Abstract
Background
Indomethacin is a prostaglandin inhibitor used to treat patent ductus arteriosus (PDA) in preterm infants. Although indomethacin produces ductal closure in the majority of cases, it is ineffective in up to 40% of patients. Furthermore, the ductus will re‐open in up to 35% of infants who initially respond to the drug. Prolonging the course of indomethacin has the potential to achieve higher rates of ductal closure.
Objectives
To determine the effect of a prolonged course of indomethacin (compared to a short course) on the rate of treatment failure without unwanted side‐effects in preterm infants with PDA.
Search methods
The search included review of personal files, abstracts of conferences, and the following electronic databases: MEDLINE (1966 to December 2006), EMBASE (1974 to December 2006), and Oxford Database of Perinatal Trials, Cochrane Central Register of Controlled Trials (The Cochrane Library, Issue 4, 2006). No language restrictions were applied.
Selection criteria
Randomized or quasi‐randomized controlled trials including preterm infants with PDA, diagnosed on clinical and/or echocardiographic examination that evaluated indomethacin treatment by any route given as a long course (four or more doses) vs. a short course (three or fewer doses) were included in the review. Trials needed to report on at least one of the following outcomes: failure of PDA to close, need for re‐treatment, PDA re‐opening, PDA ligation, mortality, duration of assisted ventilation, chronic lung disease (CLD), duration of supplemental oxygen dependence, intraventricular hemorrhage (IVH) (all and severe), diminished urine output, increased serum creatinine, necrotizing enterocolitis (NEC), bleeding diathesis, retinopathy of prematurity (ROP), and duration of hospital stay.
Data collection and analysis
The three review authors independently abstracted data from each study. Relative risk (RR) and Risk Difference (RD) with 95% confidence intervals (CI) using the fixed effect model for meta‐analysis are reported. When a statistically significant RD was found, the number needed to treat (NNT) or number needed to harm (NNH) was also calculated with 95% CIs. The I squared statistic was used to test for heterogeneity of results among included trials.
Main results
Five trials met inclusion criteria and included 431 infants. Prolonged indomethacin treatment when compared to the short course did not result in a statistically significant difference in PDA closure, re‐treatment, re‐opening, or ligation rates. The prolonged course was associated with an increased risk of NEC [typical RR 1.87 (95% CI 1.07, 3.27); typical RD 0.08 (95% CI 0.01, 0.15); NNH 13 (7, 100)] and a decreased incidence of renal function impairment, as evidenced by a lower proportion of infants having diminished urine output [typical RR 0.27 (95% CI 0.13, 0.6); typical RD ‐0.19 (95% CI ‐0.28, ‐0.09); NNT 5 (4, 11)] and increased serum creatinine level [typical RR 0.51 (95% CI 0.33, 0.77); typical RD ‐0.14 (95% CI ‐0.23, ‐0.06); NNT 7 (4, 16)].
Authors' conclusions
Implications for practice Prolonged indomethacin course does not appear to have a significant effect on improving important outcomes, such as PDA treatment failure, CLD, IVH, or mortality. The reduction of transient renal impairment does not outweigh the increased risk of NEC associated with the prolonged course. Based on these results, a prolonged course of indomethacin cannot be recommended for the routine treatment of PDA in preterm infants.
Implications for research There is a paucity of data on optimal dosing and duration of indomethacin therapy for the treatment of PDA, in particular for extremely low birth weight infants (ELBW) premature infants. It is likely that a single standard indomethacin regime is not the ideal for every premature infant. Therefore, individual patient response should be considered and evaluated, in particular in ELBW infants. Future randomized clinical trials should include this high risk population and investigate the effect of tailoring dose and duration of therapy to individual response in terms of echocardiographic findings and/or prostaglandin levels, focusing on clinically significant outcomes, including long‐term neurodevelopmental outcomes. In addition, factors that may influence treatment effect, such as birth weight, gestational age, age at the time of randomization, total fluid intake, feeding practice, and severity of PDA, need to be taken into account when designing such studies.
Plain language summary
Prolonged versus short course of indomethacin for the treatment of patent ductus arteriosus in preterm infants
The ductus arteriosus is a blood vessel that connects the aorta and pulmonary artery. The ductus arteriosus is normally present in the fetus. It allows the majority of the output of the right side of the heart to bypass the lungs and supply the body of the fetus and placenta in‐utero. In most term infants the patent ductus arteriosus (PDA) closes within days of birth, first by contraction of a muscular layer to achieve functional closure and then by endothelial remodeling. If the ductus arteriosus persists, blood is shunted from the aorta to the pulmonary circulation, which can cause overloading of the pulmonary circulation and reduced perfusion of the brain, gut and kidneys. In preterm infants, closure may be delayed or fail to occur, due in part to circulating vasodilatory prostaglandins. Indomethacin inhibits prostaglandin synthesis and it is used to treat PDA in preterm infants. Although indomethacin is successful in closing the PDA in the majority of cases, the ductus will re‐open in up to 35% of infants who initially respond to the drug and a more prolonged course of indomethacin has been studied to achieve higher rates of ductal closure. Important side effects of indomethacin include renal dysfunction, decreased platelet aggregation, and necrotizing enterocolitis (NEC). Where indomethacin fails, the ductus arteriosus may be surgically ligated if clinically indicated. Five randomized trials are included in this review. These studies were published between 1988 and 2000 and included a total of 431 preterm and low birth weight infants. Indomethacin was given intravenously in four trials and orally in one, in total amounts of 0.6 to 1.6 mg/kg for the prolonged course (six to eight doses) and 0.3 to 0.6 mg/kg for the short course (two to three doses). There was no significant benefit of prolonged indomethacin administration on failure of the PDA to close after completion of allocated treatment (four studies, 361 infants). Prolonged course of indomethacin compared to the short course did not reduce the rate of PDA re‐opening after initial closure (three studies, 322 infants), rate of PDA re‐treatment (five studies, 431 infants), or ligation rate (four studies, 310 infants). The prolonged course was associated with decreased incidence of renal function impairment (three studies, 318 infants). However, a prolonged indomethacin course increased the risk of NEC (four studies, 310 infants). The number of deaths was no different.
Background
The ductus arteriosus is a vascular connection between the fetal aorta and pulmonary artery. In‐utero, the ductus arteriosus allows the majority of the right ventricular output to bypass the high resistance pulmonary circulation and combine with the left ventricular output to supply the body and placenta. In most term infants the ductus arteriosus closes within days of birth, first by contraction of its muscular medial layer to achieve functional closure and then by endothelial ablation that leads to permanent anatomical closure (Hammerman 1995). In preterm infants closure may be delayed or fail to occur (Gersony 1983), due in part to increased circulating concentrations of and sensitivity to vasodilating prostaglandins (Brook 1995).
Persistent patent ductus arteriosus (PDA) leads to shunting of blood from the aorta to the pulmonary circulation as the pulmonary vascular resistance falls. Consequences of this "left‐to‐right" shunting are overloading of the pulmonary circulation and reduced perfusion of the brain, gut and kidneys. In preterm infants, clinical sequelae of the "ductal steal" of blood include a higher risk of adverse outcomes such as chronic lung disease (Rojas 1995), pulmonary hemorrhage (Kluckow 2000), renal hypoperfusion (Hammerman 1995), necrotizing enterocolitis and death (Cotton 1978).
The use of indomethacin, a prostaglandin synthetase inhibitor, to close the patent ductus arteriosus in premature infants was first reported in 1976 (Friedman 1976, Heymann 1976). Although indomethacin results in ductal closure in the majority of cases, it is ineffective in up to 40% of patients (Siassi 1976). In addition, in up to 35% of those infants who initially respond to the drug, the ductus will reopen (Gersony 1983). PDAs that fail to close with indomethacin may require surgical ligation (Clyman 1996).
Pharmacokinetic data indicate that preterm infants less than 1000 grams have lower indomethacin plasma levels compared to larger premature infants (Yeh 1991). It is speculated that this could be related to their higher volume of distribution, due in part to their relatively large extracellular fluid space and lower serum protein concentration (Yeh 1983). Furthermore, in preterm infants, the half‐life of indomethacin is longer in infants of lowest extrauterine age and levels are cumulative with repeated dosing (Yaffe 1980). Therefore, in this population, indomethacin is not without risk (Walters 1988). Important side effects include renal dysfunction, decreased platelet aggregation, and necrotizing enterocolitis.
It has been noted that prostaglandin levels rise 24 ‐ 72 hours after the last dose of indomethacin (Seyberth 1982). This coincides with the period of highest risk of PDA reopening. It has been suggested that permanent ductal closure is dependent on endothelial remodeling that occurs over a period of several days when the lumen is closed (Seyberth 1983). In most trials, short courses of indomethacin have been studied (Brook 1995). A prolonged course of indomethacin exposure may be more successful than a short course in achieving and maintaining ductal closure.
In the neonatal population, factors known to affect indomethacin blood levels include postnatal age, weight, gestational age, and route of administration. In addition, ductal size may influence the rate of closure after indomethacin is given.
Objectives
To determine the effect of a prolonged course of indomethacin (compared to a short course) on the rate of treatment failure without unwanted side‐effects in preterm infants with PDA.
Methods
Criteria for considering studies for this review
Types of studies
Randomized and quasi‐randomized controlled trials.
Types of participants
Preterm infants (less than 37 weeks of completed gestation) with a PDA diagnosed on clinical and / or echocardiographic examination. Planned subgroup analyses:
Gestational age: < 28 weeks versus 28 weeks and above
Birth weight: < 1000 g versus 1000 g and above
Postnatal age at commencement of treatment: < 1 wk of age versus 1 wk of age or greater
Symptomatology: asymptomatic (patency by cardiac echocardiogram/Doppler, but no attributable symptoms) versus symptomatic (hemodynamically significant PDA)
Route: oral versus intravenous
Total indomethacin dose: 0.6 mg/kg or less versus > 0.6 mg/kg
Types of interventions
Indomethacin treatment by any route given as a long course (four or more doses) versus a short course (defined as three or fewer doses).
Types of outcome measures
Primary outcome:
Failure of PDA to close after completion of allocated treatment.
Secondary outcomes:
PDA reopening after treatment (after initial closure with allocated treatment)
Need for re‐treatment for PDA (indomethacin and/or ligation) after completion of allocated treatment
PDA ligation after treatment
Mortality
Duration of assisted ventilation (defined as endotracheal tube ventilation or continuous positive airway pressure) (days)
Chronic lung disease at 36 weeks postmenstrual age (defined as need for supplemental oxygen at 36 weeks post‐menstrual age) (days)
Duration of supplemental oxygen dependence (days)
Intraventricular hemorrhage (Papile 1978): all IVH
Intraventricular hemorrhage (Papile 1978): severe IVH (grades 3 and 4)
Diminished urine output (< 1 ml/kg/hr)
Increased serum creatinine (> 0.10 micro mol/L)
Necrotizing enterocolitis as defined by Bell's criteria (Bell 1978)
Bleeding diathesis
Retinopathy of prematurity (defined by ICROP classification: any ROP and severe ROP stage 3 or worse)
Duration of hospital stay (days)
Chronic lung disease at 28 days of life (defined as need for supplemental oxygen at 28 days of life)
Pulmonary hemorrhage
Pneumothorax
Neurodevelopmental outcome
Thrombocytopenia (< 50,000 x 10^9/L)
Sepsis
Search methods for identification of studies
Studies were identified using the guidelines issued by the Cochrane Neonatal Review Group. The search included review of personal files and searches of the following electronic databases: MEDLINE (1966 to December 2006) using MeSH terms: Infant, Newborn (exp), Indomethacin (exp), EMBASE (Excerpta Medica online) (1974 to April 2006), and Oxford Database of Perinatal Trials, Cochrane Central Register of Controlled Trials (The Cochrane Library, Issue 4, 2006). No language restrictions were applied.
Data collection and analysis
Criteria and methods used to assess the methodological quality of the trials: Standard method of the Cochrane Collaboration and its Neonatal Group were used.
The three review authors independently assessed the quality of studies using the following criteria: blinding of randomization, blinding of intervention, completeness of follow‐up and blinding of the outcome measurement. Data were extracted independently by the three review authors, compared, and differences resolved.
RevMan 4.1 was used for data analysis. For categorical outcomes, results are presented as typical relative risk (RR) and typical risk difference (RD) with 95% CI's. The number needed to treat (NTT)/needed to harm (NTH) was calculated (1/RD) if there was a statistically significant effect using the RD. For continuous outcomes, weighted mean differences were used. The I squared statistic was used to detect heterogeneity of study results.
Results
Description of studies
Five randomized trials met the inclusion criteria: Hammerman 1990; Lee 2003; Rennie 1991; Rhodes 1988; and Tammela 1999. These studies included a total of 431 preterm infants and were performed in Israel, Singapore, U.K., U.S.A., and Finland. For specific details, refer to Table "Characteristics of Included Studies".
The inclusion criteria were not uniform. Hammerman 1990 included infants less than 1500 grams with clinically significant PDA confirmed by echocardiogram. Lee 2003 enrolled infants less than 1500 grams who had hemodynamically significant PDA greater than 1.5 mm diameter. Rennie 1991 studied infants less than 2500 grams with clinical signs of PDA without echocardiographic confirmation. Rhodes 1988 enrolled infants less than 1500 grams with umbilical artery catheters in place. The diagnosis of PDA was made using contrast precordial echocardiograms. Tammela 1999 included preterm infants less than 33 weeks gestation with a hemodynamically significant PDA and continuous left‐to‐right shunting diagnosed by echocardiography. Only three studies, Lee 2003, Rennie 1991, and Tammela 1999, reported data on infants at highest risk for treatment failure, i.e. those less than 28 weeks estimated gestational age and/or weighing less than 1000 at birth; however, these studies were not powered to detect differences in this group.
Indomethacin was given intravenously in all studies except in the Rhodes trial in which it was given orally. Only Lee 2003 and Hammerman 1990 reported the indomethacin preparation used (Indocid IV, Merck and Indocin, Merck Sharp & Dohme, respectively). The total amount of indomethacin for the prolonged course varied from 0.6 (Lee 2003 and Rennie 1991) to 1.6 mg/kg (Hammerman 1990), whereas for the short course it varied from 0.3 (Rhodes 1988) to 0.6 mg/kg (Hammerman 1990; Lee 2003; and Rennie 1991). The number of total doses varied from six (Lee 2003 and Rennie 1991) to eight (Hammerman 1990) for the prolonged course. All studies used three doses for the short course, except Rennie's trial which used two. The primary outcome assessments also varied: Hammerman 1990: failure of PDA to close after completion of the allocated treatment; Lee 2003: response to treatment defined by echocardiogram; Rennie 1991: response to treatment and relapse after treatment; Rhodes 1988: PDA reopening; and Tammela 1999: PDA closure rate. Not all the infants who did not respond to the allocated treatment or reopened their PDA afterwards were treated with either additional indomethacin or ligation. None of the studies report on specific criteria for retreatment of the PDA. One additional possibly eligible study was identified, VanOvermeire 2001. It was not included because it had been published only in abstract form at the time of completion of this review.
Risk of bias in included studies
The methodological quality of the included studies varied and elements of potential bias could not be completely ascertained from the published reports. In Hammerman's study, both the randomization and the intervention were blinded. Lee reported blinded randomization and echocardiographic outcome assessment, but the intervention was not described as blinded. Rennie had blinded randomization and adequate follow‐up; however, neither the intervention nor the outcome assessment were blinded. It is unclear if the studies by Rhodes and Tammela had blinded randomization. Rhodes trial had incomplete follow‐up and the outcome assessment was not blinded. Tammela's study had complete follow‐up, but it is unclear if the outcome assessment was blinded. The assessment details of individual studies are presented in the Table "Characteristics of Included Studies". Methodological quality was evaluated using the criteria described in "Effective Care of the Newborn Infant" (Sinclair 1992).
Effects of interventions
Prolonged versus short course of indomethacin for the treatment of patent ductus arteriosus in preterm infants (Comparison 01) Five randomized clinical trials met inclusion criteria. After discussion between the three review authors, there was agreement regarding the quality assessment and data extraction from these studies. They included a total of 431 preterm and/or LBW infants and reported at least one of the outcomes of interest for this systematic review. For details of results, see Tables of Analyses.
We detected statistical heterogeneity of treatment effect for outcomes relating to ductal closure, i.e., failure of PDA to close, need for re‐treatment, and PDA ligation. Heterogeneity was also observed for the outcomes mortality, CLD (defined as need for supplemental oxygen at 36 weeks postmenstrual age), IVH (all grades), NEC, and ROP. Pooled analyses for these outcomes should therefore be interpreted with caution.
Primary Outcome:
Failure of PDA to close after completion of allocated treatment (Outcome 01‐01): Four studies (including 361 infants) reported this outcome. Only the study by Tammela 1999 showed a statistically significant effect, with an increase in failure of the PDA to close after completion of the allocated treatment with a prolonged course of indomethacin [RR 5.17 (95% CI 1.23, 21.65); RD 0.27 (95% CI 0.08, 0.46)]. When all studies were combined there was no statistically significant difference in failure of PDA closure [typical RR 0.82 (95% CI 0.51, 1.33); typical RD ‐0.03 (95% CI ‐0.11, 0.04)].
Secondary Outcomes:
PDA reopening after treatment (after initial closure with allocated treatment) (Outcome 01‐02): Three studies (including 322 infants) reported this outcome. The overall effect was not statistically significant [typical RR was 0.63 (95% CI 0.39, 1.04) and typical RD was ‐0.08 (95% CI ‐0.16, 0.00)].
Need for re‐treatment for PDA (indomethacin and/or ligation) after completion of allocated treatment (Outcome 01‐03): Five studies (including 431 infants) reported this outcome. Hammerman 1990 showed a statistically significant reduction in the need for re‐treatment in patients who received the prolonged indomethacin course [(RR 0.21; 95% CI 0.05, 0.85); RD ‐0.37 (95% CI ‐0.63, ‐0.11)]. However, Tammela 1999 reported an increased incidence of re‐treatment with the prolonged course for RD (RD 0.24; 95% CI 0.01, 0.48). The overall analysis showed no significant effect of prolonged versus short indomethacin course on this outcome [typical RR 0.95 (95% CI 0.67, 1.34); typical RD ‐0.01 (95% CI ‐0.09, 0.7)].
PDA ligation after treatment (Outcome 01‐04): Four studies (including 310 infants) reported this outcome (Hammerman 1990; Lee 2003; Tammela 1999; Rhodes 1988). In the study of Tamella 1999, a prolonged course of indomethacin significantly increased the rate of PDA ligation rate [RR 4.65 (95% CI 1.09, 19.78)]. However, a prolonged course of indomethacin had the opposite effect in Hammerman's study [RR 0.14 (95% CI 0.02, 1.00)]. When all studies were combined, there was no statistically significant effect [typical RR 0.86 (95% CI 0.49, 1.51); typical RD ‐0.02 (95% CI ‐0.10, 0.05)] and a highly significant test for heterogeneity (p=0.002).
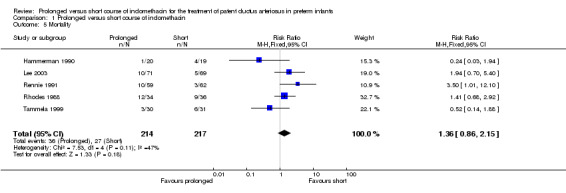
Mortality (Outcome 01‐05): All studies (including 431 infants) reported this outcome, but not all studies included specific data about timing of assessment. Only Rennie's study showed a statistically significant effect; an increase in mortality was associated with prolonged indomethacin [RR 3.5 (95% CI 1.01, 12.1); RD 0.12 (95% CI 0.01, 0.23). When all studies were combined there was no statistically significant effect on mortality [typical RR 1.36 (95% CI 0.86, 2.15); typical RD 0.05 (95% CI (‐0.02, 0.11)].
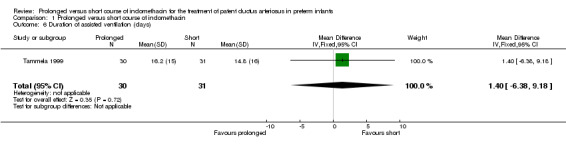
Duration of assisted ventilation (days on endotracheal tube ventilation or continuous positive airways pressure) (Outcome 01‐06): Only one study (including 61 infants) reported this outcome (Tammela 1999) and it showed no statistically significant difference. The mean difference was 1.4 days (95% CI ‐6.4, 9.2).
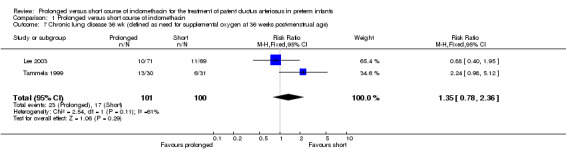
Chronic lung disease (defined as need for supplemental oxygen at 36 weeks postmenstrual age) (Outcome 01‐07): Two studies (including 201 infants) reported this outcome as defined above (Lee 2003; Tammela 1999). Tammela reported a borderline statistically significant effect, with the prolonged course increasing the risk of CLD [RR 2.24 (95% CI 0.98, 5.12); RD 0.24 (95% CI 0.01, 0.47); NNH 4 (2, 100)]. When both studies were combined, there was no statistically significant effect [typical RR 1.35 (95% CI 0.78, 2.36); typical RD 0.06 (95% CI ‐0.05, 0.17)]
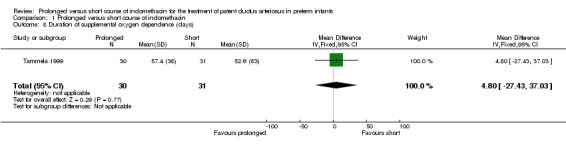
Duration of supplemental oxygen dependence (days) (Outcome 01‐08): Only one study (including 61 infants) reported mean values for this outcome (Tammela 1999). No statistically significant effect on duration of supplemental oxygen administration was noted. The mean difference was 4.8 days (95% CI ‐27.4, 37.0).
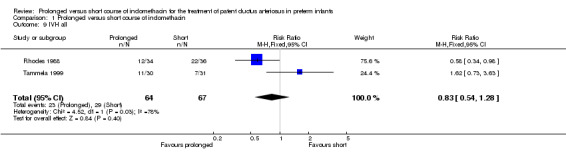
All intraventricular hemorrhage (defined by Papile 1978): all grades (Outcome 01‐09): Two studies (including 131 infants) reported on this outcome (Rhodes 1988; Tammela 1999). One study (Rhodes 1988) reported a decrease in all IVH with prolonged course of indomethacin [RR 0.58 (95% CI 0.34, 0.98); RD ‐0.26 (95% CI ‐0.48, ‐0.03). Overall, there was no significant effect of prolonged versus short course of indomethacin on this outcome [typical RR 0.83 (95% CI 0.54, 1.28); typical RD ‐0.07 (95% CI ‐0.23, 0.09)].
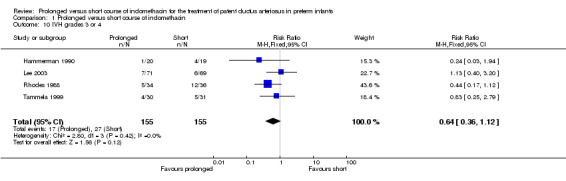
Severe intraventricular hemorrhage (defined by Papile 1978: grades 3 and 4) (Outcome 01‐10): Four studies (including 310 infants) reported on the incidence of severe IVH (Hammerman 1990; Lee 2003; Rhodes 1988; Tammela 1999). Overall, there was no statistically significant effect for this outcome [typical RR 0.64 (95% CI 0.36, 1.12); typical RD ‐0.06 (95% CI ‐0.14, 0.01].
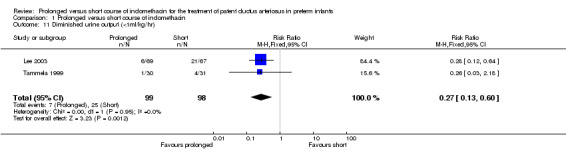
Diminished urine output (< 1 ml/kg/hr) (Outcome 01‐11): Two studies (including 197 infants) reported on this outcome (Lee 2003; Tammela 1999). There was a lower proportion of infants with decreased urine output with the prolonged indomethacin course when both studies were combined [typical RR 0.27 (95% CI 0.13, 0.6); typical RD ‐0.19 (95% CI ‐0.28, ‐0.09)].
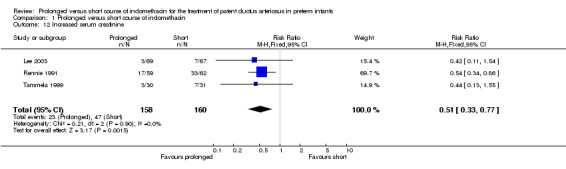
Increased serum creatinine (Outcome 01‐12): Three studies (including 318 infants) reported on increased serum creatinine: Lee 2003 (>140 Umol/L); Rennie 1991 (unspecified level); and Tammela 1999 (>150Umol/L). Rennie's study showed a significant difference, with a reduction in the proportion of infants having increased creatinine levels with the prolonged course of indomethacin [RR 0.54 (95% CI 0.34, 0.86); RD ‐0.24 (‐0.41, ‐0.07)]. The overall effect was also statistically significant [typical RR 0.51 (95% CI 0.33, 0.77); typical RD ‐0.14 (95% CI ‐0.23, ‐0.06); NNT 7 (4, 16)].
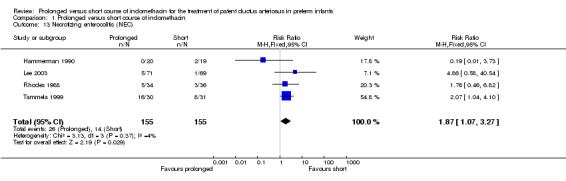
Necrotizing enterocolitis [defined by Bell's criteria (Bell 1978)] (Outcome 01‐13): Four studies (including 310 infants) reported on NEC (Hammerman 1990; Lee 2003; Rhodes 1988; Tammela 1999). Only Tammela found a statistically significant difference; an increased rate of NEC was noted with the prolonged course [RR 2.07 (95% CI 1.04, 4.1); RD 0.28 (95% CI 0.04, 0.51)]. When all the studies were combined there also was a statistically significant effect for this outcome [typical RR 1.87 (95% RR 1.07, 3.27); typical RD 0.08 (95% CI 0.01, 0.15), NNH 13 (7, 100)].
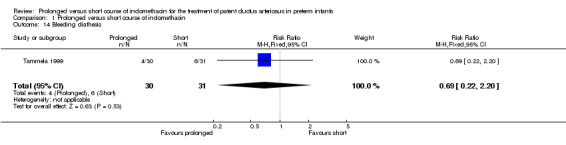
Bleeding diathesis (Outcome 01‐14): Only one study (Tammela 1999, including 61 infants) reported on this outcome, defined as "significant bleeding tendency". No significant difference was noted [RR 0.69 (95% CI 0.22, 2.20); RD ‐0.06 (‐0.24, 0.12)].
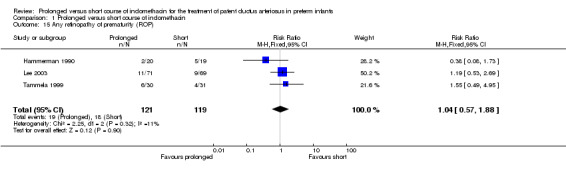
Retinopathy of prematurity (defined by ICROP classification: any ROP and severe ROP stage 3 or worse) (Outcome 01‐15): Three studies (including 240 infants) reported on this outcome (Hammerman 1990; Lee 2003; and Tammela 1999). Neither reported a significant difference in ROP. Overall, there was no statistically significant effect [typical RR 1.04 (95% CI 0.57, 1.88); typical RD 0.01 (95% CI ‐0.09, 0.1).
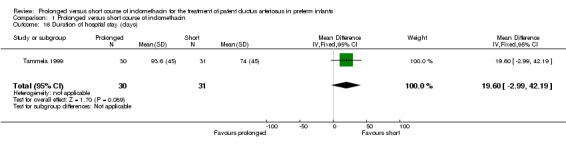
Duration of hospital stay (days) (Outcome 01‐16): Only one study (including 61 infants) reported on this outcome (Tammela 1999). It showed no significant effect on duration of hospital stay. The mean difference was 19.6 days (95% CI ‐3.0, 42.2).
Chronic lung disease (defined as need for supplemental oxygen at 28 days of life) (Outcome 01‐17): Only one study (including 140 infants) reported this outcome (Lee 2003). It showed no significant effect on CLD defined as the need for supplemental oxygen at 28 days of life [RR 0.97 (95% CI 0.58, 1.64); RD ‐0.01 (95% CI ‐0.16, 0.14)].
Pulmonary hemorrhage None of the studies reported this outcome.
Pneumothorax None of the studies reported this outcome.
Neurodevelopmental outcome None of the studies reported this outcome.
Thrombocytopenia None of the studies reported this outcome.
Sepsis None of the studies reported this outcome.
Discussion
This systematic overview revealed that a prolonged course of indomethacin compared to short course indomethacin treatment did not have a significant effect on PDA closure, re‐treatment, re‐opening, or ligation rates. The prolonged course of indomethacin was associated with an increased risk for NEC and decreased incidence of renal function impairment, as evidenced by a lower proportion of infants with oliguria and elevated creatinine level compared to the short treatment course.
We detected heterogeneity for treatment effect on the primary outcome, failure of PDA to close after allocated treatment, and for the following secondary outcomes: need for re‐treatment, PDA ligation, CLD (36 wk), IVH (all grades), NEC, and ROP. Possible sources for heterogeneity include differences in study patients' characteristics such as birth weight, gestational age, age at the time of randomization, and severity of PDA. Higher birth weight, more mature infants, earlier treatment, and small PDAs have all been associated with higher ductal closure rates and therefore may require fewer doses of indomethacin to achieve closure. Other plausible factors that could lead to increased effectiveness of indomethacin response, thereby decreasing the potential number of doses required to close the ductus, include exposure to antenatal steroids, fluid intake restriction, and the presence of respiratory distress (Qinn 2002; Itabashi 2003). Much of the heterogeneity seems to originate in the different direction of results reported by Hammerman 1990 and Tammela 1999. These two studies differ in the populations included (more mature and larger infants in Tammela's study) and methodology: Hammerman performed echocardiograms within 48 hours after completion of the seven day course of therapy and repeated it if the PDA murmur recurred; Tammela repeated echocardiograms in all patients on the third, ninth and fourteenth days after the first dose of indomethacin and when clinically indicated. The age at the first dose of indomethacin was younger for Tammela 1999 (three to four days) compared to Hammerman 1990 (nine to ten days). Also, in the study of Hammerman 1990 the intervention was blinded, whereas in the study of Tammela 1999 it was not, making the latter at much higher risk for bias. Another potentially important cause of this reported heterogeneity is the different overall doses and administration routes used in the included studies. Indeed the study that used the highest cumulative dose, Hammerman 1990 (1.6 mg/kg, IV), had the most benefit for prolonged course indomethacin when compared to Tammela 1999 (0.7 mg/kg, IV), Rhodes 1988 (0.8 mg/kg, PO), and both Lee 2003 and Rennie 1991 (0.6 mg/kg, IV). This finding should be interpreted with caution given the limited number of patients. Furthermore, most studies do not report the dosing strategy used for re‐treatment with indomethacin. In the study of Rhodes 1988, infants in whom the ductus reopened received a second two‐dose course of indomethacin, whereas in the study of Lee 2003, infants were treated with a second course (same dosing regimen as the first) when the PDA failed to close or relapsed after the first course. Regarding the heterogeneity seen with the NEC outcome, another feasible source are the particular feeding practices during treatment, as there may be a higher risk for NEC associated with feeding during indomethacin treatment secondary to potential bowel ischemia. The only study that addressed this issue is Lee 2003, reporting that feedings were not withheld during treatment with indomethacin.
Sub‐group analyses was planned to determine whether responses differed according to gestational age (< 28 weeks vs. 28 weeks and above), birth weight (< 1000 g vs. 1000 g and above), post‐natal age at commencement of treatment (< 1 week vs. 1 week or greater), asymptomatic (patency by cardiac echocardiogram/doppler, but no attributable symptoms) vs. symptomatic (hemodynamically significant) PDA, oral vs. IV route, and total indomethacin dose (0.6 mg/kg or less vs. > 0.6 mg/kg). However, these were not possible because data were not provided regarding gestational age, birth weight, and hemodynamic significance for most study patients. Furthermore, the cumulative dose for the prolonged indomethacin course was 0.6 mg/kg or more for all included studies.
Finally, it is likely that a single standard indomethacin regime may be not the ideal for every premature infant (Shaffer 2002). Therefore, individual patient response should be considered and evaluated, in particular in the high risk group of extremely low birth weight babies.
Authors' conclusions
Implications for practice.
Prolonged indomethacin course does not appear to have a significant effect on improving important outcomes, such as PDA treatment failure, CLD, IVH, or mortality. The reduction of transient renal impairment does not outweigh the increased risk of NEC associated with the prolonged course. Based on these results, prolonged course of indomethacin cannot be recommended for the routine treatment of PDA in preterm infants.
Implications for research.
There is a paucity of data on optimal dosing and duration of indomethacin therapy for the treatment of PDA, in particular for extremely low birth weight infants (ELBW) premature infants. It is likely that a single standard indomethacin regime is not the ideal for every premature infant. Therefore, individual patient response should be considered and evaluated, in particular in ELBW infants. Future randomized clinical trials should include this high risk population and investigate the effect of tailoring dose and duration of therapy to individual response in terms of echocardiographic findings and/or prostaglandin levels, focusing on clinically significant outcomes, including long‐term neurodevelopmental outcomes. In addition, factors that may influence treatment effect, such as birth weight, gestational age, age at the time of randomization, total fluid intake, feeding practice, and severity of PDA, need to be taken into account when designing such studies.
What's new
| Date | Event | Description |
|---|---|---|
| 3 November 2008 | Amended | Converted to new review format. |
History
Protocol first published: Issue 1, 2002 Review first published: Issue 1, 2004
| Date | Event | Description |
|---|---|---|
| 1 December 2006 | New citation required and conclusions have changed | Substantive amendment |
Data and analyses
Comparison 1. Prolonged versus short course of indomethacin.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Failure of PDA to close after completion of allocated treatment | 4 | 361 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.82 [0.51, 1.33] |
| 2 PDA reopening after treatment (after initial closure with allocated treatment) | 3 | 322 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.63 [0.39, 1.04] |
| 3 Need for re‐treatment for PDA (indomethacin and/or ligation) after completion of allocated treatment | 5 | 431 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.95 [0.67, 1.34] |
| 4 PDA ligation after treatment | 4 | 310 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.86 [0.49, 1.51] |
| 5 Mortality | 5 | 431 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.36 [0.86, 2.15] |
| 6 Duration of assisted ventilation (days) | 1 | 61 | Mean Difference (IV, Fixed, 95% CI) | 1.40 [‐6.38, 9.18] |
| 7 Chronic lung disease 36 wk (defined as need for supplemental oxygen at 36 weeks post‐menstrual age) | 2 | 201 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.35 [0.78, 2.36] |
| 8 Duration of supplemental oxygen dependence (days) | 1 | 61 | Mean Difference (IV, Fixed, 95% CI) | 4.80 [‐27.43, 37.03] |
| 9 IVH all | 2 | 131 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.83 [0.54, 1.28] |
| 10 IVH grades 3 or 4 | 4 | 310 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.64 [0.36, 1.12] |
| 11 Diminished urine output (<1ml/kg/hr) | 2 | 197 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.27 [0.13, 0.60] |
| 12 Increased serum creatinine | 3 | 318 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.51 [0.33, 0.77] |
| 13 Necrotizing enterocolitis (NEC) | 4 | 310 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.87 [1.07, 3.27] |
| 14 Bleeding diathesis | 1 | 61 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.69 [0.22, 2.20] |
| 15 Any retinopathy of prematurity (ROP) | 3 | 240 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.04 [0.57, 1.88] |
| 16 Duration of hospital stay (days) | 1 | 61 | Mean Difference (IV, Fixed, 95% CI) | 19.60 [‐2.99, 42.19] |
| 17 Chronic lung disease 28d (defined as need for supplemental oxygen at 28 days of life) | 1 | 140 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.97 [0.58, 1.64] |
1.1. Analysis.
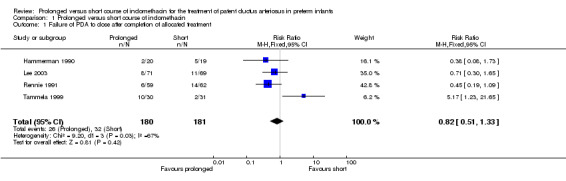
Comparison 1 Prolonged versus short course of indomethacin, Outcome 1 Failure of PDA to close after completion of allocated treatment.
1.2. Analysis.
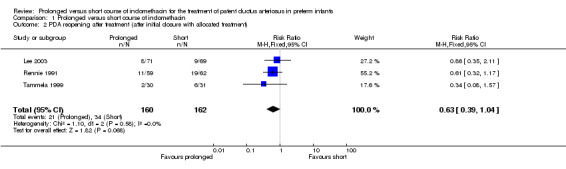
Comparison 1 Prolonged versus short course of indomethacin, Outcome 2 PDA reopening after treatment (after initial closure with allocated treatment).
1.3. Analysis.
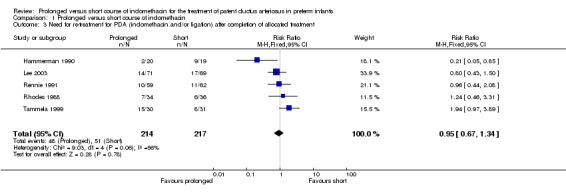
Comparison 1 Prolonged versus short course of indomethacin, Outcome 3 Need for re‐treatment for PDA (indomethacin and/or ligation) after completion of allocated treatment.
1.4. Analysis.
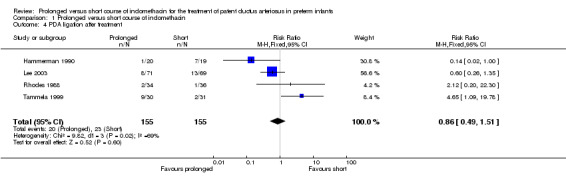
Comparison 1 Prolonged versus short course of indomethacin, Outcome 4 PDA ligation after treatment.
1.5. Analysis.
Comparison 1 Prolonged versus short course of indomethacin, Outcome 5 Mortality.
1.6. Analysis.
Comparison 1 Prolonged versus short course of indomethacin, Outcome 6 Duration of assisted ventilation (days).
1.7. Analysis.
Comparison 1 Prolonged versus short course of indomethacin, Outcome 7 Chronic lung disease 36 wk (defined as need for supplemental oxygen at 36 weeks post‐menstrual age).
1.8. Analysis.
Comparison 1 Prolonged versus short course of indomethacin, Outcome 8 Duration of supplemental oxygen dependence (days).
1.9. Analysis.
Comparison 1 Prolonged versus short course of indomethacin, Outcome 9 IVH all.
1.10. Analysis.
Comparison 1 Prolonged versus short course of indomethacin, Outcome 10 IVH grades 3 or 4.
1.11. Analysis.
Comparison 1 Prolonged versus short course of indomethacin, Outcome 11 Diminished urine output (<1ml/kg/hr).
1.12. Analysis.
Comparison 1 Prolonged versus short course of indomethacin, Outcome 12 Increased serum creatinine.
1.13. Analysis.
Comparison 1 Prolonged versus short course of indomethacin, Outcome 13 Necrotizing enterocolitis (NEC).
1.14. Analysis.
Comparison 1 Prolonged versus short course of indomethacin, Outcome 14 Bleeding diathesis.
1.15. Analysis.
Comparison 1 Prolonged versus short course of indomethacin, Outcome 15 Any retinopathy of prematurity (ROP).
1.16. Analysis.
Comparison 1 Prolonged versus short course of indomethacin, Outcome 16 Duration of hospital stay (days).
1.17. Analysis.
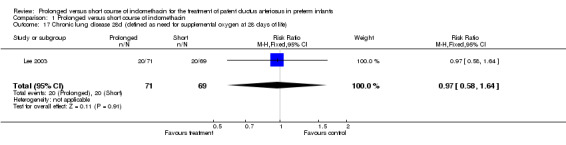
Comparison 1 Prolonged versus short course of indomethacin, Outcome 17 Chronic lung disease 28d (defined as need for supplemental oxygen at 28 days of life).
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Hammerman 1990.
| Methods | Single centre, Israel (dates not given), randomized, double‐blind, placebo‐controlled trial. I. Blinding of randomization: ‐ yes (pharmacist responsible for random assignment) II. Blinding of intervention: yes III. Complete follow‐up: yes IV. Blind outcome assessment: ‐ yes | |
| Participants | 39 premature VLBW infants (< 1500 grams). BW (mean +/‐ SD) 1099 +/‐ 435 g, GA 28 +/‐ 3 wk, age at indomethacin therapy 9 +/‐ 4 days (indomethacin group) versus BW 1040 +/‐ 394 g, GA 27 +/‐ 7 wk, age at indomethacin therapy 10 +/‐ 5 days (placebo group). Diagnosis of clinically significant PDA was made by clinicians (murmur plus any two of the following: bounding pulses, diastolic pressure equal or less than 25 mmHg, cardiomegaly or pulmonary plethora on chest X‐ray) and confirmed by echocardiogram (M mode and pulsed Doppler). Exclusion criteria: older than 21 days postnatal age, platelet count < 80,000/uL, renal failure, persistent fetal circulation, any other congenital heart disease. | |
| Interventions | Initially, all infants received standard indomethacin therapy (3 doses of 0.2 mg/kg/dose IV every 12 hours). Prolonged course: additional 0.2 mg/kg/day IV for 5 days (n=20). Short course: equivalent volume of placebo (sterile water) for 5 days (n=19). Infants received first dose of indomethacin at a mean age of 9‐10 days. Initial and subsequent dosages of indomethacin equaled 1 week of therapy. Alveolar‐arterial gas pressure difference, "cardiovascular distress score" were calculated. Measurements of 6‐keto‐prostaglandin F1 alpha were also done. Second echocardiogram was done within 48 hours after completion of the 7‐day course therapy. If PDA murmur recurred, a repeat echocardiogram was also done. | |
| Outcomes | Primary: decrease in the incidence of surgical ligation Other: non‐response to treatment, PDA re‐opening, prostaglandin levels, renal function, ROP, NEC, IVH, BPD (outcome assessed, but no definitions given), oxygen therapy at home, death. | |
| Notes | Re‐treatment after intervention included both ligation and indomethacin (dosing not reported) | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Low risk | A ‐ Adequate |
Lee 2003.
| Methods | Single centre, Singapore (April 1988 to July 2000), randomized controlled trial. I. Blinding of randomization: ‐yes (computer program). Stratified blocked randomization was used with birth weight (<1000 g and 1000‐1499 g) as the stratification factor. II. Blinding of intervention: ‐no III. Complete follow‐up: ‐yes (plan to f/u neurodevelopmental outcome at 2 years of age) IV. Blind outcome assessment: ‐yes, diagnostic echocardiograms were blinded. | |
| Participants | 140 VLBW (<1500 g) preterm infants who had a hemodynamically significant PDA, defined as the presence of any of the following: systolic murmur, hyperactive precordium, wide pulse pressure (diastolic pressure less than half of systolic), hypotension, apnea or rising pCO2, plus a PDA >1.5mm in diameter on a 2‐dimensional echocardiogram. Infants were excluded if they had any major congenital malformation or NEC. | |
| Interventions | Prolonged course: 6‐day course indomethacin (Indocid IV, Merck, West Point, PA): 0.1 mg/kg/dose IV every 24 hours) (n=71). Short course: 0.2 mg/kg per dose every 12 hours for three doses (n=69). If the ductus failed to close or relapsed after the first course and was hemodynamically significant, a second course was given after an interval of at least 48 hours from the last dose. The dosing regimen of the second course was the same as that for the first. Surgical ligation was conducted if both courses failed. Enrolled infants were followed up until death or discharge from the hospital. To document response scans were repeated 24 to 72 hours after the last dose of the drug. Additional scans were done at the discretion of the physician in charge. | |
| Outcomes | Primary: response to the first indomethacin course. It was defined echocardiographically as: 1) closed: ductus was closed (without relapse) and needed no further treatment; 2) open: ductus still open and hemodynamically significant; and 3) relapsed: ductus relapsed and was hemodynamically significant. Failure of response means that the ductus was either open or relapsed after treatment. Secondary: need for a second course of indomethacin, number of surgical ligations, and anticipated side effects: oliguria, increased serum creatinine, decreased serum sodium, brownish gastric aspirates treated with ranitidine, fresh gastric bleeding, and focal gastrointestinal perforation. Side effects were considered to be attributable to indomethacin if occurred within a week of starting treatment. Other reported outcomes: ROP (Stage III), NEC (Stage II/III), CLD (supplemental oxygen at 28 days and 36 weeks corrected gestational age), IVH (Grades III, IV). | |
| Notes | Data on potential confounder parameters were also collected: daily fluid intake for the first two weeks, surfactant use, ventilator parameters, pulmonary pathology, antenatal steroids, and culture‐proven sepsis. Feedings were started early and were not withheld during indomethacin treatment. The duration of supplemental oxygen was reported as the median, not the mean value. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Low risk | A ‐ Adequate |
Rennie 1991.
| Methods | Two centres, UK. (September 1985 to July 1989), randomized controlled trial without the use of a placebo. I. Blinding of randomization: ‐ yes (sealed envelopes) II. Blinding of intervention: no III. Complete follow‐up: yes IV. Blind outcome assessment: no | |
| Participants | 121 infants weighing less than 2,500 grams at birth with clinical signs of persistent ductus arteriosus who required indomethacin treatment. BW (mean +/‐ SD) 1116 +/‐ 340 g, GA 27 +/‐ 2.2 wk, age at indomethacin therapy: < 7 days(n = 32), >/= 7 days (n = 27 days) (prolonged low dose group) versus BW 1135 +/‐ 340 g, GA 27 +/‐ 2.2 wk, age at indomethacin therapy: < 7 days(n = 39), >/= 7 days (n = 23 days) (conventional dose group) | |
| Interventions | Prolonged course: 6‐ day course (0.1 mg/kg/dose IV) (n=59). Short course: 3 doses at intervals of 12 hours of 0.2 mg/kg IV (n=62). The course was completed unless the treatment was stopped because of a complication. | |
| Outcomes | Primary: response to treatment (PDA closure) Other: relapse after treatment (PDA re‐opening), retreatment, rise in serum urea or creatinine concentration, reduction of inspired oxygen at 6 days, death, side effects (GI hemorrhage, GI perforation, bleeding, acute renal failure). | |
| Notes | Echocardiography was not available in either centre at the time of this study. Relapse was defined as a recurrence of systolic murmur and increased pulse volume. Indomethacin dosage for retreatment was not specified. There were a total of three PDA ligations, but treatment allocation was not clear. Same total dose of indomethacin was given in two different ways. Increased mortality from BPD with prolonged course could be explained by the significantly higher baseline oxygen requirement in this group. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Low risk | A ‐ Adequate |
Rhodes 1988.
| Methods | Single centre, randomized controlled trial without the use of a placebo. 13‐ month study period (dates not given), USA. I. Blinding of randomization: ‐unclear II. Blinding of intervention: no III. Complete follow‐up: ‐ no (see notes) IV. Blind outcome assessment: no | |
| Participants | 70 VLBW (<1500 g) preterm infant with severe respiratory distress (more than 50% FiO2 and UAC in place) and left to right shunting through the PDA determined by contrast echocardiogram on the first day of life. BW (mean +/‐ SD) 975 +/‐ 234 g, GA 27 +/‐ 2.3 wk (maintenance group) versus BW 972+/‐ 245 g, GA 27 +/‐ 2.2 wk (non‐maintenance group). All infants entered the study within the first 24 hours of life and most infants did not have PDA symptomatology at this time. Exclusion criteria: serum creatinine > 2 mg/dl, oliguria, bleeding diathesis, indirect bilirubin > 12 mg/dl, platelet count < 60,000/uL, any congenital anomalies. | |
| Interventions | Initially, all infants received 2 oral doses of indomethacin at a dose of 0.15 mg/kg given 12 hours apart. Prolonged course: oral indomethacin for 5 additional days (0.1 mg/kg) (n=34). Short course: no additional indomethacin (n=36). A follow‐up contrast echocardiogram was done within 24 hours after receiving the first 2 doses of indomethacin. Each of the infants with an initially closed PDA was followed for ductal reopening. Infants were followed clinically daily by one of the investigators (not blinded) until discharge for ductal re‐opening signs. Cardiology follow ‐up (blinded) with echocardiography (left atrial size) was done if left to right shunting through the ductus was suspected. | |
| Outcomes | Primary: re‐opening of PDA Other: length of oxygen requirement, NEC, IVH, VP shunt placement, mortality, length of stay | |
| Notes | Oral administration Looked at re‐opening rates once PDA was shown to be closed after the first 2 doses; contrast echocardiogram was not done to assess closure rates after the intervention (umbilical catheter not longer clinically indicated). Contrast echocardiograms (Doppler not available during study period). Only the infants who had their ductus closed by contrast echocardiogram after the first 2 doses of indomethacin were followed for ductal reopening (there are no data concerning the infants whose ductus did not close at this time). Indomethacin was used in a near prophylactic manner, i.e. echocardiograms were performed at 24 hours of life, when ductal patency would be considered to be physiological. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Unclear risk | B ‐ Unclear |
Tammela 1999.
| Methods | Two centres, Finland (dates not given), randomized controlled trial without the use of a placebo. I. Blinding of randomization: ‐unclear II. Blinding of intervention: no III. Complete follow‐up: yes IV. Blind outcome assessment: no | |
| Participants | 61 premature infants (24 to 32 weeks estimated GA). BW (mean +/‐ SD) 1094 +/‐ 298 g, GA 27.3 +/‐ 1.9 wk, age at indomethacin therapy 3.1 +/‐ 1.7 days (prolonged low dose group) versus BW 1154 +/‐ 388 g, GA 27.9 +/‐ 2.3 wk, age at indomethacin therapy 4.3 +/‐ 4.4 days (short standard dose group). Infants had hemodynamically significant PDA (at least 3 of 6 clinical signs: heart murmur, increased precordial impulse, bounding pulses, tachycardia, unexplained deterioration of the respiratory status, and cardiomegaly or pulmonary edema by chest X‐ray ) with continuous left to right shunt by echocardiography. Indomethacin was considered the treatment of choice by the attending physician. Exclusion criteria: ductal‐dependent heart defect, pulmonary hypertension or bidirectional shunt, oliguria (urine output < 1 ml/kg/h for more than 6 hours), platelet count <60 x 10^9/L or bleeding diathesis, bilirubin level > 200 umol/L (11.7 mg/dL), clinical or radiologic evidence for NEC. | |
| Interventions | Prolonged course: IV indomethacin at 0.1 mg/kg/dose every 24 hours for 7 days (n=30). Short course: 3 doses of IV indomethacin at 12 hour intervals (0.2, 0.1, and 0.1 mg/kg/dose) (n=31). Infants received first dose of indomethacin at a mean age of 3‐4 days. Echocardiograms were obtained for all infants with clinical signs of a PDA. To detect silent PDAs, echocardiography was used for all infants treated with ventilators daily during the first 3 to 4 days of life and later in cases with an increased need for ventilatory support. Echocardiography was done 3, 9, and 14 days after the first dose of indomethacin was administered and in all cases where an unsuccessful ductal closure or PDA reopening was suspected on clinical evaluation. Side effects were also monitored. | |
| Outcomes | Primary: ductal closure confirmed by echocardiogram on the ninth day after first dose of indomethacin Secondary: PDA reopening, retreatment with indomethacin, surgical ligation, mortality, BPD (oxygen requirement and CXR changes at 36 wk PCA), ROP, IVH, cystic PVL, duration of assisted ventilation, oxygen supplementation, inotropics use, length of hospital stay, and adverse events (urine output < 1 ml/kg/h, creatinine > 150 umol/L, BUN > 3.6 mmol/L, bleeding diathesis, thrombocytopenia (<60 x 10^9/L), GI hemorrhage, NEC, severe adverse event (at least one of the following: severe bleeding, symptomatic oliguria, NEC with intestinal perforation). | |
| Notes | Echocardiograms were classified in 2 categories: presence or absence of a clinically and hemodynamically significant PDA requiring treatment. Retreatment (doses not specified) with indomethacin was given when the PDA reopened. Final closure rates were better for those infants who received the short course initially. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Unclear risk | B ‐ Unclear |
Contributions of authors
Each reviewer did the literature search, evaluated the trials for quality, and extracted the data independently. JRH entered the data and CMH wrote the text with input from the other two authors.
Sources of support
Internal sources
University of Washington, USA.
Murdoch Childrens Research Institute, Australia.
Royal Women's Hospital, Melbourne, Australia.
External sources
National Institutes of Health 1‐K23 HD44791, USA.
National Health and Medical Research Council, Australia.
Declarations of interest
None
Edited (no change to conclusions)
References
References to studies included in this review
Hammerman 1990 {published data only}
- Hammerman C, Aramburo MJ. Prolonged indomethacin therapy for the prevention of recurrences of patent ductus arteriosus. Journal of Pediatrics 1990;117:771‐6. [DOI] [PubMed] [Google Scholar]
Lee 2003 {published data only}
- Lee J, Rajadurai VS, Tan KW, Wong KY, Wong EH, Leong JY. Randomized trial of prolonged low‐dose versus conventional‐dose indomethacin for treating patent ductus arteriosus in very low birth weight infants. Pediatrics 2003;112:345‐50. [DOI] [PubMed] [Google Scholar]
Rennie 1991 {published data only}
- Rennie JM, Cooke RW. Prolonged low dose indomethacin for persistent ductus arteriosus of prematurity. Archives of Disease in Childhood 1991;66:55‐8. [DOI] [PMC free article] [PubMed] [Google Scholar]
Rhodes 1988 {published data only}
- Rhodes PG, Ferguson MG, Reddy NS, Joransen JA, Gibson J. Effects of prolonged versus acute indomethacin therapy in very low birth‐weight infants with patent ductus arteriosus. European Journal of Pediatrics 1988;147:481‐4. [DOI] [PubMed] [Google Scholar]
Tammela 1999 {published data only}
- Tammela O, Ojala R, Iivainen T, Lautamatti L, Pokela M, Janas M, et al. Short versus prolonged indomethacin therapy for patent ductus arteriosus in preterm infants. Journal of Pediatrics 1999;134:552‐7. [DOI] [PubMed] [Google Scholar]
References to studies awaiting assessment
VanOvermeire 2001 {published data only}
- Overmeire B, Rienstra M, Groore K. Short versus prolonged indomethacin therapy for retreatment of patent ductus arteriosus in preterm infants. Pediatric Research 2001;49:375 A. [Google Scholar]
Additional references
Bell 1978
- Bell MJ, Ternberg JL, Feigin RD, Keating JP, Marshall R, Barton L, Brotherton T. Neonatal necrotizing enterocolitis. Therapeutic decisions based upon clinical staging. Annals of Surgery 1978;187:1‐7. [DOI] [PMC free article] [PubMed] [Google Scholar]
Brook 1995
- Brook M, Heymann M. Patent ductus arteriosus. In: Emmanouilides GC, Riemenschneider TA, Allen HD, Gutgesell HP editor(s). Heart Disease in Infants, Children, and Adolescents Including the Fetus and Young Adult. Williams & Wilkins, 1995:746‐4. [Google Scholar]
Clyman 1996
- Clyman RI. Recommendations for the postnatal use of indomethacin: an analysis of four separate treatment strategies. Journal of Pediatrics 1996;128:601‐7. [DOI] [PubMed] [Google Scholar]
Cotton 1978
- Cotton RB, Stahlman MT, Kovar I, Catterton WZ. Medical management of small preterm infants with symptomatic patent ductus arteriousus. Journal of Pediatrics 1978;92:467‐73. [DOI] [PubMed] [Google Scholar]
Friedman 1976
- Friedman WF, Hirschklau MJ, Printz MP, Pitlick PT, Kirkpatrick SE. Pharmacologic closure of patent ductus arteriosus in the premature infant. New England Journal of Medicine 1976;295:526‐9. [DOI] [PubMed] [Google Scholar]
Gersony 1983
- Gersony WM, Peckham GJ, Ellison RC, Miettinen OS, Nadas AS. Effects of indomethacin on premature infants with patent ductus arteriosus: results of a national collaborative study. Journal of Pediatrics 1983;102:895‐906. [DOI] [PubMed] [Google Scholar]
Hammerman 1995
- Hammerman C. Patent ductus arteriosus. Clinical relevance of prostaglandins and prostaglandin inhibitors in PDA pathophysiology and treatment. Clinics in Perinatology 1995;22:457‐79. [PubMed] [Google Scholar]
Heymann 1976
- Heymann MA, Rudolph AM, Silverman NH. Closure of the ductus arteriosus in premature infants by inhibition of prostaglandin synthesis. New England Journal of Medicine 1976;295:530‐3. [DOI] [PubMed] [Google Scholar]
Itabashi 2003
- Itabashi K, Ohno T, Nishida H. Indomethacin responsiveness of patent ductus arteriosus and subsequent renal abnormalities in preterm infants treated with indomethacin. Journal of Pediatrics 2003;143:203‐7. [DOI] [PubMed] [Google Scholar]
Kluckow 2000
- Kluckow M, Evans N. Ductal shunting, high pulmonary blood flow and pulmonary hemorrhage. Journal of Pediatrics 2000;137:68‐72. [DOI] [PubMed] [Google Scholar]
Papile 1978
- Papile LA, Burstein J, Burstein R, Koffler H. Incidence and evolution of subependymal and intraventricular hemorrhage: a study of infants with birth weights less than 1,500 gm. Journal of Pediatrics 1978;92:529‐34. [DOI] [PubMed] [Google Scholar]
Qinn 2002
- Quinn D, Cooper B, Clyman RI. Factors associated with permanent closure of the ductus arteriosus: a role for prolonged indomethacin therapy. Pediatrics 2002;110:e10. [DOI] [PubMed] [Google Scholar]
Rojas 1995
- Rojas M, Gonzalez A, Bancalari E, Claure N, Poole C, Silva‐Neto G. Changing trends in the epidemiology and pathogenesis of neonatal chronic lung disease. Journal of Pediatrics 1995;126:605‐10. [DOI] [PubMed] [Google Scholar]
Schmidt 2001
- Schmidt B, Davis P, Moddemann D, Ohlsson A, Roberts R, Saigal S, et al. Long‐term effects of indomethacin prophylaxis in extremely‐low‐birth‐weight infants. New England Journal of Medicine 2001;344:1966‐72. [DOI] [PubMed] [Google Scholar]
Seyberth 1982
- Seyberth HW, Muller H, Wille L, Pluckthun H, Wolf D, Ulmer HE. Recovery of prostaglandin production associated with reopening of the ductus arteriosus after indomethacin treatment in preterm infants with respiratory distress syndrome. Pediatric Pharmacology 1982;2:127‐41. [PubMed] [Google Scholar]
Seyberth 1983
- Seyberth HW, Knapp G, Wolf D, Ulmer HE. Introduction of plasma indomethacin level monitoring and evaluation of an effective threshold level in very low birth weight infants with symptomatic patent ductus arteriosus. European Journal of Pediatrics 1983;141:71‐6. [DOI] [PubMed] [Google Scholar]
Shaffer 2002
- Shaffer CL, Gal P, Ransom JL, Carlos RQ, Smith MS, Davey AM, Dimaguila MA, Brown YL, Schall SA. Effect of age and birth weight on indomethacin pharmacodynamics in neonates treated for patent ductus arteriosus. Critical Care Medicine 2002;30:343‐8. [DOI] [PubMed] [Google Scholar]
Siassi 1976
- Siassi B, Blanco C, Cabal L, Coran A. Incidence and clinical features of patent ductus arteriosus in low birthweight infants: a prospective analysis of 150 consecutively born infants. Pediatrics 1976;57:347‐51. [PubMed] [Google Scholar]
Sinclair 1992
- Sinclair JC, Bracken MB. Effective care of the newborn infant. Oxford, Oxford University Press, 1992. [Google Scholar]
Walters 1988
- Walters M. Tolerance of intravenous indomethacin treatment for premature infants with patent ductus arteriosus. BMJ 1988;297:773‐4. [DOI] [PMC free article] [PubMed] [Google Scholar]
Yaffe 1980
- Yaffe SJ, Friedman WF, Rogers D, Lang P, Ragni M, Saccar C. The disposition of indomethacin in preterm babies. Journal of Pediatrics 1980;97:1001‐6. [DOI] [PubMed] [Google Scholar]
Yeh 1983
- Yeh T, Luken J, Raval D, Thalji A, Carr I, Pildes R. Indomethacin treatment in small versus large premature infants with ductus arteriosus. Comparison of plasma indomethacin concentration and clinical response. British Heart Journal 1983;50:27‐30. [DOI] [PMC free article] [PubMed] [Google Scholar]
Yeh 1991
- Yeh TF, Carr I. Pharmacologic closure of ductus arteriosus. Drug Therapy in the Neonate and Small Infant. 2nd Edition. Chicago: Year Book, 1991:123‐38. [Google Scholar]
References to other published versions of this review
Herrera 2004
- Herrera C, Holberton J, Davis P. Prolonged versus short course of indomethacin for the treatment of patent ductus arteriosus in preterm infants. Cochrane Database of Systematic Reviews 2004, Issue 1. [DOI] [PubMed] [Google Scholar]


