Abstract
Rationale
Reducing nicotine content of inhaled tobacco products may prevent nicotine addiction, but the threshold for nicotine reinforcement has not been systematically evaluated in controlled human laboratory studies.
Objectives
The current study uses a novel double-blind placebo-controlled intravenous (IV) nicotine self-administration (NSA) model to determine threshold for subjective effects of nicotine and nicotine reinforcement using a forced choice self-administration procedure.
Methods
Young adults (n = 34) had 5 laboratory sessions after overnight nicotine abstinence. In each session, participants sampled and rated the subjective effects of an IV dose of nicotine (0.0125, 0.025, 0.05, 0.1, or 0.2 mg nicotine/70 kg bodyweight) versus saline (placebo), then were given a total of 10 opportunities to self-administer either the IV dose of nicotine or placebo.
Results
Mixed effect models revealed a significant effect of nicotine dose for positive (i.e., “stimulatory” and “pleasurable”; p < .0001) effects, but not “aversive” effects during sampling period. Post hoc comparisons showed that higher doses (i.e., 0.1 and 0.2 mg) were associated with greater stimulatory, pleasurable, and physiological effects than placebo and lower doses. Mixed effect models revealed that only the highest dose (i.e., 0.2 mg) was consistently preferred over placebo. Sex differences were generally weak (p = .03–.05).
Conclusions
Using our IV nicotine NSA model, the threshold for detecting positive effects of nicotine in young adult smokers is about 0.1 mg, but a higher dose of nicotine, 0.2 mg, is required to produce a consistent nicotine reinforcement. Regarding the regulatory impact, our findings further support the value of nicotine reinforcement threshold as a tobacco regulatory target.
Keywords: Nicotine, Sex, Self-administration, Abuse liability, Nicotine reduction
Introduction
Nicotine is considered to be the main reinforcing ingredient in tobacco, primarily responsible for imitation and maintenance of tobacco addiction. Therefore, the nicotine content of tobacco products may serve as a logical target in the development of effective tobacco-control policies. To reduce the public health burden of tobacco smoking, the Food and Drug Administration (FDA) is considering reducing the nicotine content of tobacco products to “non-addictive” levels. This approach, initially proposed by Benowitz and Henningfield, hypothesizes that the gradual reduction of the nicotine content of cigarettes to an amount below the threshold addictive dose could prevent the development of addiction among new smokers (Benowitz and Henningfield 1994). Since the initial proposal, several clinical trials have supported the feasibility and potential efficacy of this approach in reducing nicotine intake and level of addiction. Most notably, across 6 weeks of smoking in a natural environment, Donny et al. reported a reduction in cigarette smoking among participants who were assigned to 2.4, 1.3, or 0.4 mg nicotine/g of tobacco than those who were assigned to 15.8 and 5.2 mg/g (Donny et al. 2015). In another 22-week study, immediate reduction of nicotine in cigarettes to 0.4 mg nicotine/gram was more effective than gradual reduction (Hatsukami et al. 2018). Long-term effects of such approaches beyond the duration of these clinical trials remain to be determined in future studies.
Parallel to clinical trials testing the impact of reducing nicotine in tobacco products, laboratory studies seeking to determine the threshold for nicotine reinforcement have been conducted (Sofuoglu and LeSage 2012). In a series of studies, Perkins and colleagues tested nicotine discrimination in smokers using Spectrum cigarettes with different levels of nicotine (Perkins et al. 2017a, b). They found that Spectrum cigarettes that could be discriminated by smokers induced “liking” and were preferred over ultra-low-nicotine content cigarettes (0.4 mg/g of tobacco). However, the ability to accurately deliver doses of nicotine by smoked tobacco products is challenging and the effects of nicotine are confounded by the many other chemicals in these products. For example, other conditioned reinforcers in smoked tobacco, such as the sight, taste, and smell of cigarettes, are difficult to separate from the primary reinforcing effects of nicotine. To address these limitations, we have developed a novel intravenous (IV) nicotine self-administration (NSA) model for humans (Sofuoglu et al. 2008). IV nicotine closely mimics both the pharmacokinetics and behavioral effects of smoked nicotine, including reinforcement. The IV route also has the unique advantage over other nicotine delivery systems by producing precise and reproducible dosing of nicotine, features that are essential for assessment of threshold reinforcing doses of nicotine (Jensen et al. 2016a, b).
In a previous study, we examined IV NSA at low doses of nicotine (0.1, 0.2, 0.3, or 0.4 mg), delivered over 30 s in dependent smokers (Jensen et al. 2016a, b). These nicotine doses correspond to nicotine delivered by about 1 to 3 puffs of a cigarette (Mehmet Sofuoglu et al. 2008). NSA was negatively correlated with nicotine dose in males who displayed choice preference for lower doses (0.1 and 0.2 mg doses) of nicotine over the highest tested dose (0.4 mg). However, no significant relationship between dose and choice preference was evident in females. The 0.1 and 0.2 mg nicotine doses also produced pleasurable subjective effects (e.g., like, good drug effects), suggesting that nicotine reinforcement threshold is about 0.1 mg (Jensen et al. 2016a, b). Additionally, compared to females, males report greater positive effects of IV nicotine (0.5 mg and 1.0 mg) in dependent smokers (DeVito et al. 2014). To our knowledge, the dose-effect curve of nicotine in doses less than 0.1 mg has not been examined in humans.
The main goal of this project was to estimate threshold reinforcing doses of nicotine in non-dependent young adult male and female smokers using an IV NSA procedure. To bracket the threshold reinforcing doses of nicotine, saline and five nicotine doses ranging from 0.0125 to 0.2 mg/70 kg were included. The highest dose, 0.2 mg/70 kg, corresponds to nicotine delivered by about one or two puffs of a cigarette, produces self-reported positive effects, and is self-administered by smokers when given by IV route (Djordjevic et al. 2000; Jensen et al. 2016a, b). The lowest dose represents the amount of nicotine delivered by a single puff of a cigarette with nicotine yield below the addiction threshold, as proposed by Benowitz and Henningfield (1994). We hypothesized that the threshold reinforcing doses for IV NSA would be between 0.0125 and 0.1 mg/70 kg. We further hypothesized that the dose-effect curve for NSA will differ between males and females with relatively flat curve in female smokers.
Method
Subjects
The participants were young adult smokers (ages 18 to 30) who were recruited from the New Haven, CT area. Participants had to be (1) a smoker for at least a year and report a life-time consumption of at least 100 cigarettes; (2) smoke more frequently than once a week and ≤ 5 cigarettes per day (cpd); (3) Fagerstrom Test of Nicotine Dependence (FTND) score < 3 indicating no or minimal evidence for nicotine dependence; (4) not seeking treatment for nicotine dependence at the time of the study. All participants had normal physical, laboratory and psychiatric examinations and participants had no current drug abuse or dependence for any substances other than nicotine, as established by the structured clinical interview (SCID) for DSM-5 (American Psychiatric Association 2013). A urine drug screen and breathalyzer were done before each session to assess for recent drug and alcohol use, respectively. Participants were excluded if the urine drug screen was positive for illicit substances (excluding cannabis). All participants provided informed consent prior to study participation. The study was approved by the Yale University and the VA Connecticut Healthcare System Institutional Review Boards (IRB). The study sessions were conducted in the Biostudies Unit, which is located at the VA Connecticut Healthcare System (West Haven, CT), and participants were paid up to $780 for study participation.
Laboratory study procedures
This outpatient, double-blind, crossover study included 5 experimental sessions. Participants were asked not to smoke after 10 PM before sessions and compliance was verified by breath carbon monoxide levels (≤ 8 parts-per-million [ppm]; BreathCO, Vitalograph, Inc., Lenexa, KS). Nicotine and cotinine levels were obtained before each session to quantify the level of nicotine intake. An indwelling intravenous catheter was inserted in the participant’s antecubital vein for nicotine infusion, baseline blood draw, and as a safety precaution. Cardiac rhythm was monitored during infusion, and 12-lead ECGs were obtained before and at the end of the session. The sessions started at approximately 08:00 AM and were scheduled 2 to 7 days apart to minimize any carryover effects from nicotine. Each experimental session consisted of one randomly assigned nicotine dose (0.0125, 0.025, 0.05, 0.1, and 0.2 mg/70 kg) as well as sterile saline serving as placebo. At the start of each session, subjects first sampled their assigned nicotine dose (either 0.0125, 0.025, 0.05, 0.1, and 0.2 mg/70 kg) and placebo (saline) in random order. The nicotine dose and saline were randomly labeled as “A” and “B” by a research pharmacist at VA Connecticut to maintain the study blind. Sample dose “B” was administered 15 min after sample dose “A”. Fifteen minutes after sample dose “B”, subjects were given opportunities to choose whether they wished to receive either an infusion of “A” or an infusion of “B”. There were 10 “A” versus “B” forced choice trials, each separated by 15 min. Immediately following the subject’s selection, “A” or “B” was administered over 30 s using an infusion pump activated by research staff.
Nicotine and placebo preparation
An investigational new drug (IND) application was approved by the FDA for IV nicotine. To prepare vials of nicotine stock solution, nicotine bitartrate dihydrate powder was dissolved in 0.9% sodium chloride to a concentration of 1 mg/ml and then passed through 0.22-micron filters. The amount of nicotine bitartrate dihydrate powder was adjusted by molecular weight to reflect nicotine-free base. Each batch of nicotine solution was tested for concentration, pyrogenicity, and sterility, all of which yielded satisfactory results for all batches. For each session, two 60-ml syringes, which were marked as either “A” or “B”, were prepared in a randomized, double-blinded fashion with identical-looking IV labels and equal volumes (46 ml). The syringe that contained the nicotine had enough volume to account for the line flush and 11 experimental infusions, i.e., 1 for the sample dose and the 10 optional doses for the choice trials. The placebo syringe contained 46 ml of 0.9% sodium chloride.
Outcome measures
The main outcomes were reinforcement (i.e., nicotine choice), subjective drug effects, and physiological responses (i.e., blood pressure/heart rate). Reinforcement was measured by the percentage of nicotine administrations per participant choice (out of 10 trials) during the session. Subjective drug effects were measured by the Drug Effects Questionnaire (DEQ), which comprised nine items that were grouped into three domains based on prior work showing high correlations between DEQ responses (Jensen et al. 2015a, b; Morean et al. 2013). These included (1) “stimulatory” effects comprising the average of “feel stimulated”, “feel drug effects”, and “feel high”; (2) “pleasurable” effects comprising the average of “like”, “feel good ”, and “want more”; and (3) “aversive ” effects comprising the average of “feel anxious”, “feel down”, and “feel bad”. Each response was rated on a 100 mm scale, from 0 “not at all” to 100 “extremely.” The DEQ was given before each sample dose infusion (0 min) and then 1, 3, and 5 min post-infusion. Systolic and diastolic blood pressure and heart rate were obtained just before (0 min) and at 1, 2, 3, and 5 min after each infusion.
Data analysis
For nicotine reinforcement, percent of times the active dose was chosen during forced choice (out of 10 possibilities) within test day was analyzed using mixed effects models with sex, dose, the interaction of sex and dose, and day as fixed predictors and structured variance-covariance matrix for the repeated measures within individual across days. The best-fitting structure was selected based on BIC. Least square mean comparisons were performed to explain significant effects in the model. The least square mean for each dose condition was also compared with a confidence interval to the expected percent corresponding to no preference (i.e., 50%).
For the subjective and physiological outcomes, peak subjective drug effects (i.e., stimulatory, pleasurable, and aversive) and peak systolic/diastolic blood pressure and heart rate after each infusion (i.e., A and B, each choice trial) were extracted as study outcomes. Since the subjective drug effect outcomes were skewed, log transformations were used to bring the variables more in line with the normal distribution. These outcomes were analyzed using separate mixed effects models with sex, dose during priming period (nicotine dose or placebo), the interaction between sex and dose, dose order (A or B), and day (1 through 5) as predictors, a random subject effect and structured variance-covariance matrix within day. The best-fitting structure for each outcome was selected based on the Schwartz-Bayesian criterion (BIC). Least square means and standard errors were calculated to describe the patterns of means for each outcome and pairwise comparisons among the different doses and placebo were used to evaluate dose effects. Serum nicotine and cotinine were analyzed and used in accordance with an established laboratory protocol developed in prior studies from our lab (Sofuoglu et al. 2012).
Results
Participant characteristics
A total of 34 participants completed at least one laboratory test session. The majority of participants completed all 5 experimental sessions (n = 30); the remaining participants completed 4 (n = 1), 3 (n = 1), and 2 (n = 2) experimental sessions. Demographic variables and cigarette smoking history of the 34 study participants are shown in Table 1. All participants met the < 8 ppm CO requirement before each session, which was later confirmed by low average serum nicotine levels, averaging 1.6 ng/mL (SD = 2.4), indicating compliance with overnight smoking abstinence. All but one participant reported an exclusive preference for menthol cigarettes. The analysis included all available data on 23 male and 11 female participants who completed all or some of the sessions. There were no sex differences in demographics or smoking history (all p’s > 0.1).
Table 1.
Descriptive statistics
| M (SD) | n (%) | |
|---|---|---|
| Demographics | ||
| Age (years) | 26.74 (3.1) | |
| Sex | ||
| Male | 23 (67.6) | |
| Female | 11 (32.4) | |
| Race/ethnicity | ||
| African American | 26 (76.5) | |
| Caucasian | 4 (11.8) | |
| Other | 4 (11.8) | |
| Hispanic ethnicity | 4 (11.8) | |
| Smoking severity and tobacco product use | ||
| FTND | 1.62 (1.3) | |
| Average cigarettes per day | 4.47 (1.1) | |
| Years of smoking | 8.74 (4.4) | |
| Serum cotinine level (ng/mL)a | 137.4 (104.0) |
FTND Fagerstrom Test of Nicotine Dependence
Cotinine extracted from blood serum samples taken immediately before each experimental session
Primary outcomes
Subjective drug effects
Nicotine produced an expected increase in stimulatory effects at 1 min post-infusion in a dose-dependent manner (Fig. 1a). There was a statistically significant effect of nicotine dose on stimulatory effects [F(5,231) = 13.46, p < .0001]. The highest dose of nicotine (0.2 mg) produced greater peak stimulatory effects than placebo and the lower doses of nicotine (0.0125, 0.025, and 0.05 mg), but was not different than 0.1 mg (Fig. 2a). The 0.1-mg dose of nicotine was significantly different than placebo and the 0.0125 and 0.025 mg doses. There was also a trend-level interaction between sex and nicotine dose [F(5,231) = 2.20, p = 0.05]. Females, compared to males, reported greater peak stimulatory effects for the highest dose of nicotine (0.2 mg) compared to lower nicotine doses (0.0125, 0.025, and 0.05 mg) (Fig. 2a). Ratings of pleasurable effects also demonstrated a dose-dependent increase 1 min post-infusion (Fig. 1b). There was a statistically significant effect of nicotine dose on peak pleasurable effects [F(5,231) = 8.94, p < .0001]. The highest dose of nicotine (0.2 mg) produced significantly higher peak ratings than placebo and all other doses (0.0125, 0.025, 0.05, and 0.1 mg). The 0.1-mg dose produced significantly higher pleasurable effects than placebo and the 0.0125- and 0.025-mg doses (Fig. 2b). There was also a significant interaction between sex and nicotine dose [F(5,230) = 2.42, p = 0.04]. Similar to stimulatory effects, females, compared to males, reported greater pleasurable effects at the 0.2-mg dose compared to the 0.0125-, 0.025-, 0.05-, and 0.1-mg doses (Fig. 2b). For the aversive effects, there were no significant main effects of nicotine dose (Fig. 1c), sex, or sex by nicotine dose interactions (Fig. 2c).
Fig. 1.
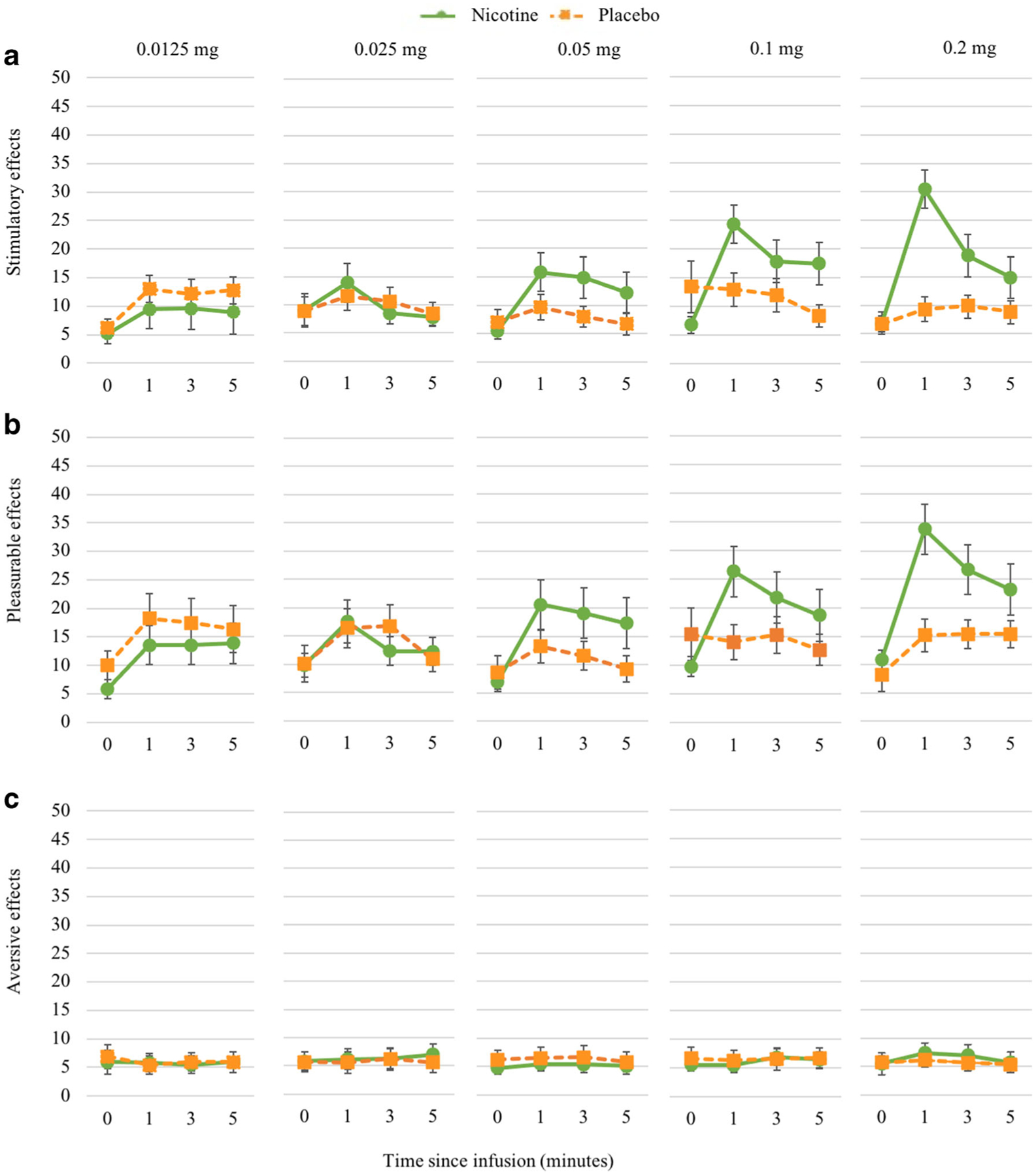
Nicotine dose response for stimulatory effects during sampling period. Subjective responses assessed with the Drug Effects Questionnaire (DEQ). Responses to stimulatory effect (panel a) of nicotine demonstrate an expected rise 1 min post-infusion at higher doses (0.1 and 0.2 mg). Dose curve was similar for pleasurable effects (panel b). Time course of aversive effects did not vary by dose (panel c)
Fig. 2.
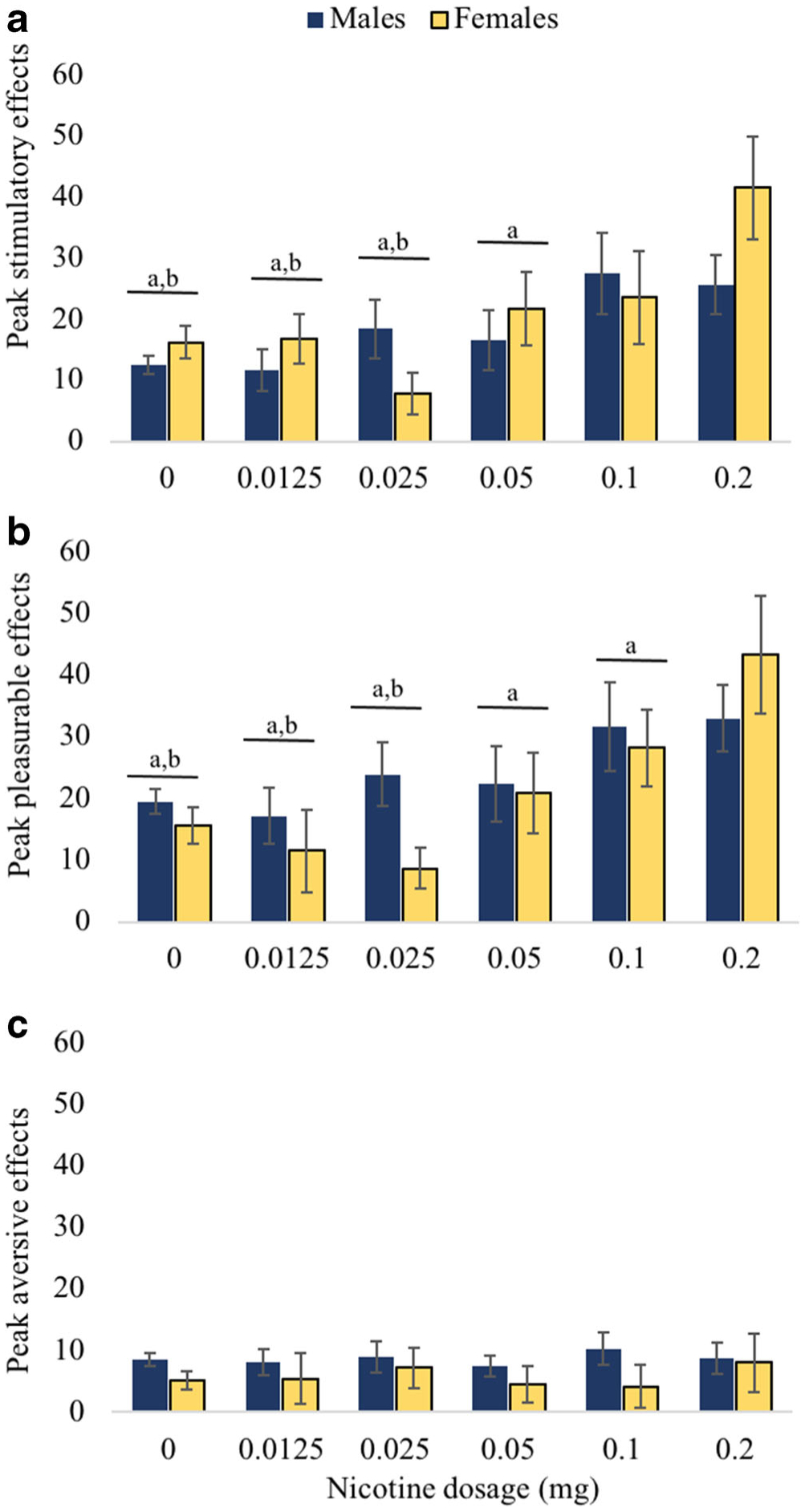
Differences in peak subjective drug effects and sex differences. Peak subjective drug effects during higher doses (i.e., 0.1 and 0.2 mg) were significantly greater than lower doses for stimulatory (panel a) and pleasurable effects (panel b). Letter “a” above doses (combined reports from male and female) signifies significant difference with 0.2 mg (minimum significance p < .05). Letter “b” above doses (combined reports from male and female) signifies significant difference with 0.1 mg (minimum significance p < .05). Females (vs. males) endorsed significantly higher stimulatory and pleasurable effects at 0.2 mg compared to lower doses. There were no significant comparisons for aversive effects (panel c)
Physiological effects
There was a significant main effect of sex on systolic blood pressure [F(1,31.6) = 5.31, p = 0.03]. Males had higher systolic blood pressure than females. No other effects were statistically significant for systolic blood pressure. There was a significant effect of nicotine dose on diastolic blood pressure [F(5,213) = 3.00, p = 0.01]. Specifically, diastolic blood pressure was significantly higher at the 0.2-mg nicotine dose than at placebo and the 0.05-mg dose. Finally, there was a significant main effect of dose on heart rate [F(5,208) = 7.39, p < 0.0001]. As expected, heart rate was significantly higher at the 0.1- and 0.2-mg nicotine doses compared to placebo and the 0.0125, 0.025, 0.05 mg nicotine. There were no significant sex differences in diastolic blood pressure or heart rate.
Nicotine reinforcement
We observed a significant main effect of nicotine dose on the choice behavior [F(4,116) = 2.66, p = 0.04] (Fig. 3). Of the five nicotine doses, only the highest dose (0.2 mg) was consistently preferred to placebo with the percent chosen over placebo equal to 65% (95% CI: 55%, 75%). The three middle doses (0.1, 0.05, and 0.025 mg) were chosen over placebo on average more than half of the time (55, 58, and 51%, respectively) but the differences were not statistically significantly different from no preference for nicotine vs. placebo (i.e., 50%). The pairwise comparisons of the nicotine doses showed that percentage of times participants chose 0.2 mg was significantly greater than 0.0125 mg, and similarly, participants chose 0.05 mg significantly greater than 0.0125 mg. The main effect of sex as well as the interaction of sex and dose were not significant.
Fig. 3.
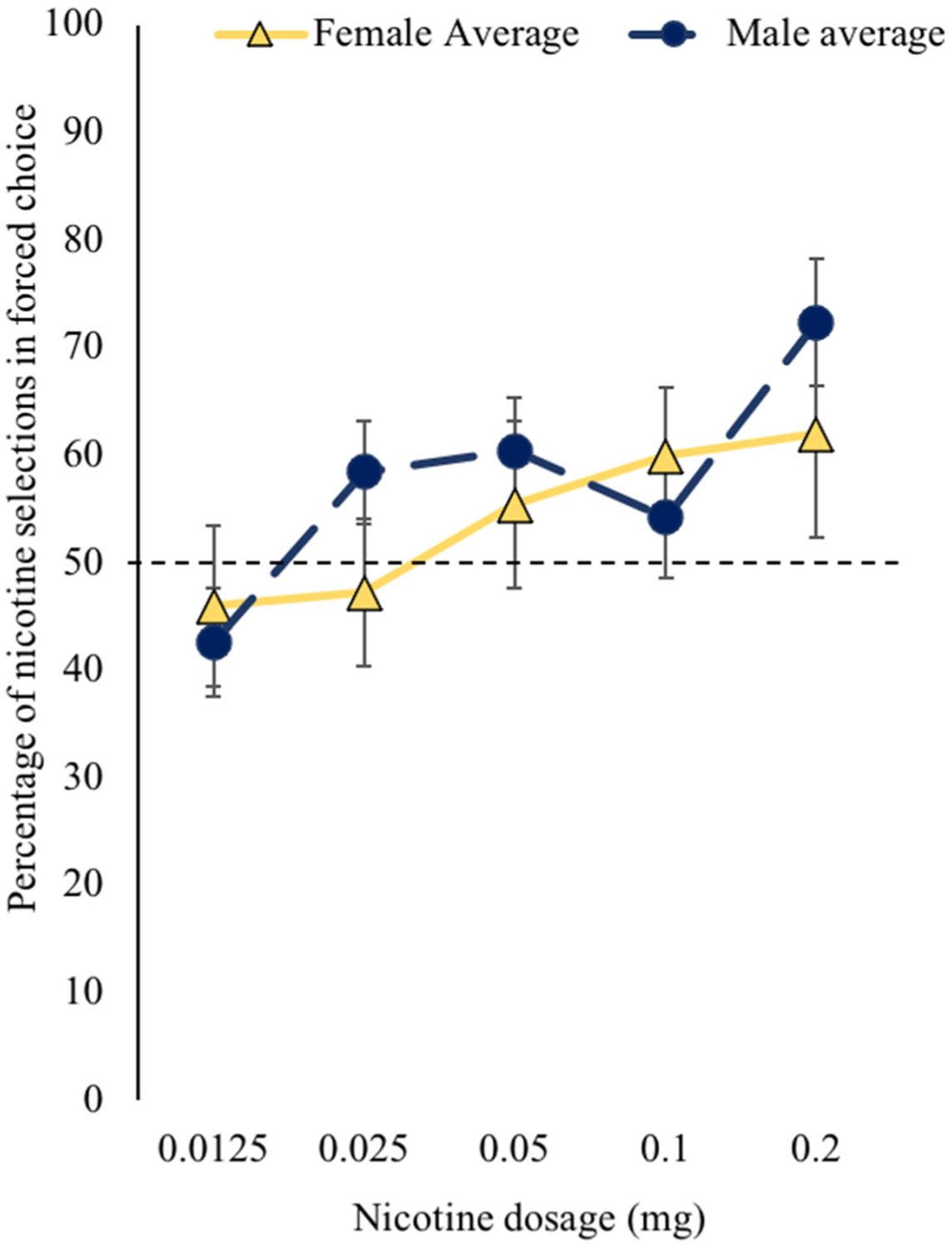
Nicotine forced choice self-administration separated by sex. In the forced choice procedure, choice of nicotine over placebo self-administration was greater than chance at the 0.2-mg dose. Dotted line represents chance (i.e., 50%). There were no sex differences with respect to nicotine self-administration
Discussion
This study examined the threshold nicotine dose for its reinforcing, subjective, and physiological effects in non-dependent smokers. Precise doses of nicotine were delivered using an IV NSA procedure with saline as a control. The selected doses were estimated to be below and above the reinforcing dose(s) of nicotine. The highest doses (0.1 and 0.2 mg) were approximately the amount of nicotine that would be delivered by smoking a puff of a cigarette. We found that the threshold doses of nicotine for the subjective and heart rate responses to nicotine were 0.1 mg/70 kg. On the other hand, the threshold for nicotine reinforcement, assessed with greater number of nicotine choices over saline, was 0.2 mg/70 kg. Women reported greater stimulatory and pleasurable subjective responses than men to 0.2 mg/70 kg of nicotine. Nicotine reinforcement threshold, on the other hand, did not differ between male and female smokers. To our knowledge, this is the first study that demonstrates nicotine reinforcement threshold by using precise IV nicotine dosing in non-dependent smokers (Table 2).
Table 2.
Threshold nicotine dose for the primary outcomes
| Outcome | Threshold dose |
|---|---|
| Subjective drug effects | |
| Stimulatory | 0.1 mg |
| Pleasurable | 0.1 mg |
| Aversive | None identified |
| Physiological effects | |
| Heart rate | 0.1 mg |
| Diastolic blood pressure | 0.2 mg |
| Systolic blood pressure | None identified |
| Nicotine reinforcement | |
| Forced choice NSA | 0.2 mg |
NSA nicotine self-administration
Note: For subjective drug effects and physiological effects, threshold refers to significantly different from placebo and lower doses. For reinforcement, threshold refers to significantly different from no preference (i.e., 50%)
Reducing the nicotine content of cigarettes is one strategy to reduce the addictive potential of cigarette smoking (Benowitz and Henningfield 2013). It is essential to first identify an average minimum threshold necessary to detect the positive subjective effects of nicotine. Specifically, it is important that detecting the threshold for reinforcement is based on the amount of nicotine per unit dose, or a puff from a cigarette (Sofuoglu and LeSage 2012). We found that the dose to detect the subjective effects of nicotine was lower than the dose associated with nicotine reinforcement. Of note, this difference could be due to greater sensitivity of continuous outcome (0 to 100) that was used to assess the subjective drug effects (0 to 100 scale) than the categorical outcome used for reinforcement (nicotine vs. placebo) (Cohen 1992). Overall, these findings support the idea that positive subjective drug effects are needed for reinforcement. For nicotine doses that were subthreshold for subjective effects, choice for nicotine vs. placebo did not differ indicating no evidence of reinforcement. These doses were below 0.1 mg, which is a level of nicotine that is considered equivalent to a puff from a cigarette. These findings are consistent with the nicotine discrimination studies conducted by Perkins and colleagues using spectrum cigarettes (Perkins et al. 2017a, b). They found that the median discrimination threshold was 11 mg nicotine/gram tobacco and cigarettes with nicotine content below this threshold did not produce “liking” and was not chosen over ultra-low-nicotine content cigarettes (0.4 mg/g of tobacco). Together, these findings support overall consistency between the threshold dose for the subjective drug effects and reinforcement.
Sex differences in the subjective responses to nicotine showed that among young adult non-dependent smokers, females demonstrated a marginally significant greater sensitivity than males for stimulation and pleasurable effects at the 0.2-mg dose. In our previous study with adult dependent smokers, we observed greater stimulatory effects from 0.3 to 0.4 mg/70 kg nicotine with no significant effects of sex (Jensen et al. 2016a, b). In contrast, there were sex differences in the threshold dose for nicotine reinforcement such that males exhibited a preference for lower doses that was not present in females. These findings are consistent with previous discrimination threshold studies in which no sex differences were observed at 0.4 mg (Perkins 2019; Perkins et al. 2017a, b). Therefore, it is possible that non-dependent females may be more sensitive to the positive effects of nicotine at lower doses (e.g., 0.1 to 0.2 mg). Additionally, it is possible that subjective effects may be necessary but not sufficient to drive reinforcement in some smokers. For example, non-nicotine stimuli may be more important for the reinforcing effects of smoking in women than in men (Perkins 2008). Additional research with comparable numbers of males and females are necessary to determine the relationship between positive effects of nicotine at lower doses and nicotine reinforcement.
In addition to possible sex differences, it is important to note that the majority of our participants were African-Americans and, as expected, menthol cigarette smokers. Both African-American race and menthol smoking have been shown to impact nicotine metabolism and withdrawal severity (Alexander et al. 2016; Benowitz et al. 2004; Valentine et al. 2018). In addition, our participants were non-dependent smokers with narrow age range of 18 to 30. As shown in a previous study, age of the smokers may impact the reinforcement from low nicotine cigarettes (Cassidy et al. 2019). Whether our findings can also be generalized to other racial and age groups warrants future studies.
The FDA is interested in reducing the nicotine content of tobacco cigarettes to minimize the addictive potential. Reducing nicotine content may have the greatest impact in preventing the development of nicotine addiction in adolescents and children and the progression to addiction in non-dependent (i.e., low level) smokers (Benowitz and Henningfield 2013). Large-scale clinical trials as well as carefully conducted human laboratory studies focusing on the individual differences in reinforcement threshold for nicotine are necessary to determine whether reducing nicotine is a feasible and acceptable treatment. Cumulating data from large clinical trials support the feasibility and potential efficacy of reducing tobacco-related harm with reduced nicotine content cigarettes. A recent two-arm, double-blind randomized clinical trial evaluated reduced nicotine content versus usual nicotine content cigarettes in treatment seeking, daily adult smokers. Individuals randomized to the reduced nicotine content group tapered nicotine content every 3 weeks to a 0.2 mg per cigarette (Krebs et al. 2020). At post-treatment, the reduced nicotine content group demonstrated significantly lower cotinine levels and lower CO levels compared to the usual nicotine group. Additionally at 3-month follow-up, 27% of those in the reduced nicotine group quit smoking compared to none in the usual smoking group (Krebs et al. 2020). Our findings suggest that a clear threshold exist for nicotine’s subjective and reinforcing effects. Below 0.1 mg, nicotine neither induces pleasurable drug effects nor greater self-administration compared to saline. The 0.1-mg dose also represents the threshold dose for the blood pressure and heart rate increasing effects of nicotine. Together, these findings support the value of nicotine reinforcement threshold as a tobacco regulatory target.
Acknowledgements
The authors thank Ms. Haleh Nadim for measurements of nicotine and cotinine.
Funding
This work was funded by a US Department of Veteran Affairs Career Development Award, Mental Illness Research Education Clinical, Centers (MIRECC), and NIDA/NIH and FDA Center for Tobacco Products (CTP) R01 DA042528 (MS). The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health or the FDA.
Footnotes
Conflict of interest The authors declare no competing interests.
References
- Alexander LA, Trinidad DR, Sakuma KL, Pokhrel P, Herzog TA, Clanton MS et al. (2016) Why we must continue to investigate menthol’s role in the African American Smoking Paradox. Nicotine Tob Res 18(Suppl 1):S91–S101. 10.1093/ntr/ntv209 [DOI] [PMC free article] [PubMed] [Google Scholar]
- American Psychiatric Association (2013) Diagnostic and statistical manual of mental disorders (DSM-5®): American Psychiatric Pub [Google Scholar]
- Benowitz NL, Henningfield JE (1994) Establishing a nicotine threshold for addiction. The implications for tobacco regulation. N Engl J Med 331(2):123–125 [DOI] [PubMed] [Google Scholar]
- Benowitz NL, Henningfield JE (2013) Reducing the nicotine content to make cigarettes less addictive. Tob Control 22(Suppl 1):i14–i17. 10.1136/tobaccocontrol-2012-050860 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benowitz NL, Herrera B, Jacob P 3rd. (2004) Mentholated cigarette smoking inhibits nicotine metabolism. J Pharmacol Exp Ther 310(3):1208–1215. 10.1124/jpet.104.066902 [DOI] [PubMed] [Google Scholar]
- Cassidy RN, Miller ME, Tidey JW, DiGuiseppi G, Denlinger-Apte R, Colby SM (2019) The impact of nicotine dose on the reinforcing value of cigarettes in adolescents. Tob Regul Sci 5(2):105–114 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen J (1992) A power primer. Psychol Bull 112(1):155–159 [DOI] [PubMed] [Google Scholar]
- DeVito EE, Herman AI, Waters AJ, Valentine GW, Sofuoglu M (2014) Subjective, physiological, and cognitive responses to intravenous nicotine: effects of sex and menstrual cycle phase. Neuropsychopharmacology 39(6):1431–1440. 10.1038/npp.2013.339 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Djordjevic MV, Stellman SD, Zang E (2000) Doses of nicotine and lung carcinogens delivered to cigarette smokers. J Natl Cancer Inst 92(2): 106–111 [DOI] [PubMed] [Google Scholar]
- Donny EC, Denlinger RL, Tidey JW, Koopmeiners JS, Benowitz NL, Vandrey RG, al’Absi M, Carmella SG, Cinciripini PM, Dermody SS, Drobes DJ, Hecht SS, Jensen J, Lane T, le CT, McClernon FJ, Montoya ID, Murphy SE, Robinson JD, Stitzer ML, Strasser AA, Tindle H, Hatsukami DK (2015) Randomized trial of reduced-nicotine standards for cigarettes. N Engl J Med 373(14):1340–1349. 10.1056/NEJMsa1502403 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hatsukami DK, Luo X, Jensen JA, al’Absi M, Allen SS, Carmella SG, … Drobes DJ (2018). Effect of immediate vs gradual reduction in nicotine content of cigarettes on biomarkers of smoke exposure: a randomized clinical trial. Jama, 320(9), 880–891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jensen KP, DeVito EE, Herman AI, Valentine GW, Gelernter J, Sofuoglu M (2015a) A CHRNA5 smoking risk variant decreases the aversive effects of nicotine in humans. Neuropsychopharmacology 40(12): 2813–2821. 10.1038/npp.2015.131 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jensen KP, Herman AI, Morean ME, Kranzler HR, Gelernter J, Sofuoglu M (2015b) FKBP5 variation is associated with the acute and chronic effects of nicotine. Pharm J 15(4):340–346. 10.1038/tpj.2014.76 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jensen KP, DeVito EE, Sofuoglu M (2016a) How intravenous nicotine administration in smokers can inform tobacco regulatory science. Tob Regul Sci 2(4):452–463 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jensen KP, DeVito EE, Valentine G, Gueorguieva R, Sofuoglu M (2016b) Intravenous nicotine self-administration in smokers: dose–response function and sex differences. Neuropsychopharmacology 41(8):2034–2040 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krebs NM, Zhu J, Wasserman E, Kuprewicz R, Martinez DJ, Veldheer S, Livelsberger C, Modesto J, Reinhart L, Trushin N, Reilly SM, Liao J, Fazzi A, Bascom R, Richie JP Jr, Foulds J, Horn K Muscat JE (2020). Switching to progressively reduced nicotine content cigarettes in smokers with low socioeconomic status: a double-blind randomized clinical trial. Nicotine Tob Res 10.1093/ntr/ntaa247 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morean ME, de Wit H, King AC, Sofuoglu M, Rueger SY, O’Malley SS (2013) The drug effects questionnaire: psychometric support across three drug types. Psychopharmacology 227(1):177–192. 10.1007/s00213-012-2954-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perkins KA (2008) Sex differences in nicotine reinforcement and reward: influences on the persistence of tobacco smoking. The motivational impact of nicotine and its role in tobacco use:143–169 [DOI] [PubMed] [Google Scholar]
- Perkins KA (2019) Research on behavioral discrimination of nicotine may inform FDA policy on setting a maximum nicotine content in cigarettes. Nicotine Tob Res 21(Suppl 1):S5–S12. 10.1093/ntr/ntz136 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perkins KA, Kunkle N, Karelitz JL (2017a) Preliminary test of cigarette nicotine discrimination threshold in non-dependent versus dependent smokers. Drug Alcohol Depend 175:36–41. 10.1016/j.drugalcdep.2017.01.033 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perkins KA, Kunkle N, Karelitz JL, Perkins KA, Kunkle N, Karelitz JL (2017b) Preliminary test of cigarette nicotine discrimination threshold in non-dependent versus dependent smokers. Drug Alcohol Depend 175:36–41. 10.1016/j.drugalcdep.2017.01.033 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sofuoglu M, LeSage MG (2012) The reinforcement threshold for nicotine as a target for tobacco control. Drug Alcohol Depend 125(1–2):1–7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sofuoglu M, Yoo S, Hill KP, Mooney M (2008) Self-administration of intravenous nicotine in male and female cigarette smokers. Neuropsychopharmacology 33(4):715–720 [DOI] [PubMed] [Google Scholar]
- Sofuoglu M, Herman AI, Nadim H, Jatlow P (2012) Rapid nicotine clearance is associated with greater reward and heart rate increases from intravenous nicotine. Neuropsychopharmacology 37(6):1509–1516. 10.1038/npp.2011.336 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Valentine GW, DeVito EE, Jatlow PI, Gueorguieva R, Sofuoglu M (2018) Acute effects of inhaled menthol on the rewarding effects of intravenous nicotine in smokers. J Psychopharmacol 32(9):986–994 [DOI] [PMC free article] [PubMed] [Google Scholar]


