Abstract
Luminex single antigen bead (SAB) assay utilizes beadsets coated with a set of cloned and purified HLA molecules, for monitoring serum anti-HLA antibodies. Particularly, the level of serum IgG against native HLA-I trimers (heavy chain (HC) and β2-microglobulin (β2 m) with a peptide), expressed in allograft tissues is correlated with graft failure. In addition to native trimeric HLA–I, the beadsets may carry HC only or the dimeric variants, peptide-free HC with β2 m and β2 m–free HC with or without peptides. Currently, three different HLA-I coated beadsets have been produced commercially. The HLA antigen density on one beadset was reported to be approximately 50% of that present on another beadset as evidenced by the binding of an anti-HLA-I mAb W6/32. To date, no efforts have been made to compare the relative distribution of HLA-I variants in these three beadsets. In this study, using monoclonal antibodies (W6/32, HC-10 and TFL-006) that can distinguish the structural variants based on their epitope specificities, the nature of the variants in the three beadsets were comparatively evaluated. One beadset (Beadset A, see Materials and methods for Brand and Manufacturer’s names) (W6/32+/HC-10+/TFL-006+) carried at least three variants, while beadset B (W6/32+/HC-10+/TFL-006−) carried two (peptide-associated and peptide-free β2 m–HC) and the beadset C (W6/32+/HC-10−/TFL-006−) carried exclusively the HLA-I trimer suggesting its usefulness for specific monitoring native HLA-I trimer antibodies. Because of the salient differences in the variants coated on the different beadsets, it would be warranted to investigate, if these differences are clinically relevant for monitoring serum anti-HLA antibodies in sensitized patients waiting for donor organs and in allograft recipients (274).
1. Introduction
The native tissue-associated HLA-I trimer consists of a folded heavy chain (HC) (40–45 kDa) non-covalently associated with β2-microglobulin (β2 m) (12 kDa) and an 8–10 amino acid long peptide in the grooves of HC (PepA-β2aHC). One of the known causes for rejection of allograft in a recipient is the presence of pre-existing or post-transplant de novo IgG antibodies against mismatched HLA-I expressed on the allograft tissues. To monitor serum HLA antibodies in allograft recipients before and after transplantation, the Luminex multiplex HLA coated single antigen beadsets were developed using a set of cloned and purified HLA antigens (Pei et al., 2003). Using one manufacturer’s beadset, Cai et al. (2009) documented in a large cohort of renal allograft recipients (n = 994) that patients with donor specific antibodies (DSA) for native HLA-I trimer had a significantly lower graft survival rate compared to those with no DSA or possessed antibodies against β2 m–free HC. In addition to native PepA-β2aHC, this beadset may carry HC only (PepF-β2fHC) or the dimeric variants such as peptide-free HC with β2 m (PepF-β2aHC) and the antibodies directed against these structural variants are not deleterious (Michel et al., 2016; Visentin et al., 2014, 2015; Otten et al., 2013). However, the presence of structural variants in the beadsets may impede the true assessment of the level of IgG against native trimeric HLA–I. Recognizing the possible interference of structural variants in a beadset, the same manufacturer developed a second version of beadset, free of a monomeric variant, β2 m–free HC. The mAb W6/32 recognized both the beadsets, but the antigen density in the second beadset was found to be lower than the first beadset (Jucaud et al., 2017). In addition, by comparing HLA-I antigens on two different beadsets from different manufacturers with W6/32, Hilton and Parham (2013) noted that the antigen density present on beadset of one manufacturer was approximately 50% of that present on the beadset of the other manufacturer. To date, neither an examination for the HLA-I molecular variants nor a comparative evaluation for the distribution of structural variants with different beadsets has been conducted. It is hypothesized that such a comparative analysis and characterization of the three different beadsets for the relative distribution of HLA-I conformational variants may elucidate whether the different reactivity of mAb W6/32 is really due to “antigen density” or due to differential distribution of conformational variant(s) or both.
To test the hypothesis, we have used three unique HLA-I-specific mAbs which distinguish β2aHC from β2fHC (W6/32 vs TFL-006) and PepA-β2aHC from PepF-β2aHC variants (W6/32 vs HC-10/TFL-006). The results confirmed that one beadset from a manufacturer carried only the HLA-I trimeric (PepA-β2aHC), in contrast to the other two beadsets from another manufacturer which carried the other structural variants (PepF-β2aHC and PepF-β2fHC or PepF-β2aHC) in addition to PepA-β2aHC.
2. Materials and methods
2.1. Monoclonal antibodies
The mAb W6/32 (IgG2a) (One Lambda, Canoga Park, CA, USA) binds to β2aHC (pepA-β2aHC) and pepF-β2aHC) but not with β2fHC (Barnstable et al., 1978). The mAb W6/32 defined epitope depends on the β2 m residues 3 (Parham et al., 1979) and 89, and on the HC residue 121 (Martayan et al., 2009; Ladasky et al., 1999). The mAb HC-10 (IgG2a) (Source: Nordic MUbio, Susteren, Netherlands, Cat#-MUB2037P) binds to pepF-β2aHC and β2fHC but not with pepA-β2aHC (Stam et al., 1986). The epitope recognized by mAb HC-10 is defined by the antigenic determinant, arginine at position 62 (R62) located in the α1 helix of the HLA-A, HLA-B and HLA–Cw HC (Perosa et al., 2003). mAb HC-10 does not bind to the epitope, if R62 is blocked by a peptide in the groove, or replaced by another amino acid. The details of the amino acid substitutions at position 62 in HLA class I alleles are presented elsewhere (Jucaud et al., 2017). Purified ascites of mAb TFL-006 (IgG2a), developed at Terasaki Foundation Laboratory by immunizing HLA-E PepF-β2fHC (Ravindranath et al., 2013a, 2013b), binds to the β2fHC conformation of all antigens of HLA–I loci, with its epitope located at 117AYDGKDY123, which is shared by all HLA-I antigens. The epitope is exposed only in β2fHC variant, as it is otherwise masked by β2 m in β2aHC.
2.2. SAB Luminex-based immunoassay
The HLA-I reactivity of the mAbs was analyzed using Luminex multiplex single antigen bead (SAB) assay (Ravindranath et al., 2010, 2011, 2013a, 2013b, 2017; Jucaud et al., 2017). Multianalyte profiling (xMAP) technology from Luminex (http://www.luminexcorp.com/) involves using dual-laser Flow cytometry to distinguish Multiplex polystyrene single antigen coated beadset, with each bead containing fluorochromes of differing intensity embedded within the bead. The nature of the antigen coated onto the bead is the most critical aspect relevant to monitoring antibodies specific to conformational HLA-I antigens.
The SAB used in this investigation are: (i) Beadset A: Brand name: Labscreen (Cat#LS1A04, Lot # 8, Manufacturer One Lambda Inc. (Thermo Fisher), Canoga Park, CA), extensively utilized for clinical monitoring of HLA antibodies in US. (ii) Beadset B: Brand name: iBeads (Manufacturer: One Lambda, Canoga Park, CA) (El-Awar et al., 2010; Otten et al., 2013; Ravindranath et al., 2013a; Visentin et al., 2015), and the manufacturing of this beadset is discontinued; and (iii) Beadset C: Brand name: Lifecodes (LSA Class I 03203F beads; Manufacturer: Immucor, Norcross, GA). The beadset is also utilized for monitoring HLA-I antibodies in allograft recipients (Oh et al., 2015, Tozkir et al., 2016, Hyun et al., 2012, Middelburg et al., 2011, Jung et al., 2009). The panel of HLA-I molecules coated on the SABs from One Lambda and Immucor are almost similar with few differences. SABs from both sources carried identical alleles of HLA-A (n = 27), HLA-B (n = 44) and HLA-Cw (n = 13). The antigen unique to each beadset is as follows:
HLA-A (One Lambda: A*02:06, A*29:01, A*20:02, A*34:01, Immucor: A*02:02, A*02:05);
HLA-B (One Lambda: B*13:01, B*15:10, B*15:11, B*40:06, B*51:02, B*57:03; Immucor: B*07:03, B*15:18, B*27:03; B*35:08); HLA-Cw (One Lambda: Cw*03:02, Cw*12:03, Cw*18:02; Immucor: Cw*04:03, Cw*07:01, Cw*08:02; Cw*12:02; Cw*18:01).
Immucor and One Lambda provide different protocols for using their respective product. The main differences between the protocols are: (i) stock concentration of the bead solution (Immucor beads are diluted 8 folds compared to One Lambda beads), where the Immucor protocol involved the incubation of 40 μL of bead mixture with 10 μL of mAbs for 30 min at RT, whereas the One Lambda protocol involved incubation of 20 μL of mAbs with 2 μL of SAB for 30 min at RT.; (ii) Immucor protocol suggests the use of filter plate to perform washes with a vacuum manifold, whereas One Lambda protocol suggests the use of V-bottom plate and the spin/flick method for washing. After incubating the bead mixtures with the mAbs, the rest of the protocols are similar and as reported earlier (Jucaud et al., 2017). Briefly, the SAB were then washed (×3) with Wash Buffer (PBS, Tween-20 and sodium azide). PE-conjugated Goat Anti-Mouse IgG2a (γ2a chain specific; Concentration: 0.5 mg/mL; Cat#1080–09; Southern Biotech, Birmingham, AL, USA) diluted 1 to 100 in wash buffer was used to monitor the mAb binding.
With the objective to remove protocol differences as a confounding variable, and since the One Lambda protocol is the standard of procedure (SOP) in our laboratory, we assessed whether there was any difference in MFI between the One Lambda protocol and the Immucor protocol using Immucor (Lifecodes) beads. As shown in Table 1, there was no difference in MFI between the Immucor and One Lambda protocol using Lifecodes beadset for HLA-A, −B and −C beads. Therefore, all the data presented subsequently represent MFI obtained with the One Lambda protocol for all beadsets tested. In addition, all mAbs were titrated (5 μg/mL and at 10 μg/mL), however only the data obtained with 10 μg/mL are presented, because this concentration is optimal for saturation of the beads. Last, for positive reaction, the MFI cutoff was 1000.
Table 1.
Comparison of the Immucor and One Lambda protocola using beadset C. For the brand names and manufacturers names of the beadset refer to Materials and methods.
| mAb tested | Beadset | Loci | Alleles examined | Protocol | MFI median ± SD | MFI range | p value |
|---|---|---|---|---|---|---|---|
| W6/32 | Beadset C | HLA-A | 29 | Immucor | 17,553 ± 1989 | 13,869–20,885 | 0.87 |
| One Lambda | 17,146 ± 2242 | 13,662–21,638 | |||||
| HLA-B | 48 | Immucor | 19,231 ± 2180 | 11,482–22,266 | 0.96 | ||
| One Lambda | 19,365 ± 2371 | 10,683–22,887 | |||||
| HLA-Cw | 18 | Immucor | 11,859 ± 2757 | 5990–15,660 | 0.53 | ||
| One Lambda | 11,364 ± 2979 | 5336–15,633 |
The Immucor protocol: 40 μL of bead mixture was incubated with 10 μL of mAbs at the concentration of 50 μg/mL for 30 min at RT.
The One Lambda protocol: 2 μL of bead mixture was incubated with 20 μL of mAbs at the concentration of 10 μg/mL for 30 min at RT.
Note that the stock concentration of the bead solution differ between beadsets A and C: Beads of Beadset C are diluted 8 folds compared to Beadset A;
2.3. Statistical analysis
All statistical analysis were performed using STATA 13. All data were tested for normality using Shapiro-Wilk and Shapiro-Francia tests. Analysis of significant was performed using the Wilcoxon matched-pairs signed-rank test. Two-tailed p-values < 0.05 were considered significant. (564)
3. Results
To assess the relative density of HLA-I variants coated on beadsets, we have used the mAbs that distinguish the HLA-I variants, using protocols of the beadset manufacturers. The protocol differences did not introduce any significant differences in the MFI values (Table 1). For sake of simplicity, only the data obtained with one manufacturer’s protocol is presented. The data obtained with the other protocol is available on request. The names of the brand and manufacturers of each of the three different beadsets (termed as Beadset A, Beadset B and Beadset C) are presented in Materials and methods.1
3.1. With mAb W6/32, the MFI with beadset A is higher than that of beadset C
mAb W6/32 recognizes a conformational epitope specifically expressed by HLA-I trimers. This epitope is defined as “protruding tip of β2m and its contact point is right below the β-sheet platform of the binding groove” (p.3614) of the HC (Martayan et al., 2009). Therefore this epitope is expressed only by β2aHC but not by β2fHC. Hence, mAb W6/32 is the best mAb to detect HLA-I trimer (β2aHC).
Comparing the reactivity (MFI) of the mAb W6/32, it was noted W6/32 reacted with 100% of HLA-A (n = 27), HLA-B (n = 44) and HLA–Cw (N = 13) alleles on both beadsets A and C (Fig. 1). However, mAb W6/32 reactivity with the beadset C is 80% of the MFI expressed by HLA-A beads, 89% of that by HLA-B beads, and 58% of that by HLA–Cw beads of the beadset A. The median MFI generated by mAb W6/32 was significantly higher (p2 < 0.002) for beadset A compared to beadset C for each HLA class I locus (Table 1).
Fig. 1.

Immunostaining of the Beadset A and C beadsets with the murine mAb W6/32, which recognizes β2 m–associated heavy chains of HLA-I with or without peptides.
Although the MFI generated by mAb W6/32 is similar to that of a previous report (Hilton and Parham, 2013), the percentage difference between the MFIs generated by mAb W6/32 with beadset A and beadset C is markedly lower than that of the previous report. This difference could be due to a lower density of the HLA antigens coated on the beads as suggested by Hilton and Parham (2013) or could be a consequence of the differential density of the HLA-I structural variants on the beadsets. To address this question, the levels of HLA-I structural variants coated on beadsets A and C were compared. For this purpose, the mAbs HC-10 and TFL-006, which recognize epitopes distinct from each other and from the epitope defined by mAb W6/32 were utilized.
3.2. With mAb HC-10, the MFI with beadset C is significantly lower than that of beadset A
The epitope recognized by mAb HC-10 is defined by the antigenic determinant, arginine at position 62 (R62) located in the α1 helix of the HLA-A, HLA-B and HLA–Cw HC (Perosa et al., 2003; Jucaud et al., 2017). mAb HC-10 does not bind to the epitope, if R62 is blocked by a peptide in the groove, or replaced by another amino acid. The details of the amino acid substitutions at position 62 in HLA class I alleles are presented elsewhere (Jucaud et al., 2017). Therefore, the MFI generated by the mAb HC-10 reactivity with beads is indicative of their coating with β2fHC as well as with PepF-β2aHC.
Distinctly, the median MFI of mAb HC-10 with beadset C is as low as 4% of that with HLA-A and HLA–Cw beads and 6% of that with HLA-B beads of the beadset A (median MFI of HLA-A: 10,645, HLA–B: 14,066, HLA-Cw: 18,322) (Table 2, Fig. 2). Indeed, the median MFI generated by the reactivity of mAb HC-10 with beadset A was significantly higher (p2 < 0.006) than that generated by the reactivity with beadset C for each of the HLA class I loci (Table 2). These results confirm low level of PepF-β2aHC as well as β2fHC coated on beadset C (Fig. 2). Therefore, the coating of beadset A with peptide-free β2aHC may account for the different binding of mAb W6/32.
Table 2.
Comparison of the reactivity of mouse mAbs W6/32, HC-10 and TFL-006 with HLA-I coated three different beadsets A, B and C. For the commercial sources and the brand names of the beadsets refer to Materials and methods.
| mAbs tested | Loci | n | Beadset | Median MFI ± SD | MFi range | p value vs Beadset C |
|---|---|---|---|---|---|---|
| W6/32 | HLA-A | 27 | A | 21,664 ± 950 | 19,008–22,513 | < 0.0001 |
| B | 17,159 ± 1978 | 11,450–19,860 | < 0.0754 | |||
| C | 17,308 ± 2240 | 13,663–21,638 | ||||
| HLA-B | 44 | A | 21,811 ± 1568 | 16,084–23,072 | < 0.0001 | |
| B | 17,481 ± 3178 | 10,433–21,406 | 0.0002 | |||
| C | 19,635 ± 2392 | 10,863–22,887 | ||||
| HLA-Cw | 13 | A | 18,797 ± 3768 | 11,436–23,742 | 0.0015 | |
| B | 13,258 ± 4196 | 4918–18,639 | 0.75 | |||
| C | 10,825 ± 3095 | 5336–15,299 | ||||
| HC-10 | HLA-A | 10 | A | 10,645 ± 4274 | 5038–17,797 | 0.0051 |
| B | 3732 ± 1882 | 1488–7112 | 0.0051 | |||
| C | 426 ± 256 | 97–905 | ||||
| HLA-B | 42 | A | 14,066 ± 3859 | 3057–19,455 | < 0.0001 | |
| B | 1202 ± 1296 | 370–6922 | 0.028 | |||
| C | 838 ± 1253 | 67–5736 | ||||
| HLA-Cw | 13 | A | 18,322 ± 3115 | 12,096–21,774 | 0.0015 | |
| B | 732 ± 686 | 455–2650 | 0.13 | |||
| C | 791 ± 423 | 231–1627 | ||||
| TFL-006 | HLA-A | 27 | A | 1172 ± 1312 | 242–6907 | < 0.0001 |
| B | 231 ± 338 | 80–1468 | < 0.0001 | |||
| C | 1 ± 1 | 0–5 | ||||
| HLA-B | 44 | A | 2257 ± 1158 | 205–4806 | < 0.0001 | |
| B | 13 ± 45 | 4–225 | < 0.0001 | |||
| C | 0 ± 1 | 0–6 | ||||
| HLA-Cw | 13 | A | 3702 ± 1932 | 1852–7733 | 0.0015 | |
| B | 18 ± 18 | 9–72 | 0.0019 | |||
| C | 2 ± 5 | 0–15 |
Fig. 2.
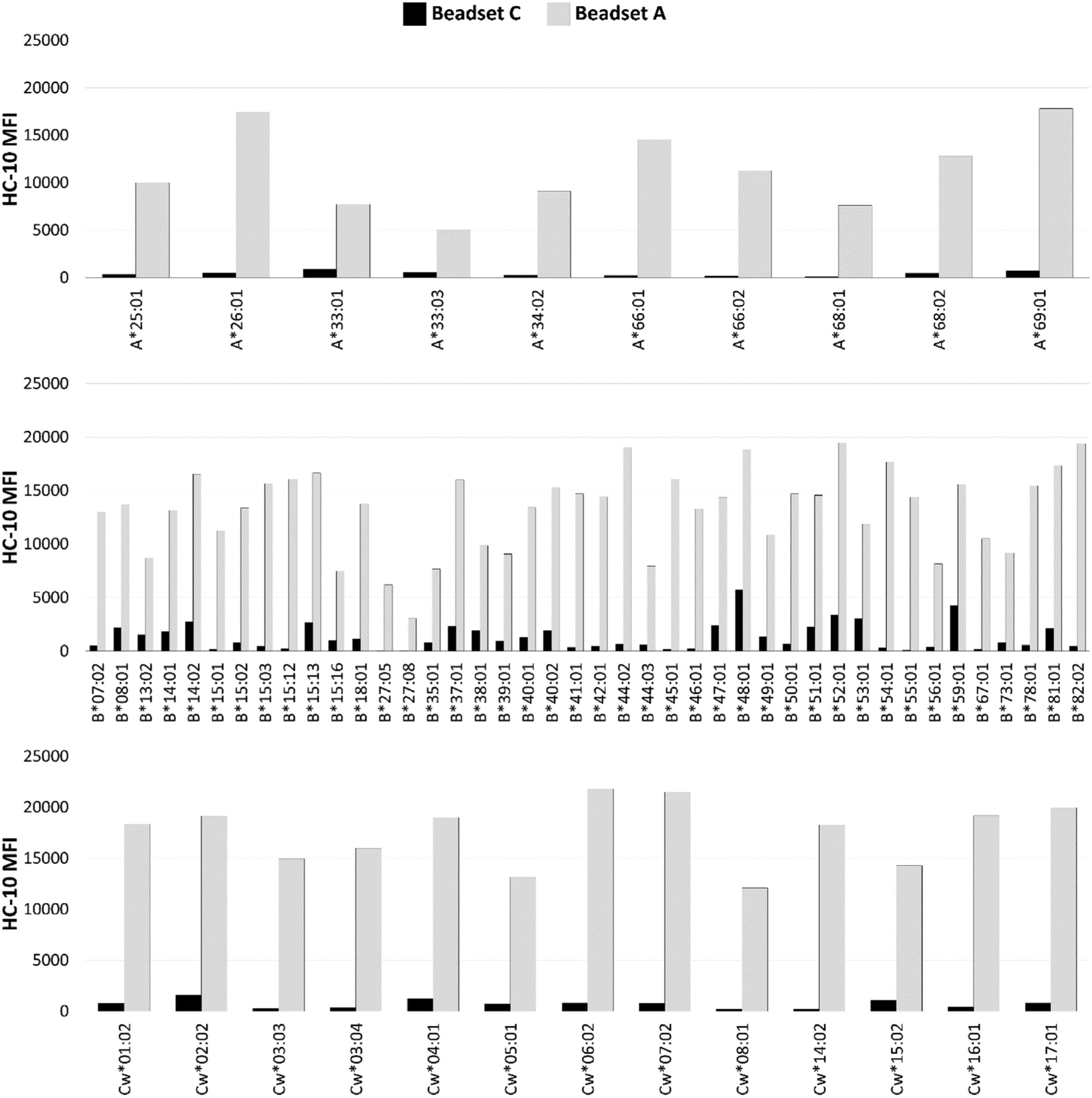
Immunostaining of the beadsets A and C with the murine mAb HC-10, which recognizes β2 m–associated heavy chains of HLA-I without peptides as well as β2 m–free heavy chains of HLA–I.
3.3. Beadset C is devoid of β2fHC
TFL-006 is utilized to assess the coating of beadsets A and C with β2fHC. The epitope recognized by mAb TFL-006 is cryptic in β2aHC but exposed in β2fHC (Ravindranath et al., 2013a, 2013b). mAb TFL-006 recognizes the peptide sequence (117AYDGKDY123) in the HC α1 helix; this sequence is present in all the gene products of the three HLA-I loci.
Lack of reactivity of beadset C with mAb TFL-006 indicate the absence of β2fHC on the beadset. The median MFI of mAb TFL-006 with beadset C is lower (< 0.09%) than the TFL-006-reactivity of beadset A for HLA-A, HLA-B and HLA–Cw beads (median MFI of HLA-A: 1172, HLA–B: 2257, HLA-Cw: 3702) (Table 2, Fig. 3). The median MFI generated by the reactivity of mAb TFL-006 with beadset A was significantly higher (p2 < 0.002) than that generated by the reactivity with beadset C for each of the HLA class I loci (Table 2). With beadset A, the MFI generated by the reactivity of mAb TFL-006 with 20 of the 27 HLA-A beads, 34 of the 44 HLA-B beads, and 13 of the 13 HLA–Cw beads was higher than 500, confirming that beadset A is coated with β2fHC. Indeed, mAb TFL-006 is a reliable quality control reagent for beadsets, to certify that the beadset is free of β2fHC.
Fig. 3.
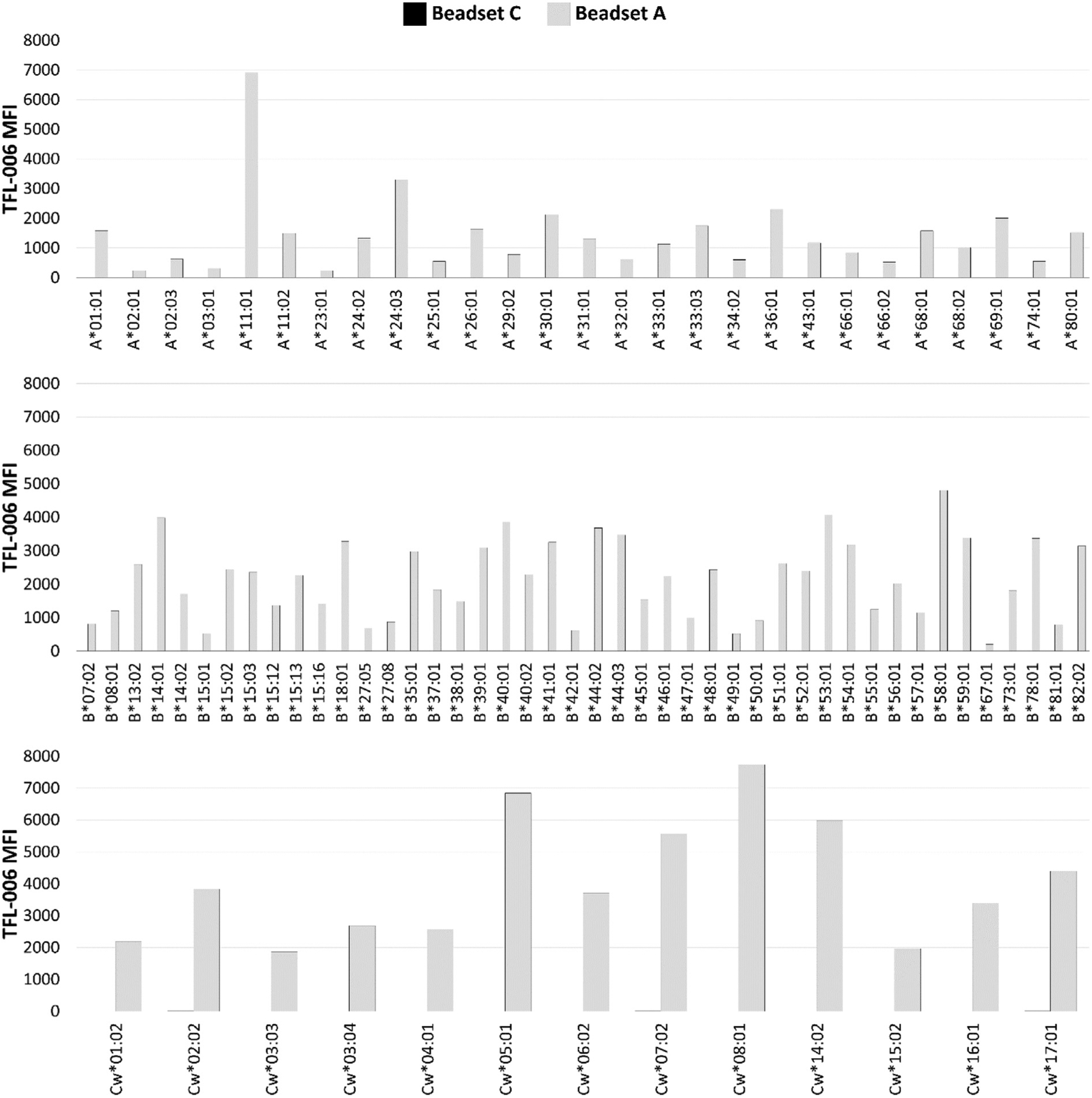
Immunostaining of the beadsets A and C with the murine mAb TFL-006, which recognizes β2 m–fHC of HLA-I [42].
3.4. The levels of PepF-β2aHC & β2fHC on the beadset C are far lower than those coated on the beadset B
The MFI generated by the reactivity of mAb W6/32 with beadset B is lower than that generated by the reactivity with beadset A. Therefore, the reactivities of mAb W6/32, HC-10 and TFL-006 with beadsets A and C were compared to those with beadset B (Fig. 4).
Fig. 4.

Differential reactivity of mAbs W6/32, HC-10 and TFL-006 with HLA class I molecule coated beadsets A, B and C. For the commercial sources and the brand names of the beadsets refer to Materials and methods.
As shown in Table 2, mAb W6/32 reactivity with the beadset C is 101% of that with HLA-A of beadset B, 111% of that with HLA-B beadset B, and 82% of that with HLA–Cw of beadset B. However, the median MFI of mAb W6/32 with HLA-B beads of beadset C was significantly higher (p2 < 0.001) than the MFI with beadset B, but not significantly different from the MFI obtained with HLA-A and HLA–Cw beads. The median MFI of mAb HC-10 with beadset C is 11% of the MFI of HLA-A of beadset B, 70% of MFI with HLA-B of beadset B, and 108% of MFI with HLA–Cw of beadset B. However, the median MFI generated by the reactivity of mAb HC-10 with HLA-A and HLA-B beads of beadset C was significantly lower (p2 < 0.03) than that with HLA-A and HLA-B iBeads. Such a significant difference was not observed with HLA–Cw beads. The median MFI generated by the reactivity of mAb TFL-006 with HLA-A, HLA-B and HLA–Cw beads of beadset C was significantly lower (p2 < 0.002) than the median MFI generated by the reactivity of mAb TFL-006 with beadset B (median MFI: < 500).
The median MFI generated by the reactivity of mAb HC-10 with HLA-A and B beads of beadset C was the lowest, suggesting that the levels of PepF-β2aHC and β2fHC coated on the beads of beadset C are lower than those coated on the beadset B. Furthermore, the lack of reactivity of mAb TFL-006 with beadset C (median MFI: 0–2) confirms that the beadset C is coated only with HLA-I trimer (PepA-β2aHC).
4. Discussion
The level of antibodies, recognizing HLA-I trimer (or also referred to as “intact HLA”), measured as MFI by Luminex multiplex single antigen bead (SAB) assay, has been directly associated with pathologic antibody mediated graft failure (Cai et al., 2009; Michel et al., 2016; Visentin et al., 2014, 2015; Otten et al., 2013). The assay is considered standard of care for detection and identification of anti-HLA antibodies in sensitized patients waiting for donor organs and DSA in allograft recipients. However it should be noted that for clinical evaluation of the HLA antibodies by the SAB assays, the antibody is measured as MFI but not as titer after serial dilution, primarily because ascertaining the titer is cost-prohibitive in Luminex multiplex assays. The investigators use either undiluted sera or sera diluted either 1/3 or 1/8 or 1/10 to measure the MFI. As a consequence, US-FDA considers the assay semiquantitative.
To date, there is scant information on the specificity of the different beadsets for testing HLA-I trimer-specific DSA. The results of this investigation is summarized in Fig. 5. The findings confirm our previous report (Jucaud et al., 2017) that not all the beadsets are the same relating to carrying PepA-β2aHC, PepF-β2aHC, and β2fHC (Fig. 5). In patients’ pre-transplant sera, the reactivity of serum antibodies to variants other than PepA-β2aHC may result in “inappropriate assignment of unacceptable antigens during transplant listing” (Michel et al., 2016). Although cell based assays, such as complement dependent cytotoxicity cross match (CDCXM) and flow crossmatch (FCXM), are considered to be specific for intact HLA-I (PepA-β2aHC) (Fig. 5), they lack sensitivity. No doubt that the Luminex multiplex assay is a highly sensitive assay with a potential for reliable monitoring clinically relevant anti-HLA-I trimer antibodies, provided that the beadset carries only HLA-I trimers (PepA-β2aHC). Without defining the HLA variants coated on the beadsets (Fig. 5), it is uncertain which beadset has high specificity for the detection of anti-HLA-I trimer antibodies.
Fig. 5.
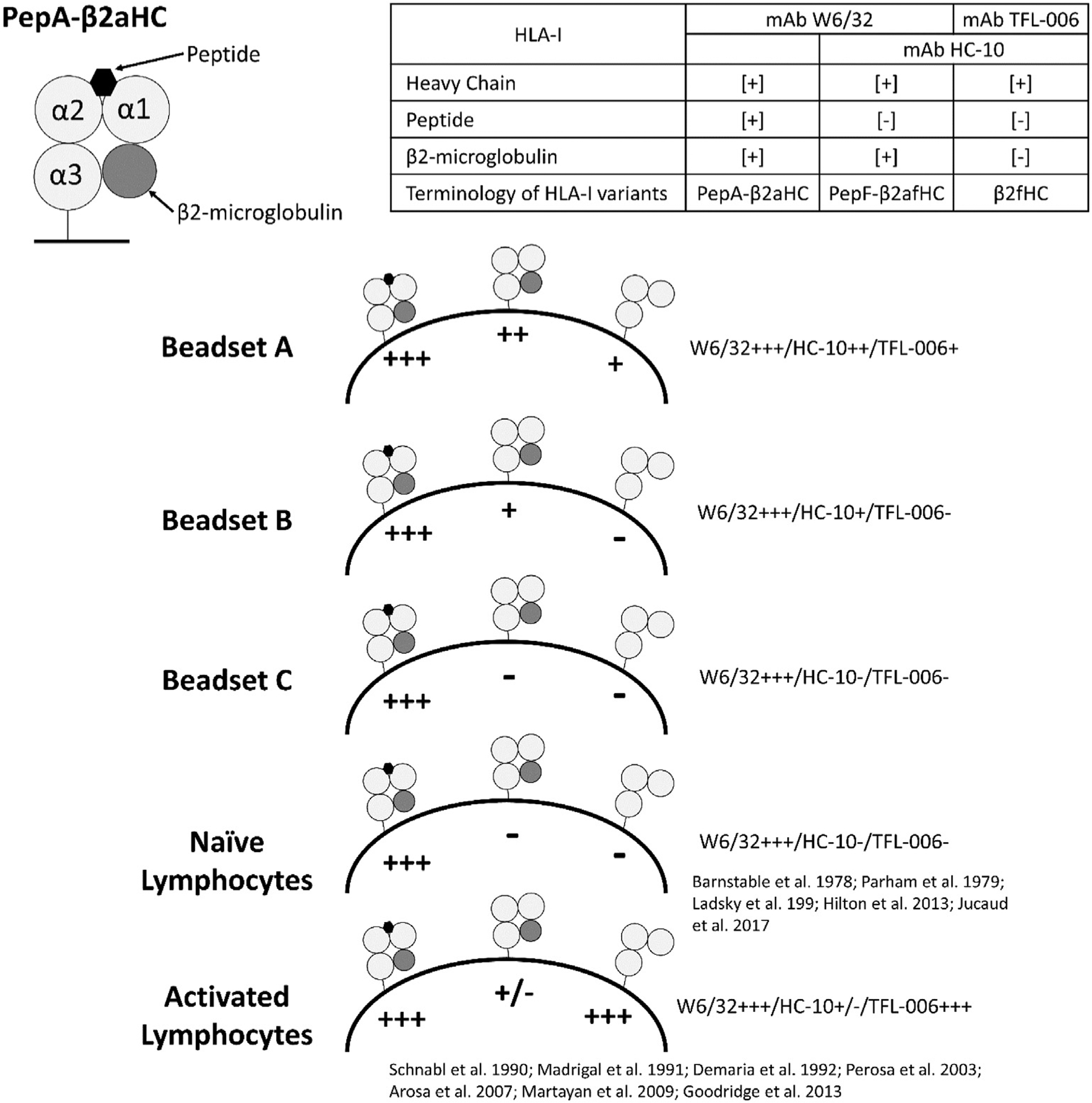
Characteristics of three different Beadsets (A, B and C) as assessed with anti-HLA variant detecting Monoclonal antibodies (W6/32, HC-10 and TFL-006). For the commercial sources and the brand names of the beadsets refer to Materials and methods.
The results of this study compared the three commercially produced beadsets used to monitor HLA-I antibodies to determine if any carries only the trimeric HLA-I or a mixture of HLA-I conformational variants, as summarized in Figs. 4 and 5. The relative coating of the three beadsets with HLA-I variants was shown by their reactivity with the mAbs W6/32, HC-10 and TFL-006 (Fig. 5). Abundancy of native form of HLA-I (PepA-β2aHC) on the beadset C is confirmed by mAb W6/32 reactivity. Very low level of mAb HC-10 reactivity with beadset C further indicates the lack of PepF-β2aHC and β2fHC. The absence of mAb TFL-006 reactivity with beadset C also point out the lack of coating with β2fHC for all HLA-I loci coated on the beadset. In contrast, the high mAb W6/32, HC-10 and TFL-006 reactivity observed with beadset A suggests the heterogeneous coating with all HLA-I conformational variants. Compared to the beadset B, the beadset C is unique in that it is free from β2fHC and PepF-β2aHC for all antigens (Table 2), thus contains only the native HLA-I trimer (PepA-β2aHC), potentially the main target of pathogenic anti-HLA-I alloantibodies in transplant patients (Cai et al., 2009; Otten et al., 2013; Visentin et al., 2015).
Beadsets coated only with PepA-β2aHC (HLA-I trimer) such as the beadset C and a beadset coated only with β2fHC only would be ideal to differentiate serum antibodies reactive with HLA-I trimer from those reactive with β2fHC. Although acid/alkali/heat treatments of the beads carrying HLA-I trimer can generate β2fHC, due to variations in the degree of denaturation, they may not parallel with naturally occurring β2fHC. A stable pool of β2 m–free HLA (β2fHC) was observed in proliferating human lymphoid cells (Schnabl et al., 1990), followed by the confirmation of β2fHC overexpression on activated human T and B cells (Madrigal et al., 1991; Demaria et al., 1992; Lee et al., 1998; Strong et al., 2003; Goodridge et al., 2010, 2013) and on trophoblasts (Gonen-Gross et al., 2005). Nevertheless, clinical transplant investigators referred to β2 m–free HLA as “denatured HLA”, since “protein misfolding is usually associated with denaturation” (Arosa et al., 2007). Usage of the terminology “denatured HLA–I” was questioned (Arosa et al., 2017) since “it does not apply to the pool of β2m -free HC identified on the cell surface,” nor to cells and tissues unexposed to any kind of treatments (acid/alkali/heat) (for further elucidation see also Ravindranath and Jucaud, 2017).
Recently, Battle et al. (2017) after comparatively examining the anti-HLA class II IgG in the sera of a single allograft rejected patient with two beadsets from different manufacturers, reported that the multifactorial “prozone effect” was observed only with one manufacturer’s beadsset but not with the other manufacturer’s beadset. Although the factor for the difference is far from clear, relative distribution of trimeric and monomeric variants of HLA class II antigens in both the beadsets deserve attention in the light of the results obtained in the present investigation.
In conclusion, the results of this investigation confirm that the beadset C is coated with native HLA-I or HLA-I trimer (PepA-β2aHC) with minimal presence or absence of other conformational variants. There is a possibility for lot-to-lot variations in any beadsets and therefore, a need arises to quality control for the presence of PepF-β2aHC and β2fHC with mAbs HC-10 and TFL-006. Since this report indicates that there is a difference between the beadsets as it relates to HLA-I conformational variants, it would be worthwhile to investigate, if these differences are clinically relevant for monitoring serum anti-HLA antibodies in sensitized patients waiting for donor organs and in allograft recipients.
Acknowledgement
The funding for the project is supported by Terasaki Family Foundation. First and Second authors have equally contributed to the investigation. First author formulated the hypothesis and wrote the manuscript and the second author carried out the Single antigen Bead assays and discussed the results on daily basis. All authors examined and analyzed the results, discussed and contributed to the manuscript preparation.
Footnotes
According to the policy of Terasaki Research Institute.
References
- Arosa FA, Santos SG, Powis SJ, 2007. Open conformers: the hidden face of MHC-I molecules. Trends Immunol. 28, 115–123. [DOI] [PubMed] [Google Scholar]
- Arosa FA, Esgalhado AJ, Padrão CA, Cardoso EM, 2017. Divide, conquer, and sense: CD8+CD28− T cells in perspective. Front. Immunol 7, 665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barnstable CJ, Bodmer WF, Brown G, Galfre G, Milstein C, Williams AF, Ziegler A, 1978. Production of monoclonal antibodies to group a erythrocytes, HLA and other human cell surface antigens-new tools for genetic analysis. Cell 14, 9–20. [DOI] [PubMed] [Google Scholar]
- Battle RK, Abel AA, Turner DM, 2017. Prozone effect can be specific to single antigen bead kit manufacturers. Am. J. Transplant 17, 1425–1426. [DOI] [PubMed] [Google Scholar]
- Cai J, Terasaki PI, Anderson N, Lachmann N, Schönemann C, 2009. Intact HLA not beta2m-free heavy chain-specific HLA class I antibodies are predictive of graft failure. Transplantation 88, 226–230. [DOI] [PubMed] [Google Scholar]
- Demaria S, Schwab R, Bushkin Y, 1992. The origin and fate of beta 2m-free MHC class I molecules induced on activated T cells. Cell. Immunol 142, 103–113. [DOI] [PubMed] [Google Scholar]
- El-Awar N, Nikaein A, Everly M, Hopefield J, Nguyen A, 2010. A novel HLA class I single antigen bead preparation eliminates false positive reactions attributed to natural antibodies – in the sera of normal males and pre-transplant patients. Hum. Immunol 71 (Sup-1), S26. [Google Scholar]
- Gonen-Gross T, Achdout H, Arnon TI, Gazit R, Stern N, Horejsí V, Goldman-Wohl D, Yagel S, Mandelboim O, 2005. The CD85J/leukocyte inhibitory receptor-1 distinguishes between conformed and beta 2-microglobulin-free HLA-G molecules. J. Immunol 175, 4866–4874. [DOI] [PubMed] [Google Scholar]
- Goodridge JP, Burian A, Lee N, Geraghty DE, 2010. HLA-F complex without peptide binds to MHC class I protein in the open conformer form. J. Immunol 184, 6199–6208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goodridge JP, Burian A, Lee N, Geraghty DE, 2013. HLA-F and MHC-I open conformers cooperate in a MHC-I antigen cross-presentation pathway. J. Immunol 191, 1567–1577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hilton HG, Parham P, 2013. Direct binding to antigen-coated beads refines the specificity and cross-reactivity of four monoclonal antibodies that recognize polymorphic epitopes of HLA class I molecules. Tissue Antigens 81, 212–220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hyun J, Park KD, Yoo Y, Lee B, Han BY, Song EY, Park MH, 2012. Effects of different sensitization events on HLA alloimmunization in solid organ transplantation patients. Transplant. Proc 44, 222–225. [DOI] [PubMed] [Google Scholar]
- Jucaud V, Ravindranath MH, Terasaki PI, 2017. Conformational variants of the individual HLA-I antigens on Luminex single antigen beads used in monitoring HLA antibodies: problems and solutions. Transplantation 101, 764–777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jung S, Oh EJ, Yang CW, Ahn WS, Kim Y, Park YJ, Han K, 2009. Comparative evaluation of ELISA and Luminex panel reactive antibody assays for HLA alloanti-body screening. Korean J. Lab. Med 29, 473–480. [DOI] [PubMed] [Google Scholar]
- Ladasky JJ, Shum BP, Canavez F, Seuanez HN, Parham P, 1999. Residue 3 of β2-microglobulin affects binding of class I MHC molecules by the W6/32 antibody. Immunogenetics 49, 312–320. [DOI] [PubMed] [Google Scholar]
- Lee N, Goodlett DR, Ishitani H, Marquardt H, Geraghty DE, 1998. HLA-E surface expression depends on binding of TAP-dependent peptides derived from certain HLA class I signal sequences. J. Immunol 160, 4951–4960. [PubMed] [Google Scholar]
- Madrigal JA, Belichm MP, Benjamin RJ, Little AM, Hildebrand WH, Mann DL, Parham P, 1991. Molecular definition of a polymorphic antigen (LA45) of free HLA-A and -B heavy chains found on the surfaces of activated B and T cells. J. Exp. Med 174, 1085–1095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martayan A, Sibilio L, Tremante E, Lo Monaco E, Mulder A, Fruci D, Cova A, Rivoltini L, Giacomini P, 2009. Class I HLA folding and antigen presentation in beta 2-microglobulin-defective Daudi cells. J. Immunol 182, 3609–3617. [DOI] [PubMed] [Google Scholar]
- Michel K, Santella R, Steers J, Sahajpal A, Downey FX, Thohan V, Oaks M, 2016. Many de novo donor-specific antibodies recognize β2 -microglobulin-free, but not intact HLA heterodimers. HLA 87, 356–366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Middelburg RA, Porcelijn L, Lardy N, Briët E, Vrielink H, 2011. Prevalence of leucocyte antibodies in the Dutch donor population. Vox Sang. 100, 327–335. [DOI] [PubMed] [Google Scholar]
- Oh EJ, Park H, Park KU, Kang ES, Kim HS, Song EY, 2015. Interlaboratory comparison of the results of Lifecodes LSA class I and class II single antigen kits for human leukocyte antigen antibody detection. Ann. Lab. Med 35, 321–328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Otten HG, Verhaar MC, Borst HP, van Eck M, van Ginkel WG, Hené RJ, van Zuilen AD, 2013. The significance of pretransplant donor-specific antibodies reactive with intact or denatured human leucocyte antigen in kidney transplantation. Clin. Exp. Immunol 173, 536–543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parham P, Barnstable CJ, Bodmer WF, 1979. Use of a monoclonal antibody (W6/32) in structural studies of HLA-A, B, C, antigens. J. Immunol 123, 342–349. [PubMed] [Google Scholar]
- Pei R, Lee JH, Shih NJ, Chen M, Terasaki PI, 2003. Single human leukocyte antigen flow cytometry beads for accurate identification of human leukocyte antigen antibody specificities. Transplantation 75, 43–49. [DOI] [PubMed] [Google Scholar]
- Perosa F, Prete M, Luccarelli G, Favoino B, Dammacco F, 2003. Beta 2-microglobulin-free HLA class I heavy chain epitope mimicry by monoclonal antibody HC-10-specific peptide. J. Immunol 171, 1918–1926. [DOI] [PubMed] [Google Scholar]
- Ravindranath MH, Jucaud V, 2017. Conformational variants of HLA-I antigens on Luminex single antigen beads for monitoring antibodies. Transplantation 101, e153–e154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ravindranath MH, Taniguchi M, Chen CW, Ozawa M, Kaneku H, El-Awar N, Cai J, Terasaki PI, 2010. HLA-E monoclonal antibodies recognize shared peptide sequences on classical HLA class Ia: relevance to human natural HLA antibodies. Mol. Immunol 47, 1121–1131. [DOI] [PubMed] [Google Scholar]
- Ravindranath MH, Pham T, El-Awar N, Kaneku H, Terasaki PI, 2011. Anti-HLA-E mAb 3D12 mimics MEM-E/02 in binding to HLA-B and HLA-C alleles: web-tools validate the immunogenic epitopes of HLA-E recognized by the antibodies. Mol. Immunol 48, 423–430. [DOI] [PubMed] [Google Scholar]
- Ravindranath MH, Terasaki PI, Pham T, Jucaud V, Kawakita S, 2013a. Therapeutic preparations of IVIg contain naturally occurring anti-HLA-E antibodies that react with HLA-Ia (HLA-A/-B/-Cw) alleles. Blood 121, 2013–2028. [DOI] [PubMed] [Google Scholar]
- Ravindranath MH, Zhu D, Pham T, Jucaud V, Hopfield J, Kawakita S, Terasaki PI, 2013b. Anti-HLA-E monoclonal antibodies reacting with HLA-la and lb alleles like IVIg as potential IVIg-immunomimetics: an evolving therapeutic concept. Clin. Transpl 2013, 293–305. [PubMed] [Google Scholar]
- Ravindranath MH, Jucaud V, Banuelos N, Everly MJ, Cai J, Nguyen A, Terasaki PI, 2017. Nature and clonality of the fluoresceinated secondary antibody in Luminex multiplex bead assays are critical factors for reliable monitoring of serum HLA antibody levels in patients for donor organ selection, desensitization therapy, and assessment of the risk for graft loss. J. Immunol 198, 4524–4538. [DOI] [PubMed] [Google Scholar]
- Schnabl E, Stockinger H, Majdic O, Gaugitsch H, Lindley IJ, Maurer D, Hajek-Rosenmayr A, Knapp W, 1990. Activated human T lymphocytes express MHC class I heavy chains not associated with beta 2-microglobulin. J. Exp. Med 171, 1431–1442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stam NJ, Spits H, Ploegh HL, 1986. Monoclonal antibodies raised against denatured HLA-B locus heavy chains permit biochemical characterization of certain HLA-C locus products. J. Immunol 137, 2299–2306. [PubMed] [Google Scholar]
- Strong RK, Holmes MA, Li P, Braun L, Lee N, Geraghty DE, 2003. HLE-E allelic variants. Correlating differential expression, peptide affinities, crystal structures and thermal stabilities. J. Biol. Chem 278, 5082–5090. [DOI] [PubMed] [Google Scholar]
- Tozkir H, Pamuk ON, Duymaz J, Gurkan H, Yazar M, Sari G, Tanrikulu H, Pamuk GE, 2016. Increased frequency of class I and II anti-human leukocyte antigen antibodies in systemic lupus erythematosus and scleroderma and associated factors: a comparative study. Int. J. Rheum. Dis 19, 1304–1309. [DOI] [PubMed] [Google Scholar]
- Visentin J, Guidicelli G, Moreau JF, Lee JH, Taupin JL, 2014. Denatured class I human leukocyte antigen antibodies in sensitized kidney recipients: prevalence, relevance, and impact on organ allocation. Transplantation 98, 738–744. [DOI] [PubMed] [Google Scholar]
- Visentin J, Guidicelli G, Nong T, Moreau JF, Merville P, Couzi L, Lee JH, Taupin JL, 2015. Evaluation of the iBeads assay as a tool for identifying class I HLA antibodies. Hum. Immunol 76 (851–656). [DOI] [PubMed] [Google Scholar]


