Abstract
Background
Elevated blood pressure among adolescents has been shown to be associated with future adverse cardiovascular outcomes and early onset diabetes. Most data regarding systolic and diastolic blood pressure trends are based on surveys of selected populations within 10–20-year periods. The goal of this study was to characterize the secular trend of blood pressure given the rising prevalence of adolescent obesity.
Methods
This nationwide population-based study included 2,785,515 Israeli adolescents (41.6% females, mean age 17.4 years) who were medically evaluated and whose weight, height and blood pressure were measured, prior to mandatory military service between 1977 and 2020. The study period was divided into 5-year intervals. Linear regression models were used to describe the P for trend along the time intervals. Analysis of covariance was used to calculate means of blood pressure adjusted for body mass index.
Results
During the study period, the mean body mass index increased by 2.1 and 1.6 kg/m2 in males and females, respectively (P for trend < 0.001 in both sexes). The mean diastolic blood pressure decreased by 3.6 mmHg in males and by 2.9 mmHg in females (P < 0.001 in both sexes). The mean systolic blood pressure increased by 1.6 mmHg in males and decreased by 1.9 mmHg in females. These trends were also consistent when blood pressure values were adjusted to body mass index.
Conclusion
Despite the increase in body mass index over the last four decades, diastolic blood pressure decreased in both sexes while systolic blood pressure increased slightly in males and decreased in females.
Supplementary Information
The online version contains supplementary material available at 10.1186/s12933-021-01433-0.
Keywords: Systolic blood pressure, Diastolic blood pressure, Hypertension, Body Mass Index, Obesity, Adolescents
Background
Elevated blood pressure (BP) among the general population is a leading contributor to cardiovascular morbidity and mortality [1, 2]. A number of studies have reported associations of adolescent elevated BP with adult hypertension and with renal and cardiovascular morbidity and mortality later in life [3–6]. Furthermore, adolescent elevated BP was found to promote early onset type 2 diabetes mellitus [5].
Weight status is a key determinant of BP in adolescents [7]. Consistent increases have been shown in childhood and adolescent obesity in most Western countries [8, 9], including Israel and the US, where adolescent obesity has nearly tripled over the last decades [10, 11]. Most relevant studies in Western countries reported decreases over the years in diastolic BP (DBP), and to a lesser extent, in systolic BP (SBP) [12–18]. Those studies were based on relatively small samples of wide age range, including both pediatric and adolescent populations. The aim of this study was to describe secular trends during 44 years, of measured SBP and DBP, while accounting for the trend in body mass index (BMI), among 2.78 million adolescents screened at age 17 years.
Methods
Databases and study population
One year prior to mandatory military service, usually at age 16–19 years, male and female Israeli adolescents are called to regional conscription centers for medical evaluation. The current study comprised adolescents examined during 1977–2020, regardless of their fitness for military service. Orthodox and ultra-orthodox Jewish women are not obligated to serve, and therefore are under-represented [19]. We excluded adolescents with missing values of BP or BMI data (0.2% in total). Figure 1 presents the study design. The Institutional Review Board of the Israel Defense Forces Medical Corps approved the study.
Fig. 1.
Study population
Data collection at regional military recruitment centers
The medical assessment included review of the examinee’s medical history, an interview with a physician, physical examination and further medical tests as appropriate [20, 21]. Height and weight were measured (barefoot and in underwear) by trained medics, and BMI was calculated (weight in kilograms divided by height squared in meters) [22]. We used the BMI classification of the US Centers for Disease Control and Prevention, which was validated and selected by the Israeli Ministry of Health as the routine reference for anthropometric data for Israeli children and adolescents [23]. Separately for males and females [24], the study population was divided into four BMI-for-age percentile groups [25]: underweight (< 5th percentile), normal weight (5–84th), overweight (85–94th) and obese (≥ 95th). SBP and DBP were routinely recorded, based on the measurement on the right arm acquired while sitting quietly for at least 5 min. Until 2005, manual sphygmomanometers were used (1977–1985, mercury sphygmomanometers; 1986–2005, aneroid sphygmomanometers). From 2006, digital oscillometric devices gradually replaced manual ones.
Data regarding years of schooling were obtained from the Ministry of Education and were dichotomized at 11 years (< 11/≥ 11 years) [10]. Data regarding immigration history and place of residence were received from the Ministry of Interior [26]. Residential socioeconomic status was classified into three groups (low/medium/high) based on criteria of the Israeli Bureau of Statistics [26, 27].
Statistical analysis
We a priori stratified analyses by sex and divided the study period into 5-year time intervals (1977–1980, 1981–1985, 1986–1990, 1991–1995, 1996–2000, 2001–2005, 2006–2010, 2011–2015 and 2016–2020). We calculated the P for trend for all the study variables including SBP, DBP and BMI using linear regression models. We plotted mean SBP, DBP and BMI values for the time intervals.
Two analytical approaches were taken to address the potential effect of the rise in adolescent BMI throughout the study period [10], in light of its tight relationship with BP levels [10]. (i) Mean SBP and DBP values were adjusted for BMI in each time interval using analysis of covariance (ANCOVA). We calculated the regression coefficients of the trends in SBP and DBP in unadjusted (βunadjusted) and BMI-adjusted (βBMI-adjusted) models, which reflected the predicted mean change in mmHg per 5-year interval. (ii) We stratified the secular trend of mean SBP and DBP by BMI categories (underweight/normal BMI/overweight/obese). Additional sensitivity analyses included the following: (1) limiting the study sample to persons with unimpaired health (apart from hypertension, a lack of chronic medical treatment, or a history of major surgery or cancer) in order to minimize confounding by coexisting comorbidities. (2) We included only Israeli-born adolescents to reduce health effects related to immigration [26]. The IBM SPSS software program version 25 was used for statistical analysis.
Results
The final study population comprised 2,785,515 adolescents; 1,157,958 (41.6%) were females. The characteristics at assessment are shown separately for males and females in Tables 1, 2, respectively. The overall mean age at assessment was 17.4 (SD; 0.6) years in both sexes. During the study period, the mean height increased by approximately 1 cm in males, and remained stable in females. There was a trend of increasing mean BMI: from 20.7 (SD; 2.8) kg/m2 for males and 21.0 (2.9) kg/m2 for females in the earliest time interval, to 22.8 (4.4) kg/m2 and 22.6 (4.4) kg/m2, respectively (P for trend < 0.001 in both sexes; Tables 1, 2). This increase was accompanied by increased prevalences of adolescent overweight and obesity (P for trend < 0.001 in both sexes; Tables 1, 2).
Table 1.
Characteristics of males by time intervals (n = 1,627,557)
| 1977–1980 (n = 97,483) | 1981–1985 (n = 1,35,678) | 1986–1990 (n = 1,57,008) | 1991–1995 (n = 2,05,252) | 1996–2000 (n = 2,07,875) | 2001–2005 (n = 2,05,692) | 2006–2010 (n = 1,93,699) | 2011–2015 (n = 2,18,790) | 2016–2020 (n = 2,06,080) | Total (n = 16,27,557) | P for trend | |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Age (mean ± SD) | 17.4 ± 0.5 | 17.4 ± 0.5 | 17.4 ± 0.5 | 17.5 ± 0.8 | 17.5 ± 0.8 | 17.3 ± 0.6 | 17.3 ± 0.6 | 17.4 ± 0.8 | 17.4 ± 0.7 | 17.4 ± 0.7 | 0.12 |
| Height (cm ± SD) | 172.9 ± 6.6 | 173.3 ± 6.7 | 173.9 ± 6.7 | 174.3 ± 6.8 | 174.2 ± 6.9 | 173.9 ± 6.8 | 174.2 ± 6.7 | 173.8 ± 6.8 | 174.2 ± 6.8 | 174 ± 6.8 | 0.046 |
| BMI (mean ± SD) | 20.7 ± 2.8 | 20.6 ± 2.9 | 21.1 ± 3.1 | 21.1 ± 3.3 | 21.1 ± 3.5 | 21.6 ± 3.8 | 21.9 ± 4.0 | 22.4 ± 4.35 | 22.8 ± 4.4 | 21.5 ± 3.7 | < 0.001 |
| CDC BMI groups (% of each BMI percentile category) | < 0.001 | ||||||||||
| Underweight | 8058 (8.3) | 12,513 (9.3) | 13,828 (8.9) | 17,000 (8.6) | 18,334 (9.1) | 15,578 (7.7) | 13,081 (6.8) | 13,472 (6.3) | 13,556 (6.7) | 1,24,420 (7.8) | |
| Normal weight | 81,228 (84.2) | 1,11,594 (82.9) | 1,25,690 (80.7) | 1,57,596 (79.5) | 1,57,312 (77.8) | 1,53,480 (75.9) | 1,41,329 (73.8) | 1,52,355 (70.9) | 1,42,770 (70.7) | 12,23,354 (76.6) | |
| Overweight | 5326 (5.4) | 7429 (5.5) | 11,223 (7.2) | 15,606 (7.9) | 16,508 (8.2) | 19,816 (9.8) | 21,381 (11.2) | 27,084 (12.6) | 24,796 (12.3) | 1,49,079 (9.3) | |
| Obese | 1986 (2.1) | 3050 (2.3) | 5030 (3.2) | 7950 (4.0) | 10,052 (5.0) | 13,375 (6.6) | 15,893 (8.2) | 22,114 (10.3) | 20,776 (10.3) | 1,00,026 (6.3) | |
| Immigrants (% of time interval) | 18,842 (19.3) | 15,576 (11.5) | 13,872 (8.8) | 37,962 (18.5) | 47,970 (23.1) | 46,705 (22.7) | 34,773 (18.0) | 24,944 (11.4) | 19,012 (9.2) | 2,59,656 (16.0) | 0.97 |
| Years of education ≥ 11 (% of time interval) | 61,361 (63.2) | 99,202 (73.4) | 12,968 (82.8) | 1,85,590 (90.6) | 1,91,457 (92.1) | 1,93,326 (94.0) | 1,81,861 (94.0) | 2,00,850 (92.1) | 1,73,019 (94.5) | 1,416,346 (88.4) | 0.004 |
| Socioeconomic status (% of each category) | 0.37 | ||||||||||
| Low | 29,456 (30.7) | 41,462 (30.9) | 45,283 (29.1) | 58,851 (29.0) | 58,187 (28.1) | 58,214 (28.4) | 57,367 (29.7) | 71,381 (32.8) | 58,985 (28.9) | 4,79,195 (29.7) | |
| Medium | 45,714 (47.6) | 64,026 (47.7) | 76,167 (49.0) | 1,02,573 (50.5) | 1,09,380 (52.9) | 1,06,996 (52.2) | 98,301 (51.0) | 1,04,099 (47.9) | 1,01,908 (50.0) | 8,09,164 (50.1) | |
| High | 20,807 (21.7) | 28,628 (21.3) | 34,051 (21.9) | 41,798 (20.6) | 39,138 (18.9) | 39,689 (19.4) | 37,255 (19.3) | 41,987 (19.3) | 43,061 (21.1) | 3,26,414 (20.2) | |
CDC US Center of Disease Control; BMI body mass index (calculated as weight in kilograms divided by height in meters squared); SD standard deviations; cm centimeters; kg kilograms
Table 2.
Characteristics of females by time intervals (n = 1,157,958)
| 1977–1980 (n = 58,181) | 1981–1985 (n = 90,198) | 1986–1990 (n = 1,09,192) | 1991–1995 (n = 1,43,745) | 1996–2000 (n = 1,50,368) | 2001–2005 (n = 1,53,276) | 2006–2010 (n = 1,45,457) | 2011–2015 (n = 1,53,623) | 2016–2020 (n = 1,53,908) | Total (n = 11,57,958) | P value for trend | |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Age (mean ± SD) | 17.5 ± 0.4 | 17.4 ± 0.4 | 17.4 ± 0.3 | 17.3 ± 0.3 | 17.3 ± 0.4 | 17.2 ± 0.4 | 17.1 ± 0.4 | 17 ± 0.6 | 17.0 ± 0.5 | 17.2 ± 2.7 | 0.48 |
| Height (cm ± SD) | 161.9 ± 5.9 | 162.1 ± 5.9 | 162.5 ± 6.0 | 162.1 ± 6.2 | 162.0 ± 6.2 | 162.1 ± 6.3 | 161.8 ± 6.2 | 162.2 ± 6.3 | 162.2 ± 6.2 | 162.2 ± 6.2 | 0.51 |
| BMI (mean ± SD) | 21.0 ± 2.9 | 20.9 ± 3.0 | 21.1 ± 3.2 | 21.1 ± 3.4 | 21.2 ± 3.5 | 21.2 ± 3.7 | 21.5 ± 3.9 | 22 ± 4.2 | 22.6 ± 4.3 | 21.5 ± 3.7 | 0.002 |
| CDC BMI groups (% of each BMI percentile category) | 0.001 | ||||||||||
| Underweight | 2229 (3.8) | 3927 (4.4) | 4581 (4.2) | 6741 (4.7) | 7127 (4.7) | 7502 (4.9) | 7045 (4.9) | 6213 (4.1) | 5806 (3.8) | 51,171 (4.4) | |
| Normal weight | 50,633 (87.3) | 77,811 (86.5) | 92,536 (84.9) | 1,19,379 (83.1) | 1,23,537 (82.3) | 1,23,963 (81.1) | 1,14,648 (79.0) | 1,17,181 (76.8) | 1,14,940 (75.2) | 9,34,628 (81.0) | |
| Overweight | 4438 (7.7) | 6917 (7.7) | 9590 (8.8) | 13,536 (9.4) | 14,506 (9.7) | 15,387 (10.1) | 16,294 (11.2) | 19,879 (13.0) | 21,142 (13.8) | 1,21,689 (10.5) | |
| Obese | 704 (1.2) | 1249 (1.4) | 2327 (2.1) | 3934 (2.7) | 4891 (3.3) | 6051 (4.0) | 7049 (4.9) | 9377 (6.1) | 10,918 (7.1) | 46,500 (4.0) | |
| Immigrants (% of time interval) | 8548 (14.7) | 8206 (9.1) | 7327 (6.7) | 16,307 (11.3) | 27,728 (18.4) | 33,836 (221) | 26,814 (18.4) | 18,207 (11.9) | 14,063 (9.1) | 1,61,036 (13.9) | 0.61 |
| Years of education ≥ 11 (% of time interval) | 48,412 (85.3) | 80,902 (91.0) | 1,04,099 (96.6) | 1,39,508 (97.6) | 1,45,783 (97.0) | 1,49,065 (97.3) | 1,42,693 (98.1) | 1,50,279 (98.1) | 1,33,567 (97.8) | 1,094,308 (96.4) | 0.02 |
| Socioeconomic status (% of each category) | 0.19 | ||||||||||
| Low | 11,500 (20.7) | 19,582 (22.2) | 21,518 (20.1) | 28,739 (20.2) | 33,465 (22.3) | 33,790 (22.1) | 32,358 (22.3) | 32,031 (20.9) | 31,633 (20.7) | 2,44,616 (21.3) | |
| Medium | 28,350 (51.1) | 45,566 (51.6) | 58,008 (54.1) | 78,144 (55.0) | 82,946 (55.3) | 85,663 (56.0) | 79,580 (54.8) | 83,523 (54.6) | 83,251 (54.4) | 6,25,031 (54.5) | |
| High | 15,622 (28.2) | 23,099 (26.2) | 27,757 (25.9) | 35,140 (24.7) | 33,483 (22.3) | 33,524 (21.9) | 33,350 (23.0) | 37,504 (24.5) | 38,052 (24.9) | 2,77,531 (24.2) | |
CDC US Center of Disease Control; BMI body mass index (calculated as weight in kilograms divided by height in meters squared); SD standard deviations; cm centimeters; kg kilograms
Mean DBP decreased in both sexes during the study period (P for trend < 0.001; Table 3; Fig. 2). The overall mean decrease was 3.6 mmHg (βunadjusted = − 0.65 mmHg/5-year, βBMI-adjusted = − 0.74 mmHg/5-year) in males and 2.9 mmHg (βunadjusted = − 0.41 mmHg/5-year, βBMI-adjusted = − 0.48 mmHg/5-year) in females. The SBP trend was not consistent between the sexes. In males, we recorded a small increase in mean SBP during the study period, of 1.6 mmHg (P for trend < 0.001; (βunadjusted = 0.39 mmHg/5-year, βBMI-adjusted = 0.17 mmHg/5-year). In females we observed a decrease of 1.9 mmHg (P for trend < 0.001 βunadjusted = − 0.10, βBMI-adjusted = − 0.25).
Table 3.
Recorded BMI, crude systolic and diastolic blood pressure values, and BMI adjusted levels, separately for males (a) and females (b)
| Years | BMI | Recorded SBP | BMI adjusted SBP | Recorded DBP | BMI adjusted DBP | |||
|---|---|---|---|---|---|---|---|---|
| Mean | SD | Mean | SD | Mean | Mean | SD | Mean | |
| A. Males (n = 1,627,557) | ||||||||
| 1977–1980 | 20.66 | 2.83 | 120.00 | 12.19 | 120.90 | 73.29 | 8.10 | 73.67 |
| 1981–1985 | 20.57 | 2.88 | 118.91 | 11.94 | 119.87 | 72.97 | 8.02 | 73.43 |
| 1986–1990 | 20.91 | 3.13 | 121.13 | 11.95 | 121.66 | 74.12 | 8.17 | 74.37 |
| 1991–1995 | 21.13 | 3.29 | 120.43 | 11.69 | 120.76 | 73.76 | 7.99 | 73.92 |
| 1996–2000 | 21.18 | 3.50 | 117.48 | 11.11 | 117.74 | 73.29 | 7.96 | 73.42 |
| 2001–2005 | 21.55 | 3.77 | 117.37 | 11.15 | 117.39 | 71.62 | 7.90 | 71.63 |
| 2006–2010 | 21.92 | 4.05 | 121.56 | 11.87 | 121.31 | 70.03 | 8.48 | 69.93 |
| 2011–2015 | 22.45 | 4.35 | 123.85 | 12.29 | 123.23 | 69.51 | 9.00 | 69.25 |
| 2016–2020 | 22.78 | 4.41 | 121.60 | 12.09 | 120.80 | 69.73 | 8.86 | 69.38 |
| B. Females (n = 1,157,958) | ||||||||
| 1977–1980 | 20.99 | 2.91 | 115.12 | 11.7 | 115.49 | 72.49 | 8.03 | 72.70 |
| 1981–1985 | 20.88 | 2.98 | 113.07 | 11.49 | 113.53 | 71.12 | 8.00 | 71.38 |
| 1986–1990 | 21.1 | 3.18 | 114.36 | 11.89 | 114.62 | 71.99 | 8.37 | 72.14 |
| 1991–1995 | 21.14 | 3.36 | 114.25 | 12.09 | 114.48 | 71.68 | 7.94 | 71.81 |
| 1996–2000 | 21.19 | 3.50 | 110.38 | 11.31 | 110.58 | 70.29 | 8.02 | 70.40 |
| 2001–2005 | 21.25 | 3.70 | 109.46 | 11.02 | 109.62 | 69.31 | 7.95 | 69.40 |
| 2006–2010 | 21.53 | 3.92 | 112.60 | 11.79 | 112.57 | 68.98 | 8.23 | 69.97 |
| 2011–2015 | 22.04 | 4.18 | 114.51 | 11.94 | 114.11 | 69.47 | 8.55 | 69.28 |
| 2016–2020 | 22.6 | 4.35 | 113.21 | 11.32 | 112.41 | 69.60 | 8.39 | 69.26 |
The recorded blood pressure values were retrieved directly from the Israeli Defense Forces Registry. BMI adjusted values of SBP and DBP were calculated by analysis of covariance (ANCOVA) using a general linear model
BMI body mass index (weight in kilograms divided by height squared in meters); SBP systolic blood pressure (mm/Hg); DBP diastolic blood pressure; SD standard deviation
Fig. 2.
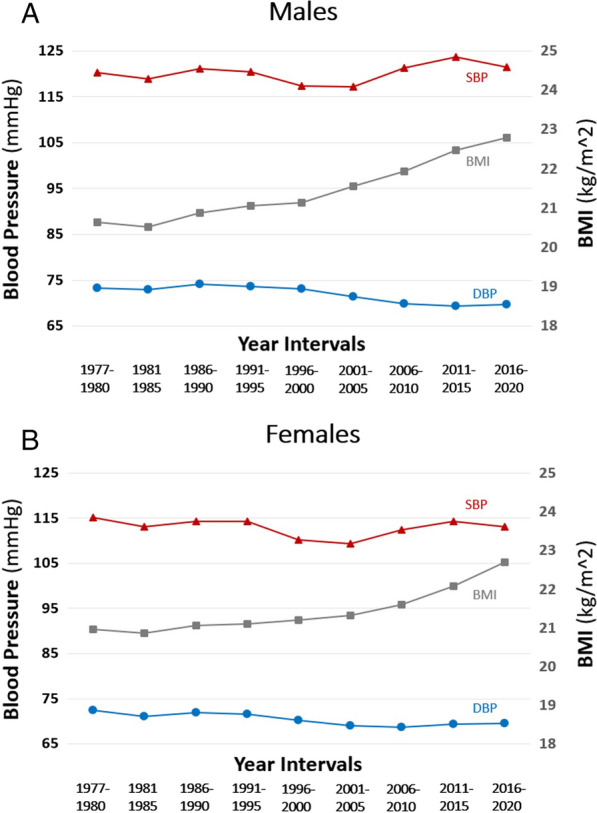
The secular trend of systolic blood pressure, diastolic blood pressure and BMI among A males (n = 1,627,557) and B females (n = 1,157,958) over the study period (1977–2020), by 5-year time intervals. BMI body mass index (weight in kilograms divided by height squared in meters); SBP systolic blood pressure (mm/Hg); DBP diastolic blood pressure; SD standard deviation
The trends of SBP and DBP levels remained stable throughout the study period, across all BMI categories (Fig. 3). For both sexes, mean SBP and DBP values in every time interval were highest in the obese group, followed by the overweight group, compared to the other BMI groups. The secular trends of BMI-adjusted DBP (Additional file 1: Figure S1) followed trends of the crude BP values in both sexes (P for trend for both sexes < 0.001, Table 3). The results persisted when the study population was limited to those with unimpaired health (Additional file 1: Figure S2), and to those born in Israel only (Additional file 1: Figure S3).
Fig. 3.
The secular trends of systolic blood pressure and diastolic blood pressure by BMI group, separately for males (A, C) and females (B, D) by 5-year time intervals. The BMI groups are defined by the US Center of Disease Control: underweight (< 5th percentile), normal weight (5–84th), overweight (85–94th) and obese (≥ 95th)
Discussion
In this nationwide study we demonstrated a trend of a slight increase in SBP, of 1.6 mmHg, among males; and a small decrease, of 1.9 mmHg, among females, over the course of four decades. Meanwhile, DBP exhibited mean decreases of 3.6 and 2.9 mmHg among males and females, respectively. These trends remained after accounting for increases in BMI throughout the study period, and in all strata of BMI categories.
A pooled analysis of 19 million adults in Western countries showed a trend of decreasing SBP and DBP [28]. Among the adolescent population, several studies from the US reported minimal decreases in SBP, of up to 0.8 mm/Hg, during 13–20 years (1974–1994 [14], 1999–2012 [17] and 1998–2018 [12]). Reports from other Western countries such as Northern Ireland (during the years 1999–2009) [18], Japan (1994–2010) [15] and Korea (1998–2008) [29] reported much larger SBP decrements, of 7–10 mmHg. DBP decrements of 2–4 mmHg were reported among American and Korean adolescents [12, 14, 17, 29], and up to 11 mmHg in the Northern Ireland population [18]. Our results concur with most reports that were based on surveys originated from the United States.
For a time interval of 44 years, we report overall increases in mean BMI of 2.1 and 1.6 kg/m2 among male and female adolescents, respectively. This concurs with studies that reported increases over recent decades in BMI in developed countries; and following the industrial process, in developing countries as well. Globally, mean BMI increases were estimated as about 0.5 kg/m2 per decade [30, 31]. As expected, throughout the period of the current study, mean BP values were significantly higher among those with overweight and obesity than normal weight, emphasizing the effect of BMI on BP [32, 33]. Yet, the trends of SBP and DBP were consistent regardless of BMI category, and most BMI-adjusted analyses demonstrated similar trends to those of the crude BP values. Therefore, it is unlikely that BMI solely accounts for the BP trends described in this study. Notably, the impact of increased BMI (overweight and obesity compared to normal weight) in regard to SBP and DBP was more pronounced in females than in males, despite a similar increase in the proportion of overweight and obesity in both sexes during the study period. This observation may be explained by the occurrence of increased BMI that represents increased muscle among a higher proportion of males than females [34, 35].
Alternatively, our results may stem from changes in demographic composition, mostly due to immigration [36]. Starting from the 1990s and continuing until 2010, the proportion of immigrants from the entire examinees’ population increased to 23% (compared to approximately 10% in the 1980s). This mostly comprised Ashkenazi Jews immigrating from the countries of the former Union of Soviet Socialist Republics. Moreover, immigration status [26] and Ashkenazi ethnicity [37] in particular, were associated with increased risk for adolescent elevated BP. This explanation is less probable since we analyzed separately Israeli-born adolescents and observed similar trends to that of the entire study population.
Our study lacks data of behavioral factors that are associated with hypertension. While dietary changes are associated with hypertension, multinational long-term cross-sectional studies have indicated stable rates of salt consumption across recent decades [38, 39]. Similarly, fruit and vegetable consumption, which are recommended to reduce blood pressure, remained stable over the last four decades in Israel [40]. Furthermore, previous reports from adolescent populations concluded that salt intake and fruit consumption could not explain BP trends [29] among adolescents. Smoking prevalence among adolescents in Western countries is decreasing [41]; however, its role was shown to be minor regarding SBP and DBP trends among adolescents [29]. Similarly, changes in physical activity [16, 29] or in daily alcohol drinking among adolescents are unlikely to explain the BP trends. Notably, alcohol abuse is more than five-fold less frequent in Israel than in other Western countries such as the US. The effects of other factors on BP are more controversial; for example, the possibility of associations of birth weight and breastfeeding with adult and adolescent hypertension [16, 18, 42].
The results of our study are of public health significance since elevated blood pressure among adolescents plays a substantial role in future cardiovascular morbidity and mortality [3, 43]. This effect is mediated through the increased risk for adulthood hypertension as well as via induction of additional cardiovascular risk factors such as chronic kidney disease and diabetes [4, 5]. Recently, adolescent hypertension has been shown to promote early onset type 2 diabetes [5] which has even more deleterious cardiovascular sequela compared to later onset diabetes [44]. Moreover, elevated blood pressure is a central factor of the metabolic syndrome [45] representing insulin resistance which is induced by obesity [46]. Nevertheless, we observed opposite trends of the BMI and the BP among adolescents throughout the study period, while other traditional environmental and behavioral risk factors are considered to remain stable throughout the years. This suggests that additional factors, that are not traditionally addressed [47], play role in the pathogenesis of elevated blood pressure among adolescents.
This study has limitations. First, throughout the study period the devices used for BP measurements changed. This, however, cannot be the sole explanation for the results as the decreasing trend in DBP started before the digital oscillometric devices were applied and continued after they were routinely implemented in the medical examination. Second, we lacked lifestyle data. Finally, our study did not include some ethnicities, such as East Asians. Yet, the genetic ancestry of the population was heterogeneous, and was shown to be related by genotyping to contemporary Middle East, North African, European and other Western populations [48, 49]. The strengths of this study include a nationwide screening setting that applied to both sexes, measured BP, weight and height within a narrow age range during a period of over four decades.
In conclusion, we report a trend of a decrement in DBP values during the last four decades in both sexes, which occurred despite significant increases in BMI and in obesity prevalence, but in the presence of more modest changes in SBP.
Supplementary Information
Additional file 1: Figure S1. The secular trends of BMI-adjusted systolic blood pressure and diastolic blood pressure among A males (n = 16,27,557) and B females (n = 11,57,958), by 5-year time intervals. Figure S2. The secular trends of systolic blood pressure, diastolic blood pressure and BMI among A males (n= 11,47,611) and B females (n = 8,17,085) with unimpaired health, by 5-year time intervals. Figure S3. The secular trends of systolic blood pressure, diastolic blood pressure and BMI among A males (n = 13,67,901) and B females (n = 9,96,922) born in Israel, by 5-year time intervals.
Acknowledgements
Not applicable.
Abbreviations
- BP
Blood pressure
- SBP
Systolic blood pressure
- DBP
Diastolic blood pressure
- BMI
Body mass index
Authors’ contributions
Concept and design: BF, EG, GT. Acquisition and analysis of data: BF, YZ, ED, DT, GT. Interpretation of the data: All authors. Drafting of the manuscript and critical revision: All authors. Statistical analysis: BF, YZ, ED, GT. Supervision: GT. All authors read and approved the final manuscript.
Funding
No funding sources.
Availability of data and materials
The databases used in our study were based on Israeli Defense Forces registries and are stored on Israeli Defense Forces computers. These computers are connected solely to the military network. These databases cannot be transferred to other computers or shared on the web, due to Israeli Defense Forces data security restrictions.
Declarations
Ethics approval and consent to participate
The study was approved by the IDF’s ethics committee, who waived the requirement for informed consent since data were obtained from medical records without the participation of patients.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Gan L, Langenickel T, Petruck J, Kode K, Rajman I, Chandra P, et al. Effects of age and sex on the pharmacokinetics of LCZ696, an angiotensin receptor neprilysin inhibitor. J Clin Pharmacol. 2016;56(1):78–86. doi: 10.1002/jcph.571. [DOI] [PubMed] [Google Scholar]
- 2.Teng H, Hu J, Ge W, Dai Q, Liu J, Xiao C, et al. Body mass index trajectories during 6–18 years old and the risk of hypertension in young adult: a longitudinal study in Chinese population. Int J Hypertens. 2021 doi: 10.1155/2021/6646868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Gray L, Lee IM, Sesso HD, Batty GD. Blood pressure in early adulthood, hypertension in middle age, and future cardiovascular disease mortality: HAHS (Harvard Alumni Health Study) J Am Coll Cardiol. 2011;58(23):2396–403. doi: 10.1016/j.jacc.2011.07.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Leiba A, Fishman B, Twig G, Gilad D, Derazne E, Shamiss A, et al. Association of adolescent hypertension with future end-stage renal disease. JAMA Intern Med. 2019 doi: 10.1001/jamainternmed.2018.7632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Fishman B, Grossman E, Zucker I, Orr O, Lutski M, Bardugo A, et al. Adolescent hypertension and risk for early-onset type 2 diabetes: a nationwide study of 1.9 million Israeli adolescents. Diabetes Care. 2020 doi: 10.2337/dc20-1752. [DOI] [PubMed] [Google Scholar]
- 6.Kelly RK, Thomson R, Smith KJ, Dwyer T, Venn A, Magnussen CG. Factors affecting tracking of blood pressure from childhood to adulthood: the childhood determinants of adult health study. J Pediatr. 2017;167(6):1422–1428.e2. doi: 10.1016/j.jpeds.2015.07.055. [DOI] [PubMed] [Google Scholar]
- 7.Song P, Zhang Y, Yu J, Zha M, Zhu Y, Rahimi K, et al. Global prevalence of hypertension in children: a systematic review and meta-analysis. JAMA Pediatr. 2019 doi: 10.1001/jamapediatrics.2019.3310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Roulet C, Bovet P, Brauchli T, Simeoni U, Xi B, Santschi V, et al. Secular trends in blood pressure in children: a systematic review. J Clin Hypertens. 2017;19(5):488–497. doi: 10.1111/jch.12955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bendor CD, Bardugo A, Pinhas-Hamiel O, Afek A, Twig G. Cardiovascular morbidity, diabetes and cancer risk among children and adolescents with severe obesity. Cardiovasc Diabetol. 2020;19(1):1–14. doi: 10.1186/s12933-020-01052-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Twig G, Reichman B, Afek A, Derazne E, Hamiel U, Furer A, et al. Severe obesity and cardio-metabolic comorbidities: a nationwide study of 2.8 million adolescents. Int J Obes. 2019;43(7):1391–9. doi: 10.1038/s41366-018-0213-z. [DOI] [PubMed] [Google Scholar]
- 11.Ogden CL, Carroll MD, Lawman HG, Fryar CD, Kruszon-Moran D, Kit BK, et al. Trends in obesity prevalence among children and adolescents in the United States, 1988–1994 through 2013–2014. JAMA J Am Med Assoc. 2016;315(21):2292–2299. doi: 10.1001/jama.2016.6361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hardy ST, Sakhuja S, Jaeger BC, Urbina EM, Suglia SF, Feig DI, et al. Trends in blood pressure and hypertension among US children and adolescents, 1999–2018. JAMA Netw Open. 2021;4(4):1–14. doi: 10.1001/jamanetworkopen.2021.3917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Xi B, Bovet P, Hong YM, Zong X, Chiolero A, Kim HS, et al. Recent blood pressure trends in adolescents from China, Korea, Seychelles and the United States of America, 1997–2012. J Hypertens. 2016;34(10):1948–1958. doi: 10.1097/HJH.0000000000001058. [DOI] [PubMed] [Google Scholar]
- 14.Freedman DS, Goodman A, Contreras OA, DasMahapatra P, Srinivasan SR, Berenson GS. Secular trends in BMI and blood pressure among children and adolescents: The Bogalusa Heart Study. Pediatrics. 2012 doi: 10.1542/peds.2011-3302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Shirasawa T, Ochiai H, Nishimura R, Morimoto A, Shimada N, Ohtsu T, et al. Secular trends in blood pressure among Japanese schoolchildren: a population-based annual survey from 1994 to 2010. J Epidemiol. 2012;22(5):448–453. doi: 10.2188/jea.JE20110137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Smpokos EA, Linardakis M, Papadaki A, Kafatos A. Secular changes in anthropometric measurements and blood pressure in children of Crete, Greece, during 1992/93 and 2006/07. Prev Med. 2011;52(3–4):213–7. doi: 10.1016/j.ypmed.2011.02.006. [DOI] [PubMed] [Google Scholar]
- 17.Xi B, Zhang T, Zhang M, Liu F, Zong X, Zhao M, et al. Trends in elevated blood pressure among US children and adolescents: 1999–2012. Am J Hypertens. 2016;29(2):217–225. doi: 10.1093/ajh/hpv091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Watkins D, Savage M, Mccarron P, Murray L, Cran G, Boreham C, et al. Trends in blood pressure over 10 years in adolescents: analyses of cross sectional surveys in the Northern Ireland Young Hearts project. BMJ. 2004;329(7458):139. doi: 10.1136/bmj.38149.510139.7C. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Bardugo A, Bendor CD, Zucker I, Lutski M, Cukierman-Yaffe T, Derazne E, et al. Adolescent nonalcoholic fatty liver disease and type 2 diabetes in young adulthood. J Clin Endocrinol Metab. 2021;106(1):e34–44. doi: 10.1210/clinem/dgaa753. [DOI] [PubMed] [Google Scholar]
- 20.Fishman B, Shlomai G, Twig G, Derazne E, Tenenbaum A, Fisman EZ, et al. Renal glucosuria is associated with lower body weight and lower rates of elevated systolic blood pressure: results of a nationwide cross-sectional study of 2.5 million adolescents. Cardiovasc Diabetol. 2019;18(1):1–8. doi: 10.1186/s12933-019-0929-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Tsur AM, Hershkovich S, Zucker I, Lutski M, Pinhas-Hamiel O, Vivante A, et al. Stuttering and incident type 2 diabetes: a population-based study of 2.2 million adolescents. J Clin Endocrinol Metab. 2021;106(4):978–87. doi: 10.1210/clinem/dgaa988. [DOI] [PubMed] [Google Scholar]
- 22.Simchoni M, Hamiel U, Pinhas-Hamiel O, Zucker I, Cukierman-Yaffe T, Lutski M, et al. Adolescent BMI and early-onset type 2 diabetes among Ethiopian immigrants and their descendants: a nationwide study. Cardiovasc Diabetol. 2020;19(1):168. doi: 10.1186/s12933-020-01143-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Goldstein A, Haelyon U, Krolik E, Sack J. Comparison of body weight and height of Israeli schoolchildren with the Tanner and Centers for Disease Control and Prevention growth charts. Pediatrics. 2001 doi: 10.1542/peds.108.6.e108. [DOI] [PubMed] [Google Scholar]
- 24.Furer A, Afek A, Orr O, Gershovitz L, Landau Rabbi M, Derazne E, et al. Sex-specific associations between adolescent categories of BMI with cardiovascular and non-cardiovascular mortality in midlife. Cardiovasc Diabetol. 2018;17(1):80. doi: 10.1186/s12933-018-0727-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kuczmarski RJ, Ogden CL, Guo SS, Grummer-Strawn LM, Flegal KM, Mei Z, et al. 2000 CDC growth charts for the United States: methods and development. Vital and health statistics, series 11. Maryland: Data from the National Health Survey, the National Health and Nutrition Examination Surveys, and the Hispanic Health and Nutrition Examination Survey; 2002. [PubMed] [Google Scholar]
- 26.Hamiel U, Pinhas-Hamiel O, Vivante A, Bendor C, Bardugo A, Afek A, et al. Impact of immigration on body mass index and blood pressure among adolescent males and females a nationwide study. Hypertension. 2019;74(6):1316–1323. doi: 10.1161/HYPERTENSIONAHA.119.13706. [DOI] [PubMed] [Google Scholar]
- 27.Bardugo A, Derazne E, Zucker I, Bendor CD, Puris G, Lutski M, et al. Adolescent thyroid disorders and risk for type 2 diabetes in young adulthood. J Clin Endocrinol Metab. 2021;106(9):e3426–35. doi: 10.1210/clinem/dgab382. [DOI] [PubMed] [Google Scholar]
- 28.Zhou B, Bentham J, Di Cesare M, Bixby H, Danaei G, Cowan MJ, et al. Worldwide trends in blood pressure from 1975 to 2015: a pooled analysis of 1479 population-based measurement studies with 19·1 million participants. Lancet. 2017;389(10064):37–55. doi: 10.1016/S0140-6736(16)31919-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Khang YH, Lynch JW. Exploring determinants of secular decreases in childhood blood pressure and hypertension. Circulation. 2011;124(4):397–405. doi: 10.1161/CIRCULATIONAHA.110.014399. [DOI] [PubMed] [Google Scholar]
- 30.Di Cesare M, Bentham J, Stevens GA, Zhou B, Danaei G, Lu Y, et al. Trends in adult body-mass index in 200 countries from 1975 to 2014: a pooled analysis of 1698 population-based measurement studies with 19.2 million participants. Lancet. 2016;387(10026):1377–96. doi: 10.1016/S0140-6736(16)30054-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Finucane MM, Stevens GA, Cowan MJ, Danaei G, Lin JK, Paciorek CJ, et al. National, regional, and global trends in body-mass index since 1980: systematic analysis of health examination surveys and epidemiological studies with 960 country-years and 9.1 million participants. Lancet. 2011;377(9765):557–67. doi: 10.1016/S0140-6736(10)62037-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Skinner AC, Perrin EM, Moss LA, Skelton JA. Cardiometabolic risks and severity of obesity in children and young adults. N Engl J Med. 2015;373(14):1307–17. doi: 10.1056/NEJMoa1502821. [DOI] [PubMed] [Google Scholar]
- 33.Naguib YM, Samaka RM, Rizk MS, Ameen O, Motawea SM. Countering adipose tissue dysfunction could underlie the superiority of telmisartan in the treatment of obesity-related hypertension. Cardiovasc Diabetol. 2021;20(1):1–19. doi: 10.1186/s12933-021-01259-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Lindsay RS, Hanson RL, Roumain J, Ravussin E, Knowler WC, Tataranni PA. Body mass index as a measure of adiposity in children and adolescents: relationship to adiposity by dual energy X-Ray absorptiometry and to cardiovascular risk factors. J Clin Endocrinol Metab. 2001;86(9):4061–7. doi: 10.1210/jcem.86.9.7760. [DOI] [PubMed] [Google Scholar]
- 35.Hwang YC, Jeon WS, Park CY, Youn BS. The ratio of skeletal muscle mass to visceral fat area is a main determinant linking circulating irisin to metabolic phenotype. Cardiovasc Diabetol. 2016;15(1):1–6. doi: 10.1186/s12933-015-0319-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Reuven Y, Dreiher J, Shvartzman P. The prevalence of diabetes, hypertension and obesity among immigrants from East Africa and the former Soviet Union: a retrospective comparative 30-year cohort study. Cardiovasc Diabetol. 2016;15(1):8–14. doi: 10.1186/s12933-016-0392-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Fishman B, Leiba A, Twig G, Shlomai G, Orr O, Landau R, et al. Ethnic variability among jews is associated with hypertension: results of a nationwide study of 1.44 million adolescents. Am J Hypertens. 2020;33(2):175–81. doi: 10.1093/ajh/hpz167. [DOI] [PubMed] [Google Scholar]
- 38.Powles J, Fahimi S, Micha R, Khatibzadeh S, Shi P, Ezzati M, et al. Global, regional and national sodium intakes in 1990 and 2010: a systematic analysis of 24 h urinary sodium excretion and dietary surveys worldwide. BMJ Open. 2013 doi: 10.1136/bmjopen-2013-003733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Bernstein AM, Willett WC. Trends in 24-h urinary sodium excretion in the United States, 1957–2003: a systematic review. Am J Clin Nutr. 2010;92(5):1172–1180. doi: 10.3945/ajcn.2010.29367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Lev B, Elliot Rosenberg TS. Health behaviors-healthful nurtition. Jerusalem: Israeli Ministry of Health; 2011. [Google Scholar]
- 41.Meza R, Jimenez-Mendoza E, Levy DT. Trends in tobacco use among adolescents by grade, sex, and race, 1991–2019. JAMA Netw Open. 2020;3(12):1–14. doi: 10.1001/jamanetworkopen.2020.27465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Jornayvaz FR, Vollenweider P, Bochud M, Mooser V, Waeber G, Marques-Vidal P. Low birth weight leads to obesity, diabetes and increased leptin levels in adults: the CoLaus study. Cardiovasc Diabetol. 2016;15(1):73. doi: 10.1186/s12933-016-0389-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Liu Y, Yan Y, Jiang T, Li S, Guo Y, Fernandez C, et al. Impact of long-term burden of body mass index and blood pressure from childhood on adult left ventricular structure and function. J Am Heart Assoc. 2020 doi: 10.1161/JAHA.120.016405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Wu H, Lau ESH, Yang A, Ma RCW, Kong APS, Chow E, et al. Trends in diabetes-related complications in Hong Kong, 2001–2016: a retrospective cohort study. Cardiovasc Diabetol. 2020;19(1):1–11. doi: 10.1186/s12933-020-01039-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Lopez-Lopez JP, Cohen DD, Ney-Salazar D, Martinez D, Otero J, Gomez-Arbelaez D, et al. The prediction of metabolic syndrome alterations is improved by combining waist circumference and handgrip strength measurements compared to either alone. Cardiovasc Diabetol. 2021;20(1):1–11. doi: 10.1186/s12933-021-01256-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Aguilar A. Hypertension: global blood pressure trends. Nat Rev Nephrol. 2017;13(1):2. doi: 10.1038/nrneph.2016.178. [DOI] [PubMed] [Google Scholar]
- 47.Kim S, Kyung C, Park JS, Lee SP, Kim HK, Ahn CW, et al. Normal-weight obesity is associated with increased risk of subclinical atherosclerosis. Cardiovasc Diabetol. 2015;14(1):1–9. doi: 10.1186/s12933-014-0162-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Ostrer H, Skorecki K. The population genetics of the Jewish people. Hum Genet. 2013;132(2):119–127. doi: 10.1007/s00439-012-1235-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Behar DM, Yunusbayev B, Metspalu M, Metspalu E, Rosset S, Parik J, et al. The genome-wide structure of the Jewish people. Nature. 2010;466(7303):238–42. doi: 10.1038/nature09103. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Additional file 1: Figure S1. The secular trends of BMI-adjusted systolic blood pressure and diastolic blood pressure among A males (n = 16,27,557) and B females (n = 11,57,958), by 5-year time intervals. Figure S2. The secular trends of systolic blood pressure, diastolic blood pressure and BMI among A males (n= 11,47,611) and B females (n = 8,17,085) with unimpaired health, by 5-year time intervals. Figure S3. The secular trends of systolic blood pressure, diastolic blood pressure and BMI among A males (n = 13,67,901) and B females (n = 9,96,922) born in Israel, by 5-year time intervals.
Data Availability Statement
The databases used in our study were based on Israeli Defense Forces registries and are stored on Israeli Defense Forces computers. These computers are connected solely to the military network. These databases cannot be transferred to other computers or shared on the web, due to Israeli Defense Forces data security restrictions.