Abstract
Background & objectives:
The elimination goal for leprosy as a public health problem at the national level was achieved in 2005 in India. However, the number of new cases reporting annually remained nearly the same during the last 10-15 years. Moreover, a substantial number of these new cases reported disabilities for the first time. Therefore, besides multidrug therapy (MDT), newer strategies with focus on effectively decreasing the number of new cases, optimizing the treatment of detected cases, averting disabilities and arresting the transmission of the disease are required. So the objective of this study was to assess the cost-effectiveness of Mycobacterium indicus pranii (MIP) vaccine implementation in National Leprosy Eradication Programme (NLEP) for newly diagnosed leprosy patients as well as their contacts to arrest/decrease the transmission and occurrence of new cases.
Methods:
This was a model-based estimation of incremental costs, total quality-adjusted life years (QALYs) gained, new cases averted, deaths averted, incremental cost-effectiveness ratio (ICER) and budget impact of the vaccination intervention. This model included the addition of MIP treatment intervention to the newly detected leprosy patients as well as vaccination with MIP to their contacts.
Results:
Using the societal perspective, discounted ICER was estimated to be ₹73,790 per QALY gained over a five-year time period. Probabilistic sensitivity analysis (PSA) was assessed by varying the values of input parameters. Majority (96%) of simulations fell in North East quadrant of cost-effectiveness plane, which were all below the willingness to pay threshold.
Interpretation & conclusions:
Introduction of MIP vaccination in the NLEP appears to be a cost-effective strategy for India. Significant health gains were reduction in the number of new leprosy cases, decreased incidence and severity of reactions during treatment, and after release from treatment, prevention of disabilities, thus reducing the cost as well as stigma of the disease.
Keywords: Cost-effectiveness, disabilities, economic evaluation, incremental cost-effectiveness ratio, Mycobacterium indicus pranii, National Leprosy Eradication Programme, quality-adjusted life years
Leprosy is a chronic infectious disease caused by Mycobacterium leprae and continues to be a public health problem in India. It has a low mortality rate but is characterized by several complications in the form of reactions, impairment and loss of sensory and/or motor function, disabilities, social stigma as well as socio-economic implications. Though India achieved the leprosy elimination goal as a public health problem in 2005, it still has a high burden of the disease and reported 120,334 new leprosy cases in 2017-20181. These constituted about 57.7 per cent of the global burden of new cases in 20182. The Indian National Leprosy Eradication Programme (NLEP) has scaled up campaigns for early case detection and implemented new initiatives and interventions to accelerate leprosy elimination goals at all sub-national levels (https://dghs.gov.in). Reporting of a nearly stable incidence of new cases in the last 10-15 yr and also increasing reports of disabilities in new cases, are a pointer that the current multidrug therapy (MDT) strategy alone may not be able to effectively control the disease and its transmission. Better treatment strategies, early diagnosis and care of reactions, reducing disabilities and sensory and motor impairments, preventing relapses and controlling the transmission of leprosy are required.
Mycobacterium indicus pranii (MIP), previously called Mw, is a rapidly growing, saprophytic, non-pathogenic Mycobacterium and shares several antigens with M. leprae3. Killed MIP is an effective immunomodulator, safe, well accepted, and has both immunotherapeutic4,5,6,7,8,9, as well as immunoprophylactic10,11,12 properties against leprosy. It is well established that household contacts are about seven times more likely to be infected, while social contacts are 3-4 times more likely to be infected, as compared to the general population13,14. Moreover, this risk further increases several folds if a co-prevalent case is also present concurrently.
Immunomodulation is a good option to arrest the transmission of diseases, due to its dual action (both on the host as well as on the infecting organism). Several related mycobacteria, which share antigens with M. leprae and mount an effective immune response, have been used and tried in leprosy, such as Bacillus Calmette–Guerin (BCG)15,16, BCG+killed M. leprae16,17, Indian Cancer Research Centre (ICRC)18, M. vaccae19, M. habana20 and MIP4,5,6,7,8,9,10,12,21,22,23,24,25,26,27. BCG being a live vaccine has its inherent disadvantages, and importantly, leprosy is still prevalent in countries, where for several years, BCG has, and is being widely used for TB prophylaxis.
MIP has been studied extensively, evaluated both in field situations and in well-controlled clinical studies in several tertiary care hospitals in India4,5,6,7,8,9,10,11,12,22,23,24,25,26,27. It is safe and well accepted and both Drugs Controller General of India (DCGI) and U.S. Food and Drug Administration (FDA) approved it as an immunomodulator assess. This study was undertaken to estimate the total cost-effectiveness of MIP immunotherapy as an adjunct to current MDT for all newly diagnosed leprosy patients in NLEP, and to estimate the MIP immuno-prophylaxis to the contacts of the newly diagnosed patients in NLEP to arrest/decrease the transmission and occurrence of new cases.
Material & Methods
Study design: This study design analysis involved a model-based simulation to estimate the incremental cost-effectiveness of implementing MIP vaccination as a new strategy under the existing NLEP in India. The proposed intervention was compared with the current MDT treatment including active follow up of leprosy patients and their contacts (Table I). The various costs included in the model were cost of vaccination to the leprosy patients and their contacts, cost of treatment for leprosy patient with MDT, cost of reconstructive surgery (RCS), provision of steroids for reactions, microcellular rubber footwear to patients with disabilities and loss of foot sensations, incentives given to healthcare workers and patients for RCS and disability correction.
Table I.
Details of the two strategies used in the study
| Strategies being compared | Target population and intervention | Implementation |
|---|---|---|
| Proposed strategy (Strategy 2) | Newly detected cases of leprosy - MDT + MIP | MDT + MIP vaccination (2 doses in PB patients and 3 doses in MB patients; each dose given at interval of six months). Follow up post MDT for five yr |
| Contacts of newly detected cases - MIP + active follow up | 1st dose consisting of 2 divided doses on both the upper arms on day zero, followed by repeat single dose after six months. Follow up postvaccination for five yr | |
| Standard comparator (Strategy 1) | Newly detected cases of leprosy - MDT + follow up | Current practice of MDT; follow up post-MDT for five yr |
| Contacts of newly detected cases - Active follow up as per Programme guidelines | Active follow up for five yr as per Programme guidelines |
MB, multibacillary leprosy; PB, paucibacillary leprosy; MDT, multidrug therapy; MIP, Mycobacterium indicus pranii
The effectiveness was estimated to include the overall health outcomes of reactions and disabilities prevented, deaths averted, life years saved and quality-adjusted life years (QALYs) gained by leprosy patients. It also includes the gains accrued by prevention of leprosy in contacts of these newly diagnosed cases.
Study population: The study population consisted of newly diagnosed leprosy patients reporting to the NLEP (to be administered MIP as an adjunct immunotherapy with MDT) and their close contacts who were at increased risk of infection (for immunoprophylaxis).
Audience: The policy makers and health departments, programme managers, hospitals/programmes treating leprosy patients, constituted the primary audience. Other audiences included the aid providing agencies, international development and lending institutions, non-governmental organizations (NGOs), private health care providers etc.
Study perspective: An abridged societal perspective which accounted for all the relevant costs to the patient who accessed NLEP health facilities for leprosy diagnosis and treatment was considered. This perspective also accounted for direct and indirect costs experienced by the patients and their families.
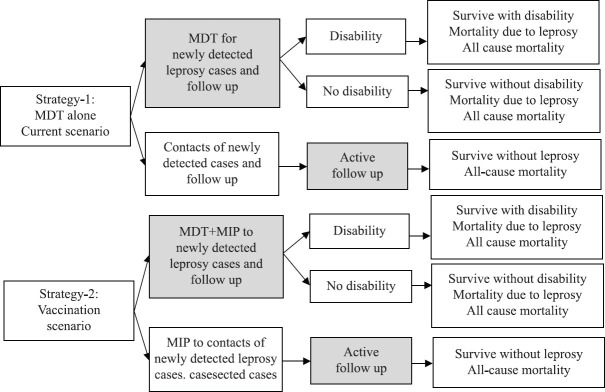
Time horizon: A five-year time horizon was considered for comparing the costs and outcomes of intervention of MIP+MDT treatment as compared to MDT alone for treatment of the new cases, and MIP administration to the contacts of these newly detected case in comparison to only active follow up (as done in the NLEP) (Tables I-III and Fig. 1).
Table III.
Total costs, health outcomes and cost-effectiveness of implementing Mycobacterium indicus pranii (MIP) vaccination in National Leprosy Eradication Programme (NLEP), over a five-year period in India
| Intervention | Total cost (₹ in million) | QALYs lived (₹ in million) | ICER in ₹ per QALYs gained Discounted | ||
|---|---|---|---|---|---|
|
|
|
||||
| Non-discounted | Discounted | Non-discounted | Discounted | ||
| MDT + MIP vaccination for all new patients + MIP vaccination for contacts of new patients | 21,151 | 19,939 | 6,772 | 6,385 | 73,790 |
| MDT alone for new patients and follow up + follow up of contacts of new patients | 18,688 | 17,618 | 6,772 | 6,385 | |
The dollar equivalent of ICER per QALY’s gained is $1054 (approximate 2019 conversion rate). MDT, multidrug therapy; ICER, Incremental cost-effectiveness ratio; QALY, Quality Adjusted Life Years
Fig. 1.
The decision tree for economic evaluation of implementing Mycobacterium indicus pranii (MIP) vaccination in National Leprosy Eradication Programme (NLEP), India.
Model assumptions: The proposed vaccination intervention involved vaccinating newly detected leprosy patients and their contacts (who provided written informed consent). Two doses of vaccination for new paucibacillary (PB) patients (one at the start of current MDT and one at the end of MDT) and three doses of vaccination for new multibacillary (MB) patients (at six monthly intervals from start to completion of treatment)8; two doses for contacts of newly detected patients, at an interval of six months10. The predictive modelling approach was used to calculate the costs and effectiveness of these two intervention strategies.
Strategy 1 was the standard MDT treatment for newly diagnosed leprosy patients, examine their contacts and follow them up for five years; Strategy 2 is the current proposed intervention consisting of MIP vaccination for newly detected patients in addition to the standard MDT treatment; and MIP vaccination for their contacts and follow up (Fig. 1). The standard guidelines for Consolidated Health Economic Evaluation Reporting (CHEERS) were followed28.
Model structure: The outcomes of the two strategies and their respective costs were evaluated by adopting a decision tree model (Fig. 1). In strategy 1, newly diagnosed leprosy patients were treated with MDT, disabilities noted and corrected as admissible, clinically followed up during and after completion of treatment for five years. The contacts of these patients were also examined and followed by healthcare workers who were incentivized by the NLEP for five years .
In strategy 2, newly diagnosed leprosy patients were administered MIP vaccine (as described above) in addition to MDT. They were followed up and reactions and disabilities were noted as per NLEP. The contacts of these patients were given MIP vaccination (two doses as described above), to prevent leprosy, and were also followed up for five years.
Model input parameters: Data on model input parameters were collected from different sources and are presented in Table IV. The parameters included, demographic, epidemiological, cost and quality of life estimates. Population data were collected from the office of the Registrar General and Census Commissioner, India29. Life expectancy was taken from the India’s life table published based on the SRS data30. Epidemiological parameters included in the analysis were incidence of leprosy, mean age of leprosy patients at diagnosis, incidence among contacts and incidence among general population (Table IV). The total patients registered for treatment under the NLEP were taken from NLEP annual report 2015-201631 and 2016-201732. Parameter estimates on vaccine efficacy10, quality of life of leprosy patients33,34, mortality due to leprosy35, relapse rate31,35, number of contacts35,36,37 and cost of leprosy treatment from health system38 were collected from the published literature as indicated (Table IV). All the costs have been adjusted to the current value using Consumer Price Index (CPI) inflator for India.
Table IV.
Input parameters used for model based economic evaluation of implementing Mycobacterium indicus pranii (MIP) vaccination in National Leprosy Eradication Programme (NLEP) India
| Parameters used | Base case | Lower limit | Upper limit | Distribution | Reference number |
|---|---|---|---|---|---|
| Demographical data | |||||
| Life expectancy at age 27 (yr) | 44.75 | 33.56 | 55.93 | NA | 30 |
| Average age of leprosy patient at diagnosis (yr) | 27 | 20.25 | 33.75 | NA | 31 |
| Epidemiological parameters | |||||
| New leprosy patients in 2016-2017 | 135,485 | 101,614 | 169,356 | NA | 33 |
| Contacts per new patient | 20 | 6 | 25 | NA | 25 35 |
| Contacts in 2016-2017 | 2,709,700 | 609,684 | 4,233,900 | NA | 32 |
| Incidence of leprosy in the population | |||||
| Incidence in contacts 2016-2017 | 0.0008 | 0.0006 | 0.0010 | Beta | 32 |
| Incidence in general population 2016-2017 | 0.00010 | 0.00007 | 0.00012 | Beta | 32 |
| Vaccine effect used in the study | |||||
| Vaccine efficacy | 0.60 | 0.45 | 0.75 | Beta | 9 10 |
| Disability rate | |||||
| Proportion of leprosy patients getting disability | 0.04 | 0.03 | 0.05 | Beta | 43 44 |
| Mortality | |||||
| Mortality due to leprosy | 0.089500 | 0.067125 | 0.111875 | Beta | 35 |
| Quality of Life (QoL) | |||||
| QoL of healthy Indian | 1 | 1 | 1 | Beta | Assumption |
| QoL of leprosy patient | 0.848 | 0.636 | 1 | Beta | 34 |
| QoL of leprosy patient with disability | 0.6021 | 0.451575 | 0.752625 | Beta | 33 |
| Relapses in patients in percentage | |||||
| Relapse rate | 0.02 | 0.015 | 0.025 | NA | 30 32 |
| Costs/expenses estimated in Indian rupees | |||||
| Cost of vaccinating per person | 96.6 | 72.45 | 120.75 | Gamma | Table III |
| Cost of leprosy treatment (health system) | 14,000 | 10,000 | 36,000 | Gamma | 38 |
| Cost of leprosy treatment (patient) | 10,680 | 8010 | 13,350 | Gamma | 39 |
| Cost of treatment (health system perspective) | 24,680 | 18,010 | 49,350 | Gamma | 32 |
| Cost of foot wear | 1400 | 1080 | 1800 | Gamma | 32 |
| Expenses incurred for RCS | 5142 | 3856 | 6427 | Gamma | 32 |
| Incentives to health workers | 750 | 563 | 938 | Gamma | 32 |
| Discounting rate as used in the study | |||||
| Discounting rate (costs) | 0.03 | 0.03 | 0.03 | Beta | 40 |
| Discounting rate (QoL) | 0.03 | 0.03 | 0.03 | Beta | 40 |
| CET (Cost effectiveness thresholds) | |||||
| Per capita in Indian rupees GDP India 2018 | 120,000 | 120,000 | 120,000 | NA | 42 |
QoL, quality of life; CET, cost-effectiveness thresholds; RCS, reconstructive surgery; GDP, gross domestic product
For strategy 2 a micro-costing was used to derive unit costs of MIP vaccination. This included cost for vaccine vial (as quoted by M/s Cadila Pharma and submitted to Department of Health Research, MoHFW, Government of India, June, 2018), number of doses required, staff training and time, storage, maintenance of cold chain and transportation (Table II). The capital costs included supervision and monitoring and overhead costs. The proportion of MB and PB patients in the country, and the average values of disabilities at the current standards of care were used. The vaccination cost in strategy 2 was added to the current MDT treatment which included the overall treatment, cost of provider and patient and incentive to healthcare workers.
Table II.
The unit cost of giving Mycobacterium indicus pranii (MIP) to new leprosy patients and their contacts in National Leprosy Eradication Programme (NLEP), India
| Parameters | Details of cost (₹) | References/comments |
|---|---|---|
| Cost of one vial of MIP vaccine | 140 | As per cost quoted by Ms Cadila Pharmaceuticals in June, 2018 |
| Number of doses from one vial | 6 | |
| Cost of vaccine/dose + syringe + needle per dose | 23.33 | |
| Cost of supply chain and other expenses/dose | 25 | Cost for human resource training and cold chain equipment strengthening |
| Total cost of giving MIP to MB patients/year | 215 | One dose thrice in a year: 0, 6 and 12 months during treatment |
| Total cost of giving MIP to PB patients/year | 143.33 | One dose at day zero and repeated after six months |
| Total cost of two doses to contacts | 143.33 | Two doses: One on both the arms (in two divided doses, on both the upper arm) at day zero and single dose after six months |
| Cost of giving MIP to patient (weighted average) | 179.17 |
MB, multibacillary leprosy; PB, paucibacillary leprosy
Vaccine efficacy for adjunct immunotherapy with multidrug therapy (MDT): Efficacy parameters were assessed using published data from an immunotherapeutic clinical trial where MIP was administered in addition to MDT for 156 MB patients who were followed up for seven years after treatment9. Based on the results, an optimal 60 per cent adjunct immunotherapy efficacy was used for the current analysis.
Vaccine efficacy for immunoprophylaxis: Vaccine efficacy parameter was used from published data, of a large-scale, double-blind immunoprophylactic trial of a leprosy vaccine conducted at Kanpur, India, among a population of 420,823 covering 272 villages. The population consisted of 1226 MB and 3757 PB cases and 24,060 household contacts10. The study population was followed up at 3, 6 and 9-10 yr after the initial vaccination. The protective efficacy (PE) reported was 68, 60 and 28 per cent at the end of the first, second and third re-surveys, respectively10.
Model outcome parameters: The outcomes of the model were expressed in terms of QALYs lived per patient and overall costs incurred per patient in both intervention and comparator arms. The projection for averted new leprosy cases and deaths was calculated based on the vaccine efficacy rate.
Base case analysis: The total cost was calculated by multiplying unit cost by expected number of persons to be vaccinated. Based on the estimated incidence rate of leprosy among contacts, and the expected number of contacts, the expected number of persons to be vaccinated was calculated. Future costs and consequences were discounted at three per cent per year38,39,40,41. A total of 1000 individuals were entered in the decision analytic model for the estimation of incremental costs and QALYs gained by including MIP vaccination. The incremental cost-effectiveness ratio (ICER) between the MIP-vaccinated and -non-vaccinated strategies was calculated. ICER was compared with the cost-effectiveness thresholds (CETs) value. The one-time Gross Domestic Product (GDP) per capita of the country was taken as ₹120,000 as in 201842. Results were expressed in terms of undiscounted QALYs gained, discounted QALYs gained, undiscounted life years gained, discounted life years gained and deaths averted (Tables III and IV).
Sensitivity analysis: To account for uncertainty, variations in key parameters such as cost of treatment, mortality due to leprosy, incidence in contacts, quality of life of leprosy patients, vaccine efficacy and cost of vaccination were considered by doing one-way sensitivity analysis. This analysis was performed to assess the impact of various individual parameters that are likely to have effect on ICER. The Tornado diagram representing the effect of parameters on ICER is depicted in Fig. 2.
Fig. 2.
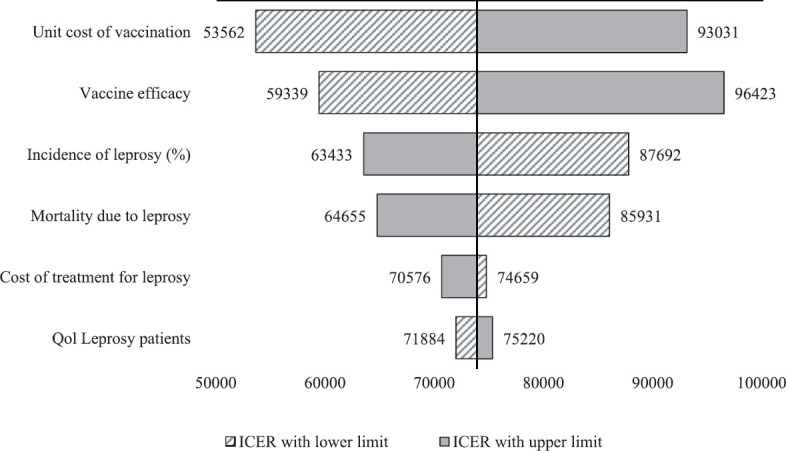
Tornado chart of one-way sensitivity analysis: Impact of variation of input parameters on incremental cost-effectiveness ratio in the study. All-cause mortality was obtained from India’s Standard Life Table30.
The probabilistic sensitivity analysis (PSA) was performed to find out the joint effect of variation in these parameters on ICER (Fig. 2). The robustness of model results was tested using Monte–Carlo simulations (1000 times) with 95 per cent confidential intervals. PSA was done by MS Excel using visual basic analysis code. The results are represented on a Cost-Effectiveness Acceptability Curve (CEAC), which indicates the model’s probabilistic response to a CET, expressed in terms of cost per QALYs (Fig. 3).
Fig. 3.
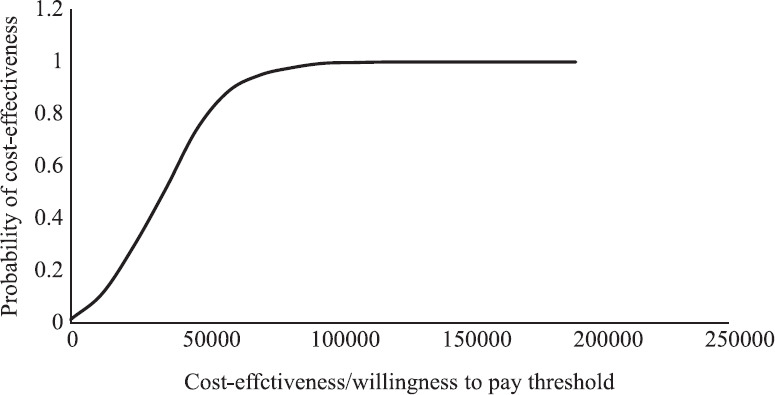
Cost-effectiveness acceptability curve derived in the study. All costs are in Indian ₹.
Budget impact analysis and net health benefit: The annual cost of nationwide implementation of incorporating MIP vaccination for newly detected leprosy patients and their contacts in the present NLEP was estimated. Based on the estimated incidence of leprosy, the incidence of leprosy among contacts, the expected number of contacts and the expected number of persons to be vaccinated, as well as the tentative budget required was calculated. However, the cost of administering single-dose rifampicin (SDR) to contacts of index case was not accounted for, in the comparator arm, as this strategy was recently introduced in the NLEP and that too only in endemic districts.
Results
With the present trend of identifying about 100,000 to 110,000 cases/year in the Programme (this includes cases also detected in the special campaigns launched by NLEP, India), about 500,000-600,000 new cases are expected to be enrolled and vaccinated in the next five years. Assuming about 20 contacts/new patients, about 1-1.2 million household contacts were assumed/estimated to be vaccinated. The budget cost of MIP vaccination to both patients and contacts was estimated to be ₹27.49 million in five years (this was estimated taking into consideration of 135,485 new cases detected and reported by the NLEP in the year 2016-2017)32. In addition, the cost of MDT and Programme costs were added and the results are summarized in Table IV.
Cost-effectiveness: The discounted and non-discounted costs, QALYs gained, life years gained and deaths averted by addition of MIP using this model are detailed in Table IV. The discounted QALYs gained was ₹73,790. The increase in cost in NLEP due to vaccination gets largely compensated by preventing new leprosy cases as well as preventing reactions and disabilities in the new cases over a five-year period. Besides this, the life-long stigma and shortcomings due to it (which have not been accounted for), it also brings down the cost burden of the Programme.
Incremental cost-effectiveness ratio (ICER): The ICER of incorporating MIP vaccine for newly diagnosed leprosy patients and their contacts was ₹73,790/- per QALYs gained, which was much less than willingness to pay [one times per capita Gross Domestic Product (GDP)] in India. It is important to mention that this estimated ICER value in this analysis does not reflect the psycho-social benefits of preventing disabilities to patients and their family members which also contribute to the economic costs besides the psycho-social aspect.
Impact of parameters on ICER values: Fig. 2 shows a Tornado diagram depicting the cost-effectiveness results of the one-way sensitivity analyses. It has been observed that the results are sensitive to the unit cost of vaccination, vaccine efficacy rate, incidence of leprosy and mortality due to leprosy.
Probability of cost-effectiveness: PSA involving 1000 random interactions of ICER value was performed. This PSA analysis highlighted that more than 96 per cent of times, ICER fell in the second quadrant indicating that the new intervention was highly cost effective (Fig. 3), even after adding the cost of vaccination. Cost-effectiveness acceptability curve indicates 100 per cent probability of this intervention being very cost-effective since none of the ICER value was higher than willingness to pay threshold of country (Fig. 4).
Fig. 4.
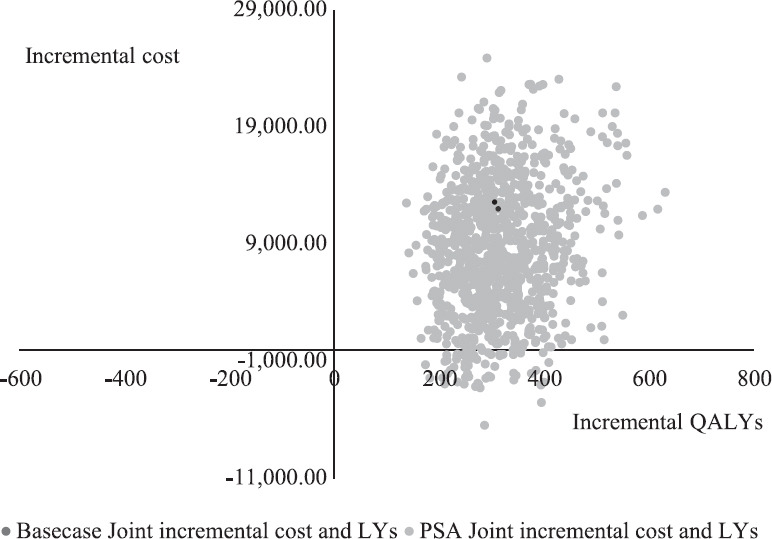
Cost-effectiveness cloud: Probabilistic sensitivity analysis simulations as compared to base case results. All costs are in ₹.
Budget and net monetary benefits: The budget required for incorporating MIP vaccination for the new leprosy patients was ₹13 million; for their contacts, it was estimated to be ₹262 million. This amounts to a total cost of ₹275 million over a five-year period. Additionally, the net monetary benefit of incorporating MIP vaccination in NLEP in India in terms of discounted QALYs gained was estimated to be ₹1450 million over a five-year period. The resulting return on investment was 1.62 times the investment on vaccination and is very cost effective.
Discussion
In the present cost effectiveness analysis, the cost of addition of MIP to the treatment was moderate and acceptable, and less than one time the GDP of our country as well as willingness to pay threshold. The gains obtained were more in terms of reducing disabilities, improvement in sensation of the affected part, better and faster treatment outcomes which have also been clinically observed and reported in biopsies and tissue sections4,5,12,24,25. Also there were no relapses and reactions in post-treatment follow up ranging from 2-12 yr5,7,8. These complications, besides, adding to the morbidity of leprosy, also have an economic burden37, as well affect the socio-psychological wellbeing of affected patients, which has also been highlighted by Global Health Action Forum43.
It is well established that close household contacts are more prone to develop leprosy as compared to the general population13,14. Besides this, age of the exposed individual and type of leprosy in the index case are independent risk factors for development of leprosy in contacts44. NLEP also has taken cognizance of this and conducts active follow up of this population to diagnose leprosy early, provide early treatment and reduce transmission of the disease. Therefore, follow up of contacts of all new cases did not involve extra cost in this study. The extra cost incurred of administering MIP was largely covered by the reduction in new cases in the population as is evident from Figs 2 and 3.
In the Kanpur study10, it was observed that the protective efficacy (PE) by administration of MIP to contacts of newly diagnosed leprosy cases was 68 per cent after three years post-vaccination, 60 per cent at the end of 5-6 yr post-vaccination and 28 per cent at the end of 9-10 yr post-vaccination. As per approved protocol, the vaccine was administered in two doses at an interval of six months. This differed from the protocol adopted by the vaccine study in Tamil Nadu, South India11, where only a single dose of vaccine was administered. Furthermore, in the South India study, surveys were started immediately after the vaccination of the general population. The PE reported for Mw (MIP) was 41 in the third re-survey (nine-year post-vaccination) in the general population11,45. On re-analysis, for observing the PE in contacts of index cases only, it was reported that MIP vaccine efficacy, using the Cochran-Mantel-Haenszel (MH) test for measuring the vaccine efficacy was 50-60 per cent in household contacts after nine years of vaccination (unpublished data, reproduced from the reply to ICMR Expert Study Group which had suggested a re-look in contacts). It was observed that the MIP provided substantial protection to contacts of the newly detected leprosy patients and decreased the incidence rate of the disease in them.
The discounted ICER was estimated as ₹73,790 per QALY gained over a five-year period. This was much less than one time of the GDP and was thus considered cost-effective. This would help not only in reducing and minimizing the number of new cases in the subsequent years but also improve the quality of life of the diagnosed patients by preventing disabilities, improvement and return of tactile sensations and also the incidence and severity of reactions, both during and after release from treatment. Fig. 3 depicts the cost effectiveness cloud and PSA simulations. More than 90 per cent of the calculations fall in the cost effectiveness quadrant and all fall in the willingness to pay threshold.
The strength of this present study was that it had utilized vaccine efficacy information from a large field-based clinical trial to model its population level implementation10. Earlier studies on cost-effectiveness of leprosy interventions measured effects in terms of leprosy cases or consequences prevented and disability-adjusted life years averted.
SDR-PEP (Single Dose Rifampicin-Post Exposure Prophylaxis) is being used/tried in NLEP in the endemic districts only, for prevention of leprosy in contacts, since the end of 2018. It is early to comment on its efficacy as the strategies to implement them are still evolving/being debated whether to repeat it after two years or replace it with single dose MDT instead of SDR46. In a modelling study reported, from India47, on long-term cost-effectiveness of SDR-PEP, it is reported that one leprosy case prevented among different contacts was found to be cost-effective with a saving of $214 to $856 over a 25 yr period. More recently, a mathematical model-based study estimate of the number of people requiring PEP to achieve significant reduction of new leprosy cases globally has been published46. The investigators opine that administration of PEP-SDR to household contacts (for India, they have estimated 4.9 contacts/index case) and administering to 4-5 such neighbouring households near the index case will be required for reducing the new case load by 50 per cent in five years46. Furthermore, SDR is still evolving and there are still no clear directives of its repetition after two years, nor of use of MDT as a single dose for post-exposure prophylaxis (PEP). Whatever may be the decided strategy, it will add to the costs in the comparator arm and will therefore, benefit the cost-effectiveness of the present intervention arm in the study.
Using different methods and statistical tools in the present study, the estimated ICER was of $1054 (₹73,790) for each discounted QALY gained by vaccinating both the newly detected case and their contacts (6-20 contacts per index case) over a five-year period. It is premature in a disease like leprosy with a long and variable incubation period, to draw comparisons between the two methods with widely different approaches and mechanisms of action of interventions used. More importantly, the present strategy outlined in the study will use a two pronged approach of better treatment outcomes and limiting the transmission by vaccinating the close contacts.
Limitations of the study: The present vaccine efficacy estimate was derived from a single, large, controlled clinical trial done in India. Meta-analysis was not possible due paucity of such studies in leprosy. Secondly, the present study has not accounted for SDR administration in contacts in the high endemic districts. Thirdly, it has not accounted for the gains likely to be accrued in mental and social well-being of the patient and their contacts by the reduction of both self-stigma and stigma prevailing in the society. Addition of these socio-economic gains may substantially increase the cost-effectiveness of the intervention proposed in the study.
In conclusion, the results of the analysis showed that the MIP vaccination among newly diagnosed leprosy patients and their contacts was safe, effective, socially acceptable, operationally feasible, cost-effective and beneficial. While MDT has undoubtedly helped in containing leprosy in India, still the pattern of disease in the last 10-15 yr emphasizes the need for multipronged approaches to overcome the challenges of the disease. The present analysis showed that addition of MIP to newly detected cases for better treatment outcomes, reducing the morbidity of the disease, stigma of the disabilities, improving the quality of life of patients, together with reducing the incidence of new cases and is cost-effective for the Programme needs
Footnotes
Financial support & sponsorship: None.
Conflicts of Interest: None.
References
- 1.Central Leprosy Division. NLEP Annual Report: 2017-18. New Delhi: Directorate General of Health Services, Ministry of Health &Family Welfare, Government of India; [Google Scholar]
- 2.World Health Organization. Global leprosy update, 2018:Moving towards a leprosy-free world. Wkly Epidemiol Rec. 2019;94:389–412. [Google Scholar]
- 3.Mustafa AS, Talwar GP. Early and late reactions in tuberculoid and lepromatous leprosy patients with lepromins from Mycobacterium leprae and five selected cultivable mycobacteria. Lepr India. 1978;50:566–71. [PubMed] [Google Scholar]
- 4.Katoch K, Katoch VM, Natrajan M, Bhatia AS, Sreevatsa, Gupta UD, et al. Treatment of bacilliferous BL/LL cases with combined chemotherapy and immunotherapy. Int J Lepr Other Mycobact Dis. 1995;63:202–12. [PubMed] [Google Scholar]
- 5.Katoch K, Katoch VM, Natrajan M, Sreevatsa, Gupta UD, Sharma VD, et al. 10-12 years follow-up of highly bacillated BL/LL leprosy patients on combined chemotherapy and immunotherapy. Vaccine. 2004;22:3649–57. doi: 10.1016/j.vaccine.2004.03.037. [DOI] [PubMed] [Google Scholar]
- 6.Zaheer SA, Beena KR, Kar HK, Sharma AK, Misra RS, Mukherjee A, et al. Addition of immunotherapy with Mycobacterium w. vaccine to multi-drug therapy benefits multibacillary leprosy patients. Vaccine. 1995;13:1102–10. doi: 10.1016/0264-410x(94)00033-j. [DOI] [PubMed] [Google Scholar]
- 7.Kaur I, Dogra S, Kumar B, Radotra BD. Combined 12-month WHO/MDT MB regimen and Mycobacterium w. vaccine in multibacillary leprosy:A follow-up of 136 patients. Int J Lepr Other Mycobact Dis. 2002;70:174–81. [PubMed] [Google Scholar]
- 8.Kamal R, Natrajan M, Katoch K, Arora M. Clinical and histopathological evaluation of the effect of addition of immunotherapy with Mw vaccine to standard chemotherapy in borderline leprosy. Indian J Lepr. 2012;84:287–306. [PubMed] [Google Scholar]
- 9.Sharma P, Misra RS, Kar HK, Mukherjee A, Poricha D, Kaur H, et al. Mycobacterium w. vaccine, a useful adjuvant to multidrug therapy in multibacillary leprosy:A report on hospital based immunotherapeutic clinical trials with a follow-up of 1-7 years after treatment. Lepr Rev. 2000;71:179–92. doi: 10.5935/0305-7518.20000020. [DOI] [PubMed] [Google Scholar]
- 10.Sharma P, Mukherjee R, Talwar GP, Sarathchandra KG, Walia R, Parida SK, et al. Immunoprophylactic effects of the anti-leprosy Mw vaccine in household contacts of leprosy patients:Clinical field trials with a follow up of 8-10 years. Lepr Rev. 2005;76:127–43. [PubMed] [Google Scholar]
- 11.Gupte MD, Vallishayee RS, Anantharaman DS, Nagaraju B, Sreevatsa, Balasubramanyam S, et al. Comparative vaccine trial in South India. Int J Lepr. 1998;70:369–88. [PubMed] [Google Scholar]
- 12.Katoch K. IAL Textbook of Leprosy. New Delhi: Jaypee Brothers Medical Publishers; 2016. Leprosy vaccines:Immunoprophylaxis and immunotherapy; pp. 496–505. [Google Scholar]
- 13.Blok DJ, De Vlas SJ, Richardus JH. Global elimination of leprosy by 2020:Are we on track? Parasit Vectors. 2015;8:548. doi: 10.1186/s13071-015-1143-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Vijayakumaran P, Jesudasan K, Mozhi NM, Samuel JD. Does MDT arrest transmission of leprosy to household contacts? Int J Lepr Other Mycobact Dis. 1998;66:125–30. [PubMed] [Google Scholar]
- 15.Fernandes JMM. Use of BCG in immunoprophylaxis of leprosy. Rev Arg Dermatol. 1939;233:435. [Google Scholar]
- 16.SAGE Working Group on BCG Vaccines and WHO Secretariat. Geneva: WHO; 2017. Report on BCG vaccine use for protection against mycobacterial infections including tuberculosis, leprosy and other non-tuberculous mycobacteria (NTM) infections. [Google Scholar]
- 17.Duthie MS, Gillis TP, Reed SG. Advances and hurdles on the way toward a leprosy vaccine. Hum Vaccin. 2011;7:1172–83. doi: 10.4161/hv.7.11.16848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Bhatki WS, Chulawala RG. Immunotherapeutic potential of ICRC vaccine:A case control study. Lepr Rev. 1992;63:358–64. [PubMed] [Google Scholar]
- 19.Stanford JL, Rook GA, Bahr GM, Dowlati Y, Ganapati R, Ghazi Saidi K, et al. Mycobacterium vaccae in immunoprophylaxis and immunotherapy of leprosy and tuberculosis. Vaccine. 1990;8:525–30. doi: 10.1016/0264-410x(90)90002-4. [DOI] [PubMed] [Google Scholar]
- 20.Wakhlu A, Gaur SP, Kaushal GP, Misra A, Asthana P, Sircar AR. Response of Mycobacterium habana vaccine in patients with lepromatous leprosy and their household contacts. A pilot clinical study. Lepr Rev. 2001;72:179–91. [PubMed] [Google Scholar]
- 21.World Health Organization. New Delhi: WHO–SEARO; 2018. Guidelines for the diagnosis, treatment and prevention of leprosy. [Google Scholar]
- 22.Talwar GP, Zaheer SA, Mukherjee R, Walia R, Misra RS, Sharma AK, et al. Immunotherapeutic effects of a vaccine based on a saprophytic cultivable mycobacterium, Mycobacterium w. in multibacillary leprosy patients. Vaccine. 1990;8:121–9. doi: 10.1016/0264-410x(90)90134-8. [DOI] [PubMed] [Google Scholar]
- 23.De Sarkar A, Kaur I, Radotra BD, Kumar B. Impact of combined Mycobacterium w. vaccine and 1 year of MDT on multibacillary leprosy patients. Int J Lepr Other Mycobact Dis. 2001;69:187–94. [PubMed] [Google Scholar]
- 24.Natarajan M, Katoch K, Bagga AK, Katoch VM. Histological changes with combined chemotherapy and immunotherapy in highly bacillated lepromatous leprosy. Acta Leprol. 1992;8:79–86. [PubMed] [Google Scholar]
- 25.Mukherjee A, Zaheer SA, Sharma AK, Misra RS, Kar HK, Mukherjee R, et al. Histopathological monitoring of an immunotherapeutic trial with Mycobacterium w. Int J Lepr Other Mycobact Dis. 1992;60:28–35. [PubMed] [Google Scholar]
- 26.Kar HK, Sharma AK, Misra RS, Beena KR, Zaheer SA, Mukherjee R, et al. Reversal reaction in multibacillary leprosy patients following MDT with and without immunotherapy with a candidate for an antileprosy vaccine, Mycobacterium w. Lepr Rev. 1993;64:219–26. doi: 10.5935/0305-7518.19930024. [DOI] [PubMed] [Google Scholar]
- 27.Zaheer SA, Misra RS, Sharma AK, Beena KR, Kar HK, Mukherjee A, et al. Immunotherapy with Mycobacterium w. vaccine decreases the incidence and severity of type 2 (ENL) reactions. Lepr Rev. 1993;64:7–14. [PubMed] [Google Scholar]
- 28.Husereau D, Drummond M, Petrou S, Carswell C, Moher D, Greenberg D, et al. Consolidated health economic evaluation reporting standards (CHEERS) statement. BMJ. 2013;346:f1049. doi: 10.1136/bmj.f1049. [DOI] [PubMed] [Google Scholar]
- 29.Office of the Registrar General &Census Commissioner, India. 2011 census data. [accessed on March 13. 2019]. Available from: http://censusindia.gov.in/2011-Common/CensusData2011.html .
- 30.Office of the Registrar General &Census Commissioner, India. SRS Based Life Table 2012-15. Ministry of Home Affairs, Government of India; [Google Scholar]
- 31.Central Leprosy Division. NLEP Annual Report 2015–2016. New Delhi: Directorate General of Health Services, Ministry of Health and Family Welfare, Government of India; [Google Scholar]
- 32.Central Leprosy Division. NLEP Annual Report 2016–2017. New Delhi: Directorate General of Health Services, Ministry of Health and Family Welfare, Government of India; [Google Scholar]
- 33.Govindharaj P, Srinivasan S, Darlong J. Quality of life of persons affected by leprosy in an endemic district, West Bengal, India. Indian J Dermatol. 2018;63:459–64. doi: 10.4103/ijd.IJD_324_18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Geetha AJ, PSS Sundar R. Impact of leprosy on the quality of life. Bull WHO. 1999;77:515–7. [PMC free article] [PubMed] [Google Scholar]
- 35.World Health Organization. Global Leprosy update on 2012 situation. Wkly Epidemiol Rec. 2013;35:365–80. [PubMed] [Google Scholar]
- 36.Kai M, Maeda Y, Maeda S, Fukutomi Y, Kobayashi K, Kashiwabara Y, et al. Active surveillance of leprosy contacts in country with low prevalence rate. Int J Lepr Other Mycobact Dis. 2004;72:50–3. doi: 10.1489/1544-581X(2004)072<0050:ASOLCI>2.0.CO;2. [DOI] [PubMed] [Google Scholar]
- 37.Johannes MF, David P, Schuring RP, Linda O, Jan HR. Physical distance, genetic relationship, age, and leprosy classification are independent risk factors for leprosy in contacts of patients with leprosy. J Infect Dis. 2006;193:346–53. doi: 10.1086/499278. [DOI] [PubMed] [Google Scholar]
- 38.Tiwari A, Blok DJ, Suryawanshi P, Raikwar A, Arif M, Richardus JH. Leprosy services in primary health care in India:Comparative economic cost analysis of two public-health settings. Trop Med Int Health. 2019;24:155–65. doi: 10.1111/tmi.13182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Chandler DJ, Hansen KS, Mahato B, Darlong J, John A, Lockwood DN. Household costs of leprosy reactions (ENL) in rural India. PLoS Negl Trop Dis. 2015;9:e0003431. doi: 10.1371/journal.pntd.0003431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Smith DH, Gravelle H. The practice of discounting in economic evaluations of healthcare interventions. Int J Technol Assess Health Care. 2001;17:236–43. doi: 10.1017/s0266462300105094. [DOI] [PubMed] [Google Scholar]
- 41.Attema AE, Brouwer WBF, Claxton K. Discounting in economic evaluations. Pharmacoeconomics. 2018;36:745–58. doi: 10.1007/s40273-018-0672-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Trading Economics. India Gross National Income. [accessed on April 20, 2019]. Available from: https://tradingeconomics.com/india/gross-national-product .
- 43.van Brakel WH, Sihombing B, Djarir H, Beise K, Kusumawardhani L, Yulihane R, et al. Disability in people affected by leprosy:The role of impairment, activity, social participation, stigma and discrimination. Glob Health Action. 2012;5:10. doi: 10.3402/gha.v5i0.18394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Moet FJ, Pahan D, Schuring RP, Oskam L, Richardus JH. Physical distance, genetic relationship, age, and leprosy classification are independent risk factors for leprosy in contacts of patients with leprosy. J Infect Dis. 2006;193:346–53. doi: 10.1086/499278. [DOI] [PubMed] [Google Scholar]
- 45.Indian Council of Medical Research. Minutes of the expert group meeting to review the results of trial on anti-leprosy candidate vaccines carried out in South India, held at ICMR headquarters, New Delhi, 1st June 2004. DO No. 5/8/3(7)/90-ECD-1(Vol X) dated 13.8.04. [Google Scholar]
- 46.Taal AT, Blok DJ, van Brakel WH, de Vlas SJ, Richardus JH. Number of people requiring post-exposure prophylaxis to end leprosy:A modeling study. PLoS Negl Trop Dis. 2021;15:e0009146. doi: 10.1371/journal.pntd.0009146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Tiwari A, Blok DJ, Arif M, Richardus JH. Leprosy post-exposure prophylaxis in the Indian health system:A cost-effectiveness analysis. PLoS Negl Trop Dis. 2020;14:e0008521. doi: 10.1371/journal.pntd.0008521. [DOI] [PMC free article] [PubMed] [Google Scholar]