Abstract
Objective:
For the second aim of the Kellogg Foundation grant, this double-blind RCT investigated the impact of plasma vitamin D metabolite, 25-hydroxyvitamin D (25(OH)D) on plasma immune-mediators during pregnancy. We hypothesized that higher 25(OH)D concentrations would associate with reduced pro-inflammatory and increased tolerogenic immune-mediator concentrations.
Method:
Pregnant women enrolled at 10–14 weeks gestation were randomized to 400 or 4400 IU vitamin D3/day. Data on health, safety, circulating 25(OH)D, and 9 immune-mediators were collected at each trimester. Associations between immune-mediators and 25(OH)D at baseline and at 2nd and 3rd trimesters were examined.
Results:
Baseline TGF-β and 2nd and 3rd trimesters IFN-γ and IL-2 were associated with baseline 25(OH)D. Baseline immune-mediators were associated with immune-mediators at 2nd and 3rd trimesters for all immune-mediators except IL-5 and IL-10. Race was associated with baseline TGF-β, VEGF and IL-10 and with IL-10 at 2nd and 3rd trimesters.
Conclusion:
Both treatment groups had increased 25(OH)D at 2nd and 3rd trimesters, greatest in the 4400 IU group. Though associations between baseline 25(OH)D and baseline TGF-β and 2nd and 3rd trimester IFN-γ and IL-2 were noted, vitamin D supplementation throughout pregnancy did not impact immune-mediators at later trimesters. Supplementing with vitamin D before conception conceivably influences immune-mediator responses during pregnancy.
Introduction
During pregnancy, the maternal immune system undergoes remarkable changes. The body increases immune suppressive cells, reduces highly activated natural killer and cytotoxic T-cells that can destroy the fetus and still has the capacity to fight off foreign pathogens to guard both mother and child from infectious pathogens. When this delicate balance is disrupted, the mother and child are not only at risk for infection but more susceptible to immune system dysregulation that can manifest as pregnancy loss, preeclampsia, and preterm labor.
Circulating 25(OH)D has been associated with pregnancy-related comorbidities, with higher concentrations protective in certain women (1). The National Institute of Child Health and Human Development (NICHD) trial found conversion of serum 25-hydroxyvitamin D (25(OH)D) to 1,25(OH)2D during pregnancy is twice that normally observed in non-pregnant women, resulting in serum concentrations of 1,25(OH)2D in pregnancy that would be considered toxic in non-pregnant individuals (1). In the NICHD trial and another pregnancy clinical trial, supplementation at lower doses of vitamin D showed worse circulating 25(OH)D status throughout pregnancy and combined higher risk of comorbidities of pregnancy compared to supplementation at higher doses (1–3). Similarly, in newborns, 25(OH)D3 deficiency is associated with hypocalcemia (4), while low circulating 25(OH)D is associated with poor bone and skeletal health (5) and other conditions such as sepsis (6) and jaundice (7).
Evidence suggests that immune-mediators also are associated with pregnancy-related comorbidities. For instance, concentrations of C-reactive protein (CRP), Granulocyte-Macrophage Colony-Stimulating Factor (GM-CSF), IL-10, IL-6, IL-8 and TNF-α were elevated in women with threatened preterm labor (8–12). Similarly, compared to women with healthy pregnancies, circulating concentrations of pro-inflammatory immune-mediator IL-2 also were elevated in women with preeclampsia (13). Anti-inflammatory factors such as IL-10 and Transforming Growth Factor-beta (TGF-β) also play an important role in maintaining a healthy pregnancy. Early reductions in IL-10 have been associated with pregnancy loss, while elevated IL-10 later in pregnancy is associated with preeclampsia (14) and elevated TGF-β later in pregnancy is associated with gestational diabetes and preeclampsia (15,16). Elevated concentrations of IL-10 (17), IL-5 (18), and IL-4 (17,18), as well as reduced concentrations of IFN-γ (17) also were associated with prenatal maternal stress, while TNF-α (18) was not. Growth factors such as VEGF play dual roles in fetal development and pregnancy. In studies of pregnant mice and women, reduced VEGF was associated with preterm labor, while extremely elevated levels were observed in those with preeclampsia (19).
While few studies have investigated how circulating 25(OH)D affects immune-mediators during pregnancy, evidence suggests that the active form of vitamin D (1,25-dihydroxyvitamin D or 1,25[OH]2D) plays a role in regulating immune responses (11,20–25). Supplementation with vitamin D3 is known to reduce VEGF in women with polycystic ovarian syndrome (PCOS) (15). Some studies of pregnant women observed a positive association between serum 25(OH)D and IL-10 (26,27). Yet, another study analyzing maternal and cord blood 25(OH)D concentration showed an inverse correlation between maternal 25(OH)D and IL-10 response (28).
High-dose vitamin D supplementation in pregnancy also has been shown to modify immune profiles in newborns, but less is known about how they affect the immune profiles of pregnant women (29). The immune profiles of newborns from mothers supplemented with high-dose vitamin D3 differed from those of newborns from mothers supplemented with low-dose vitamin D3 (29). Further studies, therefore, are needed to clarify how concentrations of pro- and anti- inflammatory immune-mediators change during pregnancy in mothers and how supplementation with vitamin D3 during pregnancy affects their immune-mediator profiles.
Some of the differences in the literature on how immune-mediator profiles change during pregnancy may be explained by racial differences; however, findings are limited and incongruous. A RCT in 328 non-pregnant Black women found that vitamin D3 supplementation did not affect CRP, IL-6 and IL-10 (30). In a cohort of women with preterm and term births at delivery, however, differences were observed in concentrations of IL-10, IL-1β, TNF-α, and sTNFR2 between Black and White women (9). Another study of Black and White pregnant women showed significant increases in IL-6, TNF-α and IL-1β from early to late pregnancy for both groups, even though inflammatory response patterns were similar between Blacks and Whites (31). Racial differences also exist in the metabolism of circulating 25(OH)D. In a cohort of African-American children who met the recommended daily allowance of vitamin D intake of 400 IU/day, higher serum 25(OH)D was observed in those with the Gc1S allele of the vitamin D (vitD)-binding protein gene compared to those with the Gc1F or Gc2 alleles (32).
The mechanisms orchestrating these complex immune-mediation processes during pregnancy are unknown. We hypothesized that vitamin D status throughout pregnancy, as measured by circulating 25(OH)D after supplementing women with 400 vs 4400 IU vitamin D3/day, would be associated with reduced concentrations of pro-inflammatory and increased concentrations of tolerogenic immune-mediators. Our study is also novel in that it evaluates the differences in immune-mediator profiles during pregnancy between racial/ethnic groups. Yet, it is important to note that circulating concentrations of 25(OH)D do not necessarily reflect tissue concentrations, and therefore, circulating 25(OH)D becomes an imprecise proxy of vitamin D status and interactions at the cellular level. In the following sections of the manuscript, the term ‘vitamin D status’ is used interchangeably with circulating 25(OH)D concentration.
Materials and Methods
Study Overview and Design
This study was part of a randomized, double-blind, placebo-controlled clinical trial (clinicaltrials.gov NCT01932788) in which women provided written informed consent and were followed from enrollment through delivery (January 2013-May 2016). The Institutional Review Board at the Medical University of South Carolina approved this study protocol (Pro00020570). Details on inclusion/exclusion criteria for participants can be found elsewhere (33). The analysis performed in this paper is part of the second aim of the Kellogg Foundation grant, “Preventing Health Disparities during Pregnancy through Vitamin D supplementation.”
As per protocol, 300 women were enrolled (100 Hispanic, 100 African-American, and 100 Caucasian) and half of each ethnicity was randomized to 400 IU vitamin D3/day and to 4400 IU vitamin D3/day. It was anticipated that at least 90% of women in the 4400 IU arm and 50% of women in the 400 IU arm would attain repletion total circulating 25(OH)D at delivery of 30 ng/mL. Assuming 30% of women in each group would be lost to follow-up, approximately 35 women in each ethnicity group and treatment arm were anticipated to complete the study. A sample size of 35 women per group provided 80% power to detect an increase in the proportion achieving vitamin D repletion to 87% in the 4400 IU arm compared to 50% in the 400 IU at Bonferroni adjust significance of α = 0.0167. At the completion of the study, we were able to retain 217 pregnant women in whom circulating immune-mediator analyses were performed.
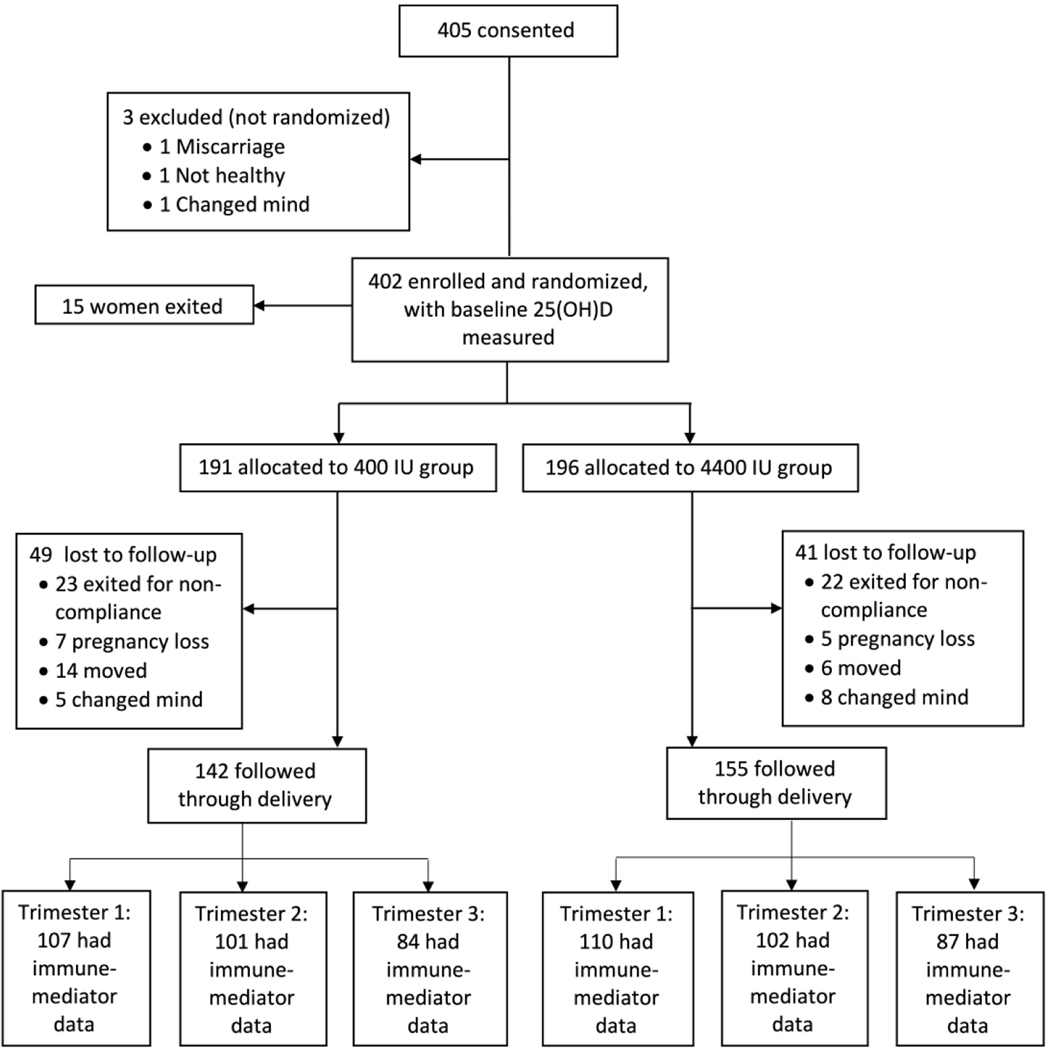
To evaluate the impact of vitamin D on immune-mediator responses during pregnancy, longitudinal data were collected from 217 pregnant women, randomized to receive either 400 or 4400 IU vitamin D3/day (Church & Dwight, Inc, Princeton, NJ). Figure 1 presents the CONSORT flow diagram for this RCT. Data on plasma 25(OH)D and plasma immune-mediators were collected at 1st, 2nd and 3rd trimesters (month 2, 5 and 8 of gestation).
Figure 1:
CONSORT flow diagram for Kellogg Pregnancy study
Maternal Sociodemographic and Clinical Characteristics
Questionnaires were completed monthly to assess baseline and longitudinal health characteristics. Information collected included maternal age, race, BMI at first visit, smoking status, time-variant sun exposures, and 25(OH)D and immune-mediator concentrations. Clinical characteristics were confirmed through review of the electronic medical record using a standardized questionnaire from prior studies (1,2).
Sun Exposure Measurement
Sun exposure was measured using the SmartProbe-400 (IMS, Portland, ME), which returned a degree of skin pigmentation (DSP) value on a scale of 0–100, with lower numbers indicating darker skin pigmentation. Participant DSP was measured monthly using two standardized points (dorsal forearm and buttock) on the body. Sun exposure was calculated as the difference between buttock and arm probe measurements. DSP values from two, five and seven months of gestation were used as exposure measurements for 1st, 2nd and 3rd trimesters, respectively.
Laboratory Measurements
Plasma immune-mediators were measured using specific ELISA kits according to manufacturers’ guidelines for IFN-γ (BD biosciences, San Jose, CA), CRP (R&D Systems, Minneapolis, MN), TGF-β (R&D Systems, Minneapolis, MN), TNF-α (R&D Systems, Minneapolis, MN), VEGF (R&D Systems, Minneapolis, MN), IL-2 (BD biosciences, San Jose, CA), IL-4 (BD biosciences, San Jose, CA), IL-5 (BD biosciences, San Jose, CA), IL-6 (BD biosciences, San Jose, CA), IL-8 (BD biosciences, San Jose, CA), IL-10 (BD biosciences, San Jose, CA), IL-17 (R&D Systems, Minneapolis, MN), GM-CSF (BD biosciences, San Jose, CA), antimicrobial peptide LL-37 (Hycult, Wayne, PA), β-defensin (β-def) (Genway, San Diego, CA), immunoglobulin E (IgE) (Genway, San Diego, CA), and basic fibroblast growth factor (bFGF) (R&D Systems, Minneapolis, MN). These immune-mediators were chosen based on prior studies of both pregnant and non-pregnant population that had been associated with vitamin D status or pregnancy related comorbidities (34–39). All 17 immune-mediators were measured in the first 50 women; however, because immune-mediator concentrations for IL-6, IL-8, IL-17, GM-CSF, LL-37, β-def, IgE and bFGF could not be detected or could not be differentiated between participants, only the remaining nine plasma immune-mediators were measured in all participants. Total circulating 25(OH)D was measured in plasma samples using a commercially available radioimmunoassay (Diasorin Corporation, Stillwater, MN) in the laboratory of Dr. Bruce Hollis as described previously (33).
Statistical analysis
Descriptive statistics were calculated for all baseline covariates, baseline 25(OH)D and immune-mediator concentrations, stratified by treatment group. For all tests of association, immune-mediators with values below the limit of detection were imputed using LDLimmune − mediator, where LDLimmune − mediator is the lower limit of detection for a given immune-mediator. LDLimmune − mediator values used for imputation are: 4.7 pg/mL for IFN-γ; 1.6 pg/mL for TNF-α; 31.3 pg/mL for VEGF and 7.8 pg/mL for IL-2, IL-4, IL-5 and IL-10. CRP and TGF-β did not have observations below LDLimmune − mediator.
Outcomes of interest included 25(OH)D and immune-mediator concentrations during each trimester of pregnancy. We examined univariate and multivariable associations between 25(OH)D at 2nd and 3rd trimesters with treatment group and other covariates using a linear mixed regression approach. The multivariable model included treatment, trimester, race, maternal BMI at first visit, maternal age, smoking status, sun exposure and an interaction between treatment and trimester. The covariates were selected based on prior research and knowledge (2).
For evaluation of the associations between immune-mediator and 25(OH)D, we first assessed the association between baseline immune-mediators and 25(OH)D, controlling for patient characteristics using a linear regression approach. Models for each baseline immune-mediator included baseline 25(OH)D, maternal age, maternal BMI at first visit, race and smoking status. Next, we assessed the association between immune-mediator concentrations at 2nd and 3rd trimesters and 25(OH)D at those time points, adjusting for baseline 25(OH)D and baseline immune-mediator using a series of linear mixed models. Models also included trimester, race, maternal BMI at first visit, maternal age and smoking status as fixed effects and a random subject effect to account for repeated measures on subjects at 2nd and 3rd trimesters. We did not consider sun exposure as a covariate in models of immune-mediators because 25(OH)D concentration, a presumed proxy for sun exposure, was included in these models. Multi-collinearity between covariates was examined using baseline covariates/outcomes. All immune-mediators were log-transformed to meet model assumptions. All analyses were performed using SAS 9.4 (SAS Institute, Cary, NC).
Results
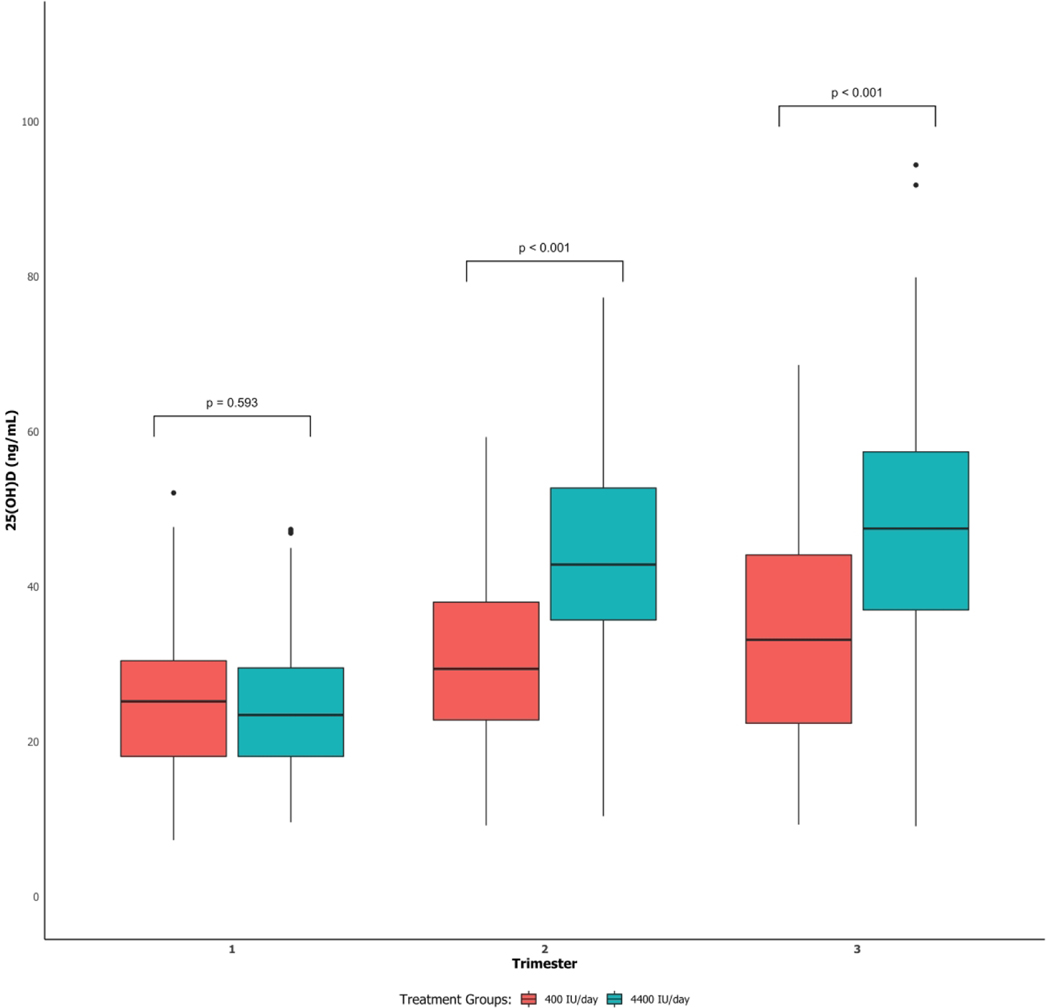
Patient characteristics were similar in the two treatment groups (Table 1). Plasma 25(OH)D concentrations were higher at 2nd and 3rd trimesters in the 4400 IU group than the 400 IU group, but they were also significantly higher for the 400 IU group in 2nd and 3rd trimesters than the 1st trimester (Figure 2). Median immune-mediator concentrations for the two treatment groups, stratified by trimester, are reported in Supplemental Table S1 (online).
Table 1:
Baseline characteristics of the study population, stratified by treatment groups (400 and 4400 IU).
| Characteristics | 400 IU Group (n = 107) |
4400 IU Group (n = 110) |
|---|---|---|
|
| ||
| Maternal age, years, mean (sd) | 28.9 (5.29) | 28.3 (4.69) |
|
| ||
| BMI at first visit*, kg/m2, mean (sd) | 29.5 (7.95) | 28.5 (6.80) |
|
| ||
| 25(OH)D*, ng/mL, mean (sd) | 25.2 (9.55) | 24.5 (9.03) |
|
| ||
| Parity, median (IQR) | 1 (2) | 1 (1) |
|
| ||
| Race | ||
| White/Caucasian, n (%) | 38 (35.5) | 35 (31.8) |
| Black/African American, n (%) | 37 (34.6) | 38 (34.6) |
| Hispanic, n (%) | 32 (29.9) | 37 (33.6) |
|
| ||
| Education | ||
| Less than high school, n (%) | 21 (19.6) | 13 (11.8) |
| High school, n (%) | 58 (54.2) | 70 (63.6) |
| Some college, n (%) | 28 (26.2) | 27 (24.6) |
|
| ||
| Smoking status | ||
| Never, n (%) | 86 (80.4) | 92 (83.6) |
| Current/Former, n (%) | 21 (19.6) | 18 (16.4) |
|
| ||
| Insurance Status | ||
| Private, n (%) | 38 (35.5) | 41 (37.3) |
| Medicaid/none, n (%) | 69 (64.5) | 69 (62.7) |
Figure 2:
Boxplots of 25(OH)D concentrations at the 1st, 2nd, and 3rd trimesters for 400 and 4400 IU vitamin D3/day groups. P-values are for significant difference in 25(OH)D between the 400 and 4400 IU groups at each trimester.
Treatment Groups and Plasma 25(OH)D Concentration at 2nd and 3rd trimesters
In univariate models, 25(OH)D concentrations at 2nd and 3rd trimesters were positively associated with trimester of pregnancy, maternal age, White race and sun exposure and were negatively associated with maternal BMI at first visit (p<0.05; Table 2). In the multivariable model, treatment, trimester, race, and the interaction between treatment and trimester were significant (p<0.05; Table 2). 25(OH)D concentrations at 2nd and 3rd trimesters were higher in Whites than Blacks and Hispanics, controlling for other covariates, and higher in 2nd and 3rd trimesters in the 4400 IU group than the 400 IU group after controlling for other covariates.
Table 2:
Univariate and multivariable associations between 25(OH)D concentrations and covariates with Bonferroni-corrected specific comparison list.
| Covariates | Univariate | Multivariable (n = 364) | ||
|---|---|---|---|---|
|
| ||||
| Mean difference (95% CI) |
P | Mean difference (95% CI) |
P | |
|
| ||||
| Treatment | ||||
| 4400 IU/day vs 400 IU/day | 0.54 (−1.91, 2.99) | 0.666 | * | <0.001 |
|
| ||||
| Trimester | ||||
| Trimester 1 vs. Trimester 2 | −12.5 (−14.6, −10.5) | < 0.001 | * | < 0.001 |
| Trimester 1 vs. Trimester 3 | −16.4 (−19.2, −13.7) | < 0.001 | * | < 0.001 |
| Trimester 2 vs. Trimester 3 | −3.93 (−6.22, −1.65) | < 0.001 | * | 0.001 |
|
| ||||
| Maternal age (5-year increase) | 1.51 (0.29, 2.73) | 0.015 | 0.63 (−0.79, 2.06) | 0.384 |
|
| ||||
| BMI at first visit (1-unit increase) | −0.35 (−0.51, −0.19) | < 0.001 | −0.27 (−0.09, 0.39) | 0.214 |
|
| ||||
| Race | ||||
| Black vs. White | −10.2 (−13.4, −6.92) | < 0.001 | −7.53 (−12.3, −2.76) | <0.001 |
| Black vs. Hispanic | −4.78 (−8.08, −1.48) | 0.002 | −1.15 (−6.01, 3.71) | 1 |
| White vs. Hispanic | 5.39 (2.07, 8.70) | <0.001 | 6.38 (1.89, 10.9) | 0.002 |
|
| ||||
| Smoking status | ||||
| Current/Former vs. Never | 0.42 (−2.77,3.61) | 0.797 | 1.35 (−2.58, 5.27) | 0.500 |
|
| ||||
| Sun exposure (1-unit increase) | 0.31 (0.06, 0.57) | 0.017 | 0.15 (−0.09, 0.39) | 0.214 |
|
| ||||
| Treatment * Trimester | ||||
| Trimester 1: 4400 IU/day vs 400 IU/day | −0.69 (−4.42, 3.05) | 1 | −0.07 (−5.87, 5.72) | 1 |
| Trimester 2: 4400 IU/day vs 400 IU/day | 13.8 (8.54, 19.0) | <0.001 | 14.2 (8.94, 19.4) | <0.001 |
| Trimester 3: 4400 IU/day vs 400 IU/day | 14.0 (7.53, 20.5) | <0.001 | 12.6 (5.74, 19.5) | <0.001 |
| 4400 IU/day: Trimester 1 vs Trimester 2 | −19.7 (−22.6, −16.8) | <0.001 | −21.6 (−26.3, −16.8) | <0.001 |
| 4400 IU/day: Trimester 1 vs Trimester 3 | −23.7 (−28.0, −19.4) | <0.001 | −24.9 (−30.9, −18.9) | <0.001 |
| 4400 IU/day: Trimester 2 vs Trimester 3 | −4.00 (−7.94, −0.06) | 0.043 | −3.32 (−8.07, 1.42) | 0.581 |
| 400 IU/day: Trimester 1 vs Trimester 2 | −5.26 (−8.12, −2.39) | <0.001 | −7.31 (−12.2, −2.37) | <0.001 |
| 400 IU/day: Trimester 1 vs Trimester 3 | −9.02 (−13.3, −4.70) | <0.001 | −12.2 (−18.2, −6.21) | <0.001 |
| 400 IU/day: Trimester 2 vs Trimester 3 | −3.76 (−7.76, 0.25) | 0.087 | −4.92 (−9.62, −0.22) | 0.032 |
The mean difference for main effects of treatment and visit are not presented since the interaction between the two groups are significant in the model, and therefore, they must be interpreted in the context of one another.
Baseline Immune-mediator and Baseline Plasma 25(OH)D Concentrations
Baseline 25(OH)D was associated with baseline TGF-β controlling for maternal age, BMI at first visit, race and smoking status (p<0.05; Table 3). Specifically, a five-unit increase in baseline 25(OH)D was associated with a 6.7% increase in baseline TGF-β. Patient BMI at first visit was associated with baseline CRP; every 1 kg/m2 increase in BMI was associated with a 5.5% increase in CRP (p<0.001; Supplemental Table S2 (online)). Additionally, race was associated with baseline TGF-β, VEGF and IL-10. Whites had significantly lower baseline TGF-β and IL-10 and higher baseline VEGF than Hispanics (p<0.05; Table 3), but no notable differences were observed between Blacks and Whites or Blacks and Hispanics. Having ever smoked was associated with increased baseline TGF-β and IL-10 and decreased baseline VEGF (p<0.05 respectively; Table 3).
Table 3:
Results of multivariable linear regression models of immune-mediators with significant associations with baseline 25(OH)D concentrations, race, smoking status, maternal age, or BMI at visit 1. Values are reported as percent change in differences (95% CI) with the specific comparison list. CIs including 0 are non-significant. For example, the coefficient relating baseline 25(OH)D to baseline TGF-β indicates that for a 5-unit increase in baseline 25(OH)D, average baseline TGF-β increased by 6.68%, adjusting for maternal race, smoking status, age and BMI at visit 1. Complete results are presented in Supplemental Table S2 (online).
| Baseline Immune-mediators | |||
|---|---|---|---|
|
| |||
| Covariates | TGF-β | VEGF | IL-10 |
|
| |||
|
Baseline 25(OH)D
(5-unit increase) |
6.68** (2.36, 10.8) |
0.52 (−7.70, 8.10) |
4.45 (−4.21, 12.4) |
|
| |||
| Race | |||
| Black vs. White | 7.89 (−12.9, 24.8) |
−23.1 (−76.5, 14.2) |
25.8 (−9.98, 49.9) |
| Black vs. Hispanic | −13.4 (−38.0, 6.88) |
23.1 (−8.90, 45.7) |
−21.5 (−77.4, 16.8) |
| White vs Hispanic | −23.1* (−49.7, −1.21) |
37.5** (11.9, 55.7) |
−63.7* (−138, −12.5) |
|
| |||
| Smoking status | |||
| Current/Former vs. Never | 26.9** (10.7, 40.1) |
−55.0* (−121, −8.65) |
42.1** (14.7, 60.7) |
p < 0.10
p < 0.05
p < 0.01
p < 0.001
Immune-mediator and Plasma 25(OH)D Concentrations at 2nd and 3rd trimesters
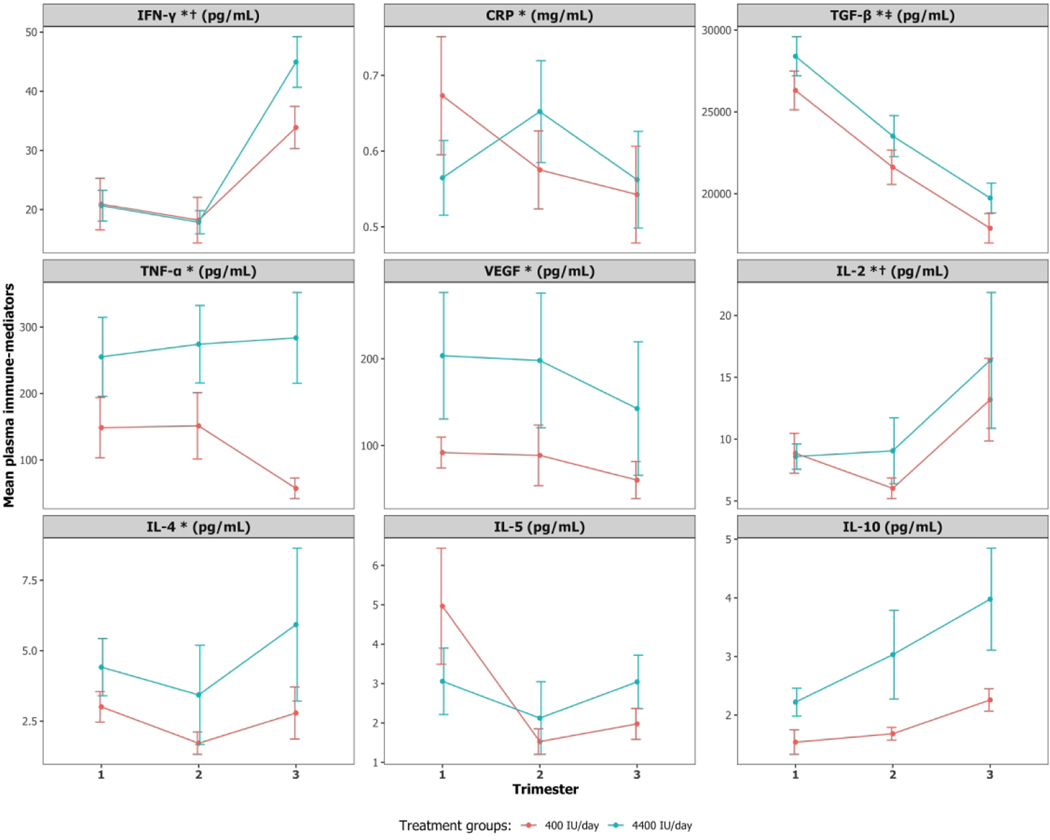
Plasma 25(OH)D concentrations in 2nd and 3rd trimesters were not associated with any immune-mediators in 2nd and 3rd trimesters after adjusting for the respective baseline immune-mediator, baseline 25(OH)D and other covariates. Baseline 25(OH)D was associated with IFN-γ and IL-2 in 2nd and 3rd trimesters (p<0.05; Table 4). Specifically, a 5 ng/mL increase in baseline 25(OH)D was associated with a 10.4% and 12.2% increase in IFN-γ and IL-2 concentrations, respectively, during the 2nd and 3rd trimesters. Baseline immune-mediator concentrations were associated with immune-mediator concentrations in 2nd and 3rd trimesters for IFN-γ, CRP, TGF-β, TNF-α, VEGF, IL-2 and IL-4. Trimester of pregnancy was also associated with IFN-γ and CRP: IFN-γ, increased by 54.4% and CRP decreased by 17.9% in the 3rd vs. the 2nd trimester. Maternal BMI at first visit was associated with increased CRP in 2nd and 3rd trimesters (p<0.05; Supplemental Table S3 (online)). Additionally, Whites and Blacks had higher IL-10 concentrations than Hispanics; and smoking status was significantly associated with increased TGF-β and decreased TNF-α in 2nd and 3rd trimesters (p<0.05; Table 4). Figure 3 shows the observed mean immune-mediator concentrations in the three trimesters by treatment groups. Supplemental Figure S1 provides subject-level profile plots for observed plasma immune-mediator concentrations in the three trimesters separated by the two treatment groups: 400 and 4400 IU vitamin D3/day. No discernable differences were observed between the treatment groups based on this plot.
Table 4:
Results of mixed effects models of immune-mediators at 2nd and 3rd trimesters with significant associations with baseline 25(OH)D, respective baseline immune-mediator, 25(OH)D concentrations at 2nd and 3rd trimesters, race, smoking status or trimester. Values are reported as percent change in difference (95% CI) with the specific comparison list. CIs including 0 are non-significant. For example, the coefficient relating baseline TGF-β to over time TGF-β indicates that for a 5-unit increase in baseline TGF-β, TGF-β at 2nd and 3rd trimesters increased by 81.3%, adjusting for baseline 25(OH)D, maternal race, smoking status, age, trimester and BMI at visit 1. Complete results are presented in Supplemental Table S3 (online).
| Covariates | Immune-mediators | |||||||
|---|---|---|---|---|---|---|---|---|
|
| ||||||||
| IFN-γ | CRP | TGF-β | TNF-α | VEGF | IL-2 | IL-4 | IL-10 | |
|
| ||||||||
| 25(OH)D (5-unit increase) | 1.67 (−2.11, 5.31) |
−1.02 (−4.24, 2.09) |
−0.16 (−2.35, 1.97) |
0.78 (−1.98, 3.46) |
0.11 (−3.02, 3.14) |
−3.41 (−8.44, 1.40) |
1.35 (−2.21, 4.78) |
1.42 (−2.11, 4.82) |
|
| ||||||||
|
Baseline immune-mediator
(5-unit increase) |
90.6*** (82.2, 95.1) |
94.4*** (91.1, 96.4) |
81.3*** (66.0, 89.7) |
98.1*** (97.1, 98.7) |
98.3*** (97.0, 99.0) |
68.1* (21.4, 87.0) |
36.7* (0.35, 59.8) |
29.9 (−11.6, 56.0) |
|
| ||||||||
|
Baseline 25(OH)D (5-unit increase) |
10.4** (4.00, 16.5) |
1.60 (−4.19, 7.07) |
3.62† (−0.52, 7.58) |
0.35 (−6.67, 6.91) |
1.01 (−5.37, 7.01) |
12.2** (4.05, 19.6) |
−3.66 (−10.5, 2.80) |
−1.54 (−8.12, 4.63) |
|
| ||||||||
| Race | ||||||||
| Black vs. White | 12.1 (−17.1, 34.0) |
−2.66 (−29.9, 18.9) |
4.51 (−13.7, 19.8) |
2.79 (−30.5, 27.6) |
4.78 (−24.2, 27.0) |
3.97 (−38.2, 33.3) |
−11.5 (−45.3, 14.5) |
−5.02 (−36.0, 18.9) |
| Black vs. Hispanic | 23.5† (−1.04, 42.1) |
−15.8 (−45.5, 7.90) |
−9.97 (−30.7, 7.49) |
25.1† (−0.25, 44.0) |
10.1 (−16.8, 30.9) |
13.8 (−22.8, 39.5) |
13.2 (−12.2, 32.8) |
24.9* (3.51, 41.6) |
| White vs Hispanic | 13.0 (−14.9, 34.1) |
−12.8 (−41.6, 10.2) |
−15.2† (−35.8, 2.35) |
22.9† (−2.61, 42.1) |
5.63 (−21.6, 26.8) |
10.2 (−27.8, 36.9) |
22.1† (−0.46, 39.6) |
28.5** (8.32, 44.3) |
|
| ||||||||
|
Smoking status Current/Former vs. Never |
−29.2† (−72.0, 2.97) |
−9.76 (−38.9, 13.2) |
21.3** (6.38, 33.8) |
−50.9** (−101, −13.1) |
−4.86 (−35.5, 18.8) |
−1.01 (−42.6, 31.3) |
−20.8 (−56.6, 6.73) |
15.2 (−9.15, 34.1) |
p < 0.10
p < 0.05
p < 0.01
p < 0.001
Figure 3:
Mean profiles for observed plasma immune-mediator concentrations at the 1st, 2nd, and 3rd trimesters for 400 and 4400 IU vitamin D3/day groups. Bars represent standard error.
* Baseline immune-mediator is associated with immune-mediator concentrations at 2nd and 3rd trimesters
‡ Baseline 25(OH)D is associated with baseline immune-mediator
† Baseline 25(OH)D is associated with immune-mediator concentrations at 2nd and 3rd trimesters
Discussion
Principal findings
Our study demonstrated that supplementation with vitamin D during pregnancy positively impacts 25(OH)D concentrations throughout pregnancy, with the greatest increase in the 4400 IU group. The impact of supplementation on 25(OH)D concentrations in 2nd and 3rd trimesters remained significant after adjusting for additional covariates known to be associated with 25(OH)D. Contrary to our hypothesis, these increases were not associated with changes in immune-mediator concentrations in 2nd and 3rd trimesters. Maternal vitamin D status at baseline (i.e. before supplementation), however, was associated with concentrations of TGF-β at baseline and IFN-γ and IL-2 in 2nd and 3rd trimesters. Baseline immune-mediator concentrations for most immune-mediators were positively associated with their concentrations during later pregnancy. Also, of note, race was associated with baseline TGF-β, VEGF and IL-10 and with IL-10 in 2nd and 3rd trimesters. While some subjects did not achieve ‘immune sufficiency’ throughout pregnancy as their 25(OH)D concentration was below 30 ng/mL, racial differences were observed in immune response even after controlling for 25(OH)D, which suggests that racial differences in immune response are not entirely due to 25(OH)D.
Comparison with other relevant studies
Our data suggest that vitamin D sufficiency in early pregnancy, possibly even before pregnancy, may be crucial for modulation of clinical and immune outcomes. Recent reports have suggested that high-dose supplementation during pregnancy may not be sufficient and that higher maternal vitamin D status in the 1st trimester is also important to significantly impact clinical outcomes. For example, the VDAART clinical trial demonstrated that high-dose vitamin D supplementation, initiated at 10–18 weeks of gestation, increased circulating 25(OH)D but did not impact the risk of preeclampsia (40); however, serum 25(OH)D levels ≥30 ng/mL during both early (10–18 weeks) and late (32–38 weeks) pregnancy were associated with a decreased risk of preeclampsia (40). In the same cohort, supplementing mothers with high-dose vitamin D did not significantly reduce the risk of recurrent wheeze and asthma in their children at three years of age (41). However, the risk of wheezing and asthma was significantly lower in children of mothers with initial 25(OH)D levels ≥30 ng/mL randomized to 4400 IU than in children of mothers with initial 25(OH)D levels ≤20 ng/mL randomized to 400 IU (42).
Several reports have shown allelic differences in single-nucleotide polymorphisms for TGF-β and IL-10 between Blacks and Whites (43–45). Yet, to our knowledge, our study is the first to examine how TGF-β, VEGF and IL-10 concentrations change during pregnancy across racial/ethnic groups after vitamin D supplementation.
Research implications
Evaluating the relationship between circulating 25(OH)D and inflammatory markers, we examined whether or not a dose response exists. As noted in our results (Table 4), this was only observed with baseline cytokine levels and may suggest that immune response as measured by cytokine blood concentrations during pregnancy is unaffected by increased circulating 25(OH)D or that the latency period for immune response to increased circulating 25(OH)D during pregnancy is longer than the observation window for these participants.
While we did not observe associations between 25(OH)D and plasma immune-mediators in 2nd and 3rd trimesters, an in vivo study (46) has shown conventional associations between vitamin D and immune-mediators collected from placenta in an animal model. Therefore, the placenta could be a better site for studying immune-mediator regulation by vitamin D and its metabolites. Future studies focused on associations for immune-mediators collected from placenta will be needed for a holistic picture of immune-mediators during pregnancy.
The ability of higher 25(OH)D to affect immune-mediator levels could also be conditional on the mother having a robust immune status during early pregnancy. In an in vivo study (46), altered vitamin D status alone had little impact on serum concentrations of immune-mediators in pregnant mice. More immune-mediator differences were observed, however, when pregnant mice were immune-challenged during pregnancy (46). Our study showed an association between baseline immune-mediator concentrations and both baseline vitamin 25(OH)D and immune-mediator concentrations in 2nd and 3rd trimesters, suggesting that both higher baseline 25(OH)D and robust baseline immune status are crucial in immune-mediator regulation.
Further complicating the examination of 25(OH)D and immune-mediators during pregnancy is the difficulty of determining which immune-mediators are linked to vitamin D. For example, multiplex analysis in an in vivo study (46) revealed that IL-1β and IL-18 were suppressed only in non-pregnant mice while grown hormone (GH) was elevated in only vitamin D-deficient pregnant mice. Thus, it is plausible that profiles for immune-mediators associated with vitamin D status in non-pregnant populations are not linked to vitamin D in pregnant populations. Future studies evaluating additional immune-mediators are needed to further elucidate the role of immune-mediators during pregnancy.
It is also important to note that circulating immune-mediators do not provide information on what is happening at the cellular, tissue or organ level, but rather give us a broader perspective on changes that occur during pregnancy. Although we did not evaluate the impact of changing immune-mediator concentrations on comorbidities of pregnancy, other studies (11–19) have shown that changing immune-mediator concentrations can impact comorbidities of pregnancy. Future studies that focus on associations for immune mediators collected from cellular and tissue levels can be useful in understanding the overall impact of immune-mediators during pregnancy.
These findings hint that vitamin D supplementation before or early during pregnancy may help regulate immune-mediator profiles during pregnancy. Future RCTs that supplement women before pregnancy are needed to confirm these findings.
Strengths and Limitations
Previous studies have examined immune-mediator response for certain immune-mediators, such as IL-2 and IFN-γ in small cohort studies (47), but none has considered the impact of vitamin D or examined the racial/ethnic differences in the regulation process. Our study is novel in that it assesses the impact of vitamin D supplementation on multiple immune-mediators in pregnant women of different racial/ethnic groups using longitudinal data from a relatively large RCT. Other strengths of our study are its randomization of women to two different doses of vitamin D supplementation, a relatively large sample size, and repeated evaluation of plasma immune-mediator concentrations in participants throughout pregnancy.
One limitation of the study, however, was that not all participants were evaluated for immune-mediator profiles at every trimester. Also, concentrations for the nine immune-mediators were undetectable in 0–34% of participants at any trimester. While values below the limit of detection were imputed, we acknowledge the limitation of this approach and the impact on the power of our study to detect associations between 25(OH)D concentration and immune-mediator response in later pregnancy. As such, the analyses performed here are exploratory and need to be verified by additional studies. Also, a previous study showed that uptake and function of 25(OH)D by monocytes is inhibited by vitamin D binding protein (DBP) (48). Although it is possible that DBP could influence immune responses to 25(OH)D, the current study did not include analysis of circulating levels of DBP. Moreover, it is unclear whether during pregnancy measurement of DBP at a single time point is adequate to assess cellular bioavailability of 25(OH)D. Current strategies for determining the bioavailable or ‘free’ concentrations of 25(OH)D are based on the measurement of serum DBP and 25(OH)D concentration and subsequent calculations based on established equations. However, it is known that DBP measurements increase during pregnancy and so ‘free’ 25(OH)D may vary significantly. At present, direct measurement of free 25(OH)D does not appear to be truly reproducible, and so we cannot be certain whether DBP influences 25(OH)D function during pregnancy or not. Also, the mechanisms of vitamin D supplementation leading to altered immune function during pregnancy was not explored in this study. These, however, are important questions that we plan to consider in future studies.
Finally, because we assessed only circulating plasma concentrations of immune-mediators, we could not investigate whether significant effects are observed in response to immune stimulation or in relevant tissues such as the placenta.
Conclusions and clinical implications
This is the first study examining the impact of vitamin D supplementation throughout pregnancy on immune-mediator profiles in pregnant women. While we observed improved vitamin D status in both treatment and control groups after supplementation, the observed increase in 25(OH)D did not significantly impact immune-mediator profiles. However, baseline immune-mediator concentrations were associated with baseline 25(OH)D and with immune-mediator concentrations in 2nd and 3rd trimesters.
It is therefore plausible that higher baseline 25(OH)D concentrations, along with a robust baseline immune status, are beneficial in modulating immune-mediator profiles and maintaining a healthy pregnancy. These findings suggest that vitamin D supplementation before or early during pregnancy rather than at the first prenatal visit may be more beneficial in regulating immune-mediator profiles during pregnancy. Future RCTs focusing on the impact of vitamin D supplementation before conception and during early pregnancy on immune-mediator profiles will further our understanding of how vitamin D affects not only immune-mediator profiles, but also pregnancy-related comorbidities. We also observed novel ethnic/racial differences in immune-mediator concentrations at baseline and in 2nd and 3rd trimesters that merit further investigation in future genetic studies.
Supplementary Material
IMPACT:
In this vitamin D supplementation clinical trial, baseline (1st trimester) but not increasing plasma 25(OH)D concentration impacted select plasma immune-mediator profiles in pregnant women.
Baseline 25(OH)D was associated with baseline TGF-β and with IFN-γ and IL-2 at 2nd and 3rd trimesters.
Baseline IFN-γ, CRP, TGF-β, TNF-α, VEGF, IL-2 and IL-4 were associated with concentrations at 2nd and 3rd trimesters for respective immune-mediators; however, 25(OH)D concentration at 2nd and 3rd trimesters were not.
Some racial differences existed in immune-mediator concentrations at baseline and at 2nd and 3rd trimesters.
What this study adds to the existing literature:
This study assesses the impact of vitamin D supplementation on multiple immune-mediators in pregnant women of different racial/ethnic groups using longitudinal data from a relatively large randomized controlled trial.
This study found that race was associated with baseline TGF-β, VEGF and IL-10 and with IL-10 at 2nd and 3rd trimesters, a novel finding that sheds light where relationships were less well-defined.
Impact of this study:
The results of this study suggest that vitamin D supplementation before conception or early in pregnancy, rather than during pregnancy may be necessary to significantly impact immune-mediator response.
This study sets premise for future clinical trials to evaluate the effect of vitamin D supplementation before conception or prior to pregnancy.
Acknowledgments
We would like to thank Patrick Simpson, MS, of the W. K. Kellogg Foundation and Myla D. Ebeling, data manager in the Department of Pediatrics at the Medical University of South Carolina (MUSC), for their support during the conduct of this study. We also would like to thank the women who participated in this study, without whose participation we could not have learned what we did. We also are thankful to Church & Dwight, Inc. (Princeton, NJ), who provided the vitamin D and placebo gummies for this study. This publication also was supported, in part, by the Kellogg Foundation and the South Carolina Clinical & Translational Research Institute (SCTR), MUSC’s Clinical and Translational Science Awards Hub, NIH/NCRR Grant Number 1UL1TR001450. The contents are solely the responsibility of the authors and do not necessarily represent the official views of the Kellogg Foundation, NIH or NCRR.
Funding: W. K. Kellogg Foundation Grant P3020828, “Preventing Health Disparities during Pregnancy through Vitamin D supplementation”; South Carolina Clinical & Translational Research Institute (SCTR), NIH/NCRR 1UL1TR001450
Footnotes
Disclosure: None of the authors have a conflict of interest to declare.
Patient Consent: Patient consent was required for this study and all participants provided written, informed consent prior to their enrollment in the study.
Category of study: Clinical research article
References
- 1.Hollis BW, Johnson D, Hulsey TC, Ebeling M. & Wagner CL Vitamin D supplementation during pregnancy: Double-blind, randomized clinical trial of safety and effectiveness. J. Bone Miner. Res 26, 2341–2357 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Wagner CL et al. A randomized trial of vitamin D supplementation in 2 community health center networks in South Carolina. Am. J. Obstet. Gynecol 208, 137.e1–137.e13 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Wagner CL et al. Health characteristics and outcomes of two randomized vitamin D supplementation trials during pregnancy: A combined analysis. J. Steroid Biochem. Mol. Biol 136, 313–320 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Yılmaz B, Aygün C. & Çetinoğlu E. Vitamin D levels in newborns and association with neonatal hypocalcemia. J. Matern. Neonatal Med 31, 1889–1893 (2018). [DOI] [PubMed] [Google Scholar]
- 5.Antonucci R, Locci C, Clemente MG, Chicconi E. & Antonucci L. Vitamin D deficiency in childhood: old lessons and current challenges. J. Pediatr. Endocrinol. Metab 31, 247–260 (2018). [DOI] [PubMed] [Google Scholar]
- 6.Ozdemir AA & Cag Y. Neonatal Vitamin D status and the risk of neonatal sepsis. Pakistan J. Med. Sci 35, 420–425 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Aletayeb SMH, Dehdashtiyan M, Aminzadeh M, Malekyan A. & Jafrasteh S. Comparison between maternal and neonatal serum vitamin D levels in term jaundiced and nonjaundiced cases. J. Chinese Med. Assoc 79, 614–617 (2016). [DOI] [PubMed] [Google Scholar]
- 8.Herrera-Muñoz A, Fernández-Alonso AM, Fischer-Suárez N, Chedraui P. & Pérez-López FR Maternal serum cytokine levels in pregnancies complicated with threatened preterm labour. Gynecol. Endocrinol 33, 408–412 (2017). [DOI] [PubMed] [Google Scholar]
- 9.Velez DR et al. Patterns of cytokine profiles differ with pregnancy outcome and ethnicity. Hum. Reprod 23, 1902–1909 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ragab D, Soliman D, Samaha D. & Yassin A. Vitamin D status and its modulatory effect on interferon gamma and interleukin-10 production by peripheral blood mononuclear cells in culture. Cytokine 85, 5–10 (2016). [DOI] [PubMed] [Google Scholar]
- 11.Stoffels K. et al. Immune Regulation of 25-Hydroxyvitamin-D3–1α-Hydroxylase in Human Monocytes. J. Bone Miner. Res 21, 37–47 (2005). [DOI] [PubMed] [Google Scholar]
- 12.Ruiz R. et al. Second trimester maternal plasma levels of cytokines Il-1Ra, IL-6 and IL-10 and preterm birth. J. Perinatol 32, 483–490 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Szarka A, Rigó J, Lázár L, Beko G. & Molvarec A. Circulating cytokines, chemokines and adhesion molecules in normal pregnancy and preeclampsia determined by multiplex suspension array. BMC Immunol. 11, 1–9 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lau SY et al. Tumor necrosis factor-alpha, interleukin-6, and interleukin-10 levels are altered in preeclampsia: A systematic review and meta-analysis. Am. J. Reprod. Immunol 70, 412–427 (2013). [DOI] [PubMed] [Google Scholar]
- 15.Lygnos MC et al. Changes in maternal plasma levels of VEGF, bFGF,TGF-β1, ET-1 and sKL during uncomplicated pregnancy, hypertensive pregnancy and gestational diabetes. In Vivo (Brooklyn). 20, 157–164 (2006). [PubMed] [Google Scholar]
- 16.Clausen T. et al. Altered plasma concentrations of leptin, transforming growth factor-β1 and plasminogen activator inhibitor type 2 at 18 weeks of gestation in women destined to develop pre-eclampsia. Circulating markers of disturbed placentation? Placenta 23, 380–385 (2002). [DOI] [PubMed] [Google Scholar]
- 17.Entringer S. et al. Influence of Prenatal Psychosocial Stress on Cytokine Production in Adult Women. Dev. Psychol 50, 579–587 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Andersson NW et al. Influence of prenatal maternal stress on umbilical cord blood cytokine levels. Arch. Womens. Ment. Health 19, 761–767 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Irani M. et al. Vitamin D decreases serum VEGF correlating with clinical improvement in vitamin D-deficient women with PCOS: A randomized placebo-controlled trial. Nutrients 9, (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Zhang Y. et al. Vitamin D Inhibits Monocyte/Macrophage Proinflammatory Cytokine Production by Targeting MAPK Phosphatase-1. J. Immunol 188, 2127–2135 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Heine G. et al. 1,25-dihydroxyvitamin D 3 promotes IL-10 production in human B cells. Eur. J. Immunol 38, 2210–2218 (2008). [DOI] [PubMed] [Google Scholar]
- 22.Wei R. & Christakos S. Mechanisms Underlying the Regulation of Innate and Adaptive Immunity by Vitamin D. Nutrients 7, 8251–8260 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Barker T. et al. Supplemental vitamin D increases serum cytokines in those with initially low 25-hydroxyvitamin D: A randomized, double blind, placebo-controlled study. Cytokine 71, 132–138 (2015). [DOI] [PubMed] [Google Scholar]
- 24.Jeffery LE et al. 1,25-Dihydroxyvitamin D3 and IL-2 Combine to Inhibit T Cell Production of Inflammatory Cytokines and Promote Development of Regulatory T Cells Expressing CTLA-4 and FoxP3. J. Immunol 183, 5458–5467 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Gubatan J. et al. Higher serum vitamin D levels are associated with protective serum cytokine profiles in patients with ulcerative colitis. Cytokine 103, 38–45 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Vijayendra Chary A. et al. Vitamin D deficiency in pregnant women impairs regulatory T cell function. J. Steroid Biochem. Mol. Biol 147, 48–55 (2015). [DOI] [PubMed] [Google Scholar]
- 27.Cantorna M, Snyder L, Lin Y-D & Yang L. Vitamin D and 1,25(OH)2D Regulation of T cells. Nutrients 7, 3011–3021 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Liao S. et al. Maternal Vitamin D Level Is Associated with Viral Toll-Like Receptor Triggered IL-10 Response but Not the Risk of Infectious Diseases in Infancy. Mediators Inflamm. 2016, 1–8 (2016). [DOI] [PMC free article] [PubMed]
- 29.Hornsby E. et al. Vitamin D supplementation during pregnancy: Effect on the neonatal immune system in a randomized controlled trial. J. Allergy Clin. Immunol 141, 269–278.e1 (2018). [DOI] [PubMed] [Google Scholar]
- 30.Chandler PD et al. Impact of Vitamin D Supplementation on Inflammatory Markers in African Americans: Results of a Four-Arm, Randomized, Placebo-Controlled Trial. Cancer Prev. Res 7, 218–225 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Gillespie SL, Porter K. & Christian LM Adaptation of the inflammatory immune response across pregnancy and postpartum in Black and White women. J. Reprod. Immunol 114, 27–31 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Newton DA et al. Vitamin D binding protein polymorphisms significantly impact vitamin D status in children. Pediatr. Res (2019) doi: 10.1038/s41390-019-0322-y. [DOI] [PMC free article] [PubMed]
- 33.Jefferson KK et al. Relationship between vitamin D status and the vaginal microbiome during pregnancy. J. Perinatol 1–13 (2019) doi: 10.1038/s41372-0190343-8. [DOI] [PMC free article] [PubMed]
- 34.Ozkan ZS et al. Plasma IL-17, IL-35, interferon-γ, SOCS3 and TGF-β levels in pregnant women with preeclampsia, and their relation with severity of disease. J. Matern. Neonatal Med 27, 1513–1517 (2014). [DOI] [PubMed] [Google Scholar]
- 35.Yusupov E. et al. Vitamin D and serum cytokines in a randomized clinical trial. Int. J. Endocrinol. 2010, (2010). [DOI] [PMC free article] [PubMed]
- 36.Scott MG, Davidson DJ, Gold MR, Bowdish D. & Hancock REW The Human Antimicrobial Peptide LL-37 Is a Multifunctional Modulator of Innate Immune Responses. J. Immunol 169, 3883–3891 (2002). [DOI] [PubMed] [Google Scholar]
- 37.Mitchell C, Gottsch ML, Liu C, Fredricks DN & Nelson DB Associations between vaginal bacteria and levels of vaginal defensins in pregnant women. Am. J. Obstet. Gynecol 208, 132.e1–132.e7 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Perry LM et al. Differences in total and allergen specific IgE during pregnancy compared with 1 month and 1 year post partum. Ann. Allergy, Asthma Immunol. 103, 342–347 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Hohlagschwandtner M. et al. Basic fibroblast growth factor and hypertensive disorders in pregnancy. Hypertens. Pregnancy 21, 235–241 (2002). [DOI] [PubMed] [Google Scholar]
- 40.Mirzakhani H. et al. Early pregnancy vitamin D status and risk of preeclampsia. J. Clin. Invest 126, 4702–4715 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Litonjua AA et al. Effect of Prenatal Supplementation With Vitamin D on Asthma or Recurrent Wheezing in Offspring by Age 3 Years. JAMA 315, 362 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Wolsk HM et al. Vitamin D supplementation in pregnancy, prenatal 25(OH)D levels, race, and subsequent asthma or recurrent wheeze in offspring: Secondary analyses from the Vitamin D Antenatal Asthma Reduction Trial. J. Allergy Clin. Immunol 140, 1423–1429.e5 (2017). [DOI] [PubMed] [Google Scholar]
- 43.Van Dyke AL, Cote ML, Wenzlaff AS, Land S. & Schwartz AG Cytokine SNPs: Comparison of allele frequencies by race and implications for future studies☆. Cytokine 46, 236–244 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Hoffmann SC et al. Ethnicity greatly influences cytokine gene polymorphism distribution. Am. J. Transplant 2, 560–567 (2002). [DOI] [PubMed] [Google Scholar]
- 45.Hassan MI, Aschner Y, Manning CH, Xu J. & Aschner JL Racial differences in selected cytokine allelic and genotypic frequencies among healthy, pregnant women in North Carolina. Cytokine 21, 10–16 (2003). [DOI] [PubMed] [Google Scholar]
- 46.Liu NQ et al. Vitamin D-deficiency and sex-specific dysregulation of placental inflammation. J. Steroid Biochem. Mol. Biol 177, 223–230 (2018). [DOI] [PubMed] [Google Scholar]
- 47.Denney JM et al. Longitudinal modulation of immune system cytokine profile during pregnancy. Cytokine 53, 170–177 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Chun RF et al. Vitamin D-binding protein directs monocyte responses to 25-hydroxy- and 1,25-dihydroxyvitamin D. J. Clin. Endocrinol. Metab 95, 3368–3376 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.