Abstract
Objectives:
To evaluate the effect of vision-related quality of life on depression and anxiety in patients with Behçet uveitis.
Materials and Methods:
The Beck Depression Inventory (BDI), the State-Trait Anxiety Inventory (STAI) I-II, and the Visual Functioning Questionnaire (VFQ)-25 were used to evaluate 105 patients being followed for Behçet uveitis. Sociodemographic data and VFQ-25 scores were compared between the groups with and without depression and anxiety. Regression analysis was performed to determine the relationship between the variables.
Results:
Forty-eight (82.8%) men and 10 (17.2%) women who completed the questionnaires were included in the study. The mean age of the patients was 37.76±11.14 (18-65) years and the mean duration of uveitis was 8.57±7.43 (1-27) years. The mean VFQ-25 composite, BDI, STAI-I, and STAI-II scores were 74.90±18.50 (18.79-97.04), 10.76±8.90 (0-43), 42.52±6.23 (25-55), and 46.53±6.80 (27-58), respectively. Of 58 patients, 31% had depressive symptoms and 58.6% had anxiety symptoms. VFQ-25 composite score was lower in the depressive group than in the group with no depression (p=0.030), while there was no significant difference in this score between the groups with and without anxiety. Regression analysis revealed a negative relationship between total VFQ-25 composite score and depression.
Conclusion:
In our study, high rates of depression and anxiety were detected in patients with Behçet uveitis. Patient-reported visual functioning was associated with depression. In patients with Behçet uveitis, it is important to evaluate vision-related quality of life as well as visual acuity.
Keywords: Behçet uveitis, depression, anxiety disorder, vision-related quality of life
Introduction
Behçet’s disease is a chronic inflammatory disease that can involve the mucocutaneous, ocular, vascular, pulmonary, gastrointestinal, genitourinary, and central nervous systems and is characterized by recurrent episodes and spontaneous remission. More than two-thirds of patients have sight-threatening ocular involvement, which usually presents with attacks of bilateral panuveitis and retinal vasculitis.1,2,3
Ocular inflammatory diseases are known to affect both physical and mental health.4,5 In addition to the chronic and sight-threatening course of the disease, systemic therapies6 and inflammatory cytokines that cross the blood-brain barrier have also been reported to lead to behavioral changes.7
Behçet’s disease can lead to a significant deterioration in quality of life and even mental problems such as depression and anxiety disorder due to its recurrent course and threat to vision, the need for treatment with immunosuppressive and biological agents, and other systemic symptoms.8,9
The aim of this study was to screen patients being followed for Behçet uveitis in our center for depression and anxiety and to evaluate the effect of age, education level, uveitis duration, visual acuity, and vision-related quality of life on depression and anxiety levels.
Materials and Methods
Between October 2016 and October 2017, 105 patients with Behçet uveitis who were followed in the Ophthalmology Uvea-Behçet outpatient clinic of Ondokuz Mayıs University and had clinically inactive disease or were in remission were given the Beck Depression Inventory (BDI), the State-Trait Anxiety Inventory (STAI-I and -II), and the Visual Functioning Questionnaire (VFQ-25). The patients’ sociodemographic characteristics were evaluated with a semi-structured sociodemographic form. The patients were asked to complete the questionnaires at the clinic or to fill them out at home and bring them to their next visit. Uveitis duration and localization, systemic treatment received, and best corrected visual acuity in the better-seeing eye and fellow eye were obtained from the patients’ records. In patients with bilateral involvement and a difference in visual acuity of at least one Snellen line between the eyes, the eye with better visual acuity was included in the better-seeing eye group and the fellow eye was included in the worse-seeing eye group. If both eyes had equal visual acuity, data from a single eye was evaluated in the better-seeing eye group. In patients with unilateral involvement, the affected eye was included in the worse-seeing eye group. Clinical inactivity was defined as the presence of up to grade 0.5+ cells in the anterior chamber and vitreous, the absence of vitreous haze, and the absence of posterior segment inflammation findings such as retinitis, retinal vasculitis, and papillitis. Remission was defined as disease inactivity lasting more than three months despite discontinuation of treatment.10
The study was approved by the Clinical Research Ethics Committee of Ondokuz Mayıs University and conducted in accordance with the Declaration of Helsinki.
Assessment Tools
Visual Functioning Questionnaire (VFQ-25)
The VFQ-25 is a 25-question self-report instrument used to assess vision-related quality of life. Patients grade the severity of their visual symptoms or the difficulty of various activities. The items aim to measure the effects of visual impairment in the domains of general health, general vision, ocular pain, distance activities, near activities, vision-specific social functioning, vision-specific mental health, vision-specific role difficulties, vision-specific dependency, driving, color vision, and peripheral vision. A score for each subscale is calculated by averaging the scores for the relevant items. The VFQ-25 total score is calculated as the sum of the vision-related scores (excluding the general health subscale) and ranges from 0 to 100. Higher scores are associated with better quality of life.11 Toprak et al.12 translated the VFQ-25 into Turkish and conducted the validity study of the Turkish version.
Beck Depression Inventory (BDI)
The BDI is a 21-item self-report scale used to assess symptoms of depression. Each item has four response options scored between 0 and 3. Total scores of 0-13 are interpreted as no depression, 14-19 as mild depression, 20-28 as moderate depression, and 29-63 as severe depression.13 Using a cut-off value of 13, the BDI was reported to have a sensitivity of 90% and a specificity of 99% in depression screening.14 A validity-reliability study was conducted among university students in our country.15
State-Trait Anxiety Inventory (STAI-I and -II)
The STAI-I and STAI-II are self-report scales, each comprising 20 items containing positively or negatively worded statements that are scored between 1 and 4. The total score varies between 20 and 80, with higher scores reflecting higher anxiety. Studies indicate that a score of 40 or above indicates a clinical level of anxiety.16 Oner17 translated the STAI into Turkish and performed the validity-reliability study.
Statistical Analysis
The data were analyzed using SPSS version 21.0 (IBM Corp, Armonk, NY) software. For quantitative variables, mean, standard deviation, minimum, and maximum values were presented. Mann-Whitney U test was used for intergroup comparisons. Regression analyses were done to determine relationships between variables.
Results
Five of the 105 patients surveyed did not return the questionnaires. Thirty-two patients who did not complete the questionnaires fully and 10 patients still receiving interferon therapy were excluded from the study. Therefore, the data of 58 patients consisting of 48 men (82.8%) and 10 women (17.2%) with a mean age of 34.76±11.14 (18-65) years were included in the statistical analysis.
The mean duration of uveitis was 8.57±7.43 (1-27) years. Fifty-three (91.4%) of the patients had posterior segment involvement. Highest level of education completed was elementary school for 16 patients (27.6%), middle school for 9 patients (15.5%), high school for 22 patients (37.9%), and university for 11 patients (19%). Seven patients were not receiving any systemic treatment, 30 patients were being treated with conventional agents, and 21 patients were being treated with anti-TNF-a agents. Three patients were currently using antidepressants, while 14 patients had a history of antidepressant drug use. Ten of the patients reported receiving emotional support from their spouse, three patients received emotional support from friends, and three patients said they had seen a psychiatrist, while 42 patients stated that they did not receive any emotional support.
The mean visual acuity was 0.86±0.22 (0.2-1.0) Snellen in the better-seeing eye and 0.40±0.31 (0.0-0.9) Snellen in the worse-seeing eye. The patients’ mean BDI, STAI-I, and STAI-II scores were 10.76±8.90 (0-43), 42.52±6.23 (25-55) and 46.53±6.80 (27-58), respectively. Mean VFQ-25 scores were 74.90±18.50 (18.79-97.04) for total score, 40.08±19.27 (0-100) for general health, 66.55±12.64 (40-80) for general vision, 67.67±21.72 (25-75) for ocular pain, 77.87±21.72 (25-100) for distance activities, 74.07±22.33 (16.66-100) for near activities, 86.85±21.26 (25-100) for vision-specific social functioning, 62.09±28.69 (12.50-100) for vision-specific mental health, 64.87±28.62 (12.50-100) for vision-specific role difficulties, 77.15±28.85 (8.33-100) for vision-specific dependency, 85.64±13.60 (50-100) for driving, 90.94±17.95 (25-100) for color vision, and 78.44±23.62 (0-100) for peripheral vision.
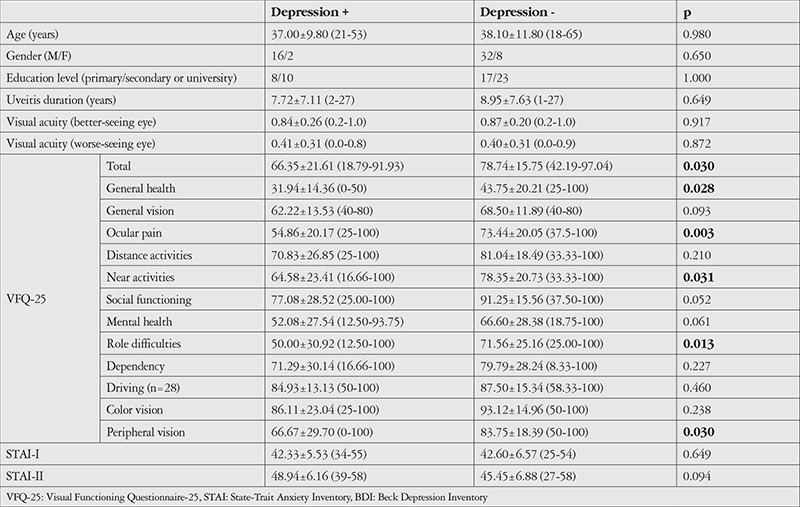
The patients were grouped according to their BDI scores. Eighteen patients (31.0%) whose BDI score was above 13 were included in the depression group and 40 patients (68.9%) whose BDI score was 13 or lower were included in the no depression group. When evaluated according to BDI score, depressive symptoms were mild in 10 patients (17.2%), moderate in 5 patients (8.6%), and severe in 3 patients (5.2%). There was no significant difference between patients with and without depression in terms of age, sex, education, uveitis duration, or visual acuity. There was also no significant difference between the two groups in terms of anxiety scores measured by STAI-I and II. The depression group had significantly lower VFQ-25 total score and general health, ocular pain, vision-specific role difficulties, and peripheral vision subscale scores (Table 1).
Table 1. Comparison of sociodemographic characteristics, visual acuities, and VFQ-25, STAI-I, and STAI-II scores between patients with and without depressive symptoms.
Thirty-four patients (58.6%) scored above the recommended cut-off for the STAI-I (≥40) and 46 patients (79.3%) scored above the recommended cut-off for the STAI-II (≥40). We evaluated whether patients whose anxiety scores were above and below the cut-off differed in terms of VFQ-25 subscale scores. When grouped according to the STAI-I, the only significant differences were in the general health (p=0.047) and dependency (p=0.040) domains; when grouped according to the STAI-II, there was no significant difference in any VFQ-25 score.
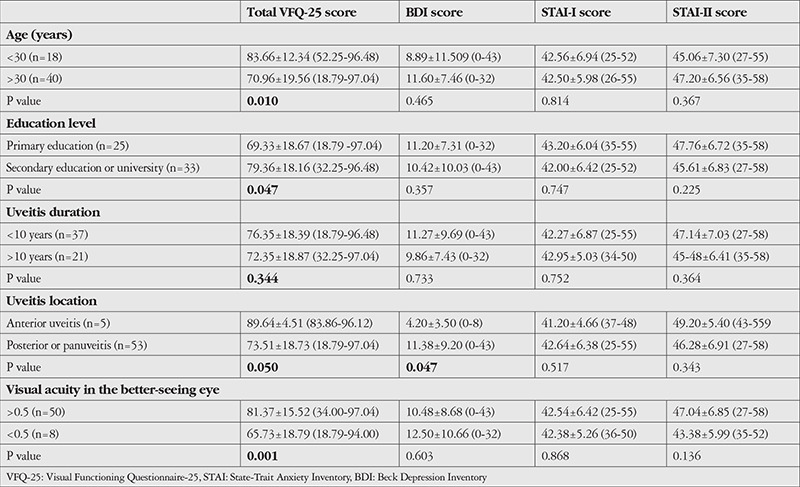
VFQ-25 total score and general health, general vision, distance activities, near activities, color vision, and peripheral vision subscale scores were lower in patients over 30 years of age (p=0.027, p=0.021, p=0.003, p=0.008, p=0.001, p=0.036, and p=0.007, respectively). VFQ-25 total score and scores for distance vision, vision-specific social functioning, and vision-specific mental health were significantly lower in patients with lower education (p=0.047, p=0.018, p=0.027, p=0.045, respectively). Patients with visual acuity of 0.5 or worse in the better-seeing eye had longer uveitis duration (mean 17.00±7.91 years for ≤0.5 and mean 7.22±6.47 years for >0.5) and significantly lower VFQ-25 total score and distance activities, near activities, social functioning, mental health, dependency, driving, and color vision subscale scores (p=0.003, p=0.019, p=0.020, p=0.042, p=0.005, p=0.023, p=0.031, p=0.008, and p=0.033, respectively). There was no difference in depression and anxiety scores in any group. Patients with posterior segment involvement had significantly lower VFQ-25 total score and distance activities score (p=0.050 and p=0.009, respectively). Depression scores were higher in patients with posterior segment involvement, while anxiety scores did not differ significantly (Table 2).
Table 2. Comparison of VFQ-25, BDI, STAI-I, and STAI-II scores in terms of age, education level, uveitis duration, uveitis location, and visual acuity.
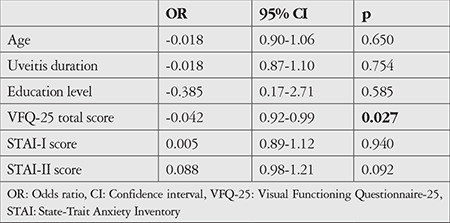
The linear regression analysis performed to determine the relationship between VFQ-25 total score and depression score revealed a significant negative correlation. There was a 1.5-point decrease in BDI score for each 10-point increase in VFQ-25 total score (odds ratio: -0.15, 95% confidence interval: -0.26 to -0.04, p=0.009). Logistic regression analysis was performed to evaluate predictors of depression. We observed an association between VFQ-25 total score and depression. There was no statistically significant relationship between depression and patient age, uveitis duration, education level, anxiety scores, or visual acuity in the better-seeing eye (Table 3).
Table 3. Logistic regression analysis of predictors of depression in patients with Behçet uveitis.
Discussion
In this study, we screened patients with Behçet uveitis for depression and anxiety disorder and examined the effects of vision-related quality of life on depression and anxiety scores.
In depression screening performed with the BDI, we determined that 18 (31%) of the patients scored above the cut-off score of 13 points. This rate has varied between 8.1% and 54% in survey studies of patients with uveitis.18,19,20,21,22 This wide range may be related to cultural differences, as well as the differences in disease activity, assessment tools used, and cut-off points specified in the studies. Maca et al.19 found that BDI scores were above the normal limit in 31.6% of the patients in their study of HLA-B27+ patients. They also reported that patients assessed during an acute attack had higher scores. In a study comparing patients experiencing acute anterior uveitis attacks with healthy controls, BDI score was above the cut-off value in 54% of the patients and 9% of control subjects. Pain caused by the attack and the decrease in visual acuity were shown to be the main reasons for this increase in depressive state.18 Their assessment of patients during an acute attack as well as using a cut-off point of 10 may explain the high depression rate in their study. In a study conducted in Thailand, which had the lowest reported rate of depression (8.1%), the authors attributed the low rate to cultural differences and their use of a different assessment tool.22 The presence of chronic disease, bilateral involvement, oral corticosteroid use, and treatment with multiple immunomodulatory drugs have been shown to affect the rate of depression.21 Onal et al.20 from Turkey reported a depression rate of 37.3% in patients with active uveitis. Tanriverdi et al.23 conducted a depression screening in Behçet’s patients with ocular involvement and determined that patients with uveitis had a higher mean BDI score than the control group.
Studies also indicate that vision-related quality of life is impaired in patients with uveitis.5,24 Schiffman et al.5 reported that patients with uveitis had lower VFQ-25 total scores than all test groups (patients with age-related macular degeneration, diabetic retinopathy, glaucoma, cataract, and cytomegalovirus infection) except patients with low vision. In multivariate regression analyses, they reported that poor visual acuity, bilateral involvement, and more intense immunosuppressive therapy were associated with lower visual functioning scores.5 In a study by Onal et al.24 conducted in patients with Behçet uveitis, VFQ-25 domain scores differed significantly according to age, education level, uveitis activity, uveitis severity, and visual acuity. In our study, we found that older age, low education level, posterior segment involvement, and low visual acuity were associated with lower VFQ-25 total score.
In this study aiming to elucidate the effect of vision-related quality of life on the psychological state of patients with Behçet uveitis, we observed that VFQ-25 total score and general health, ocular pain, near activities, role difficulties, and peripheral vision subscale scores were significantly lower in the depression group. Similarly, previous studies have demonstrated significantly lower scores in all VFQ-25 domains except for driving and color vision scores in patients with depression.20,21 Onal et al.20 reported a relationship between depression and visual acuity in the better-seeing eye and attributed this to the fact that vision in the better-seeing eye was an indicator of visual potential. However, in our study, there was no difference in visual acuity between the depression and no depression groups. Qian et al.21 showed that decreased visual acuity was associated with an increase in BDI scores in bivariate analyses. However, they reported that this effect disappeared in regression analyses and that VFQ-25 total score was a better indicator of depression. In other studies in the literature, VFQ-25 scores were found to be associated with depression, whereas visual acuity was not as strongly related.25,26 This is because Snellen visual acuity does not fully reflect vision, but represents only one parameter of visual function. The fact that only patients with active disease were included in the study by Onal et al.20 may explain the significant results in their analyses.
According to the logistic regression analysis in our study, only VFQ-25 total score was a significant predictor of depression. Qian et al.21 identified VFQ-25 total score, inadequate emotional support, change in immunomodulatory therapy, and oral corticosteroid use as predictors of depression in their study. In our study, we observed no significant difference between patients with and without depression in terms of emotional support. In addition, because our study did not include patients with active disease and none of our patients were using steroids at doses higher than the maintenance dose, we did not evaluate the steroid effect.
Consistent with other studies, we detected no statistically significant relationship between sociodemographic characteristics and depression in our study.20,21,22
We determined that 34 patients (58.6%) had state anxiety scores above the cut-off according to the STAI-I and 46 patients (79.3%) had trait anxiety scores above the cut-off according to the STAI-II. Onal et al.20 found that approximately 50% of patients had high state anxiety scores. Tanriverdi et al.23 reported that Behçet’s patients with ocular involvement had higher depression scores as well as higher anxiety scores than healthy individuals.
We observed that patients evaluated as having anxiety disorder according to their state anxiety scores had statistically significantly lower scores only in the general health and dependency subscales. Onal et al.20 reported that VFQ-25 total score and nearly all subscale scores were lower in the anxiety disorder group compared to the group without anxiety disorder. Considering that disease activity may cause an increase in state anxiety and further impairment of vision-related quality of life, the inclusion of patients with active uveitis in their study may have led to a significant difference in more subscale scores.
Study Limitations
The main limitation of our study is the small patient sample. Furthermore, the patients were only evaluated using questionnaire screening, with no psychiatric evaluation for depression and anxiety disorder. In addition, as only patients in remission or with inactive disease were included in our study, the effect of disease activity on their scores was not evaluated.
Conclusion
This study emphasizes the need to evaluate subjective loss of function associated with vision loss in patients with Behçet’s uveitis in addition to performing visual acuity tests. Our findings suggest that patient-reported vision-related quality of life is an important predictor of depression and is more valuable than Snellen visual acuity. Using the same instruments to assess patients with non-Behçet uveitis involving the posterior segment and comparing with the data of patients with Behçet uveitis will aid in determining the relationship between our results and Behçet’s disease. Ophthalmologists should be aware that visual functioning is associated with depressive symptoms and anxiety levels in patients with Behçet uveitis and should refer these patients for appropriate psychosocial support to increase their quality of life.
Acknowledgments
We would like to thank Özlem Terzi, MD, from the Public Health Department of Ondokuz Mayıs University Faculty of Medicine for her assistance with statistical analyses.
Footnotes
Ethics
Ethics Committee Approval: Ondokuz Mayıs University Clinical Research Ethics Committee OMU KAEK 2019/605.
Informed Consent: Obtained.
Peer-review: Internally peer reviewed.
Authorship Contributions
Surgical and Medical Practices: H.E.Ö., V.Y., A.K., Y.S., Concept: H.E.Ö., V.Y., A.K., Y.S., Design: H.E.Ö., V.Y., A.K., Y.S., Data Collection or Processing: H.E.Ö., A.K., Analysis or Interpretation: H.E.Ö., V.Y., A.K., Y.S., Literature Search: H.E.Ö., V.Y., A.K., Y.S., Writing: H.E.Ö., A.K.
Conflict of Interest: No conflict of interest was declared by the authors.
Financial Disclosure: The authors declared that this study received no financial support.
References
- 1.Deuter CM, Kötter I, Wallace GR, Murray PI, Stübiger N, Zierhut M. Behcet’s disease: ocular effects and treatment. Prog Retin Eye Res. 2008;27:111–136. doi: 10.1016/j.preteyeres.2007.09.002. [DOI] [PubMed] [Google Scholar]
- 2.Tugal-Tutkun I. Behcet’s Uveitis. Middle East Afr J Ophthalmol. 2009;16:219–224. doi: 10.4103/0974-9233.58425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Tugal-Tutkun I, Onal S, Altan-Yaycioglu R, Huseyin Altunbas H, Urgancioglu M. Uveitis in Behcet disease: an analysis of 880 patients. Am J Ophthalmol. 2004;138:373–380. doi: 10.1016/j.ajo.2004.03.022. [DOI] [PubMed] [Google Scholar]
- 4.Miserocchi E, Modorati G, Mosconi P, Colucci A, Bandello F. Quality of life in patients with uveitis on chronic systemic immunosuppressive treatment. Ocul Immunol Inflamm. 2010;18:297–304. doi: 10.3109/09273941003637510. [DOI] [PubMed] [Google Scholar]
- 5.Schiffman RM, Jacobsen G and Whitcup SM. Visual functioning and general health status in patients with uveitis. Arch Ophthalmol. 2001;119:841–849. doi: 10.1001/archopht.119.6.841. [DOI] [PubMed] [Google Scholar]
- 6.Warrington TP and Bostwick JM. Psychiatric adverse effects of corticosteroids. Mayo Clin Proc. 2006;81:1361–1367. doi: 10.4065/81.10.1361. [DOI] [PubMed] [Google Scholar]
- 7.Dantzer R, O’Connor JC, Freund GG, Johnson RW, Kelley KW. From inflammation to sickness and depression: when the immune system subjugates the brain. Nat Rev Neurosci. 2008;9:46–56. doi: 10.1038/nrn2297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Calikoglu E, Onder M, Cosar B, Candansayar S. Depression, anxiety levels and general psychological profile in Behcet’s disease. Dermatology. 2001;203:238–240. doi: 10.1159/000051756. [DOI] [PubMed] [Google Scholar]
- 9.Can Sandikci S, Colak S, Omma A, Enecik ME. An evaluation of depression, anxiety and fatigue in patients with Behcet’s disease. Int J Rheum Dis. 2019;22:974–979. doi: 10.1111/1756-185X.13411. [DOI] [PubMed] [Google Scholar]
- 10.Jabs DA, Nussenblatt RB, Rosenbaum JT; Standardization of Uveitis Nomenclature (SUN) Working Group. Nussenblatt RB, Rosenbaum JT; Standardization of Uveitis Nomenclature (SUN) Working Group. Standardization of uveitis nomenclature for reporting clinical data. Results of the First International Workshop. Am J Ophthalmol. 2005;140:509–516. doi: 10.1016/j.ajo.2005.03.057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Mangione CM, Lee PP, Gutierrez PR, Spritzer K, Berry S, Hays RD; National Eye Institute Visual Function Questionnaire Field Test Investigators. Development of the 25-item National Eye Institute Visual Function Questionnaire. Arch Ophthalmol. 2001;119:1050–1058. doi: 10.1001/archopht.119.7.1050. [DOI] [PubMed] [Google Scholar]
- 12.Toprak AB, Eser E, Guler C, Baser FE, Mayali H. Cross-validation of the Turkish version of the 25-item National Eye Institute Visual Functioning Questionnaire (NEI-VFQ 25) Ophthalmic Epidemiol. 2005;12:259–269. doi: 10.1080/09286580590967763. [DOI] [PubMed] [Google Scholar]
- 13.Beck A, Steer RA, Brown GK. Manual for the Beck Depression Inventory. San Antonio, Texas. 1996. [Google Scholar]
- 14.Lasa L, Ayuso-Mateos JL, Vázquez-Barquero JL, Díez-Manrique FJ, Dowrick CF. The use of the Beck Depression Inventory to screen for depression in the general population: a preliminary analysis. J Affect Disord. 2000;57:261–265. doi: 10.1016/s0165-0327(99)00088-9. [DOI] [PubMed] [Google Scholar]
- 15.Hisli N. Validity and reliability of Beck Depression Inventory in university students. Psikoloji Dergisi. 1989;7:3–13. [Google Scholar]
- 16.Emons WH, Habibovic M, Pedersen SS. Prevalence of anxiety in patients with an implantable cardioverter defibrillator: measurement equivalence of the HADS-A and the STAI-S. Qual Life Res. 2019;28:3107–3116. doi: 10.1007/s11136-019-02237-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Oner N. State and Trait Anxiety Inventory: Manual Book. İstanbul, Turkey: Bogazici University Press. 1985. [Google Scholar]
- 18.Maca SM, Wagner J, Weingessel B, Vécsei-Marlovits PV, Gruber K, Schiesser AW. Acute anterior uveitis is associated with depression and reduction of general health. Br J Ophthalmol. 2013;97:333–337. doi: 10.1136/bjophthalmol-2012-302304. [DOI] [PubMed] [Google Scholar]
- 19.Maca SM, Schiesser AW, Sobala A, Gruber K, Pakesch G, Prause C, Barisani-Asenbauer T. Distress, depression and coping in HLA-B27-associated anterior uveitis with focus on gender differences. Br J Ophthalmol. 2011;95:699–704. doi: 10.1136/bjo.2009.174839. [DOI] [PubMed] [Google Scholar]
- 20.Onal S, Oray M, Yasa C, Akman M, Uludag G, Koc Akbay A, Tugal-Tutkun I. Screening for Depression and Anxiety in Patients with Active Uveitis. Ocul Immunol Inflamm. 2018;26:1078–1093. doi: 10.1080/09273948.2017.1319959. [DOI] [PubMed] [Google Scholar]
- 21.Qian Y, Glaser T, Esterberg E, Acharya NR. Depression and visual functioning in patients with ocular inflammatory disease. Am J Ophthalmol. 2012;153:370–378, e372. doi: 10.1016/j.ajo.2011.06.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Sittivarakul W and Wongkot P. Anxiety and Depression among Patients with Uveitis and Ocular Inflammatory Disease at a Tertiary Center in Southern Thailand: Vision-Related Quality of Life, Sociodemographics, and Clinical Characteristics Associated. Ocul Immunol Inflamm. 2019;27:731–742. doi: 10.1080/09273948.2018.1484495. [DOI] [PubMed] [Google Scholar]
- 23.Tanriverdi N, Dürü C, Ozdal P, Ortaç S, Firat E. Health-related quality of life in Behcet patients with ocular involvement. Jpn J Ophthalmol. 2003;47:85–92. doi: 10.1016/s0021-5155(02)00647-0. [DOI] [PubMed] [Google Scholar]
- 24.Onal S, Savar F, Akman M, Kazokoglu H. Vision- and health-related quality of life in patients with Behcet uveitis. Arch Ophthalmol. 2010;128:1265–1271. doi: 10.1001/archophthalmol.2010.209. [DOI] [PubMed] [Google Scholar]
- 25.Jampel HD, Frick KD, Janz NK, Wren PA, Musch DC, Rimal R, Lichter PR CIGTS Study Group. Depression and mood indicators in newly diagnosed glaucoma patients. Am J Ophthalmol. 2007;144:238–244. doi: 10.1016/j.ajo.2007.04.048. [DOI] [PubMed] [Google Scholar]
- 26.Hahm BJ, Shin YW, Shim EJ, Jeon HJ, Seo JM, Chung H, Yu HG. Depression and the vision-related quality of life in patients with retinitis pigmentosa. Br J Ophthalmol. 2008;92:650–654. doi: 10.1136/bjo.2007.127092. [DOI] [PubMed] [Google Scholar]


