranted.
Third, although the intervention was successfully blinded to the treating clinicians, members of treatment teams were likely to have participated in the care of enrolled infants at some time during hospitalization, raising the possibility of performance bias.
Conclusions
Among preterm infants with respiratory distress syndrome supported with CPAP, minimally invasive surfactant therapy compared with sham (control) treatment did not significantly reduce the incidence of the composite outcome of death or bronchopulmonary dysplasia at 36 weeks’ postmenstrual age. However, given the statistical uncertainty reflected in the 95% CI, a clinically important effect cannot be excluded.
Trial protocol
Statistical analysis plan
eTable 1. Baseline characteristics by gestation strata
eTable 2. Primary outcome and its components: extended generalized linear model
eTable 3. Primary outcome and its components: as treated and per protocol analyses
eTable 4. Primary outcome and its components: gestation strata
eTable 5. Key clinical and safety outcomes: gestation strata
eTable 6. Secondary outcomes: gestation strata
eTable 7. Primary outcome and its components: subgroup analysis by FiO2 at randomization
eTable 8. Key clinical and safety outcomes: subgroup analysis by FiO2 at randomization
eTable 9. MIST intervention – procedural details and safety outcomes
eTable 10. Serious adverse events and cause of death
eTable 11. Clinical and procedural details by treatment group and survival to hospital discharge
eFigure 1. Requirement for intubation <72 h
eFigure 2. Primary outcome treatment effects in regional subgroups
eReference
OPTIMIST-A trial investigators
Data sharing statement
References
- 1.Committee on Fetus and Newborn; American Academy of Pediatrics . Respiratory support in preterm infants at birth. Pediatrics. 2014;133(1):171-174. doi: 10.1542/peds.2013-3442 [DOI] [PubMed] [Google Scholar]
- 2.Sweet DG, Carnielli V, Greisen G, et al. European consensus guidelines on the management of respiratory distress syndrome: 2019 update. Neonatology. 2019;115(4):432-450. doi: 10.1159/000499361 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Thébaud B, Goss KN, Laughon M, et al. Bronchopulmonary dysplasia. Nat Rev Dis Primers. 2019;5(1):78. doi: 10.1038/s41572-019-0127-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Morley CJ, Davis PG, Doyle LW, Brion LP, Hascoet JM, Carlin JB; COIN Trial Investigators . Nasal CPAP or intubation at birth for very preterm infants. N Engl J Med. 2008;358(7):700-708. doi: 10.1056/NEJMoa072788 [DOI] [PubMed] [Google Scholar]
- 5.Finer NN, Carlo WA, Walsh MC, et al. ; SUPPORT Study Group of the Eunice Kennedy Shriver NICHD Neonatal Research Network . Early CPAP versus surfactant in extremely preterm infants. N Engl J Med. 2010;362(21):1970-1979. doi: 10.1056/NEJMoa0911783 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Sandri F, Plavka R, Ancora G, et al. ; CURPAP Study Group . Prophylactic or early selective surfactant combined with nCPAP in very preterm infants. Pediatrics. 2010;125(6):e1402-e1409. doi: 10.1542/peds.2009-2131 [DOI] [PubMed] [Google Scholar]
- 7.Dunn MS, Kaempf J, de Klerk A, et al. ; Vermont Oxford Network DRM Study Group . Randomized trial comparing 3 approaches to the initial respiratory management of preterm neonates. Pediatrics. 2011;128(5):e1069-e1076. doi: 10.1542/peds.2010-3848 [DOI] [PubMed] [Google Scholar]
- 8.Schmölzer GM, Kumar M, Pichler G, Aziz K, O’Reilly M, Cheung PY. Non-invasive versus invasive respiratory support in preterm infants at birth: systematic review and meta-analysis. BMJ. 2013;347:f5980. doi: 10.1136/bmj.f5980 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Jobe AH. Why, when, and how to give surfactant. Pediatr Res. 2019;86(1):15-16. doi: 10.1038/s41390-019-0372-1 [DOI] [PubMed] [Google Scholar]
- 10.Ammari A, Suri M, Milisavljevic V, et al. Variables associated with the early failure of nasal CPAP in very low birth weight infants. J Pediatr. 2005;147(3):341-347. doi: 10.1016/j.jpeds.2005.04.062 [DOI] [PubMed] [Google Scholar]
- 11.Dargaville PA, Aiyappan A, De Paoli AG, et al. Continuous positive airway pressure failure in preterm infants: incidence, predictors and consequences. Neonatology. 2013;104(1):8-14. doi: 10.1159/000346460 [DOI] [PubMed] [Google Scholar]
- 12.Dargaville PA, Gerber A, Johansson S, et al. ; Australian and New Zealand Neonatal Network . Incidence and outcome of CPAP failure in preterm infants. Pediatrics. 2016;138(1):e20153985. doi: 10.1542/peds.2015-3985 [DOI] [PubMed] [Google Scholar]
- 13.Kribs A, Pillekamp F, Hünseler C, Vierzig A, Roth B. Early administration of surfactant in spontaneous breathing with nCPAP: feasibility and outcome in extremely premature infants (postmenstrual age ≤27 weeks). Paediatr Anaesth. 2007;17(4):364-369. doi: 10.1111/j.1460-9592.2006.02126.x [DOI] [PubMed] [Google Scholar]
- 14.Dargaville PA, Aiyappan A, Cornelius A, Williams C, De Paoli AG. Preliminary evaluation of a new technique of minimally invasive surfactant therapy. Arch Dis Child Fetal Neonatal Ed. 2011;96(4):F243-F248. doi: 10.1136/adc.2010.192518 [DOI] [PubMed] [Google Scholar]
- 15.Vento M, Bohlin K, Herting E, Roehr CC, Dargaville PA. Surfactant administration via thin catheter: a practical guide. Neonatology. 2019;116(3):211-226. doi: 10.1159/000502610 [DOI] [PubMed] [Google Scholar]
- 16.Dargaville PA, Kamlin CO, De Paoli AG, et al. The OPTIMIST-A trial: evaluation of minimally-invasive surfactant therapy in preterm infants 25-28 weeks gestation. BMC Pediatr. 2014;14:213. doi: 10.1186/1471-2431-14-213 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Schmidt B, Roberts RS, Davis P, et al. ; Caffeine for Apnea of Prematurity Trial Group . Caffeine therapy for apnea of prematurity. N Engl J Med. 2006;354(20):2112-2121. doi: 10.1056/NEJMoa054065 [DOI] [PubMed] [Google Scholar]
- 18.Dargaville PA, Aiyappan A, De Paoli AG, et al. Minimally-invasive surfactant therapy in preterm infants on continuous positive airway pressure. Arch Dis Child Fetal Neonatal Ed. 2013;98(2):F122-F126. doi: 10.1136/archdischild-2011-301314 [DOI] [PubMed] [Google Scholar]
- 19.Walsh MC, Yao Q, Gettner P, et al. ; National Institute of Child Health and Human Development Neonatal Research Network . Impact of a physiologic definition on bronchopulmonary dysplasia rates. Pediatrics. 2004;114(5):1305-1311. doi: 10.1542/peds.2004-0204 [DOI] [PubMed] [Google Scholar]
- 20.Papile LA, Burstein J, Burstein R, Koffler H. Incidence and evolution of subependymal and intraventricular hemorrhage: a study of infants with birth weights less than 1,500 gm. J Pediatr. 1978;92(4):529-534. doi: 10.1016/S0022-3476(78)80282-0 [DOI] [PubMed] [Google Scholar]
- 21.Schmidt B, Asztalos EV, Roberts RS, Robertson CM, Sauve RS, Whitfield MF; Trial of Indomethacin Prophylaxis in Preterms (TIPP) Investigators . Impact of bronchopulmonary dysplasia, brain injury, and severe retinopathy on the outcome of extremely low-birth-weight infants at 18 months: results from the trial of indomethacin prophylaxis in preterms. JAMA. 2003;289(9):1124-1129. doi: 10.1001/jama.289.9.1124 [DOI] [PubMed] [Google Scholar]
- 22.Zou G. A modified poisson regression approach to prospective studies with binary data. Am J Epidemiol. 2004;159(7):702-706. doi: 10.1093/aje/kwh090 [DOI] [PubMed] [Google Scholar]
- 23.Jobe AH. Animal models, learning lessons to prevent and treat neonatal chronic lung disease. Front Med (Lausanne). 2015;2:49. doi: 10.3389/fmed.2015.00049 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Laughon MM, Langer JC, Bose CL, et al. ; Eunice Kennedy Shriver National Institute of Child Health and Human Development Neonatal Research Network . Prediction of bronchopulmonary dysplasia by postnatal age in extremely premature infants. Am J Respir Crit Care Med. 2011;183(12):1715-1722. doi: 10.1164/rccm.201101-0055OC [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Göpel W, Kribs A, Ziegler A, et al. ; German Neonatal Network . Avoidance of mechanical ventilation by surfactant treatment of spontaneously breathing preterm infants (AMV): an open-label, randomised, controlled trial. Lancet. 2011;378(9803):1627-1634. doi: 10.1016/S0140-6736(11)60986-0 [DOI] [PubMed] [Google Scholar]
- 26.Isayama T, Iwami H, McDonald S, Beyene J. Association of noninvasive ventilation strategies with mortality and bronchopulmonary dysplasia among preterm infants: a systematic review and meta-analysis. JAMA. 2016;316(6):611-624. doi: 10.1001/jama.2016.10708 [DOI] [PubMed] [Google Scholar]
- 27.Abdel-Latif ME, Davis PG, Wheeler KI, De Paoli AG, Dargaville PA. Surfactant therapy via thin catheter in preterm infants with or at risk of respiratory distress syndrome. Cochrane Database Syst Rev. 2021;5:CD011672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kanmaz HG, Erdeve O, Canpolat FE, Mutlu B, Dilmen U. Surfactant administration via thin catheter during spontaneous breathing: randomized controlled trial. Pediatrics. 2013;131(2):e502-e509. doi: 10.1542/peds.2012-0603 [DOI] [PubMed] [Google Scholar]
- 29.Kribs A, Roll C, Göpel W, et al. ; NINSAPP Trial Investigators . Nonintubated surfactant application vs conventional therapy in extremely preterm infants: a randomized clinical trial. JAMA Pediatr. 2015;169(8):723-730. doi: 10.1001/jamapediatrics.2015.0504 [DOI] [PubMed] [Google Scholar]
- 30.Roberts KD, Leone TA, Edwards WH, Rich WD, Finer NN. Premedication for nonemergent neonatal intubations: a randomized, controlled trial comparing atropine and fentanyl to atropine, fentanyl, and mivacurium. Pediatrics. 2006;118(4):1583-1591. doi: 10.1542/peds.2006-0590 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Trial protocol
Statistical analysis plan
eTable 1. Baseline characteristics by gestation strata
eTable 2. Primary outcome and its components: extended generalized linear model
eTable 3. Primary outcome and its components: as treated and per protocol analyses
eTable 4. Primary outcome and its components: gestation strata
eTable 5. Key clinical and safety outcomes: gestation strata
eTable 6. Secondary outcomes: gestation strata
eTable 7. Primary outcome and its components: subgroup analysis by FiO2 at randomization
eTable 8. Key clinical and safety outcomes: subgroup analysis by FiO2 at randomization
eTable 9. MIST intervention – procedural details and safety outcomes
eTable 10. Serious adverse events and cause of death
eTable 11. Clinical and procedural details by treatment group and survival to hospital discharge
eFigure 1. Requirement for intubation <72 h
eFigure 2. Primary outcome treatment effects in regional subgroups
eReference
OPTIMIST-A trial investigators
Data sharing statement