Abstract
The susceptibility of 30 clinical isolates belonging to six different species of filamentous fungi (Aspergillus fumigatus, Aspergillus flavus, Scedosporium prolificans, Scedosporium apiospermum, Fusarium solani, and Fusarium oxysporum) was tested against six antifungal drugs (miconazole, voriconazole, itraconazole, UR9825, terbinafine, and amphotericin B) with the microdilution method recommended by the National Committee for Clinical Laboratory Standards (NCCLS) (M38-P). The MICs were compared with the MICs obtained by a colorimetric method measuring the reduction of the dye 3-(4,5-dimethyl-2-thiazyl)-2,5-diphenyl-2H-tetrazolium bromide (MTT) to formazan by viable fungi. The levels of agreement between the two methods were 96 and 92% for MIC-0 (clear wells) and MIC-1 (75% growth reduction), respectively. The levels of agreement were always higher for Aspergillus spp. (97% ± 2.5%), followed by Scedosporium spp. (87% ± 10.3%) and Fusarium spp. (78% ± 7.8%). The NCCLS method was more reproducible than the MTT method: 98 versus 95% for MIC-0 and 97 versus 90% for MIC-1. However, the percentage of hyphal growth as determined visually by the NCCLS method showed several discrepancies when they were compared with the percentages of MTT reduction. A new simplified assay that incorporates the dye MTT with the initial inoculum and in which the fungi are incubated with the dye for 48 h or more was developed, showing comparable levels of agreement and reproducibility with the other two methods. Furthermore, the new assay was easier to perform and more sensitive than the MTT method.
Filamentous fungi may cause invasive mycoses in both immunocompromised and nonimmunocompromised individuals. These infections require prompt systemic antifungal therapy, the effectiveness of which depends, among other things, on the in vitro susceptibility of the fungus to antifungal drugs. Therefore, there is a demand for reproducible and reliable methods of antifungal susceptibility testing of filamentous fungi. Many variables that decrease the inter- and intralaboratory agreement are now defined (2, 4, 5, 16, 19). Broth macrodilution methods are time-consuming and labor-intensive (1), and therefore, efforts have focused on microdilution methods that are more practical and more user-friendly in the clinical laboratory (18). Based on these principles, the Subcommittee on Antifungal Susceptibility Testing of the National Committee for Clinical Laboratory Standards (NCCLS) has proposed the M38-P standard for the antifungal susceptibility testing of conidium-forming fungi (14).
The determination of MICs for filamentous fungi can be facilitated with a method which overcomes observer bias and quantifies the hyphal growth of molds. Because turbidity measurements and colony counts (9) are not useful in the case of filamentous fungi, colorimetric methods based on the measurement of metabolic activity may facilitate determination of the MIC. One of these is an assay using the dye 3-(4,5-dimethyl-2-thiazyl)-2,5-diphenyl-2H-tetrazolium bromide (MTT). This yellow tetrazolium salt is cleaved by dehydrogenases inside mitochondria or in other cellular locations possessing dehydrogenase activity (7) to form its purple formazan derivative (11), which can be measured spectrophotometrically at 550 nm. MTT is cleaved by all living, metabolically active fungi independent of proliferation and irrespective of unicellular or multicellular growth and thus is a measure of metabolic activity.
A method based on MTT has been used for susceptibility testing of fungi. The MTT method demonstrated excellent agreement with the standard macrodilution method for the antifungal susceptibility testing of yeasts (1, 9). For filamentous fungi, the MTT method has been used for Aspergillus fumigatus, resulting in interpretable data (10). In order to overcome the shortcomings of visual MIC determination for filamentous fungi and to develop a reliable, subjective, and less variable method for the antifungal susceptibility testing of filamentous fungi, we evaluated the performance of a method using MTT and compared the results with those obtained with the proposed standard of the NCCLS. The correlation between the metabolic activity and the growth of fungi was examined. Furthermore, a simplified method using MTT was developed and compared with the previous methods.
MATERIALS AND METHODS
Test isolates.
Thirty clinical isolates of filamentous fungi (part of our private collection) were tested. These included five isolates of each of the following species: A. fumigatus (AZN7151, AZN7275, AZN8249, AZN8248, and AZN7272), Aspergillus flavus (AZN501, AZN6837, AZN6803, AZN6686, and AZN6578), Scedosporium prolificans (AZN7886, AZN7903, AZN7946, AZN7921, and AZN7895), Scedosporium apiospermum (AZN1252, AZN7309, AZN7110, AZN7111, and AZN6474), Fusarium solani (AZN729, AZN646, AZN2106, AZN7844, and AZN2784), and Fusarium oxysporum (AZN685, AZN441, AZN1859, AZN8263, and AZN1188). Candida parapsilosis (ATCC 22019) and Candida krusei (ATCC 6258) were used for quality controls.
Isolates were passaged twice at an interval of 5 to 7 days at 30 to 37°C. All isolates were tested three times on three different days. All the solutions were prepared ex novo with powders from the same lot. Conidia of the isolates were obtained from fresh cultures each time.
NCCLS method.
A broth microdilution method was performed according to NCCLS guidelines (M38-P) (14). The drugs that were used included miconazole (Janssen Research Foundation, Beerse, Belgium), terbinafine (Novartis, Basel, Switzerland), itraconazole (Janssen Research Foundation), UR9825 (Ureach, Madrid, Spain), voriconazole (Pfizer Central Research, Sandwich, United Kingdom), and amphotericin B (Bristol-Myers Squibb, Woerden, The Netherlands). The drug dilutions were made in RPMI 1640 medium (with l-glutamine, without bicarbonate) (GIBCO BRL, Life Technologies, Woerden, The Netherlands) buffered to pH 7.0 with 0.165 M morpholinepropanesulfonic acid (MOPS) (Sigma-Aldrich Chemie GmbH, Steinheim, Germany). The final concentrations of the antifungal agents ranged from 0.015 to 16 mg of amphotericin B/liter, 0.06 to 64 mg of miconazole/liter, and 0.03 to 32 mg of itraconazole, terbinafine, UR9825, and voriconazole per liter.
The tests were performed in 96-well flat-bottom microtitration plates, which were kept at −70°C until the day of testing. Spores were collected with a cotton stick and suspended in sterile water. After the heavy particles were allowed to settle, the turbidity of the supernatants were measured spectrophotometrically (Spectronic 20D; Milton Roy, Rochester, N.Y.) at 530 nm and transmission was adjusted to 68 to 70% (Scedosporium spp. and Fusarium spp.) or to 80 to 82% (Aspergillus spp.). Each suspension was diluted 1:50 in RPMI 1640 to obtain two times the desired inoculum. The inoculum size was confirmed by plating serial dilutions on Sabouraud dextrose agar (SDA) plates, with final inocula ranging from 1 × 104 to 5 × 104 CFU/ml. After agitation, the plates were incubated at 37°C for 48 h (Aspergillus spp. and Fusarium spp.) and 72 h (Scedosporium spp.). The growth was assessed by visual observation with the aid of a concave mirror. Growth was scaled according to the NCCLS guidelines as follows: 4, no reduction in growth; 3, slight reduction in growth, or approximately 75% of the growth control; 2, prominent reduction in growth, or approximately 50% of the growth control; 1, slight growth, or approximately 25% of growth control; and 0, optically clear, or the absence of growth. MIC-1 and MIC-0 were determined to be the lowest concentrations of drug showing slight growth (25%) and absence of growth (100% visible-growth inhibition) compared with that of the drug-free control, respectively. For amphotericin B, only the MIC-0 was determined.
MTT method (MTT-3h).
The MICs were also determined by a colorimetric method using the dye MTT (Sigma Chemical, St. Louis, Mo.). After the MICs were visually determined on each of the microtitration plates, 25 μl of RPMI 1640 containing 5 mg of MTT/ml was added to each well. Incubation was continued at 37°C for 3 h. The content of each well was removed (centrifugation is not required for filamentous fungi due their adherence to the wells), and 200 μl of isopropanol containing 5% 1 M HCl was added to extract the dye. After 30 min of incubation at room temperature and gentle agitation, the optical density (OD) was measured with a microtitration plate spectrophotometer (MS2 reader, Titertekplus; ICN Biomedical Ltd., B.V., Zoetermeer, The Netherlands) at 550 nm. The OD of the blank, which consisted of an uninoculated plate incubated together with the inoculated plates, was subtracted from the ODs of the inoculated plates. The percentage of MTT conversion to its formazan derivative for each well was calculated by comparing the OD at 550 nm (OD550) of the wells with that of the drug-free control based on the following equation: (A550 of wells that contained the drug/A550 of the drug-free well) × 100%. MIC-0 and MIC-1 were considered to be the lowest concentrations of drug showing at least 95 and 75% reductions in the OD compared with that of the drug-free well, respectively.
Toxicity test.
The dye MTT was tested for possible inhibitory effects on the growth of filamentous fungi. The isolates were incubated in RPMI 1640 with different concentrations of MTT ranging from 4 to 0.06 mg/ml, for 72 h at 37°C. A final inoculum of 104 CFU/ml was used. Growth inhibition was assessed by visual observation of the wells containing MTT and compared with the MTT-free wells. The level of inhibition of the fungal growth was assessed and scored as described above.
Modified MTT method.
The MICs for each of the isolates were also determined by a modification of the MTT method previously described. For this method, conidia suspensions were prepared as described above. Each suspension was diluted 1:50 in RPMI 1640 containing 0.2 mg of MTT/ml to obtain two times the desired inoculum. Then, the inoculum that contained MTT was added to microtitration plates, which resulted in a final concentration of MTT of 0.1 mg/ml, and the microtitration plates were incubated for 72 h (Scedosporium spp.) and 48 h (other species). After incubation, the formed formazan was extracted and the MICs were determined as described above.
Analysis of results.
Each of the 30 isolates was tested three times by the three methods against the six drugs. For each isolate, there was one reading per test. The MICs derived from the three readings by the NCCLS method and the two MTT methods were compared for each drug-isolate combination.
(i) Agreement between NCCLS and MTT methods.
The percentage of agreement between the NCCLS method and the MTT methods was defined as the proportion of MIC-0 and MIC-1 determined by MTT methods which fell within 1 dilution of the MIC-0 and MIC-1 determined by the NCCLS method, respectively, for each reading. The high and low off-scale MICs were included in the analysis by converting to the next higher or lower drug concentration, respectively. The differences were analyzed by two-way analysis of variance and were considered not statistically significant when the probability (P) exceeded 0.05.
(ii) Correlation of visual reading of growth with OD reduction.
The correlation of the visual reading of growth with the OD reduction was estimated for each MIC endpoint by comparing the MIC-0 and MIC-1, as these were determined visually by the NCCLS method and spectrophotometrically by the MTT method (MTT-3h). The frequency of visual MICs, which fulfilled the criteria defined above for the MIC endpoints of the MTT method (at least 95 and 75% reductions of the OD for MIC-0 and MIC-1, respectively) as well as the average of percentage of OD reduction compared with the visual MIC endpoints were calculated.
(iii) Reproducibility of methods.
For each method, the median of the three MICs measured was calculated and the proportion of the MICs that fell within 1 dilution of the median was estimated. Based on these calculations, the reproducibility of each method for determining the drug-MIC endpoint was determined.
RESULTS
A total of 1,620 MICs obtained by the three methods were evaluated in this study. All the isolates produced detectable growth at 48 and 72 h. The MICs of quality control strains were within the reference ranges for the three replicates.
MIC data.
The MIC ranges for both MIC endpoints obtained by the NCCLS method for six antifungal drugs against the six fungal species are summarized in Table 1. Table 1 also provides the median MIC of the three readings. In most of the endpoint-drug-species combinations, the MIC range and the median MIC were the same for the two methods, with very few exceptions with the Fusarium spp. The MICs were distributed over all the drug concentrations used.
TABLE 1.
The MIC-0 and MIC-1 of six antifungal drugs against 30 clinical isolates of filamentous fungi obtained by the NCCLS method
| Druga | MIC (mg/liter) range (median)b
|
|||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
A. fumigatus
|
A. flavus
|
S. apiospermum
|
S. prolificans
|
F. oxysporum
|
F. solani
|
|||||||
| MIC-0c | MIC-1d | MIC-0 | MIC-1 | MIC-0 | MIC-1 | MIC-0 | MIC-1 | MIC-0 | MIC-1 | MIC-0 | MIC-1 | |
| MCZ | 4–16 (4) | 4–16 (4) | 1–16 (4) | 0.125–8 (2) | 1–8 (2) | 0.5–2 (1) | 16–>64 (>64) | 4–>64 (64) | 1–>64 (16) | 0.25–>64 (8) | 4–>64 (16) | 2–>64 (8) |
| TB | 2–4 (4) | 0.5–4 (1) | 0.03–0.5 (0.06) | 0.03–0.125 (0.03) | >32 | >32 | >32 | >32 | 0.125–>32 (8) | 0.03–8 (2) | 1–>32 (4) | 0.5–8 (2) |
| ICZ | 0.5–2 (1) | 0.5–1 (0.5) | 0.125–1 (1) | 0.06–1 (0.5) | 2–>32 (32) | 0.5–4 (1) | >32 | >32 | 0.5–>32 (32) | 0.125–>32 (32) | 4–>32 (32) | 2–>32 (16) |
| UR | 0.25–1 (0.5) | 0.125–0.25 (0.25) | 0.125–0.25 (0.25) | 0.06–0.125 (0.125) | 1–8 (2) | 0.25–2 (1) | 8–32 (16) | 1–8 (2) | 0.5–>32 (8) | 0.125–16 (4) | 4–>32 (8) | 2–>32 (4) |
| VCZ | 0.25–1 (0.5) | 0.125–0.5 (0.25) | 0.25–1 (0.5) | 0.06–0.5 (0.25) | 0.5–2 (0.5) | 0.125–1 (0.25) | 16–>32 (32) | 1–8 (4) | 0.5–16 (4) | 0.125–8 (2) | 2–32 (4) | 1–16 (2) |
| AB | 0.5–1 (0.5) | NDe | 0.5–1 (1) | ND | 2–4 (2) | ND | 0.5–>16 (16) | ND | 0.25–2 (1) | ND | 0.5–2 (1) | ND |
MCZ, miconazole; TB, terbinafine; ICZ, itraconazole; UR, UR9825; VCZ, voriconazole; AB, amphotericin B.
There were five isolates per species.
MIC-0, the lowest concentration of drug showing complete inhibition of growth (NCCLS method).
MIC-1, the lowest concentration of drug showing slight growth (25%) (NCCLS method).
ND, not determined.
Toxicity of MTT.
MTT did not have any effect on the growth of the fungi at concentrations of 0.125 mg/ml and lower (Fig. 1). At the higher concentrations, MTT was toxic for Scedosporium spp. and Fusarium spp. since the growth was completely inhibited. By contrast, Aspergillus spp. appeared resistant to MTT even at higher concentrations.
FIG. 1.
Toxicity of MTT for six species of filamentous fungi. The bars for each species represent each of five individual strains tested and indicate the lowest concentration of MTT at which no inhibition of growth was observed. The dotted line shows the concentration of MTT used for MIC determination (0.1 mg/ml) by the modified MTT method.
Agreement between methods.
The percentages of agreement within 1 dilution between MICs obtained by the NCCLS and MTT methods for each species-drug-MIC endpoint combination are presented in Table 2. The results were stratified by antifungal agents and MIC endpoint. High levels of agreement (96% for MIC-0 and 92% for MIC-1) were found for the comparison of the NCCLS method with the MTT method. Lower levels of agreement were found when results of the modified MTT method were compared with those of the NCCLS method and the MTT method (MTT-3h). The agreement for MIC-0 was always higher than that for MIC-1. The observed differences among the methods were not statistically significant except for those derived from the comparison of the modified MTT method with the other two methods for both MIC endpoints of voriconazole and for MIC-1 of UR9825.
TABLE 2.
Agreement between NCCLS- and MTT-derived MICs
| Drug | % Agreement within 1 dilution (P value)
|
|||||
|---|---|---|---|---|---|---|
| NCCLS-MTT
|
NCCLS-modified MTT
|
MTT-modified MTT
|
||||
| MIC-0 | MIC-1 | MIC-0 | MIC-1 | MIC-0 | MIC-1 | |
| Miconazole | 99 (0.94) | 93 (0.79) | 89 (0.36) | 88 (0.66) | 86 (0.36) | 83 (0.48) |
| Terbinafine | 92 (0.82) | 84 (0.45) | 93 (0.85) | 78 (0.36) | 94 (0.96) | 75 (0.89) |
| Itraconazole | 97 (1) | 95 (0.43) | 90 (0.66) | 81 (0.18) | 91 (0.66) | 82 (0.46) |
| UR9825 | 99 (0.98) | 96 (0.53) | 76 (0.04) | 77 (0.09) | 86 (0.03) | 76 (0.25) |
| Voriconazole | 94 (0.91) | 94 (0.21) | 83 (0.006) | 69 (0.003) | 86 (0.01) | 81 (0.05) |
| Amphotericin B | 94 (0.60) | 88 (0.16) | 87 (0.35) | |||
| Overall agreement | 95.8 | 92.3 | 86.6 | 78.5 | 88.4 | 79.4 |
The levels of agreement for each drug-MIC endpoint combination between the methods were always the highest for the Aspergillus spp. (97% ± 2.5%), followed by Scedosporium spp. (87% ± 10.3%). For Fusarium spp., the levels of agreement were relatively low (78% ± 7.8%) (data not shown).
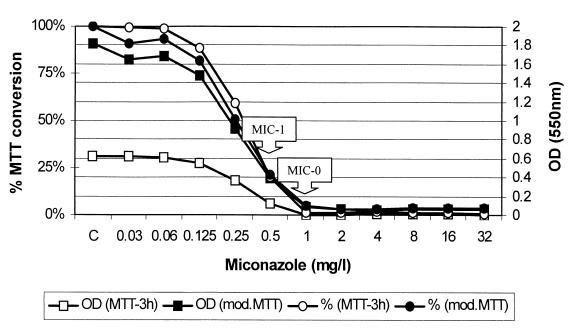
Representative curves of the OD and percentage of MTT conversion of miconazole against S. apiospermum are presented in Fig. 2. MIC-0 and MIC-1 determined visually by the NCCLS method were 1 and 0.5 mg/liter, respectively.
FIG. 2.
Testing of susceptibility of an S. apiospermum strain to miconazole. Representative curves were generated by the MTT method (MTT-3h) and its modification (mod.MTT). The curves with the circles represent the percentage of OD reduction compared with the OD of the drug-free well, and the curves with the squares represent the absorption of formed formazan at 550 nm. The MIC-0 and MIC-1 determined by the NCCLS method were 1 and 0.5 mg/liter, respectively.
Correlation between visual reading of growth and OD reduction.
Visual reading of growth was correlated with the colorimetric assessment of metabolic activity of fungi by comparing the results of the NCCLS method with the results of the MTT method (MTT-3h) (Table 3). The off-scale MICs were excluded from the analysis. For the MIC-0 endpoint, 84.7% of the MIC-0s of all drugs determined by the NCCLS method corresponded to a 95% or greater reduction of the OD. For the MIC-1 endpoint, 63.9% of the MIC-1s obtained by the NCCLS method corresponded to a 75% or greater reduction of the OD.
TABLE 3.
Correlation of visually determined MICs (NCCLS MICs) with the percentage of MTT conversion, as calculated by the MTT methoda
| Drug | MTT conversion of isolates with NCCLS MIC-0
|
MTT conversion of isolates with NCCLS MIC-1
|
||||
|---|---|---|---|---|---|---|
| % of isolates with <5% conversion | % Conversion [median (range)]b | % of isolates with:
|
% Conversion [median (range)]b | |||
| <25% conversion | 26–50% conversion | 51–75% conversion | ||||
| Miconazole | 89.5 | 0 (0–36) | 74.7 | 21.5 | 3.8 | 13 (0–75) |
| Terbinafine | 76.9 | 2 (0–24) | 53.6 | 31.9 | 14.5 | 24 (0–75) |
| Itraconazole | 84.9 | 1 (0–30) | 68.8 | 25.0 | 6.2 | 15.5 (0–75) |
| UR9825 | 83.3 | 1 (0–36) | 61.3 | 31.2 | 7.5 | 21 (0–75) |
| Voriconazole | 87.8 | 1 (0–35) | 61.7 | 27.7 | 10.6 | 21 (0–71) |
| Amphotericin B | 84.3 | 0 (0–28) | ||||
| Overall conversion | 84.7 | 1 (0–36) | 63.9 | 27.6 | 8.5 | 20 (0–75) |
Percentage of MTT conversion was calculated by comparing the OD of the drug-containing well with that of the drug-free well.
The median and range of percentage of MTT conversion calculated by the MTT method were corresponded to MIC endpoints determined by the NCCLS method.
Reproducibility of methods.
Overall, the three methods were very reproducible, since the levels of the reproducibility were higher than 89% for both MIC endpoints. The NCCLS method was more reproducible than the MTT methods, since the reproducibility for MIC-0 and MIC-1 were 94.8 and 93.4% for the NCCLS method compared with 94.8 and 89.5% for the MTT method and 92.8 and 89.6% for the modified MTT method, respectively. Between the two MIC endpoints, MIC-0 was more reproducible than MIC-1. For all drugs tested, the reproducibility of MIC-0 ranged from 90 to 100% and that of MIC-1 ranged from 82 to 99%. The lowest reproducibility among the drug-MIC endpoint-method combinations was exhibited by MIC-1 of terbinafine determined by the MTT method and the modified MTT method (82 and 84%, respectively).
DISCUSSION
There are several problems in the determination of the MICs of antifungal agents, such as trailing effect caused by partial inhibition of fungal growth (8) and the subjectivity of visual reading with the aid of a magnifying mirror (16). These shortcomings are increased for filamentous fungi, which produce hyphae, making quantification of growth difficult. Moreover, the unequal growth, clumping, and adherence (13) of molds in assay tubes makes establishing a clear endpoint impossible both by visual reading as well as by spectrophotometer.
The colorimetric method using the dye MTT has been investigated as an alternative method for the NCCLS method for in vitro susceptibility testing of fungi. Its applicability was investigated for the MIC of fluconazole against 101 yeast isolates, and high levels of agreement with the NCCLS method were reported (1). In the same study, the authors found that the MICs could be determined 24 h earlier with the MTT method. Although previous studies have shown that the MTT method resulted in interpretable dose-response curves with both amphotericin B and itraconazole against A. fumigatus (9, 10), the applicability of the MTT method for the antifungal susceptibility testing of various filamentous fungi against different antifungal drugs and the comparison of this method with the NCCLS method (M-38P) had not been studied.
Overall, the levels of agreement between the MTT and NCCLS methods within 1 dilution were 95.8% based on MIC-0 and 92.3% based on MIC-1 (Table 2). The MTT method relies on metabolic activity of mycelia (6, 11) and, thus, not directly on growth. Any factor which influences the metabolic rate might have an effect on reduction of MTT even if the biomass remains the same. The high levels of agreement with the NCCLS method might indicate that growth corresponds directly with the metabolic status of fungi. However, different levels of agreement were found between the genera, with Aspergillus spp. showing higher levels of agreement (97% ± 2.5%) than Scedosporium spp. (87% ± 10.3%) and Fusarium spp. (78% ± 7.8%). This could be explained by the different rates and manner of sporulation and, if true, indicates potential difficulties in developing a general, accurate, comprehensive assay for testing the susceptibility of conidium-forming filamentous fungi in vitro.
Although the microtitration plates were prepared ex novo, the two methods showed high reproducibility since the levels of agreement among the replicates were 97.6 and 96.7% (NCCLS) and 94.8 and 89.5% (MTT) based on MIC-0 and MIC-1, respectively. The lower reproducibility of MIC-1 compared with that of MIC-0 in the MTT method could be explained by the high sensitivity of the assay. Different variables in the conditions of incubation, such as batch of medium, temperature, and evaporation, might have an effect in the reduction of MTT influencing the less-stable MIC-1 endpoint to a higher degree.
The correlation of the MIC endpoints in two methods showed some discrepancies since only 84.7% of the MIC-0s determined by the NCCLS method corresponded with metabolic activity lower than 5%, as assessed by MTT conversion, and 63.9% of visually determined MIC-1s by the NCCLS method corresponded to MTT conversion lower than 25% (Table 3). The discrepancies that occurred from the comparison of MIC endpoints were expected since the accurate method of MTT precisely quantifies the growth in each well, which is very difficult to achieve by visual reading.
In order to increase the reduction of MTT and diminish the incubation period required for the MIC determination, it has been suggested to use menadione, an electron-coupling agent that increases the reduction of MTT (6, 9, 10). However, we were able to increase the reduction of MTT just by adding the dye to the inoculum and by incubating the fungi with the dye for 48 and 72 h. This resulted in a 1.5- to 5-fold increase of absorption due to the higher production of formazan (Fig. 2). To ensure that MTT does not inhibit the growth of fungi, we incubated the fungi with different concentrations of MTT. All fungi were inhibited by a high concentration of MTT, although the susceptibility varied among the species. No inhibition was observed at a concentration of 0.125 mg/ml or lower when all the isolates were incubated for 72 h with the dye (Fig. 1). Therefore, a final concentration of 0.1 mg of MTT/ml was used in the modified MTT assay. The agreement of this new assay with the NCCLS method was lower than that of the MTT method (86.6% for MIC-0 and 78.5% for MIC-1), probably due to interexperimental variability (different plates) and its higher sensitivity, since small differences in the growth rate can result in large differences in the amount of formazan that is produced. The discrepancies for UR9825 and voriconazole were statistically significant. This could be related to the trailing effect, which might influence the results obtained by the modified MTT method more than those derived by the MTT assay. However, the modified assay was very reproducible (92.8% for MIC-0 and 89.6% for MIC-1), compared to the MTT method. The MTT method incorporates several advantages. It is easy to perform and objective (1). The MICs of antifungal drugs can be determined rapidly since it can produce results after 24 h for Aspergillus spp. and 48 h for Scedosporium spp., although the reduction of MTT increased during prolonged incubation (unpublished observations). The MTT method is reproducible and reliable. It provides good agreement with the NCCLS method while overcoming some of its drawbacks. The main advantage of the MTT method is that it enables precise quantification of hyphal proliferation of molds and can assess even very small differences. This property is very important in cases that interactions between combinations of antifungal agents are studied (12).
A drawback of the MTT method is the requirement of high numbers of cells, especially if they have low metabolic activity (6), in order to achieve measurable MTT reduction. Furthermore, the high sensitivity can decrease the reproducibility in some experimental designs. The extraction of formazan crystals is performed by dissolution in organic solvents (15), which precludes direct spectrophotometric measurement of absorption (20) and prevents the assessment of fungicidal concentration of the drugs. Furthermore, an additional extraction step is required to visualize the reduction of MTT (20). Alternatively, 2,3-bis(2-methoxy-4-nitro-5-sulfophenyl)-5-[(phenylamino)carbonyl]-2H-tetrazolium hydroxide (XTT) could be considered an alternative since it is reduced to a water-soluble formazan product (8, 20; J. Meletiadis, unpublished observations).
In conclusion, the MTT method can be used as an alternative method to the NCCLS method since it shows high levels of agreement with the NCCLS method. The spectrophotometric reading provides more detailed information on the antifungal activity in each well. Furthermore, optimalization of the MTT method may overcome the above-mentioned drawbacks. The modified assay we described in this study could be the starting point for further optimization of the MTT method.
ACKNOWLEDGMENTS
This study was supported by the EC-TMR-EUROFUNG network (ERBFMXR-CT970145) and by the Mycology Research Center of Nijmegen.
REFERENCES
- 1.Clancy C J, Nguyen M H. Comparison of a photometric method with standardized methods of antifungal susceptibility testing of yeasts. J Clin Microbiol. 1997;35:2878–2882. doi: 10.1128/jcm.35.11.2878-2882.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Cormican M G, Pfaller M A. Standardization of antifungal susceptibility testing. J Antimicrob Chemother. 1996;38:561–578. doi: 10.1093/jac/38.4.561. [DOI] [PubMed] [Google Scholar]
- 3.Denizot F, Lang R. Rapid colorimetric assay for cell growth and survival. Modifications to the tetrazolium dye procedure giving improved sensitivity and reliability. J Immunol Methods. 1986;89:271–277. doi: 10.1016/0022-1759(86)90368-6. [DOI] [PubMed] [Google Scholar]
- 4.Espinel-Ingroff A, Dawson K, Pfaller M, Anaissie E, Breslin B, Dixon D, Fothergill A, Paetznick V, Peter J, Rinaldi M G, Walsh T. Comparative and collaborative evaluation of standardization of antifungal susceptibility testing for filamentous fungi. Antimicrob Agents Chemother. 1995;39:314–319. doi: 10.1128/aac.39.2.314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Espinel-Ingroff A, Bartlett M, Bowden R, Chin N X, Cooper C, Jr, Fothergill A, McGinnis M R, Menezes P, Messer S A, Nelson P W, Odds F C, Pasarell L, Peter J, Pfaller M A, Rex J H, Rinaldi M G, Shankland G S, Walsh T J, Weitzman I. Multicenter evaluation of proposed standardized procedure for antifungal susceptibility testing of filamentous fungi. J Clin Microbiol. 1997;35:139–143. doi: 10.1128/jcm.35.1.139-143.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Garn H, Krause H, Enzmann V, Drossler K. An improved MTT assay using the electron-coupling agent menadione. J Immunol Methods. 1990;168:253–256. doi: 10.1016/0022-1759(94)90062-0. [DOI] [PubMed] [Google Scholar]
- 7.Hansen M B, Nielsen S E, Berg K. Re-examination and further development of a precise and rapid dye method for measuring cell growth/cell kill. J Immunol Methods. 1989;119:203–210. doi: 10.1016/0022-1759(89)90397-9. [DOI] [PubMed] [Google Scholar]
- 8.Hawser S P, Norris H, Jessup C J, Ghannoum M A. Comparison of a 2,3-bis(2-methoxy-4-nitro-5-sulfophenyl)-5-[(phenylamino)carbonyl]-2H-tetrazolium hydroxide (XTT) colorimetric method with the standardized National Committee for Clinical Laboratory Standards method of testing clinical yeast isolates for susceptibility to antifungal agents. J Clin Microbiol. 1998;36:1450–1452. doi: 10.1128/jcm.36.5.1450-1452.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Jahn B, Martin E, Stueben A, Bhakdi S. Susceptibility testing of Candida albicans and Aspergillus species by a simple microtiter menadione-augmented 3-(4,5-dimethyl-2-thiazolyl)-2,5-diphenyl-2H-tetrazolium bromide assay. J Clin Microbiol. 1995;33:661–667. doi: 10.1128/jcm.33.3.661-667.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Jahn B, Stueben A, Bhakdi S. Colorimetric susceptibility testing for Aspergillus fumigatus: comparison of menadione-augmented 3-(4,5-dimethyl-2-thiazolyl)-2,5-diphenyl-2H-tetrazolium bromide and Alamar blue tests. J Clin Microbiol. 1996;34:2039–2041. doi: 10.1128/jcm.34.8.2039-2041.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Levitz S M, Diamond R D. A rapid colorimetric assay of fungal viability with the tetrazolium salt MTT. J Infect Dis. 1985;152:938–945. doi: 10.1093/infdis/152.5.938. [DOI] [PubMed] [Google Scholar]
- 12.Meletiadis J, Mouton J W, Rodriguez-Tudela J L, Meis J F G M, Verweij P E. In vitro interaction of terbinafine with itraconazole against clinical isolates of Scedosporium prolificans. Antimicrob Agents Chemother. 2000;44:470–472. doi: 10.1128/aac.44.2.470-472.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Meshulam T, Levitz S M, Christin L, Diamond R D. A simplified new assay for assessment of fungal cell damage with the tetrazolium dye, (2,3)-bis-(2-methoxy-4-nitro-5-sulphenyl)-(2H)-tetrazolium-5-carboxanilide (XTT) J Infect Dis. 1995;172:1153–1156. doi: 10.1093/infdis/172.4.1153. [DOI] [PubMed] [Google Scholar]
- 14.National Committee for Clinical Laboratory Standards. Reference method for broth dilution antifungal susceptibility testing of conidium forming filamentous fungi. Proposed standard M38-P. Wayne, Pa: National Committee for Clinical Laboratory Standards; 1998. [Google Scholar]
- 15.Niks M, Otto M. Towards an optimized MTT assay. J Immunol Methods. 1990;130:149–151. doi: 10.1016/0022-1759(90)90309-j. [DOI] [PubMed] [Google Scholar]
- 16.Pfaller M A, Rinaldi M G. Antifungal susceptibility testing. Current state of technology, limitations, and standardization. Infect Dis Clin N Am. 1993;7:435–444. [PubMed] [Google Scholar]
- 17.Pfaller M A, Vu Q, Lancaster M, Espinel-Ingroff A, Fothergill A, Grant C, McGinnis M R, Pasarell L, Rinaldi M G, Steele-Moore L. Multisite reproducibility of colorimetric broth microdilution method for antifungal susceptibility testing of yeast isolates. J Clin Microbiol. 1994;32:1625–1628. doi: 10.1128/jcm.32.7.1625-1628.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Pujol I, Guarro J, Llop C, Soler L, Fernandez-Ballart J. Comparison study of broth macrodilution and microdilution antifungal susceptibility tests for the filamentous fungi. Antimicrob Agents Chemother. 1996;40:2106–2110. doi: 10.1128/aac.40.9.2106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Rex J H, Pfaller M A, Rinaldi M G, Polak A, Galgiani J N. Antifungal susceptibility testing. Clin Microbiol Rev. 1993;6:367–381. doi: 10.1128/cmr.6.4.367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Roehm N W, Rodgers G H, Hatfield S M, Glasebrook A L. An improved colorimetric assay for cell proliferation and viability utilizing the tetrazolium salt XTT. J Immunol Methods. 1991;142:257–265. doi: 10.1016/0022-1759(91)90114-u. [DOI] [PubMed] [Google Scholar]