Abstract
In line with the substantial increase in the broiler industry worldwide, Clostridium perfringens-induced necrotic enteritis (NE) became a continuous challenge leading to high economic losses, especially after banning antimicrobial growth promoters in feeds by many countries. The disease is distributed worldwide in either clinical or subclinical form, causing a reduction in body weight or body weight gain and the feed conversion ratio, impairing the European Broiler Index or European Production Efficiency Factor. There are several predisposing factors in the development of NE. Clinical signs varied from inapparent signs in case of subclinical infection (clostridiosis) to obvious enteric signs (morbidity), followed by an increase in mortality level (clostridiosis or clinical infection). Clinical and laboratory diagnoses are based on case history, clinical signs, gross and histopathological lesions, pathogenic agent identification, serological testing, and molecular identification. Drinking water treatment is the most common route for the administration of several antibiotics, such as penicillin, bacitracin, and lincomycin. Strict hygienic management practices in the farm, careful selection of feed ingredients for ration formulation, and use of alternative antibiotic feed additives are all important in maintaining broiler efficiency and help increase the profitability of broiler production. The current review highlights NE caused by C. perfringens and explains the advances in the understanding of C. perfringens virulence factors involved in the pathogenesis of NE with special emphasis on the use of available antibiotic alternatives such as herbal extracts and essential oils as well as vaccines for the control and prevention of NE in broiler chickens.
Key words: Antibiotic alternatives, broiler chickens, Clostridium perfringens, necrotic enteritis, organic poultry
INTRODUCTION
Digestive tract infections are a major concern in the poultry industry and have led to severe economic losses (Salem and Attia, 2021). Necrotic enteritis (NE) in either clinical or subclinical form is a major enteric poultry disease that has a detrimental effect on profitability in the broiler industry (Bansal et al., 2021; Salem et al., 2021). A survey by Van der Sluis (2000) estimated that the cost of subclinical NE can be as high as 5 cents per bird, and NE outbreaks have the potential to cost the global broiler industry approximately $2 billion per year.
Moreover, Timbermont et al. (2011) estimated the annual cost of NE to the global poultry industry is approximately $6 billion, which included the cost of output losses and control steps. Before the current name, Clostridium perfringens was first called Bacillus welchii and then renamed Clostridium welchii, which was isolated from intestinal lesions of Black Orpington pullets in Australia (Bennetts, 1930). Thereafter, the disease was induced by feeding C. perfringens culture to chickens and called the “six-day disease,” as the bacteria were observed to infect the intestinal mucosa of birds (Johansson and Sarles, 1948). However, the term “necrotic enteritis” was coined and reported by Parish in 1961 in England. Several methods had been developed to control NE following its discovery. Antibiotics were first used for NE prevention and treatment and then used as growth promoters to increase weight gain and feed efficiency, which was believed to have reduced the incidence of NE (Prescott et al., 1978).
Antibiotic growth promoters (AGPs) have been widely used in food animal production for decades (Stokstad and Jukes, 1950). The United States Food and Drug Administration (FDA) approved the use of antibiotics in animal feed in 1951 (Jones and Ricke, 2003). Antibacterial drugs, such as avoparcin, lincomycin, amoxicillin, tylosin, virginiamycin, and bacitracin, were commonly used for the treatment and prevention of NE (Craven et al., 2001; McDevitt et al., 2006). However, after observing the effects of AGPs on the development and expansion of drug-resistant bacteria, people started to understand its potential threat, and there was increased pressure to decrease antimicrobial use in poultry production. Even though the primary threat was the dramatic increase in the emergence of bacterial antibiotic resistance, the effect of drug withdrawal was not negligible (Hershberger et al., 2005). The European Union implemented Regulation (EC) No. 1831/2003 in 2006 to outlaw the use of antimicrobial growth promoters in food. The FDA recently announced that it will implement legislation called the Veterinary Feed Directive (VFD) drugs (Department of Health and Human Services, 2015). This legislation will ensure that the use of antimicrobials, which are known as important for human health, is completely prohibited for production purposes and require veterinarian authorization for control and treatment of disease. Large-scale businesses always source meat of animals raised without antimicrobial input, which puts pressure on poultry producers to reduce the use of in-feed antimicrobials in their systems.
In the broiler meat industry, the removal of AGPs from commercial farms dramatically increased the incidence of economically important diseases, such as NE (Casewell et al., 2003). For example, after the withdrawal of avoparcin as an antibacterial feed additive in Norway, an increase in NE cases was observed. Moreover, 0% of flocks raised in the conventional system had NE, whereas 27% of drug-free flocks had clinical NE and 49% had subclinical NE (Gaucher et al., 2015). These results support the idea that removal of these feed additives increases the incidence of intestinal disorders, specifically NE (Sarson et al., 2009).
Although strict biosecurity practices without AGPs can maintain production in some farms (Engster et al., 2002), it is difficult to rely on this approach for every farm. Therefore, AGP alternatives are much needed to control NE and other diseases. The drug-free program used essential oil alternatives rather than in-feed antimicrobials and coccidiostats, which were used in conventional systems (Grave et al., 2004; El-Shall et al., 2022). Therefore, this review throws light on C. perfringens virulence factors involved in the pathogenesis of NE, available vaccines and emphasizes the use of herbal medicine as an alternative to antibiotics for the control and prevention of NE in broiler chickens.
CLOSTRIDIUM PERFRINGENS RISK FACTORS
The C. perfringens is responsible for the enteric disorder in animals and humans (Songer, 1996; Khelfa et al., 2012b). It can be found anywhere, commonly in wastewater, dust, air, and healthy human and animal intestinal tracts (Khelfa et al., 2012a). NE mostly occurs in broiler chicks between 2 and 6 wk of age and is caused by C. perfringens, anaerobic, Gram-positive, endospore-forming, nonmotile bacteria, which could form resistant endospores, allowing it to survive and remain persistent in extreme environmental conditions, such as decaying organic matter and soil (Novak et al., 2003; Khelfa et al., 2015). Since C. perfringens cannot synthesize several essential amino acids, it releases enzymes that degrade host tissue to meet its demand (Shimizu et al., 2002).
In humans and animals, the ability of C. perfringens to release toxins, grow very fast in a wide range of temperatures, and form endospores enable them to induce disease. C. perfringens induces NE by adhering to the small intestine villi, multiplying, and releasing toxins that cause necrosis (Shimizu et al., 2002).
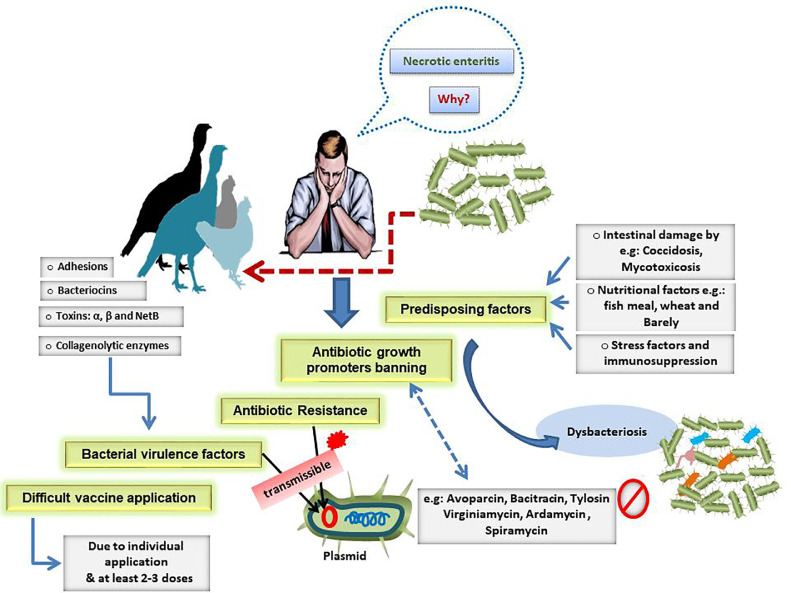
The risk factors or causes of the current worldwide high prevalence necrotic enteritis are illustrated in Figure 1.
Figure 1.
Risk factors or causes of the current worldwide high prevalence necrotic enteritis.
TYPES OF TOXINS PRODUCED BY C. PERFRINGENS
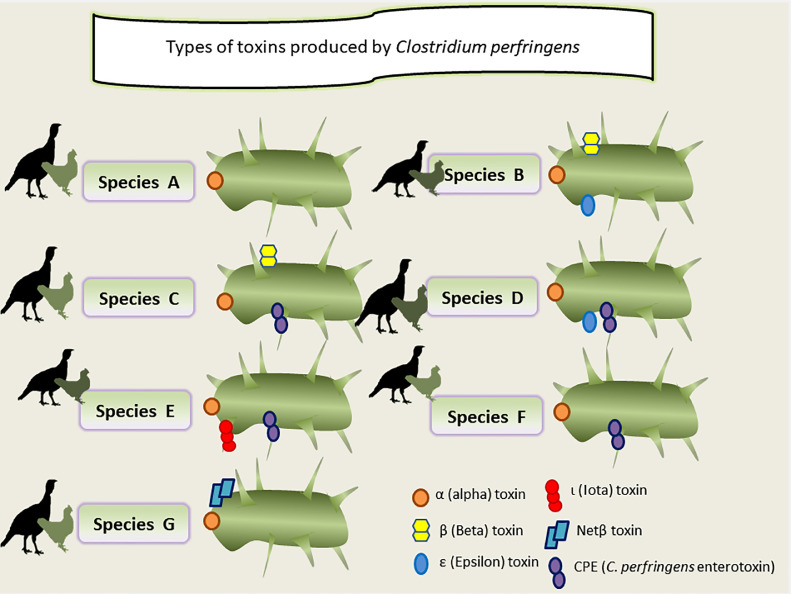
Toxins released by C. perfringens play a vital function in the incidence of NE. To date, more than 20 different types of C. perfringens toxins have been studied (Li et al., 2013). Due to the existence of encoding genes for C. perfringens alpha [α] (CPA or α (cpa), C. perfringens beta [β] (CPB or β (cpb), ETX or ε (etx) (epsilon [ε]), and ITX or ι (cpI) (iota [ι]) toxins, and the enterotoxin that was recently added, CPE or (cpe), β2 (cpb2), and NE B-like toxin (NetB), poultry C. perfringens is categorized into 7 types (A–G) (Figure 2) (Songer, 1996; Opengart, 2020).
Figure 2.
Classification of Clostridium perfringens according to the production of major exotoxins.
C. perfringens type A produces the α-toxin; type B produces the α-, β-, and ε-toxins; type C produces the α- and β-toxins; type D produces the α- and ε-toxins; type E produce the α- and ι-toxins; and all types can produce the enterotoxin and β2-toxin. Each toxin type is associated with a particular human or animal disease, which suggests that C. perfringens virulence is linked to toxin and enterotoxin development (Smedley et al., 2004).
The differences between genotypes and their associated toxins explain the wide spectrum of diseases associated with C. perfringens. For example, C. perfringens type A can cause gas gangrene in humans and intestinal diseases in both humans and animals, while in domestic animals and humans, type C can cause mucosal necrosis of the small intestine (Petit et al., 1999). C. perfringens types A, C, and G are of particular interest to the poultry industry because they have been associated with diseases in avian species (Opengart, 2020). The classification and types and toxins produced by C. perfringens are illustrated in Figure 2.
Alpha Toxins
Of all types of toxins, α-toxin is the only main toxin produced by all types of C. perfringens. Genes of C. perfringens toxins can be found in both chromosomes and plasmids. Alpha toxin isolated from C. perfringens was shown in early work to have enzymatic activity, indicating that it can catalyze reactions at its site of action (MacFarlane and Knight, 1941).
Thus, the α-toxin is considered as the first known enzyme and main fatal bacterial toxin. The α-toxin is a phospholipase C sphingomyelinase enzyme that hydrolyzes the cell membrane's phospholipids, causing cell death. Thus, the biological activity of α-toxin has been described as cytolytic, hemolytic, dermonecrotic, and lethal (Petit et al., 1999; Sakurai et al., 2004). Even though the α-toxin is responsible for myonecrosis of gas gangrene, its effect on NE is controversial (Awad et al., 1995). Of the known seven C. perfringens types, both A and C were the main prevalent causative agents of NE (Cooper et al., 2010).
In the type A strains, the α-toxin is essential in the pathogenesis of C. perfringens. In another study, infected chickens had shown higher α-toxin levels compared to uninfected chickens (Hofshagen and Stenwig, 1992). Inoculation of germ-free chickens with either a broth culture supernatant or purified α-toxin of C. perfringens type A-induced mortality and intestinal lesions, whereas with the use of an α-toxin and neutralization with an anti-α-toxin serum did not result in deaths in chickens (Fukata et al., 1988).
Although C. perfringens type A strains are usually found in the environment and healthy chicken gut, virulent strains produce much higher α-toxin levels. Rehman et al. (2009) determined in their in vitro study that the intestinal mucosal barrier function was impaired by α-toxin. However, since these studies were based on crude or partially purified toxins, the toxins can still have other co-purifying proteins (Keyburn et al., 2006). Cooper and Songer (2009) have shown that the development of NE lesions was not associated with the volume of in vitro α-toxin production, even though immunization with recombinant α-toxin provided partial protection against C. perfringens-induced NE (Cooper and Songer, 2009).
However, another in vitro study using C. perfringens isolated from diseased and healthy chickens showed no difference in α-toxin production (Keyburn et al., 2006). Until 2006, the major virulence factor was thought to be α-toxin, but it has since been shown that mutant isolates without the α-toxin gene can also produce NE in broilers (Keyburn et al., 2006). Therefore, it remains uncertain whether α-toxin is the main virulence factor of NE.
The α-toxin binds with GM1a, a ganglioside on the cell membrane (Oda et al., 2012). This induces an accumulation of diacylglycerol molecules in the cell membrane, leading to activation of tyrosine kinase A, which induces the release of interleukin-8 (Oda et al., 2012). The action of α-toxin produces a source of cholesterol for the binding of another toxin, perfringolysin O (PFO), also known as theta-toxin (Moe and Heuck, 2010). Perfringolysin O belongs to the family of cholesterol-dependent cytolysins. Members of this toxin family share 40 to 80% of their structural identity and have similar biological properties (Popoff, 2014).
Perfringolysin O is also part of a toxin family known as the thiol-activated cytolysin family, which are produced by Gram-positive bacteria and may work to synergize the effects of α-toxin (Billington et al., 2000). A total of 40 to 50 monomer subunits of PFO, produced by C. perfringens, oligomerize on the cell surface where there is a source of cholesterol and then insert a transmembrane domain, creating a pore (Shepard et al., 2000). This allows for the passage of ions and macromolecules in and out of the cell (Billington et al., 2000).
Perfringolysin O from C. perfringens type A was shown in experimental models of gas gangrene to destroy host tissue and inflammatory cells in the area. As the toxin spreads, it can diffuse into the systemic circulation where adhesion molecules are altered on polymorphonuclear leukocytes. This is thought to cause vascular leukostasis and regional tissue hypoxia (Bryant et al., 1993).
Necrotic Enteritis B-like Toxins
NE B-like toxin is a member of the α-hemolysin family of β-pore-forming toxins, and it was isolated from C. perfringens type A strain in NE birds. The name NetB is given because of the similarity to C. perfringens β-toxin. The pore-forming toxin NetB, not α-toxin, produced by some C. perfringens type A and G isolates was believed to be an indispensable virulence factor in NE pathogenesis (Keyburn et al., 2008).
Researchers found similar induced NE between the wild-type strain and α-toxin negative mutants isolated from NE birds (Cheung et al., 2010). Moreover, in vitro experiment using LMH cells showed that NetB toxin causes cell rounding and lysing. These results challenged the view that α-toxin is the only virulence factor in NE pathogenesis. However, NetB negative bacteria were also detected in NE birds (Chalmers et al., 2008a), and in another experimental NE model, NetB negative C. perfringens failed to induce NE (Timbermont et al., 2011).
Broilers infected with an α-toxin mutant were NE positive after the experimental challenge, indicating that this toxin was not solely responsible for virulence in chickens (Keyburn et al., 2006). The discovery of NetB has initiated novel lines of enquiry into NE in broilers. This toxin has been identified in several field cases (Chalmers et al., 2008b; Martin and Smyth, 2009; Johansson et al., 2010). NetB has a limited sequence like other pore-forming toxins but does share sequence identity with β- and δ-toxins from C. perfringens (38 and 40%, respectively) and α- and γ-hemolysin from Staphylococcus aureus (Nagahama et al., 2015). Seven subunits of NetB come together at the cell membrane to form a pore, which is similar to the assembly and action of α-hemolysin (Savva et al., 2013). NetB is enhanced when there is a source of cholesterol present, but the receptor that this toxin utilizes to bind with the cell is still unknown (Popoff, 2014).
The percentage of isolates containing the NetB toxin (in healthy and infected birds) varies between countries. In Australia, 70% of C. perfringens isolated from diseased birds have been shown to possess the NetB gene, and all of these isolates produced NetB in vitro (Keyburn et al., 2010). In the United States, 58% of NE isolates were found in birds, and 9% of isolates from healthy birds were positive for NetB toxin (Martin and Smyth, 2009). However, 35% of isolates from healthy birds also contained the NetB gene (Chalmers et al., 2008b). A study in Denmark showed that 61% of C. perfringens isolates from healthy birds contained NetB. The prevalence of the NetB gene in diseased birds was also low, with 52% of tested isolates containing the gene.
The existence of the gene does not preclude the bacteria from producing NetB, as not all isolates positive for the NetB gene produced the toxin in vitro (Abildgaard et al., 2010). However, isolates from diseased birds were more likely to produce this toxin than isolates from healthy birds (Abildgaard et al., 2010).
The activation of many virulence genes within C. perfringens and release of virulence factors are controlled by a two component signal transduction system. This consists of a sensor molecule, VirS, and a responder molecule, VirR. VirS is a transmembrane protein. The extracellular domain senses the external environment of the cell and promotes autophosphorylation of the intracellular domain. This, in turn, causes phosphorylation of VirR in the cytoplasm. NE B-like toxin is regulated by the VirSR system. It is produced after 4 h of inoculation in the late logarithmic phase of bacterial growth (Cheung et al., 2010).
The genes encoding for VirSR were originally discovered for their role in regulating PFO, α-toxin, and sialidase release, but they also regulate several genes involved in macromolecule degradation, which provide nutrients for the bacteria. Other genes that seem to be controlled via this system are involved in nutrient import and metabolism (Shimizu et al., 1994). It remains unclear what activates VirS to promote the release of virulence factors, but inhibition of toxin release could prevent necrotic damage in NE.
C. perfringens has been shown to contain three pathogenicity loci, which may contribute to NE pathogenesis. Locus 1, NELoc1, is located on a plasmid that holds the NetB gene and is approximately 42 kb in length. The other two loci, NELoc2 and NELoc3, are shorter at 11.2 and 5.6 kb, respectively. NELoc3 is also located on a plasmid, but NELoc2 is located in a chromosome (Lepp et al., 2010).
Other Toxins Produced by C. perfringens
The β-toxin has been associated with hemorrhagic mucosal ulceration in humans and animals (Petit et al., 1999). The biological activity of its β-toxin has been described as cytolytic, dermonecrotic, and lethal; however, its mode of action has not yet been defined. The β2 toxin gene is located on the plasmid (Petit et al., 1999).
It was discovered for the first time when a piglet with necrotizing enterocolitis was isolated from C. perfringens type C (Bacciarini et al., 2003). Since its discovery, the β2 toxin has been isolated from healthy and diseased avian species, ruminants (Lebrun et al., 2007), and horses (Herholz et al., 1999). The function of the β2-toxin is still controversial in poultry (Allaart et al., 2012). However, the role of the β2-toxin in intestinal diseases in animals is still controversial (van Asten et al., 2008). In 2007, a study in the Netherlands suggested that the presence of C. perfringens with atypical cpb2 might be associated with subclinical NE in laying hens (Allaart et al., 2012).
The enterotoxin present in some isolates of C. perfringens is linked to gastrointestinal (GI) disease in humans. The etx gene is located on the plasmid (Petit et al., 1999), and its ε-toxin is secreted as an inactive form and converted to a toxic form by proteolytic enzymes (Petit et al., 1999). This toxin binds with claudin molecules, which are components of tight junctions. Enterotoxin is another pore-forming toxin and forms a pre-pore on the cell surface before insertion into the cell (Gao and McClane, 2012).
The formation of these pores allows calcium entry into the cell. At low levels, this induces cell apoptosis, and at high levels, this can cause oncosis, where cells increase in volume and induce inflammatory cell death (McClane and Chakrabarti, 2004). This toxic form is dermonecrotizing, causing diarrhea-related disease in dogs, pigs, horses, and humans (Songer, 1997). The enterotoxin is produced during sporulation and activated after proteolysis, a process that involves removing 24 N-termino amino acids from the molecule (Songer, 1997). Its biological activity has been described as cytotoxic, erythematous, and lethal (Petit et al., 1999). The ε-toxin is essential for the virulence of type D isolate in sheep, goats, and mice (Garcia et al., 2013) but can also be present on type B isolates and is considered one of the most virulent bacterial toxins produced (Alves et al., 2014).
The binary toxin family includes ι-toxin and consists of two separate polypeptides, Ia and Ib, which work together to disrupt the actin cytoskeleton and cause cell death (Songer, 1997; Rood, 1998). These proteins are secreted as promolecules and require activation via proteolytic cleavage of the N-terminal region. Ib interacts with host cell lipoproteins. Once bound, Ia interacts with Ib to promote endocytosis of ι-toxin. In turn, the cytoskeleton of the host cell collapses (Adams et al., 2014).
Other toxins produced by C. perfringens may facilitate the pathogenesis of NE, such as perfrin, TpeL, and mu toxin (Keyburn et al., 2008). Perfrin, a recently discovered bacteriocin, has been described as a possible virulence factor for NE. Bacteriocins are molecules that have antibacterial properties (Nishie et al., 2012). The gene encoding this antimicrobial protein (bacteriocin) was present in several netB positive isolates but not found in netB negative isolates. Perfrin has bactericidal activity against other C. perfringens isolates. It exerts this activity on all isolates without the perfrin gene, indicating that it could be important in promoting the growth of poultry virulent isolates that possess it (Timbermont et al., 2014).
The TpeL toxin, which was originally found in C. perfringens type C, was found in type A. It is a member of the large clostridial cytotoxins, which have been shown to increase the severity of NE. These toxins have at least four active domains, “ABCD”, where the B domain binds to the cell. The toxin is endocytosed, and the D domain inserts into the endosome membrane. The components of the cytosol activate the protease C domain, which results in cleavage of the toxin and release of the A domain. The A domain can inactivate GTPases in the cytosol. TpeL modifies Rac1 and Ras to mediate its cytotoxic effects (Timbermont et al., 2011).
These small GTPases have roles in actin cytoskeleton reorganization and cell proliferation (Nagahama et al., 2011). Type A strains of C. perfringens may also contain a mu toxin. It is hyaluronidase and degrades hyaluronic acid in the extracellular matrix. It is thought to increase the virulence of C. perfringens by potentiating the effects of other toxins by increasing cellular permeability (Canard et al., 1994).
EPIDEMIOLOGY OF C. PERFRINGENS IN POULTRY
Given its ubiquitous nature, the main source of C. perfringens is the environment (Petit et al., 1999). C. perfringens usually spreads through horizontal transmission as vertical transmission is rare. It is extremely rare for chickens not to have C. perfringens in their intestines. Approximately 75 to 95% of broiler chickens have C. perfringens as part of their normal intestinal microflora (Svobodova et al., 2007).
C. perfringens contamination can originate inside broiler barns during grow-out or from sources outside the barn. Chickens can be exposed to the pathogen by ingesting litter or drinking from a contaminated source. Insects, including darkling beetles and flies, staff footwear, dirt from the barn entrance area, and stagnant water outside the barn have been identified as potential sources of contamination. Furthermore, various pathogens can be spread by insects, wild birds, and mammals shedding feces around the barn. The incidence of C. perfringens in the environment might vary with the hatchery, farm, season, age of the birds, and sample type (Craven et al., 2001).
PREDISPOSING FACTORS, TYPES, AND PATHOGENESIS OF NE
NE is a multifactorial disease. The ubiquitous nature of C. perfringens makes it difficult to attribute a single cause to the development of NE. C. perfringens is a serious pathogen which attacks the intestinal cells and disturbs the ecosystem within the intestine resulting in dysbiosis (Yang et al., 2021). McDevitt et al. (2006) estimated that C. perfringens colonize over three-quarters of birds in any flock at any given time, but only small percentages develop NE. The overgrowth of C. perfringens in the intestines has been suggested to occur because of a combination of events, including damage to the intestinal mucosa, low pH level in the intestine (Baba et al., 1992), and co-infection with coccidia, breed, sex, and age (Prescott et al., 2016).
Factors that might contribute to the development of NE include thickening of the digesta due to consumption of water-soluble and hard-to-digest carbohydrates (Kocher et al., 2003), damage to the intestinal lining because of rough ration and different farm operations (Craven et al., 2001), and seasonal variation (Kaldhusdal and Skjerve, 1996). Further, the severity of NE in chickens might vary by dietary content (wheat and barley or fishmeal, antimicrobial and anticoccidial content, and animal protein and soya been content) (Prescott et al., 2016). Additional environmental factors might include wet litter, use of ammonia as a disinfectant, and plasterboard walls (Hermans and Morgan, 2007), overcrowding, and stress (Hoerr, 2010).
The overgrowth of C. perfringens, specifically type A, and its related toxins in the small intestine cause this disorder, which is characterized by sudden diarrhea and mucosal necrosis (Porter Jr, 1998). NE is categorized as clinical (clostridiosis) or subclinical (clostridiosis) disease. Broilers develop NE approximately 3 to 4 wk after hatching (Løvland and Kaldhusdal, 2001). In broiler flocks, the clinical type can cause fast, rapid mortality, with 1% loss per day for several days in a row during the final days of grow-out (Kaldhusdal and Løvland, 2000).
The clinical signs of clostridiosis include dehydration, depression, orange colored frothy diarrhea and, in some cases, tinged with blood, and ruffled feathers. Large sections of the intestine are necrotic and coated in a yellow brown pseudo-membrane filled with necrotic cells, bacterial colonies, and tissue fragments postmortem (Lee et al., 2011; Timbermont et al., 2011). Subclinical forms are more difficult to diagnose and have a higher economic effect (despite the absence of mortality) compared to clinical forms (Stutz and Lawton, 1984). Signs of clostridiosis are very limited and difficult to detect. They may include decreased nutrient absorption and digestion, impaired feed conversion ratio (FCR), and reduced body weight gain (BWG) (Stutz and Lawton, 1984).
Moreover, the presence of mild to moderate necrotic lesions in the small intestines is indicators of subclinical NE. The diagnosis is confirmed through bacterial analysis and genotyping of isolates (Kaldhusdal and Hofshagen, 1992). Subclinical NE can also be associated with deterioration in the litter material, increasing the risk of foot pad dermatitis and hock burn, two conditions that are large welfare problems for the industry (Allain et al., 2009).
In clostridiosis, the bacteria can transfer to and colonize in the liver, via the bile duct, to cause cholangiohepatitis and ascites, an accumulation of fluid in the peritoneal cavity (Kaldhusdal et al., 2001). Birds that have C. perfringens-associated lesions in the liver can be condemned at slaughter (Løvland and Kaldhusdal, 1999). Histologically, the lamina propria of the gut becomes hyperemic, but the epithelium is relatively normal in experimental birds with subclinical NE. Lymphocytes, granulocytes, plasma cells, macrophages, and some eosinophils infiltrate the lamina propria. At the site of interaction, the basal domain, enterocytes, and lamina propria become edematous. Villi are shortened, and crypts become distended. Necrosis of epithelial cells can be characterized by chromatin condensation, karyorrhexis, and karyolysis (Engberg et al., 2002).
The financial cost to the industry comes from the extra feeding required due to reduced efficiency, the housing of birds that will be condemned at slaughter, and any treatment required for restoring health in the flock. Although the disease pathogenesis is not fully understood, it appears that C. perfringens antigens and toxins alone are insufficient to cause the disease in the absence of predisposing factors (Cooper and Songer, 2009).
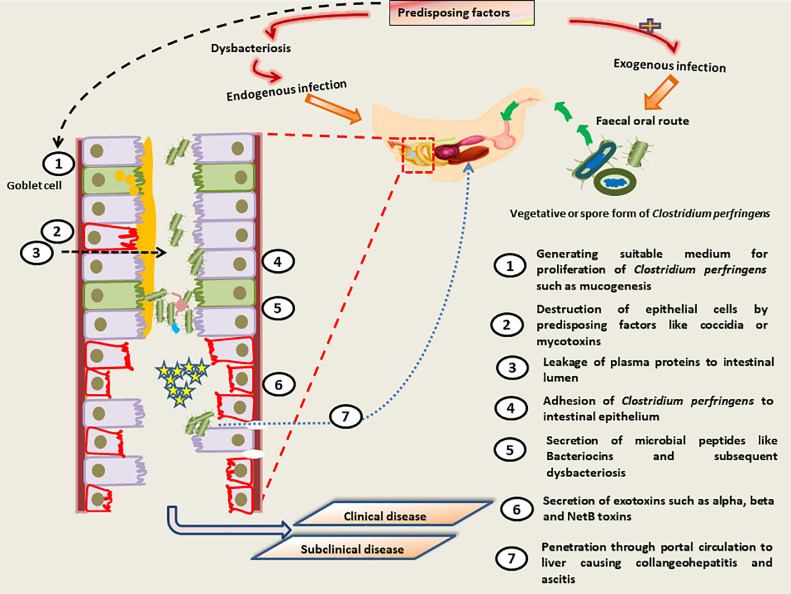
The predisposing factors and pathogenesis of NE are illustrated in Figure 3.
Figure 3.
Pathogenesis of necrotic enteritis.
Coccidial Infection
Coccidia is a protozoan parasite that propagates inside the host intestine and causes enteric disease. So far, seven species of Eimeria (E. acervulina, E. maxima, E. mitis, E. praecox, E. necatrix, E. tenella, and E. brunetti) have been known to infect birds (Williams, 2005). Eimeria species, such as E. acervulina and E. maxima, are the most known predisposing factors for NE in chickens (Al-Sheikhly and Al-Saieg, 1980).
Even though coccidiosis and NE are associated and have similar symptoms, coccidiosis usually develops before or during the NE phase. Moreover, the Eimeria vaccine was shown to increase the incidence of NE (Pedersen et al., 2008).
There is no clear mechanism showing how coccidia induces NE, and possible causes are proposed. Damage caused to the intestinal lumen, usually during coccidia propagation, leads to bleeding; thus, the leaked plasma proteins become a source of growth substrate for C. perfringens. Furthermore, coccidiosis induced mucus production and provides a suitable environment for C. perfringens growth (Collier et al., 2008; Kaldhusdal et al., 2021). Genomic sequencing data shows that C. perfringens cannot produce enzymes required for essential amino acid biosynthesis (Shimizu et al., 2002).
Baba et al. (1992) showed that C. perfringens was less likely to remain in the cecal mucosa of chicks that were not infected with Eimeria than infected chicks. A co-infection with E. maxima is commonly associated with NE incidence in broilers. It is often used in experimental models of NE with C. perfringens to produce NE lesions as it alters the microbiota composition (Wu et al., 2014). E. maxima is an intracellular protozoan parasite that causes coccidiosis which produces intestinal lesions and causes destruction of the intestinal epithelium during the intracellular stages of its life cycle. Plasma proteins leak into the lumen, supplying C. perfringens with a source of nutrients (Collier et al., 2008).
It has been found that animals infected with E. maxima have lower levels of liver-expressed antimicrobial peptide-2. This may help NE-causing C. perfringens to proliferate within a gut that has already been damaged. In an experimental model of NE with Eimeria and C. perfringens co-infection, the immunosuppressive cytokine, interleukin-10 (IL-10), was found to be significantly increased, but with C. perfringens infection alone, no changes were detected, suggesting that the parasite may allow a greater opportunity for C. perfringens to proliferate (Park et al., 2008). Other immune mediators, such as IFN-α, IFN-γ, and IL-1β, are all downregulated in co-infection models compared with C. perfringens alone in the days after infection. Interleukin-8 is upregulated along with IL-10 (Parvizi et al., 2010).
Broilers infected with Eimeria (10 × Paracox vaccine) and C. perfringens fed ad libitum had increased lesion scores compared to birds that were feed restricted for 12 h per night in the infection protocol (Tsiouris et al., 2014).
Nutritional Factors
In poultry, diet composition has a significant impact on the development and pathogenesis of NE. Diets with high levels of indigestible materials, non-starch polysaccharides (NSP) feeds, such as wheat, barley, rye, and oats, are shown to be a predisposing factor for NE in birds (Kaldhusdal et al., 1999). Broilers fed a wheat-based diet, as well as a wheat-based diet supplemented with complex carbohydrates and additional fiber, developed more lesions than those fed a corn diet (Annett et al., 2002). This may be associated with the presence of arabinoxylans and β-glucans, which are not easily digested by birds’ digestive system; however, they can be used as a growth substrate for both the microbiota and pathogenic bacteria, such as C. perfringens (Annett et al., 2002).
The inclusion of large amounts of cereals, which are often rich in water-soluble NSP, is a predisposing factor for NE. With increasing concentrations of carboxymethyl cellulose, an NSP, the feed transit time decreases, leading to higher gut viscosity, which acts as a very suitable environment or substrate for clostridial infection (Timbermont et al., 2011). The presence of undigested protein in the lower gut of broilers has been associated with NE. The percentage of protein in the diet and nature of the protein has been linked to NE outbreaks (Fernando et al., 2011).
Fernando et al. (2011) showed that birds on a potato protein diet (which is poorly digested) produced a higher titer of α-toxin antibody and had a significant increase in hepatic lesions and higher mean incidence of intestinal necrosis compared with birds fed a soya bean protein diet. Protein from an animal source has also been implicated in predisposing NE. C. perfringens counts in the ileum and cecum were higher in broilers fed a fish or meat/bone meal-based diet and increased when crude protein levels were increased (Williams, 2005). Fishmeal is a good source of zinc, glycine, and methionine (Dahiya et al., 2006). The availability of zinc is linked to α-toxin production by C. perfringens and can prevent trypsin from destroying the α-toxin (Baba et al., 1992).
The gizzard can be triggered by the presence of animal protein to increase the pH that favors C. perfringens growth. Animal fat, such as tallow or lard, are believed to increase ileal C. perfringens count more than plant oils (Knarreborg et al., 2002). The quality of protein in this diet is high and is not always fully digestible, allowing some to pass through the lower GI tract. Different protein sources can have different amino acid concentrations. Some amino acids may accelerate the growth of C. perfringens and, therefore, contribute to NE incidence. An increasing number of C. perfringens cells are detected in the ileum and cecum when increased glycine levels are included in broiler diets. Feeding with a fishmeal protein source can alter the dynamics of the broiler intestinal microbiota, which may establish the correct conditions for C. perfringens to colonize the intestine (Wu et al., 2014).
Antinutritional factors, such as protease inhibitors, lectins, and tannins, can make birds more susceptible to NE. By leaving undigested proteins in the lower gut, trypsin inhibitors commonly found in soybean meal provide an ideal environment for bacterial development (Clarke and Wiseman, 2007). In addition to the chemical nature of the feed, the existence of gut microbes may depend on the physical nature of the feed (Smith and Macfarlane, 1998). Roller-mill ground wheat has been shown to minimize NE-induced death of chickens compared to hammer ground wheat. Coarsely ground mash may initiate HCl secretion and increase feed retention time in the gizzard and proventriculus (Engberg et al., 2004).
Moreover, the form of diet offered to broilers has also been implicated with NE pathogenesis. Engberg et al. (2002) found decreased C. perfringens counts when broilers were fed a diet in pellet form compared with those fed a mash diet, but their study did not score intestinal lesions. Feed restriction in C. perfringens-infected broilers had significantly reduced intestinal pH and cecal C. perfringens counts (change the intestinal ecosystem) compared to their ad libitum counterparts. The mechanisms for reduced lesions scores in restricted fed birds were unclear, but it was hypothesized that increased glucocorticoid levels caused by the feed restriction may, in turn, reduce prostaglandin levels and suppress inflammation (Tsiouris et al., 2014).
Higher BW and BWG in broiler chickens were found to be a predisposing factor for NE in a recent report. Since their study revealed an association between BWG and the development of NE lesions, the authors investigated whether lowering BWG (at specific stages of the growth cycle) could be part of a strategy to prevent NE (Dierick et al., 2019).
Stress and Immunosuppression
Stress is considered another predisposing factor for NE. This could be due to the change in the gut (McDevitt et al., 2006). Chicken stressors, such as ammonia, physiological stress, high stocking density, heat stress, and mycotoxicosis, have been demonstrated to suppress immunity and predispose chickens to NE (Tsiouris et al., 2015). They also showed the effect of environmental stress, like cold stress, to cause immunosuppressive induced NE. Likewise, Burkholder et al. (2008) reported that heat stress changes the intestinal structure and disrupts the microbiota community, acting as a predisposing factor before infection (Lee et al., 2011).
A survey of broiler farms in the United Kingdom showed that farms with wet litter or plasterboard walls or those that used ammonia as a disinfectant were found to have a higher prevalence of NE (Hermans and Morgan, 2007). Increased stocking density (overcrowding) has also been implicated as a predisposing factor that reduces broiler welfare and affects gut health to favor NE development (Tsiouris et al., 2015). Immunosuppressive diseases, such as infectious bursal disease, chick infectious anemia virus (CIAV), and Marek's disease, have been recommended to exacerbate the progress of C. perfringens-induced NE (Lee et al., 2011).
Marek's disease virus causes B cell cytolysis and T cell transformation, which leads to immunosuppression and lymphomas (Müller et al., 2003). Infectious bursal disease virus targets lymphoid cells in the bursa of Fabricius, resulting in lymphoid depletion and immunosuppression in birds, increasing their risk to other infections (Hailemariam et al., 2008). Anemia, bone marrow aplasia, thymus atrophy, and immunosuppression characterize CIAV and enhance NE incidence (Flores-Diaz et al., 2005).
BROILER IMMUNE RESPONSE AGAINST NE
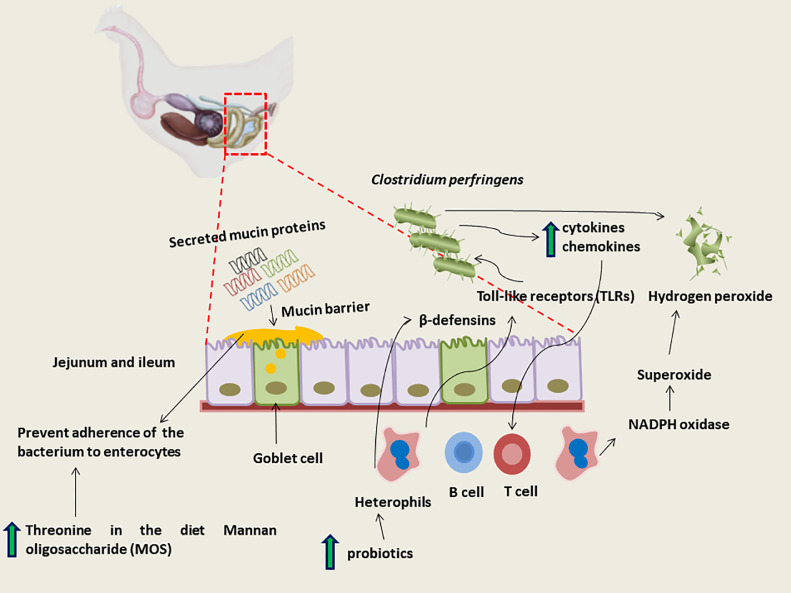
Until recently, the use of in-feed antimicrobials and anticoccidiostats were thought to have prevented NE from becoming a significant problem for the poultry industry, so the immune response to C. perfringens in chickens has not been well characterized (Figure 4). Like mammals, chickens have both the general arms of innate and adaptive immunity. However, there are differences in the avian system (Salem and Attia, 2021).
Figure 4.
Chickens immune response to Clostridium perfringens infection.
Mainly the avian immune system appears to have fewer receptors and effector molecules than the mammalian system. Innate immunity provides an early line of defense against pathogens and consists of antigen recognition receptors, phagocytic cells, and secreted barrier molecules (Müller et al., 2003). Conversely, the adaptive response is pathogen-specific and mediated by T and B cells. In relation to NE, the immune system in the intestine is of interest. The chicken gut develops rapidly in the last few days before and just after hatching for nutrient digestion and absorption (Hailemariam et al., 2008).
After hatching, the gut quickly becomes colonized with bacteria, and it must adapt to balance digestive functions with the protection of the host from pathogens. Initial protection arises from physical measures, which actively inhibit pathogen attachment to the epithelium, and chemical measures, which can disrupt microbial cell membranes (Tsiouris et al., 2015).
Microbial colonization of the intestinal tract in birds is required for the development of the immune system. Birds kept in a germ-free environment have poorly developed lymphoid follicles in the cecal tonsil with no IgG or IgA positive cells detected at 4 wk of age compared to their conventionally housed counterparts. Germ-free birds also had fewer T cells (CD3+) in the villus regions of the cecal tonsil compared with conventional birds (Honjo et al., 1993).
The expression of the CD3 gene, which is a marker for T cells, is detected at low levels in the first days of life but increases substantially at d 4, indicating increased development of the T cell population in time throughout the intestine (Bar-Shira et al., 2003). The intestinal immune system must develop to distinguish between commensal and pathogenic bacteria so that effective responses can clear organisms likely to invade and destroy host tissues (Bar-Shira et al., 2003).
Innate Responses
Barrier Molecules
One of the first lines of defense in the chicken intestine is the mucin barrier, and these mucin molecules are likely to be some of the first molecules exposed to C. perfringens. Eight mucin genes have been discovered in the chicken genome; five of these are secreted proteins (Muc2, Muc5ac, Muc5b, Muc6, and ovomucin) and three are transmembrane molecules (Muc13, Muc16, and Muc1) (Lang et al., 2006).
Secreted mucins are predominantly released from goblet cells. These cells are part of the epithelial layer that separates the lumen from the lamina propria, which produces a mucous layer that is predominantly made up of mucin glycoproteins. These can be released by baseline secretion or compound exocytosis (Kim and Khan, 2013). Baseline secretion is the continuous release of mucin molecules, and compound exocytosis is the release of central mucin stores after stimulations from hormones, neuropeptides, and inflammatory mediators. Alternatively, transmembrane mucins are found on the surface of enterocytes (Kim and Khan, 2013).
These molecules form part of the glycocalyx, a region at the apical end of enterocytes that prevents bacterial attachment (Pelaseyed et al., 2014). Muc13 has been identified in the chicken, and the structure identified indicates that this molecule can be produced in larger and smaller lengths, providing a barrier at different regions from the cell surface (Lang et al., 2006). Mucins as part of the mucous layer prevent damage from the contents of the lumen and stop the adherence of pathogens to the intestinal wall. Its composition can be altered by nutrients and antimicrobial compounds. Crude mucin increases with the inclusion of increasing amounts of threonine in the diet (Horn et al., 2009).
In ovo administration of mannan oligosaccharide (MOS) 3 d before hatching increased the Muc2 levels (Cheled-Shoval et al., 2011). Similar results were also observed in broilers fed a diet supplemented with MOS. Muc-2 is generally expressed at higher levels in the jejunum in birds supplemented with MOS compared with birds without supplementation. Some studies have investigated mucin mRNA expression after NE challenge and detected variations in Muc2, Muc5ac, and Muc13 transcripts in the post-infection days, but these are not always consistent between studies (Collier et al., 2008). On the final day of infection and after 2 d, Collier et al. (2008) found increased Muc2 mRNA expression in the ileum of birds challenged with C. perfringens and co-infection with Eimeria.
Forder et al. (2012) detected increased Muc5ac mRNA 3 d after an NE co-infection challenge and reduced Muc2 and Muc13 mRNA expression at the same time point. Kitessa et al. (2014) found changes in Muc5ac and Muc13 expression with predisposing factors, but mRNA levels were like controls when C. perfringens was added to the experimental challenge. It is possible that these inconsistencies could be attributed to differences in the challenge models as all inoculated the birds on different days, but not all used the same predisposing factors, and there could also be differences in mucin expression between regions of the small intestine.
Other defense molecules present in the avian intestine are β-defensins. These are antimicrobial peptides that are produced by heterophils and found in other tissues in the bird from the early stage of development (Meade et al., 2009). So far, 14 genes for β-defensins have been identified in the chicken genome, AvBD1-14. A co-infection model in Ross and Cobb broilers with E. maxima and C. perfringens showed altered expression of defensin genes in the crop and jejunum (Hong et al., 2012).
There were few changes in the defensin levels detected in the crop between the infected birds and uninfected controls; AvBD1 and 6 and 7 mRNA levels were increased in infected Cobb broilers, while AvBD11 was reduced 2 d after infection. Infected Ross broilers had increased AvBD2 and reduced AvBD6 compared to uninfected controls at the same time point (Hong et al., 2012). In comparison, there were more changes in AvBD mRNA expression in the jejunum. AvBD8 was the only defensin where increased mRNA was detected in infected broilers of both breeds, and AvBD12 was reduced in both. AvBD8, AvBD10, and AvBD13 were highly expressed in the jejunum; however, the expression of AvBD13 was not significantly higher in infected broilers compared with control birds (Hong et al., 2012). Higher levels of these β-defensins were noted in Ross broilers compared to those in Cobb broilers, which may imply some genetic differences in their ability to mount an immune response in NE (Hong et al., 2012).
Intestinal Epithelial and Immune Cells
Pathogens that can disrupt the mucous barrier and evade these antimicrobial peptides will then interact with the epithelium of the chicken intestine. Goblet cells, enterocytes, and intraepithelial lymphocytes (IELs) are some of the cells that make up the epithelial layer (Brisbin et al., 2008). The IEL populations are comprised of NK cells, T cells, and B cells (Gobel et al., 2001).
Interactions with the epithelium can activate pathogen recognition receptors, such as Toll-like receptors (TLRs), and their pathways. Pathogen recognition receptors are found on various cell types, such as dendritic cells, heterophils, and endothelial cells. TLRs are important in the recognition of pathogen-associated molecular patterns, which are found on the surface of bacteria. These molecules on the surface of cells trigger pathways that upregulate the expression of inflammatory molecules, which attract increased numbers of inflammatory cells to the site of infection, such as cytokines and chemokines (Kaiser, 2010).
TLRs genes are expressed in the entire intestine, which may reflect the wide array of pathogens that can be detected in this tissue (Iqbal et al., 2005). Toll-like receptor gene expression in the ileum has been shown to be altered in the first few days after C. perfringens challenge on d 18 of life (Lu et al., 2009). Changes in TLRs are also detected later in challenge models with differences being described 1 wk after C. perfringens challenge (Yitbarek et al., 2012).
TLR4 mRNA was not differentially expressed in experiments in either the ileum or cecal tonsil. Yitbarek et al. (2012) detected increased TLR2.2 levels in the cecal tonsil but not in the ileum. Conversely, TLR2.2 mRNA levels increased after C. perfringens challenge in the ileal mucosa in a separate study. Again, differences in the detection of TLR genes between these different studies could in part be related to the levels of the challenge given to the broilers and time between the C. perfringens challenge and sampling. In addition to epithelial cells, a number of immune cells also express TLRs, including heterophils, macrophages, and IELs. These cells have different functions during host responses to bacterial infections (Kogut et al., 2006).
Heterophils are part of the innate response, are the avian equivalent of neutrophils, and are located in the lamina propria of the small intestine. They are polymorphonuclear cells that phagocytose invading pathogens. Once pathogens have been internalized by a heterophil, they can be killed by a respiratory burst or degranulation (Kogut et al., 2006).
The respiratory burst involves NADPH oxidase being activated to produce superoxide, which in turn is converted to hydrogen peroxide. This is converted to hypochlorous acid, which seemed to have bactericidal activity. Degranulation refers to the killing of microbes by the release of proteins into the phagosome (Genovese et al., 2013). Activation of different TLRs on heterophils produces different cytokine and chemokine responses. Moreover, heterophils from genetically different broiler lines vary in their responses to TLR activation (Kogut et al., 2006).
Early exposure to certain bacteria may improve heterophil responses in broilers. Heterophils from broilers that received probiotic treatment on the day of the hatch had improved oxidative burst and degranulation responses than birds that did not receive probiotic treatment (Farnell et al., 2006). Heterophils invade NE lesions in the intestine, so it is unclear how they help fight the disease.
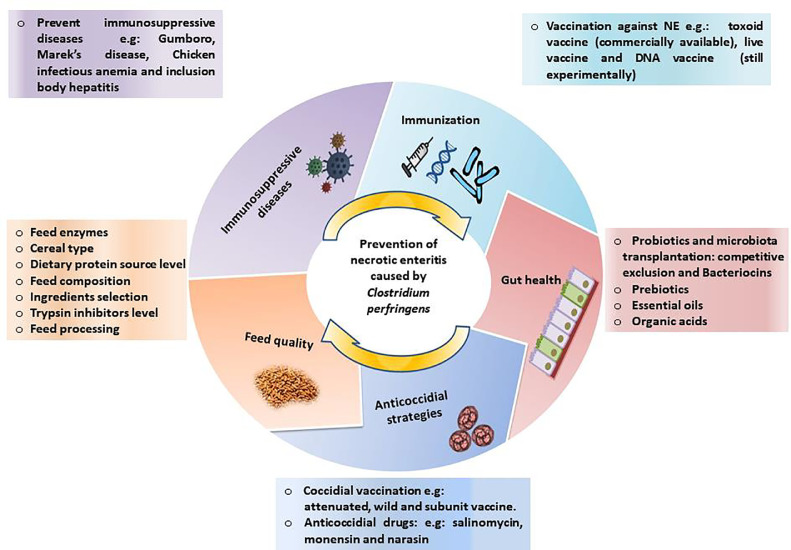
INTERVENTION STRATEGIES FOR NE
The prevention and control principles of NE are mainly based on biosecurity, sanitation, flock management, antimicrobial use, and prevention of predisposing factors (Figure 5). Cleaning and disinfection (using bactericidal and sporicidal disinfectants) are one of the most effective methods for reducing microbial load in general and pathogen levels in poultry farms. The best litter management and ventilation practices should be followed. Moreover, all methods should be applied to minimize stress and follow good animal husbandry practices (Brennan et al., 2001).
Figure 5.
Prevention strategies against necrotic enteritis.
The Role of Antimicrobials
Antimicrobials were used to control disease and enhance production either in feed or water. They can directly reduce the risk of subclinical infections by decreasing the number of opportunistic pathogens in the gut microflora and enhance nutrient digestibility (Dibner and Richards, 2005). The term antimicrobial refers to natural, synthetic, and semisynthetic substances used to inhibit growth or kill microorganisms (Giguere, 2006).
The main concern with preventative antimicrobials is that human bacterial pathogens might acquire antimicrobial resistance from animal pathogens (World Health Organization, 2001). Furthermore, environmentalists fear that manure waste, containing arsenic, heavy metals, and antibiotics, can spread in the environment and contribute to the spread of pathogens with antimicrobial-resistant genes (Osterberg and Wallinga, 2004). After a study suggested a lack of consumer interest in meat safety due to large amounts of antimicrobials used in food processing, Sweden became the first country to prohibit the use of antimicrobial growth promoters in animal operations in 1986 (Wierup, 2001).
Other countries in the European Union, including the United Kingdom and Denmark, soon followed the Swedish initiative (Wierup, 2001). In the 1990s, the use of avoparcin in poultry feed was associated with establishing a reservoir of vancomycin-resistant Enterococcus spp. (VRE) in food animals, leading some countries in the European Union to ban the use of antimicrobial classes used for human medicine in animal production (Bates et al., 1994). In 1999, tylosin, zinc bacitracin, virginiamycin, spiramycin, and avoparcin were all banned by the European Union (Casewell et al., 2003). The European ban on growth-promoting antimicrobials led to an increase in NE outbreaks and a subsequent increase in the use of therapeutic antimicrobials (Casewell et al., 2003). After implementing a ban on avoparcin, Germany, Netherlands, and Italy reported a decrease in the prevalence of VRE in humans (Emborg et al., 2001). The use of therapeutic antimicrobials returned to approximately the same amount after the introduction of narasin into the feed (Grave et al., 2004).
Antimicrobial Susceptibility
The antimicrobial resistance of C. perfringens has been determined using a number of methods. The minimum inhibitory concentration (MIC) required to inhibit the growth of 50 or 90% of C. perfringens isolates was defined by some researchers as MIC50 or MIC90, respectively (Gharaibeh et al., 2010; Slavic et al., 2011). Gharaibeh et al. (2010) described the activity of each antimicrobial on C. perfringens isolates by ranking the MIC50 and MIC90 from smallest to largest. Other researchers used epidemiological cutoff values obtained by assessing the distribution of the MIC data (Johansson et al., 2004; Slavic et al., 2011). When MICs showed a mono-modal distribution, all isolates were classified as susceptible or resistant. When MICs of antimicrobials showed a bimodal distribution, isolates with high MICs were classified as resistant, and isolates with low MICs were deemed susceptible (Johansson et al., 2004).
When the MICs showed a multimodal distribution, isolates between the two modes were classified as intermediate. Gad et al. (2011, 2012) classified C. perfringens isolates obtained from commercial layer and turkey flocks in Germany as susceptible or resistant using the Clinical and Laboratory Standards Institute guidelines, European Committee on Antimicrobial Susceptibility Testing, and Arbeitskreis Veterinärmedizinische Infektionsdiagnostik. In Belgium, the MIC of C. perfringens isolates from pigs, cattle, and chickens was investigated. All strains were susceptible to avoparcin, furazolidone, monensin, nitrofuran, penicillin G, ronidazole, and tiamulin but resistant to flavomycin. Chicken isolates were resistant to carbadox, chloramphenicol, erythromycin, and virginiamycin (Dutta and Devriese, 1980).
Watkins et al. (1997) conducted an analysis to determine whether 48 C. perfringens isolates from chickens and turkeys are susceptible to antibiotics. In the United States, C. perfringens isolates were obtained from 26 commercial broiler chicken farms and 22 commercial turkey farms (Watkins et al., 1997). They reported low MIC for avilamycin, avoparcin, monensin, narasin, and penicillin; moderate MIC for tilmicosin, tylosin, and virginiamycin; and high MIC for bacitracin and lincomycin in broiler chickens (Watkins et al., 1997).
Johansson et al. (2004) investigated the antimicrobial susceptibility of 102 C. perfringens isolates from 89 healthy or diseased broilers, nine layers, and four turkeys. A total of 59 isolates originated from 12 Swedish farms, 20 isolates from 16 Danish farms, and 22 isolates from 21 Norwegian farms. Isolates were isolated from 1986 to 2002. All isolates from all poultry sources were susceptible to ampicillin, narasin, avilamycin, erythromycin, and vancomycin. Moreover, 3 and 15% of isolates from Sweden and Denmark, respectively, were resistant to bacitracin. Thirteen percent of isolates from Norway were resistant to virginiamycin. In 2002, Martel et al. (2004) investigated the antimicrobial resistance of 47 isolates of C. perfringens collected from 31 broiler chicken farms in Belgium. All isolates were susceptible to monensin, lasalocid, salinomycin, maduramycin, narasin, avilamycin, tylosin, and amoxicillin. Low-level acquired resistance to chlortetracycline and oxytetracycline was detected in 66% of isolates (Martel et al., 2004).
In one study, Chalmers et al. (2008a) found that 28 of 61 isolates (45.9%) were resistant to bacitracin; where 17 of 41 isolates (41.5%) and 11 of 20 isolates (55%) were obtained from diseased and healthy birds, respectively. In another study, Chalmers et al. (2008b) found that 39 of 41 isolates (95.1%) were resistant to bacitracin; all resistant isolates were obtained from birds that received bacitracin. The two susceptible isolates were obtained from birds that did not receive bacitracin. The determinants for the high prevalence of bacitracin resistance were unknown. It is possible that bacitracin resistance genes spread horizontally between strains, or resistant strains have a selective advantage over nonresistant strains. Sixteen of 41 isolates (41.4%) were resistant to tetracycline using a breakpoint of 4 µg/mL as suggested by Johansson et al. (2004).
Gharaibeh et al. (2010) investigated the antimicrobial susceptibility of C. perfringens among 155 broiler chickens in Jordan with a history of enteritis. MIC showed varied susceptibility to antimicrobials. Reduced susceptibility of some antimicrobials was attributed to antimicrobial use at poultry operations. Gad et al. (2011) tested 100 C. perfringens isolates collected from turkey flocks in Germany between March 2008 and March 2009 for antimicrobial susceptibility. Lactam antimicrobials, as well as combinations of lincomycin, spectinomycin, and tylosin, were all effective against all isolates. The majority of isolates were sensitive to enrofloxacin (98%), oxacillin (83%), tiamulin (80%), tilmicosin (80%), and trimethoprim/sulfamethoxazole (72%). The majority of isolates were resistant to spectinomycin (74%), neomycin (94%), and colistin (100%).
Slavic et al. (2011) investigated the MICs of 100 C. perfringens isolates obtained from Ontario broiler chickens and found resistance to bacitracin (64%), virginiamycin (25%), tetracycline (62%), erythromycin (2%), clindamycin (2%), and metronidazole (1%) and no resistance to salinomycin (0.0%) and florfenicol (0%). They suggested that there is a pattern of increased resistance of C. perfringens against certain antimicrobial agents commonly used in disease control and treatment. Reduced susceptibility to several antimicrobials was reported. Gad et al. (2012) tested 46 C. perfringens isolates collected from commercial layer chicken flocks between 2008 and 2009 for antimicrobial resistance to 16 antimicrobials. Lactam antimicrobials, tylosin, doxycycline, tetracycline, enrofloxacin, trimethoprim/sulfamethoxazole, lincomycin, and tilmicosin were all effective against all isolates. Erythromycin (17.4%) and tiamulin resistance were found in isolates (19.6%).
Alternative Strategies for the Control of NE
Different alternatives to antimicrobials in feed have been suggested. Caly et al. (2015) provided a review of different strategies used to control C. perfringens. Generally, microbial infections harm poultry productivity by colonizing the digestive tract and affecting the final BW, intestinal health, and meat quality of broiler chickens (Abd El-Hack et al., 2020a). Antibiotics effectively repress and inhibit microorganisms until antibiotic-resistant bacteria appear. The tendency to use alternative eco-friendly compounds solves this problem (Abd El-Hack et al., 2020b,c; Abo Ghanima et al., 2021; Dosoky et al., 2021; Mohamed et al., 2021), such as phenolic compounds in herbal extracts (El-Saadony et al., 2021a; Saad et al., 2021a,b), prebiotics (Abd El‐Hack et al., 2021a,b,c; Yaqoob et al., 2021), probiotics (Abd El-Hack et al., 2020b; Alagawany et al., 2021a; El-Saadony et al., 2021b,c), essential oils (Alagawany et al., 2021b; El-Tarabily et al., 2021), various plant extracts (El-Saadony et al., 2021b), phytogenic feed additives (Abdelnour et al., 2020a,b; Ashour et al., 2021; Raza et al., 2021; Seidavi et al., 2021a,b), hen egg antibodies (Wilkie et al., 2006), feed enzymes (Llamas-Moya et al., 2020), vaccination (Kulkarni et al., 2007), diet formulation with ingredient selection, cereal type, feed processing, and dietary protein source level (Caly et al., 2015).
A probiotic is defined as “a live microbial food supplement that beneficially affects the host by improving the intestinal microbial balance” (Fuller, 1999). Probiotics have been shown to play a critical role in the metabolism, immune-stimulation, and disease prevention of the host (Edens, 2003). Probiotics can improve feed conversion efficiency, nutrient digestibility, and absorption by modulating gut microbiota (Kan et al., 2021).
Moreover, probiotics have been shown to prevent different GI diseases in animal production (La Ragione et al., 2004). Hence, they have been one of the candidates for an alternative strategy to antibiotics. They can be administered via drinking water, feed, or spray. Even though the mechanism of action is not very clear, a possible mechanism can be direct action by competing with the pathogenic bacteria for nutrient and niche establishment or indirect action by stimulating the immune response and mucosal barrier formation. Probiotics have also been shown to change enterocyte morphology by enlarging nuclei and active impetus in cell mitosis (Kabir, 2009).
Chichlowski et al. (2007) observed that probiotics enhance villus length and cell mitosis after adding Bacillus subtilis to chicken feed (Chichlowski et al., 2007). The most widely used probiotics for chickens are Bacillus, Aspergillus, Lactobacillus, Bifidobacterium, Candida, and Streptomyces. Lactobacillus and Bacillus are the most widely used probiotics against C. perfringens-induced NE (Jadamus et al., 2001). Birds supplemented with probiotics showed higher antibody titer compared to control birds. They have also shown to prevent bacterial infection by producing bacteriocins (antibacterial activity) and inhibiting bacterial toxin production. B. subtilis PB6 has been shown to protect birds from C. perfringens-induced NE and improve gut health (Jayaraman et al., 2013).
Microbiota and its Metabolites
The microbiota has been shown to prevent and treat various diseases. Microbiota transferred from adult healthy birds to two-day-old birds has shown to protect birds against cecal colonization of Salmonella infantis (Rantala and Nurmi, 1973). Human GI diseases, such as recurrent Clostridium difficile infection, were successfully treated by microbiota transplantation (Lawley et al., 2012).
Microbiota uses different mechanisms to prevent and treat diseases in animals. One of them is competitive exclusion (CE) which indicates that microbiota is competing for either nutrient or physical attachment (colonization) with the pathogenic bacteria. Zinc is an important mineral for both the microbiota and pathogenic bacteria. Healthy beneficial microbiota prevents young chicks from pathogenic bacterial colonization by competing for nutrients in the gut (Schneitz, 2005).
Another CE mechanism is bacteriocin production. Bacteriocin secreted from the microbiota has bactericidal property; hence, it can be used for both prevention and treatment against pathogenic bacteria (Caly et al., 2015). The host digestive system, including the intestinal epithelium and immune system, matures and develops in part because of lymphoid organ formation, antimicrobial peptide and immunoglobulin A production, and lymphocyte activation and differentiation, all of which are mediated by the gut microbiota.
Forder et al. (2007) demonstrated the effect of microbiota on the goblet and mucosal cell architecture after hatching. Compared with germ-free animals, the conventionally grown animals have shown better development in intestinal morphology, such as mucus layer, epithelial layer, lamina propria, villi length, and crypt depth (Deplancke and Gaskins, 2001). Moreover, the beneficial bacteria have shown strong effects on the intestinal T cell repertoire and cytokine expression (Mwangi et al., 2010). Gut microbiota plays an essential role in the growth and performance of the animal. Even though it is controversial whether microbiota penetrates the egg and inhabits the embryo before hatch. Many studies suggest the important effect of feed, water, hatching environment, and transportation on the microbiota profile and colonization in post-hatch chickens (Kers et al., 2018).
Gut microbiota and their metabolic products improve nutrient digestion, absorption and metabolism, as well as the performance of broiler chickens. This is believed to be mainly through the production of short-chain fatty acid (SCFAs): acetate, propionate, butyrate, and lactate), amino acids, and vitamins B and K (Yadav and Jha, 2019). Short-chain fatty acids are produced through fermentation of indigestible NSP and other polysaccharides. They provide energy to the intestinal epithelial cell and increases villus height and absorption (De Vadder et al., 2014). This has been supported by the study using germ-free and conventional mice. The germ-free mice have shown less villus thickness and intestinal surface area and impaired microvilli growth compared to the conventional mice (Deplancke and Gaskins, 2001). Moreover, SCFAs have also played a role in glucose regulation, energy and lipid metabolism (Den Besten et al., 2013).
Phytogenic Compounds Used for Intervention of C. perfringens-Induced NE
There are many effective antibiotic alternatives (Table 1) which are available for the control of NE as herbal extracts (Abou-Kassem et al., 2021; Reda et al., 2021a), bioactive peptides (El-Saadony et al., 2021b; Saad et al., 2021c), phytogenic feed additives in poultry diets (Ashour et al., 2020a,b), biological synthesized nanoparticles (Reda et al., 2020; Sheiha et al., 2020; Abd El-Ghany et al., 2021), probiotics (Abd El‐Hack et al., 2021c), prebiotics, symbiotics and enzymes (Llamas-Moya et al., 2020). The effectiveness of many phytogenic compounds in reducing the incidence and severity of NE has been studied (Table 1). Turmeric powder (Ali et al., 2020); Yucca schidigera extract (Calik et al., 2019); Origanum vulgare and Thymus vulgaris (Timbermont et al., 2010); ginger oil and carvacrol; Capsicum or Curcuma longa oleoresin (Lee et al., 2013); peppermint oil (Sorour et al., 2021); and essential oil blends have been shown to successfully prevent C. perfringens and improve broiler efficiency (Gharaibeh et al., 2021).
Table 1.
Effect of different plant extracts and essential oils on Clostridium perfringens inducing necrotic enteritis (NE).
| Type of plant and essential oils | Doses | Infectious dose and pattern | Main effects | References |
|---|---|---|---|---|
| Turmeric (Curcuma longa) powder | 2 g/kg | No experimental infection. | Significantly decreases loads of C. perfringens and chick's mortality. Significantly decreases feed conversion ratio. | Ali et al., 2020 |
| Mojave yucca (Yucca schidigera) extract | 567 g/ton for starter, 454 g/ton for grower, and 340 g/ton for finisher | Naturally occurring NE model by indirect challenge with Coccivac-B52 vaccine on d 7. | Significantly improves broilers performance during the early NE challenge phase, as well as in the grower period but had no effect on NE lesions in the small intestine. | Calik et al., 2019 |
| Muscadine grape (Vitis rotundifolia) | 5, 20, and 50 (g/kg) | 2 × 3 107 CFU/mL on d 19, d 20, and day 21 proceeded by Eimeria acervulina and Eimeria maxima on d 14. | Extracts at 5 and 20 g/kg improves body weight gain after challenge with C. perfringens and reduces mortality and lesion scores. | McDougald et al., 2008 |
| Chestnut (Castanea sp.) and quebracho | 0.03 to 8 (mg/mL) | In vitro. | Inhibits growth of C. perfringens types A, B, C, D, and E in a dose-dependent manner in the presence of all tannins extracts. Quebracho tannins shows partial bactericidal activity, whereas chestnut tannin activity was stronger. Both tannins reduces alpha toxin lecithinase activity and epsilon toxin cytotoxicity. | Elizondo et al., 2010 |
| Oregano (Origanum vulgare) and thyme (Thymus vulgaris) | 100 ppm (15% thymol and 15% carvacrol) & (30% thymol) | No experimental infection. | Reduces C. perfringens counts in the gastrointestinal tract and feces. | Mitsch et al., 2004 |
| Pepper (Capsicum ) and turmeric (Curcuma longa) | 4 mg/kg | 1 × 109 CFU of C. perfringens /bird on d 18 proceeded by oral infection on d 14 with Eimeria maxima. | Increases body weight. Decreases NE-related inflammatory response and macroscopic intestinal lesion score. | Lee et al., 2013 |
| Sweet sagewort (Artemisia annua) | 10 g/kg | Administration of fish meal diets with 200 mL of 106 of C. perfringens, on d 17, 18, 19, and 20 with administration of a 10-fold overdose of paracox-5 vaccine. | Decreases body weight gain and feed intake but improves feed conversion ratio. Reduces macroscopic intestinal lesion score. Reduces C. perfringens counts in large intestine | Engberg et al., 2012 |
| Commercial product (25% carvacrol and 25% thymol) | 60, 120, or 240 mg/kg | Wheat-based diet with oral gavage of C. perfringens from d 14 to 21. | Shows no effect on body weight gain and feed intake but reduces macroscopic intestinal lesion score. | Du et al., 2015 |
| A mixture of thymol, Cinnamaldehyde and eucalyptus | 150 g/ton | 4.108 CFU of C. perfringens on d 19, 20, 21 and 22 (3 times a day) with using wheat/rye-based (43%/7.5%) diet, with soybean meal and fishmeal (30%) as a protein source followed by a 10-fold dose of paracox-5 on d 20. | Reduces macroscopic intestinal lesion score induced by C. perfringens | Timbermont et al., 2010 |
| Ginger oil and carvacrol 1% | 1.5 g/kg | 2 mL of C. perfringens A suspension (6–8 × 108 CFU) orally on d 18, 19, 20, and 21 3 times a day (at 0800 h, 1,200 h, and 1,600 h) that proceeded by bursal disease vaccine via drinking water on day 16 to induce immunosuppression and followed by a 10-fold dose of Paracox-5 vaccine on d 19. | Improves growth performance, reduces macrsoscopic and microscopic intestinal lesion score. Increases epithelial villus lengths and villus: crypt ratio. | Jerzsele et al., 2012 |
| Peppermint oil and peppermint oil micro emulsion (15% oil/water) | Peppermint oil (0.5 mL/mL water) micro emulsion (0.25 mL/mL water) | 1 mL (1 × 108) C. perfringens as 3 successive doses for d 14, 15, and 16. | Induces lower gross lesions and mortality. Reduces colony-forming units and improved growth performance. | Sorour et al., 2021 |
| Oregano (Origanum vulgare subsp. hirtum) | 10 g/kg | No experimental infection. | Improves growth performance. | Cross et al., 2007 |
| Rosemary (Rosmarinus officinalis) | 10 g/kg | No experimental infection. | Reduces C. perfringens bacterial counts in ceca and feces and had no effect on lactic acid bacteria. | Cross et al., 2007 |
| Essential oils and organic acids | 500 mg/kg | 2.2 × 108 CFU/day orally at 18–20 day of age proceeded by Eimeria maxima and Eimeria necatrix at d 14 | Improves feed conversion ratio. Increases villus height and villus: crypt ratio. Reduces intestinal C. perfringens counts, liver C. perfringens carriage, gut lesion scores and serum fluoresce in isothiocyanate dextran. | Pham et al., 2021 |
| Yarrow (Achillea millefolium var. alba) | 10 g/kg | No experimental infection. | Improves growth performance, reduces C. perfringens bacterial counts in ceca and feces and had no effect on lactic acid bacteria. | Cross et al., 2007 |
| Lippia origanoides essential oils | 37 ppm | Challenged with ST (d 1),Eimeria maxima (d 18), and CP 1 × 109 CFU per bird (d 22 and 23) orally | Significantly reduces the harmful effects of induced infection/dysbiosis and a significantly reduces NE lesion scores, morbidity and mortality. Significantly reduces FITC-d, IFN-γ and IgA. | Coles et al., 2021 |
| Essential oil mixture (25% thymol and 25% carvacrol) | 120 mg/kg | 0.1 mL of 1.0 × 108 CFU/mL from day 14 to d 20 orally | Decreases the mortality, reduces the gut lesions and the liver enterobacteriaceae carriage. Increases the villus height of the ileum. Proliferation of C. perfringens in the ileum was not inhibited. | Du and Guo, 2021 |
| A mixture of thyme and star anise 17.0% and 17.0% | 250mg/kg | 5 mL of (107 CFU/mL)/chick on d 21–23 orally | Improves growth performance and digestibility of dry matter. Decreases gross lesion score in the intestine C. perfringens bacterial counts in small and large intestines. | Cho et al., 2014 |
| Clove, Oregano and thyme (individual administration) | 0.062, 0.125, 0.25, 0.5, 1 and 2 mg/mL-1 | In vitro. | Strongly inhibits the bacterial growth in vitro through the micro broth dilution method. | Mzabi et al., 2019 |
Vaccination for NE
Even though vaccination offers an alternate option to antimicrobial medications for disease control, immunity to NE is not completely understood (Kulkarni et al., 2007). Alphatoxin, the most immunogenic protein produced by C. perfringens stimulates immunity to produce antibodies (Kulkarni et al., 2007). Comparing healthy birds with clinical NE birds, healthy birds showed higher levels of antibodies (IgY) against both NetB and α-toxin (Lee et al., 2012). This observation suggests the importance of these antibodies in protecting broiler chickens against NE (Lee et al., 2012).
Several studies have been conducted on preventing NE by using vaccines. Some of them include inactivated toxins (toxoid vaccines); live attenuated, protein-based, or attenuated live vectors expressing C. perfringens proteins; and DNA vaccines. These vaccines have been administered through feed, water, spraying on the hatchery, and in ovo injection (Mot et al., 2014).
Keyburn et al. (2013b) immunized broiler chickens subcutaneously with purified recombinant NetB (rNetB), formalin treated bacteria and cell free toxoid with or without rNetB supplementation. They found that vaccination with NetB did not protect the birds from NE in the field, but it can protect chickens from NE when administrated in combination with other cellular or cell-free antigens. Maternal immunization with a NetB-enhanced toxoid vaccine is a useful technique for the control of NE in chicks (Keyburn et al., 2013a). Mishra and Smyth (2017) developed an oral vaccination for broiler chickens against NE using a non-virulent NetB positive strain of C. perfringens type A and the vaccine provoked a protective immunity in vaccinated group in comparison with the control group.
Vaccination with modified toxin or other secreted immunogenic proteins seems to be a logical method to defend against toxin-producing bacteria. Immunization requires a combination of various immunogenic antigens and protective proteins and multiple-dose vaccination regimens that are not applicable in the broiler industry because single vaccination regimens for 1-day-old chicks tend to be nonprotective. An inactivated vaccine, containing a toxoid of C. perfringens type A α-toxin combined with an oil adjuvant, was used in chickens (2 doses at 10–14 wk of age and 4–10 wk later) to provide passive protection to chicks against NE (Mot et al., 2014). It was not protective against a heavy challenge administered in the feed. Studies have demonstrated that NE can be controlled by live attenuated vaccines (Jiang et al., 2015). Recombinant attenuated Salmonella vaccines orally administered to broiler chickens provide cost-effective protection against C. perfringens (Jiang et al., 2015).
Despite studies on partial protection by these vaccines, there is still no effective commercial vaccine against C. perfringens-induced NE. Moreover, future vaccine development should confirm protection against NE and toxins, thereby improving bird performance and optimizing vaccine delivery to broiler chickens.
CONCLUSION
NE infection represents a devastating threat to the poultry industry that can cause severe economic losses. There is a critical need for searching effective antibiotic alternatives to control NE infection after the global ban on antibiotics usage. C. perfringens requires periodic molecular monitoring to update the data about its virulence factors and trace its molecular classification based on the virulence factors and toxins produced. Probiotics, prebiotics, symbiotics, essential oils, herbal extracts, and protective vaccines are efficient antibiotic alternatives that could be used along with biosecurity practice to mitigate the negative effect of NE in poultry.
ACKNOWLEDGMENTS
Prof. Khaled A. El-Tarabily thanks library at Murdoch University, Australia for the valuable online resources and comprehensive databases. Author contributions: All authors were equally contributed in writing this review article. All authors reviewed and approved the final version of the manuscript.
DISCLOSURES
Authors declare no conflict of interests.
REFERENCES
- Abd El-Ghany W.A., Shaalan M., Salem H.M. Nanoparticles applications in poultry production: an updated review. Worlds Poult. Sci. 2021;77:1–25. [Google Scholar]
- Abd El-Hack M.E., Alaidaroos B.A., Farsi R.M., Abou-Kassem D.E., El-Saadony M.T., Saad A.M., Shafi M.E., Albaqami N.M., Taha A.E., Ashour E.A. Impacts of supplementing broiler diets with biological curcumin, zinc nanoparticles and Bacillus licheniformis on growth, carcass traits, blood indices, meat quality and cecal microbial load. Animals. 2021;11:1878. doi: 10.3390/ani11071878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Abd El-Hack M.E., El-Saadony M.T., Swelum A.A., Arif M., Abo Ghanima M.M., Shukry M., Noreldin A., Taha A.E., El-Tarabily K.A. Curcumin, the active substance of turmeric: its effects on health and ways to improve its bioavailability. J. Sci. Food Agric. 2021;101:5747–5762. doi: 10.1002/jsfa.11372. [DOI] [PubMed] [Google Scholar]
- Abd El-Hack M.E., El-Saadony M.T., Shehata A.M., Arif M., Paswan V.K., Batiha G.E., Khafaga A.F., Elbestawy A.R. Approaches to prevent and control Campylobacter spp. colonization in broiler chickens: a review. Environ. Sci. Pollut. Res. Int. 2021;28:4989–5004. doi: 10.1007/s11356-020-11747-3. [DOI] [PubMed] [Google Scholar]
- Abd El-Hack M.E., El-Saadony M.T., Shafi M.E., Zabermawi N.M., Arif M., Batiha G.E., Khafaga A.F., Abd El-Hakim Y.M., Al-Sagheer A.A. Antimicrobial and antioxidant properties of chitosan and its derivatives and their applications: a review. Int. J. Biol. Macromol. 2020;1:2726–2744. doi: 10.1016/j.ijbiomac.2020.08.153. [DOI] [PubMed] [Google Scholar]
- Abd El-Hack M.E., El-Saadony M.T., Shafi M.E., Qattan S.Y., Batiha G.E., Khafaga A.F., Abdel-Moneim A.M.E., Alagawany M. Probiotics in poultry feed: a comprehensive review. J. Anim. Physiol. Anim. Nutr. 2020;104:1835–1850. doi: 10.1111/jpn.13454. [DOI] [PubMed] [Google Scholar]
- Abd El-Hack M.E., Abdelnour S.A., Taha A.E., Khafaga A.F., Arif M., Ayasan T., Swelum A.A., Aleya L., Abdel Daim M., Alkahtani S., Abukhalil M.H. Herbs as thermoregulatory agents: an overview. Sci. Total Environ. 2020;703 doi: 10.1016/j.scitotenv.2019.134399. [DOI] [PubMed] [Google Scholar]
- Abdelnour S.A., Swelum A.A., Salama A., Al-Ghadi M.Q., Qattan S.Y.A., Abd El-Hack M.E., Khafaga A.F., Alhimaidi A.R., Almutairi B.O., Ammari A.A., El-Saadony M.T. The beneficial impacts of dietary phycocyanin supplementation on growing rabbits under high ambient temperature. Ital. J. Anim. Sci. 2020;19:1046–1056. [Google Scholar]
- Abdelnour S.A., El-Saadony M.T., Saghir S.A.M., Abd El-Hack M.E., Al-shargi O.Y.A., Al- Gabri N., Salama A. Mitigating negative impacts of heat stress in growing rabbits via dietary prodigiosin supplementation. Livest. Sci. 2020;240 [Google Scholar]
- Abildgaard L., Sondergaard T.E., Engberg R.M., Schramm A., Højberg O. In vitro production of necrotic enteritis toxin B, NetB, by netB-positive and netB-negative Clostridium perfringens originating from healthy and diseased broiler chickens. Vet. Microbiol. 2010;144:231–235. doi: 10.1016/j.vetmic.2009.12.036. [DOI] [PubMed] [Google Scholar]
- Abo Ghanima M.M., Swelum A.A., Shukry M., Ibrahim S.A., Abd El-Hack M.E., Khafaga A.F., Alhimaidi A.R., Ammari A.A., El-Tarabily K.A., Younis M.E.M. Impacts of tea tree or lemongrass essential oils supplementation on growth, immunity, carcass traits, and blood biochemical parameters of broilers reared under different stocking densities. Poult. Sci. 2021;100 doi: 10.1016/j.psj.2021.101443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Abou-Kassem D.E., Mahrose K.M., El-Samahy R.A., Shafi M.E., El-Saadony M.T., Abd El-Hack M.E., Emam M., El-Sharnouby M., Taha A.E., Ashour E.A. Influences of dietary herbal blend and feed restriction on growth, carcass characteristics and gut microbiota of growing rabbits. Ital. J. Anim. Sci. 2021;20:896–910. [Google Scholar]
- Adams V., Li J., Wisniewski J.A., Uzal F.A., Moore R.J., McClane B.A., Rood J.I. Virulence plasmids of spore-forming bacteria. Microbiol. Spectr. 2014;2:1–24. doi: 10.1128/microbiolspec.PLAS-0024-2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alagawany M., Madkour M., El-Saadony M.T., Reda F.M. Paenibacillus polymyxa (LM31) as a new feed additive: antioxidant and antimicrobial activity and its effects on growth, blood biochemistry, and intestinal bacterial populations of growing Japanese quail. Anim. Feed Sci. Technol. 2021;276 [Google Scholar]
- Alagawany M., El-Saadony M.T., Elnesr S.S., Farahat M., Attia G., Madkour M., Reda F. Use of lemongrass essential oil as a feed additive in quail's nutrition: its effect on growth, carcass, blood biochemistry, antioxidant and immunological indices, digestive enzymes and intestinal microbiota. Poult. Sci. 2021;100 doi: 10.1016/j.psj.2021.101172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ali M.Z., Islam M.M., Zaman S. Effects of turmeric powder on Clostridium perfringens load in broiler chickens. SAARC J. Agric. 2020;18:209–218. [Google Scholar]
- Allaart J.G., de Bruijn N.D., van Asten A.J., Fabri T.H., Grone A. NetB-producing and Beta2-producing Clostridium perfringens associated with subclinical necrotic enteritis in laying hens in the Netherlands. Avian Pathol. 2012;41:541–546. doi: 10.1080/03079457.2012.729809. [DOI] [PubMed] [Google Scholar]
- Allain V., Mirabito L., Arnould C., Colas M., Bouquin S.Le, Lupo C., Michel V. Skin lesions in broiler chickens measured at the slaughterhouse: relationships between lesions and between their prevalence and rearing factors. Br. Poult. Sci. 2009;50:407–417. doi: 10.1080/00071660903110901. [DOI] [PubMed] [Google Scholar]
- Al-Sheikhly F., Al-Saieg A. Role of coccidia in the occurrence of necrotic enteritis of chickens. Avian Dis. 1980;24:324–333. [PubMed] [Google Scholar]
- Alves G.G., Machado de Ávila R.A., Chávez-Olórtegui C.D., Lobato F.C. Clostridium perfringens epsilon toxin: the third most potent bacterial toxin known. Anaerobe. 2014;30:102–107. doi: 10.1016/j.anaerobe.2014.08.016. [DOI] [PubMed] [Google Scholar]
- Annett C.B., Viste J.R., Chirino-Trejo M., Classen H.L., Middleton D.M., Simko E. Necrotic enteritis: effect of barley, wheat and corn diets on proliferation of Clostridium perfringens type A. Avian Pathol. 2002;31:598–601. doi: 10.1080/0307945021000024544. [DOI] [PubMed] [Google Scholar]
- Ashour E.A., Abd El-Hack M.E., Shafi M.E., Alghamdi W.Y., Taha A.E., Swelum A.A., Tufarelli V., Mulla Z.S., El-Ghareeb W.R., El-Saadony M.T. Impacts of green coffee powder supplementation on growth performance, carcass characteristics, blood indices, meat quality and gut microbial load in broilers. Agriculture. 2020;10:457. [Google Scholar]
- Ashour E.A., Farsi R.M., Alaidaroos B.A., Abdel-Moneim A.M.E., El-Saadony M.T., Osman A.O., Abou Sayed-Ahmed E.T., Albaqami N.M., Shafi M.E., Taha A.E., Abd El-Hack M.E. Impacts of dietary supplementation of pyocyanin powder on growth performance, carcase traits, blood chemistry, meat quality and gut microbial activity of broilers. Ital. J. Anim. Sci. 2021;20:1357–1372. [Google Scholar]
- Ashour E.A., Abd El-Hack M.E., Swelum A.A., Osman A.O., Taha A.E., Alhimaidi A.R., Ismail I.E. Does the dietary graded levels of herbal mixture powder impacts growth, carcass traits, blood indices and meat quality of the broilers? Ital. J. Anim. Sci. 2020;19:1228–1237. [Google Scholar]
- Awad M.M., Bryant A.E., Stevens D.L., Rood J.I. Virulence studies on chromosomal α-toxin and Θ-toxin mutants constructed by allelic exchange provide genetic evidence for the essential role of α-toxin in Clostridium perfringens-mediated gas gangrene. Mol. Microbiol. 1995;15:191–202. doi: 10.1111/j.1365-2958.1995.tb02234.x. [DOI] [PubMed] [Google Scholar]
- Baba E., Wakeshima H., Fukui K., Fukata T., Arakawa A. Adhesion of bacteria to the cecal mucosal surface of conventional and germ-free chickens infected with Eimeria tenella. Am. J. Vet. Res. 1992;53:194–197. [PubMed] [Google Scholar]
- Bacciarini L.N., Boerlin P., Straub R., Frey J., Grone A. Immunohistochemical localization of Clostridium perfringens beta2-toxin in the gastrointestinal tract of horses. Vet. Pathol. 2003;40:376–381. doi: 10.1354/vp.40-4-376. [DOI] [PubMed] [Google Scholar]
- Bansal M., Alenezi T., Fu Y., Almansour A., Wang H., Gupta A., Liyanage R., Graham D.B., Hargis B.M., Sun X. Specific secondary bile acids control chicken necrotic enteritis. Pathogens. 2021;10:1041. doi: 10.3390/pathogens10081041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bar-Shira E., Sklan D., Friedman A. Establishment of immune competence in the avian GALT during the immediate post-hatch period. Dev. Comp. Immunol. 2003;27:147–157. doi: 10.1016/s0145-305x(02)00076-9. [DOI] [PubMed] [Google Scholar]
- Bates J., Jordens J.Z., Griffiths D.T. Farm animals as a putative reservoir for vancomycin-resistant enterococcal infection in man. J. Antimicrob. Chemother. 1994;34:507–514. doi: 10.1093/jac/34.4.507. [DOI] [PubMed] [Google Scholar]
- Bennetts H.W. Bacillus welchii and bowel lesions: with special reference to a case of coccidiosis. Aust. Vet. J. 1930;6:153–154. [Google Scholar]
- Billington S.J., Jost B.H., Songer J.G. Thiol-activated cytolysins: structure, function and role in pathogenesis. FEMS Microbiol. Lett. 2000;182:197–205. doi: 10.1016/s0378-1097(99)00536-4. [DOI] [PubMed] [Google Scholar]
- Brennan J., Moore G., Poe S.E., Zimmermann A., Vessie G., Barnum D.A., Wilson J. Efficacy of in-feed tylosin phosphate for the treatment of necrotic enteritis in broiler chickens. Poult. Sci. 2001;80:1451–1454. doi: 10.1093/ps/80.10.1451. [DOI] [PubMed] [Google Scholar]
- Brisbin J.T., Gong J., Sharif S. Interactions between commensal bacteria and the gut-associated immune system of the chicken. Anim. Health Res. Rev. 2008;9:101–110. doi: 10.1017/S146625230800145X. [DOI] [PubMed] [Google Scholar]
- Bryant A.E., Bergstrom R., Zimmerman G.A., Salyer J.L., Hill H.R., Tweten R.K., Sato H., Stevens D.L. Clostridium perfringens invasiveness is enhanced by effects of theta toxin upon PMNL structure and function: the role of leukocytotoxicity and expression of CD11/CD18 adherence glycoprotein. FEMS Immunol. Med. Microbiol. 1993;7:321–336. doi: 10.1111/j.1574-695X.1993.tb00414.x. [DOI] [PubMed] [Google Scholar]
- Burkholder K.M., Thompson K.L., Einstein M.E., Applegate T.J., Patterson J.A. Influence of stressors on normal intestinal microbiota, intestinal morphology, and susceptibility to Salmonella enteritidis colonization in broilers. Poult. Sci. 2008;87:1734–1741. doi: 10.3382/ps.2008-00107. [DOI] [PubMed] [Google Scholar]
- Calik A., Omara I.I., White M.B., Evans N.P., Karnezos T.P., Dalloul R.A. Dietary non-drug feed additive as an alternative for antibiotic growth promoters for broilers during a necrotic enteritis challenge. Microorganisms. 2019;7:257. doi: 10.3390/microorganisms7080257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caly D.L., D'Inca R., Auclair E., Drider D. Alternatives to antibiotics to prevent necrotic enteritis in broiler chickens: a microbiologist's perspective. Front. Microbiol. 2015;6:1336. doi: 10.3389/fmicb.2015.01336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Canard B., Garnier T., Saint-Joanis B., Cole S.T. Molecular genetic analysis of the nagH gene encoding a hyaluronidase of Clostridium perfringens. Mol. Gen. Genet. 1994;243:215–224. doi: 10.1007/BF00280319. [DOI] [PubMed] [Google Scholar]
- Casewell M., Friis C., Marco E., McMullin P., Phillips I. The European ban on growth-promoting antibiotics and emerging consequences for human and animal health. J. Antimicrob. Chemother. 2003;52:159–161. doi: 10.1093/jac/dkg313. [DOI] [PubMed] [Google Scholar]
- Chalmers G., Bruce H.L., Hunter D.B., Parreira V.R., Kulkarni R.R., Jiang Y.F., Prescott J.F., Boerlin P. Multilocus sequence typing analysis of Clostridium perfringens isolates from necrotic enteritis outbreaks in broiler chicken populations. J. Clin. Microbiol. 2008;46:3957–3964. doi: 10.1128/JCM.01548-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chalmers G., Martin S.W., Hunter D.B., Prescott J.F., Weber L.J., Boerlin P. Genetic diversity of Clostridium perfringens isolated from healthy broiler chickens at a commercial farm. Vet. Microbiol. 2008;127:116–127. doi: 10.1016/j.vetmic.2007.08.008. [DOI] [PubMed] [Google Scholar]
- Cheled-Shoval S.L., Amit-Romach E., Barbakov M., Uni Z. The effect of in ovo administration of mannan oligosaccharide on small intestine development during the pre- and posthatch periods in chickens. Poult. Sci. 2011;90:2301–2310. doi: 10.3382/ps.2011-01488. [DOI] [PubMed] [Google Scholar]
- Cheung J.K., Keyburn A.L., Carter G.P., Lanckriet A.L., Van Immerseel F., Moore R.J., Rood J.I. The VirSR two-component signal transduction system regulates NetB toxin production in Clostridium perfringens. Infect. Immun. 2010;78:3064–3072. doi: 10.1128/IAI.00123-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chichlowski M., Croom W.J., Edens F.W., McBride B.W., Qiu R., Chiang C.C., Daniel L.R., Havenstein G.B., Koci M.D. Microarchitecture and spatial relationship between bacteria and ileal, cecal, and colonic epithelium in chicks fed a direct-fed microbial, PrimaLac, and salinomycin. Poult. Sci. 2007;86:1121–1132. doi: 10.1093/ps/86.6.1121. [DOI] [PubMed] [Google Scholar]
- Cho J.H., Kim H.J., Kim I.H. Effects of phytogenic feed additive on growth performance, digestibility, blood metabolites, intestinal microbiota, meat color and relative organ weight after oral challenge with Clostridium perfringens in broilers. Livest. Sci. 2014;160:82–88. [Google Scholar]
- Clarke E., Wiseman J. Effects of extrusion conditions on trypsin inhibitor activity of full fat soybeans and subsequent effects on their nutritional value for young broilers. Br. Poult. Sci. 2007;48:703–712. doi: 10.1080/00071660701684255. [DOI] [PubMed] [Google Scholar]
- Coles M.E., Forga A.J., Señas-Cuesta R., Graham B.D., Selby C.M., Uribe A.J., Martínez B.C., Angel-Isaza J.A., Vuong C.N., Hernandez-Velasco X., Hargis B.M., Tellez-Isaias G. Assessment of Lippia origanoides essential oils in a Salmonella typhimurium, Eimeria maxima, and Clostridium perfringens challenge model to induce necrotic enteritis in broiler chickens. Animals. 2021;11:1111. doi: 10.3390/ani11041111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Collier C.T., Hofacre C.L., Payne A.M., Anderson D.B., Kaiser P., Mackie R.I., Gaskins H.R. Coccidia-induced mucogenesis promotes the onset of necrotic enteritis by supporting Clostridium perfringens growth. Vet. Immunol. Immunopathol. 2008;122:104–115. doi: 10.1016/j.vetimm.2007.10.014. [DOI] [PubMed] [Google Scholar]
- Cooper K.K., Songer J.G. Necrotic enteritis in chickens: a paradigm of enteric infection by Clostridium perfringens type A. Anaerobe. 2009;15:55–60. doi: 10.1016/j.anaerobe.2009.01.006. [DOI] [PubMed] [Google Scholar]
- Cooper K.K., Theoret J.R., Stewart B.A., Trinh H.T., Glock R.D., Songer J.G. Virulence for chickens of Clostridium perfringens isolated from poultry and other sources. Anaerobe. 2010;16:289–292. doi: 10.1016/j.anaerobe.2010.02.006. [DOI] [PubMed] [Google Scholar]
- Craven S.E., Stern N.J., Bailey J.S., Cox N.A. Incidence of Clostridium perfringens in broiler chickens and their environment during production and processing. Avian Dis. 2001;45:887–896. [PubMed] [Google Scholar]
- Cross D.E., McDevitt R.M., Hillman K., Acamovic T. The effect of herbs and their associated essential oils on performance, dietary digestibility and gut microflora in chickens from 7 to 28 days of age. Br. Poult. Sci. 2007;48:496–506. doi: 10.1080/00071660701463221. [DOI] [PubMed] [Google Scholar]
- Dahiya J.P., Wilkie D.C., Van Kessel A.G., Drew M.D. Potential strategies for controlling necrotic enteritis in broiler chickens in post-antibiotic era. Anim. Feed Sci. Technol. 2006;129:60–88. [Google Scholar]
- De Vadder F., Kovatcheva-Datchary P., Goncalves D., Vinera J., Zitoun C., Duchampt A., Bäckhed F., Mithieux G. Microbiota-generated metabolites promote metabolic benefits via gut-brain neural circuits. Cell. 2014;156:84–96. doi: 10.1016/j.cell.2013.12.016. [DOI] [PubMed] [Google Scholar]
- Den Besten G., Van Eunen K., Groen A.K., Venema K., Reijngoud D.J., Bakker B.M. The role of short-chain fatty acids in the interplay between diet, gut microbiota, and host energy metabolism. J. Lipid Res. 2013;54:2325–2340. doi: 10.1194/jlr.R036012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Department of Health and Human Services (HHS). 2015. Accessed Oct. 2021. https://obamaadministration.archives.performance.gov/agency/department-health-and-human-services.html
- Deplancke B., Gaskins H.R. Microbial modulation of innate defense: goblet cells and the intestinal mucus layer. Am. J. Clin. Nutr. 2001;73:1131S–1141S. doi: 10.1093/ajcn/73.6.1131S. [DOI] [PubMed] [Google Scholar]
- Dibner J.J., Richards J.D. Antibiotic growth promoters in agriculture: history and mode of action. Poult. Sci. 2005;84:634–643. doi: 10.1093/ps/84.4.634. [DOI] [PubMed] [Google Scholar]
- Dierick E., Hirvonen O.P., Haesebrouck F., Ducatelle R., Van Immerseel F., Goossensa E. Rapid growth predisposes broilers to necrotic enteritis. Avian Pathol. 2019;48:416–422. doi: 10.1080/03079457.2019.1614147. [DOI] [PubMed] [Google Scholar]
- Dosoky W.M., Zeweil H.S., Ahmed M.H., Zahran S.M., Shaalan M.M., Abdelsalam N.R., Abdel-Moneim A.E., Taha A.E., El-Tarabily K.A., Abd El-Hack M.E. Impacts of onion and cinnamon supplementation as natural additives on the performance, egg quality and immunity in laying Japanese quail. Poult. Sci. 2021;100 doi: 10.1016/j.psj.2021.101482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Du E., Guo Y. Dietary supplementation of essential oils and lysozyme reduces mortality and improves intestinal integrity of broiler chickens with necrotic enteritis. Anim. Sci. J. 2021;92:e13499. doi: 10.1111/asj.13499. [DOI] [PubMed] [Google Scholar]
- Du E., Gan L., Li Z., Wang W., Liu D., Guo Y. In vitro antibacterial activity of thymol and carvacrol and their effects on broiler chickens challenged with Clostridium perfringens. J. Anim. Sci. Biotechnol. 2015;6:58. doi: 10.1186/s40104-015-0055-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dutta G.N., Devriese L.A. Susceptibility of Clostridium perfringens of animal origin to fifteen antimicrobial agents. J. Vet. Pharmacol. Ther. 1980;3:227–236. [Google Scholar]
- Edens F. An alternative for antibiotic se in poultry: probiotics. Braz. J. Poult. Sci. 2003;5:75–97. [Google Scholar]
- Elizondo A.M., Mercado E.C., Rabinovitz B.C., Fernandez-Miyakawa M.E. Effect of tannins on the in vitro growth of Clostridium perfringens. Vet. Microbiol. 2010;145:308–314. doi: 10.1016/j.vetmic.2010.04.003. [DOI] [PubMed] [Google Scholar]
- El-Saadony M.T., Alkhatib F.M., Alzahrani S.O., Shafi M.E., Abdel-Hamid S.E., Taha T.F., Aboelenin S.M., Soliman M.M., Ahmed N.H. Impact of mycogenic zinc nanoparticles on performance, behavior, immune response, and microbial load in Oreochromis niloticus. Saudi J. Biol. Sci. 2021;28:4592–4604. doi: 10.1016/j.sjbs.2021.04.066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- El-Saadony M.T., Alagawany M., Patra A.K., Kar I., Tiwari R., Dawood M.A., Dhama K., Abdel-Latif H.M.R. The functionality of probiotics in aquaculture: an overview. Fish Shellfish Immunol. 2021;117:36–52. doi: 10.1016/j.fsi.2021.07.007. [DOI] [PubMed] [Google Scholar]
- El-Saadony M.T., Zabermawi N.M., Burollus M.A., Shafi M.E., Alagawany M., Yehia N., Askar A.M., Alsafy S.A., Noreldin A.E., Khafaga A.F., Dhama K., Elnesr S.S., Elwan H.A.M., Di Cerbo A., El-Tarabily K.A., Abd El-Hack M.E. Nutritional aspects and health benefits of bioactive plant compounds against infectious diseases: a review. Food Rev. Int. 2021 doi: 10.1080/87559129.2021.1944183. In press. [DOI] [Google Scholar]
- El-Shall N.A., Abd El-Hack M.E., Albaqami N.M., Khafaga A.F., Taha A.E., Swelum A.A., El-Saadony M.T., Salem H.M., El-Tahan A.M., AbuQamar S.F., El-Tarabily K.A., Elbestawy A.R. Phytochemical control of poultry coccidiosis: A review. Poult. Sci. 2022;101 doi: 10.1016/j.psj.2021.101542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- El-Tarabily K.A., El-Saadony M.T., Alagawany M., Arif M., Batiha G.E., Khafaga A.F., Elwan H.A., Elnesr S.S., Abd El-Hack M.E. Using essential oils to overcome bacterial biofilm formation and their antimicrobial resistance. Saudi J. Biol. Sci. 2021;28:5145–5156. doi: 10.1016/j.sjbs.2021.05.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Emborg H., Ersboll A.K., Heuer O.E., Wegener H.C. The effect of discontinuing the use of antimicrobial growth promoters on the productivity in the Danish broiler production. Prev. Vet. Med. 2001;50:53–70. doi: 10.1016/s0167-5877(01)00218-5. [DOI] [PubMed] [Google Scholar]
- Engberg R.M., Hedemann M.S., Jensen B.B. The influence of grinding and pelleting of feed on the microbial composition and activity in the digestive tract of broiler chickens. Br. Poult. Sci. 2002;43:569–579. doi: 10.1080/0007166022000004480. [DOI] [PubMed] [Google Scholar]
- Engberg R.M., Hedemann M.S., Steenfeldt S., Jensen B.B. Influence of whole wheat and xylanase on broiler performance and microbial composition and activity in the digestive tract. Poult. Sci. 2004;83:925–938. doi: 10.1093/ps/83.6.925. [DOI] [PubMed] [Google Scholar]
- Engster H.M., Marvil D., Stewart-Brown B. The effect of withdrawing growth promoting antibiotics from broiler chickens: a long-term commercial industry study. J. Appl. Poult. Res. 2002;11:431–436. [Google Scholar]
- Farnell M.B., Donoghue A.M., Solis de los Santos F., Blore P.J., Hargis B.M., Tellez G., Donoghue D.J. Upregulation of oxidative burst and degranulation in chicken heterophils stimulated with probiotic bacteria. Poult. Sci. 2006;85:1900–1906. doi: 10.1093/ps/85.11.1900. [DOI] [PubMed] [Google Scholar]
- Fernando P.S., Rose S.P., Mackenzie A.M., Silva S.S. Effect of diets containing potato protein or soya bean meal on the incidence of spontaneously-occurring subclinical necrotic enteritis and the physiological response in broiler chickens. Br. Poult. Sci. 2011;52:106–114. doi: 10.1080/00071668.2010.549105. [DOI] [PubMed] [Google Scholar]
- Flores-Diaz M., Alape-Giron A., Clark G., Catimel B., Hirabayashi Y., Nice E., Gutiérrez J.M., Titball R., Thelestam M. A cellular deficiency of gangliosides causes hypersensitivity to Clostridium perfringens phospholipase C. J. Biol. Chem. 2005;280:26680–26689. doi: 10.1074/jbc.M500278200. [DOI] [PubMed] [Google Scholar]
- Forder R.E.A., Howarth G.S., Tivey D.R., Hughes R.J. Bacterial modulation of small intestinal goblet cells and mucin composition during early posthatch development of poultry. Poult. Sci. 2007;86:2396–2403. doi: 10.3382/ps.2007-00222. [DOI] [PubMed] [Google Scholar]
- Forder R.E.A., Nattrass G.S., Geier M.S., Hughes R.J., Hynd P.I. Quantitative analyses of genes associated with mucin synthesis of broiler chickens with induced necrotic enteritis. Poult. Sci. 2012;91:1335–1341. doi: 10.3382/ps.2011-02062. [DOI] [PubMed] [Google Scholar]
- Fukata T., Hadate Y., Baba E., Uemura T., Arakawa A. Influence of Clostridium perfringens and its toxin in germ-free chickens. Res. Vet. Sci. 1988;44:68–70. [PubMed] [Google Scholar]
- Fuller R. Horizon Scientific Press; Wymondham, UK: 1999. Probiotics for Farm Animals. [Google Scholar]
- Gad W., Hauck R., Krüger M., Hafez M. Determination of antibiotic sensitivities of Clostridium perfringens isolates from commercial turkeys in Germany in vitro. Arch. Geflugelkunde. 2011;75:80–83. [Google Scholar]
- Gad W., Hauck R., Kruger M., Hafez M. In vitro determination of antibiotic sensitivities of Clostridium perfringens isolates from layer flocks in Germany. Arch. Geflugelkunde. 2012;76:234–238. [Google Scholar]
- Gao Z., McClane B.A. Use of Clostridium perfringens enterotoxin and the enterotoxin receptor-binding domain (C-CPE) for cancer treatment: opportunities and challenges. J. Toxicol. 2012;2012:1–9. doi: 10.1155/2012/981626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garcia J.P., Adams V., Beingesser J., Hughes M.L., Poon R., Lyras D., Hill A., McClane B.A., Rood J.I., Uzal F.A. Epsilon toxin is essential for the virulence of Clostridium perfringens type D infection in sheep, goats, and mice. Infect. Immun. 2013;81:2405–2414. doi: 10.1128/IAI.00238-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gaucher M.L., Quessy S., Letellier A., Arsenault J., Boulianne M. Impact of a drug-free program on broiler chicken growth performances, gut health, Clostridium perfringens and Campylobacter jejuni occurrences at the farm level. Poult. Sci. 2015;94:1791–1801. doi: 10.3382/ps/pev142. [DOI] [PubMed] [Google Scholar]
- Genovese K.J., He H., Swaggerty C.L., Kogut M.H. The avian heterophil. Dev. Comp. Immunol. 2013;41:334–340. doi: 10.1016/j.dci.2013.03.021. [DOI] [PubMed] [Google Scholar]
- Gharaibeh M.H., Khalifeh M.S., Nawasreh A.N., Hananeh W.M., Awawdeh M.S. Assessment of immune response and efficacy of essential oils application on controlling necrotic enteritis induced by Clostridium perfringens in broiler chickens. Molecules. 2021;26:4527. doi: 10.3390/molecules26154527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gharaibeh S., Al Rifai R., Al-Majali A. Molecular typing and antimicrobial susceptibility of Clostridium perfringens from broiler chickens. Anaerobe. 2010;16:586–589. doi: 10.1016/j.anaerobe.2010.10.004. [DOI] [PubMed] [Google Scholar]
- Giguere S. Pages 1-9 in Antimicrobial Therapy in Veterinary Medicine. Blackwell, IA; Hoboken, NJ: 2006. Antimicrobial drug action and interaction: an introduction. S. Giguere, J. F. Prescott, J. D. Baggot, R. D. Walker, and P. M. Dowling, eds. Blackwell, IA, Hoboken, NJ. [Google Scholar]
- Gobel T.W., Kaspers B., Stangassinger M. NK and T cells constitute two major, functionally distinct intestinal epithelial lymphocyte subsets in the chicken. Int. Immunol. 2001;13:757–762. doi: 10.1093/intimm/13.6.757. [DOI] [PubMed] [Google Scholar]
- Grave K., Kaldhusdal M., Kruse H., Harr L.M.F., Flatlandsmo K. What has happened in Norway after the ban of avoparcin? Consumption of antimicrobials by poultry. Prev. Vet. Med. 2004;62:59–72. doi: 10.1016/j.prevetmed.2003.08.009. [DOI] [PubMed] [Google Scholar]
- Hailemariam Z., Omar A.R., Hair-Bejo M., Giap T.C. Detection and characterization of chicken anemia virus from commercial broiler breeder chickens. Virol. J. 2008;5:128. doi: 10.1186/1743-422X-5-128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herholz C., Miserez R., Nicolet J., Frey J., Popoff M., Gibert M., Gerber H., Straub R. Prevalence of beta2-toxigenic Clostridium perfringens in horses with intestinal disorders. J. Clin. Microbiol. 1999;37:358–361. doi: 10.1128/jcm.37.2.358-361.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hermans P.G., Morgan K.L. Prevalence and associated risk factors of necrotic enteritis on broiler farms in the United Kingdom; a cross-sectional survey. Avian Pathol. 2007;36:43–51. doi: 10.1080/03079450601109991. [DOI] [PubMed] [Google Scholar]
- Hershberger E., Oprea S.F., Donabedian S.M., Perri M., Bozigar P., Bartlett P., Zervos M.J. Epidemiology of antimicrobial resistance in enterococci of animal origin. J. Antimicrob. Chemother. 2005;55:127–130. doi: 10.1093/jac/dkh508. [DOI] [PubMed] [Google Scholar]
- Hoerr F.J. Clinical aspects of immunosuppression in poultry. Avian Dis. 2010;54:2–15. doi: 10.1637/8909-043009-Review.1. [DOI] [PubMed] [Google Scholar]
- Hofshagen M., Stenwig H. Toxin production by Clostridium perfringens isolated from broiler chickens and capercaillies (Tetrao urogallus) with and without necrotizing enteritis. Avian Dis. 1992;36 837–843. [PubMed] [Google Scholar]
- Hong Y.H., Song W., Lee S.H., Lillehoj H.S. Differential gene expression profiles of -defensins in the crop, intestine, and spleen using a necrotic enteritis model in 2 commercial broiler chicken lines. Poult. Sci. 2012;91:1081–1088. doi: 10.3382/ps.2011-01948. [DOI] [PubMed] [Google Scholar]
- Honjo K., Hagiwara T., Itoh K., Takahashi E., Hirota Y. Immunohistochemical analysis of tissue distribution of B and T cells in germfree and conventional chickens. J. Vet. Med. Sci. 1993;55:1031–1034. doi: 10.1292/jvms.55.1031. [DOI] [PubMed] [Google Scholar]
- Horn N.L., Donkin S.S., Applegate T.J., Adeola O. Intestinal mucin dynamics: response of broiler chicks and White Pekin ducklings to dietary threonine. Poult. Sci. 2009;88:1906–1914. doi: 10.3382/ps.2009-00009. [DOI] [PubMed] [Google Scholar]
- Iqbal M., Philbin V.J., Smith A.L. Expression patterns of chicken Toll-like receptor mRNA in tissues, immune cell subsets and cell lines. Vet. Immunol. Immunopathol. 2005;104:117–127. doi: 10.1016/j.vetimm.2004.11.003. [DOI] [PubMed] [Google Scholar]
- Jadamus A., Vahjen W., Simon O. Growth behavior of a spore forming probiotic strain in the gastrointestinal tract of broiler chicken and piglets. Arch. Tierernahr. 2001;54:1–17. doi: 10.1080/17450390109381962. [DOI] [PubMed] [Google Scholar]
- Jayaraman S., Thangavel G., Kurian H., Mani R., Mukkalil R., Chirakkal H. Bacillus subtilis PB6 improves intestinal health of broiler chickens challenged with Clostridium perfringens-induced necrotic enteritis. Poult. Sci. 2013;92:370–374. doi: 10.3382/ps.2012-02528. [DOI] [PubMed] [Google Scholar]
- Jerzsele A., Szeker K., Csizinszky R., Gere E., Jakab C., Mallo J.J., Galfi P. Efficacy of protected sodium butyrate, a protected blend of essential oils, their combination, and Bacillus amyloliquefaciens spore suspension against artificially induced necrotic enteritis in broilers. Poult. Sci. 2012;91:837–843. doi: 10.3382/ps.2011-01853. [DOI] [PubMed] [Google Scholar]
- Jiang Y., Mo H., Willingham C., Wang S., Park J.Y., Kong W., Roland K.L., Curtiss R. Protection against necrotic enteritis in broiler chickens by regulated delayed lysis Salmonella vaccines. Avian Dis. 2015;59:475–485. doi: 10.1637/11094-041715-Reg. [DOI] [PubMed] [Google Scholar]
- Johansson A., Aspan A., Kaldhusdal M., Engstrom B.E. Genetic diversity and prevalence of netB in Clostridium perfringens isolated from a broiler flock affected by mild necrotic enteritis. Vet. Microbiol. 2010;144:87–92. doi: 10.1016/j.vetmic.2009.12.017. [DOI] [PubMed] [Google Scholar]
- Johansson A., Greko C., Engstrom B.E., Karlsson M. Antimicrobial susceptibility of Swedish, Norwegian and Danish isolates of Clostridium perfringens from poultry, and distribution of tetracycline resistance genes. Vet. Microbiol. 2004;99:251–257. doi: 10.1016/j.vetmic.2004.01.009. [DOI] [PubMed] [Google Scholar]
- Johansson K.R., Sarles W.B. Bacterial population changes in the ceca of young chickens infected with Eimeria tenella. J. Bacteriol. 1948;56:635–647. doi: 10.1128/jb.56.5.635-647.1948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones F.T., Ricke S.C. Observations on the history of the development of antimicrobials and their use in poultry feeds. Poult. Sci. 2003;82:613–617. doi: 10.1093/ps/82.4.613. [DOI] [PubMed] [Google Scholar]
- Kabir S.M.L. The role of probiotics in the poultry industry. Int. J. Mol. Sci. 2009;10:3531–3546. doi: 10.3390/ijms10083531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaiser P. Advances in avian immunology—prospects for disease control: a review. Avian Pathol. 2010;39:309–324. doi: 10.1080/03079457.2010.508777. [DOI] [PubMed] [Google Scholar]
- Kaldhusdal M., Løvland A. The economical impact of Clostridium perfringens is greater than anticipated. World Poult. 2000;16:50–51. [Google Scholar]
- Kaldhusdal M., Schneitz C., Hofshagen M., Skjerve E. Reduced incidence of Clostridium perfringens - associated lesions and improved performance in broiler chickens treated with normal intestinal bacteria from adult fowl. Avian Dis. 2001;45:149–156. [PubMed] [Google Scholar]
- Kaldhusdal M., Skjerve E. Association between cereal contents in the diet and incidence of necrotic enteritis in broiler chickens in Norway. Prev. Vet. Med. 1996;28:1–16. [Google Scholar]
- Kaldhusdal M., Hofshagen M. Barley inclusion and avoparcin supplementation in broiler diets.: 2. clinical, pathological, and bacteriological findings in a mild form of necrotic enteritis. Poult. Sci. 1992;71:1145–1153. doi: 10.3382/ps.0711145. [DOI] [PubMed] [Google Scholar]
- Kaldhusdal M., Skjerve E., Hansen M.K., Hamnes I.S., David B., Hanssen S.A., Løvland A. The incidence of necrotic enteritis in turkeys is associated with farm, season and faecal Eimeria oocyst counts. BMC Vet. Res. 2021;17:292. doi: 10.1186/s12917-021-03003-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaldhusdal M., Hofshagen M., Løvland A., Langstrand H., Redhead K. Necrotic enteritis challenge models with broiler chickens raised on litter: evaluation of preconditions, Clostridium perfringens strains and outcome variables. FEMS Immunol. Med. Microbiol. 1999;24:337–343. doi: 10.1111/j.1574-695X.1999.tb01303.x. [DOI] [PubMed] [Google Scholar]
- Kan L., Guo F., Liu Y., Pham V.H., Guo Y., Wang Z. Probiotics Bacillus licheniformis improves intestinal health of subclinical necrotic enteritis-challenged broilers. Front. Microbiol. 2021;12 doi: 10.3389/fmicb.2021.623739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kers J.G., Velkers F.C., Fischer E.A.J., Hermes G.D.A., Stegeman J.A., Smidt H. Host and environmental factors affecting the intestinal microbiota in chickens. Front. Microbiol. 2018;9:235. doi: 10.3389/fmicb.2018.00235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keyburn A.L., Boyce J.D., Vaz P., Bannam T.L., Ford M.E., Parker D., Di Rubbo A., Rood J.I., Moore R.J. NetB, a new toxin that is associated with avian necrotic enteritis caused by Clostridium perfringens. PLoS Pathog. 2008;4:e26. doi: 10.1371/journal.ppat.0040026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keyburn A.L., Portela R.W., Sproat K., Ford M.E., Bannam T.L., Yan X., Rood J.I., Moore R.J. Vaccination with recombinant NetB toxin partially protects broiler chickens from necrotic enteritis. Vet. Res. 2013;44:54. doi: 10.1186/1297-9716-44-54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keyburn A.L., Portela R.W., Ford M.E., Bannam T.L., Yan X., Rood J.I., Moore R.J. Maternal immunization with vaccines containing recombinant NetB toxin partially protects progeny chickens from necrotic enteritis. Vet. Res. 2013;44:108. doi: 10.1186/1297-9716-44-108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keyburn A.L., Sheedy S.A., Ford M.E., Williamson M.M., Awad M.M., Rood J.I., Moore R.J. Alpha-toxin of Clostridium perfringens is not an essential virulence factor in necrotic enteritis in chickens. Infect. Immun. 2006;74:6496–6500. doi: 10.1128/IAI.00806-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keyburn A.L., Yan X.X., Bannam T.L., Van Immerseel F., Rood J.I., Moore R.J. Association between avian necrotic enteritis and Clostridium perfringens strains expressing NetB toxin. Vet. Res. 2010;41:21. doi: 10.1051/vetres/2009069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khelfa D.G., Madian K., El-Meneisy A.A., Faten F.M., Salem H.M. Field and laboratory diagnosis of C. perfringens enteric infection among rabbit flocks in Egypt. Middle East J. Appl. Sci. 2015;5:252–261. [Google Scholar]
- Khelfa D.E., Abd El-Ghany W.A., Salem H.M. Recent status of Clostridial enteritis affecting early weaned rabbits in Egypt. Life Sci. J. 2012;9:2272–2279. [Google Scholar]
- Khelfa D.E., Abd El-Ghany W., Salem H.M. Serological and molecular typing of Clostridium perfringens and its toxins recovered from weaned rabbit's flocks in Egypt. Life Sci. J. 2012;9:2263–2271. [Google Scholar]
- Kim J.J., Khan W.I. Goblet cells and mucins: role in innate defense in enteric infections. Pathogens. 2013;2:55–70. doi: 10.3390/pathogens2010055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kitessa S.M., Nattrass G.S., Forder R.E.A., McGrice H.A., Wu S.B., Hughes R.J. Mucin gene mRNA levels in broilers challenged with Eimeria and/or Clostridium perfringens. Avian Dis. 2014;58:408–414. doi: 10.1637/10757-122313-Reg.1. [DOI] [PubMed] [Google Scholar]
- Knarreborg A., Simon M.A., Engberg R.M., Jensen B.B., Tannock G.W. Effects of dietary fat source and subtherapeutic levels of antibiotic on the bacterial community in the ileum of broiler chickens at various ages. Appl. Environ. Microbiol. 2002;68:5918–5924. doi: 10.1128/AEM.68.12.5918-5924.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kocher A., Choct M., Ross G., Broz J., Chung T.K. Effects of enzyme combinations on apparent metabolizable energy of corn-soybean meal-based diets in broilers. J. Appl. Poult. Res. 2003;12:275–283. [Google Scholar]
- Kogut M.H., Swaggerty C., He H., Pvzner I., Kaiser P. Toll-like receptor agonists stimulate differential functional activation and cytokine and chemokine gene expression in heterophils isolated from chickens with differential innate responses. Microbes Infect. 2006;8:1866–1874. doi: 10.1016/j.micinf.2006.02.026. [DOI] [PubMed] [Google Scholar]
- Kulkarni R.R., Parreira V.R., Sharif S., Prescott J.F. Immunization of broiler chickens against Clostridium perfringens-induced necrotic enteritis. Clin. Vaccine. Immunol. 2007;14:1070–1077. doi: 10.1128/CVI.00162-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- La Ragione R.M., Narbad A., Gasson M.J., Woodward M.J. In vivo characterization of Lactobacillus johnsonii FI9785 for use as a defined competitive exclusion agent against bacterial pathogens in poultry. Lett. Appl. Microbiol. 2004;38:197–205. doi: 10.1111/j.1472-765x.2004.01474.x. [DOI] [PubMed] [Google Scholar]
- Lang T., Hansson G.C., Samuelsson T. An inventory of mucin genes in the chicken genome shows that the mucin domain of Muc13 is encoded by multiple exons and that ovomucin is part of a locus of related gel-forming mucins. BMC Genomics. 2006;7:197. doi: 10.1186/1471-2164-7-197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lawley T.D., Clare S., Walker A.W., Stares M.D., Connor T.R., Raisen C., Goulding D., Rad R., Schreiber F., Brandt C., Deakin L.J., Pickard D.J., Duncan S.H., Flint H.J., Clark T.G., Parkhill J., Dougan G. Targeted restoration of the intestinal microbiota with a simple, defined bacteriotherapy resolves relapsing Clostridium difficile disease in mice. PLoS Pathog. 2012;8 doi: 10.1371/journal.ppat.1002995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lebrun M., Filee P., Mousset B., Desmecht D., Galleni M., Mainil J.G., Linden A. The expression of Clostridium perfringens consensus Beta2 toxin is associated with bovine enterotoxaemia syndrome. Vet. Microbiol. 2007;120:51–157. doi: 10.1016/j.vetmic.2006.10.020. [DOI] [PubMed] [Google Scholar]
- Lee K.W., Lillehoj H.S., Jeong W., Jeoung H.Y., An D.J. Avian necrotic enteritis: experimental models, host immunity, pathogenesis, risk factors, and vaccine development. Poult. Sci. 2011;90:1381–1390. doi: 10.3382/ps.2010-01319. [DOI] [PubMed] [Google Scholar]
- Lee S.H., Lillehoj H.S., Jang S.I., Lillehoj E.P., Min W., Bravo D.M. Dietary supplementation of young broiler chickens with Capsicum and turmeric oleoresins increases resistance to necrotic enteritis. Br. J. Nutr. 2013;110:840–847. doi: 10.1017/S0007114512006083. [DOI] [PubMed] [Google Scholar]
- Lee K.W., Lillehoj H.S., Park M.S., Jang S.I., Ritter G.D., Hong Y.H., Jeong W., Jeoung H.Y., An D.J., Lillehoj E.P. Clostridium perfringens α-Toxin and NetB toxin antibodies and their possible role in protection against necrotic enteritis and gangrenous dermatitis in broiler chickens. Avian Dis. 2012;56:230–233. doi: 10.1637/9847-070711-ResNote.1. [DOI] [PubMed] [Google Scholar]
- Lepp D., Roxas B., Parreira V.R., Marri P.R., Rosey E.L., Gong J., Songer J.G., Vedantam G., Prescott J.F. Identification of novel pathogenicity loci in Clostridium perfringens strains that cause avian necrotic enteritis. PLoS One. 2010;5:e10795. doi: 10.1371/journal.pone.0010795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li J., Adams V., Bannam T.L., Miyamoto K., Garcia J.P., Uzal F.A., Rood J.I., McClane B.A. Toxin plasmids of Clostridium perfringens. Microbiol. Mol. Biol. Rev. 2013;77:08–233. doi: 10.1128/MMBR.00062-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Llamas-Moya S., Girdler C.P., Shalash S.M.M., Atta A.M., Gharib H.B., Morsy E.A., Salim H.M., Awaad M.H.H., Elmenawey M. Effect of a multicarbohydrase containing α-galactosidase enzyme on the performance, carcass yield, and humoral immunity of broilers fed corn–soybean meal–based diets of varying energy density. J. Appl. Poult. Res. 2020;29:142–151. [Google Scholar]
- Løvland A., Kaldhusdal M. Liver lesions seen at slaughter as an indicator of necrotic enteritis in broiler flocks. FEMS Immunol. Med. Microbiol. 1999;24:345–351. doi: 10.1111/j.1574-695X.1999.tb01304.x. [DOI] [PubMed] [Google Scholar]
- Løvland A., Kaldhusdal M. Severely impaired production performance in broiler flocks with high incidence of Clostridium perfringens-associated hepatitis. Avian Pathol. 2001;30:73–81. doi: 10.1080/03079450020023230. [DOI] [PubMed] [Google Scholar]
- Lu Y., Sarson A.J., Gong J., Zhou H., Zhu W., Kang Z., Yu H., Sharif S., Han Y. Expression profiles of genes in toll-like receptor mediated signaling of broilers infected with Clostridium perfringens. Clin. Vaccine Immunol. 2009;16:1639–1647. doi: 10.1128/CVI.00254-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacFarlane M.G., Knight B.C. The biochemistry of bacterial toxins: the lecithinase activity of Cl. welchii toxins. Biochem. J. 1941;35:884–902. doi: 10.1042/bj0350884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martel A., Devriese L.A., Cauwerts K., De Gussem K., Decostere A., Haesebrouck F. Susceptibility of Clostridium perfringens strains from broiler chickens to antibiotics and anticoccidials. Avian Pathol. 2004;33:3–7. doi: 10.1080/0307945031000163291. [DOI] [PubMed] [Google Scholar]
- Martin T.G., Smyth J.A. Prevalence of netB among some clinical isolates of Clostridium perfringens from animals in the United States. Vet. Microbiol. 2009;136:202–205. doi: 10.1016/j.vetmic.2008.10.026. [DOI] [PubMed] [Google Scholar]
- McClane B.A., Chakrabarti G. New insights into the cytotoxic mechanisms of Clostridium perfringens enterotoxin. Anaerobe. 2004;10:107–114. doi: 10.1016/j.anaerobe.2003.11.004. [DOI] [PubMed] [Google Scholar]
- McDevitt R.M., Brooker J.D., Acamovic T., Sparks N.H.C. Necrotic enteritis; a continuing challenge for the poultry industry. Worlds Poult. Sci. J. 2006;62:221–247. [Google Scholar]
- McDougald L.R., Hofacre C., Mathis G., Fuller L., Hargrove J.L., Greenspan P., Hartle D.K. Enhancement of resistance to coccidiosis and necrotic enteritis in broiler chickens by dietary muscadine pomace. Avian Dis. 2008;52:646–651. doi: 10.1637/8306-041508-Reg.1. [DOI] [PubMed] [Google Scholar]
- Meade K.G., Higgs R., Lloyd A.T., Giles S., O'Farrelly C. Differential antimicrobial peptide gene expression patterns during early chicken embryological development. Dev. Comp. Immunol. 2009;33:516–524. doi: 10.1016/j.dci.2008.10.003. [DOI] [PubMed] [Google Scholar]
- Mishra N., Smyth J.A. Oral vaccination of broiler chickens against necrotic enteritis using a non-virulent NetB positive strain of Clostridium perfringens type A. Vaccine. 2017;35:6858–6865. doi: 10.1016/j.vaccine.2017.10.030. [DOI] [PubMed] [Google Scholar]
- Mitsch p., Zitterl-Eglseer K., Köhler B., Gabler C., Losa R., Zimpernik I. The effect of two different blends of essential oil components on the proliferation of Clostridium perfringens in the intestines of broiler chickens. Poult. Sci. 2004;83:669–675. doi: 10.1093/ps/83.4.669. [DOI] [PubMed] [Google Scholar]
- Moe P.C., Heuck A.P. Phospholipid hydrolysis caused by Clostridium perfringens α-toxin facilitates the targeting of perfringolysin O to membrane bilayers. Biochemistry. 2010;49:9498–9507. doi: 10.1021/bi1013886. [DOI] [PubMed] [Google Scholar]
- Mohamed S.H., Attia A.I., Reda F.M., Abd El-Hack M.E., Ismail I.E. Impacts of dietary supplementation of Boswellia serrata on growth, nutrients digestibility, immunity, antioxidant status, carcass traits and caecum microbiota of broilers. Ital. J. Anim. Sci. 2021;20:205–214. [Google Scholar]
- Mot D., Timbermont L., Haesebrouck F., Ducatelle R., Van Immerseel F. Progress and problems in vaccination against necrotic enteritis in broiler chickens. Avian Pathol. 2014;43:290–300. doi: 10.1080/03079457.2014.939942. [DOI] [PubMed] [Google Scholar]
- Müller H., Islam M.R., Raue R. Research on infectious bursal disease–the past, the present and the future. Vet. Microbiol. 2003;97:153–165. doi: 10.1016/j.vetmic.2003.08.005. [DOI] [PubMed] [Google Scholar]
- Mwangi W.N., Beal R.K., Powers C., Wu X., Humphrey T., Watson M., Bailey M., Friedman A., Smith A.L. Regional and global changes in TCRalphabeta T cell repertoires in the gut are dependent upon the complexity of the enteric microflora. Dev. Comp. Immunol. 2010;34:406–417. doi: 10.1016/j.dci.2009.11.009. [DOI] [PubMed] [Google Scholar]
- Mzabi A., Tanghort M., Chefchaou H., Moussa H., Chami N., Chami F., Remmal A. A comparative study of the anticlostridial activity of selected essential oils, their major components and a natural product with antibiotics. Int. J. Poult. Sci. 2019;18:187–194. [Google Scholar]
- Nagahama M., Ohkubo A., Oda M., Kobayashi K., Amimoto K., Miyamoto K., Sakurai J. Clostridium perfringens TpeL glycosylates the Rac and Ras subfamily proteins. Infect. Immun. 2011;79:905–910. doi: 10.1128/IAI.01019-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nagahama M., Ochi S., Oda M., Miyamoto K., Takehara M., Kobayashi K. Recent insights into Clostridium perfringens beta-toxin. Toxins. 2015;7:396–406. doi: 10.3390/toxins7020396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nishie M., Nagao J., Sonomoto K. Antibacterial peptides “bacteriocins”: an overview of their diverse characteristics and applications. Biocontrol Sci. 2012;17:1–16. doi: 10.4265/bio.17.1. [DOI] [PubMed] [Google Scholar]
- Novak J.S., Juneja V.K., McClane B.A. An ultrastructural comparison of spores from various strains of Clostridium perfringens and correlations with heat resistance parameters. Int. J. Food Microbiol. 2003;86:239–247. doi: 10.1016/s0168-1605(02)00550-0. [DOI] [PubMed] [Google Scholar]
- Oda M., Kabura M., Takagishi T., Suzue A., Tominaga K., Urano S., Nagahama M., Kobayashi K., Furukawa K., Furukawa K., Sakurai J. Clostridium perfringens alpha-toxin recognizes the GM1a-TrkA complex. J. Biol. Chem. 2012;287:33070–33079. doi: 10.1074/jbc.M112.393801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Opengart K. Necrotic enteritis. In: D. E. Swayne, M. Boulianne, C. M. Logue, L. R. McDougald, V. Nair, and D. L. Suarez. Pages 972-976 in Diseases of Poultry. Wiley-Blackwell; Hoboken, NJ: 2020. [Google Scholar]
- Osterberg D., Wallinga D. Addressing externalities from swine production to reduce public health and environmental impacts. Am. J. Public Health. 2004;94:1703–1708. doi: 10.2105/ajph.94.10.1703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park S.S., Lillehoj H.S., Allen P.C., Park D.W., FitzCoy S., Bautista D.A., Lillehoje E.P. Immunopathology and cytokine responses in broiler chickens coinfected with Eimeria maxima and Clostridium perfringens with the use of an animal model of necrotic enteritis. Avian Dis. 2008;52:14–22. doi: 10.1637/7997-041707-Reg. [DOI] [PubMed] [Google Scholar]
- Parvizi P., Abdul-Careem M.F., Haq K., Thanthrige-Don N., Schat K.A., Sharif S. Immune responses against Marek's disease virus. Anim. Health. Res. Rev. 2010;11:123–134. doi: 10.1017/S1466252310000022. [DOI] [PubMed] [Google Scholar]
- Pedersen K., Bjerrum L., Heuer O.E., Lo Fo Wong D.M., Nauerby B. Reproducible infection model for Clostridium perfringens in broiler chickens. Avian Dis. 2008;52:34–39. doi: 10.1637/7955-022307-Reg. [DOI] [PubMed] [Google Scholar]
- Pelaseyed T., Bergström J.H., Gustafsson J.K., Ermund A., Birchenough G.M., Schütte A., van der Post S., Svensson F., Rodríguez-Piñeiro A.M., Nyström E.E., Wising C., Johansson M.E., Hansson G.C. The mucus and mucins of the goblet cells and enterocytes provide the first defense line of the gastrointestinal tract and interact with the immune system. Immunol. Rev. 2014;260:8–20. doi: 10.1111/imr.12182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petit L., Gibert M., Popoff M.R. Clostridium perfringens: toxinotype and genotype. Trends Microbiol. 1999;7:104–110. doi: 10.1016/s0966-842x(98)01430-9. [DOI] [PubMed] [Google Scholar]
- Pham V.H., Abbas W., Huang J., He O., Zhen W., Guo Y., Wang Z. Effect of blending encapsulated essential oils and organic acids as an antibiotic growth promoter alternative on growth performance and intestinal health in broilers with necrotic enteritis. Poult. Sci. 2021;101:101563. doi: 10.1016/j.psj.2021.101563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Popoff M.R. Clostridial pore-forming toxins: powerful virulence factors. Anaerobe. 2014;30:220–238. doi: 10.1016/j.anaerobe.2014.05.014. [DOI] [PubMed] [Google Scholar]
- Porter R.E., Jr Bacterial enteritides of poultry. Poult. Sci. 1998;77:1159–1165. doi: 10.1093/ps/77.8.1159. [DOI] [PubMed] [Google Scholar]
- Prescott J.F., Smyth J.A., Shojadoost B., Vince A. Experimental reproduction of necrotic enteritis in chickens: a review. Avian Pathol. 2016;45:317–322. doi: 10.1080/03079457.2016.1141345. [DOI] [PubMed] [Google Scholar]
- Prescott J.F., Sivendra R., Barnum D.A. The use of bacitracin in the prevention and treatment of experimentally-induced necrotic enteritis in the chicken. Can. Vet. J. 1978;19:181–183. [PMC free article] [PubMed] [Google Scholar]
- Rantala M., Nurmi E. Prevention of the growth of Salmonella infantis in chicks by the flora of the alimentary tract of chickens. Br. Poult. Sci. 1973;14:627–630. doi: 10.1080/00071667308416073. [DOI] [PubMed] [Google Scholar]
- Raza S.H.A., Naqvi S.R.Z., Abdelnour S.A., Schreurs N., Mohammedsaleh Z.M., Khan I., Shater A.F., Abd El-Hack M.E., Khafaga A.F., Quan G., Khan R., Wang S., Cheng G., Zan L. Beneficial effects and health benefits of astaxanthin molecules on animal production: a review. Res. Vet. Sci. 2021;138:69–78. doi: 10.1016/j.rvsc.2021.05.023. [DOI] [PubMed] [Google Scholar]
- Reda F.M., El-Saadony M.T., Elnesr S.S., Alagawany M., Tufarelli V. Effect of dietary supplementation of biological curcumin nanoparticles on growth and carcass traits, antioxidant status, immunity and caecal microbiota of Japanese quails. Animals. 2020;10:754. doi: 10.3390/ani10050754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reda F., El-Saadony M.T., El-Rayes T.K., Farahat M., Attia G., Alagawany M. Dietary effect of licorice (Glycyrrhiza glabra) on quail performance, carcass, blood metabolites and intestinal microbiota. Poult. Sci. 2021;100 doi: 10.1016/j.psj.2021.101266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rehman H., Ijaz A., Specht A., Dill D., Hellweg P., Männer K., Zentek J. In vitro effects of alpha toxin from Clostridium perfringens on the electrophysiological parameters of jejunal tissues from laying hens preincubated with inulin and N-acetyl-L-cysteine. Poult. Sci. 2009;88:199–204. doi: 10.3382/ps.2008-00054. [DOI] [PubMed] [Google Scholar]
- Rood J.I. Virulence genes of Clostridium perfringens. Annu. Rev. Microbiol. 1998;52:333–360. doi: 10.1146/annurev.micro.52.1.333. [DOI] [PubMed] [Google Scholar]
- Saad A.M., Mohamed A.S., El-Saadony M.T., Sitohy M.Z. Palatable functional cucumber juices supplemented with polyphenols-rich herbal extracts. LWT-Food Sci. Technol. 2021;148 [Google Scholar]
- Saad A.M., El-Saadony M.T., El-Tahan A.M., Sayed S., Moustafa M.A., Taha A.E., Taha T.F., Ramadan M.M. Polyphenolic extracts from pomegranate and watermelon wastes as substrate to fabricate sustainable silver nanoparticles with larvicidal effect against Spodoptera littoralis. Saudi J. Biol. Sci. 2021;28:5674–5683. doi: 10.1016/j.sjbs.2021.06.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saad A.M., Sitohy M.Z., Ahmed A.I., Rabie N.A., Amin S.A., Aboelenin S.M., Soliman M.M., El-Saadony M.T. Biochemical and functional characterization of kidney bean protein alcalase-hydrolysates and their preservative action on stored chicken meat. Molecules. 2021;26:4690. doi: 10.3390/molecules26154690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sakurai J., Nagahama M., Oda M. Clostridium perfringens alpha-toxin: characterization and mode of action. J. Biochem. 2004;136:569–574. doi: 10.1093/jb/mvh161. [DOI] [PubMed] [Google Scholar]
- Salem H.M., Attia M.M. Accidental intestinal myiasis caused by Musca domestica L. (Diptera: Muscidae) larvae in broiler chickens: a field study. Int. J. Trop. Insect Sci. 2021;41:2549–2554. [Google Scholar]
- Salem H.M., Ismael E., Shaalan M. Evaluation of the effects of silver nanoparticles against experimentally induced necrotic enteritis in broiler chickens. Int. J. Nanomed. 2021;16:6783–6796. doi: 10.2147/IJN.S319708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sarson A.J., Wang Y., Kang Z., Dowd S.E., Lu Y., Yu H., Han Y., Zhou H., Gong J. Gene expression profiling within the spleen of Clostridium perfringens-challenged broilers fed antibiotic-medicated and non-medicated diets. BMC Genomics. 2009;10:260. doi: 10.1186/1471-2164-10-260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Savva C.G., Fernandes da Costa S.P., Bokori-Brown M., Naylor C.E., Cole A.R., Moss D.S., Titball R.W., Basak A.K. Molecular architecture and functional analysis of NetB, pore-forming toxin from Clostridium perfringens. J. Biol. Chem. 2013;288:3512–3522. doi: 10.1074/jbc.M112.430223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schneitz C. Competitive exclusion in poultry - 30 years of research. Food Control. 2005;16:657–667. [Google Scholar]
- Seidavi A., Azizi M., Swelum A.A., Abd El-Hack M.E., Naiel M.A.E. Practical application of some common agro-processing wastes in poultry diets. Worlds Poult. Sci. 2021 doi: 10.1080/00439339.2021.1960461. , accessed December 8, 2021. [DOI] [Google Scholar]
- Seidavi A., Tavakoli M., Slozhenkina M., Gorlov I., Hashem N.M., Asroush F., Taha A.E., Abd El-Hack M.E., Swelum A.A. The use of some plant-derived products as effective alternatives to antibiotic growth promoters in organic poultry production: a review. Environ. Sci. Poll. Res. 2021;28:47856–47868. doi: 10.1007/s11356-021-15460-7. [DOI] [PubMed] [Google Scholar]
- Sheiha A.M., Abdelnour S.A., Abd El-Hack M.E., Khafaga A.F., Metwally K.A., Ajarem J.S., Maodaa S.N., Allam A.A., El-Saadony M.T. Effects of dietary biological or chemical-synthesized nanoselenium supplementation on growing rabbits exposed to thermal stress. Animals. 2020;10:430. doi: 10.3390/ani10030430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shepard L.A., Shatursky O., Johnson A.E., Tweten R.K. The mechanism of pore assembly for a cholesterol-dependent cytolysin: formation of a large prepore complex precedes the insertion of the transmembrane β-hairpins. Biochemistry. 2000;39:10284–10293. doi: 10.1021/bi000436r. [DOI] [PubMed] [Google Scholar]
- Shimizu T., Ohtani K., Hirakawa H., Ohshima K., Yamashita A., Shiba T., Ogasawara N., Hattori M., Kuhara S., Hayashi H. Complete genome sequence of Clostridium perfringens, an anaerobic flesh-eater. PNAS. 2002;99:996–1001. doi: 10.1073/pnas.022493799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shimizu T., Ba-Thein W., Tamaki M., Hayashi H. The virR gene, a member of a class of two-component response regulators, regulates the production of perfringolysin O, collagenase, and hemagglutinin in Clostridium perfringens. J. Bacteriol. 1994;176:616–1623. doi: 10.1128/jb.176.6.1616-1623.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Slavic D., Boerlin P., Fabri M., Klotins K.C., Zoethout J.K., Weir P.E., Bateman D. Antimicrobial susceptibility of Clostridium perfringens isolates of bovine, chicken, porcine, and turkey origin from Ontario. Can. J. Vet. Res. 2011;75:89–97. [PMC free article] [PubMed] [Google Scholar]
- Smedley J.G., Fisher D.J., Sayeed S., Chakrabarti G., McClane B.A. The enteric toxins of Clostridium perfringens. Rev. Physiol. Biochem. Pharmacol. 2004;152:183–204. doi: 10.1007/s10254-004-0036-2. [DOI] [PubMed] [Google Scholar]
- Smith E.A., Macfarlane G.T. Enumeration of amino acid fermenting bacteria in the human large intestine: effects of pH and starch on peptide metabolism and dissimilation of amino acids. FEMS Microbiol. Ecol. 1998;25:355–368. [Google Scholar]
- Songer J.G. Clostridial enteric diseases of domestic animals. Clin. Microbiol. Rev. 1996;9:216–234. doi: 10.1128/cmr.9.2.216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Songer J.G. Bacterial phospholipases and their role in virulence. Trends Microbiol. 1997;5:156–161. doi: 10.1016/S0966-842X(97)01005-6. [DOI] [PubMed] [Google Scholar]
- Sorour H.K., Hosny R.A., Elmasry D.M.A. Effect of peppermint oil and its microemulsion on necrotic enteritis in broiler chickens. Vet. World. 2021;14:483–491. doi: 10.14202/vetworld.2021.483-491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stokstad E.L.R., Jukes T.H. Further observations on the “animal protein factor. Proc. Soc. Exp. Biol. Med. 1950;73:523–528. [Google Scholar]
- Stutz M.W., Lawton G.C. Effects of diet and antimicrobials on growth, feed efficiency, intestinal Clostridium perfringens, and ileal weight of broiler chicks. Poult. Sci. 1984;63:2036–2042. doi: 10.3382/ps.0632036. [DOI] [PubMed] [Google Scholar]
- Svobodova I., Steinhauserova I., Nebola M. Incidence of Clostridium perfringens in broiler chickens in Czech Republic. Acta Vet. Brno. 2007;76:25–30. [Google Scholar]
- Timbermont L., Lanckriet A., Dewulf J., Nollet N., Schwarzer K., Haesebrouck F., Ducatelle R., Van Immerseel F. Control of Clostridium perfringens-induced necrotic enteritis in broilers by target-released butyric acid, fatty acids and essential oils. Avian Pathol. 2010;39:117–121. doi: 10.1080/03079451003610586. [DOI] [PubMed] [Google Scholar]
- Timbermont L., Haesebrouck F., Ducatelle R., Van Immerseel F. Necrotic enteritis in broilers: an updated review on the pathogenesis. Avian Pathol. 2011;40:341–347. doi: 10.1080/03079457.2011.590967. [DOI] [PubMed] [Google Scholar]
- Timbermont L., De Smet L., Van Nieuwerburgh F., Parreira V.R., Van Driessche G., Haesebrouck F., Ducatelle R., Prescott J., Deforce D., Devreese B., Van Immerseel F. Perfrin, a novel bacteriocin associated with netB positive Clostridium perfringens strains from broilers with necrotic enteritis. Vet. Res. 2014;45:40. doi: 10.1186/1297-9716-45-40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsiouris V., Georgopoulou I., Batzios C., Pappaioannou N., Ducatelle R., Fortomaris P. Temporary feed restriction partially protects broilers from necrotic enteritis. Avian Pathol. 2014;43:139–145. doi: 10.1080/03079457.2014.889278. [DOI] [PubMed] [Google Scholar]
- Tsiouris V., Georgopoulou I., Batzios C., Pappaioannou N., Ducatelle R., Fortomaris P. High stocking density as a predisposing factor for necrotic enteritis in broiler chicks. Avian Pathol. 2015;44:59–66. doi: 10.1080/03079457.2014.1000820. [DOI] [PubMed] [Google Scholar]
- van Asten A.J., Allaart J.G., Meeles A.D., Gloudemans P.W., Houwers D.J., Grone A.A. A new PCR followed by MboI digestion for the detection of all variants of the Clostridium perfringens cpb2 gene. Vet. Microbiol. 2008;127:412–416. doi: 10.1016/j.vetmic.2007.08.035. [DOI] [PubMed] [Google Scholar]
- Van der Sluis W. Clostridial enteritis a syndrome emerging worldwide. World Poult. 2000;16:56–57. [Google Scholar]
- Watkins K.L., Shryock T.R., Dearth R.N., Saif Y.M. In vitro antimicrobial susceptibility of Clostridium perfringens from commercial turkey and broiler chicken origin. Vet. Microbiol. 1997;54:195–200. doi: 10.1016/s0378-1135(96)01276-x. [DOI] [PubMed] [Google Scholar]
- Wierup M. The Swedish experience of the 1986-year ban of antimicrobial growth promoters, with special reference to animal health, disease prevention, productivity, and usage of antimicrobials. Microb. Drug Resist. 2001;7:183–190. doi: 10.1089/10766290152045066. [DOI] [PubMed] [Google Scholar]
- Wilkie D.C., Van Kessel A.G., Dumonceaux T.J., Drew M.D. The effect of hen-egg antibodies on Clostridium perfringens colonization in the gastrointestinal tract of broiler chickens. Prev. Vet. Med. 2006;74:279–292. doi: 10.1016/j.prevetmed.2005.12.004. [DOI] [PubMed] [Google Scholar]
- Williams R.B. Intercurrent coccidiosis and necrotic enteritis of chickens: rational, integrated disease management by maintenance of gut integrity. Avian Pathol. 2005;34:159–180. doi: 10.1080/03079450500112195. [DOI] [PubMed] [Google Scholar]
- World Health Organization (WHO). 2001. WHO Global Strategy for Containment of Antimicrobial Resistance. The World Health Organization, Geneva, Switzerland. https://www.who.int/drugresistance/WHO_Global_Strategy_English.pdf.
- Wu S.B., Stanley D., Rodgers N., Swick R.A., Moore R.J. Two necrotic enteritis predisposing factors, dietary fishmeal and Eimeria infection, induce large changes in the caecal microbiota of broiler chickens. Vet. Microbiol. 2014;169:188–197. doi: 10.1016/j.vetmic.2014.01.007. [DOI] [PubMed] [Google Scholar]
- Yadav S., Jha R. Strategies to modulate the intestinal microbiota and their effects on nutrient utilization, performance, and health of poultry. J. Anim. Sci. Biotechnol. 2019;10:2. doi: 10.1186/s40104-018-0310-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang Q., Liu J., Wang X., Robinson K., Whitmore M.A., Stewart S.N., Zhao J., Zhang G. Identification of an intestinal microbiota signature associated with the severity of necrotic enteritis. Front. Microbiol. 2021;12 doi: 10.3389/fmicb.2021.703693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yaqoob M., Abd El-Hack M.E., Hassan F., El-Saadony M.T., Khafaga A., Batiha G., Yehia N., Elnesr S., Alagawany M., El-Tarabily K.A., Wang M. The potential mechanistic insights and future implications for the effect of prebiotics on poultry performance, gut microbiome, and intestinal morphology. Poult. Sci. 2021;100 doi: 10.1016/j.psj.2021.101143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yitbarek A., Echeverry H., Brady J., Hernandez-Doria J., Camelo-Jaimes G., Sharif S., Guenter W., House J.D., Rodriguez-Lecompte J.C. Innate immune response to yeast-derived carbohydrates in broiler chickens fed organic diets and challenged with Clostridium perfringens. Poult. Sci. 2012;91:1105–1112. doi: 10.3382/ps.2011-02109. [DOI] [PubMed] [Google Scholar]