Abstract
Importance
COVID-19 is associated with clinically significant symptoms despite resolution of the acute infection (i.e., post-COVID-19 syndrome). Fatigue and cognitive impairment are amongst the most common and debilitating symptoms of post-COVID-19 syndrome.
Objective
To quantify the proportion of individuals experiencing fatigue and cognitive impairment 12 or more weeks following COVID-19 diagnosis, and to characterize the inflammatory correlates and functional consequences of post-COVID-19 syndrome.
Data sources
Systematic searches were conducted without language restrictions from database inception to June 8, 2021 on PubMed/MEDLINE, The Cochrane Library, PsycInfo, Embase, Web of Science, Google/Google Scholar, and select reference lists.
Study selection
Primary research articles which evaluated individuals at least 12 weeks after confirmed COVID-19 diagnosis and specifically reported on fatigue, cognitive impairment, inflammatory parameters, and/or functional outcomes were selected.
Data extraction & synthesis
Two reviewers independently extracted published summary data and assessed methodological quality and risk of bias. A meta-analysis of proportions was conducted to pool Freeman-Tukey double arcsine transformed proportions using the random-effects restricted maximum-likelihood model.
Main outcomes & measures
The co-primary outcomes were the proportions of individuals reporting fatigue and cognitive impairment, respectively, 12 or more weeks following COVID-19 infection. The secondary outcomes were inflammatory correlates and functional consequences associated with post-COVID-19 syndrome.
Results
The literature search yielded 10,979 studies, and 81 studies were selected for inclusion. The fatigue meta-analysis comprised 68 studies, the cognitive impairment meta-analysis comprised 43 studies, and 48 studies were included in the narrative synthesis. Meta-analysis revealed that the proportion of individuals experiencing fatigue 12 or more weeks following COVID-19 diagnosis was 0.32 (95% CI, 0.27, 0.37; p < 0.001; n = 25,268; I2 = 99.1%). The proportion of individuals exhibiting cognitive impairment was 0.22 (95% CI, 0.17, 0.28; p < 0.001; n = 13,232; I2 = 98.0). Moreover, narrative synthesis revealed elevations in proinflammatory markers and considerable functional impairment in a subset of individuals.
Conclusions & relevance
A significant proportion of individuals experience persistent fatigue and/or cognitive impairment following resolution of acute COVID-19. The frequency and debilitating nature of the foregoing symptoms provides the impetus to characterize the underlying neurobiological substrates and how to best treat these phenomena.
Study registration
PROSPERO (CRD42021256965).
Abbreviation: PCS, Post-COVID-19 syndrome
Keywords: Long COVID, Post-COVID-19 syndrome, Post-COVID-19 condition, Brain fog, Cognitive impairment, Fatigue, Inflammation, Functional outcomes, Population health, Depression, Cognition, Bipolar, COVID-19, Immunology, Mental illness, Anhedonia, Brain
1. Introduction
The global confirmed case count of coronavirus disease 2019 (COVID-19) surpassed 275 million as of December 2021 (Coronavirus disease (COVID-19), 2021). The actual case positive rate, however, is estimated to be much higher with multiple models predicting the actual number to be 10 (3 to 24) times greater than the number of confirmed cases (Wu et al., 2020, Havers et al., 2020, Aizenman, 2021). In keeping with this view, a projection of over 2.75 billion people may have been infected by COVID-19.
>30% of individuals affected by COVID-19 (Tenforde et al., 2020), including asymptomatic cases (Huang et al., 2021), and approximately 80% of patients hospitalized for COVID-197 may experience post-COVID sequelae. Fatigue and cognitive impairment, along with other enduring neuropsychiatric (e.g., depression) (Renaud-Charest et al., 2021) and physical (e.g., dyspnea) manifestations, comprise ‘post-acute sequelae of SARS-CoV-2′ (i.e., symptoms persisting for at least 4 weeks following infection) (Nalbandian et al., 2021), colloquially referred to as ‘long COVID’ (https://www.ncbi.nlm.nih.gov/pmc/articles/PMC7992371/). The National Institute for Health and Care Excellence (NICE) defines ‘post-COVID-19 syndrome’ (PCS) as a constellation of symptoms which develop during or following COVID-19 infection, persist for >12 weeks, and are not sufficiently explained by alternative diagnoses (https://www.nice.org.uk/guidance/ng188). Towards the aim of identifying a common nomenclature in case definition, the World Health Organization (WHO) has recently proposed the moniker ‘post COVID-19 condition’ (https://www.who.int/publications/i/item/WHO-2019-nCoV-Post_COVID-19_condition-Clinical_case_definition-2021.1). Post COVID-19 condition is defined as persistent symptoms usually occurring 3 months from onset in individuals with past confirmed or probable SARS-CoV-2 infection and persisting for at least 2 months which cannot be explained by an alternative diagnosis (hq) WH. A clinical case definition of post COVID-19 condition by a Delphi consensus, 6 October, 2021).
Research efforts into PCS were originated by online patient advocacy groups, who have reported substantial detriments to quality of life and daily functioning as a consequence of persistent symptoms, lack of formal diagnosis, and effective established treatments (Siegelman, 2020, Rubin, 2020). Fatigue and cognitive impairment have been consistently reported to be some of the most common and debilitating features of PCS (Davis et al., 2020, Marshall, 2020, Report: What does COVID-19 recovery actually look like - patient-led research collaborative. Published June 10, 2020). Chronic fatigue (Sabes-Figuera et al., 2010) and cognitive impairment (Winston, 2020, Xu et al., 2017) constitute a significant global economic burden, respectively. Unlike other common symptoms of PCS including dyspnea and depression, there are no established and effective treatments for post-viral fatigue and cognitive impairment, as well as related conditions such as Myalgic Encephalomyelitis/Chronic Fatigue Syndrome (ME/CFS). The global incidence of COVID-19 infection and the potential economic burden and quality of life diminution provide the impetus for identifying neurobiological substrates subserving PCS-related fatigue and cognitive impairment, associated factors and determinants, as well as safe and effective treatments. Herein, we sought to determine the proportion of individuals exhibiting fatigue and cognitive impairment 12 or more weeks following COVID-19 diagnosis, including amongst age, sex, and clinical subgroups. We additionally aimed to characterize the inflammatory correlates and functional consequences of PCS.
2. Methods
2.1. Data Sources and searches
The protocol pertaining to this systematic review and meta-analysis was registered on PROSPERO (CRD42021256965). This study followed the Meta-analysis of Observational Studies in Epidemiology (MOOSE) reporting guidelines (Stroup et al., 2000). In accordance with the 2020 Preferred Reporting Items for Systematic Reviews and Meta-analyses (PRISMA) guidelines (Page et al., 2020), a systematic search was conducted on PubMed/MEDLINE, Cochrane Library, PsycInfo, EMBASE, and Web of Science from database inception to June 8, 2021. The search string implemented was: “long covid” OR “persistent covid” OR “post covid” OR “post-acute sequelae of SARS-CoV-2 PASC” OR “enduring COVID-19 sequelae” OR “long-haul covid” OR “long-tail covid”. We manually searched the references of relevant articles, as well as Google Scholar/Google, for additional studies. No language or publication date restrictions were imposed.
Titles and abstracts were independently screened by two review authors (FC and SL) using the Covidence platform. (Better systematic review management, 2020) Articles identified as potentially relevant by at least one reviewer were retrieved, and duplicates were removed. Full text-articles were independently screened by two reviewers (FC and SL), with discrepancies resolved through discussion. Authors of potentially eligible studies were contacted to provide clarification and/or supplementary data where necessary.
2.2. Study selection
We sought articles reporting on the incidence of any primary outcome (i.e., fatigue or cognitive impairment) and/or secondary outcome (i.e., inflammatory markers or functional outcomes/quality of life measures), as defined in Table 1 , in individuals with confirmed COVID-19 12 or more weeks following diagnosis. At the outset, we planned to determine the secondary outcomes solely for fatigue and cognitive impairment in PCS. However, due to the paucity of data concerning the foregoing, we subsequently included inflammatory correlates and functional outcomes associated with PCS more broadly. Inclusion criteria were established prior to article review and were as follows:
-
1.
Complete summary estimates (i.e., exact proportions) pertaining to at least one primary outcome, and/or qualitative or quantitative data pertaining to at least one secondary outcome, as defined in Table 1, for individuals previously diagnosed with COVID-19 of any age, sex, or ethnicity.
-
2.
Median or mean follow-up time of at least 12 weeks (84 days) since COVID-19 diagnosis, to serve as a proxy for time since infection, in accordance with the NICE definition of PCS. If the index date was hospital admission or discharge, resolution of acute illness, or onset of symptoms, it was assumed that these events occurred either concurrently or subsequent to diagnosis.
-
3.
COVID-19 (any severity) ascertained according to laboratory testing, diagnostic code linkage, and/or clinical diagnosis.
-
4.
Primary research.
-
5.
Presentation as full-text article (including preprints).
Table 1.
Definition of study variables.
| PRIMARY OUTCOMES | Objective Ascertainment | Subjective Ascertainment |
|---|---|---|
| Fatigue (asthenia) | Fatigue ascertained via any validated tool (e.g., FACIT fatigue scale, FSS), or clinical diagnosis of CFS/EM. | Self-report or non-validated measure of fatigue, tiredness/low energy, muscular fatigue/muscular weakness (myasthenia), malaise. |
| Cognitive Impairment | Cognitive impairment ascertained via any validated tool for performance-based cognitive function (e.g., MoCA, TICS, SCIP), or clinical diagnosis of cognitive impairment. | Self-report or non-validated measure of cognitive impairment/’brain fog’, mental slowness, deficits in attention, executive, processing, memory, learning, articulation, and/or psychomotor coordination. |
| SECONDARY OUTCOMES | ||
| Inflammatory Parameters | Abnormal levels of circulating or intracellular cytokines, CRP, D-dimer, and/or procalcitonin, in accordance with thresholds determined by study investigators, or relative to control group or established standard. | N/A |
| Functional Outcomes/Quality of Life | Functional impairment (including activity, occupational, and social limitations) (Ustün and Kennedy, 2009) ascertained via any validated tool for quality of life or functional outcomes (e.g., EQ-5D, mRS). | Self-report or non-validated assessment of functional impairment (including activity, occupational, and social limitations), as well as general vitality/quality of life (Ustün and Kennedy, 2009). |
Acronyms: FACIT: Functional Assessment of Chronic Illness Therapy, FSS: Fatigue Severity Scale, CFS/EM: chronic fatigue syndrome/myalgic encephalomyelitis, MoCA: Montreal Cognitive Assessment, TICS: Telephone interview for cognitive status, SCIP: Screen for Cognitive Impairment for Psychiatry CRP: C-reactive peptide, D-dimer: domain dimer, N/A: not applicable, EQ-5D: European Quality of Life 5 Dimension Scale, mRS: modified Rankin Scale.
Exclusion criteria were:
-
1.
Incomplete or inexact quantitative data (i.e., no exact proportions provided for primary outcomes).
-
2.
Outcomes precede exposure (i.e., it is stated that fatigue, cognitive impairment, inflammation, and/or functional impairment were present prior to COVID-19 infection, and/or did not markedly increase in severity following COVID-19 infection at 12 or more weeks follow-up).
-
3.
Study solely reports new symptoms arising following resolution of acute COVID-19 (i.e., not persisting since diagnosis).
-
4.
Outcomes of interest reported solely in the general population, or in persons without a confirmed prior COVID-19 diagnosis.
-
5.
Median/mean follow-up time of <12 weeks (84 days) since COVID-19 infection or diagnosis.
-
6.
COVID-19 is not verified by laboratory testing or ICD-10 linkage, or is not clinically diagnosed.
-
7.
Post-mortem study of COVID-19 patients.
-
8.
Case series, or any study design wherein participants are selected for inclusion based on the presence of PCS symptoms (i.e., outcomes of interest).
-
9.
Unpublished study, abstract, case report, study with a sample size of <10 persons, or protocol.
-
10.
Non-primary research.
2.3. Data extraction
Published summary data from included articles were independently extracted by two reviewers (FC and SL) using a piloted data extraction form, then corroborated, with discrepancies resolved through discussion. Information to be extracted was established a priori and included study characteristics, participant characteristics and subgroups, sample size and source, modes of ascertainment, follow-up period, exact proportions (including subgroup-specific data where available) pertaining to primary or secondary outcomes, qualitative data pertaining to secondary outcomes, and factors reportedly associated with PCS across individual analyses.
2.4. Quality assessment
Methodological quality and risk of bias was assessed using the Newcastle-Ottawa Scale (NOS) (Stang, 2010), modified for applicable cohort and case-control studies, as well as adapted for cross-sectional studies. Studies wherein the design was unclear were assessed according to the prospective cohort NOS. Cohort studies were penalized for failing to include a non-exposed cohort. All component studies were independently rated by two reviewers (FC and LMWL) and results were corroborated, with discrepancies resolved through discussion. Modified NOSs and methodological quality rankings for each study type are provided (supplementary material).
2.5. Data synthesis and analysis
A meta-analysis of proportions was conducted using R version 4.1.0 (R Foundation for Statistical Computing). An α level of 0.05 was chosen to indicate statistical significance. In anticipation of marked heterogeneity, the meta::metaprop function (Balduzzi et al., 2019) was used to pool proportions, indicated as the number of cases exhibiting fatigue or cognitive impairment (events) divided by the size of the sample (observations), via the random-effects restricted maximum-likelihood model (REML) (Kenward and Roger, 1997, Miller, 1978). Where one study reported multiple proportions qualifying as a primary outcome measure (e.g., concentration impairment and memory impairment, which can both be subsumed under cognitive impairment), only the largest proportion was included to prevent data duplication or skewing of true effect size. Where studies provided data for multiple qualifying follow-up periods, the earliest follow-up was used in the main analyses. Single proportions were transformed via the Freeman-Tukey Double arcsine method to stabilize variances (Miller, 1978), Clopper-Pearson 95% confidence intervals (CIs) were calculated for individual studies, and Wald 95% CIs were calculated for pooled proportions. Forest plots for each primary outcome were created via the meta::forest function. Random effects subgroup analyses, established a priori, for sex, COVID-19 hospitalization status, age group (children vs adults, defined as median/mean age <18 and ≥18 years, respectively), follow-up duration (<6 months vs ≥6 months), and mode of ascertainment (objective vs subjective) were conducted using the byvar argument, assuming separate estimates of between-study variance for each subgroup. Study populations were classified as comprising hospitalized populations if ≥80% of the participants had been hospitalized for COVID-19 (and vice versa for outpatients). Post hoc sensitivity analyses according to NOS quality rating groupings, as well as by study design, were undertaken. Statistically significant differences in inter-group effect sizes were calculated via the Wald-type χ2 test.
Heterogeneity was quantified using the I2 statistic, where the cut-offs 30.0%, 50.0%, and 75.0% denote moderate, substantial, and considerable heterogeneity, respectively, as recommended by GRADE (Grading of Recommendations, Assessment, Development and Evaluations) criteria and the Cochrane Handbook’s interpretation of heterogeneity scores (Deeks et al., 2008, Schünemann et al., 2019). The Egger regression intercept test and the Begg and Mazumdar rank correlation test, as well as visual inspection of funnel plots for asymmetry, were used to assess publication bias via the meta::metabias and meta::funnel functions, respectively. Qualitative analysis via narrative synthesis was performed for secondary outcomes, which were not sufficiently homogenous to meta-analyze.
3. Results
3.1. Search results
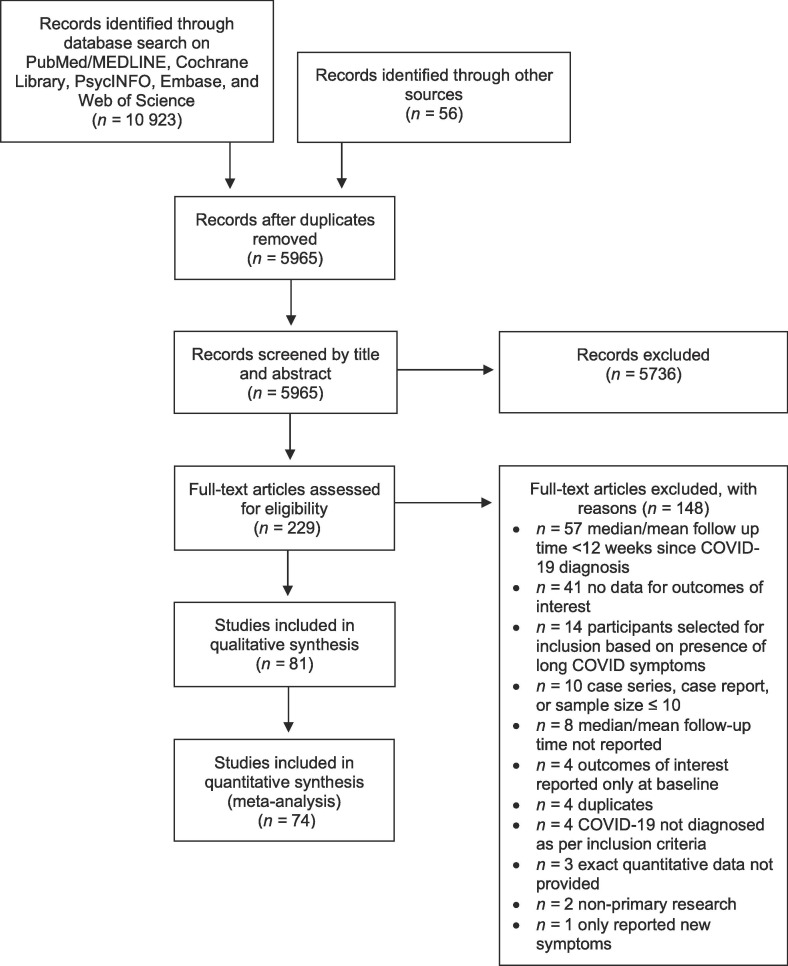
The literature search yielded 10,979 studies. Following the removal of duplicates, 5965 studies were screened by title and abstract, producing 229 eligible studies. 148 studies were further excluded following full-text screening. Details of study selection are provided in Fig. 1 . In total, 81 studies were included in the review: 56 prospective cohort studies, 14 cross-sectional studies, 10 retrospective cohort studies, and 1 retrospective case-control study. Component studies are grouped by design in the supplementary material. The quantitative synthesis primarily evaluating the effect of COVID-19 exposure on fatigue (i.e., fatigue meta-analysis) included 68 studies, whereas the quantitative synthesis primarily evaluating the effect of COVID-19 exposure on cognition (i.e., cognitive impairment meta-analysis) included 43 studies. 48 studies were qualitatively analyzed via narrative synthesis, including 7 which were excluded from quantitative analyses (Breton et al., 2021, Shuwa et al., 2021, Sonnweber et al., 2021, Taboada et al., 2021, Todt et al., 2021, Wong et al., 2020, Zhou et al., 2021).
Fig. 1.
Flow diagram of study selection.
3.2. Study characteristics
Ten studies analyzed data from Italy, nine from Spain, eight from the US, seven from China, six from the UK, three from Denmark, France, and Norway, respectively, two from Australia, Austria, Brazil, Canada, Egypt, Germany, Israel, Russia, and the Netherlands, respectively, and one from Belgium, the Czech Republic, England, Faroe Islands, Iran, Japan, Mexico, Pakistan, Singapore, Sweden, Switzerland, and Turkey, respectively. Sample sizes ranged from 23 to 2649, and median or mean follow-up periods ranged from 2.8 to 11.2 months. Six study authors provided confirmation regarding mode of ascertainment of COVID-19 (Ghosn et al., 2021, Say et al., 2021, Pilotto et al., 2021, Liyanage-Don et al., 2021, Miyazato et al., 2020, González et al., 2021), and one author (Fernández-de-las-Peñas et al., 2021, Fernández-de-Las-Peñas et al., 2021) confirmed that there was no sample duplication amongst two component studies, as well as advised the exclusion of a third study on the basis of possible duplication. Table 2 provides detailed characteristics and summaries of applicable findings for all 81 component studies.
Table 2.
Characteristics and results of studies (n = 81) examining individuals with confirmed COVID-19 12 or more weeks following diagnosis.
| Study | Country | Study Design | Sample Source | Sample Characteristics | Follow-up Duration | Ascertainment of COVID-19 | Ascertainment of Outcomes | Results | Factors Associated with persistent symptoms |
|---|---|---|---|---|---|---|---|---|---|
| Abdallah et al., 2021 | Canada | Prospective Cohort | The Ottawa Hospital |
N = 63 (including 25 previously hospitalized cases and 38 previously non-hospitalized cases)
|
Mean 119.9 ± 16.2 days following first positive test for hospitalized patients, and mean 129 ± 16.5 days for non-hospitalized patients | RT-PCR | Subjective self-report via clinical follow-up |
|
|
| Amin-Chowdhury et al., 2021a | England | Prospective Cohort | Public Health England (ESCAPE study) |
N = 140 (clinical and non-clinical healthcare workers)
|
Median 7.5 (7.1–7.8) months following COVID-19 diagnosis | Serologyand RT-PCR | Subjective self-report via online questionnaire |
|
|
| Arnold et al., 2020 | United Kingdom | Prospective Cohort | Diagnostic and Severity markers of COVID-19 to Enable Rapid triage (DISCOVER) study (Bristol) |
N = 110 (all previously hospitalized cases)
|
Median 90 (80–97) days following onset of symptoms | RT-PCR or clinico-radiological diagnosis | Objective assessment via laboratory testing (inflammatory parameters), SF-36 (quality of life), as well as subjective self-report via questionnaire |
|
|
| Augustin et al., 2021 | Germany | Prospective Cohort | University Hospital Cologne (recruited through public media) |
N = 353
|
Median 6.8 (6–8) months following onset of symptoms | RT-PCR | Subjective self-report via systematic questionnaires and evaluation by physician |
|
|
| Breton et al., 2021 | USA | Retrospective Cohort | Residents of greater New York City Tristate Region |
N = 41 cases (including 8 previously hospitalized)
|
Mean 6.1 months following COVID-19 infection | RT-PCR | Objective assessment via laboratory testing (flow cytometry; intracellular cytokine staining) |
|
|
| Buonsenso et al., 2021 | Italy | Cross-sectional | Fondazione Policlinico Universitario Agostino Gemelli (part of ISARIC) |
N = 68 children (including 6 previously hospitalized and 3 in pediatric ICU)
|
Mean 162.5 ± 113.7 days following diagnosis | RT-PCR | Subjective self-report via phone interview or outpatient assessment |
|
|
| Cirulli et al., 2020a | USA | Prospective Cohort | Helix DNA Discovery Project and the Healthy NevadaProject |
N = 357 (including 9 previously hospitalized cases)
|
90 days following onset of symptoms | Laboratory test | Subjective self-report via online questionnaires |
|
|
| Darley et al., 2021a | Australia | Prospective Cohort | St. Vincent’s Hospital Sydney (ADAPT study) |
N = 99
|
Median 240 (227–256) days following infection | RT-PCR | Objective assessment via SPHERE-34 (fatigue, cognitive function) |
|
|
| Elkan et al., 2021a | Israel | Retrospective Case-control | Shamir (Assaf Harofeh) Medical Center |
N = 66 (all previously hospitalized cases)
|
Median 9 (6–9) months following discharge | RT-PCR | Objective assessment via RAND-36 (quality of life), as well as subjective self-report |
|
|
| Evans et al., 2021a | United Kingdom | Prospective Cohort | 53 National Health Service hospitals (PHOSP-COVID study) |
N = 1077 (all previously hospitalized cases)
|
Median 159 (120–189) days following discharge | RT-PCR or clinician-diagnosed | Objective assessment via FACIT (fatigue), MoCA (cognitive function), EQ-5D-5L, WG-SS (quality of life and functioning), laboratory testing (serology), and subjective self-report via research visit and clinical follow-up questionnaire |
|
|
| Evlice et al., 2021 | Turkey | Retrospective Cohort | Hospital in Turkey |
N = 266 (all previously hospitalized cases, including 11 in ICU)
|
Mean 99.80 ± 26.16 days following discharge | RT-PCR or CT | Subjective self-report via telephone survey |
|
|
| Fernández-de-Las-Peñas (1) et al., 2021 | Spain | Retrospective Cohort | Four public hospitals in Madrid |
N = 1142 (all previously hospitalized cases)
|
Mean 7.0 ± 0.6 months following discharge | RT-PCR | Subjective self-report via systematic telephone interview conducted by trained researchers |
|
|
| Fernández-de-Las-Peñas (2) et al., 2021 | Spain | Retrospective Cohort | Three public hospitals in Madrid |
N = 1950 (all previously hospitalized cases, including 129 in the ICU)
|
Mean 11.2 ± 0.5 months after hospital discharge | RT-PCR and radiological findings | Self-report via systematic telephone interview conducted by trained healthcare professionals |
|
|
| Ferrucci et al., 2021 | Italy | Prospective Cohort | Non-intensive COVID units of the ASST Santi Paolo e Carlo hospitals |
N = 38 (all previously hospitalized cases in non-intensive wards)
|
Mean 4.43 ± 1.22 months following discharge | RT-PCR | Objectively assessed via MoCA (cognitive function), BRB-NT (neurological battery of tests for cognition), SSD (fatigue) |
|
|
| Fortini et al., 2021 | Italy | Prospective Cohort | San Giovanni di Dio Hospital |
N = 59 (all previously hospitalized cases in non-intensive ward)
|
Median 123 (116–145) days following discharge | RT-PCR | Objective assessment via laboratory testing (inflammatory parameters), as well as subjective self-report via self-administered questionnaire |
|
|
| Froidure et al., 2021 | Belgium | Prospective Cohort | Hospital in Belgium |
N = 126 patients (all previously hospitalized and/or ICU cases)
|
Median 95 (86–107) days following infection | RT-PCR and lung HRTC or chest X-ray | Subjective self-report via clinical assessment |
|
|
| Frontera et al., 2021 | USA | Prospective Cohort | Four NYC area hospitals |
N = 382 (all previously hospitalized cases including 196 neurologic cases, 67 of which were admitted to the ICU, and 186 cases without neurological disorders during hospitalization, 54 of which were admitted to the ICU)
|
Median 6.7 (6.5–6.8) months following onset of symptoms | RT-PCR | Objective assessment via MoCA (cognitive function), Barthel index (functional impairment), as well as subjective self-report via telephone questionnaire |
|
|
| García-Abellán et al., 2021a | Spain | Prospective Cohort | Hospital General Universitario de Elche |
N = 116 (all previously hospitalized cases, including 15 previously admitted to ICU)
|
6 months following discharge | RT-PCR and serology | Objective assessment via laboratory testing (immunological parameters), and subjective self-report via CSQ (fatigue) during clinical visit |
|
|
| Garrigues et al., 2020 | France | Retrospective Cohort | Beaujon Hospital, COVID-19 Unit |
N = 120 (all previously hospitalized cases, including 24 in ICU that underwent mechanical ventilation)
|
Mean 110.9 days ± 11.1 following hospital admission | RT-PCR and/or chest CT | Objective assessment via EQ-5D-5L (quality of life), as well as subjective self-report via telephone questionnaire conducted by trained physicians |
|
|
| Ghosn et al., 2021 | France | Prospective Cohort | Institut National de la Santé Et de la Recherche Médicale |
N = 1137 (all previously hospitalized cases, including 288 in ICU)
|
3 and 6 (median 194 [188–205] days) months following hospital admission | RT-PCR | Subjective self-report via physician visit |
|
|
| González et al., 2021 | Spain | Prospective Cohort | Hospital Universitary Arnau de Vilanova |
N = 62 (all previously ICU cases)
|
3 months following discharge | RT-PCR | Objective assessment via SF-12 (quality of life), as well as subjective self-report |
|
|
| González-Hermosillo et al., 2021 | Mexico | Prospective Cohort | Instituto Nacional de Cardiología Ignacio Chávez |
N = 130 (all previously hospitalized cases)
|
3 and 6 (mean 270 ± 32 days) months following discharge | RT-PCR | Subjective self-report via telephone questionnaire based on ME/CFS International Consensus Criteria |
|
|
| Havervall et al., 2021 | Sweden | Prospective Cohort | Danderyd Hospital |
N = 1395 health care professionals (mild cases, hospitalization status not specified)
|
8 months following onset of symptoms | Serology | Objective assessment via Sheehan Disability Scale (functional outcomes), as well as subjective self-report via smartphone app questionnaire |
|
|
| Huang et al., 2021 | China | Ambidirectional Cohort | Jin Yin-tan Hospital |
N = 1733 patients (all previously hospitalized cases, including 76 previously admitted to ICU)
|
Median 186 (175–199) days following onset of symptoms | Laboratory testing | Objective assessment viaEQ-5D-3L (quality of life), as well as subjective self-report via questionnaire |
|
|
| Jacobson et al., 2021 | USA | Prospective Cohort | Patients from enrolled clinical trials at Stanford University |
N = 118 participants (including 22 previously hospitalized cases, of which 11 were previously admitted to ICU)
|
Mean 119.3 ± 33.0 days following diagnosis | RT-PCR | Objective assessment via WPAI (functional outcomes), as well as subjective self-report via questionnaire |
|
|
| Johnsen et al., 2021 | Denmark | Cross-sectional | Copenhagen University Hospital at Bispebjerg |
N = 57 (34 previously hospitalized cases and 34 non-hospitalized cases)
|
3 months following discharge/resolution of acute disease | RT-PCR | Objective assessment via WPAI, PCFS (functional outcomes), EQ-5D-5L (quality of life), and CFQ, SCIP-D, and TMT-B (cognitive function) |
|
|
| Kashif et al., 2021a | Pakistan | Prospective Cohort | Hameed Latif Hospital |
N = 242 (including hospitalized cases and non-hospitalized cases who sought healthcare at hospital)
|
3 months following discharge or onset of symptoms | RT-PCR | Subjective self-report via telephone interview |
|
|
| Klein et al., 2021 | Israel | Retrospective Cohort | Israeli residents recruited through social media and word of mouth |
N = 103 (all mild symptomatic cases; asymptomatic excluded, hospitalization status not specified)
|
6 months following onset of symptoms | RT-PCR | Subjective self-report via telephone questionnaire |
|
|
| Latronico et al., 2021a | Italy | Prospective Cohort | 3 ICUs of the Spedali Civili University Hospital |
N = 55 (all cases which were previously admitted to ICU with ARDS)
|
3 and 6 months following discharge | RT-PCR | Objective assessment via FSS (fatigue), PICS, MoCA (cognitive function), SF-36 (quality of life), Barthel Index (functional outcomes) |
|
|
| Leth et al., 2021 | Denmark | Prospective Cohort | Department of Infectious Diseases, Aarhus University Hospital |
N = 49 (all previously hospitalized cases, including 6 previously admitted to ICU)
|
Median 128 (98–148) days following discharge | RT-PCR | Objective assessment via OMC (cognitive function), as well as subjective self-report via in person or telephonequestionnaire |
|
|
| Liang et al., 2020 | China | Prospective Cohort | Wuhan Union Hospital |
N = 76 (all previously hospitalized, including 65 healthcare worker cases, and 7 previously admitted to ICU)
|
3 months following discharge | RT-PCR | Subjective self-report via questionnaire |
|
|
| Liyanage-Don et al., 2021 | USA | Prospective Cohort | 2 Columbia University Hospitals |
N = 153 (all previously hospitalized cases)
|
Median 3.7 (2.6–5.7) months following discharge | RT-PCR | Subjective self-report via online or telephone questionnaire |
|
|
| Logue et al., 2021 | USA | Prospective Cohort | University of Washington |
N = 177 (145 previously outpatient cases, 16 previously hospitalized)
|
Median 169 (range 31–300) days following onset of acute COVID-19 | RT-PCR | Subjective self-report via electronic questionnaire |
|
|
| Mattioli et al., 2021 | Italy | Prospective Cohort | Unit of Occupational Health, General University Hospital of Brescia |
N = 150 (120 healthcare workers cases, including 2 with previous respiratory failure requiring hospitalization) and 30 healthcare worker healthy controls)
|
4 months following first COVID-19 diagnosis | RT-PCR | Objective assessment via MMSE (cognitive function), as well as subjective self-report via clinical diagnostic assessment (including questionnaire) |
|
|
| Mazza et al., 2021 | Italy | Prospective Cohort | IRCCS San Raffaele Hospital |
N = 226 (including 177 hospitalized cases and 49 cases treated at home)
|
Mean 90.1 ± 13.4 days following discharge | RT-PCR | Objective assessment via BACS (cognitive function), as well as subjective self-report via questionnaire |
|
|
| Mei et al., 2021 | China | Prospective Cohort | Wuhan No.1 Hospital, Wuchang Hospital, Zhongshang Hospital, and Hubei Province Hospital |
N = 3,677 (all previously hospitalized cases)
|
Median 144 (135–157) days following discharge | RT-PCR | Subjective self-report during clinical follow-up |
|
|
| Menges et al., 2021a | Switzerland | Cross-sectional | General Population of Zurich (Zurich SARS-CoV-2 Cohort Study) |
N = 431 (including 81 previously hospitalized cases, of which 10 were in ICU, and 350 non-hospitalized cases)
|
Median 220 (181–232) days following diagnosis | RT-PCR | Objective assessment via FAS (fatigue), EQ-5D-5L (quality of life), as well as subjective self-report via online survey conducted via REDcap |
|
|
| Miskowiak et al., 2021 | Denmark | Prospective Cohort | Bispebjerg Hospital (IMPACT-COVID study) |
N = 129 (29 previously hospitalized cases, and 100 matched healthy controls)
|
3–4 months following hospital discharge | RT-PCR and serology | Objective assessment via SCIP-D, TMT-B, and CFQ (cognitive function), and EQ-5D-5L (quality of life), as well as subjective self-report via questionnaire |
|
|
| Miyazato et al., 2021 | Japan | Cross-sectional | Disease Control and Prevention Center and National Center for Global Health and Medicine |
N = 63 (all previously hospitalized cases)
|
Mean 129 ± 21 days following onset of symptoms | RT-PCR | Subjective self-report via structured telephone interview conducted by the investigators |
|
|
| Morin et al., 2021 | France | Prospective Cohort | Bicêtre Hospital (Paris-Saclay University hospitals) |
N = 478 (all previously hospitalized cases, including 142 previously admitted to ICU)
|
Median 113 (94–128) days following discharge | RT-PCR and/or CT scan | Objective assessment via Q3PC, MoCA, d-2R (cognitive function), MFI (fatigue) during in-clinic/ ambulatory assessment, as well as subjective self-report via telephone questionnaire |
|
|
| Munblit et al., 2021a | Russia | Prospective Cohort | Sechenov University Hospital Network |
N = 2649 (all previously hospitalized cases)
|
Median 217.5 (200.4–235.5) days following discharge | RT-PCR or clinically diagnosed | Objective assessment via EQ-5D-5L (quality of life), as well as subjective self-report via telephone interview performed by medical students using |
|
|
| O’Keefe et al., 2021a | USA | Cross-sectional | Emory Healthcare’s Virtual Outpatient Management Clinic (VOMC) |
N = 198 participants discharged from outpatient telemedicine program for COVID-19(including 35 previously hospitalized cases)
|
Median 119 (range 26–220) days following diagnosis | RT-PCR | Subjective self-report via e-mail survey |
|
|
| Ong et al., 2021 | Singapore | Prospective Cohort | 4 public hospitals in Singapore |
|
Median 181 (103–191) days following discharge | RT-PCR | Objective assessed via immunoassay (inflammatory parameters), as well as subjective self-report |
|
|
| Orrù et al., 2021 | Italy | Cross-sectional | Individuals living in Italy (recruited through the web) |
N = 152 (hospitalization status not specified)
|
At least 3 months following positive test | RT-PCR | Objective assessment via EQ-5D-3L (quality of life), as well as subjective self-report via online survey |
|
|
| Osmanov et al., 2021a | Russia | Prospective Cohort | Z.A. Bashlyaeva Children’s Municipal Clinical Hospital |
N = 518 children (all previously hospitalized cases)
|
Median 256 (223–271) days following discharge | RT-PCR | Subjective self-report via telephone surveyconducted by medical students |
|
|
| Peghin et al., 2021 | Italy | Prospective Cohort | Udine Hospital |
N = 599 (442 outpatient cases, 157 previously hospitalized, including 23 in ICU)
|
Median 191 (172–204) days following onset of acute COVID-19 | Nucleic acid amplification tests and/or clinical diagnosis | Subjective self-report via telephone questionnaire administered by trained nurses |
|
|
| Pereira et al., 2021 | United Kingdom | Prospective Cohort | Hospital in North West London |
N = 38 hospital staff (35 symptomatic and 3 asymptomatic cases, all not requiring hospitalization)
|
7–8 months following symptom onset | RT-PCR | Subjective self-report via questionnaire based on NICE guidelines |
|
|
| Petersen et al., 2021 | Faroe Islands | Prospective Cohort | The Faroese Hospital System |
N = 180 (all outpatient cases)
|
Mean 125 ± 17 days following symptom onset | RT-PCR | Objective assessment via fatigue impact scale (fatigue) |
|
|
| Pilotto et al., 2021a | Italy | Prospective Cohort | Spedali Civili Brescia Hospital |
N = 165 (all previously hospitalized non-neurological cases)
|
6 months following hospitalization | RT-PCR | Objective assessment via MoCA (cognitive function), as well as subjective self-report via clinical follow-up checklist |
|
|
| Qu et al., 2021 | China | Prospective Cohort | 6 Hospitals in Anhui Province and Hubei Province |
N = 540 (all previously hospitalized cases)
|
3 months following discharge | RT-PCR | Objective assessment via SF-36 (quality of life), as well as subjective self-report via electronic survey form |
|
|
| Rass et al., 2021 | Austria | Prospective Cohort | Department of Internal Medicine II, Medical University of Innsbruck, Zams, and Muenster |
N = 135 (31 cases previously admitted to ICU, 72 previously admitted to ward, 32 previously received mild outpatient care)
|
Median 102 (91–110) days following onset of symptoms | RT-PCR | Objective assessment via MoCA (cognitive function), SF-36-v2 (quality of life), GOSE and mRS (functional outcome), as well as subjective self-report via clinical follow-up |
|
|
| Rauch et al., 2021a | Germany | Prospective Cohort | Life&Covid Online Cohort Study (Ludwig-Maximilians- Universität) |
N = 127 (including 116 outpatient cases and 11 inpatients)
|
6 months following infection | RT-PCR or Serology | Subjective self-report via e-mail survey |
|
|
| Romero-Duarte et al., 2021 | Spain | Retrospective Cohort | Four hospitals in Spain |
N = 797 (all previously hospitalized cases, including 81 previously admitted to ICU)
|
6 months following discharge | RT-PCR | Subjective self-report via questionnaire |
|
|
| Savarraj et al., 2021a | USA | Prospective Cohort | University of Texas Health Science Center |
N = 48 (all previously hospitalized cases)
|
3 months following discharge | RT-PCR | Objective assessment via mRS (functional outcomes), BNST (cognitive function), FSS (fatigue) |
|
|
| Say et al., 2021 | Australia | Prospective Cohort | Royal Children's Hospital |
N = 151 children (including 54 asymptomatic, 91 mostly mild symptomatic cases, and 14 previously hospitalized)
|
3–6 months following diagnosis | RT-PCR | Subjective self-report via follow-up clinic proforma |
|
|
| Shang et al., 2021 | China | Prospective Cohort | Zhongnan Hospital of Wuhan University, No. 7 Hospital of Wuhan, Leishenshan Hospital |
N = 796 (all previously hospitalized cases, including 38 in ICU)
|
6 months following discharge | RT-PCR | Subjective self-report via telephone interview |
|
|
| Shendy et al., 2021 | Egypt | Prospective Cohort | Ministry of Health and Population |
N = 81 (11 previously hospitalized cases, 70 non-hospitalized cases)
|
3–5 months following recovery from COVID-19 | RT-PCR | Objective assessment via MFIS (fatigue) |
|
|
| Shuwa et al., 2021 | United Kingdom | Prospective Cohort | Coronavirus Immune Response and Clinical Outcomes (CIRCO) study based at 4 hospitals in greater Manchester |
N = 83 (all previously hospitalized cases)
|
Median 158 (116.5–184.5) days following hospital admission | RT-PCR or clinical diagnosis | Objective assessment via cell culture and flow cytometry (immune parameters) |
|
|
| Simani et al., 2021 | Iran | Prospective Cohort | University-affiliated hospital of Tehran |
N = 120 (all previously hospitalized, including 9 in ICU)
|
6 months following COVID-19 infection | RT-PCR or CT | Objective assessment via previously validated questionnaire based on Fukuda guidelines for CFS/EM (fatigue) |
|
|
| Skala et al., 2021 | Czech Republic | Prospective Cohort | Hradec Kralove District |
N = 102 (including 15 previously hospitalized cases and 87 outpatient cases)
|
3 months following COVID-19 diagnosis | RT-PCR | Objective assessment via laboratory testing (inflammatory parameters), as well as subjective self-report via questionnaires administered by physician |
|
|
| Soldati et al., 2021 | Brazil | Prospective Cohort | ICU unit, Complexo Hospitalar de Niterói |
N = 23 (all previously admitted to ICU)
|
Median 83 (37–115) days following discharge | RT-PCR | Objective assessment viaTICS (cognitive function), EuroQol (quality of life) |
|
|
| Sonnweber et al., 2021 | Austria | Prospective Cohort | Department of Internal Medicine II, Medical University of Innsbruck, and two additional medical centres in Zams and Münster (CovILD study) |
N = 134 (including 109 previously hospitalized, of which 29 were previously admitted to ICU)
|
Mean 103 ± 21 days following diagnosis | RT-PCR | Objective assessment via laboratory testing (serology) |
|
|
| Soraas et al., 2021a | Norway | Prospective Cohort | Conducted online in Norway |
N = 588 (all previously non-hospitalized cases)
|
Mean 248 ± 18 days from baseline (mean 15.9 ± 9 days from testing to baseline) | RT-PCR | Objective assessment via RAND-36 (quality of life), as well as subjective self-report via online questionnaire |
|
|
| Stavem et al., 2021 | Norway | Cross-sectional | Akershus University Hospital (Ahus) and Østfold Hospital |
N = 458 (all non-hospitalized cases)
|
Median 117.5 (105–135) days following first COVID-19 symptom | RT-PCR | Objective assessment via CFQ-11 and RAND-36 (fatigue) administered via web or post |
|
|
| Suárez-Robles et al., 2020 | Spain | Cross-sectional | Hospital Clínico San Carlos |
N = 134 (all previously hospitalized, including 2 previously admitted to ICU)
|
90 days following discharge | RT-PCR | Subjective self-report via telephone structured interview |
|
|
| Sykes et al., 2021 | UK | Prospective Cohort | Hull University Teaching Hospitals NHS Trust |
N = 134 (all previously hospitalized, including 27 previously admitted to ICU)
|
Median 113 (range 46–167) days following discharge | RT-PCR | Objective assessment via EQ-5D-5L (quality of life), as well as subjective self-report via standardised clinical assessment by a specialist nurse and/or physiotherapist |
|
|
| Taboada (1) et al., 2020 | Spain | Prospective Cohort | Seven hospitals located in northwestern Spain |
N = 91 (all cases previously admitted to ICU)
|
6 months following ICU treatment | RT-PCR | Objectively assessed via EQ-5D-3L (quality of life) and PCFS (functional outcomes), as well as subjective self-report via structured interview conducted by trained research coordinators |
|
|
| Taboada (2) et al., 2021 | Spain | Cross-sectional | University Hospital of Santiago |
N = 183 (all previously hospitalized cases, including 32 to ICU)
|
6 months following hospitalization | RT-PCR | Objective assessment via PCFS (functional status), as well as subjective self-reportvia surveys conducted by trained study investigators |
|
|
| Tawfik et al., 2021 | Egypt | Retrospective Cohort | Ain-Shams University and Ministry of Health and Population hospitals |
N = 120 healthcare workers (including 18 previously hospitalized)
|
3 months following COVID-19 infection | RT-PCR and CT | Subjective self-report via questionnaire |
|
|
| Todt et al., 2021 | Brazil | Prospective Cohort | Hospital Municipal Dr. Moyses Deutsch |
N = 251 patients (all previously hospitalized, including 42 in ICU)
|
3 months following discharge | RT-PCR | Objective assessment viaEQ-5D-3L (quality of life) |
|
|
| Valiente-De Santis et al., 2020a | Spain | Prospective Cohort | Outpatients’ office of Regional University Hospital of Málaga |
N = 108 (all outpatient cases; both symptomatic and asymptomatic, including 30 healthcare workers)
|
12 weeks following acute COVID-19 | Serology | Subjective self-report via telephone survey |
|
|
| van den Borst et al., 2020 | The Netherlands | Prospective Cohort | Radboud University Medical Center (POSTCOVER study) |
N = 124 (all previously hospitalized cases)
|
Mean 13.0 ± 2.2 weeks following onset of symptoms | RT-PCR or clinically diagnosed | Objective assessment via laboratory testing (serological parameters), TICS, CFQ (cognitive function), SF-36 and NCSI (quality of life, fatigue) |
|
|
| Van Veenendaal et al., 2021a | The Netherlands | Prospective Cohort | University Medical Center Groningen, ICU (COFICS) |
N = 60 (all previously admitted cases to ICU) [50 at 6 months]
|
6 months following ICU discharge | RT-PCR | Objective assessment via SF-20 (quality of life), FAD-GF6+ (social functioning), as well as subjective self-report via telephone questionnaire conducted by research nurses (at 3 months), and mail questionnaire (at 6 months) |
|
|
| Venturelli et al., 2021 | Italy | Prospective Cohort | Papa Giovanni XXIII Hospital |
N = 767 (all previously hospitalized cases, including 66 in ICU)
|
Median 105 (84–127) days following onset of symptoms | RT-PCR or Serology | Objective assessment via laboratory testing (serology), MoCA (cognitive function), Barthel index (functional impairment), and Brief Fatigue inventory (fatigue), as well as subjective self-report via questionnaire |
|
|
| Walle-Hansen et al., 2021 | Norway | Retrospective Cohort | Four general hospitals in South-Eastern Norway |
N = 106 (all previously hospitalized cases, including 28 in ICU)
|
6 months following hospitalization | RT-PCR | Objective assessment via MoCA (cognitive function), and EQ 5D-5L (quality of life) |
|
|
| Wong et al., 2020 | Canada | Prospective Cohort | Post-COVID-19 Respiratory Clinic in Vancouver |
N = 78 (all previously hospitalized cases)
|
Median 13 (11–14) weeks following onset of symptoms | Laboratory test | Objective assessment via EQ-5D-5L (quality of life) |
|
|
| Woo et al., 2020 | United Kingdom | Cross-sectional | University Medical Centre Hamburg-Eppendorf |
N = 28 (11 previously hospitalized cases, 6 previously outpatient cases, 1 case receiving no medical care, and 10 healthy controls)
|
Median 85 (range 20–105) days following recovery | RT-PCR | Objective assessment via TICS-M (cognitive function), as well as subjective self-report via questionnaire |
|
|
| Xiong et. al., 2020 | China | Prospective cohort | Renmin Hospital of Wuhan University |
N = 722 (538 previously hospitalized cases, and 184 healthy controls)
|
Median 97 (95–102) days following discharge | COVID-19 diagnosis according to WHO interim guidance | Subjective self-report via telephone survey conducted by three experienced clinicians |
|
|
| Zhao et al., 2020 | China | Retrospective cohort | 3 Tertiary Hospitals in Henan Province |
N = 55 (all previously hospitalized cases)
|
3 months following discharge | RT-PCR | Self-report via clinical follow-up |
|
|
| Zhou et al., 2021 | China | Cross-sectional | 4 Hospitals in Wuhan |
N = 72 (55 cases, including 16 asymptomatic, and 17 healthy controls)
|
Mean 139.79 ± 7.41 days following illness onset for severe cohort, mean 133.75 ± 9.64 days following illness onset for mild cohort | RT-PCR or serology | Objective assessment via mesoscale-discovery (MSD) multiplexed immunoassay(immunological parameters) |
|
|
Proportions are reported as cases/total study sample size. ‘Cases’ refers to previous confirmed COVID-19 cases. Medians are reported as median (interquartile range), if the interquartile range was provided, or unless otherwise specified. Means are reported as mean ± standard deviation, if the standard deviation was provided. ‘Previously hospitalized’/‘admitted to ICU’ refers to COVID-19 treatment. ‘Admission’ and ‘discharge’ refer to COVID-19 inpatient treatment. ‘Infection’ refers to infection with SARS-CoV-2. Ages are given in years. ‘%F/%M’ refers to percentage of study sample which is female/percentage of study sample which is male.
Acronyms: COVID-19: Coronavirus disease 2019, SARS-CoV-2: Severe acute respiratory syndrome coronavirus 2, ICU: Intensive care unit, USA: United States of America, RT-PCR: Reverse transcription polymerase chain reaction, ESCAPE: Evaluation Study of Congestive Heart Failure and Pulmonary Artery Catheterization Effectiveness, DISCOVER: Diagnostic and Severity Markers of COVID-19 to Enable Rapid Triage, ISARIC: International Severe Acute Respiratory and Emerging Infection Consortium, SF-36: 36-Item Short Form Survey, CRP: C-reactive protein, IgG: Immunoglobulin G, CD4+: Cluster of differentiation 4+, CD8+: Cluster of differentiation 8+, IL-2: Interleukin-2, IFN-γ: Interferon gamma, TNF-α: Tumor necrosis factor alpha, ADAPT: Australians’ Drug Use: Adapting to Pandemic Threats, SPHERE-34: 34-Item Somatic and Psychological Health Report, RAND-36: Rand 36-Item Health Survey, PHOSP-COVID: Post-hospitalization COVID-19 study, IQR: Interquartile range, FACIT: Functional Assessment of Chronic Illness Therapy, CFS/ME: Chronic Fatigue Syndrome/Myalgic Encephalomyelitis, MoCA: Montreal Cognitive Assessment, EuroQol: European Quality of Life Scale, EQ-VAS: EuroQol visual analog scale, EQ-5D-5L: EuroQol-5 Dimension-5 levels, EQ-5D-3L: EuroQol-5-Dimension-3 levels, EQ-5D-5L VAS: EuroQol-5 Dimension-5 levels visual analog scale, WG-SS: Washington Group Short Set on Functioning, CT: Computerized tomography, BRB-NT: Brief Repeatable Battery of Neuropsychological Tests, SSD: Subjective Scale of Damage, SDMT: Symbol Digit Modalities Test, ARDS: Acute respiratory distress syndrome, IL-6: Interleukin-6, NYC: New York City, CSQ: COVID-19 Symptom Questionnaire, SF-12: 12-Item Short Form Survey, SF-23: 23-Item Short Form Survey, ME/CFS: Myalgic encephalomyelitis/chronic fatigue syndrome, WPAI: Work Productivity and Activity Impairment Questionnaire, PCFS: Post-COVID-19 Functional Status, CFQ: Cognitive Failures Questionnaire, HRTC: High Resolution Computed Tomography, SCIP-D: Screen for Cognitive Impairment in Psychiatry, TMT-B: Trail Making Test Part B, FSS: Fatigue Severity Scale, PICS: Post-intensive care syndrome, SF-35: 35-Item Short Form Survey, SD: Standard deviation, OMC: Orientation-Memory-Concentration Test, OR: Odds ratio, PTSD: Post-traumatic stress disorder, MMSE: Mini-Mental State Examination, BACS: Brief Assessment of Cognition in Schizophrenia, REDCap: Research Electronic Data Capture, FAS: Fatigue Assessment Scale, Q3PC: questionnaire of cognitive complaints, d-2R: D2 Test of Attention, MFI: Multidimensional fatigue inventory, WHO CRF: World Health Organization’s Post COVID Case Report form, MIP-1β: Macrophage inflammatory protein-1 beta, SDF-1α: Stromal Cell Derived Factor 1 alpha, IL-12p70: Interleukin-12p70, SCF: Stem cell factor, IL-17A: Interleukin-17A, BDNF: Brain-derived neurotrophic factor, VEGF: Vascular endothelial growth factor, IP-10: Interferon-inducible protein 10, IL-18: Interleukin-18, MCP-1: Monocyte chemoattractant protein-1, ELISA: Enzyme-linked immunosorbent assay, GOSE: Glasgow Outcome Scale-Extended, mRS: Modified Rankin Scale, BNST: Brief neurocognitive screening test, MFIS: Modified Fatigue Impact Scale, CIRCO: Coronavirus Immune Response and Clinical Outcome, TICS: Telephone interview for cognitive status, CFQ-11: Chalder Fatigue Scale 11, BMI: Body mass index, POSTCOVER: Post-COVID-19 Recovery Study, SF-20: 20-Item Short Form Survey, FAD-GF6+: McMaster Family Assessment Device-General Functioning subscale, MSD: Mesoscale-discovery multiplexed immunoassay.
This article is a pre-print as of June 8, 2021.
3.3. Methodological quality and risk of bias
Taken together, the NOS rating of the component studies was moderate, evidenced by mean scores of 6.0 out of 9.0 for prospective/ambidirectional cohort studies, 4.1 out of 6.0 for retrospective cohort studies, and 5.6 out of 9.0 for cross-sectional studies. Common methodological limitations were the failure to include a non-exposed group in cohort studies, failure to ascertain whether outcomes were present prior to COVID-19 infection, and a lack of sample size justification in cross-sectional studies. NOS scores within each category for all component studies organised by design are included (Table S1 in the supplementary material).
We conducted sensitivity analyses a posteriori to compare pooled proportions across high, moderate, and low NOS-ranked studies. Studies of moderate NOS rank reported higher proportions of individuals exhibiting cognitive impairment when compared to low and high NOS-ranked studies (p = 0.035; Table 3 ). However, the proportion of individuals experiencing fatigue did not significantly differ across NOS ranking categories (p = 0.885; Table 3).
Table 3.
Subgroup and sensitivity analyses for the primary outcomes.
| No. of Studies | Proportion | 95% CI | p | I2 | Q | psubgroup (χ2 test) | |
|---|---|---|---|---|---|---|---|
| FATIGUE | |||||||
| Sex | |||||||
| Females | 7 | 0.46 | (0.32, 0.60) | <0.01 | 96.0% | 3.36 | 0.067 |
| Males | 7 | 0.30 | (0.22, 0.39) | <0.01 | 92.6% | ||
| Age Groupa | |||||||
| Adults (≥18 years) | 65 | 0.32 | (0.26, 0.37) | <0.001 | 98.3% | 13.83 | <0.001 |
| Children (<18 years) | 3 | 0.07 | (0.03, 0.16) | <0.01 | 78.5% | ||
| COVID-19 Hospitalization Status | |||||||
| Hospitalized | 45 | 0.36 | (0.30, 0.43) | <0.001 | 99.4% | 1.76 | 0.185 |
| Non-Hospitalized | 10 | 0.44 | (0.34, 0.55) | <0.01 | 92.9% | ||
| Follow-up Duration | |||||||
| <6 Months | 46 | 0.33 | (0.26, 0.39) | <0.001 | 99.1% | 0.10 | 0.755 |
| ≥6 Months | 26 | 0.31 | (0.24, 0.37) | <0.001 | 99.0% | ||
| Mode of Ascertainmentb | |||||||
| Subjective | 55 | 0.29 | (0.24, 0.35) | <0.001 | 99.2% | 7.56 | 0.006 |
| Objective | 13 | 0.45 | (0.35, 0.55) | <0.01 | 96.4% | ||
| NOS Rating Category | |||||||
| High | 24 | 0.28 | (0.20, 0.37) | <0.001 | 98.9% | 0.59 | 0.750 |
| Moderate | 27 | 0.32 | (0.25, 0.40) | <0.01 | 96.6% | ||
| Low | 17 | 0.30 | (0.17, 0.46) | <0.01 | 98.4% | ||
| Study Design | |||||||
| Prospective Cohort | 48 | 0.28 | (0.22, 0.34) | <0.001 | 97.6% | 94.84 | < 0.001 |
| Retrospective Cohort | 8 | 0.31 | (0.17, 0.49) | <0.01 | 98.7% | ||
| Cross-sectional | 10 | 0.36 | (0.21, 0.53) | <0.01 | 97.1% | ||
| Ambidirectional Cohort | 1 | 0.63 | (0.60, 0.65) | N/A | N/A | ||
| Retrospective Case-control | 1 | 0.50 | (0.38, 0.62) | N/A | N/A | ||
| COGNITIVE IMPAIRMENT | |||||||
| Sex | |||||||
| Females | 2 | 0.56 | (0.46, 0.66) | 0.960 | 0.0% | 3.46 | 0.063 |
| Males | 2 | 0.36 | (0.19, 0.55) | 0.020 | 82.5% | ||
| Age Groupa | |||||||
| Adults (≥18 years) | 42 | 0.19 | (0.14, 0.26) | <0.01 | 97.0% | 1.77 | 0.182 |
| Children (<18 years) | 1 | 0.12 | (0.06, 0.22) | N/A | N/A | ||
| COVID-19 Hospitalization Status | |||||||
| Hospitalized | 24 | 0.30 | (0.22, 0.38) | <0.01 | 96.7% | 2.77 | 0.096 |
| Non-Hospitalized | 5 | 0.20 | (0.12, 0.29) | <0.01 | 70.8% | ||
| Follow-up Duration | |||||||
| <6 Months | 31 | 0.22 | (0.15, 0.30) | <0.001 | 98.2% | 0.07 | 0.794 |
| ≥6 Months | 14 | 0.21 | (0.13, 0.30) | <0.01 | 97.3% | ||
| Mode of Ascertainmentb | |||||||
| Subjective | 31 | 0.18 | (0.12, 0.24) | <0.01 | 97.9% | 9.97 | 0.002 |
| Objective | 12 | 0.36 | (0.27, 0.46) | <0.01 | 94.9% | ||
| NOS Rating Category | |||||||
| High | 12 | 0.18 | (0.10, 0.29) | <0.01 | 95.7% | 10.95 | 0.004 |
| Moderate | 17 | 0.32 | (0.21, 0.44) | <0.01 | 92.6% | ||
| Low | 14 | 0.10 | (0.05, 0.18) | <0.01 | 97.4% | ||
| Study Design | |||||||
| Prospective Cohort | 31 | 0.18 | (0.12, 0.26) | <0.01 | 97.4% | 2.01 | 0.366 |
| Retrospective Cohort | 5 | 0.16 | (0.06, 0.35) | <0.01 | 92.5% | ||
| Cross-sectional | 7 | 0.26 | (0.16, 0.44) | <0.01 | 92.9% | ||
Acronyms: NOS: Newcastle-Ottawa Scale, N/A: not applicable.
Statistically significant subgroup effect sizes, ascertained as psubgroup (χ2 test) <0.05, are bolded.
Studies categorized by age group depending on mean or median age.
Refers to ascertainment of outcomes (see Table 1).
3.4. Synthesis of results
Meta-analyses of the two primary outcomes indicated that 32% of individuals experienced fatigue and 22% of individuals exhibited cognitive impairment 12 or more weeks following COVID-19 diagnosis. Furthermore, 13 of 14 studies examining inflammatory parameters reported elevations in proinflammatory markers (i.e., proinflammatory cytokines, C-reactive peptide, D-dimer, and procalcitonin) in a subset of patients. All studies investigating functional outcomes reported marked functional impairment in a sample subset.
3.4.1. Fatigue Meta-Analysis
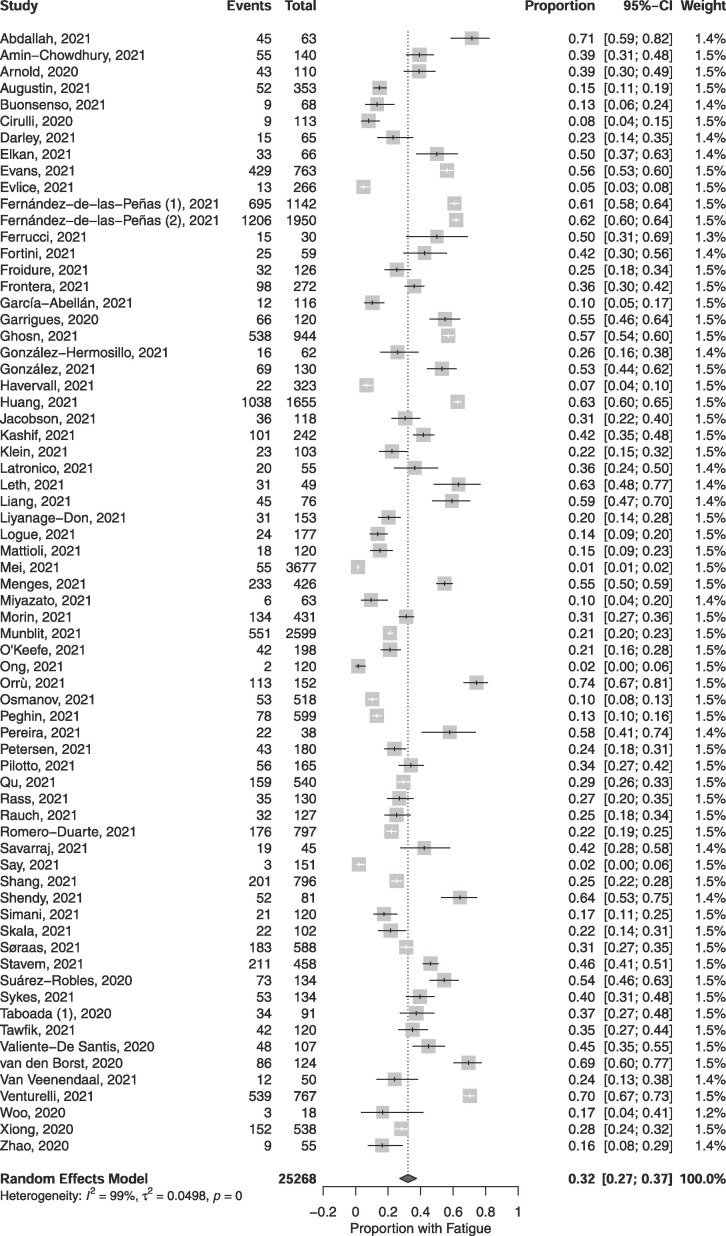
The pooled proportion of individuals experiencing fatigue amongst COVID-19 patients 12 or more weeks following diagnosis was 0.32 (95% CI, 0.27, 0.37; p < 0.001; n = 25,268; Fig. 2 ). A larger proportion of females reported fatigue as compared to males, but the inter-subgroup difference was not statistically significant (0.46 of females vs 0.30 of males; psubgroup = 0.067; Table 3). Subgroup analysis for age category revealed that a significantly greater proportion of adults experienced fatigue as compared to children (0.32 of adults vs 0.07 of children; psubgroup < 0.001; Table 3). Moreover, studies which objectively assessed fatigue reported significantly greater proportions of individuals experiencing fatigue as compared to subjective modes of ascertainment (0.45 when assessed objectively vs 0.29 when assessed subjectively; psubgroup = 0.006; Table 3). However, there was no statistically significant difference in the proportion of persons reporting fatigue between hospitalized and non-hospitalized respondents (0.36 hospitalized vs 0.44 non-hospitalized; psubgroup = 0.185; Table 3). Likewise, there was no significant difference in the proportions of persons experiencing fatigue at <6 months follow-up since COVID-19 diagnosis compared to ≥6 months (0.33 when <6 months vs 0.31 when ≥ 6 months; psubgroup = 0.755; Table 3). Sensitivity analyses revealed that stratifying studies by design produced statistically significant differences in effect size, although the effect sizes between prospective cohort, retrospective cohort, cross-sectional studies (i.e., the 3 most common study designs) did not significantly differ.
Fig. 2.
Pooled proportions of individuals experiencing fatigue 12 or more weeks following COVID-19 diagnosis.
3.4.2. Cognitive Impairment Meta-Analysis
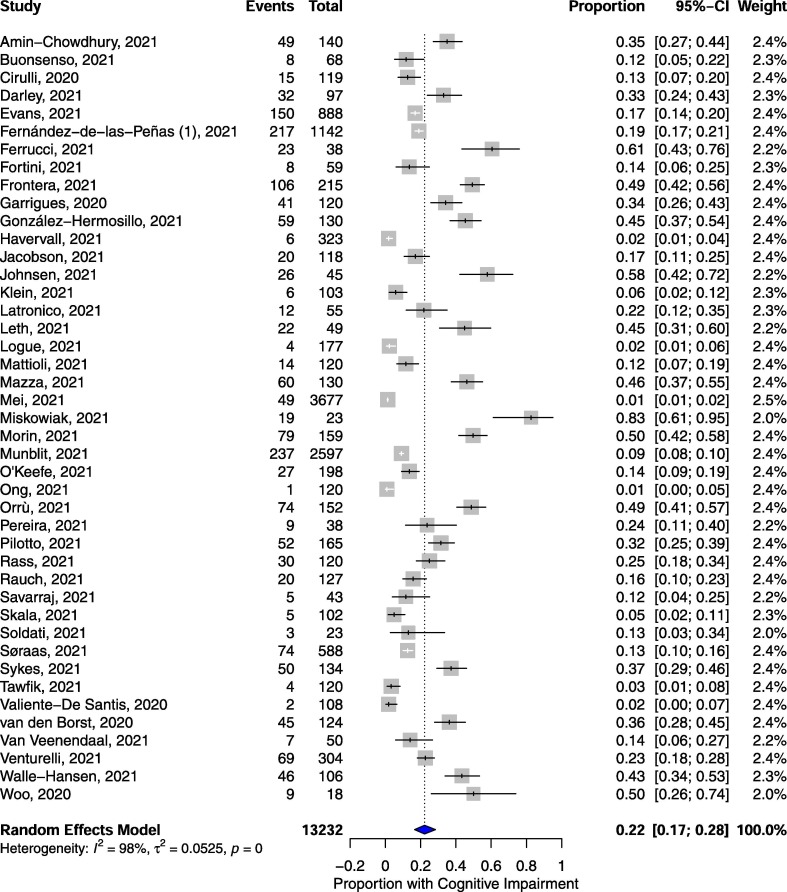
The pooled proportion of individuals exhibiting cognitive impairment amongst COVID-19 patients 12 or more weeks following diagnosis was 0.22 (95% CI, 0.17, 0.28; p <0.001; n = 13,232; Fig. 3 ). There was a non-significant trend towards a greater proportion of females than males who exhibited cognitive impairment, (0.56 of females vs 0.36 of males; psubgroup = 0.063; Table 3), however, the subgroup proportion for females was not statistically significant (0.56; 95% CI, 0.46, 0.66; p = 0.960; Table 3). Only one study reported on cognitive impairment in children (Buonsenso et al., 2021), thus, significant differences between child and adult subgroups were not determined (psubgroup = 0.182; Table 3). Studies which objectively assessed cognitive impairment reported significantly greater proportions of individuals with cognitive impairment as compared to those employing subjective modes of ascertainment (0.36 objectively assessed vs 0.18 subjectively assessed; psubgroup = 0.002; Table 3). There was no statistically significant difference between hospitalized and non-hospitalized subgroup proportions reporting post-COVID cognitive impairment (0.30 hospitalized vs 0.20 non-hospitalized; psubgroup = 0.096; Table 3). Likewise, there was no significant difference in the proportions reporting cognitive impairment at <6 and ≥6 months follow-up (0.22 when <6 months vs 0.21 when ≥6 months; psubgroup = 0.794; Table 3). Unlike for fatigue, stratifying studies by design did not produce any statistically significant differences in effect sizes (psubgroup = 0.366; Table 3).
Fig. 3.
Pooled proportions of individuals exhibiting cognitive impairment 12 or more weeks following COVID-19 diagnosis.
3.4.3. Heterogeneity
The fatigue (I2 = 99.1%) and cognitive impairment (I2 = 98.0%) meta-analyses exhibited considerable heterogeneity. Select subgroup analyses resulted in a reduction of heterogeneity (Table 3).
3.4.4. Publication Bias
Visual inspection of funnel plot asymmetry for the fatigue meta-analysis did not suggest the presence of publication bias (Supplementary, Fig. S1), and neither the Egger regression intercept test (intercept = 0.538; SE = 0.061; p = 0.390) nor the Begg and Mazumdar rank correlation test (p = 0.857) were statistically significant. Conversely, visual inspection of funnel plot asymmetry for the cognitive impairment meta-analysis suggested the presence of publication bias (Supplementary, Fig. S2); the Egger regression intercept test was statistically significant (intercept = 0.187; SE = 0.040; p < 0.001), whereas the Begg and Mazumdar rank correlation test was not significant (p = 0.818).
3.4.5. Inflammatory Parameters
14 studies investigated peripheral inflammatory parameters in COVID-19 patients 12 or more weeks following diagnosis (Zhou et al., 2021, Breton et al., 2021, Shuwa et al., 2021, Sonnweber et al., 2021, Arnold et al., 2021, Fortini et al., 2021, García-Abellán et al., 2021, Ong et al., 2021, PHOSP-COVID Collaborative Group et al., 2021, Skala et al., 2021, Sykes et al., 2021, Santis et al., 2020, van den Borst et al., 2020, Venturelli et al., 2021). Of those which quantified intracellular cytokine levels, Breton et al. reported marked increases in the numbers of CD4+ T cells expressing the inflammatory cytokines interleukin (IL)-2, interferon (IFN)-γ, and tumor necrosis factor (TNF)-α in individuals with prior COVID-19 as compared with healthy donors (Breton et al., 2021). Ong et al. reported that previously-hospitalized patients exhibited elevated levels of proinflammatory cytokines, including but not limited to macrophage inflammatory protein 1β, IL-1β, and IL-17A (Ong et al., 2021). Ong et al. additionally reported that concentrations of the proinflammatory factors IL-1β, IL-17A, IL-12p70, stem cell factor (SCF), and MIP-1β were significantly higher in prior COVID-19 patients compared to healthy controls, regardless of acute phase severity (Ong et al., 2021). Moreover, Shuwa et al. determined that patients with prior moderate to severe acute illness demonstrated an elevation in most cytokine-producing T cells, as well as increased production of cytokines, whereas no significant differences in the frequency of TNF-α+ B cells were observed (Shuwa et al., 2021). Zhou et al. reported significant increases in serum amyloid A, TNF-α, and IL-1RA in the severe COVID-19 cohort, higher than normal levels of IL-17A and IL-17D, and decreased IL-7 (Zhou et al., 2021). Between 3.9% and 32.2% of post-COVID individuals exhibited elevated IL-6 levels (Sonnweber et al., 2021, Fortini et al., 2021, Santis et al., 2020), and García-Abellán et al. reported a median serum IL-6 level of 3 pg/mL (García-Abellán et al., 2021), which exceeds the standard reference value of ≤ 1.8 pg/mL (IL6 - Clinical: Interleukin 6, 2021). Breton et al. similarly reported that post-COVID patients exhibited systemic cytokine profiles distinct from uninfected controls (Breton et al., 2021), whereas Zhou et al. reported normal levels of IL-6 and IL-10 across all groups (Zhou et al., 2021).
Between 1.8% and 24.5% of patients exhibited elevated CRP levels (Sonnweber et al., 2021, Arnold et al., 2021, PHOSP-COVID Collaborative Group et al., 2021, Skala et al., 2021, Santis et al., 2020), operationalized as >10 mg/L or >2.9 mg/dL. Median CRP levels ranged between 0.6 and 2.9 mg/L (Zhou et al., 2021, García-Abellán et al., 2021, Sykes et al., 2021, van den Borst et al., 2020). Moreover, 9.8% to 38.0% of patients exhibited elevated levels of D-dimer (i.e., ≥500 ng/mL) (Sonnweber et al., 2021, Fortini et al., 2021, PHOSP-COVID Collaborative Group et al., 2021, Skala et al., 2021, Santis et al., 2020, Venturelli et al., 2021). Conversely, Zhou et al. reported that CRP and D-dimer levels were not significantly elevated in their post-COVID sample compared to healthy controls (p > 0.05), but that cytokines such as TNF-α were correlated with abnormal clinical features (Zhou et al., 2021). In addition, Sonnweber et al. reported elevated procalcitonin levels in 9.0% of patients (mean 0.07) (Sonnweber et al., 2021).
Taken together, 13 of 14 studies (all except van den Borst et al.) (van den Borst et al., 2020) examining inflammatory parameters report elevation in at least one measure of inflammation in a subset of patients or across the whole post-COVID sample (as a median/mean, compared to healthy controls or standard reference values). It should be noted that nine of 14 studies reported both the presence of proinflammatory markers as well as persistent fatigue and/or cognitive impairment within their sample (Fortini et al., 2021, García-Abellán et al., 2021, Ong et al., 2021, PHOSP-COVID Collaborative Group et al., 2021, Skala et al., 2021, Sykes et al., 2021, Santis et al., 2020), and several studies noted an association between elevation in measures of inflammation and PCS symptoms (PHOSP-COVID Collaborative Group et al., 2021, Skala et al., 2021, Sykes et al., 2021).
3.4.6. Functional Outcomes/Quality of Life
34 studies investigated functional outcomes, frequently subsumed under quality of life (QOL) measures, in COVID-19 patients 12 or more weeks following diagnosis (Huang et al., 2021, Ghosn et al., 2021, González et al., 2021, Fernández-de-Las-Peñas et al., 2021, Arnold et al., 2021, PHOSP-COVID Collaborative Group et al., 2021, Sykes et al., 2021, van den Borst et al., 2020, Venturelli et al., 2021, Taboada et al., 2021, Todt et al., 2021, Wong et al., 2020, Elkan et al., 2021, Frontera et al., 2021, Garrigues et al., 2020, Havervall et al., 2021, Jacobson et al., 2021, Johnsen et al., 2021, Latronico et al., 2020, Logue et al., 2021, Menges et al., 2021, Miskowiak et al., 2021, Munblit et al., 2021, Orrù et al., 2021, Osmanov et al., 2021, Pereira et al., 2021, Qu et al., 2021, Rass et al., 2021, Savarraj et al., 2020, Soldati et al., 2021, Soraas et al., 2021). Nine studies measured QOL using the European Quality of Life 5 dimension 5 levels (EQ-5D-5L) scale (Wong et al., 2020, PHOSP-COVID Collaborative Group et al., 2021, Garrigues et al., 2020, Johnsen et al., 2021, Miskowiak et al., 2021, Munblit et al., 2021), four used the EQ-5D-3L scale, (Huang et al., 2021, Todt et al., 2021, Orrù et al., 2021, Taboada et al., 2021) seven used the 36-Item Short Form Survey (SF-36/RAND-36) (Arnold et al., 2021, van den Borst et al., 2020, Elkan et al., 2021, Latronico et al., 2020, Qu et al., 2021, Rass et al., 2021, Soraas et al., 2021), three used the Barthel Index (Venturelli et al., 2021, Frontera et al., 2021, Latronico et al., 2020), 11 used another scale (Taboada et al., 2021, González et al., 2021, PHOSP-COVID Collaborative Group et al., 2021, van den Borst et al., 2020, Havervall et al., 2021, Jacobson et al., 2021, Osmanov et al., 2021, Pereira et al., 2021, Rass et al., 2021, Savarraj et al., 2020, Soldati et al., 2021), and three via self-report (Ghosn et al., 2021, Fernández-de-Las-Peñas et al., 2021, Logue et al., 2021). with some studies implementing multiple assessment tools. All studies demonstrated functional impairment or reduction in at least one QOL dimension (in up to 72% of patients) (van den Borst et al., 2020) compared to regional norms, uninfected controls, or pre-COVID status, and four studies reported decrements across all QOL dimensions in their post-COVID sample (González et al., 2021, Arnold et al., 2021, van den Borst et al., 2020, Munblit et al., 2021).
Functional impairment post-COVID was exhibited by 21% to 63% of individuals (Taboada et al., 2021, Savarraj et al., 2020, Taboada et al., 2021); activity impairment (including difficulties with performing daily tasks, self-care, and mobility) in 1.0% to 68.4% (Huang et al., 2021, Todt et al., 2021, Frontera et al., 2021, Menges et al., 2021, Miskowiak et al., 2021, Orrù et al., 2021, Havervall et al., 2021, Jacobson et al., 2021, Johnsen et al., 2021, Soraas et al., 2021, Walle-Hansen et al., 2021, Taboada et al., 2021), social impairment in 5% to 15% (Elkan et al., 2021, Havervall et al., 2021, Latronico et al., 2020, Van Veenendaal et al., 2021), and 16.0% to 28.2% reportedly unable to partake in a sport/recreational activity (Fernández-de-Las-Peñas et al., 2021, Garrigues et al., 2020, Pereira et al., 2021). One in five previously hospitalized persons reached the threshold for an additional disability on the Washington Group Short Set on Functioning (WG-SS) scale (PHOSP-COVID Collaborative Group et al., 2021). Moreover, between 29.0% and 47.4% of those who were employed premorbidly were not able to return to work (Ghosn et al., 2021, Frontera et al., 2021, Garrigues et al., 2020, Jacobson et al., 2021, Latronico et al., 2020), 5% to 90% were unable to reach their pre-COVID employment level (PHOSP-COVID Collaborative Group et al., 2021, Latronico et al., 2020, Van Veenendaal et al., 2021), and between 8.0% and 38.9% reported disruption in work life (Havervall et al., 2021, Jacobson et al., 2021, Miskowiak et al., 2021, Soraas et al., 2021). Comprehensive results, including mean and median scores on QOL scales where reported, are available in Table 2. EQ-5D population norms are provided elsewhere (Szende et al., 2013).
3.4.7. Reported Factors Associated With Post-COVID-19 Syndrome
Overall, 53 of out 81 studies reported factors associated with increased incidence of PCS symptoms according to their respective analyses. Female sex was associated with an increased risk of developing PCS symptoms (including fatigue and cognitive impairment, in some instances), a greater number of persistent symptoms, or decrements in QOL dimensions in 24 studies (Taboada et al., 2021, Todt et al., 2021, Ghosn et al., 2021, Fernández-de-las-Peñas et al., 2021, García-Abellán et al., 2021, PHOSP-COVID Collaborative Group et al., 2021, Sykes et al., 2021, Venturelli et al., 2021, Menges et al., 2021, Munblit et al., 2021, Qu et al., 2021, Amin-Chowdhury et al., 2021, Augustin et al., 2021, Cirulli et al., 2020, Darley et al., 2021, Ferrucci et al., 2021, Kashif et al., 2021, O’Keefe et al., 2021, Peghin et al., 2021, Shang et al., 2021, Simani et al., 2021, Stavem et al., 2021, Xiong et al., 2021), while male sex predicted decreased functional status/QOL in one study (Taboada et al., 2021).
Increased age was associated with more reports of PCS symptoms or QOL diminution in 14 studies (Huang et al., 2021, Sonnweber et al., 2021, Todt et al., 2021, Ong et al., 2021, Jacobson et al., 2021, Qu et al., 2021, Savarraj et al., 2020, Walle-Hansen et al., 2021, Ferrucci et al., 2021, Xiong et al., 2021, González-Hermosillo et al., 2021, Petersen et al., 2021, Tawfik et al., 2021;18(3):em291., Mei et al., 2021), whereas Menges et al. noted that younger individuals more frequently reported fatigue (Menges et al., 2021), and Valiente-De Santis et al. reported that age ≥65 years was protective against COVID-19 symptom persistence (Santis et al., 2020). Pre-existing comorbidities were associated with PCS symptoms or QOL decrements in 9 studies (Sonnweber et al., 2021, Todt et al., 2021, Fernández-de-las-Peñas et al., 2021, PHOSP-COVID Collaborative Group et al., 2021, Menges et al., 2021, Osmanov et al., 2021, Amin-Chowdhury et al., 2021, Stavem et al., 2021, Evlice et al., 2021). Furthermore, greater severity of acute disease, hospitalization, or increased length of hospital stay were associated with PCS symptoms or QOL decrements in 19 studies (Huang et al., 2021, Pilotto et al., 2021, Fernández-de-las-Peñas et al., 2021, Arnold et al., 2021, Ong et al., 2021, Taboada et al., 2021, Todt et al., 2021, Elkan et al., 2021, Jacobson et al., 2021, Menges et al., 2021, Taboada et al., 2021, Darley et al., 2021, Ferrucci et al., 2021, O’Keefe et al., 2021, Peghin et al., 2021, González-Hermosillo et al., 2021, Rauch et al., 2021). Interestingly, Jacobson et al. reported that the presence of fatigue was associated with long-term activity impairment (Jacobson et al., 2021), Miskowiak et al. reported that greater global cognitive impairment and executive dysfunction both correlated with greater difficulty within the EQ-5D ‘usual activity’ and ‘anxiety and depression’ domains (Miskowiak et al., 2021), and Soldati et al. reported that patients with mild cognitive impairment tended to have a low QOL score (Soldati et al., 2021). Moreover, Woo et al. reported that persistent neurocognitive deficits were independent from fatigue and mood alterations, and may thus differ from the classical post-viral syndrome (Woo et al., 2020). Table 2 details all factors reportedly associated with PCS symptoms across individual component studies.
4. Discussion
Herein we identified that approximately a third of individuals experienced persistent fatigue and over a fifth of individuals exhibited cognitive impairment 12 or more weeks following confirmed COVID-19 diagnosis. Similar incidences of fatigue and cognitive impairment, respectively, were observed amongst hospitalized and non-hospitalized populations. Furthermore, in contradistinction to other persistent symptoms which may be self-limiting (e.g., anosmia) (Hopkins et al., 2020), fatigue and cognitive impairment appear to endure and may potentially worsen over time in susceptible individuals (Jason et al., 2021), as evidenced by similar proportions of affected individuals at <6 and ≥6 months follow-up. A lower incidence of fatigue and cognitive impairment, respectively, were identified amongst children as compared to adults. Moreover, we established that persistent inflammation was consistently reported in a subset of patients, and that symptoms of PCS (including fatigue and cognitive impairment) are associated with marked functional impairment. Frequently reported factors associated with a greater incidence of PCS symptoms amongst component studies included female sex, older age, greater severity of acute illness, and pre-existing comorbidities.
Fatigue and cognitive impairment in PCS comprise a form of postinfectious fatigue syndrome, and exhibit phenotypic similarity to ME/CFS, which is often precipitated by an infectious agent (Taboada et al., 2021). Similar incidence rates of fatigue, as well as decreased QOL measures, have been reported in the aftermath of previous coronavirus epidemics, including severe acute respiratory syndrome coronavirus (SARS) and Middle East respiratory syndrome coronavirus (MERS) (Lam et al., 2009, Rogers et al., 2020). Furthermore, PCS shares overlapping symptoms with the encephalitis lethargica epidemic (von Economo's encephalitis) of the 1920 s (e.g., fatigue, cognitive impairment, headache), which was hypothesized to be causally related to the 1918 Spanish influenza pandemic (Hoffman and Vilensky, 2017).
There are multiple mechanisms whereby SARS-CoV-2 infection can engender or exacerbate persistent fatigue and/or cognitive impairment. Neurological dysfunction can ensue due to non-mutually exclusive factors including but not limited to direct viral encephalitis, neuroinflammation (including damage to blood–brain barrier integrity) (Nalbandian et al., 2021, Lam et al., 2009, Rogers et al., 2020), hypoxia, and cerebrovascular disease (Nalbandian et al., 2021, Komaroff and Lipkin, 2021, Higgins et al., 2021). Multiple studies have identified neuroanatomical alterations and neurodegeneration (Douaud et al., 2021), cerebral microvascular injury (Lee et al., 2021), and metabolic aberrations (including hypometabolism in areas associated with motivation, such as the dorsolateral prefrontal cortex) (Guedj et al., 2021) in the brains of COVID-19 patients.
It is also recognized that systemic sequelae including endothelial dysfunction (Libby and Lüscher, 2020), hyperinflammation, autoimmunity, latent viral reactivation (Oronsky et al., 2021), multi-organ pathology, and autonomic nervous system dysfunction can interact with the foregoing in a synergistic manner (Yong, 2021). The causal relationship between specific pro-inflammatory cytokines, mood symptoms, and cognitive decline is firmly established (Sartori et al., 2012, Rosenblat et al., 2014). We report that a subset of individuals consistently exhibited markers of inflammation following the resolution of acute COVID-19 infection, suggesting hyperinflammation is an amenable cause of fatigue and/or cognitive impairment in PCS. Indeed, other post-infectious syndromes (e.g., post-infectious encephalitis) (Sonneville et al., 2009) have been previously associated with elevations in inflammatory parameters.
Several non-mutually exclusive hypotheses regarding the pathogenesis of PCS would suggest that anti-inflammatory pharmacologic approaches may be of some utility in select patients. Moreover, psychotropic medications (e.g., selective serotonin reuptake inhibitors) (Gałecki et al., 2018) may modulate pro-inflammatory cytokine levels, and possibly exert salutary effects on mood and cognition in COVID-19 survivors (Heckenberg et al., 2018). In addition to pharmacologic strategies, disparate psychosocial treatments (e.g., cognitive remediation) may also be effective in treating symptoms of PCS. There is also the need to determine whether the type and frequency of vaccines (i.e., boosters) influence the risk for incident, persistent and/or severity of PCS. Reports of breakthrough infections in persons previously receiving a full vaccination schedule invites the need for characterization of risk in that subset of individuals. Moreover, the influence of both medical and psychiatric comorbidity on the risk for PCS, notably cognitive impairment, as well as the moderating influence of social, economic, and spatial determinants of health should be evaluated.
5. Limitations
The results presented herein should be interpreted within the context of several limitations. First, component studies were observational, therefore causal relationships cannot be inferred. Second, the majority of studies did not ascertain whether outcomes were present prior to COVID-19 infection (i.e., period prevalence rather than incidence), although this is a limitation accounted for by the NOS. Thus, we cannot exclude the possibility that fatigue and cognitive impairment preceded SARS-CoV-2 infection (although infection may have exacerbated symptoms). Third, as most component studies were based on hospitalized individuals, the results may not be representative for the majority of individuals affected by COVID-19. Furthermore, it is firmly established that the incidence of depressive and anxious symptoms in the general population has increased since the pandemic onset (Xiong et al., 2020); fatigue and cognitive impairment may be consequences of chronic stress and/or depression resulting from social and economic challenges of COVID-19, rather than a result of infection, in a proportion of PCS patients. It is also noteworthy that social consequences may be exacerbated for infected individuals. The majority of cohort studies failed to include a non-exposed control group, providing no basis for comparison (see Table S1 in supplementary material).
With respect to selection bias, there is an overrepresentation of hospitalized cases in the analyses herein, and a subset of reports may be attributable to post-intensive care syndrome, comorbid conditions, and/or medications (Heckenberg et al., 2018). Conversely, PCS patients not receiving healthcare for their symptoms are underrepresented. Moreover, fatigue and cognitive impairment may be secondary to other sequelae of SARS-CoV-2 infection, including but not limited to major depressive disorder (Taquet et al., 2021). Another limitation in most studies of objective cognitive functions was the use of dementia screening tools (e.g., MoCA, TICS) that have limited sensitivity to cognitive decline in younger populations and may thus have led to underestimation of cognitive impairments (McIntyre et al., 2019). Future studies would thus be recommended to apply more sensitive tools devoid of ceiling effects, for example the Screen for Cognitive Impairment for Psychiatry (SCIP) (Miskowiak et al., 2021, Miskowiak et al., 2017) and THINC-integrated tool (THINC-it) (McIntyre et al., 2020, McIntyre et al., 2017, McIntyre et al., 2013).
Another methodological limitation is the considerable level of heterogeneity exhibited in both meta-analyses, which may be attributable to variation in methods of data collection and sample characteristics amongst component studies. For example, the various subjective and objective assessment tools utilized differential scales (e.g., dichotomous Yes/No vs. Likert), providing varying levels of granularity. Stratifying studies by design, mode of ascertainment, and quality did not markedly reduce heterogeneity (Table 3). We also included preprints (Table 2), which have not undergone peer review.
6. Conclusion
In this systematic review and meta-analysis of 81 studies, we established that approximatelt a third of the included individuals experienced persistent fatigue and over a fifth of individuals exhibited cognitive impairment 12 or more weeks following COVID-19 diagnosis. Furthermore, a subset of individuals exhibited markers of systemic inflammation, and PCS was associated with marked levels of functional impairment. Limited evidence suggested a possible association between elevated pro-inflammatory markers and fatigue or cognitive impairment in PCS. Future research should endeavour to identify the underlying mechanisms, develop standardized diagnostic criteria, and establish therapies to prevent and treat fatigue and cognitive impairment in PCS patients.
Author contributions
RSM and FC conceptualized and designed study. FC and SL conducted literature search, study selection, and data extraction. FC and LMWL conducted quality and bias assessment of component studies. FC and YL conducted statistical analyses. FC interpreted statistical results, conducted qualitative analysis, and wrote the first draft of the manuscript with input from RSM, KWM, and MV. All authors provided critical review for important intellectual content, and approved the final version of the manuscript.
Funding
This research did not receive any grants or funding.
Financial disclosures
RSM has received research grant support from CIHR/GACD/Chinese National Natural Research Foundation; speaker/consultation fees from Lundbeck, Janssen, Purdue, Pfizer, Otsuka, Takeda, Neurocrine, Sunovion, Bausch Health, Novo Nordisk, Kris, Sanofi, Eisai, Intra-Cellular, NewBridge Pharmaceuticals, Abbvie. RSM is a CEO of Braxia Scientific Corp. JDR is the medical director of Braxia Health (formally known as the Canadian Rapid Treatment Center of Excellence and is a fully owned subsidiary of Braxia Scientific Corp) which provides ketamine and esketamine treatment for depression; he has received research grant support from the American Psychiatric Association, the American Society of Psychopharmacology, the Canadian Cancer Society, the Canadian Psychiatric Association, the Joseph M. West Family Memorial Fund, the Timeposters Fellowship, the University Health Network Centre for Mental Health, and the University of Toronto and speaking, consultation, or research fees from Allergan, COMPASS, Janssen, Lundbeck, and Sunovion. YL has received personal fees from Braxia Scientific Corp. LMWL has received personal fees from Braxia Scientific Corp and honoraria Medscape. KWM has received personal fees from Lundbeck and Janssen Cilag in the past three years and is supported by a five-year Lundbeck Foundation Fellowship (grant no. R215-2015-4121). MV has received a consultancy fee from Lundbeck, Janssen-Cilag and Sunovion. VM has received speaking/consulting and fees from: AbbVie/Allergan, Acadia Pharmaceuticals, Inc., Alfasigma, USA, Inc., Alkermes, Inc., Eisai-Purdue, Intra-Cellular Therapies, Ironshore. Janssen, Lundbeck A/S, Jazz Pharmaceuticals, Noven Pharmaceuticals Inc, Otsuka America Pharmaceutical, Inc., Sage Pharmaceuticals, Sunovion Pharmaceuticals Inc., Supernus Pharmaceuticals, Inc., Takeda Pharmaceutical Company Limited. KMT has received personal fees from Braxia Scientific Corp. All other authors declare no conflicts of interest or financial disclosures.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.bbi.2021.12.020.
Appendix A. Supplementary data
The following are the Supplementary data to this article:
References
- 1. Coronavirus disease (COVID-19) – World Health Organization. Accessed June 27, 2021. https://www.who.int/emergencies/diseases/novel-coronavirus-2019.
- Wu S.L., Mertens A.N., Crider Y.S., Nguyen A., Pokpongkiat N.N., Djajadi S., Seth A., Hsiang M.S., Colford J.M., Reingold A., Arnold B.F., Hubbard A., Benjamin-Chung J. Substantial underestimation of SARS-CoV-2 infection in the United States. Nat. Commun. 2020;11(1) doi: 10.1038/s41467-020-18272-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Havers F.P., Reed C., Lim T., Montgomery J.M., Klena J.D., Hall A.J., Fry A.M., Cannon D.L., Chiang C.-F., Gibbons A., Krapiunaya I., Morales-Betoulle M., Roguski K., Rasheed M.A.U., Freeman B., Lester S., Mills L., Carroll D.S., Owen S.M., Johnson J.A., Semenova V., Blackmore C., Blog D., Chai S.J., Dunn A., Hand J., Jain S., Lindquist S., Lynfield R., Pritchard S., Sokol T., Sosa L., Turabelidze G., Watkins S.M., Wiesman J., Williams R.W., Yendell S., Schiffer J., Thornburg N.J. Seroprevalence of antibodies to SARS-CoV-2 in 10 sites in the United States, March 23-may 12, 2020. JAMA Intern Med. 2020;180(12):1576. doi: 10.1001/jamainternmed.2020.4130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aizenman N. Why the pandemic is 10 times worse than you think. NPR. https://www.npr.org/sections/health-shots/2021/02/06/964527835/why-the-pandemic-is-10-times-worse-than-you-think. Published February 6, 2021. Accessed May 19, 2021.
- Tenforde M.W., Kim S.S., Lindsell C.J., Billig Rose E., Shapiro N.I., Files D.C., Gibbs K.W., Erickson H.L., Steingrub J.S., Smithline H.A., Gong M.N., Aboodi M.S., Exline M.C., Henning D.J., Wilson J.G., Khan A., Qadir N., Brown S.M., Peltan I.D., Rice T.W., Hager D.N., Ginde A.A., Stubblefield W.B., Patel M.M., Self W.H., Feldstein L.R., Hart K.W., McClellan R., Dorough L., Dzuris N., Griggs E.P., Kassem A.M., Marcet P.L., Ogokeh C.E., Sciarratta C.N., Siddula A., Smith E.R., Wu M.J. Symptom duration and risk factors for delayed return to usual health among outpatients with COVID-19 in a multistate health care systems network - United States, march-June 2020. MMWR Morb. Mortal. Wkly Rep. 2020;69(30):993–998. doi: 10.15585/mmwr.mm6930e1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang, Y., Pinto, M.D., Borelli, J.L., et al. COVID symptoms, symptom clusters, and predictors for becoming a long-hauler: Looking for clarity in the haze of the pandemic. medRxiv. Published online March 5, 2021:2021.03.03.2125208doi:10.1101/2021.03.03.21252086. [DOI] [PMC free article] [PubMed]
- Huang C., Huang L., Wang Y., Li X., Ren L., Gu X., Kang L., Guo L.i., Liu M., Zhou X., Luo J., Huang Z., Tu S., Zhao Y., Chen L.i., Xu D., Li Y., Li C., Peng L.u., Li Y., Xie W., Cui D., Shang L., Fan G., Xu J., Wang G., Wang Y., Zhong J., Wang C., Wang J., Zhang D., Cao B. 6-month consequences of COVID-19 in patients discharged from hospital: a cohort study. Lancet. 2021;397(10270):220–232. doi: 10.1016/S0140-6736(20)32656-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Renaud-Charest O., Lui L.M.W., Eskander S., Ceban F., Ho R., Di Vincenzo J.D., Rosenblat J.D., Lee Y., Subramaniapillai M., McIntyre R.S. Onset and frequency of depression in post-COVID-19 syndrome: A systematic review. J. Psychiatr. Res. 2021;144:129–137. doi: 10.1016/j.jpsychires.2021.09.054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nalbandian A., Sehgal K., Gupta A., Madhavan M.V., McGroder C., Stevens J.S., Cook J.R., Nordvig A.S., Shalev D., Sehrawat T.S., Ahluwalia N., Bikdeli B., Dietz D., Der-Nigoghossian C., Liyanage-Don N., Rosner G.F., Bernstein E.J., Mohan S., Beckley A.A., Seres D.S., Choueiri T.K., Uriel N., Ausiello J.C., Accili D., Freedberg D.E., Baldwin M., Schwartz A., Brodie D., Garcia C.K., Elkind M.S.V., Connors J.M., Bilezikian J.P., Landry D.W., Wan E.Y. Post-acute COVID-19 syndrome. Nat. Med. 2021;27(4):601–615. doi: 10.1038/s41591-021-01283-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Accessed December 9, 2021. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC7992371/.
- Overview | COVID-19 rapid guideline: managing the long-term effects of COVID-19 | Guidance | NICE. Accessed June 29, 2021. https://www.nice.org.uk/guidance/ng188.
- (hq) WH. A clinical case definition of post COVID-19 condition by a Delphi consensus, 6 October 2021. Published October 6, 2021. Accessed October 15, 2021. https://www.who.int/publications/i/item/WHO-2019-nCoV-Post_COVID-19_condition-Clinical_case_definition-2021.1.
- Siegelman J.N. Reflections of a COVID-19 Long Hauler. JAMA. 2020;324(20):2031–2032. doi: 10.1001/jama.2020.22130. [DOI] [PubMed] [Google Scholar]
- Rubin R. As Their Numbers Grow, COVID-19 “Long Haulers” Stump Experts. JAMA. 2020;324(14):1381–1383. doi: 10.1001/jama.2020.17709. [DOI] [PubMed] [Google Scholar]
- Davis, H.E., Assaf, G.S., McCorkell, L., et al. Characterizing Long COVID in an international cohort: 7 months of symptoms and their impact. bioRxiv. Published online December 26, 2020:2020.12.24.20248802. doi:10.1101/2020.12.24.20248802. [DOI] [PMC free article] [PubMed]
- Marshall M. The lasting misery of coronavirus long-haulers. Nature. 2020;585(7825):339–341. doi: 10.1038/d41586-020-02598-6. [DOI] [PubMed] [Google Scholar]
- Report: What does COVID-19 recovery actually look like? - patient-led research collaborative. Published June 10, 2020. Accessed July 7, 2021. https://patientresearchcovid19.com/research/report-1/.
- Sabes-Figuera R., McCrone P., Hurley M., King M., Donaldson A.N., Ridsdale L. The hidden cost of chronic fatigue to patients and their families. BMC Health Serv. Res. 2010;10(1) doi: 10.1186/1472-6963-10-56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winston Wong P. Economic burden of Alzheimer disease and managed care considerations. AJMC. Published August 17, 2020. Accessed December 7, 2021. https://www.ajmc.com/view/economic-burden-of-alzheimer-disease-and-managed-care-considerations. [DOI] [PubMed]
- Xu J., Zhang Y., Qiu C., Cheng F. Global and regional economic costs of dementia: a systematic review. Lancet. 2017;390:S47. doi: 10.1016/s0140-6736(17)33185-9. [DOI] [Google Scholar]
- Stroup D.F., Berlin J.A., Morton S.C., et al. Meta-analysis of observational studies in epidemiology: a proposal for reporting. Meta-analysis Of Observational Studies in Epidemiology (MOOSE) group. JAMA. 2000;283(15):2008–2012. doi: 10.1001/jama.283.15.2008. [DOI] [PubMed] [Google Scholar]
- Page, M.J., McKenzie, J.E., Bossuyt, P.M., et al. The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. BMJ. 2021;372:n71. doi:10.1136/bmj.n71. [DOI] [PMC free article] [PubMed]
- Better systematic review management. Published June 11, 2020. Accessed July 8, 2021. https://www.covidence.org.
- Stang A. Critical evaluation of the Newcastle-Ottawa scale for the assessment of the quality of nonrandomized studies in meta-analyses. Eur. J. Epidemiol. 2010;25(9):603–605. doi: 10.1007/s10654-010-9491-z. [DOI] [PubMed] [Google Scholar]
- Balduzzi S., Rücker G., Schwarzer G. How to perform a meta-analysis with R: a practical tutorial. Evid. Based. Ment Health. 2019;22(4):153–160. doi: 10.1136/ebmental-2019-300117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kenward M.G., Roger J.H. Small sample inference for fixed effects from restricted maximum likelihood. Biometrics. 1997;53(3):983–997. doi: 10.2307/2533558. [DOI] [PubMed] [Google Scholar]
- Miller J.J. The Inverse of the Freeman – Tukey Double Arcsine Transformation. Am. Stat. 1978;32(4):138. doi: 10.1080/00031305.1978.10479283. 138. [DOI] [Google Scholar]
- Deeks J.J., Higgins J.PT., Altman D.G. Cochrane Handbook for Systematic Reviews of Interventions. John Wiley & Sons, Ltd; Chichester, UK: 2008. pp. 243–296. [DOI] [Google Scholar]
- Schünemann H.J., Higgins J.PT., Vist G.E., Glasziou P., Akl E.A., Skoetz N., Guyatt G.H. Cochrane Handbook for Systematic Reviews of Interventions. Wiley; 2019. pp. 375–402. [DOI] [Google Scholar]
- Breton G., Mendoza P., Hägglöf T., Oliveira T.Y., Schaefer-Babajew D., Gaebler C., Turroja M., Hurley A., Caskey M., Nussenzweig M.C. Persistent cellular immunity to SARS-CoV-2 infection. J. Exp. Med. 2021;218(4) doi: 10.1084/jem.20202515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shuwa H.A., Shaw T.N., Knight S.B., Wemyss K., McClure F.A., Pearmain L., Prise I., Jagger C., Morgan D.J., Khan S., Brand O., Mann E.R., Ustianowski A., Bakerly N.D., Dark P., Brightling C.E., Brij S., Felton T., Simpson A., Grainger J.R., Hussell T., Konkel J.E., Menon M., Ahmed R., Avery M., Birchall K., Charsley E., Chenery A., Chew C., Clark R., Connolly E., Connolly K., Dawson S., Durrans L., Durrington H., Egan J., Filbey K., Fox C., Francis H., Franklin M., Glasgow S., Godfrey N., Gray K.J., Grundy S., Guerin J., Hackney P., Hayes C., Hardy E., Harris J., John A., Jolly B., Kästele V., Kerry G., Lui S., Lin L., Mathioudakis A.G., Mitchell J., Moizer C., Moore K., Moss S., Baker S.M., Oliver R., Padden G., Parkinson C., Phuycharoen M., Saha A., Salcman B., Scott N.A., Sharma S., Shaw J., Shaw J., Shepley E., Smith L., Stephan S., Stephens R., Tavernier G., Tudge R., Wareing L., Warren R., Williams T., Willmore L., Younas M. Alterations in T and B cell function persist in convalescent COVID-19 patients. Med (N Y). 2021;2(6):720–735.e4. doi: 10.1016/j.medj.2021.03.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sonnweber T., Sahanic S., Pizzini A., Luger A., Schwabl C., Sonnweber B., Kurz K., Koppelstätter S., Haschka D., Petzer V., Boehm A., Aichner M., Tymoszuk P., Lener D., Theurl M., Lorsbach-Köhler A., Tancevski A., Schapfl A., Schaber M., Hilbe R., Nairz M., Puchner B., Hüttenberger D., Tschurtschenthaler C., Aßhoff M., Peer A., Hartig F., Bellmann R., Joannidis M., Gollmann-Tepeköylü C., Holfeld J., Feuchtner G., Egger A., Hoermann G., Schroll A., Fritsche G., Wildner S., Bellmann-Weiler R., Kirchmair R., Helbok R., Prosch H., Rieder D., Trajanoski Z., Kronenberg F., Wöll E., Weiss G., Widmann G., Löffler-Ragg J., Tancevski I. Cardiopulmonary recovery after COVID-19: an observational prospective multicentre trial. Eur. Respir. J. 2021;57(4):2003481. doi: 10.1183/13993003.03481-2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taboada M., Cariñena A., Moreno E., Rodríguez N., Domínguez M.J., Casal A., Riveiro V., Diaz-Vieito M., Valdés L., Álvarez J., Seoane-Pillado T. Post-COVID-19 functional status six-months after hospitalization. J. Infect. 2021;82(4):e31–e33. doi: 10.1016/j.jinf.2020.12.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Todt B.C., Szlejf C., Duim E., Linhares A.O.M., Kogiso D., Varela G., Campos B.A., Baghelli Fonseca C.M., Polesso L.E., Bordon I.N.S., Cabral B.T., Amorim V.L.P., Piza F.M.T., Degani-Costa L.H. Clinical outcomes and quality of life of COVID-19 survivors: A follow-up of 3 months post hospital discharge. Respir. Med. 2021;184:106453. doi: 10.1016/j.rmed.2021.106453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wong A.W., Shah A.S., Johnston J.C., Carlsten C., Ryerson C.J. Patient-reported outcome measures after COVID-19: a prospective cohort study. Eur. Respir. J. 2020;56(5):2003276. doi: 10.1183/13993003.03276-2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou, M., Yin, Z., Xu, J., et al. Inflammatory profiles and clinical features of COVID-19 survivors three months after discharge in Wuhan, China. J Infect Dis. Published online April 4, 2021. doi:10.1093/infdis/jiab181. [DOI] [PMC free article] [PubMed]
- Ghosn, J., Piroth, L., Epaulard, O., et al. Persistent COVID-19 symptoms are highly prevalent 6 months after hospitalization: results from a large prospective cohort. Clin Microbiol Infect. Published online April 30, 2021. doi:10.1016/j.cmi.2021.03.012. [DOI] [PMC free article] [PubMed]
- Say D., Crawford N., McNab S., Wurzel D., Steer A., Tosif S. Post-acute COVID-19 outcomes in children with mild and asymptomatic disease. The Lancet Child & Adolescent Health. 2021;5(6):e22–e23. doi: 10.1016/S2352-4642(21)00124-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pilotto, A., Cristillo, V., Piccinelli, S.C., et al. Long-term neurological manifestations of COVID-19: prevalence and predictive factors. bioRxiv. Published online January 2, 2021. doi:10.1101/2020.12.27.20248903. [DOI] [PMC free article] [PubMed]
- Liyanage-Don N.A., Cornelius T., Sanchez J.E., Trainor A., Moise N., Wainberg M., Kronish I.M. Psychological Distress, Persistent Physical Symptoms, and Perceived Recovery After COVID-19 Illness. J. Gen. Intern. Med. 2021;36(8):2525–2527. doi: 10.1007/s11606-021-06855-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miyazato Y., Morioka S., Tsuzuki S., Akashi M., Osanai Y., Tanaka K., Terada M., Suzuki M., Kutsuna S., Saito S., Hayakawa K., Ohmagari N. Prolonged and Late-Onset Symptoms of Coronavirus Disease 2019. Open Forum Infect Dis. 2020;7(11) doi: 10.1093/ofid/ofaa507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- González J., Benítez I.D., Carmona P., Santisteve S., Monge A., Moncusí-Moix A., Gort-Paniello C., Pinilla L., Carratalá A., Zuil M., Ferrer R., Ceccato A., Fernández L., Motos A., Riera J., Menéndez R., Garcia-Gasulla D., Peñuelas O., Bermejo-Martin J.F., Labarca G., Caballero J., Torres G., de Gonzalo-Calvo D., Torres A., Barbé F., Villar R.A., Añón J.M., Barberà C., Barberán J., Ortiz A.B., Bustamante-Munguira E., Caballero J., Carbajales C., Carbonell N., Catalán-González M., Galbán C., Gumucio V.D., de la Torre M.D.C., Díaz E., Estella Á., Gallego E., García Garmendia J.L., Garnacho-Montero J., Gómez J.M., Huerta A., Jorge García R.N., Loza-Vázquez A., Marin-Corral J., de la Gándara A.M., Varela I.M., Messa J.L., Albaiceta G.M., Novo M.A., Peñasco Y., Pozo-Laderas J.C., Martí P.R., Roche-Campo F., Sánchez-Miralles A., Chinesta S.S., Socias L., Solé-Violan J., Sipmann F.S., Lomas L.T., Trenado J. Pulmonary function and radiologic features in survivors of critical COVID-19: A 3-month prospective cohort. Chest. 2021;160(1):187–198. doi: 10.1016/j.chest.2021.02.062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fernández-de-las-Peñas C., Palacios-Ceña D., Gómez-Mayordomo V., Rodríuez-Jiménez J., Palacios-Ceña M., Velasco-Arribas M., Guijarro C., de-la-Llave-Rincón A.I., Fuensalida-Novo S., Elvira-Martínez C.M., Cuadrado M.L., Arias-Navalón J.A., Florencio L.L., Ortega-Santiago R., Molina-Trigueros L.J., Sebastián-Viana T., Torres-Macho J., Canto-Diez G., Plaza-Canteli S., Cigarán-Méndez M., Ambite-Quesada S., Hernández-Barrera V., Arias-Buría J.L., Arendt-Nielsen L. Long-term post-COVID symptoms and associated risk factors in previously hospitalized patients: A multicenter study. J. Infect. 2021;83(2):237–279. doi: 10.1016/j.jinf.2021.04.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fernández-de-Las-Peñas C., Guijarro C., Plaza-Canteli S., Hernández-Barrera V., Torres-Macho J. Prevalence of post-COVID-19 cough one year after SARS-CoV-2 infection: A multicenter study. Lung. 2021;199(3):249–253. doi: 10.1007/s00408-021-00450-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arnold D.T., Hamilton F.W., Milne A., Morley A.J., Viner J., Attwood M., Noel A., Gunning S., Hatrick J., Hamilton S., Elvers K.T., Hyams C., Bibby A., Moran E.d., Adamali H.I., Dodd J.W., Maskell N.A., Barratt S.L. Patient outcomes after hospitalisation with COVID-19 and implications for follow-up: results from a prospective UK cohort. Thorax. 2021;76(4):399–401. doi: 10.1136/thoraxjnl-2020-216086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fortini, A., Torrigiani, A., Sbaragli, S., et al. COVID-19: persistence of symptoms and lung alterations after 3-6 months from hospital discharge. Infection. Published online June 6, 2021. doi:10.1007/s15010-021-01638-1. [DOI] [PMC free article] [PubMed]
- García-Abellán J, Padilla S, Fernández-González M, et al. Long-term clinical, virological and immunological outcomes in patients hospitalized for COVID-19: antibody response predicts long COVID. bioRxiv. Published online March 8, 2021:2021.03.08.21253124. doi:10.1101/2021.03.08.21253124.
- Ong S.W.X., Fong S.-W., Young B.E., Chan Y.-H., Lee B., Amrun S.N., Chee R.-L., Yeo N.-W., Tambyah P., Pada S., Tan S.Y., Ding Y., Renia L., Leo Y.-S., Ng L.F.P., Lye D.C. Persistent symptoms and association with inflammatory cytokine signatures in recovered Coronavirus disease 2019 patients. Open Forum Infect Dis. 2021;8(6) doi: 10.1093/ofid/ofab156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- PHOSP-COVID Collaborative Group, Evans RA, McAuley H, et al. Physical, cognitive and mental health impacts of COVID-19 following hospitalisation – a multi-centre prospective cohort study. bioRxiv. Published online March 24, 2021:2021.03.22.21254057. doi:10.1101/2021.03.22.21254057.
- Skala M., Svoboda M., Kopecky M., Kocova E., Hyrsl M., Homolac M., Chrobok V., Bostik P., Fajfr M., Prasil P., Plisek S., Sleha R., Koblizek V. Heterogeneity of post-COVID impairment: interim analysis of a prospective study from Czechia. Virol J. 2021;18(1) doi: 10.1186/s12985-021-01546-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sykes D.L., Holdsworth L., Jawad N., Gunasekera P., Morice A.H., Crooks M.G. Post-COVID-19 symptom burden: What is long-COVID and how should we manage it? Lung. 2021;199(2):113–119. doi: 10.1007/s00408-021-00423-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Santis, L.V.-D., Pérez-Camacho, I., Sobrino, B., et al. Clinical and immunoserological status 12 weeks after infection with COVID-19: prospective observational study. bioRxiv. Published online October 8, 2020. doi:10.1101/2020.10.06.20206060.
- van den Borst, B., Peters, J.B., Brink, M., et al. Comprehensive health assessment three months after recovery from acute COVID-19. Clin Infect Dis. Published online November 21, 2020. doi:10.1093/cid/ciaa1750. [DOI] [PMC free article] [PubMed]
- Venturelli S., Benatti S.V., Casati M., Binda F., Zuglian G., Imeri G., Conti C., Biffi A.M., Spada M.S., Bondi E., Camera G., Severgnini R., Giammarresi A., Marinaro C., Rossini A., Bonaffini P.A., Guerra G., Bellasi A., Cesa S., Rizzi M. Surviving COVID-19 in Bergamo province: a post-acute outpatient re-evaluation. Epidemiol. Infect. 2021;149 doi: 10.1017/S0950268821000145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- IL6 - Clinical: Interleukin 6, Plasma. Accessed June 26, 2021. https://www.mayocliniclabs.com/test-catalog/Clinical+and+Interpretive/63020.
- Elkan, M., Dvir, A., Zaidenstein, R., et al. Patient-reported outcome measures after hospitalization during the COVID-19 pandemic, a survey among COVID-19 and non-COVID-19 patients. Research Square. Published online June 7, 2021. doi:10.21203/rs.3.rs-559099/v1. [DOI] [PMC free article] [PubMed]
- Frontera J.A., Yang D., Lewis A., Patel P., Medicherla C., Arena V., Fang T., Andino A., Snyder T., Madhavan M., Gratch D., Fuchs B., Dessy A., Canizares M., Jauregui R., Thomas B., Bauman K., Olivera A., Bhagat D., Sonson M., Park G., Stainman R., Sunwoo B., Talmasov D., Tamimi M., Zhu Y., Rosenthal J., Dygert L., Ristic M., Ishii H., Valdes E., Omari M., Gurin L., Huang J., Czeisler B.M., Kahn D.E., Zhou T., Lin J., Lord A.S., Melmed K., Meropol S., Troxel A.B., Petkova E., Wisniewski T., Balcer L., Morrison C., Yaghi S., Galetta S. A prospective study of long-term outcomes among hospitalized COVID-19 patients with and without neurological complications. J. Neurol. Sci. 2021;426:117486. doi: 10.1016/j.jns.2021.117486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garrigues E., Janvier P., Kherabi Y., Le Bot A., Hamon A., Gouze H., Doucet L., Berkani S., Oliosi E., Mallart E., Corre F., Zarrouk V., Moyer J.-D., Galy A., Honsel V., Fantin B., Nguyen Y. Post-discharge persistent symptoms and health-related quality of life after hospitalization for COVID-19. J. Infect. 2020;81(6):e4–e6. doi: 10.1016/j.jinf.2020.08.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Havervall S., Rosell A., Phillipson M., Mangsbo S.M., Nilsson P., Hober S., Thålin C. Symptoms and functional impairment assessed 8 months after mild COVID-19 among health care workers. JAMA. 2021;325(19):2015. doi: 10.1001/jama.2021.5612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacobson, K.B., Rao, M., Bonilla, H., et al. Patients with uncomplicated COVID-19 have long-term persistent symptoms and functional impairment similar to patients with severe COVID-19: a cautionary tale during a global pandemic. Clin Infect Dis. Published online February 7, 2021. doi:10.1093/cid/ciab103. [DOI] [PMC free article] [PubMed]
- Johnsen, S., Sattler, S.M., Miskowiak, K.W., et al. Descriptive analysis of long COVID sequela identified in a multidisciplinary clinic serving hospitalised and non-hospitalised patients. ERJ Open Res. Published online April 29, 2021:00205-02021. doi:10.1183/23120541.00205-2021. [DOI] [PMC free article] [PubMed]
- Latronico, N., Peli, E., Rodella F, et al. Six-month outcome in survivors of COVID-19 associated acute respiratory distress syndrome. SSRN Electron J. Published online December 29, 2020. doi:10.2139/ssrn.3756865.
- Logue J.K., Franko N.M., McCulloch D.J., McDonald D., Magedson A., Wolf C.R., Chu H.Y. Sequelae in adults at 6 months after COVID-19 infection. JAMA Netw Open. 2021;4(2):e210830. doi: 10.1001/jamanetworkopen.2021.0830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Menges D, Ballouz T, Anagnostopoulos A, et al. Burden of post-COVID-19 syndrome and implications for healthcare service planning: A population-based cohort study. bioRxiv. Published online March 1, 2021. doi:10.1101/2021.02.27.21252572. [DOI] [PMC free article] [PubMed]
- Miskowiak K.W., Johnsen S., Sattler S.M., Nielsen S., Kunalan K., Rungby J., Lapperre T., Porsberg C.M. Cognitive impairments four months after COVID-19 hospital discharge: Pattern, severity and association with illness variables. Eur. Neuropsychopharmacol. 2021;46:39–48. doi: 10.1016/j.euroneuro.2021.03.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Munblit D, Bobkova P, Spiridonova E, et al. Risk factors for long-term consequences of COVID-19 in hospitalised adults in Moscow using the ISARIC Global follow-up protocol: StopCOVID cohort study. bioRxiv. Published online February 19, 2021:2021.02.17.21251895. doi:10.1101/2021.02.17.21251895.
- Orrù G., Bertelloni D., Diolaiuti F., Mucci F., Di Giuseppe M., Biella M., Gemignani A., Ciacchini R., Conversano C. Long-COVID syndrome? A study on the persistence of neurological, psychological and physiological symptoms. Healthcare (Basel). 2021;9(5):575. doi: 10.3390/healthcare9050575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Osmanov IM, Spiridonova E, Bobkova P, et al. Risk factors for long covid in previously hospitalised children using the ISARIC Global follow-up protocol: A prospective cohort study. medRxiv. Published online April 26, 2021:2021.04.26.21256110. doi:10.1101/2021.04.26.21256110.
- Pereira C., Harris B.H.L., Di Giovannantonio M., Rosadas C., Short C.-E., Quinlan R., Sureda-Vives M., Fernandez N., Day-Weber I., Khan M., Marchesin F., Katsanovskaja K., Parker E., Taylor G.P., Tedder R.S., McClure M.O., Dani M., Fertleman M. The association between antibody response to severe acute respiratory syndrome Coronavirus 2 infection and post-COVID-19 syndrome in healthcare workers. J. Infect. Dis. 2021;223(10):1671–1676. doi: 10.1093/infdis/jiab120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qu G., Zhen Q.i., Wang W., Fan S., Wu Q., Zhang C., Li B., Liu G., Yu Y., Li Y., Yong L., Lu B., Ding Z., Ge H., Mao Y., Chen W., Xu Q., Zhang R., Cao L.u., Chen S., Li H., Zhang H., Hu X., Zhang J., Wang Y., Zhang H., Liang C., Sun L., Sun Y. Health-related quality of life of COVID-19 patients after discharge: A multicenter follow-up study. J. Clin. Nurs. 2021;30(11-12):1742–1750. doi: 10.1111/jocn.15733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rass V., Beer R., Schiefecker A.J., et al. Neurological outcome and quality of life 3 months after COVID-19: A prospective observational cohort study. Eur. J. Neurol. 2021:14803. doi: 10.1111/ene.14803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Savarraj JPJ, Burkett AB, Hinds SN, et al. Three-month outcomes in hospitalized COVID-19 patients. bioRxiv. Published online October 18, 2020. doi:10.1101/2020.10.16.20211029.
- Soldati A.B., Almeida C., Lima M., Araujo A., Araujo-Leite M.A., Silva M.T.T. Telephone Screening of Cognitive Status (TICS) in severe COVID-19 patients: Utility in the era of social isolation. eNeurologicalSci. 2021;22:100322. doi: 10.1016/j.ensci.2021.100322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soraas A, Ro R, Kalleberg KT, Ellingjord-Dale M, Landro NI. Self-reported memory problems eight months after non-hospitalized COVID-19 in a large cohort. bioRxiv. Published online February 26, 2021. doi:10.1101/2021.02.25.21252151.
- Walle-Hansen M.M., Ranhoff A.H., Mellingsæter M., Wang-Hansen M.S., Myrstad M. Health-related quality of life, functional decline, and long-term mortality in older patients following hospitalisation due to COVID-19. BMC Geriatr. 2021;21(1):199. doi: 10.1186/s12877-021-02140-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taboada M., Moreno E., Cariñena A., Rey T., Pita-Romero R., Leal S., Sanduende Y., Rodríguez A., Nieto C., Vilas E., Ochoa M., Cid M., Seoane-Pillado T. Quality of life, functional status, and persistent symptoms after intensive care of COVID-19 patients. Br. J. Anaesth. 2021;126(3):e110–e113. doi: 10.1016/j.bja.2020.12.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Veenendaal N, Meulen IV der, Onrust M, Paans W, Dieperink W, Voort PV der. Long-term outcomes in COVID-19 ICU patients: A prospective cohort study. Research Square. Published online April 7, 2021. doi:10.21203/rs.3.rs-395294/v1. [DOI] [PMC free article] [PubMed]
- Szende A, Janssen B, Cabases J, eds. Self-Reported Population Health: An International Perspective Based on EQ-5D. 2014th ed. Springer; 2013. [PubMed]
- Amin-Chowdhury Z, Harris RJ, Aiano F, et al. Characterising post-COVID syndrome more than 6 months after acute infection in adults; prospective longitudinal cohort study, England. bioRxiv. Published online March 24, 2021. doi:10.1101/2021.03.18.21253633.
- Augustin M., Schommers P., Stecher M., Dewald F., Gieselmann L., Gruell H., Horn C., Vanshylla K., Cristanziano V.D., Osebold L., Roventa M., Riaz T., Tschernoster N., Altmueller J., Rose L., Salomon S., Priesner V., Luers J.C., Albus C., Rosenkranz S., Gathof B., Fätkenheuer G., Hallek M., Klein F., Suárez I., Lehmann C. Post-COVID syndrome in non-hospitalised patients with COVID-19: a longitudinal prospective cohort study. Lancet Reg Health Eur. 2021;6:100122. doi: 10.1016/j.lanepe.2021.100122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cirulli ET, Schiabor Barrett KM, Riffle S, et al. Long-term COVID-19 symptoms in a large unselected population. bioRxiv. Published online October 11, 2020. doi:10.1101/2020.10.07.20208702.
- Darley DR, Dore GJ, Byrne A, et al. Limited recovery from post-acute sequelae of SARS-CoV-2 (PASC) at eight months in a prospective cohort. bioRxiv. Published online March 31, 2021. doi:10.1101/2021.03.29.21254211. [DOI] [PMC free article] [PubMed]
- Ferrucci R., Dini M., Groppo E., Rosci C., Reitano M.R., Bai F., Poletti B., Brugnera A., Silani V., D’Arminio Monforte A., Priori A. Long-lasting cognitive abnormalities after COVID-19. Brain Sci. 2021;11(2):235. doi: 10.3390/brainsci11020235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kashif A, Chaudhry M, Fayyaz T, et al. Follow-up of COVID-19 recovered patients with mild disease. Research Square. Published online February 17, 2021. doi:10.21203/rs.3.rs-120819/v1. [DOI] [PMC free article] [PubMed]
- O’Keefe JB, Minton HC, Johnson C, et al. High proportion of post-acute sequelae of SARS-CoV-2 infection in individuals 1-6 months after illness and association with disease severity in an outpatient telemedicine population. Published online April 27, 2021. doi:10.1101/2021.04.24.21256054.
- Peghin M, Palese A, Venturini M, et al. Post-COVID-19 symptoms 6 months after acute infection among hospitalized and non-hospitalized patients. Clin Microbiol Infect. Published online June 7, 2021. doi:10.1016/j.cmi.2021.05.033. [DOI] [PMC free article] [PubMed]
- Shang Y.F., Liu T., Yu J.N., Xu X.R., Zahid K.R., Wei Y.C., Wang X.H., Zhou F.L. Half-year follow-up of patients recovering from severe COVID-19: Analysis of symptoms and their risk factors. J. Intern. Med. 2021;290(2):444–450. doi: 10.1111/joim.13284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simani L., Ramezani M., Darazam I.A., Sagharichi M., Aalipour M.A., Ghorbani F., Pakdaman H. Prevalence and correlates of chronic fatigue syndrome and post-traumatic stress disorder after the outbreak of the COVID-19. J Neurovirol. 2021;27(1):154–159. doi: 10.1007/s13365-021-00949-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stavem K., Ghanima W., Olsen M.K., Gilboe H.M., Einvik G. Prevalence and determinants of fatigue after COVID-19 in non-hospitalized subjects: A population-based study. Int. J. Environ. Res. Public Health. 2021;18(4):2030. doi: 10.3390/ijerph18042030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xiong Q., Xu M., Li J., Liu Y., Zhang J., Xu Y.u., Dong W. Clinical sequelae of COVID-19 survivors in Wuhan, China: a single-centre longitudinal study. Clin. Microbiol. Infect. 2021;27(1):89–95. doi: 10.1016/j.cmi.2020.09.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- González-Hermosillo J.A., Martínez-López J.P., Carrillo-Lampón S.A., Ruiz-Ojeda D., Herrera-Ramírez S., Amezcua-Guerra L.M., Martínez-Alvarado M.D.R. Post-acute COVID-19 symptoms, a potential link with Myalgic Encephalomyelitis/Chronic Fatigue Syndrome: A 6-month survey in a Mexican cohort. Brain Sci. 2021;11(6):760. doi: 10.3390/brainsci11060760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petersen M.S., Kristiansen M.F., Hanusson K.D., Danielsen M.E., á Steig B., Gaini S., Strøm M., Weihe P. Long COVID in the Faroe Islands: A Longitudinal Study Among Nonhospitalized Patients. Clin. Infect. Dis. 2021;73(11):e4058–e4063. doi: 10.1093/cid/ciaa1792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tawfik H.M., Shaaban H.M., Tawfik A.M. Post-COVID-19 syndrome in Egyptian healthcare staff: Highlighting the carers sufferings. Electron J Gen Med. 2021;18(3):em291.;18(3):em291. doi: 10.29333/ejgm/10838. [DOI] [Google Scholar]
- Mei Q.i., Wang F., Yang Y., Hu G., Guo S., Zhang Q., Bryant A., Zhang L., Kurts C., Wei L.i., Yuan X., Li J. Health issues and immunological assessment related to wuhan’s COVID-19 survivors: A multicenter follow-up study. Front Med (Lausanne). 2021;8 doi: 10.3389/fmed.2021.617689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Evlice O., Kuş F., Bektas M. Persistent symptoms after discharge of COVID-19 patients. Infect Dis Clin Microbiol. 2021;3(1):22–29. doi: 10.36519/idcm.2021.40. [DOI] [Google Scholar]
- Rauch B, Kern-Matschilles S, Haschka SJ, et al. COVID-19-related symptoms 6 months after the infection - Update on a prospective cohort study in Germany. bioRxiv. Published online February 13, 2021. doi:10.1101/2021.02.12.21251619.
- Woo M.S., Malsy J., Pöttgen J., Seddiq Zai S., Ufer F., Hadjilaou A., Schmiedel S., Addo M.M., Gerloff C., Heesen C., Schulze Zur Wiesch J., Friese M.A. Frequent neurocognitive deficits after recovery from mild COVID-19. Brain Commun. 2020;2(2) doi: 10.1093/braincomms/fcaa205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hopkins C., Surda P., Whitehead E., Kumar B.N. Early recovery following new onset anosmia during the COVID-19 pandemic - an observational cohort study. J Otolaryngol Head Neck Surg. 2020;49(1):26. doi: 10.1186/s40463-020-00423-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jason L.A., Islam M.F., Conroy K., Cotler J., Torres C., Johnson M., Mabie B. COVID-19 symptoms over time: comparing long-haulers to ME/CFS. Fatigue: Biomedicine, Health & Behavior. 2021;9(2):59–68. doi: 10.1080/21641846.2021.1922140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lam M.-H.-B., Wing Y.-K., Yu M.-W.-M., et al. Mental morbidities and chronic fatigue in severe acute respiratory syndrome survivors: long-term follow-up. Arch. Intern. Med. 2009;169(22):2142–2147. doi: 10.1001/archinternmed.2009.384. [DOI] [PubMed] [Google Scholar]
- Rogers J.P., Chesney E., Oliver D., Pollak T.A., McGuire P., Fusar-Poli P., Zandi M.S., Lewis G., David A.S. Psychiatric and neuropsychiatric presentations associated with severe coronavirus infections: a systematic review and meta-analysis with comparison to the COVID-19 pandemic. Lancet Psychiatry. 2020;7(7):611–627. doi: 10.1016/S2215-0366(20)30203-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoffman L.A., Vilensky J.A. Encephalitis lethargica: 100 years after the epidemic. Brain. 2017;140(8):2246–2251. doi: 10.1093/brain/awx177. [DOI] [PubMed] [Google Scholar]
- Komaroff A.L., Lipkin W.I. Insights from myalgic encephalomyelitis/chronic fatigue syndrome may help unravel the pathogenesis of postacute COVID-19 syndrome. Trends Mol. Med. 2021;27(9):895–906. doi: 10.1016/j.molmed.2021.06.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Higgins V., Sohaei D., Diamandis E.P., Prassas I. COVID-19: from an acute to chronic disease? Potential long-term health consequences. Crit. Rev. Clin. Lab. Sci. 2021;58(5):297–310. doi: 10.1080/10408363.2020.1860895. [DOI] [PubMed] [Google Scholar]
- Douaud G, Lee S, Alfaro-Almagro F, et al. Brain imaging before and after COVID-19 in UK Biobank. bioRxiv. Published online June 15, 2021:2021.06.11.21258690. doi:10.1101/2021.06.11.21258690.
- Lee M.-H., Perl D.P., Nair G., Li W., Maric D., Murray H., Dodd S.J., Koretsky A.P., Watts J.A., Cheung V., Masliah E., Horkayne-Szakaly I., Jones R., Stram M.N., Moncur J., Hefti M., Folkerth R.D., Nath A. Microvascular injury in the brains of patients with covid-19. N. Engl. J. Med. 2021;384(5):481–483. doi: 10.1056/NEJMc2033369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guedj E., Campion J.Y., Dudouet P., Kaphan E., Bregeon F., Tissot-Dupont H., Guis S., Barthelemy F., Habert P., Ceccaldi M., Million M., Raoult D., Cammilleri S., Eldin C. 18F-FDG brain PET hypometabolism in patients with long COVID. Eur. J. Nucl. Med. Mol. Imaging. 2021;48(9):2823–2833. doi: 10.1007/s00259-021-05215-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Libby P., Lüscher T. COVID-19 is, in the end, an endothelial disease. Eur. Heart J. 2020;41(32):3038–3044. doi: 10.1093/eurheartj/ehaa623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oronsky B, Larson C, Hammond TC, et al. A review of persistent post-COVID syndrome (PPCS). Clin Rev Allergy Immunol. Published online February 20, 2021. doi:10.1007/s12016-021-08848-3. [DOI] [PMC free article] [PubMed]
- Yong S.J. Long COVID or post-COVID-19 syndrome: putative pathophysiology, risk factors, and treatments. Infectious Diseases. 2021;53(10):737–754. doi: 10.1080/23744235.2021.1924397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sartori A.C., Vance D.E., Slater L.Z., Crowe M. The impact of inflammation on cognitive function in older adults: implications for healthcare practice and research. J. Neurosci. Nurs. 2012;44(4):206–217. doi: 10.1097/JNN.0b013e3182527690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosenblat J.D., Cha D.S., Mansur R.B., McIntyre R.S. Inflamed moods: a review of the interactions between inflammation and mood disorders. Prog. Neuro-Psychopharmacol. Biol. Psychiatry. 2014;53:23–34. doi: 10.1016/j.pnpbp.2014.01.013. [DOI] [PubMed] [Google Scholar]
- Sonneville R., Klein I., de Broucker T., Wolff M. Post-infectious encephalitis in adults: diagnosis and management. J. Infect. 2009;58(5):321–328. doi: 10.1016/j.jinf.2009.02.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gałecki P., Mossakowska-Wójcik J., Talarowska M. The anti-inflammatory mechanism of antidepressants – SSRIs. SNRIs. Prog Neuropsychopharmacol Biol Psychiatry. 2018;80:291–294. doi: 10.1016/j.pnpbp.2017.03.016. [DOI] [PubMed] [Google Scholar]
- Heckenberg R.A., Eddy P., Kent S., Wright B.J. Do workplace-based mindfulness meditation programs improve physiological indices of stress? A systematic review and meta-analysis. J. Psychosom. Res. 2018;114:62–71. doi: 10.1016/j.jpsychores.2018.09.010. [DOI] [PubMed] [Google Scholar]
- Xiong J., Lipsitz O., Nasri F., Lui L.M.W., Gill H., Phan L., Chen-Li D., Iacobucci M., Ho R., Majeed A., McIntyre R.S. Impact of COVID-19 pandemic on mental health in the general population: A systematic review. J. Affect. Disord. 2020;277:55–64. doi: 10.1016/j.jad.2020.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taquet M., Geddes J.R., Husain M., Luciano S., Harrison P.J. 6-month neurological and psychiatric outcomes in 236 379 survivors of COVID-19: a retrospective cohort study using electronic health records. Lancet Psychiatry. 2021;8(5):416–427. doi: 10.1016/S2215-0366(21)00084-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McIntyre R.S., Anderson N., Baune B.T., Brietzke E., Burdick K., Fossati P., Gorwood P., Harmer C., Harrison J., Harvey P., Mansur R.B., Medalia A., Miskowiak K., Ramey T., Rong C., Rosenblat J.D., Young A., Stahl S.M. Expert Consensus on Screening and Assessment of Cognition in Psychiatry. CNS Spectr. 2019;24(1):154–162. doi: 10.1017/S1092852918001189. [DOI] [PubMed] [Google Scholar]
- Miskowiak K.W., Burdick K.E., Martinez-Aran A., Bonnin C.M., Bowie C.R., Carvalho A.F., Gallagher P., Lafer B., López-Jaramillo C., Sumiyoshi T., McIntyre R.S., Schaffer A., Porter R.J., Torres I.J., Yatham L.N., Young A.H., Kessing L.V., Vieta E. Methodological recommendations for cognition trials in bipolar disorder by the International Society for Bipolar Disorders Targeting Cognition Task Force. Bipolar Disord. 2017;19(8):614–626. doi: 10.1111/bdi.12534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McIntyre R.S., Subramaniapillai M., Park C., Zuckerman H., Cao B., Lee Y., Iacobucci M., Nasri F., Fus D., Bowie C.R., Tran T., Rosenblat J.D., Mansur R.B. The THINC-it Tool for Cognitive Assessment and Measurement in Major Depressive Disorder: Sensitivity to Change. Front. Psychiatry. 2020;11 doi: 10.3389/fpsyt.2020.00546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McIntyre R.S., Best M.W., Bowie C.R., Carmona N.E., Cha D.S., Lee Y., Subramaniapillai M., Mansur R.B., Barry H., Baune B.T., Culpepper L., Fossati P., Greer T.L., Harmer C., Klag E., Lam R.W., Wittchen H.-U., Harrison J. The THINC-Integrated Tool (THINC-it) Screening Assessment for Cognitive Dysfunction: Validation in Patients With Major Depressive Disorder. J. Clin. Psychiatry. 2017;78(7):873–881. doi: 10.4088/JCP.16m11329. [DOI] [PubMed] [Google Scholar]
- McIntyre R.S., Cha D.S., Soczynska J.K., Woldeyohannes H.O., Gallaugher L.A., Kudlow P., Alsuwaidan M., Baskaran A. Cognitive deficits and functional outcomes in major depressive disorder: determinants, substrates, and treatment interventions. Depress Anxiety. 2013;30(6):515–527. doi: 10.1002/da.22063. [DOI] [PubMed] [Google Scholar]
- Ustün B., Kennedy C. What is “functional impairment”? Disentangling disability from clinical significance. World Psychiatry. 2009;8(2):82–85. doi: 10.1002/j.2051-5545.2009.tb00219.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Abdallah S.J., Voduc N., Corrales-Medina V.F., McGuinty M., Pratt A., Chopra A., Law A., Garuba H.A., Thavorn K., Reid R.E.R., Lavoie K.L., Crawley A., Chirinos J.A., Cowan J. Symptoms, pulmonary function and functional capacity four months after COVID-19. Ann Am Thorac Soc. 2021;18(11):1912–1917. doi: 10.1513/AnnalsATS.202012-1489RL. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buonsenso D., Munblit D., De Rose C., Sinatti D., Ricchiuto A., Carfi A., Valentini P. Preliminary evidence on long COVID in children. Acta Paediatr. 2021;110(7):2208–2211. doi: 10.1111/apa.15870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Froidure A., Mahsouli A., Liistro G., De Greef J., Belkhir L., Gérard L., Bertrand A., Koenig S., Pothen L., Yildiz H., Mwenge B., Aboubakar F., Gohy S., Pilette C., Reychler G., Coche E., Yombi J.-C., Ghaye B. Integrative respiratory follow-up of severe COVID-19 reveals common functional and lung imaging sequelae. Respir. Med. 2021;181:106383. doi: 10.1016/j.rmed.2021.106383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klein H., Asseo K., Karni N., Benjamini Y., Nir-Paz R., Muszkat M., Israel S., Niv M.Y. Onset, duration and unresolved symptoms, including smell and taste changes, in mild COVID-19 infection: a cohort study in Israeli patients. Clin. Microbiol. Infect. 2021;27(5):769–774. doi: 10.1016/j.cmi.2021.02.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leth S., Gunst J.D., Mathiasen V., Hansen K., Søgaard O., Østergaard L., Jensen-Fangel S., Storgaard M., Agergaard J. Persistent symptoms in patients recovering from COVID-19 in Denmark. Open Forum. Infect Dis. 2021;8(4) doi: 10.1093/ofid/ofab042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liang L., Yang B., Jiang N., Fu W., He X., Zhou Y., Ma W.-L., Wang X. Three-month follow-up study of survivors of Coronavirus disease 2019 after discharge. J. Korean Med. Sci. 2020;35(47) doi: 10.3346/jkms.2020.35.e418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mattioli F., Stampatori C., Righetti F., Sala E., Tomasi C., De Palma G. Neurological and cognitive sequelae of Covid-19: a four month follow-up. J. Neurol. 2021;268(12):4422–4428. doi: 10.1007/s00415-021-10579-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mazza M.G., Palladini M., De Lorenzo R., Magnaghi C., Poletti S., Furlan R., Ciceri F., Rovere-Querini P., Benedetti F. Persistent psychopathology and neurocognitive impairment in COVID-19 survivors: Effect of inflammatory biomarkers at three-month follow-up. Brain Behav. Immun. 2021;94:138–147. doi: 10.1016/j.bbi.2021.02.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morin L., Savale L., Pham T., Colle R., Figueiredo S., Harrois A., Gasnier M., Lecoq A.-L., Meyrignac O., Noel N., Baudry E., Bellin M.-F., Beurnier A., Choucha W., Corruble E., Dortet L., Hardy-Leger I., Radiguer F., Sportouch S., Verny C., Wyplosz B., Zaidan M., Becquemont L., Montani D., Monnet X. Four-month clinical status of a cohort of patients after hospitalization for COVID-19. JAMA. 2021;325(15):1525. doi: 10.1001/jama.2021.3331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Romero-Duarte Á., Rivera-Izquierdo M., Guerrero-Fernández de Alba I., Pérez-Contreras M., Fernández-Martínez N.F., Ruiz-Montero R., Serrano-Ortiz Á., González-Serna R.O., Salcedo-Leal I., Jiménez-Mejías E., Cárdenas-Cruz A. Sequelae, persistent symptomatology and outcomes after COVID-19 hospitalization: the ANCOHVID multicentre 6-month follow-up study. BMC Med. 2021;19(1) doi: 10.1186/s12916-021-02003-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- W, Shendy, Ezzat MM, Md DAE, Elsherif AA. Prevalence of fatigue in patients post covid-19. European Journal of Molecular & Clinical Medicine. 2021;8(3):1330-1340. Accessed July 8, 2021. https://ejmcm.com/article_9929.html.
- Suárez-Robles M., Iguaran-Bermúdez M.D.R., García-Klepizg J.L., Lorenzo-Villalba N., Méndez-Bailón M. Ninety days post-hospitalization evaluation of residual COVID-19 symptoms through a phone call check list. Pan Afr Med J. 2020;37:289. doi: 10.11604/pamj.2020.37.289.27110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao Y.-M., Shang Y.-M., Song W.-B., Li Q.-Q., Xie H., Xu Q.-f., Jia J.-l., Li L.-M., Mao H.-l., Zhou X.-M., Luo H., Gao Y.-F., Xu A.-G. Follow-up study of the pulmonary function and related physiological characteristics of COVID-19 survivors three months after recovery. EClinicalMedicine. 2020;25:100463. doi: 10.1016/j.eclinm.2020.100463. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.