Abstract
Macrophages are heterogeneous cells that present as different functional phenotypes due to their plasticity. They can be classified into two categories, namely M1- and M2-like macrophages, which are involved in processes as diverse as anti-tumor activity and immunosuppressive tumor promotion. Tumor-associated macrophages (TAMs) are defined as being of an M2-type and are considered as the active component in tumor microenvironment. TAMs are involved in multiple processes of tumor progression through the expression of cytokines, chemokines, growth factors, protein hydrolases and more, which lead to enhance tumor cell proliferation, angiogenesis, and immunosuppression, which in turn supports invasion and metastasis. It is assumed that the abundance of TAMs in major solid tumors is correlated to a negative patient prognosis. Because of the currently available data of the TAMs’ role in tumor development, these cells have emerged as a promising target for novel cancer treatment strategies. In this paper, we will briefly describe the origins and types of TAMs and will try to comprehensively show how TAMs contribute to tumorigenesis and disease progression. Finally, we will present the main TAM-based therapeutic strategies currently available.
Keywords: Tumor-associated macrophages, Tumor progression, Therapeutic strategies
Introduction
Macrophages are present in various tissues and are involved in both innate and adaptive immunity. It is known that macrophages are phenotypically heterogeneous and functionally diverse [1]. At least two different populations can be distinguished: M1-(pro-inflammatory) and M2-like macrophages (anti-inflammatory and immunoregulatory) [2]. Their functional diversity is due to differences in phagocytosis, antigen presentation, and release of cytokines and complement components [3, 4].
Tumor-infiltrating macrophages (TAMs) are important cellular components of the tumor microenvironment (TME) [5]. The term “TAMs” mainly refers to macrophages differentiated into an M2-like phenotype because their functional characteristics more closely resemble an M2- rather than an M1-like phenotype [6, 7]. Tumor-derived cytokines and non-coding RNAs attribute to this transformation [8, 9]. Interestingly, Lu et al. found that OCT-4 not only induced stemness of lung cancer cells but also promoted M2 macrophage polarization through M-CSF secretion [10]. Pancreatic ductal adenocarcinoma (PDAC) cells with endothelial-mesenchymal transition (EMT) secreted HSP90 to induce macrophage M2-polarization and increase tumor growth through feedback regulation [11]. The recruitment of macrophages and activation in the TME is regulated via signals generated by tumor cells as well as host cells. Among the signals are C–C chemokine ligand 2 (CCL2), CCL5, colony-stimulating factor-1 (CSF-1), and granulocyte–macrophage colony-stimulating factor (GM-CSF) [12–15]. Communication within the TME is bidirectional because TAM signaling is involved in tumor initiation, accelerated disease progression and metastasis [16]. There is increasing evidence that TAMs influence various aspects of tumor progression such as tumor initiation, tumor angiogenesis, immunosuppression, invasion, and metastasis through the release of cytokines or growth factors into the TME [15, 17, 18].
A series of studies have shown that the abundance of TAMs was associated with negative prognosis in patients with solid tumors, except non-small cell lung cancer (NSCLC) [19]. Therefore, TAMs are considered a new potential target in cancer treatment. Elucidating the role of TAMs can provide useful insights for studying tumor pathogenesis and developing new therapeutic strategies. Currently, the main therapeutic strategies of targeting TAMs include targeting tumor angiogenesis, promoting TAM phagocytosis, reprogramming TAMs, blocking immune checkpoint inhibitors, and inhibiting TAM recruitment or direct depletion of TAMs [20, 21]. The dynamic changes, phenotypes, signaling pathways and functional states of TAMs in the process of tumorigenesis and development make TAMs a hot topic of research. In this paper, we briefly describe the origins and types of TAMs and will try to comprehensively show how TAMs contribute to tumorigenesis and disease progression. Finally, we will present the currently main TAM-based therapeutic strategies.
TAM origins and types
Origin
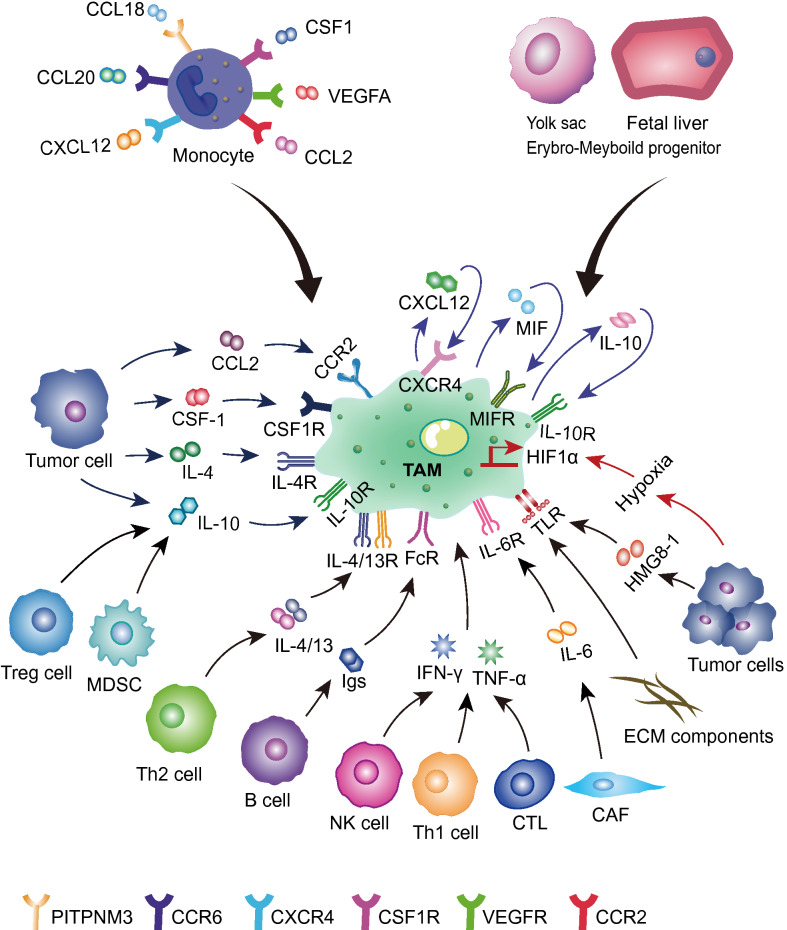
Macrophages were first discovered in the late nineteenth century by Metchnikoff and were described as immune cells derived from the mononuclear phagocytic lineage present in most animals [22]. Due to their existence in many tissues, macrophages are commonly referred to according to their tissue contexts, such as microglia, Kupffer cells, and epidermal Langerhans cells. A more general term indicating their origin has also been coined: tissue-resident macrophages [23]. Macrophages are derived from both erythroid-myeloid progenitors (EMP) in the yolk sac and fetal liver and monocyte-producing macrophage/dendritic progenitors (MDP) of the bone marrow [24]. Macrophages have been shown to be present in many solid tumors and have therefore been termed tumor-associated macrophages [5]. Franklin et al. proposed two developmental pathways that may explain the occurrence of TAMs in tumor tissues: (1) tissue-resident macrophages of embryonic or monocyte origin may change their phenotype/activation status during tumorigenesis and have therefore been termed tissue-resident TAMs; (2) monocytes undergo a distinct stage of differentiation during tumor growth and eventually become macrophages which is why these cells have been termed tumor-induced TAMs. These two cell populations may coexist in a particular tumor of which tissue-resident TAMs may predominate in the early stages of tumor growth while tumor-induced TAMs are predominant in the later stages of the tumor. In addition, monocytes entering the tumor tissue may phenotypically change in response to TME without differentiating into TAMs which is why these cells are called tumor-induced effector monocytes [25]. In the mouse model, TAMs were mainly derived from bone marrow monocytes. These monocytes were recruited via inflammatory signals (e.g. CCL2, CCL18, CCL20, C-X-C motif chemokine ligand 12 (CXCL12), CSF1, and vascular endothelial growth factor A (VEGFA)) released by cancer cells to primary or metastasis tumors where they differentiated into TAMs, further facilitating disease progression and metastasis [26, 27] (Fig. 1).
Fig. 1.
Origin of TAMs and their interaction with the TME. TAMs are mainly derived from bone marrow monocytes or erythroid myeloid progenitor cells in the yolk sac or fetal liver. Bone marrow monocytes are recruited and differentiated into TAMs by chemokines or cytokines released from tumor cells or stromal cells in the tumor microenvironment, such as CCL2, CSF-1, VEGFA, etc. TAMs are stimulated by CCL2, IL-4, and IL-10, secreted by tumor cells, and Igs, IL-10, and IL-4/13, secreted by immune cells (B cells, Treg cells, Th cells). Moreover, they can be activated by hypoxia, tumor-derived HMGB-1, and factors released by TAMs themselves (IL-10, MIF, and CXCL12). TAMs tumor-associated macrophages, CCL2 C–C chemokine ligand 2, CSF-1 colony-stimulating factor-1, VEGFA vascular endothelial growth factor A, IL-4 interleukin-4, IL-10 interleukin-10, Treg cells regulatory T cells, Th cells helper T cells, MIF macrophage migration inhibitory factor, CXCL12 C-X-C motif chemokine ligand 12
Types
According to the activation type and the different roles in TME, macrophages are usually divided into two types, M1 with a classical activation and M2 with an alternate activation pathway [1, 28]. Once M1-phenotype macrophages have activated themselves through cytokines such as interferon (IFN)-γ, tumor necrosis factor (TNF)-α, or lipopolysaccharide (LPS) [29, 30], they further produce pro-inflammatory and immune-stimulating cytokines and participate in the anti-infection response together with helper T cells 1 (Th1). In addition, M1-type cells can kill target cells by phagocytosis [31–33]. Finally, M1 cells also express nitric oxide synthase (iNOS), reactive oxygen species (ROS) [34–36], and cytokines such as interleukin-12 (IL-12) [37]. M2-type cells are mainly activated by Th2-related cytokines (e.g. IL-4, IL-10, and IL-13) and suppress T cell responses as well as promote tumor cell growth, invasion, and metastasis [1, 31–33]. In addition, they express scavenger receptors or cell differentiation (CD) markers (CD68, CD163, CD206) [38] that are associated with a high expression of IL-10, IL-1β, VEGF, and matrix metalloproteinases (MMP) [39, 40]. It is worth noting that M2 cells can be divided into more subtypes (M2a, M2b, M2c, M2d) [38, 41]. Recent research has shown that TAMs correspond to a state located between M1 and M2 [42], however, based on the role in TME, they more closely resemble an M2-phenotype [1, 43].
The role of TAMs in tumor progression
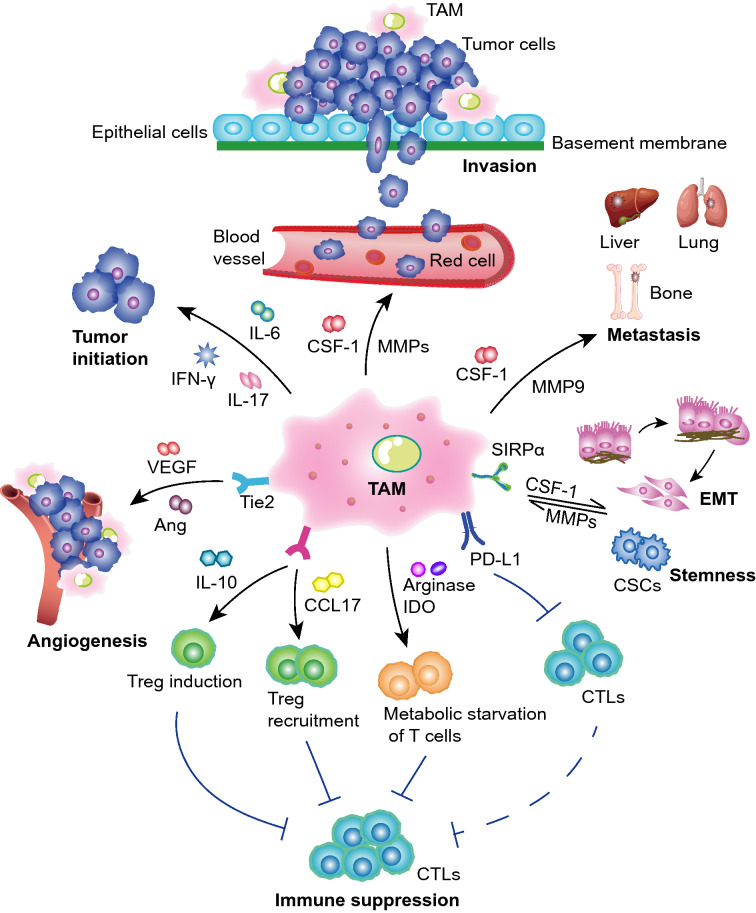
Immune cells are among the main components of TME and include macrophages, T cells, natural killer cells (NK cells), dendritic cells, and more. TAMs, as the major immunosuppressive cells, have a wide range of effects on TME through the synthesis and secretion of various cellular factors [44] (Fig. 2).
Fig. 2.
The effects of TAMs on tumor progression. The schematic diagram shows that TAMs promote tumorigenesis, angiogenesis, invasion, metastasis, epithelial-mesenchymal transformation (EMT) and the acquisition of stem cell characteristics. TAMs suppress the immune response through secretion of certain factors or proteases. TAMs tumor-associated macrophages, IL-6 interleukin-6, IL-17 interleukin-17, IFN-γ Interferon-γ, VEGF vascular endothelial growth factor, Ang angiotensin, IL-10 interleukin-10, CCL17 C–C chemokine ligand 17, IDO 1/2 indoleamine 2,3-dioxygenase 1/2, PD-L1, programmed cell death ligand 1, CTLs CD8+ cytotoxic T lymphocytes, CSCs cancer stem cells, MMPs metalloproteinases, CSF-1 colony-stimulating factor-1, EMT epithelial mesenchymal transformation, SIRP α signal-regulatory protein α, MMP2/3/7/9 metalloproteinase 2/3/7/9
Promotion of cancer initiation
Researchers found abundant inflammatory cells in tumor biopsy samples which renders it likely that chronic inflammation may be associated with tumor initiation [45, 46]. Expectedly, this has been shown in cases of colon and gastric cancer [47]. This can be explained by findings showing that chronic inflammation (persistent infection, repeated exposure to irritants, autoimmune diseases) or oncogene activation can lead to the expression of pro-inflammatory transcription factors such as nuclear factor-κB (NF-κB), signal transducer and activator of transcription 3 (STAT3), and hypoxia inducible factor 1 α (HIF-1α). After these factors have been activated, they could lead to the recruitment of macrophages mediated by the expression of cytokines and chemokines (TNF-α and IL-6) of cancer cells [48]. Macrophages can produce proinflammatory mediators such as IL-6, TNF, IFN-γ, growth factors, including epidermal growth factor (EGF) and Wnt, proteases, ROS, and nitrogen substances that may produce a mutagenic microenvironment, further facilitating cancer initiation [20, 49, 50]. Grivennikov et al. proved that TAM-derived IL-17 and IL-23 were connected to the growth and progression of colon cancer [51]. The study by Kong et al. suggested that TAM-derived IL-6 could facilitate the initiation and development of hepatocellular carcinoma (HCC) by activating the STAT3 signaling pathway [52]. In summary, TAMs may have a plethora of effects in the course of the development and occurrence of cancer.
Promotion of angiogenesis
Besides their capability of promoting cancer-related inflammation processes, TAMs can also directly affect tumor growth via the promotion of angiogenesis. Angiogenesis is necessary to meet the cancer cells’ growth demands reflected by an increased need for oxygen and nutrients [53]. The neovascularization is also crucial for tumor invasion and metastasis, referring to many factors, such as hypoxia, hyperosmotic pressure and angiogenic factors such as VEGF, transforming growth factor β (TGF-β), cyclooxygenase-2 (COX-2), placenta growth factor (PGF), fibroblast growth factor (FGF) [54], epidermal growth factor (EGF), platelet-derived growth factor (PDGF), angiotensin (Ang), and chemokines [55–57]. HIF expression was associated with the stimulation of neovascularization and caused tumor cells to produce pro-angiogenic factors (e.g. VEGF-A, FGF-2) in hypoxic areas. In line with these findings is the fact that HIF-1ɑ could upregulate the expression of VEGF in highly hypoxic glioma [58]. Yin et al. reported that EGF secreted by TAMs could activate the tumor cells' surface EGFR and further upregulate VEGF/VEGFR signaling which supported ovarian cancer cell proliferation and invasion [56]. Cui et al. showed that glioblastoma-induced TAMs could promote tumor angiogenesis through elevating the secretion of TGF-β1 and IL-10 resulting in endothelial cell proliferation [59]. TAMs express proteases, such as metalloproteinase-9 (MMP-9), MMP-2, and MMP-3, and are able to degrade extracellular matrix (ECM), thereby further indirectly facilitating angiogenic invasion [60]. The Wnt/β-catenin signaling pathway is involved in proliferation, apoptosis, invasion, and metastasis [61] and it has been proved that an aberrant Wnt/β-catenin signaling cascade facilitated cancer development [62]. Wnt7b (Wnt family ligand) expression was upregulated by TAMs which could promote tumor neovascularization [63]. Yeo et al. could show that Wnt7b could stimulate VEGF production that in turn triggered angiogenesis switches and further targeted endothelial cells to contribute to tumor growth, tumor invasion, and metastasis [64]. It has been demonstrated that an upregulated expression of VEGFA in TIE2(hi)-derived macrophages induced the proliferation of endothelial cells and by this, led to tumor neovascularization [65]. The above studies show that the TAMs-driven tumor angiogenesis and the associated promotion in tumor progression provide a theoretical basis for an anti-angiogenesis therapy strategy by targeting the TAMs.
Promotion of invasion and metastasis
Cancer cell invasion and metastasis are the major causes of death. Cancer cells acquire the ability to move and release degradative enzymes which permit tumor cells to break away from the primary tumor, they then colonize distant sites where they establish new tumors [66]. EMT is a process during which epithelial cells acquire mesenchymal character and through which malignant biological features are conferred, including invasion and metastasis [67, 68]. Recently, a series of studies demonstrated that TAMs take part in the regulation of the EMT process and facilitate metastasis [69–72]. The work by Wei et al. indicated that TAMs interfere with JAK2/STAT3/miR-506-3p/FoxQ1 regulation which increases colorectal cancer (CRC) cell invasion and metastasis capability via EMT induction. Moreover, the activation of this axis could result in the generation of CCL2 and thereby facilitate the recruitment of macrophages [73]. Tu et al. also found that a high TCF4 expression was associated with recruitment and polarization of macrophages in the metastasis sites. Moreover, the CCL2/CCR2 signaling pathway was shown to enhance metastasis [74]. Lee et al. performed a coculture study of non-neoplastic MCF10A human breast epithelial cells with TAMs. The results showed that TAMs could secrete CCL2 that led to the upregulation of endoplasmic reticulum oxidoreductase (ERO)1-α as well as MMP-9, and further inducing MCF10A acquired EMT invasive phenotype [75]. Similarly, CCL5 released by TAMs could significantly promote invasion, metastasis, and EMT of prostate cancer cells by activating the β-catenin/STAT3 signaling pathway [76]. Malignant phyllodes tumor recruited and repolarized TAMs through CCL5 binding to CCR5 in macrophages and then activated the AKT signaling pathway. Moreover, TAM-generated CCL18 bound PIPTNM3 (the receptor of myofibroblast) that further facilitated differentiation and the invasion of myofibroblast [77]. Lan et al. found that CCL26 together with CCR3 could induce the infiltration of TAMs. Further analysis showed that phosphatase of regenerating liver-3 (PRL-3) upregulated CCL26 which led to TAM infiltration and promotion of invasion and metastasis in CRC [78]. The conditioned medium from TAMs co-cultured with NSCLC cells facilitated tumor cells invasion via EMT and the upregulation of αB-Crystallin (CRYAB), and further inducing lung cancer metastasis in vivo [79]. Han et al. suggested that TAMs facilitated osteosarcoma metastasis and invasion through upregulating the expression of COX-2, MMP9, and phosphorylated STAT3 leading to EMT induction [80]. TAMs can express a variety of factors to induce EMT, such as TGF-β and IL-6. It has also been shown that TAMs secreted EGF to activate the EGFR/ERK1/2 signal pathway in cancer cells which might promote EMT [81].
Immune suppression
TAMs express programmed cell death 1 (PD-1) and CTLA-4 receptor ligands, such as PD-L1 and B7-H1 (CD80), which have been shown to inhibit the cytotoxicity of T cells and NK cells [82, 83]. TAM-born chemokines and cytokines can inhibit the antitumor effect of tumor-infiltrating T cells and NK cells, and cooperate with bone marrow-derived suppressor cells (MDSCs), tumor-related dendritic cells, and neutrophils to form an inhibitory TME [84, 85]. The TAM-produced chemokines IL-10 and TNF-α induce the expression of PD-L1 and further inhibit the antitumor T cell activity [86]. TAMs can also inhibit the proliferative ability of CD8+ T cells via the release of arginase1, iNOS, oxygen radicals, or nitrogen species [87–89]. TAMs secrete anti-inflammatory chemokines to recruit regulatory T (Treg) cells, such as CCL2, CCL3, CCL4, CCL5, and CCL20 [90]. Moreover, TAMs mediate Tregs via generating CCL22 to suppress T cell-specific activity and further promote the growth of cancer cells [91]. Microenvironment characterization by multi-omics signatures performed by Liu et al. proved that TAM enriched HCC tissues were associated with immunosuppression [92]. Eisinger et al. demonstrated that targeting an immune-suppressive TAM subtype by specific antibodies against the scavenger receptor MARCO resulted in the phenotypic conversion of TAMs into proinflammatory TAMs that recruited and activated more NK cells to enhance the TNF-related apoptosis-inducing ligand (TRAIL) mediated effect of tumor cell killing [93]. Petty et al. stressed that hedgehog (Hh) signaling induced the polarization of TAMs through inhibiting the TAM generation of CXCL9 and CXCL10 which suppressed the recruitment of CD8+ T cells [94]. In conclusion, these findings support the immunomodulatory role of TAMs that may promote tumor progression by modulating the immune response and promoting immune evasion.
Sustaining the activity of cancer stem cells
A cancer stem cell (CSC) is defined as a cell in tumor tissue that can self-renew to generate a heterogeneous set of tumor cells [95, 96]. Wan et al. found that TAMs could secrete IL-6 by STAT3 signaling to facilitate the expansion of HCC stem cells [97]. TAMs produce chemokines, such as CXCL8 and CXCL12, that are able to program cancer cells in a way that they acquire a CSC-like character and that maintain stemness in oral squamous cell carcinoma, HCC, and renal cell carcinoma [98–100]. Gomez et al. proved that head and neck squamous cell carcinoma-derived TAMs upregulated the interaction of hyaluronic acid (HA) (the ligand of CD44)-CD44 by HAS2. Once HA was bound to CD44, it increased stemness through activating the PI3K-4EBP1-SOX2 signaling pathway [101, 102]. Milk-fat globule-epidermal growth factor-VIII (MFG-E8) was released by TAMs which activated the STAT3 and the Sonic Hedgehog signaling pathway in CSCs, whereupon the cells, endowed with these new capabilities, displayed drug resistance and increased tumorigenicity [103]. TAMs could significantly upregulate the S100 calcium-binding protein A9, an inflammation-related secreted protein linked to poor survival in HCC patients that reinforced stem cell-like properties by activating NF-kB signaling [104]. Moreover, in pancreatic cancer, it was shown that TAMs could support the stemness of cancer cells by inducing the TGF-β1/Smad2/3 [105], and activating ERK1/2 pathway in glioblastoma [106]. A significant amount of in vivo and in vitro experiments suggests that the inhibition of the WNT/β-catenin pathway could weaken the TAM-induced upregulation of CSC stemness in HCC [107] and lymphoma [108]. These results strongly support the notion that TAMs support the induction, maintenance, and expansion of CSC and other stem cell subtypes (such as mesenchymal stem cells) in TME.
Chemotherapeutic and radiotherapeutic resistance of TAMs
Radiotherapy and chemotherapy are common cancer treatments and the roles of TAMs in these therapies have been studied extensively. It was reported that TAMs could reduce the efficacy of cancer chemotherapy. TAM-born CCL5, which induced the activation of STAT3 and further mediated the upregulation of transcription factor Nanog, finally resulted in chemotherapeutic drug resistance [109]. Guan et al. found that prostate cancer cells increased the secretion of CXCL12 by TAMs after combined therapy of docetaxel together with androgen deprivation, which further facilitated cancer cell survival and led to a decreased response towards chemotherapy via CXCR4 activation [110]. EGFR-TKI is a new method for the treatment of advanced NSCLC [111]. Chung et al. investigated 206 cases of NSCLC patients who received EGFR-TKI treatment and found that TAM counts were significantly higher in patients with progressive disease than in those with non-progressive disease. Moreover, high TAM counts were significantly associated with lower progression-free survival and overall survival, suggesting that TAMs are related to reduced treatment responsiveness after EGFR-TKI administration [112].
In contrast, TAMs could enhance the effect of radiotherapy. TAMs are increasingly recruited into tumors after radiotherapy and can modulate the response of tumor cells to treatment [113]. Stafford et al. clarified that the CSF-1R inhibitor PLX3397 could prevent myeloid monocyte differentiation into TAMs, and improve the response of glioblastoma towards ionizing radiation treatment which further delays the recurrence of glioblastoma [114]. Rahal et al. found that PM37 could block IL-4/IL-13-mediated STAT6 tyrosine phosphorylation and decreased TAM–mediated radioresistance of inflammatory breast cancer cells by down-regulation of TAM–induced protein kinase C zeta [115]. Moreover, other studies demonstrated that targeting TAM or TAM-related signaling pathways could improve the radiotherapy effect [116–119].
In summary, TAMs are a double-edged sword since sometimes they promote tumor clearance whereas sometimes they accelerate cancer progression and treatment resistance. Therefore, the elucidation of their functions in tumorigenesis requires further exploration.
Treatment strategies against TAMs
Many studies have shown that TAMs are correlated with a negative tumor prognosis in most human tumors [42] such as breast cancer, gastric cancer, and Hodgkin lymphoma [120–123]. Therefore, targeting TAMs is a potential strategy for cancer treatment (Table 1). In the following paragraphs, we summarized some aspects of this strategy.
Table 1.
Clinical trials on TAMs (from https://clinicaltrials.gov/)
| Action | Agent | Combination | Target | Status | Tumor type | Clinical trial number |
|---|---|---|---|---|---|---|
| Inhibiting the recruitment of TAMs | Carlumab | NA | CCL2 | Completed II | Prostate cancer | NCT00992186 |
| CNTO 888(mAb) |
Gemcitabine Paclitaxel and carboplatin docetaxel |
CCL2 | Completed I | Solid tumors | NCT01204996 | |
| CNTO 888(mAb) | NA | CCL2 | Completed I | Solid tumors | NCT00537368 | |
| PF-04136309 | FOLFIRINOX* | CCR2 | Completed I | Pancreatic neoplasms | NCT01413022 | |
| MLN1202 | NA | CCR2 | Completed II | Bone metastases | NCT01015560 | |
| Activating TAMs | MCS110 | Carboplatin and Gemcitabine | CSF-1 | Completed II | Triple-negative breast cancer | NCT02435680 |
| MCS110 | Placebo | CSF-1 | Completed II | Cancer | NCT01643850 | |
| MCS110 | NA | CSF-1 | Terminated I/II | Prostate cancer, Bone Metastases | NCT00757757 | |
|
IMC-CS4 (LY3022855) |
NA | CSF-1R | Completed I | Solid tumors | NCT01346358 | |
| IMC-CS4 | NA | CSF-1R | Completed I | Breast or Prostate cancer | NCT02265536 | |
| AMG 820 | NA | CSF-1R | Completed I | Solid tumors | NCT01444404 | |
| AMG 820 | pembrolizumab | CSF-1R | Completed I/II | Pancreatic cancer, Colorectal cancer, Non-small cell lung cancer | NCT02713529 | |
| Emactuzumab | Atezolizumab |
CSF-1R PD-L1 |
Completed Ib | Solid tumors | NCT02323191 | |
| ARRY-382 | Pembrolizumab |
CSF-1R PD-1 |
Completed I b/II | Solid tumors | NCT02880371 | |
| Pexidartinib | Durvalumab |
CSF-1R PD-L1 |
Completed I | Pancreatic or Colorectal cancers | NCT02777710 | |
| SNDX-6352 | Durvalumab |
CSF-1R PD-L1 |
Active, not recruiting I | Solid tumors | NCT03238027 | |
| BLZ945 | PDR001 |
CSF-1R PD-1 |
Active, not recruiting I/II | Solid tumors | NCT02829723 | |
|
Cabiralizumab (BMS-986227, FPA008) |
Nivolumab (BMS-936558) |
CSF-1R PD-1 |
Completed I | Malignancies | NCT03158272 | |
| Trabectedin | Durvalumab (MEDI4736) | PD-L1 | Active, not recruiting Ib | Soft-tissue sarcomas and ovarian carcinomas | NCT03085225 | |
| PLX7486 | NA | CSF-1R | Terminated I | Solid tumors | NCT01804530 | |
|
PLX3397 (Pexidartinib) |
NA | CSF-1R | Terminated II | Glioblastoma | NCT01349036 | |
| PLX3397 | NA | CSF-1R | Completed II | Hodgkin lymphoma | NCT01217229 | |
| PLX3397 | NA | CSF-1R | Terminated II | Prostate cancer | NCT01499043 | |
| PLX3397 | Sirolimus | CSF-1R |
Recruiting I Recruiting II |
Sarcoma Malignant Peripheral Nerve Sheath Tumors |
NCT02584647 | |
| PLX3397 | Pembrolizumab | CSF-1R | Terminated I/II | Tumors* | NCT02452424 | |
| PLX3397 | Eribulin | CSF-1R | Completed I b/II | Breast cancer | NCT01596751 | |
| PLX3397(Turalio) | NA | Recruiting I/II | Leukemias, Solid tumors | NCT02390752 | ||
|
PLX3397 (Pexidartinib) |
Paclitaxel (Onxol) |
Completed I | Solid tumors | NCT01525602 | ||
| Alemtuzumab | NA | CD52 | Terminated I | Ovarian/primary peritoneal cancer | NCT00637390 | |
| Alemtuzumab | fludarabine and cyclophosphamide | CD52 | Completed II | Kidney cancer | NCT00073879 | |
| Reprogramming TAMs | Chi Lob 7/4 | NA | CD40 | Completed I | Malignancies | NCT01561911 |
| GM.CD40L* Vaccine | CCL21 | CD40 | Completed I/II | Lung cancer, Adenocarcinoma | NCT01433172 | |
| CP-870,893* | NA | CD40 | Completed I | Advanced solid tumors | NCT02225002 | |
| CP-870,893 | Tremelimumab* | CD40 | Completed I | Melanoma | NCT01103635 | |
| CP-870,893 | Paclitaxel + Carboplatin | CD40 | Completed I | Neoplasms | NCT00607048 | |
| CP-870,893 | Gemcitabine | CD40 | Completed I | Adenocarcinoma pancreas | NCT01456585 | |
| RO7009789 | Emactuzumab (RO5509554) | CD40 | Completed I | Neoplasms | NCT02760797 | |
| RO7009789 | nab-paclitaxel, gemcitabine | CD40 | Completed I | Pancreatic cancer | NCT02588443 | |
| RO7009789 | Vanucizumab, Bevacizumab | CD40 | Completed I | Solid tumors | NCT02665416 | |
| Selicrelumab (RO7009789) | Atezolizumab |
CD40 PD-1 |
Completed I | Solid tumors | NCT02304393 | |
| APX005M | Nivolumab | CD40 | Completed I/II | Non-small cell lung cancer, Metastatic Melanoma | NCT03123783 | |
| IPI-549 | Nivolumab |
PI3K-γ PD-1 |
Active, not recruiting I | Solid tumors, non-small cell lung cancer, melanoma, breast cancer | NCT02637531 | |
| TTI-621 |
PD-1/PD-L1 Inhibitor* pegylated interferon-α2a talimogene laherparepvec (T-Vec) radiation |
SIRPα-IgG1 Fc | Terminated I | Solid Tumors, Mycosis fungoides, Melanoma, Merkel-cell carcinoma, Squamous cell carcinoma, Breast carcinoma, Human papillomavirus-related malignant neoplasm, Soft tissue sarcoma | NCT02890368 | |
| Hu5F9-G4 | Atezolizumab | CD47 | Completed I | Acute myeloid leukemia | NCT03922477 | |
| CC-90002 | NA | CD47 | Terminated I |
Leukemia, Myeloid, Acute myelodysplastic syndromes |
NCT02641002 | |
| CC-90002 | Rituximab | CD47 | Completed I | Hematologic neoplasms | NCT02367196 | |
| GSK3145095 | pembrolizumab | RIP | Terminated II | Neoplasms, Pancreatic | NCT03681951 | |
| NKTR-262 |
Bempegaldesleukin (NKTR-214) Nivolumab |
TLR7/8 PD-1 |
Active, not recruiting I/II | Solid tumors | NCT03435640 | |
| WP1066 | NA | STAT3 | Active, not recruiting I | Glioma and Brain metastasis from melanoma | NCT01904123 | |
|
AZD9150 (ISIS 481464) |
NA | STAT3 | Completed I/Ib | Advanced/Metastatic Hepatocellular carcinoma | NCT01839604 | |
| Imprime PGG | Cetuximab | MAPK | Completed II | Colorectal Cancer | NCT00912327 | |
| Immunological Adjuvant OPT-821 | β-glucan | Active, not recruiting I/II | Neuroblastoma | NCT00911560 | ||
| β-Glucan | Anti-GD2 Monoclonal Antibody 3F8 | Active, not recruiting I | Neuroblastoma | NCT00492167 |
FOLFIRINOX*: fluorouracil, leucovorin calcium, irinotecan hydrochloride, and oxaliplatin
Tumors*: Melanoma, Non-small Cell Lung Cancer, Squamous Cell Carcinoma of the Head and Neck, Gastrointestinal Stromal Tumor (GIST), Ovarian Cancer
GM.CD40L*: GM-CSF-Producing and CD40L-Expressing Bystander Cell Line
Tremelimumab*: Blocking Anti-CTLA-4 Antibody
CP-870,893*: Agonist Anti-CD40 Antibody
PD-1/PD-L1 Inhibitor*: nivolumab, pembrolizumab, durvalumab, avelumab, or atezolizumab
Targeting angiogenesis
It could be shown that dual inhibition of VEGF/Ang-2 could prolong the survival of preclinical glioblastoma models through decreasing tumor burden, improving the vascular morphological normalization, and reprogramming TAMs to an M1-phenotype [124]. The HIF-1 pathway is stimulated by hypoxia and treatment with HIF-1 inhibitors led to the influx of CD11b+ bone marrow monocytes as well as the development of a tumor vascular system, both of which ultimately inhibited tumor cell regeneration [125]. However, understanding its specific anti-angiogenesis mechanism is useful to develop additional therapeutic strategies.
Targeting phagocytosis of TAMs
CD47, a transmembrane glycoprotein expressed on cancer cells, serves as a “don't eat me" signal. CD47 combined with the signal-regulatory protein (SIRP) α (CD172a or SHPS-1) on macrophages could inhibit TAM phagocytosis [126, 127]. A large number of studies have shown that monoclonal antibodies were able to disrupt the CD47-SIRP 1α signaling pathway. These antibodies were capable of enhancing the phagocytosis of TAMs, increasing the number of tumor-infiltrating immune cells, suppressing the progression of tumor cells and hematological malignancies [128–130]. When the blockade of this signaling pathway was combined with mAbs targeting different tumor antigens, such as cetuximab, trastuzumab, and rituximab, the effect of cancer immunotherapies was even more pronounced [131, 132]. Clinical trials were developed using anti-CD47 antibodies (Hu5F-G4 [133], CC-90002, SRF231, and IBI188) and SIRFα-Fc fusion proteins (TTI-621, ALX148, SHR-1603) [134]. Golubovskaya et al. designed a CD47+-CAR (chimeric antigen receptor)-T cell able of targeting various cancer cells. It could be demonstrated that the CAR T cells could effectively kill ovarian, pancreatic, and cervical cancer cell lines, and moreover were able to generate IL-2 that is positively associated with the expression of CD47 [135]. In addition, Ferlin et al. suggested that targeting CD47 with a bispecific antibody might reinforce the anti-tumor activity and limit the toxicity in vivo [136]. To reduce off-target toxicity, engineered high-affinity CD47 extracellular domain-targeted blocking SIRPα, such as engineered SIRPα without Fc fusion, is now being developed [137].
Targeting TAM-associated immune checkpoints
Nowadays, checkpoint blockade inhibitors as a part of cancer immunotherapy are widely accepted by clinicians [138]. It is known that clinically relevant targets comprise PD-1/PD-L1 and CTLA-4. Anti-PD-1/PD-L1 mainly exert their effects by facilitating the activation of tumor-specific cytotoxic T cells [139–141].
The expression of PD-1 is negatively related to the capability of TAM phagocytosis and blocking PD-1/PD-L1 in vivo increased the phagocytosis of TAMs, decreased the growth of the tumor, and further prolonged the survival time of the mice by depending on macrophages [142]. Xiao et al. reported that TAMs in HCC tissues had a high expression of Siglec-10 with an associated predicted poor prognosis. Blocking Siglec-10high TAMs improved immunotherapy against HCC [143]. Xiong et al. proved that anti-PD-L1 treatment led to a transformation of TAMs to an M1-phenotype by increasing the IFN-γ levels [144]. Su et al. found that macrophages following antibody-dependent cellular phagocytosis (ADCP) could inhibit the antibody-dependent cellular cytotoxicity (ADCC) that was mediated by NK cells, and also suppressed T cell-mediated cytotoxicity in breast cancer and lymphoma. Moreover, they also found that after anti-HER2 antibody administration that was combined with PD-L1 and indoleamine 2,3-dioxygenase (IDO) inhibitors, reinforced the therapeutic efficacy of anti-tumor immunity and anti-HER2 efficacy in mouse models [145].
Viitala et al. proposed that the macrophage scavenger receptor common lymphatic endothelial and vascular endothelial receptor-1 (Clever-1, also named Stabilin-1 or Feel-1) expressed by TAMs can be defined as a checkpoint receptor because Clever-1 gene defects cause immune stimulation of TAMs and concomitant activation of endogenous anti-tumor CD8+ T cells. Therefore, an immunotherapeutic blockage of Clever-1 could achieve a similar effect as a PD-1 checkpoint inhibitor administration. Moreover, the combination of an anti-Clever-1/anti-PD-1 inhibitor has a synergistic effect on aggressive tumors [146]. Zhou et al. hypothesized that blocking the phagocytic receptor MerTK leads to an apoptotic cell accumulation in tumors and triggers an IFN-α response, leading to a further increase in the anti-tumor immune response. The treatment of tumor-bearing mice showed that administration of an anti-MerTK antibody resulted in T cell activation which creates synergies in combination with an anti-PD-1/ PD-L1 administration [147]. Clinical trials evaluating the treatment with checkpoint inhibitors and anti-TAM agents (for example, anti-CSF-1R antibodies) are ongoing [20].
TAM reprogramming
M1-like macrophages display anti-tumor activity, therefore, reprogramming tumor-promoting M2-like macrophages into tumor-killing M1-like macrophages seems to be a potential strategy in cancer therapy. This strategy mainly involves the activation of CD206 and Toll-like receptors (TLRs). RP-182-mediated activation of CD206 (mannose receptor) in M2-like TAMs induced endocytosis, phagosome lysosome formation, and autophagy, which resulted in a transformation of M2-like TAMs into M1-like TAMs and an associated enhancement of cancer cell phagocytosis [148]. Wang et al. reported that upregulation of serine/threonine-protein kinase 1 in TAMs of PDA tissues contributed to immune tolerance. Administration of RIP1 inhibitor in a mouse model led to the activation of CTL and the differentiation of Th cells to a hybrid Th1/ Th17 phenotype. Further mechanistic analysis proved that RIP1 inhibitor reprogrammed TAMs to an MHCIIhiTNFα+IFNγ+ phenotype via STAT1 [149].
Toll-like receptors (TLRs), a receptor of transmembrane pattern recognition, play their roles in innate immunity via recognizing pathogen-associated molecular patterns (PAMPs) [150]. TLR agonist administration (TLR7 agonist imiquimod, TLR9 CpG-oligonucleotide) resulted in the polarization of pro-inflammatory M1-macrophages by activating NF-κB [151]. So far, the TLR7 agonist imiquimod is the FDA-approved TLR agonist for clinical application which has shown significant anti-tumor activity in preclinical models of melanoma [152, 153]. Oya et al. showed that imiquimod induced an anti-tumor response via upregulating interferon γ (IFN-γ) expression in CD8+ T cells, however, imiquimod also enhanced the PD-1 inhibitory signaling resulting in T cell exhaustion. Combined treatment of imiquimod together with anti-PD-1 antibodies revealed a more obvious anti-tumor effect than each monotherapy [154]. The TLR7/8 agonist 3M-052 enhanced anti-tumor immunity by reprogramming TAMs to an M1-phenotype and the combined therapy with 3 M-052 and anti-CTLA-4 or anti-PD-L1 strengthened the treatment effect of the checkpoint blockade [155]. It has been shown that the simultaneous blockage of PI3k-γ and CSF-1R was able to reprogram TAMs into the M1-phenotype, activate the anti-tumor immune response, and further enhance the effect of anti-pancreatic cancer therapy [156]. Monophosphoryl lipid A (MPLA) is a TLR4 agonist. Recent studies reported that MPLA+IFNγ, both FDA-approved biological agents, reprogrammed TAMs to an M1-phenotype by stimulating type I IFN signaling and activation of cytotoxic T cells by IL-12 and TNFα secreted by macrophages. This led to a decreased tumor growth and inhibited metastasis in a mouse model of breast cancer and also enhanced the chemotherapy response in an ovarian cancer model [157].
Inhibiting the recruitment or proliferation of TAMs and depletion of TAMs
Inhibiting monocyte recruitment into tumor tissue is a strategy to target TAMs. There are various ways for inhibiting a TAM recruitment and/or induce a TAM exhaustion, including inhibition of CSF-1R, blocking CCL2/CCR2, targeting CD40, and others. CSF-1, also named macrophage-CSF (M-CSF), stimulates colony formation [158, 159]. The administration of CSF-1R facilitated the progression and metastasis of tumors [160], the administration of CSF-1R inhibitor could defer tumor growth by changing the TAM polarization [161]. Therefore, CSF-1R could be explored as one of the molecules to target macrophages for cancer treatment [162]. CSF-1R inhibitors under clinical research or development include PLX3397, JNJ-40346527, ARRY-382, and BLZ945 [163–166]. Moreover, anti-CSF-1R antibodies including RG7155, IMC-CS4, and FPA008 are in a clinical evaluation stage [167]. In mouse glioma models, administration of a CSF-1R inhibitor (BLZ945) prolonged mouse survival and led to a shrinkage of established tumors [168]. Akkari et al. demonstrated in a mouse model that combining a CSF-1R inhibitor with radiotherapy to target glioma TAM populations, leads to a significant increase in the survival time [169]. Moreover, combining a CSF-1R inhibitor with a CXCR antagonist also resulted in a significant anti-tumor effect but more importantly, avoided granulocytes from infiltrating into tumors [170]. In addition, treatment with CSF-1R inhibitors and anti-PD-1 antibodies could induce melanoma to regress in a transplantation mouse model [171]. The results by Shi et al. indicate that a combination of a CSF-1R inhibitor (PLX3397), oncolytic viruses, and anti-PD-1 antibodies could enhance CD8+ T cell anti-tumor functions and prolong the survival of mice suffering from colon cancer [172]. Chai et al. showed that miR-26a expression inhibited the expression of CSF-1 and the infiltration of macrophages in HCC [173].
Targeting the CCL2/CCR2 axis is a perspective therapy for cancer. Blocking the CCL2/CCR2 axis by CCL2 knockdown or the administration of CCL2 inhibitors could significantly decrease tumor morbidity via preventing the recruitment and polarization of TAMs, thus enhancing the antitumor effect of CD8+ T cells in TME [174, 175]. Pienta et al. indicated that CCL2 blockade by administration of carlumab (CNTO88), inhibited the growth of prostate cancer cells and that the drug was well-tolerated by patients suffering from metastatic castration-resistant prostate cancer as shown in a clinical trial. Each treatment led to a transient decrease of serum CCL2 levels and some patients acquired a stable disease status [176]. Brana et al. found that a combination treatment of carlumab and standard chemotherapy was well-tolerated with only mild responses of side effects were observed [177]. Moreover, targeting CCR2 is also is an effective therapeutic strategy. The CCR2 inhibitor PF-04136309 could deplete the primary or premetastatic liver of TAMs by inhibiting CCR2+ monocytes mobilized from bone marrow to tumors, leading to a further enhanced anti-tumor immunity and the reduction of growth and metastasis of the tumor [178]. The study by Wu et al. illustrated that CCR2 inhibitors combined with the anti-PD-1 drug to treat cutaneous T-cell lymphoma reduced tumor growth [179].
CD40, a member of the TNF receptor superfamily, is expressed in antigen-presenting cells (such as dendritic cells) and facilitates the activation of anti-tumor T cells and the polarization of M1-phenotype cells [180]. The combination of CD40 agonists and anti-CSF-1R antibodies resulted in an increase in pro-inflammatory macrophages, dendritic cell maturation and differentiation. Moreover, this combination was able to eliminate the population that elicited the inhibitory immune response, thereby enhancing the antitumor response [181]. In addition, the results by Xiong et al. demonstrated that combining anti-CD40 treatment with anti-PD-L1 therapy, significantly increased the anti-tumor activity [144]. Anti-CD40 antibodies and recombinant CD40 ligands are currently being examined in clinical trials as a single agent or combination with chemotherapy/immunotherapy, for the latter e.g. with CP-870,893, RO7009789, APX005M, ADC-1013, SGN-40, and SEA-CD40 [182].
Trabectedin, through the TRAIL (also named TNFRSF10B) receptor (ET743, Yondelis®), targets TAMs by activating the caspase 8 cascade [183]. Trabectedin leads to a selective depletion of TAMs and is accompanied by a reduction in angiogenesis [183]. The drug was approved by the FDA in 2015 for the treatment of unresectable or metastatic liposarcomas or smooth muscle sarcomas [184]. Therefore, TAM reduction by stimulating TAM apoptosis through TRAIL receptors is a potential therapeutic strategy [185].
Decreased tumor burden and lung metastasis were observed after treatment with histone deacetylase inhibitor TMP195 that led to the recruiting of anti-tumor TAMs. Moreover, combination therapies of TMP195 together with chemotherapy or check-in point inhibitors could dramatically reduce the tumor burden compared to monotherapy with e.g. Carboplatin, Paclitaxel, and anti-PD-1 drugs [186].
Others
A number of studies have demonstrated that nanoparticles can be employed as an effective therapeutic approach that may also be suitable as a new TAM-targeted strategy [187]. Hyaluronic acid-coated, mannan-conjugated MnO2 nanoparticles (Man-HA-MnO2 NPs) enabled repolarization of TAMs into an M1 phenotype (an anti-tumor phenotype) that significantly enhanced tumor oxygenation. At the same time, HIF-1 α and VEGF levels were downregulated, leading to a further relief of the tumor hypoxia and therefore the enhancement of the chemotherapy response. In addition, doxorubicin bound to Man-HA-MnO2 NPs was capable of synergistically checking the growth and proliferation of tumor cells [188]. The study by Penn et al. showed that G5-dendrimer nanoparticles loaded with methotrexate (as a ligand and toxin) was able to selectively target the folate receptor-2 (high expression in ovarian TAMs) on TAMs, leading to the depletion of TAMs in ovarian cancer tissues. Moreover, these results demonstrated that nanoparticles had a better therapeutic effect than cisplatin in cancer treatment [189]. Interestingly, investigators have also found that extracellular vesicles (e.g. exosomes) produced by M1-type macrophages can also reprogram TAMs to M1-like macrophages [187, 190]. It has been reported that M1-derived exosomes could strengthen the effect of delivering anti-tumor drugs and also improve the effectiveness of the treatment by releasing Th1-type cytokines [191]. Moreover, because of the pro-inflammatory character of M1-derived exosome content, they could be employed as a vaccine adjuvant [192]. However, due to the small number of extracellular vesicles produced by M1 macrophages and the complexity of isolation and purification, it is still a challenge at present [190, 193].
Zhang et al. designed a ultrasmall copper nanoparticles (Cu@CuOx) targeting CCR2, which could be loaded with the chemotherapy drug gemcitabine, and that could be delivered to PDAC tissues. Cu@CuOx was shown to significantly inhibit tumor progression and also to prolong survival in a syngeneic xenograft mouse model [194].
The immunosuppressive phenotype of TAMs is mainly determined by the unsaturated fatty acid metabolism which converts bone-marrow-derived macrophages to the M2 phenotype. Lipid droplets enriched in this process could therefore serve as a target for chemical inhibitors that block TAM polarization and tumor growth [195].
The above results indicate that targeted TAM therapies are potential strategies for cancer treatment. However, the development of an appropriate treatment strategy depends on the tumor type and the role of targeted TAMs as well as the application of appropriate therapeutic agents.
Conclusion and perspective
It is commonly agreed upon that macrophages are crucial for the initiation and progression of tumors. These macrophages are divided into two types: M1-like and M2-like phenotypes. M1 has been characterized as being pro-inflammatory and able to phagocytize tumor cells, while M2 has been characterized as being anti-inflammatory. TAMs are considered as belonging to the M2-phenotype that can facilitate tumor initiation, angiogenesis, invasion and metastasis. An increasing number of studies have revealed the influence of the TME on tumorigenesis and development. TAMs as a major component of the TME, are a complex heterogeneous cell population that contributes to the malignant character of solid tumors. The heterogeneity and specific characteristics of TAMs in tumors lay a foundation for the development of personalized TMA-based treatment methods. We summarized the current major therapeutic approaches for targeting TAMs, for example, targeting angiogenesis, inhibiting the recruitment of TAMs, and reprogramming TAMs. It is worth mentioning that nanoparticle development is becoming increasingly popular and that these nanosystems can be employed as drug carriers. In this paper, we briefly mentioned the carrier role of nanoparticles or extracellular vesicles in TAMs and presented the current limitations. Given the complex role of TAMs in tumorigenesis, we need more in-depth studies on their functions and the regulatory mechanisms to discover effective anti-tumor targets. Targeting TAMs may result in reversing the TME with regard to tumor promotion and immune inhibition, a promising novel therapeutic modality for future precision tumor treatments. Besides, experimental, preclinical, and clinical studies on TAMs are ongoing and we believe that targeting TAMs will be a valuable therapeutic strategy in the future. There are still many unanswered questions about the key molecules or signals functionally reprogramming TAMs. Further understanding of the regulation of TAMs is required to answer these questions. In conclusion, TAMs are functionally diverse and play complex roles in the TME. Targeted mono-therapy of TAMs or in combination with conventional therapeutic approaches may shed more light on future options of cancer treatment.
Acknowledgements
Not applicable.
Abbreviations
- TAM
Tumor-associated macrophage
- TME
Tumor microenvironment
- CCL2
C–C chemokine ligand 2
- CCL5
C–C chemokine ligand 5
- CSF-1
Colony-stimulating factor-1
- GM-CSF
Granulocyte–macrophage colony-stimulating factor
- NSCLC
Non-small cell lung cancer
- EMP
Erythroid-myeloid progenitors
- MDP
Monocyte-producing macrophage/dendritic progenitors
- VEGFA
Vascular endothelial growth factor A
- CCL18
C–C chemokine ligand 18
- CCL20
C–C chemokine ligand 20
- HCC
Hepatocellular carcinoma
- CXCL12
C-X-C motif chemokine ligand 12
- IFN-γ
Interferon-γ
- TNF-α
Tumor necrosis factor-α
- LPS
Lipopolysaccharide
- Th
Helper T cell
- iNOS
Nitric oxide synthase
- ROS
Reactive oxygen species
- IL-12
Interleukin-12
- IL-4
Interleukin-4
- IL-10
Interleukin-10
- IL-13
Interleukin-13
- CD68
Cell differentiation 68
- CD163
Cell differentiation 163
- CD206
Cell differentiation 206
- NK cells
Natural killer cells
- NF- κB
Nuclear factor- κB
- STAT3
Signal transducer and activator of transcription 3
- HIF1 α
Hypoxia inducible factor 1 α
- EGF
Epidermal growth factor
- TGF-β
Transforming growth factor
- COX-2
Cyclooxygenase-2
- PGF
Placenta growth factor
- FGF
Fibroblast growth factor
- PDGF
Platelet-derived growth factor
- Ang
Angiotensin
- IDO
Indoleamine 2,3-dioxygenase
- MMP-9
Metalloproteinase-9
- ECM
Extracellular matrix
- EMT
Epithelial-mesenchymal transformation
- CRC
Colorectal cancer
- PRL-3
Phosphatase of regenerating liver-3
- ERO 1-α
Endoplasmic reticulum oxidoreductase 1-α
- CRYAB
αB-Crystallin
- MDSCs
Bone marrow-derived suppressor cells
- Treg cell
Regulatory T cell
- PD-1
Programmed cell death 1
- PD-L1
Programmed cell death ligand 1
- ADCP
Antibody-dependent cellular phagocytosis
- ADCC
Antibody-dependent cellular cytotoxicity
- TRAIL
TNF-related apoptosis-inducing ligand
- CSC
Cancer stem cell
- HA
Hyaluronic acid
- MFG-E8
Milk-fat globule-epidermal growth factor-VIII
- SIRP α
Signal-regulatory protein α
- CAR-T cells
Chimeric antigen receptor T cells
- TLRs
Toll-like receptors
- MPLA
Monophosphoryl lipid A
- PAMPs
Pathogen-associated molecular patterns
- PDAC
Pancreatic ductal adenocarcinoma
- Man-HA-MnO2 NPs
Mannanconjugated MnO2 nanoparticles
Authors’ contributions
SZ drafted the manuscript and prepared the figures. MY and YW collected the related references and participated in the discussion. BD and KW designed this review and revised the manuscript. All authors contributed to this manuscript. All authors read and approved the final manuscript.
Funding
This work was supported by the National Natural Science Foundation of China (No.81874120, 82073370) and the Henan Medical Science and Technology Program Joint Commitment Project (LHGJ20200175).
Availability of data and materials
Not applicable.
Declarations
Ethics approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Footnotes
The original online version of this article was revised: The typo in given name of first author name has been corrected
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Change history
2/3/2022
A Correction to this paper has been published: 10.1186/s40164-022-00258-1
Contributor Information
Shuangli Zhu, Email: 1667985477@qq.com.
Ming Yi, Email: 1978135000@qq.com.
Yuze Wu, Email: wuyz0304@163.com.
Bing Dong, Email: dongbing2015@126.com.
Kongming Wu, Email: wukm_lab@163.com.
References
- 1.Pan Y, Yu Y, Wang X, Zhang T. Tumor-associated macrophages in tumor immunity. Front Immunol. 2020;11:583084. doi: 10.3389/fimmu.2020.583084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Huang X, Li Y, Fu M, Xin HB. Polarizing macrophages in vitro. Methods Mol Biol. 2018;1784:119–126. doi: 10.1007/978-1-4939-7837-3_12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Shapouri-Moghaddam A, Mohammadian S, Vazini H, Taghadosi M, Esmaeili SA, Mardani F, et al. Macrophage plasticity, polarization, and function in health and disease. J Cell Physiol. 2018;233(9):6425–6440. doi: 10.1002/jcp.26429. [DOI] [PubMed] [Google Scholar]
- 4.Qiu Y, Chen T, Hu R, Zhu R, Li C, Ruan Y, et al. Next frontier in tumor immunotherapy: macrophage-mediated immune evasion. Biomark Res. 2021;9(1):72. doi: 10.1186/s40364-021-00327-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Shu Y, Cheng P. Targeting tumor-associated macrophages for cancer immunotherapy. Biochim Biophys Acta Rev Cancer. 2020;1874(2):188434. doi: 10.1016/j.bbcan.2020.188434. [DOI] [PubMed] [Google Scholar]
- 6.Komohara Y, Jinushi M, Takeya M. Clinical significance of macrophage heterogeneity in human malignant tumors. Cancer Sci. 2014;105(1):1–8. doi: 10.1111/cas.12314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Mantovani A, Bottazzi B, Colotta F, Sozzani S, Ruco L. The origin and function of tumor-associated macrophages. Immunol Today. 1992;13(7):265–270. doi: 10.1016/0167-5699(92)90008-U. [DOI] [PubMed] [Google Scholar]
- 8.Yi M, Xu L, Jiao Y, Luo S, Li A, Wu K. The role of cancer-derived microRNAs in cancer immune escape. J Hematol Oncol. 2020;13(1):25. doi: 10.1186/s13045-020-00848-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Zhao S, Mi Y, Guan B, Zheng B, Wei P, Gu Y, et al. Tumor-derived exosomal miR-934 induces macrophage M2 polarization to promote liver metastasis of colorectal cancer. J Hematol Oncol. 2020;13(1):156. doi: 10.1186/s13045-020-00991-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lu CS, Shiau AL, Su BH, Hsu TS, Wang CT, Su YC, et al. Oct4 promotes M2 macrophage polarization through upregulation of macrophage colony-stimulating factor in lung cancer. J Hematol Oncol. 2020;13(1):62. doi: 10.1186/s13045-020-00887-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Fan CS, Chen LL, Hsu TA, Chen CC, Chua KV, Li CP, et al. Endothelial-mesenchymal transition harnesses HSP90α-secreting M2-macrophages to exacerbate pancreatic ductal adenocarcinoma. J Hematol Oncol. 2019;12(1):138. doi: 10.1186/s13045-019-0826-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Franklin RA, Liao W, Sarkar A, Kim MV, Bivona MR, Liu K, et al. The cellular and molecular origin of tumor-associated macrophages. Science. 2014;344(6186):921–925. doi: 10.1126/science.1252510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Datar I, Qiu X, Ma HZ, Yeung M, Aras S, de la Serna I, et al. RKIP regulates CCL5 expression to inhibit breast cancer invasion and metastasis by controlling macrophage infiltration. Oncotarget. 2015;6(36):39050–39061. doi: 10.18632/oncotarget.5176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Frankenberger C, Rabe D, Bainer R, Sankarasharma D, Chada K, Krausz T, et al. Metastasis suppressors regulate the tumor microenvironment by blocking recruitment of prometastatic tumor-associated macrophages. Cancer Res. 2015;75(19):4063–4073. doi: 10.1158/0008-5472.CAN-14-3394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ruffell B, Affara NI, Coussens LM. Differential macrophage programming in the tumor microenvironment. Trends Immunol. 2012;33(3):119–126. doi: 10.1016/j.it.2011.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Wang L, He T, Liu J, Tai J, Wang B, Chen Z, et al. Pan-cancer analysis reveals tumor-associated macrophage communication in the tumor microenvironment. Exp Hematol Oncol. 2021;10(1):31. doi: 10.1186/s40164-021-00226-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Chen Y, Jin H, Song Y, Huang T, Cao J, Tang Q, et al. Targeting tumor-associated macrophages: a potential treatment for solid tumors. J Cell Physiol. 2021;236(5):3445–3465. doi: 10.1002/jcp.30139. [DOI] [PubMed] [Google Scholar]
- 18.Liu Y, Xu R, Gu H, Zhang E, Qu J, Cao W, et al. Metabolic reprogramming in macrophage responses. Biomark Res. 2021;9(1):1. doi: 10.1186/s40364-020-00251-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kim DW, Min HS, Lee KH, Kim YJ, Oh DY, Jeon YK, et al. High tumour islet macrophage infiltration correlates with improved patient survival but not with EGFR mutations, gene copy number or protein expression in resected non-small cell lung cancer. Br J Cancer. 2008;98(6):1118–1124. doi: 10.1038/sj.bjc.6604256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Cassetta L, Pollard JW. Targeting macrophages: therapeutic approaches in cancer. Nat Rev Drug Discov. 2018;17(12):887–904. doi: 10.1038/nrd.2018.169. [DOI] [PubMed] [Google Scholar]
- 21.Zins K, Abraham D. Cancer immunotherapy: targeting tumor-associated macrophages by gene silencing. Methods Mol Biol. 2020;2115:289–325. doi: 10.1007/978-1-0716-0290-4_17. [DOI] [PubMed] [Google Scholar]
- 22.Metchnikoff E. Leçons sur la pathologie comparée de l’inflammation. Dtsch Med Wochenschr. 1893;19(2):37–39. [Google Scholar]
- 23.Davies LC, Jenkins SJ, Allen JE, Taylor PR. Tissue-resident macrophages. Nat Immunol. 2013;14(10):986–995. doi: 10.1038/ni.2705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Cassetta L, Pollard JW. Tumor-associated macrophages. Curr Biol. 2020;30(6):R246–R248. doi: 10.1016/j.cub.2020.01.031. [DOI] [PubMed] [Google Scholar]
- 25.Franklin RA, Li MO. Ontogeny of tumor-associated macrophages and its implication in cancer regulation. Trends Cancer. 2016;2(1):20–34. doi: 10.1016/j.trecan.2015.11.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Arwert EN, Harney AS, Entenberg D, Wang Y, Sahai E, Pollard JW, et al. A unidirectional transition from migratory to perivascular macrophage is required for tumor cell intravasation. Cell Rep. 2018;23(5):1239–1248. doi: 10.1016/j.celrep.2018.04.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Qian BZ, Li J, Zhang H, Kitamura T, Zhang J, Campion LR, et al. CCL2 recruits inflammatory monocytes to facilitate breast-tumour metastasis. Nature. 2011;475(7355):222–225. doi: 10.1038/nature10138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Murray PJ, Allen JE, Biswas SK, Fisher EA, Gilroy DW, Goerdt S, et al. Macrophage activation and polarization: nomenclature and experimental guidelines. Immunity. 2014;41(1):14–20. doi: 10.1016/j.immuni.2014.06.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.O'Neill LA, Kishton RJ, Rathmell J. A guide to immunometabolism for immunologists. Nat Rev Immunol. 2016;16(9):553–565. doi: 10.1038/nri.2016.70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Hao Z, Li R, Meng L, Han Z, Hong Z. Macrophage, the potential key mediator in CAR-T related CRS. Exp Hematol Oncol. 2020;9:15. doi: 10.1186/s40164-020-00171-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ngambenjawong C, Gustafson HH, Pun SH. Progress in tumor-associated macrophage (TAM)-targeted therapeutics. Adv Drug Deliv Rev. 2017;114:206–221. doi: 10.1016/j.addr.2017.04.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Italiani P, Boraschi D. From monocytes to M1/M2 macrophages: phenotypical vs functional differentiation. Front Immunol. 2014;5:514. doi: 10.3389/fimmu.2014.00514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Grivennikov SI, Greten FR, Karin M. Immunity, inflammation, and cancer. Cell. 2010;140(6):883–899. doi: 10.1016/j.cell.2010.01.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Liu XL, Pan Q, Cao HX, Xin FZ, Zhao ZH, Yang RX, et al. Lipotoxic hepatocyte-derived exosomal microRNA 192–5p activates macrophages through Rictor/Akt/Forkhead box transcription factor O1 signaling in nonalcoholic fatty liver disease. Hepatology. 2020;72(2):454–469. doi: 10.1002/hep.31050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Xu Y, Cui K, Li J, Tang X, Lin J, Lu X, et al. Melatonin attenuates choroidal neovascularization by regulating macrophage/microglia polarization via inhibition of RhoA/ROCK signaling pathway. J Pineal Res. 2020;69(1):e12660. doi: 10.1111/jpi.12660. [DOI] [PubMed] [Google Scholar]
- 36.Kim TH, Kang MS, Mandakhbayar N, El-Fiqi A, Kim HW. Anti-inflammatory actions of folate-functionalized bioactive ion-releasing nanoparticles imply drug-free nanotherapy of inflamed tissues. Biomaterials. 2019;207:23–38. doi: 10.1016/j.biomaterials.2019.03.034. [DOI] [PubMed] [Google Scholar]
- 37.Perry CJ, Muñoz-Rojas AR, Meeth KM, Kellman LN, Amezquita RA, Thakral D, et al. Myeloid-targeted immunotherapies act in synergy to induce inflammation and antitumor immunity. J Exp Med. 2018;215(3):877–893. doi: 10.1084/jem.20171435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Kotwal GJ, Chien S. Macrophage differentiation in normal and accelerated wound healing. Results Probl Cell Differ. 2017;62:353–364. doi: 10.1007/978-3-319-54090-0_14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Annamalai RT, Turner PA, Carson WFT, Levi B, Kunkel S, Stegemann JP. Harnessing macrophage-mediated degradation of gelatin microspheres for spatiotemporal control of BMP2 release. Biomaterials. 2018;161:216–227. doi: 10.1016/j.biomaterials.2018.01.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Hao S, Meng J, Zhang Y, Liu J, Nie X, Wu F, et al. Macrophage phenotypic mechanomodulation of enhancing bone regeneration by superparamagnetic scaffold upon magnetization. Biomaterials. 2017;140:16–25. doi: 10.1016/j.biomaterials.2017.06.013. [DOI] [PubMed] [Google Scholar]
- 41.Chanmee T, Ontong P, Konno K, Itano N. Tumor-associated macrophages as major players in the tumor microenvironment. Cancers (Basel) 2014;6(3):1670–1690. doi: 10.3390/cancers6031670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Qian BZ, Pollard JW. Macrophage diversity enhances tumor progression and metastasis. Cell. 2010;141(1):39–51. doi: 10.1016/j.cell.2010.03.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Yang L, Zhang Y. Tumor-associated macrophages: from basic research to clinical application. J Hematol Oncol. 2017;10(1):58. doi: 10.1186/s13045-017-0430-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Kaneda MM, Messer KS, Ralainirina N, Li H, Leem CJ, Gorjestani S, et al. PI3Kγ is a molecular switch that controls immune suppression. Nature. 2016;539(7629):437–442. doi: 10.1038/nature19834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Balkwill F, Mantovani A. Inflammation and cancer: back to Virchow? Lancet. 2001;357(9255):539–545. doi: 10.1016/S0140-6736(00)04046-0. [DOI] [PubMed] [Google Scholar]
- 46.Aggarwal BB, Vijayalekshmi RV, Sung B. Targeting inflammatory pathways for prevention and therapy of cancer: short-term friend, long-term foe. Clin Cancer Res. 2009;15(2):425–430. doi: 10.1158/1078-0432.CCR-08-0149. [DOI] [PubMed] [Google Scholar]
- 47.Balkwill FR, Mantovani A. Cancer-related inflammation: common themes and therapeutic opportunities. Semin Cancer Biol. 2012;22(1):33–40. doi: 10.1016/j.semcancer.2011.12.005. [DOI] [PubMed] [Google Scholar]
- 48.Crusz SM, Balkwill FR. Inflammation and cancer: advances and new agents. Nat Rev Clin Oncol. 2015;12(10):584–596. doi: 10.1038/nrclinonc.2015.105. [DOI] [PubMed] [Google Scholar]
- 49.Moore RJ, Owens DM, Stamp G, Arnott C, Burke F, East N, et al. Mice deficient in tumor necrosis factor-alpha are resistant to skin carcinogenesis. Nat Med. 1999;5(7):828–831. doi: 10.1038/10552. [DOI] [PubMed] [Google Scholar]
- 50.Canli Ö, Nicolas AM, Gupta J, Finkelmeier F, Goncharova O, Pesic M, et al. Myeloid cell-derived reactive oxygen species induce epithelial mutagenesis. Cancer Cell. 2017;32(6):869–83.e5. doi: 10.1016/j.ccell.2017.11.004. [DOI] [PubMed] [Google Scholar]
- 51.Grivennikov SI, Wang K, Mucida D, Stewart CA, Schnabl B, Jauch D, et al. Adenoma-linked barrier defects and microbial products drive IL-23/IL-17-mediated tumour growth. Nature. 2012;491(7423):254–258. doi: 10.1038/nature11465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Kong L, Zhou Y, Bu H, Lv T, Shi Y, Yang J. Deletion of interleukin-6 in monocytes/macrophages suppresses the initiation of hepatocellular carcinoma in mice. J Exp Clin Cancer Res. 2016;35(1):131. doi: 10.1186/s13046-016-0412-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Rapisarda A, Melillo G. Overcoming disappointing results with antiangiogenic therapy by targeting hypoxia. Nat Rev Clin Oncol. 2012;9(7):378–390. doi: 10.1038/nrclinonc.2012.64. [DOI] [PubMed] [Google Scholar]
- 54.Jetten N, Verbruggen S, Gijbels MJ, Post MJ, De Winther MP, Donners MM. Anti-inflammatory M2, but not pro-inflammatory M1 macrophages promote angiogenesis in vivo. Angiogenesis. 2014;17(1):109–118. doi: 10.1007/s10456-013-9381-6. [DOI] [PubMed] [Google Scholar]
- 55.Zhao Y, Adjei AA. Targeting angiogenesis in cancer therapy: moving beyond vascular endothelial growth factor. Oncologist. 2015;20(6):660–673. doi: 10.1634/theoncologist.2014-0465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Yin M, Li X, Tan S, Zhou HJ, Ji W, Bellone S, et al. Tumor-associated macrophages drive spheroid formation during early transcoelomic metastasis of ovarian cancer. J Clin Invest. 2016;126(11):4157–4173. doi: 10.1172/JCI87252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Dirkx AE, Oude Egbrink MG, Wagstaff J, Griffioen AW. Monocyte/macrophage infiltration in tumors: modulators of angiogenesis. J Leukoc Biol. 2006;80(6):1183–1196. doi: 10.1189/jlb.0905495. [DOI] [PubMed] [Google Scholar]
- 58.Du R, Lu KV, Petritsch C, Liu P, Ganss R, Passegué E, et al. HIF1alpha induces the recruitment of bone marrow-derived vascular modulatory cells to regulate tumor angiogenesis and invasion. Cancer Cell. 2008;13(3):206–220. doi: 10.1016/j.ccr.2008.01.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Cui X, Morales RT, Qian W, Wang H, Gagner JP, Dolgalev I, et al. Hacking macrophage-associated immunosuppression for regulating glioblastoma angiogenesis. Biomaterials. 2018;161:164–178. doi: 10.1016/j.biomaterials.2018.01.053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Anghelina M, Krishnan P, Moldovan L, Moldovan NI. Monocytes and macrophages form branched cell columns in matrigel: implications for a role in neovascularization. Stem Cells Dev. 2004;13(6):665–676. doi: 10.1089/scd.2004.13.665. [DOI] [PubMed] [Google Scholar]
- 61.Zhang Y, Wang X. Targeting the Wnt/β-catenin signaling pathway in cancer. J Hematol Oncol. 2020;13(1):165. doi: 10.1186/s13045-020-00990-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Zhang M, Weng W, Zhang Q, Wu Y, Ni S, Tan C, et al. The lncRNA NEAT1 activates Wnt/β-catenin signaling and promotes colorectal cancer progression via interacting with DDX5. J Hematol Oncol. 2018;11(1):113. doi: 10.1186/s13045-018-0656-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Lobov IB, Rao S, Carroll TJ, Vallance JE, Ito M, Ondr JK, et al. WNT7b mediates macrophage-induced programmed cell death in patterning of the vasculature. Nature. 2005;437(7057):417–421. doi: 10.1038/nature03928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Yeo EJ, Cassetta L, Qian BZ, Lewkowich I, Li JF, Stefater JA, 3rd, et al. Myeloid WNT7b mediates the angiogenic switch and metastasis in breast cancer. Cancer Res. 2014;74(11):2962–2973. doi: 10.1158/0008-5472.CAN-13-2421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Harney AS, Arwert EN, Entenberg D, Wang Y, Guo P, Qian BZ, et al. Real-time imaging reveals local, transient vascular permeability, and tumor cell intravasation stimulated by TIE2hi macrophage-derived VEGFA. Cancer Discov. 2015;5(9):932–943. doi: 10.1158/2159-8290.CD-15-0012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Carr I, Orr FW. Invasion and metastasis. Can Med Assoc J. 1983;128(10):1164–1167. [PMC free article] [PubMed] [Google Scholar]
- 67.Pastushenko I, Blanpain C. EMT transition states during tumor progression and metastasis. Trends Cell Biol. 2019;29(3):212–226. doi: 10.1016/j.tcb.2018.12.001. [DOI] [PubMed] [Google Scholar]
- 68.Savagner P. The epithelial-mesenchymal transition (EMT) phenomenon. Ann Oncol. 2010;21(Suppl 7):vii89–92. doi: 10.1093/annonc/mdq292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Su S, Liu Q, Chen J, Chen J, Chen F, He C, et al. A positive feedback loop between mesenchymal-like cancer cells and macrophages is essential to breast cancer metastasis. Cancer Cell. 2014;25(5):605–620. doi: 10.1016/j.ccr.2014.03.021. [DOI] [PubMed] [Google Scholar]
- 70.Fu XT, Dai Z, Song K, Zhang ZJ, Zhou ZJ, Zhou SL, et al. Macrophage-secreted IL-8 induces epithelial-mesenchymal transition in hepatocellular carcinoma cells by activating the JAK2/STAT3/Snail pathway. Int J Oncol. 2015;46(2):587–596. doi: 10.3892/ijo.2014.2761. [DOI] [PubMed] [Google Scholar]
- 71.Ravi J, Elbaz M, Wani NA, Nasser MW, Ganju RK. Cannabinoid receptor-2 agonist inhibits macrophage induced EMT in non-small cell lung cancer by downregulation of EGFR pathway. Mol Carcinog. 2016;55(12):2063–2076. doi: 10.1002/mc.22451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Lin Y, Xu J, Lan H. Tumor-associated macrophages in tumor metastasis: biological roles and clinical therapeutic applications. J Hematol Oncol. 2019;12(1):76. doi: 10.1186/s13045-019-0760-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Wei C, Yang C, Wang S, Shi D, Zhang C, Lin X, et al. Crosstalk between cancer cells and tumor associated macrophages is required for mesenchymal circulating tumor cell-mediated colorectal cancer metastasis. Mol Cancer. 2019;18(1):64. doi: 10.1186/s12943-019-0976-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Tu W, Gong J, Zhou Z, Tian D, Wang Z. TCF4 enhances hepatic metastasis of colorectal cancer by regulating tumor-associated macrophage via CCL2/CCR2 signaling. Cell Death Dis. 2021;12(10):882. doi: 10.1038/s41419-021-04166-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Lee S, Lee E, Ko E, Ham M, Lee HM, Kim ES, et al. Tumor-associated macrophages secrete CCL2 and induce the invasive phenotype of human breast epithelial cells through upregulation of ERO1-α and MMP-9. Cancer Lett. 2018;437:25–34. doi: 10.1016/j.canlet.2018.08.025. [DOI] [PubMed] [Google Scholar]
- 76.Huang R, Wang S, Wang N, Zheng Y, Zhou J, Yang B, et al. CCL5 derived from tumor-associated macrophages promotes prostate cancer stem cells and metastasis via activating β-catenin/STAT3 signaling. Cell Death Dis. 2020;11(4):234. doi: 10.1038/s41419-020-2435-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Nie Y, Huang H, Guo M, Chen J, Wu W, Li W, et al. Breast phyllodes tumors recruit and repolarize tumor-associated macrophages via secreting CCL5 to promote malignant progression, which can be inhibited by CCR5 inhibition therapy. Clin Cancer Res. 2019;25(13):3873–3886. doi: 10.1158/1078-0432.CCR-18-3421. [DOI] [PubMed] [Google Scholar]
- 78.Lan Q, Lai W, Zeng Y, Liu L, Li S, Jin S, et al. CCL26 participates in the PRL-3-induced promotion of colorectal cancer invasion by stimulating tumor-associated macrophage infiltration. Mol Cancer Ther. 2018;17(1):276–289. doi: 10.1158/1535-7163.MCT-17-0507. [DOI] [PubMed] [Google Scholar]
- 79.Guo Z, Song J, Hao J, Zhao H, Du X, Li E, et al. M2 macrophages promote NSCLC metastasis by upregulating CRYAB. Cell Death Dis. 2019;10(6):377. doi: 10.1038/s41419-019-1618-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Han Y, Guo W, Ren T, Huang Y, Wang S, Liu K, et al. Tumor-associated macrophages promote lung metastasis and induce epithelial-mesenchymal transition in osteosarcoma by activating the COX-2/STAT3 axis. Cancer Lett. 2019;440–441:116–125. doi: 10.1016/j.canlet.2018.10.011. [DOI] [PubMed] [Google Scholar]
- 81.Gao L, Zhang W, Zhong WQ, Liu ZJ, Li HM, Yu ZL, et al. Tumor associated macrophages induce epithelial to mesenchymal transition via the EGFR/ERK1/2 pathway in head and neck squamous cell carcinoma. Oncol Rep. 2018;40(5):2558–2572. doi: 10.3892/or.2018.6657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Santarpia M, Karachaliou N. Tumor immune microenvironment characterization and response to anti-PD-1 therapy. Cancer Biol Med. 2015;12(2):74–78. doi: 10.7497/j.issn.2095-3941.2015.0022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Buchbinder EI, Desai A. CTLA-4 and PD-1 pathways: similarities, differences, and implications of their inhibition. Am J Clin Oncol. 2016;39(1):98–106. doi: 10.1097/COC.0000000000000239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Kim J, Bae JS. Tumor-associated macrophages and neutrophils in tumor microenvironment. Mediators Inflamm. 2016;2016:6058147. doi: 10.1155/2016/6058147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Hao Z, Li R, Wang Y, Li S, Hong Z, Han Z. Landscape of myeloid-derived suppressor cell in tumor immunotherapy. Biomark Res. 2021;9(1):77. doi: 10.1186/s40364-021-00333-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Kuang DM, Zhao Q, Peng C, Xu J, Zhang JP, Wu C, et al. Activated monocytes in peritumoral stroma of hepatocellular carcinoma foster immune privilege and disease progression through PD-L1. J Exp Med. 2009;206(6):1327–1337. doi: 10.1084/jem.20082173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Movahedi K, Laoui D, Gysemans C, Baeten M, Stangé G, Van den Bossche J, et al. Different tumor microenvironments contain functionally distinct subsets of macrophages derived from Ly6C(high) monocytes. Cancer Res. 2010;70(14):5728–5739. doi: 10.1158/0008-5472.CAN-09-4672. [DOI] [PubMed] [Google Scholar]
- 88.Molon B, Ugel S, Del Pozzo F, Soldani C, Zilio S, Avella D, et al. Chemokine nitration prevents intratumoral infiltration of antigen-specific T cells. J Exp Med. 2011;208(10):1949–1962. doi: 10.1084/jem.20101956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Lu T, Ramakrishnan R, Altiok S, Youn JI, Cheng P, Celis E, et al. Tumor-infiltrating myeloid cells induce tumor cell resistance to cytotoxic T cells in mice. J Clin Invest. 2011;121(10):4015–4029. doi: 10.1172/JCI45862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Noy R, Pollard JW. Tumor-associated macrophages: from mechanisms to therapy. Immunity. 2014;41(1):49–61. doi: 10.1016/j.immuni.2014.06.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Curiel TJ, Coukos G, Zou L, Alvarez X, Cheng P, Mottram P, et al. Specific recruitment of regulatory T cells in ovarian carcinoma fosters immune privilege and predicts reduced survival. Nat Med. 2004;10(9):942–949. doi: 10.1038/nm1093. [DOI] [PubMed] [Google Scholar]
- 92.Liu F, Qin L, Liao Z, Song J, Yuan C, Liu Y, et al. Microenvironment characterization and multi-omics signatures related to prognosis and immunotherapy response of hepatocellular carcinoma. Exp Hematol Oncol. 2020;9:10. doi: 10.1186/s40164-020-00165-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Eisinger S, Sarhan D, Boura VF, Ibarlucea-Benitez I, Tyystjärvi S, Oliynyk G, et al. Targeting a scavenger receptor on tumor-associated macrophages activates tumor cell killing by natural killer cells. Proc Natl Acad Sci U S A. 2020;117(50):32005–32016. doi: 10.1073/pnas.2015343117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Petty AJ, Li A, Wang X, Dai R, Heyman B, Hsu D, et al. Hedgehog signaling promotes tumor-associated macrophage polarization to suppress intratumoral CD8+ T cell recruitment. J Clin Invest. 2019;129(12):5151–5162. doi: 10.1172/JCI128644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Batlle E, Clevers H. Cancer stem cells revisited. Nat Med. 2017;23(10):1124–1134. doi: 10.1038/nm.4409. [DOI] [PubMed] [Google Scholar]
- 96.Dong B, Li S, Zhu S, Yi M, Luo S, Wu K. MiRNA-mediated EMT and CSCs in cancer chemoresistance. Exp Hematol Oncol. 2021;10(1):12. doi: 10.1186/s40164-021-00206-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Wan S, Zhao E, Kryczek I, Vatan L, Sadovskaya A, Ludema G, et al. Tumor-associated macrophages produce interleukin 6 and signal via STAT3 to promote expansion of human hepatocellular carcinoma stem cells. Gastroenterology. 2014;147(6):1393–1404. doi: 10.1053/j.gastro.2014.08.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Li X, Bu W, Meng L, Liu X, Wang S, Jiang L, et al. CXCL12/CXCR4 pathway orchestrates CSC-like properties by CAF recruited tumor associated macrophage in OSCC. Exp Cell Res. 2019;378(2):131–138. doi: 10.1016/j.yexcr.2019.03.013. [DOI] [PubMed] [Google Scholar]
- 99.Atala A. Re: infiltrating macrophages increase RCC epithelial mesenchymal transition (EMT) and stem cell-like populations via AKT and mTOR signaling. J Urol. 2017;197(1):51–52. doi: 10.1016/j.juro.2016.10.008. [DOI] [PubMed] [Google Scholar]
- 100.Wang H, Yang M, Lin L, Ren H, Lin C, Lin S, et al. HepG2 cells acquire stem cell-like characteristics after immune cell stimulation. Cell Oncol (Dordr) 2016;39(1):35–45. doi: 10.1007/s13402-015-0249-1. [DOI] [PubMed] [Google Scholar]
- 101.Gomez KE, Wu F, Keysar SB, Morton JJ, Miller B, Chimed TS, et al. Cancer cell CD44 mediates macrophage/monocyte-driven regulation of head and neck cancer stem cells. Cancer Res. 2020;80(19):4185–4198. doi: 10.1158/0008-5472.CAN-20-1079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Xu H, Niu M, Yuan X, Wu K, Liu A. CD44 as a tumor biomarker and therapeutic target. Exp Hematol Oncol. 2020;9(1):36. doi: 10.1186/s40164-020-00192-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Jinushi M, Chiba S, Yoshiyama H, Masutomi K, Kinoshita I, Dosaka-Akita H, et al. Tumor-associated macrophages regulate tumorigenicity and anticancer drug responses of cancer stem/initiating cells. Proc Natl Acad Sci U S A. 2011;108(30):12425–12430. doi: 10.1073/pnas.1106645108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Wei R, Zhu WW, Yu GY, Wang X, Gao C, Zhou X, et al. S100 calcium-binding protein A9 from tumor-associated macrophage enhances cancer stem cell-like properties of hepatocellular carcinoma. Int J Cancer. 2021;148(5):1233–1244. doi: 10.1002/ijc.33371. [DOI] [PubMed] [Google Scholar]
- 105.Zhang B, Ye H, Ren X, Zheng S, Zhou Q, Chen C, et al. Macrophage-expressed CD51 promotes cancer stem cell properties via the TGF-β1/smad2/3 axis in pancreatic cancer. Cancer Lett. 2019;459:204–215. doi: 10.1016/j.canlet.2019.06.005. [DOI] [PubMed] [Google Scholar]
- 106.Zhang X, Chen L, Dang WQ, Cao MF, Xiao JF, Lv SQ, et al. CCL8 secreted by tumor-associated macrophages promotes invasion and stemness of glioblastoma cells via ERK1/2 signaling. Lab Invest. 2020;100(4):619–629. doi: 10.1038/s41374-019-0345-3. [DOI] [PubMed] [Google Scholar]
- 107.Chen Y, Wen H, Zhou C, Su Q, Lin Y, Xie Y, et al. TNF-α derived from M2 tumor-associated macrophages promotes epithelial-mesenchymal transition and cancer stemness through the Wnt/β-catenin pathway in SMMC-7721 hepatocellular carcinoma cells. Exp Cell Res. 2019;378(1):41–50. doi: 10.1016/j.yexcr.2019.03.005. [DOI] [PubMed] [Google Scholar]
- 108.Wei X, Yang S, Pu X, He S, Yang Z, Sheng X, et al. Tumor-associated macrophages increase the proportion of cancer stem cells in lymphoma by secreting pleiotrophin. Am J Transl Res. 2019;11(10):6393–6402. [PMC free article] [PubMed] [Google Scholar]
- 109.Ma J, Shayiti F, Ma J, Wei M, Hua T, Zhang R, et al. Tumor-associated macrophage-derived CCL5 promotes chemotherapy resistance and metastasis in prostatic cancer. Cell Biol Int. 2021;45(10):2054–2062. doi: 10.1002/cbin.11630. [DOI] [PubMed] [Google Scholar]
- 110.Guan W, Li F, Zhao Z, Zhang Z, Hu J, Zhang Y. Tumor-associated macrophage promotes the survival of cancer cells upon docetaxel chemotherapy via the CSF1/CSF1R-CXCL12/CXCR4 axis in castration-resistant prostate cancer. Genes (Basel) 2021;12(5):773. doi: 10.3390/genes12050773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Huang L, Jiang S, Shi Y. Tyrosine kinase inhibitors for solid tumors in the past 20 years (2001–2020) J Hematol Oncol. 2020;13(1):143. doi: 10.1186/s13045-020-00977-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Chung FT, Lee KY, Wang CW, Heh CC, Chan YF, Chen HW, et al. Tumor-associated macrophages correlate with response to epidermal growth factor receptor-tyrosine kinase inhibitors in advanced non-small cell lung cancer. Int J Cancer. 2012;131(3):E227–E235. doi: 10.1002/ijc.27403. [DOI] [PubMed] [Google Scholar]
- 113.Timaner M, Bril R, Kaidar-Person O, Rachman-Tzemah C, Alishekevitz D, Kotsofruk R, et al. Dequalinium blocks macrophage-induced metastasis following local radiation. Oncotarget. 2015;6(29):27537–27554. doi: 10.18632/oncotarget.4826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Stafford JH, Hirai T, Deng L, Chernikova SB, Urata K, West BL, et al. Colony stimulating factor 1 receptor inhibition delays recurrence of glioblastoma after radiation by altering myeloid cell recruitment and polarization. Neuro Oncol. 2016;18(6):797–806. doi: 10.1093/neuonc/nov272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Rahal OM, Wolfe AR, Mandal PK, Larson R, Tin S, Jimenez C, et al. Blocking interleukin (IL)4- and IL13-mediated phosphorylation of STAT6 (Tyr641) decreases M2 polarization of macrophages and protects against macrophage-mediated radioresistance of inflammatory breast cancer. Int J Radiat Oncol Biol Phys. 2018;100(4):1034–1043. doi: 10.1016/j.ijrobp.2017.11.043. [DOI] [PubMed] [Google Scholar]
- 116.Russell JS, Brown JM. The irradiated tumor microenvironment: role of tumor-associated macrophages in vascular recovery. Front Physiol. 2013;4:157. doi: 10.3389/fphys.2013.00157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Chiang CS, Fu SY, Wang SC, Yu CF, Chen FH, Lin CM, et al. Irradiation promotes an m2 macrophage phenotype in tumor hypoxia. Front Oncol. 2012;2:89. doi: 10.3389/fonc.2012.00089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Xu J, Escamilla J, Mok S, David J, Priceman S, West B, et al. CSF1R signaling blockade stanches tumor-infiltrating myeloid cells and improves the efficacy of radiotherapy in prostate cancer. Cancer Res. 2013;73(9):2782–2794. doi: 10.1158/0008-5472.CAN-12-3981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Ager EI, Kozin SV, Kirkpatrick ND, Seano G, Kodack DP, Askoxylakis V, et al. Blockade of MMP14 activity in murine breast carcinomas: implications for macrophages, vessels, and radiotherapy. J Natl Cancer Inst. 2015;107(4):djv017. doi: 10.1093/jnci/djv017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Yin S, Huang J, Li Z, Zhang J, Luo J, Lu C, et al. The prognostic and clinicopathological significance of tumor-associated macrophages in patients with gastric cancer: a meta-analysis. PLoS ONE. 2017;12(1):e0170042. doi: 10.1371/journal.pone.0170042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Zhao X, Qu J, Sun Y, Wang J, Liu X, Wang F, et al. Prognostic significance of tumor-associated macrophages in breast cancer: a meta-analysis of the literature. Oncotarget. 2017;8(18):30576–30586. doi: 10.18632/oncotarget.15736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Mei J, Xiao Z, Guo C, Pu Q, Ma L, Liu C, et al. Prognostic impact of tumor-associated macrophage infiltration in non-small cell lung cancer: a systemic review and meta-analysis. Oncotarget. 2016;7(23):34217–34228. doi: 10.18632/oncotarget.9079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Guo B, Cen H, Tan X, Ke Q. Meta-analysis of the prognostic and clinical value of tumor-associated macrophages in adult classical Hodgkin lymphoma. BMC Med. 2016;14(1):159. doi: 10.1186/s12916-016-0711-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Peterson TE, Kirkpatrick ND, Huang Y, Farrar CT, Marijt KA, Kloepper J, et al. Dual inhibition of Ang-2 and VEGF receptors normalizes tumor vasculature and prolongs survival in glioblastoma by altering macrophages. Proc Natl Acad Sci U S A. 2016;113(16):4470–4475. doi: 10.1073/pnas.1525349113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Kioi M, Vogel H, Schultz G, Hoffman RM, Harsh GR, Brown JM. Inhibition of vasculogenesis, but not angiogenesis, prevents the recurrence of glioblastoma after irradiation in mice. J Clin Invest. 2010;120(3):694–705. doi: 10.1172/JCI40283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Okazawa H, Motegi S, Ohyama N, Ohnishi H, Tomizawa T, Kaneko Y, et al. Negative regulation of phagocytosis in macrophages by the CD47-SHPS-1 system. J Immunol. 2005;174(4):2004–2011. doi: 10.4049/jimmunol.174.4.2004. [DOI] [PubMed] [Google Scholar]
- 127.Barclay AN, Van den Berg TK. The interaction between signal regulatory protein alpha (SIRPα) and CD47: structure, function, and therapeutic target. Annu Rev Immunol. 2014;32:25–50. doi: 10.1146/annurev-immunol-032713-120142. [DOI] [PubMed] [Google Scholar]
- 128.Hayat SMG, Bianconi V, Pirro M, Jaafari MR, Hatamipour M, Sahebkar A. CD47: role in the immune system and application to cancer therapy. Cell Oncol (Dordr) 2020;43(1):19–30. doi: 10.1007/s13402-019-00469-5. [DOI] [PubMed] [Google Scholar]
- 129.Eladl E, Tremblay-LeMay R, Rastgoo N, Musani R, Chen W, Liu A, et al. Role of CD47 in hematological malignancies. J Hematol Oncol. 2020;13(1):96. doi: 10.1186/s13045-020-00930-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Pan Y, Lu F, Fei Q, Yu X, Xiong P, Yu X, et al. Single-cell RNA sequencing reveals compartmental remodeling of tumor-infiltrating immune cells induced by anti-CD47 targeting in pancreatic cancer. J Hematol Oncol. 2019;12(1):124. doi: 10.1186/s13045-019-0822-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Zhao XW, van Beek EM, Schornagel K, Van der Maaden H, Van Houdt M, Otten MA, et al. CD47-signal regulatory protein-α (SIRPα) interactions form a barrier for antibody-mediated tumor cell destruction. Proc Natl Acad Sci U S A. 2011;108(45):18342–18347. doi: 10.1073/pnas.1106550108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Chao MP, Alizadeh AA, Tang C, Myklebust JH, Varghese B, Gill S, et al. Anti-CD47 antibody synergizes with rituximab to promote phagocytosis and eradicate non-Hodgkin lymphoma. Cell. 2010;142(5):699–713. doi: 10.1016/j.cell.2010.07.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Feng R, Zhao H, Xu J, Shen C. CD47: the next checkpoint target for cancer immunotherapy. Crit Rev Oncol Hematol. 2020;152:103014. doi: 10.1016/j.critrevonc.2020.103014. [DOI] [PubMed] [Google Scholar]
- 134.Zhang W, Huang Q, Xiao W, Zhao Y, Pi J, Xu H, et al. Advances in anti-tumor treatments targeting the CD47/SIRPα axis. Front Immunol. 2020;11:18. doi: 10.3389/fimmu.2020.00018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Golubovskaya V, Berahovich R, Zhou H, Xu S, Harto H, Li L, et al. CD47-CAR-T cells effectively kill target cancer cells and block pancreatic tumor growth. Cancers (Basel) 2017;9(10):139. doi: 10.3390/cancers9100139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Ferlin W, Masternak K, Shang L. Selective CD47 targeting with a bispecific antibody. Cancer Immunol Immunother. 2021;70(4):1161–1162. doi: 10.1007/s00262-020-02812-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Weiskopf K, Ring AM, Ho CC, Volkmer JP, Levin AM, Volkmer AK, et al. Engineered SIRPα variants as immunotherapeutic adjuvants to anticancer antibodies. Science. 2013;341(6141):88–91. doi: 10.1126/science.1238856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Sharma P, Allison JP. The future of immune checkpoint therapy. Science. 2015;348(6230):56–61. doi: 10.1126/science.aaa8172. [DOI] [PubMed] [Google Scholar]
- 139.Wei SC, Duffy CR, Allison JP. Fundamental mechanisms of immune checkpoint blockade therapy. Cancer Discov. 2018;8(9):1069–1086. doi: 10.1158/2159-8290.CD-18-0367. [DOI] [PubMed] [Google Scholar]
- 140.Wu K, Yi M, Qin S, Chu Q, Zheng X, Wu K. The efficacy and safety of combination of PD-1 and CTLA-4 inhibitors: a meta-analysis. Exp Hematol Oncol. 2019;8:26. doi: 10.1186/s40164-019-0150-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Ribas A, Wolchok JD. Cancer immunotherapy using checkpoint blockade. Science. 2018;359(6382):1350–1355. doi: 10.1126/science.aar4060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Gordon SR, Maute RL, Dulken BW, Hutter G, George BM, McCracken MN, et al. PD-1 expression by tumour-associated macrophages inhibits phagocytosis and tumour immunity. Nature. 2017;545(7655):495–499. doi: 10.1038/nature22396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Xiao N, Zhu X, Li K, Chen Y, Liu X, Xu B, et al. Blocking siglec-10(hi) tumor-associated macrophages improves anti-tumor immunity and enhances immunotherapy for hepatocellular carcinoma. Exp Hematol Oncol. 2021;10(1):36. doi: 10.1186/s40164-021-00230-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Xiong H, Mittman S, Rodriguez R, Moskalenko M, Pacheco-Sanchez P, Yang Y, et al. Anti-PD-L1 treatment results in functional remodeling of the macrophage compartment. Cancer Res. 2019;79(7):1493–1506. doi: 10.1158/0008-5472.CAN-18-3208. [DOI] [PubMed] [Google Scholar]
- 145.Su S, Zhao J, Xing Y, Zhang X, Liu J, Ouyang Q, et al. Immune checkpoint inhibition overcomes ADCP-induced immunosuppression by macrophages. Cell. 2018;175(2):442–57.e23. doi: 10.1016/j.cell.2018.09.007. [DOI] [PubMed] [Google Scholar]
- 146.Viitala M, Virtakoivu R, Tadayon S, Rannikko J, Jalkanen S, Hollmén M. Immunotherapeutic blockade of macrophage clever-1 reactivates the CD8(+) T-cell response against immunosuppressive tumors. Clin Cancer Res. 2019;25(11):3289–3303. doi: 10.1158/1078-0432.CCR-18-3016. [DOI] [PubMed] [Google Scholar]
- 147.Zhou Y, Fei M, Zhang G, Liang WC, Lin W, Wu Y, et al. Blockade of the phagocytic receptor MerTK on tumor-associated macrophages enhances P2X7R-dependent STING activation by tumor-derived cGAMP. Immunity. 2020;52(2):357–73.e9. doi: 10.1016/j.immuni.2020.01.014. [DOI] [PubMed] [Google Scholar]
- 148.Jaynes JM, Sable R, Ronzetti M, Bautista W, Knotts Z, Abisoye-Ogunniyan A, et al. Mannose receptor (CD206) activation in tumor-associated macrophages enhances adaptive and innate antitumor immune responses. Sci Transl Med. 2020;12(530):eaax6337. doi: 10.1126/scitranslmed.aax6337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Wang W, Marinis JM, Beal AM, Savadkar S, Wu Y, Khan M, et al. RIP1 kinase drives macrophage-mediated adaptive immune tolerance in pancreatic cancer. Cancer Cell. 2018;34(5):757–74.e7. doi: 10.1016/j.ccell.2018.10.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Brennan JJ, Gilmore TD. Evolutionary origins of toll-like receptor signaling. Mol Biol Evol. 2018;35(7):1576–1587. doi: 10.1093/molbev/msy050. [DOI] [PubMed] [Google Scholar]
- 151.Tang X, Mo C, Wang Y, Wei D, Xiao H. Anti-tumour strategies aiming to target tumour-associated macrophages. Immunology. 2013;138(2):93–104. doi: 10.1111/imm.12023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Wang Y, Zhang S, Li H, Wang H, Zhang T, Hutchinson MR, et al. Small-molecule modulators of toll-like receptors. Acc Chem Res. 2020;53(5):1046–1055. doi: 10.1021/acs.accounts.9b00631. [DOI] [PubMed] [Google Scholar]
- 153.Narayan R, Nguyen H, Bentow JJ, Moy L, Lee DK, Greger S, et al. Immunomodulation by imiquimod in patients with high-risk primary melanoma. J Invest Dermatol. 2012;132(1):163–169. doi: 10.1038/jid.2011.247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Oya K, Nakamura Y, Zhenjie Z, Tanaka R, Okiyama N, Ichimura Y, et al. Combination treatment of topical imiquimod plus anti-PD-1 antibody exerts significantly potent antitumor effect. Cancers (Basel) 2021;13(16):3948. doi: 10.3390/cancers13163948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Singh M, Khong H, Dai Z, Huang XF, Wargo JA, Cooper ZA, et al. Effective innate and adaptive antimelanoma immunity through localized TLR7/8 activation. J Immunol. 2014;193(9):4722–4731. doi: 10.4049/jimmunol.1401160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Li M, Li M, Yang Y, Liu Y, Xie H, Yu Q, et al. Remodeling tumor immune microenvironment via targeted blockade of PI3K-γ and CSF-1/CSF-1R pathways in tumor associated macrophages for pancreatic cancer therapy. J Control Release. 2020;321:23–35. doi: 10.1016/j.jconrel.2020.02.011. [DOI] [PubMed] [Google Scholar]
- 157.Sun L, Kees T, Almeida AS, Liu B, He XY, Ng D, et al. Activating a collaborative innate-adaptive immune response to control metastasis. Cancer Cell. 2021;39(10):1361–74.e9. doi: 10.1016/j.ccell.2021.08.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Stanley ER, Heard PM. Factors regulating macrophage production and growth. Purification and some properties of the colony stimulating factor from medium conditioned by mouse L cells. J Biol Chem. 1977;252(12):4305–4312. [PubMed] [Google Scholar]
- 159.Stanley ER, Chen DM, Lin HS. Induction of macrophage production and proliferation by a purified colony stimulating factor. Nature. 1978;274(5667):168–170. doi: 10.1038/274168a0. [DOI] [PubMed] [Google Scholar]
- 160.Stanley ER, Chitu V. CSF-1 receptor signaling in myeloid cells. Cold Spring Harb Perspect Biol. 2014;6(6):a021857. doi: 10.1101/cshperspect.a021857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Ao JY, Zhu XD, Chai ZT, Cai H, Zhang YY, Zhang KZ, et al. Colony-stimulating factor 1 receptor blockade inhibits tumor growth by altering the polarization of tumor-associated macrophages in hepatocellular carcinoma. Mol Cancer Ther. 2017;16(8):1544–1554. doi: 10.1158/1535-7163.MCT-16-0866. [DOI] [PubMed] [Google Scholar]
- 162.Aharinejad S, Abraham D, Paulus P, Abri H, Hofmann M, Grossschmidt K, et al. Colony-stimulating factor-1 antisense treatment suppresses growth of human tumor xenografts in mice. Cancer Res. 2002;62(18):5317–5324. [PubMed] [Google Scholar]
- 163.Tap WD, Wainberg ZA, Anthony SP, Ibrahim PN, Zhang C, Healey JH, et al. Structure-guided blockade of CSF1R kinase in tenosynovial giant-cell tumor. N Engl J Med. 2015;373(5):428–437. doi: 10.1056/NEJMoa1411366. [DOI] [PubMed] [Google Scholar]
- 164.von Tresckow B, Morschhauser F, Ribrag V, Topp MS, Chien C, Seetharam S, et al. An open-label, multicenter, phase I/II study of JNJ-40346527, a CSF-1R inhibitor, in patients with relapsed or refractory hodgkin lymphoma. Clin Cancer Res. 2015;21(8):1843–1850. doi: 10.1158/1078-0432.CCR-14-1845. [DOI] [PubMed] [Google Scholar]
- 165.Edwards VD, Sweeney DT, Ho H, Eide CA, Rofelty A, Agarwal A, et al. Targeting of colony-stimulating factor 1 receptor (CSF1R) in the CLL microenvironment yields antineoplastic activity in primary patient samples. Oncotarget. 2018;9(37):24576–24589. doi: 10.18632/oncotarget.25191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Quail DF, Bowman RL, Akkari L, Quick ML, Schuhmacher AJ, Huse JT, et al. The tumor microenvironment underlies acquired resistance to CSF-1R inhibition in gliomas. Science. 2016;352(6288):aad3018. doi: 10.1126/science.aad3018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Ries CH, Cannarile MA, Hoves S, Benz J, Wartha K, Runza V, et al. Targeting tumor-associated macrophages with anti-CSF-1R antibody reveals a strategy for cancer therapy. Cancer Cell. 2014;25(6):846–859. doi: 10.1016/j.ccr.2014.05.016. [DOI] [PubMed] [Google Scholar]
- 168.Pyonteck SM, Akkari L, Schuhmacher AJ, Bowman RL, Sevenich L, Quail DF, et al. CSF-1R inhibition alters macrophage polarization and blocks glioma progression. Nat Med. 2013;19(10):1264–1272. doi: 10.1038/nm.3337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Akkari L, Bowman RL, Tessier J, Klemm F, Handgraaf SM, de Groot M, et al. Dynamic changes in glioma macrophage populations after radiotherapy reveal CSF-1R inhibition as a strategy to overcome resistance. Sci Transl Med. 2020; 12(552). [DOI] [PubMed]
- 170.Kumar V, Donthireddy L, Marvel D, Condamine T, Wang F, Lavilla-Alonso S, et al. Cancer-Associated Fibroblasts Neutralize the Anti-tumor Effect of CSF1 Receptor Blockade by Inducing PMN-MDSC Infiltration of Tumors. Cancer Cell. 2017;32(5):654–68.e5. doi: 10.1016/j.ccell.2017.10.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Neubert NJ, Schmittnaegel M, Bordry N, Nassiri S, Wald N, Martignier C, et al. T cell-induced CSF1 promotes melanoma resistance to PD1 blockade. Sci Transl Med. 2018; 10(436). [DOI] [PMC free article] [PubMed]
- 172.Shi G, Yang Q, Zhang Y, Jiang Q, Lin Y, Yang S, et al. Modulating the tumor microenvironment via oncolytic viruses and CSF-1R inhibition synergistically enhances anti-PD-1 immunotherapy. Mol Ther. 2019;27(1):244–260. doi: 10.1016/j.ymthe.2018.11.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Chai ZT, Zhu XD, Ao JY, Wang WQ, Gao DM, Kong J, et al. microRNA-26a suppresses recruitment of macrophages by down-regulating macrophage colony-stimulating factor expression through the PI3K/Akt pathway in hepatocellular carcinoma. J Hematol Oncol. 2015;8:56. doi: 10.1186/s13045-015-0150-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Yang H, Zhang Q, Xu M, Wang L, Chen X, Feng Y, et al. CCL2-CCR2 axis recruits tumor associated macrophages to induce immune evasion through PD-1 signaling in esophageal carcinogenesis. Mol Cancer. 2020;19(1):41. doi: 10.1186/s12943-020-01165-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Li X, Yao W, Yuan Y, Chen P, Li B, Li J, et al. Targeting of tumour-infiltrating macrophages via CCL2/CCR2 signalling as a therapeutic strategy against hepatocellular carcinoma. Gut. 2017;66(1):157–167. doi: 10.1136/gutjnl-2015-310514. [DOI] [PubMed] [Google Scholar]
- 176.Pienta KJ, Machiels JP, Schrijvers D, Alekseev B, Shkolnik M, Crabb SJ, et al. Phase 2 study of carlumab (CNTO 888), a human monoclonal antibody against CC-chemokine ligand 2 (CCL2), in metastatic castration-resistant prostate cancer. Invest New Drugs. 2013;31(3):760–768. doi: 10.1007/s10637-012-9869-8. [DOI] [PubMed] [Google Scholar]
- 177.Brana I, Calles A, LoRusso PM, Yee LK, Puchalski TA, Seetharam S, et al. Carlumab, an anti-C-C chemokine ligand 2 monoclonal antibody, in combination with four chemotherapy regimens for the treatment of patients with solid tumors: an open-label, multicenter phase 1b study. Target Oncol. 2015;10(1):111–123. doi: 10.1007/s11523-014-0320-2. [DOI] [PubMed] [Google Scholar]
- 178.Sanford DE, Belt BA, Panni RZ, Mayer A, Deshpande AD, Carpenter D, et al. Inflammatory monocyte mobilization decreases patient survival in pancreatic cancer: a role for targeting the CCL2/CCR2 axis. Clin Cancer Res. 2013;19(13):3404–3415. doi: 10.1158/1078-0432.CCR-13-0525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Wu X, Singh R, Hsu DK, Zhou Y, Yu S, Han D, et al. A Small Molecule CCR2 antagonist depletes tumor macrophages and synergizes with anti-PD-1 in a murine model of cutaneous T-cell lymphoma (CTCL) J Invest Dermatol. 2020;140(7):1390–400.e4. doi: 10.1016/j.jid.2019.11.018. [DOI] [PubMed] [Google Scholar]
- 180.Vonderheide RH. CD40 agonist antibodies in cancer immunotherapy. Annu Rev Med. 2020;71:47–58. doi: 10.1146/annurev-med-062518-045435. [DOI] [PubMed] [Google Scholar]
- 181.Wiehagen KR, Girgis NM, Yamada DH, Smith AA, Chan SR, Grewal IS, et al. Combination of CD40 agonism and CSF-1R blockade reconditions tumor-associated macrophages and drives potent antitumor immunity. Cancer Immunol Res. 2017;5(12):1109–1121. doi: 10.1158/2326-6066.CIR-17-0258. [DOI] [PubMed] [Google Scholar]
- 182.Pathria P, Louis TL, Varner JA. Targeting tumor-associated macrophages in cancer. Trends Immunol. 2019;40(4):310–327. doi: 10.1016/j.it.2019.02.003. [DOI] [PubMed] [Google Scholar]
- 183.Germano G, Frapolli R, Belgiovine C, Anselmo A, Pesce S, Liguori M, et al. Role of macrophage targeting in the antitumor activity of trabectedin. Cancer Cell. 2013;23(2):249–262. doi: 10.1016/j.ccr.2013.01.008. [DOI] [PubMed] [Google Scholar]
- 184.Gordon EM, Sankhala KK, Chawla N, Chawla SP. Trabectedin for soft tissue sarcoma: current status and future perspectives. Adv Ther. 2016;33(7):1055–1071. doi: 10.1007/s12325-016-0344-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185.Liguori M, Buracchi C, Pasqualini F, Bergomas F, Pesce S, Sironi M, et al. Functional TRAIL receptors in monocytes and tumor-associated macrophages: a possible targeting pathway in the tumor microenvironment. Oncotarget. 2016;7(27):41662–41676. doi: 10.18632/oncotarget.9340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186.Guerriero JL, Sotayo A, Ponichtera HE, Castrillon JA, Pourzia AL, Schad S, et al. Class IIa HDAC inhibition reduces breast tumours and metastases through anti-tumour macrophages. Nature. 2017;543(7645):428–432. doi: 10.1038/nature21409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187.Xia Y, Rao L, Yao H, Wang Z, Ning P, Chen X. Engineering macrophages for cancer immunotherapy and drug delivery. Adv Mater. 2020;32(40):e2002054. doi: 10.1002/adma.202002054. [DOI] [PubMed] [Google Scholar]
- 188.Song M, Liu T, Shi C, Zhang X, Chen X. Bioconjugated manganese dioxide nanoparticles enhance chemotherapy response by priming tumor-associated macrophages toward M1-like phenotype and attenuating tumor hypoxia. ACS Nano. 2016;10(1):633–647. doi: 10.1021/acsnano.5b06779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189.Penn CA, Yang K, Zong H, Lim JY, Cole A, Yang D, et al. Therapeutic impact of nanoparticle therapy targeting tumor-associated macrophages. Mol Cancer Ther. 2018;17(1):96–106. doi: 10.1158/1535-7163.MCT-17-0688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190.Zhu S, Li S, Yi M, Li N, Wu K. Roles of microvesicles in tumor progression and clinical applications. Int J Nanomedicine. 2021;16:7071–7090. doi: 10.2147/IJN.S325448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191.Wang P, Wang H, Huang Q, Peng C, Yao L, Chen H, et al. Exosomes from M1-polarized macrophages enhance paclitaxel antitumor activity by activating macrophages-mediated inflammation. Theranostics. 2019;9(6):1714–1727. doi: 10.7150/thno.30716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192.Cheng L, Wang Y, Huang L. Exosomes from M1-polarized macrophages potentiate the cancer vaccine by creating a pro-inflammatory microenvironment in the lymph node. Mol Ther. 2017;25(7):1665–1675. doi: 10.1016/j.ymthe.2017.02.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 193.Jang SC, Kim OY, Yoon CM, Choi DS, Roh TY, Park J, et al. Bioinspired exosome-mimetic nanovesicles for targeted delivery of chemotherapeutics to malignant tumors. ACS Nano. 2013;7(9):7698–7710. doi: 10.1021/nn402232g. [DOI] [PubMed] [Google Scholar]
- 194.Zhang X, Detering L, Sultan D, Luehmann H, Li L, Heo GS, et al. CC chemokine receptor 2-targeting copper nanoparticles for positron emission tomography-guided delivery of gemcitabine for pancreatic ductal adenocarcinoma. ACS Nano. 2021;15(1):1186–1198. doi: 10.1021/acsnano.0c08185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 195.Wu H, Han Y, Rodriguez Sillke Y, Deng H, Siddiqui S, Treese C, et al. Lipid droplet-dependent fatty acid metabolism controls the immune suppressive phenotype of tumor-associated macrophages. EMBO Mol Med. 2019;11(11):e10698. doi: 10.15252/emmm.201910698. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Not applicable.