Abstract
While obesity has been shown to be associated with elevated risk for Sudden Cardiac Death (SCD), studies examining its effect on outcomes in SCD victims have shown conflicting results. We aimed to describe the body mass index (BMI) distribution in a nationwide cohort of patients admitted for an out of hospital SCD (OHSCD), and the relationship between BMI and in‐hospital mortality. We drew data from the U.S. National Inpatient Sample (NIS), to identify cases of OHSCD. Patients were divided into six groups based on their BMI (underweight, normal weight, overweight, obese I, obese II, extremely obese). Socio‐demographic and clinical data were collected, mortality and length of stay were analyzed. Multivariate analysis was performed to identify predictors of mortality. Among a weighted total of 2330 hospitalizations for OHSCD in patients with documented BMI, the mean age was 62.3 ± 29 years, 52.4% were male and 62% were white. The overall rate of in‐hospital mortality was 69.3%. A U‐shaped relationship between the BMI and mortality was documented, as patients with 25 < BMI < 40 exhibited significantly lower mortality (60.7%) compared to the other BMI groups (75.2%), p < .001. BMI of 25 kg/m2 and below or 40 kg/m2 and above, were independent predictors of in‐hospital mortality in a multivariate analysis along with prior history of congestive heart failure and Deyo Comorbidity Index of ≥2. A U‐shaped relationship between the BMI and in‐hospital mortality was documented in patients hospitalized for an out of hospital sudden cardiac death in the United States in the recent years.
Keywords: BMI, body mass index, obesity paradox, sudden cardiac death
1. INTRODUCTION
The effect of excess weight on morbidity and mortality has been acknowledged from over 2000 years ago. Hippocrates recognized that “sudden death is more common in those who are naturally fat than in the lean”. 1 Over the last few decades, the prevalence of obesity in the United States has increased significantly, bearing dramatic social, clinical and economic implications. 2 , 3 , 4 , 5 , 6 Elevated body mass index (BMI) has been proven over the years as an independent risk factor for various cardio‐vascular conditions such as ischemic heart disease, acute coronary syndrome, congestive heart failure, atrial and ventricular arrhythmia and sudden cardiac death. 7 , 8
Sudden cardiac death (SCD) is responsible for about 50% of the mortality from cardiovascular disease in the United States and other developed countries. 9 , 10 Different clinical parameters including age, co‐morbidities, initial cardiac rhythm, and time to return of spontaneous circulation were investigated as predictors of survival in SCD. 11 While obesity has been shown to be associated with increased incidence and severity of major cardiovascular risk factors and elevated risk for SCD, 8 , 12 , 13 studies examining its effect on outcomes in SCD victims have shown conflicting results. 14 , 15 , 16 , 17 , 18 , 19 , 20 Some studies showed increased mortality in patients with BMI > 30 kg/m2 admitted to the hospital following a sudden cardiac death. 17 , 18 At the same time, several other studies have implied that the “obesity paradox”, described in various cardio‐vascular conditions such as acute myocardial infarction (AMI) and heart failure, applies to patients admitted after a sudden cardiac death, showing lower mortality in obese patients. 14 , 16 , 18 , 19
We aimed at describing the BMI distribution and baseline characteristics in a nationwide cohort of patients, admitted for an out of hospital sudden cardiac death (OHSCD) in the United States, and the relationship between BMI and in‐hospital mortality.
2. METHODS
2.1. Data source
The data were drawn from the National Inpatient Sample (NIS), the Healthcare Cost and Utilization Project (HCUP), and Agency for Healthcare Research and Quality (AHRQ) 21 , 22 datasets, consisting only of de‐identified information; therefore, this study was deemed exempt from institutional review by the Human Research Committee.
The NIS is the largest collection of all‐payer data on inpatient hospitalizations in the United States. The dataset represents an approximate 20% stratified sample of all inpatient discharges from U.S. hospitals. 23 This information includes patient‐level and hospital‐level factors such as patient demographic characteristics, primary and secondary diagnoses and procedures, co‐morbidities, length of stay (LOS), hospital region, hospital teaching status, hospital bed size, and cost of hospitalization. National estimates can be calculated using the patient‐level and hospital‐level sampling weights that are provided by the HCUP.
For the purpose of this study, we obtained data for the years 2015 (last quarter) and 2016. International Classification of Diseases, 10th Revision, Clinical Modification (ICD‐10‐CM) was used from the last quarter of 2015 and thereafter for reporting diagnoses and procedures in the NIS database during the study period. For each index hospitalization, the database provides a principal discharge diagnosis and a maximum of 29 additional diagnoses, in addition to a maximum of 15 procedures. The reason we only included the data coded with ICD‐10 codes is that the ICD‐10 system includes individual codes for BMI values and ranges.
2.2. Study population and variables
We identified patients 18 years of age or older with a primary diagnosis of sudden cardiac death based on ICD‐10‐CM codes I46.2, I46.8, or I46.9, who had one of the BMI, Z68.x, codes, among the secondary diagnoses. Of notice, these represented only the successfully resuscitated OHSCD patients, since those who were not successfully resuscitated in the field or died in the emergency departments, were not hospitalized. To have the “cleanest” possible data on patients admitted for successfully resuscitated out of hospital cardiac arrest, we avoided including patients with a secondary diagnosis of a cardiac arrest in our analysis due to the fact that these could represent patients who underwent an in‐hospital sudden cardiac death or had a prior history of cardiac arrest included as a secondary diagnosis.
The following codes represent the six BMI subgroups we have created for our study: Z68.1, BMI ≤19 kg/m2, under‐weight group; Z68.20–25, BMI 20–25 kg/m2, normal‐weight group; Z68.26–30, BMI 26–30 kg/m2, over‐weight group; Z68.31–35, BMI 31–35 kg/m2, obese I group; Z68.36–39 kg/m2, BMI 36–39, obese II group; Z68.4, BMI ≥40 kg/m2, extremely obese group. In addition to analyzing the individual BMI subgroups mentioned above, we combined the overweigh, Obese I and Obese II groups to compare the outcomes of these patients to the combined group of all the underweight, normal weight and extremely obese patients.
The following patient demographics were collected from the database: age, sex, and race. Prior comorbidities were identified by measures from the AHRQ. For the purposes of calculating Deyo‐Charlson Comorbidity Index (Deyo‐CCI), additional comorbidities were identified from the database using ICD‐10‐CM codes. Deyo‐CCI is a modification of the Charlson Comorbidity Index, containing 17 comorbidity conditions with differential weights, with a total score ranging from 0 to 33. (Detailed information on Deyo‐CCI provided in the Appendix A table). Higher Deyo‐CCI scores indicate a greater burden of comorbid diseases and are associated with mortality, 1 year after admission. 24 The index has been used extensively in studies from administrative databases, with proved validity in predicting short‐ and long‐term outcomes. 25 , 26 Our primary outcome in this study was in‐hospital mortality. Length of stay was the secondary outcome we analyzed.
2.3. Statistical analysis
The chi‐square (χ 2) test and Wilcoxon Rank Sum test were used to compare categorical variables and continuous variables, respectively. The NIS provides discharge sample weights that are calculated within each sampling stratum as the ratio of discharges in the universe to discharges in the sample. 27 We generated a weighted logistic regression model to identify independent predictors of in‐hospital mortality. Candidate variables included patient‐level characteristics, Deyo‐CCI and hospital‐level factors. We retained all predictor variables that were associated with our primary and secondary outcome with p < .05 in our final multivariable regression model. For all analyses, we used SAS® software version 9.4 (SAS Institute Inc., Cary, NC.) A p value <.05 was considered statistically significant.
3. RESULTS
3.1. Study cohort
A total of 466 hospitalizations for successfully resuscitated out of hospital sudden cardiac death patients across the United States during 2015 (last quarter) and 2016 were included in the analysis. After implementing the weighting method, these represented an estimated total of 2330 hospitalizations for OHSCD, in patients with documented BMI during the index hospitalization. The majority of patients (52.4%) were male and the mean age of the cohort was 62.3 ± 29 years.
As shown in Table 1, 62% of the study population were white, majority of 56% had Medicare coverage, 82.3% were in the lower 75th income percentile. As to the clinical characteristics, 39.5% of the study population had history of hypertension, 40.1% had diabetes mellitus, 33.3% had chronic pulmonary disease, 9.9% of the patients had a prior history of an AMI, 10.9% had peripheral vascular disease. The median BMI in the study was 38 (IQR: 31–41) with 85.6% of the patients with BMI above the normal (>25 kg/m2). The data reveal that 16.3% of all hospitalizations for successfully resuscitated OHSCD included patients with a diagnosis of VT/VF and 9.2% of the patients were diagnosed with an acute myocardial infraction (STEMI or NSTEMI). Twelve percent of the patients underwent a percutaneous coronary angiography and 2.8% of the patients required percutaneous coronary intervention.
TABLE 1.
Baseline characteristics of the study population (total and per BMI groups)
| <20 | 20–25 | 26–30 | 31–35 | 36–39 | ≥40 | Total | P Value | |
|---|---|---|---|---|---|---|---|---|
| Patients, n | ||||||||
| Unweighted | 42 | 25 | 48 | 74 | 66 | 211 | 466 | |
| Weighted | 210 | 125 | 240 | 370 | 330 | 1055 | 2330 | |
| Age group, % | <.001 | |||||||
| 18–44 | 9.5 | 8.0 | 10.4 | 8.1 | 7.6 | 10.4 | 9.4 | |
| 45–59 | 23.8 | 16.0 | 20.8 | 21.6 | 25.8 | 34.1 | 27.7 | |
| 60–74 | 31.0 | 36.0 | 58.3 | 52.7 | 56.1 | 45.0 | 47.4 | |
| 75 or older | 35.7 | 40.0 | 10.4 | 17.6 | 10.6 | 10.4 | 15.5 | |
| Gender, % | <.001 | |||||||
| Male | 57.1 | 64.0 | 56.2 | 60.8 | 43.9 | 48.8 | 52.4 | |
| Female | 42.9 | 36.0 | 43.8 | 37.8 | 56.1 | 51.2 | 47.4 | |
| Missing | 0.0 | 0.0 | 0.0 | 1.4 | 0.0 | 0.0 | 0.2 | |
| Race, % | <.001 | |||||||
| White | 69.0 | 52.0 | 47.9 | 63.5 | 69.7 | 63.0 | 62.4 | |
| Non‐White | 31.0 | 44.0 | 41.7 | 16.2 | 19.7 | 26.5 | 26.8 | |
| Other/Missing | 0.0 | 4.0 | 10.4 | 20.3 | 10.6 | 10.4 | 10.7 | |
| Comorbidity, % | ||||||||
| Hypertension | 26.2 | 36.0 | 41.7 | 50.0 | 47.0 | 36.0 | 39.5 | <.001 |
| Congestive heart failure | 11.9 | 12.0 | 20.8 | 16.2 | 27.3 | 37.0 | 27.0 | <.001 |
| Diabetes Mellitus | 9.5 | 20.0 | 35.4 | 55.4 | 45.5 | 42.7 | 40.1 | <.001 |
| Renal Failure | 19.0 | 36.0 | 31.3 | 33.8 | 34.8 | 38.9 | 34.8 | <.001 |
| Peripheral Vascular Disease | 14.3 | 16.0 | 4.2 | 12.2 | 9.1 | 11.4 | 10.9 | .004 |
| Prior MI | 4.8 | 8.0 | 18.7 | 12.2 | 9.1 | 8.5 | 9.9 | <.001 |
| VT/VF | 9.5 | 16.0 | 14.6 | 27.0 | 19.7 | 13.3 | 16.3 | <.001 |
| Deyo‐CCI, % | .008 | |||||||
| 0 | 9.5 | 12.0 | 14.6 | 12.2 | 15.2 | 10.9 | 12.0 | |
| 1 | 23.8 | 16.0 | 20.8 | 14.9 | 13.6 | 13.7 | 15.7 | |
| 2 or higher | 66.7 | 72.0 | 64.6 | 73.0 | 71.2 | 75.4 | 72.3 | |
| Primary payer, % | <.001 | |||||||
| Medicare | 61.9 | 72.0 | 56.3 | 58.1 | 53.0 | 53.1 | 56.0 | |
| Medicaid | 11.9 | 12.0 | 14.6 | 10.8 | 13.6 | 19.9 | 15.9 | |
| Private insurance | 11.9 | 16.0 | 14.6 | 23.0 | 21.2 | 22.7 | 20.4 | |
| Self‐pay | 7.1 | 0.0 | 10.4 | 4.1 | 3.0 | 1.9 | 3.6 | |
| No charge | 2.4 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.2 | |
| Other/missing | 4.8 | 0.0 | 4.2 | 4.1 | 9.1 | 2.4 | 3.9 | |
| Income percentile, % | <.001 | |||||||
| 0 to 25th percentile | 33.3 | 52.0 | 27.1 | 31.1 | 31.8 | 38.9 | 35.6 | |
| 26th to 50th percentile | 26.2 | 12.0 | 18.8 | 28.4 | 34.8 | 26.1 | 26.2 | |
| 51st to 75th percentile | 21.4 | 12.0 | 20.8 | 24.3 | 18.2 | 22.7 | 21.5 | |
| 76th to 100th percentile | 19.0 | 20.0 | 29.2 | 13.5 | 12.1 | 10.9 | 14.6 | |
| Missing | 0.0 | 4.0 | 4.2 | 2.7 | 3.0 | 1.4 | 2.1 | |
| Hospital status, % | <.001 | |||||||
| Urban teaching | 69.0 | 80.0 | 66.7 | 67.6 | 80.3 | 63.0 | 68.0 | |
| Urban nonteaching | 23.8 | 16.0 | 31.2 | 23.0 | 12.1 | 28.9 | 24.7 | |
| Rural | 7.1 | 4.0 | 2.1 | 9.5 | 7.6 | 8.1 | 7.3 | |
| Hospital region, % | <.001 | |||||||
| South | 42.9 | 40.0 | 52.1 | 39.2 | 47.0 | 45.0 | 44.6 | |
| West | 23.8 | 32.0 | 14.6 | 17.6 | 15.2 | 14.7 | 17.0 | |
| Midwest | 21.4 | 16.0 | 18.8 | 29.7 | 19.7 | 28.9 | 25.3 | |
| Northeast | 11.9 | 12.0 | 14.6 | 13.5 | 18.2 | 11.4 | 13.1 | |
| Hospital bed size, % | .159 | |||||||
| Large | 57.1 | 52.0 | 52.1 | 56.8 | 48.5 | 56.9 | 54.9 | |
| Small/medium | 42.9 | 48.0 | 47.9 | 43.2 | 51.5 | 43.1 | 45.1 | |
Note: p Values were generated using Chi‐square test and refer to differences between BMI groups within baseline characteristics.
3.2. Patients characteristics by BMI group
Baseline characteristics of the study population in the different BMI groups is presented in detail in Table 1. The distribution of the BMI groups varied significantly based on the country regions as well as income percentiles (p < .001). While about three quarters of the OHSCD patients (74.9%) across the country were obese (BMI > 30 kg/m2), the prevalence of obesity among the study population was highest in the Midwest (81.4%) and lowest in the West coast of the United States (68.3%), p < .001. Female predominance was documented in the obese II and the extremely obese groups. Younger age and higher prevalence of comorbidities including hypertension, diabetes and congestive heart failure were documented in the obese patients (Table 1). Among the obese patients, only 12.1% had an annual income in the highest income quartile, compared to 22.7% among nonobese patients (p < .001).
3.3. Length of stay and mortality by BMI groups
The average LOS in the hospital for the study population was 5.51 ± 0.42 days. As shown in Figure 1; the trend of the correlation between BMI and length of stay was linear in nature with longer hospital stay in obese patients, p < .001 (Figure 1).
FIGURE 1.
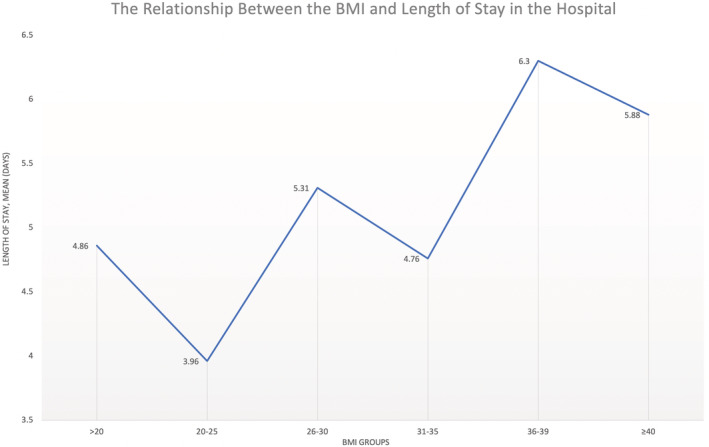
The relationship between BMI and the length of hospitalization in the study
The overall rate of in‐hospital mortality during the study period was documented at 69.3%. A U‐shaped relationship between the BMI and the in‐hospital mortality was documented, as described in Figure 2. Following the observation that the over‐weight, obese I and obese II patient subgroups (BMI 26–39) exhibited significantly lower in‐hospital mortality (61%) compared to the other BMI groups (75%), we performed an additional statistical analysis dividing the patients into these two subgroups (Table 2).
FIGURE 2.
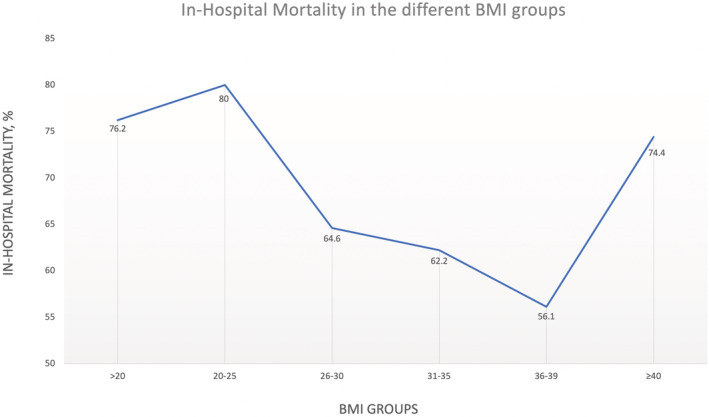
The relationship between BMI and in‐hospital mortality in the study population
TABLE 2.
In‐hospital outcomes for the total study population and per BMI group
| BMI, kg/m2 | ≤20 | 20–25 | 26–30 | 31–35 | 36–39 | ≥40 | Total | p Value |
|---|---|---|---|---|---|---|---|---|
| Mortality, % | 76.2 | 80.0 | 64.6 | 62.2 | 56.1 | 74.4 | 69.3 | <.0001 |
| Length of Stay (days), Mean ± SEM | 4.86 ± 0.85 | 3.96 ± 0.41 | 5.31 ± 0.27 | 4.76 ± 0.58 | 6.30 ± 0.57 | 5.88 ± 0.56 | 5.51 ± 0.42 | <.0001 |
Note: p Values were generated using Chi‐square test and refer to differences between BMI groups.
3.4. Predictors of in‐hospital mortality
In an unadjusted analysis, we found several parameters that significantly increased the odds of in‐hospital mortality (Table 3). These included: white race and history of congestive heart failure (all with p < .01). In addition, BMI 26–30, BMI 31–35, BMI 36–39 (compared to BMI 20–25) and having BMI between 26 and 39 compared to the other groups combined (below 25 kg/m2 or above 40 kg/m2), decreased the risk for in‐hospital mortality (all with p < .01). Personal history of hypertension and diabetes were found as predictors of improved outcomes in a univariate analysis, before adjusting for potential confounders. While admission with a diagnosis of STEMI or NSTEMI did not predict improved outcomes, the small proportion of patients who underwent coronary intervention (2.8%) were found to have lower mortality, Odds Ratio (OR) –0.21, 95% Confidence Interval (95% CI), (0.17–0.28). After adjusting for potential confounders, BMI below 25 kg/m2 or above 40 kg/m2, compared to BMI 26–39 kg/m2, remained an independent predictor of in‐hospital mortality in a multivariate analysis (Table 4). Hypertension and diabetes were not found to be independent predictors in a multivariate analysis, implying an interaction between them and other clinical parameters directly effecting the primary outcome. Congestive heart failure, OR −1.29 (1.01–1.65), and Deyo Comorbidity index of ≥2, OR −1.64 (1.19–2.25), were also found to be independent predictors of mortality.
TABLE 3.
Univariate analysis for predictors of in‐hospital mortality
| Predictor | Probability (95% CI) | Odds Ratio (95% CI) | p Value |
|---|---|---|---|
| Age group, years | .487 | ||
| 18–44 | 72.73% (66.47,78.20) | 1.00 (reference) | N/A |
| 45–59 | 70.54% (66.91,73.93) | 0.90 (0.64,1.26) | .537 |
| 60–74 | 68.33% (65.52,71.00) | 0.81 (0.59,1.12) | .198 |
| 75 or older | 68.06% (63.06,72.67) | 0.80 (0.55,1.16) | .235 |
| Race | .008 | ||
| Non‐White | 65.60% (61.79,69.22) | 1.00 (reference) | N/A |
| White | 71.48% (69.10,73.74) | 1.31 (1.08,1.61) | 0.008 |
| BMI Sub‐groups | <.001 | ||
| Below 20 | 76.19% (69.96,81.47) | 0.80 (0.47,1.37) | .419 |
| 20–25 | 80.00% (72.07,86.11) | 1.00 (reference) | N/A |
| 26–30 | 64.58% (58.33,70.38) | 0.46 (0.27,0.76) | .003 |
| 31–35 | 62.16% (57.11,66.96) | 0.41 (0.25,0.67) | <.001 |
| 36–39 | 56.06% (50.66,61.33) | 0.32 (0.20,0.52) | <.001 |
| 40 and above | 74.41% (71.69,76.95) | 0.73 (0.46,1.15) | .174 |
| BMI 2 groups | <.001 | ||
| 25–39 | 61.02% (57.92,64.04) | 1.00 (reference) | N/A |
| Below 25 or 40 and above | 75.28% (72.91,77.50) | 1.94 (1.63,2.32) | <.001 |
| Gender | .172 | ||
| Male | 70.49% (67.87,72.99) | 1.00 (reference) | N/A |
| Female | 67.87% (65.06,70.56) | 0.88 (0.74,1.05) | .172 |
| Hospital bed size | <.001 | ||
| Large | 72.27% (69.75,74.65) | 1.00 (reference) | N/A |
| Small/medium | 65.71% (62.79,68.53) | 0.74 (0.62,0.88) | <.001 |
| Diabetes mellitus | .010 | ||
| No | 71.33% (68.90,73.64) | 1.00 (reference) | N/A |
| Yes | 66.31% (63.22,69.27) | 0.79 (0.66,0.95) | .010 |
| Hypertension | .011 | ||
| No | 71.28% (68.86,73.58) | 1.00 (reference) | N/A |
| Yes | 66.30% (63.19,69.29) | 0.79 (0.66,0.95) | .011 |
| Congestive heart failure | .004 | ||
| No | 67.65% (65.38,69.83) | 1.00 (reference) | N/A |
| Yes | 73.81% (70.23,77.09) | 1.35 (1.10,1.65) | .004 |
| Deyo‐CCI | .075 | ||
| 0 | 64.29% (58.50,69.68) | 1.00 (reference) | N/A |
| 1 | 72.60% (67.80,76.93) | 1.47 (1.05,2.06) | .024 |
| 2 or higher | 69.44% (67.19,71.59) | 1.26 (0.97,1.65) | .086 |
| Clinical course | |||
| PCI | <.001 | ||
| No | 70.20% (68.28,72.05) | 1.00 (reference) | N/A |
| Yes | 38.46% (27.49,50.74) | 0.27 (0.16,0.44) | <.001 |
Abbreviations: CI, confidence interval; Deyo‐CCI, Deyo Comorbidity Index.
Note: p Values in bold refer to the global null hypothesis of no difference between the subgroups. p Values not in bold refer to the pairwise comparison of each subgroup with the reference subgroup.
TABLE 4.
Multivariate analysis for predictors of in‐hospital mortality
| Predictor | Probability (95% CI) | Odds ratio (95% CI) | p Value |
|---|---|---|---|
| Age group, years | .078 | ||
| 18–44 | 72.05% (64.03,78.86) | 1.00 (reference) | N/A |
| 45–59 | 63.50% (56.80,69.72) | 0.68 (0.46,1.00) | .050 |
| 60–74 | 64.08% (58.03,69.70) | 0.69 (0.47,1.02) | .061 |
| 75 or older | 59.41% (52.04,66.39) | 0.57 (0.37,0.87) | .010 |
| Gender | .325 | ||
| Male | 66.04% (60.54,71.14) | 1.00 (reference) | N/A |
| Female | 63.76% (58.07,69.09) | 0.90 (0.74,1.10) | .325 |
| Race | .002 | ||
| Non‐White | 60.68% (53.94,67.04) | 1.00 (reference) | N/A |
| White | 68.92% (64.33,73.16) | 1.44 (1.15,1.80) | .002 |
| BMI sub‐groups | <.001 | ||
| Below 20 | 72.62% (64.82,79.24) | 0.84 (0.48,1.47) | .546 |
| 20–25 | 75.88% (65.77,83.74) | 1.00 (reference) | N/A |
| 26–30 | 61.64% (53.40,69.26) | 0.51 (0.30,0.88) | .016 |
| 31–35 | 52.20% (44.46,59.84) | 0.35 (0.21,0.58) | <.001 |
| 36–39 | 54.16% (46.19,61.91) | 0.38 (0.22,0.64) | <.001 |
| 40 and above | 69.84% (64.67,74.55) | 0.74 (0.45,1.20) | .218 |
| BMI 2 groups | <.001 | ||
| 25–39 | 56.04% (50.17,61.74) | 1.00 (reference) | N/A |
| Below 25 or 40 and above | 71.48% (66.82,75.73) | 1.97 (1.61,2.40) | <.001 |
| Hospital bed size | <.001 | ||
| Large | 68.86% (63.60,73.68) | 1.00 (reference) | N/A |
| Small/medium | 60.74% (54.90,66.29) | 0.70 (0.57,0.85) | <.001 |
| Deyo‐CCI | .003 | ||
| 0 | 56.02% (47.81,63.92) | 1.00 (reference) | N/A |
| 1 | 70.46% (63.48,76.59) | 1.87 (1.27,2.75) | 0.001 |
| 2 or higher | 67.57% (62.81,71.99) | 1.64 (1.19,2.25) | 0.003 |
| Congestive heart failure | .043 | ||
| No | 64.35% (59.21,69.18) | 1.00 (reference) | N/A |
| Yes | 69.93% (63.04,76.03) | 1.29 (1.01,1.65) | .043 |
| Percutaneous coronary intervention | <.001 | ||
| No | 65.34% (60.28,70.08) | 1.00 (reference) | N/A |
| Yes | 33.50% (20.98,48.88) | 0.27 (0.15,0.49) | <.001 |
Abbreviations: CI, confidence interval; Deyo‐CCI, Deyo Comorbidity Index.
Note: p Values in bold refer to the global null hypothesis of no difference between the subgroups. p Values not in bold refer to the pairwise comparison of each subgroup with the reference subgroup.
4. DISCUSSION
Utilizing data from the NIS, the largest all‐payer inpatient database in the United States, we analyzed a weighted total of 2330 hospitalizations between October 2015 and December 2016, after an out of hospital sudden cardiac death. This nationwide data analysis documented a U‐shaped relationship between the BMI and in‐hospital mortality in OHSCD patients hospitalized in the United States during the study period. BMI between 26 and 39 m/kg2 was found to be independent predictor of lower in‐hospital mortality, in patients hospitalized for a successfully resuscitated OHSCD.
Some of the prior studies, investigating the relationship between BMI and survival after a sudden cardiac death, analyzed specific patient subgroups like post MI sudden cardiac death patients 28 or patients treated with therapeutic hypothermia. 17 , 18 Other studies, analyzing larger populations, investigated long‐term outcomes of patients who survived hospitalization following an out of hospital cardiac arrest, 14 or in‐hospital outcomes in large cohorts of patients who underwent resuscitation for in‐hospital cardiac arrest only. 16 , 19 In a recent meta‐analysis, Ma et al. combined seven prospective and retrospective studies, involving 25 035, including out of hospital and in‐hospital sudden cardiac death patients. 29 The patients were divided into four BMI groups: underweight (BMI < 18.5), normal weight (18.5 ≤ BMI < 25), overweight (25 ≤ BMI < 30) and obese (BMI ≥ 30) and the meta‐analysis showed that underweight patients had the worst survival rates compared to normal weight patients (OR 1.35; 95% CI 1.10–1.66; p = .004), while overweight patients did better (OR −0.8 [0.65–0.98]; p = .03) and obese had similar survival as normal weight patients. 29
Our goal was to assess the relationship between BMI and in‐hospital course and outcomes in a nationwide, general population of patients, hospitalized following a successfully resuscitated out of hospital cardiac death in the US, overcoming the potential “selection bias” in prior studies. We also aimed to better differentiate the different subgroups of patients suffering from obesity, dividing the obese patients into mild (30 < BMI≤35), moderate (35 < BMI < 40) and extremely obese (BMI ≥ 40) groups.
The clinical characteristics of the patient population in this study were consistent with prior publications on SCD in regard to the median age and different comorbidities such as diabetes mellitus (DM), hypertension (HTN) and history of a myocardial infarction (MI). 14 , 16 , 28 , 30 The in‐hospital survival to discharge rates, reported in prior studies, ranged between 20.4% and 44%. 15 , 19 , 31 This study documented 30.7% survival to discharge rate in a nationwide population of patients hospitalized after successfully resuscitated OHSCD, within the range of the previously reported outcomes. The above‐mentioned similarities in the patient population characteristics and outcomes provide external validation to our results as we show the relationship between the patients BMI and study outcomes.
Our data revealed a U‐shaped relationship between the BMI and the in‐hospital mortality, while the over‐weight, obese I and obese II patient subgroups (BMI 26–39) exhibited significantly lower in‐hospital mortality. Several other studies have implied that the “obesity paradox”, described in various cardio‐vascular conditions such as acute myocardial infarction and heart failure, applies to patients admitted after a sudden cardiac death, showing a lower mortality in obese patients. 14 , 16 , 18 , 19 Importantly, majority of these studies combined all the patients with BMI > 30 in the same study group showing improved outcomes. We subdivided these patients in three groups of mild (30 < BMI ≤ 35), moderate (35 < BMI < 40) and extremely obese (BMI ≥ 40) groups, and showed that only in the first two, the mortality decreased, while extremely obese patients' the mortality was higher.
As shown in Table 1, the overweight and obese patients in our study were younger, a finding that could have contributed to their improved survival. This observation was described previously as well, while 20 out of 26 reports included in a 2014 meta‐analysis of the “obesity paradox” in acute myocardial infarction studies by Niedziela et al., documented younger patients in the overweight and obese populations. 32
Not surprisingly, patients in the higher BMI groups suffered from increased prevalence of cardiovascular risk factors such as DM and HTN. Some prior studies reported better outcomes in patients with hypertension, 14 , 18 while diabetes was associated with worse outcomes post OHSCD. 14 In our analysis, in a multivariate regression analysis, neither diabetes nor hypertension, were not found to independently predict increased mortality in this nationwide cohort, when corrected for other risk factors including BMI. It is possible that the adverse effect of these comorbidities on patient's outcomes is counterbalanced by the protective mechanisms that improve the survival in obese patients, many suffering from hypertension and diabetes. Several such protective pathophysiological mechanisms were suggested to play a role in improving survival of critically ill patients, including the benefit of nutritional and caloric reserves in obese patients 8 , 33 , 34 and neurohormonal mechanisms related to higher leptin levels in obese patients. 35 , 36 , 37 We assume that similar mechanisms may play a role in patients who are exposed to an intense continuous metabolic stress associated with an OHSCD event and hospitalization.
This association of obesity with favorable survival after OHSCD should not be interpreted as supporting weight gain. Elevated BMI has been proven over the years as an independent risk factor for various cardio‐vascular conditions such as ischemic heart disease, acute coronary syndrome, congestive heart failure, atrial and ventricular arrhythmia and sudden cardiac death. 7 , 8 Obese patients suffered their cardiac arrest event years earlier than nonobese patients, providing unequivocal evidence to support preventive strategies to reduce the prevalence of obesity.
Our study should be interpreted in the context of several limitations. First, the NIS database is a retrospective administrative database that contains discharge‐level records and as such is susceptible to coding errors. Second, the lack of patient identifiers in the NIS database prevented us from using other outcome variables and mortality measures such as at 30 days. We could only capture events that occurred in the same index hospitalization. The NIS database also does not include detailed information about patients' clinical characteristics, medication, blood tests etc. Therefore, we cannot rule out residual confounding of the association we observed. These limitations are counterbalanced by the real world, nationwide nature of the data, lack of selection bias as well as absence of reporting bias introduced by selective publication of results from specialized centers.
4.1. Conclusion
A U‐shaped relationship between BMI and in‐hospital mortality was documented in patients hospitalized for out of hospital sudden cardiac death in the United States in the recent years. These findings support the existence of an “obesity paradox” in OHSCD, associated with improved in‐hospital survival.
Appendix A.
| ICD‐10 CM codes | Condition | Score |
|---|---|---|
| I21.x, I22.x, I25.2 | Myocardial infarction | 1 |
| I09.9, I11.0, I13.0, I13.2, I25.5, I42.0, I42.5–I42.9, I43.x, I50.x, P29.0 | Congestive heart failure | 1 |
| I70.x, I71.x, I73.1, I73.8, I73.9, I77.1, I79.0, I79.2, K55.1, K55.8, K55.9, Z95.8, Z95.9 | Peripheral vascular disease | 1 |
| G45.x, G46.x, H34.0, I60.x–I69.x | Cerebrovascular disease | 1 |
| F00.x–F03.x, F05.1, G30.x, G31.1 | Dementia | 1 |
| I27.8, I27.9, J40.x–J47.x, J60.x–J67.x, J68.4, J70.1, J70.3 | Chronic pulmonary disease | 1 |
| M05.x, M06.x, M31.5, M32.x–M34.x, M35.1, M35.3, M36.0 | Rheumatologic disease | 1 |
| K25.x–K28.x | Peptic ulcer disease | 1 |
| B18.x, K70.0–K70.3, K70.9, K71.3–K71.5, K71.7, K73.x, K74.x, K76.0, K76.2–K76.4, K76.8, K76.9, Z94.4 | Mild liver disease | 1 |
| E10.0, E10.l, E10.6, E10.8, E10.9, E11.0, E11.1, E11.6, E11.8, E11.9, E12.0, E12.1, E12.6, E12.8, E12.9, E13.0, E13.1, E13.6, E13.8, E13.9, E14.0, E14.1, E14.6, E14.8, E14.9 | Diabetes | 1 |
| E10.2–E10.5, E10.7, E11.2–E11.5, E11.7, E12.2–E12.5, E12.7, E13.2–E13.5, E13.7, E14.2–E14.5, E14.7 | Diabetes with chronic complications | 2 |
| G04.1, G11.4, G80.1, G80.2, G81.x, G82.x, G83.0–G83.4, G83.9 | Hemiplegia or paraplegia | 2 |
| I12.0, I13.1, N03.2–N03.7, N05.2–N05.7, N18.x, N19.x, N25.0, Z49.0–Z49.2, Z94.0, Z99.2 | Renal disease | 2 |
| C00.x–C26.x, C30.x–C34.x, C37.x–C41.x, C43.x, C45.x–C58.x, C60.x–C76.x, C81.x–C85.x, C88.x, C90.x–C97.x | Any malignancy including leukemia and lymphoma | 2 |
| I85.0, I85.9, I86.4, I98.2, K70.4, K71.1, K72.1, K72.9, K76.5, K76.6, K76.7 | Moderate or severe liver disease | 3 |
| C77.x–C80.x | Metastatic solid tumor | 6 |
| B20.x–B22.x, B24.x | Acquired immunodeficiency syndrome (AIDS) | 6 |
Rozen G, Elbaz‐Greener G, Marai I, et al. The relationship between the body mass index and in‐hospital mortality in patients admitted for sudden cardiac death in the United States. Clin Cardiol. 2021;44:1673-1682. doi: 10.1002/clc.23730
Guy Rozen and Gabby Elbaz‐Greener contributed equally to this study and manuscript preparation.
DATA AVAILABILITY STATEMENT
Data availability statement The data from the national database used for this study will not be made available to other researchers for purposes of reproducing the results or replicating the procedure due to restrictions on the sharing of data in the Healthcare Cost and Utilization Project (HCUP) Data Use Agreement. The National Inpatient Sample (NIS) database is publicly available for purchase and the transparent and detailed methods that are described below make it possible for anyone who wishes to do so to reproduce our results.
REFERENCES
- 1. Chadwick JMWN. Medical Works of Hippocrates. Vol 154. Blackwell Scientific Publications; 1950. [Google Scholar]
- 2. Bhattacharya J, Bundorf MK. The incidence of the healthcare costs of obesity. J Health Econ. 2009;28(3):649‐658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Gortmaker SL, Must A, Perrin JM, Sobol AM, Dietz WH. Social and economic consequences of overweight in adolescence and young adulthood. N Engl J Med. 1993;329(14):1008‐1012. [DOI] [PubMed] [Google Scholar]
- 4. Flegal KM, Kruszon‐Moran D, Carroll MD, Fryar CD, Ogden CL. Trends in obesity among adults in the United States, 2005 to 2014. JAMA. 2016;315(21):2284‐2291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Flegal KM, Carroll MD, Ogden CL, Curtin LR. Prevalence and trends in obesity among US adults, 1999‐2008. JAMA. 2010;303(3):235‐241. [DOI] [PubMed] [Google Scholar]
- 6. Flegal KM, Carroll MD, Kuczmarski RJ, Johnson CL. Overweight and obesity in the United States: prevalence and trends, 1960‐1994. Int J Obes Relat Metab Disord. 1998;22(1):39‐47. [DOI] [PubMed] [Google Scholar]
- 7. Muller MJ, Braun W, Enderle J, Bosy‐Westphal A. Beyond BMI: conceptual issues related to overweight and obese patients. Obes Facts. 2016;9(3):193‐205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Lavie CJ, Milani RV, Ventura HO. Obesity and cardiovascular disease: risk factor, paradox, and impact of weight loss. J Am Coll Cardiol. 2009;53(21):1925‐1932. [DOI] [PubMed] [Google Scholar]
- 9. Deo R, Albert CM. Epidemiology and genetics of sudden cardiac death. Circulation. 2012;125(4):620‐637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Zipes DP, Wellens HJ. Sudden cardiac death. Circulation. 1998;98(21):2334‐2351. [DOI] [PubMed] [Google Scholar]
- 11. Harhash AA, May TL, Hsu CH, et al. Risk stratification among survivors of cardiac arrest considered for coronary angiography. J Am Coll Cardiol. 2021;77(4):360‐371. [DOI] [PubMed] [Google Scholar]
- 12. Khan SS, Ning H, Wilkins JT, et al. Association of body mass index with lifetime risk of cardiovascular disease and compression of morbidity. JAMA Cardiol. 2018;3(4):280‐287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Thorgeirsson G, Thorgeirsson G, Sigvaldason H, Witteman J. Risk factors for out‐of‐hospital cardiac arrest: the Reykjavik study. Eur Heart J. 2005;26(15):1499‐1505. [DOI] [PubMed] [Google Scholar]
- 14. Matinrazm S, Ladejobi A, Pasupula DK, et al. Effect of body mass index on survival after sudden cardiac arrest. Clin Cardiol. 2018;41(1):46‐50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Gil E, Na SJ, Ryu JA, et al. Association of body mass index with clinical outcomes for in‐hospital cardiac arrest adult patients following extracorporeal cardiopulmonary resuscitation. PLoS One. 2017;12(4):e0176143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Gupta T, Kolte D, Mohananey D, et al. Relation of obesity to survival after in‐hospital cardiac arrest. Am J Cardiol. 2016;118(5):662‐667. [DOI] [PubMed] [Google Scholar]
- 17. Geri G, Savary G, Legriel S, et al. Influence of body mass index on the prognosis of patients successfully resuscitated from out‐of‐hospital cardiac arrest treated by therapeutic hypothermia. Resuscitation. 2016;109:49‐55. [DOI] [PubMed] [Google Scholar]
- 18. Breathett K, Mehta N, Yildiz V, Abel E, Husa R. The impact of body mass index on patient survival after therapeutic hypothermia after resuscitation. Am J Emerg Med. 2016;34(4):722‐725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Jain R, Nallamothu BK, Chan PS. American Heart Association National Registry of cardiopulmonary resuscitation i. body mass index and survival after in‐hospital cardiac arrest. Circ Cardiovasc Qual Outcomes. 2010;3(5):490‐497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Badheka AO, Rathod A, Kizilbash MA, et al. Influence of obesity on outcomes in atrial fibrillation: yet another obesity paradox. Am J Med. 2010;123(7):646‐651. [DOI] [PubMed] [Google Scholar]
- 21. HCUP National Inpatient Sample (NIS). Healthcare Cost and Utilization Project (HCUP) . 2012. –2013. Agency for Healthcare Research and Quality R, MD. www.hcup-us.ahrq.gov/nisoverview.jsp. [PubMed]
- 22. Healthcare Cost and Utilization Project (HCUP) . 2000. –2011. Agency for Healthcare Research and Quality R, MD. www.hcup-us.ahrq.gov/nisoverview.jsp. [PubMed]
- 23. Steiner C, Elixhauser A, Schnaier J. The healthcare cost and utilization project: an overview. Eff Clin Pract. 2002;5(3):143‐151. [PubMed] [Google Scholar]
- 24. Deyo RA, Cherkin DC, Ciol MA. Adapting a clinical comorbidity index for use with ICD‐9‐CM administrative databases. J Clin Epidemiol. 1992;45(6):613‐619. [DOI] [PubMed] [Google Scholar]
- 25. Chu YT, Ng YY, Wu SC. Comparison of different comorbidity measures for use with administrative data in predicting short‐ and long‐term mortality. BMC Health Serv Res. 2010;10:140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Radovanovic D, Seifert B, Urban P, et al. Validity of Charlson comorbidity index in patients hospitalised with acute coronary syndrome. Insights from the nationwide AMIS plus registry 2002‐2012. Heart. 2014;100(4):288‐294. [DOI] [PubMed] [Google Scholar]
- 27. Hosseini SM, Moazzami K, Rozen G, et al. Utilization and in‐hospital complications of cardiac resynchronization therapy: trends in the United States from 2003 to 2013. Eur Heart J. 2017;38(27):2122‐2128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Shiga T, Kohro T, Yamasaki H, et al. Body mass index and sudden cardiac death in Japanese patients after acute myocardial infarction: data from the JCAD study and HIJAMI‐II registry. J Am Heart Assoc. 2018;7(14):e008633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Ma Y, Huang L, Zhang L, Yu H, Liu B. Association between body mass index and clinical outcomes of patients after cardiac arrest and resuscitation: a meta‐analysis. Am J Emerg Med. 2018;36(7):1270‐1279. [DOI] [PubMed] [Google Scholar]
- 30. Khan AJ, Jan Liao C, Kabir C, et al. Etiology and determinants of in‐hospital survival in patients resuscitated after out‐of‐hospital cardiac arrest in an Urban medical center. Am J Cardiol. 2020;130:78‐84. [DOI] [PubMed] [Google Scholar]
- 31. Leary M, Cinousis MJ, Mikkelsen ME, Gaieski DF, Abella BS, Fuchs BD. The association of body mass index with time to target temperature and outcomes following post‐arrest targeted temperature management. Resuscitation. 2014;85(2):244‐247. [DOI] [PubMed] [Google Scholar]
- 32. Niedziela J, Hudzik B, Niedziela N, et al. The obesity paradox in acute coronary syndrome: a meta‐analysis. Eur J Epidemiol. 2014;29(11):801‐812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Kalantar‐Zadeh K, Horwich TB, Oreopoulos A, et al. Risk factor paradox in wasting diseases. Curr Opin Clin Nutr Metab Care. 2007;10(4):433‐442. [DOI] [PubMed] [Google Scholar]
- 34. Oliveros H, Villamor E. Obesity and mortality in critically ill adults: a systematic review and meta‐analysis. Obesity (Silver Spring). 2008;16(3):515‐521. [DOI] [PubMed] [Google Scholar]
- 35. Iikuni N, Lam QL, Lu L, Matarese G, La Cava A. Leptin and inflammation. Curr Immunol Rev. 2008;4(2):70‐79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. DeLany J. Leptin hormone and other biochemical influences on systemic inflammation. J Bodyw Mov Ther. 2008;12(2):121‐132. [DOI] [PubMed] [Google Scholar]
- 37. Bornstein SR, Licinio J, Tauchnitz R, et al. Plasma leptin levels are increased in survivors of acute sepsis: associated loss of diurnal rhythm, in cortisol and leptin secretion. J Clin Endocrinol Metab. 1998;83(1):280‐283. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data availability statement The data from the national database used for this study will not be made available to other researchers for purposes of reproducing the results or replicating the procedure due to restrictions on the sharing of data in the Healthcare Cost and Utilization Project (HCUP) Data Use Agreement. The National Inpatient Sample (NIS) database is publicly available for purchase and the transparent and detailed methods that are described below make it possible for anyone who wishes to do so to reproduce our results.


