Abstract
Brain-computer interface controlled prosthetic arms can enable people with tetraplegia to perform functional movements. However, vision provides limited feedback as information about grasping objects is best relayed through tactile feedback. We supplemented vision with tactile percepts evoked using a bidirectional brain-computer interface that records neural activity from motor cortex and generates tactile sensations through intracortical microstimulation of somatosensory cortex. This enabled a person with tetraplegia to substantially improve performance with a robotic limb; trial times on a clinical upper-limb assessment were reduced by half, from a median time of 20.9 to 10.2 s. Faster times were primarily due to less time spent attempting to grasp objects, revealing that mimicking known biological control principles results in task performance that is closer to healthy human abilities.
One Sentence Summary:
Creating artificial tactile feedback through intracortical microstimulation greatly improves the ability of a person with tetraplegia to manipulate objects with a brain-controlled robotic limb.
There are approximately 169,000 people in the United States living with tetraplegia due to spinal cord injury (SCI) (1). Of those with cervical SCI, nearly half desire improved arm and hand function over all other rehabilitation priorities (2, 3). Brain-computer interfaces (BCIs) that measure movement-related neural activity with implanted electrodes can restore some of this lost arm and hand function (4–6) as the cortex remains capable of generating neural activity that controls arm and hand motion. BCIs can therefore bypass the injured spinal cord to control prosthetic limbs (4–6), functional electrical stimulation systems (7, 8) or other devices (9, 10).
We previously developed a BCI-controlled robotic arm that enables reaching and grasping movements (6) in up to 10 continuously and simultaneously controlled dimensions (5). This high-dimensional continuous control has enabled participants to complete clinical assessments of upper-limb function such as the Action Research Arm Test (ARAT) (11). However, this BCI control relied on vision alone and lacks a critical sensory dimension. When able-bodied people interact with the environment, tactile feedback from the skin is essential to effectively explore and manipulate objects; without tactile somatosensory feedback, even simple manipulation tasks become clumsy and slow (12, 13).
Neural prosthetics that restore some somatosensation for amputees are becoming more common (14–17). However, these peripheral stimulation approaches cannot translate to individuals with tetraplegia; stimulation below the level of the lesion is unable to relay information to the somatosensory cortex for processing and perception. While stimulation in the somatosensory cortex has long been known to evoke detectable sensations (18), it is only in recent studies of humans with chronically implanted microelectrode arrays that the perceptual characteristics of microstimulation have been elucidated (19, 20). The potential benefits of a bidirectional BCI–a system in which tactile sensations are evoked through cortical stimulation while neural recordings during attempted movement are decoded to control a robotic prosthesis–have remained unexplored in humans.
Here, we show that a bidirectional BCI (Fig. 1A,B) that evokes tactile percepts substantially improves performance on functional tasks. These artificial tactile percepts were driven in real-time by sensors in a robotic hand that responded to object contact and grasp force (Fig. 1C,D, Fig. S1, Fig. S2), were evoked through intracortical microstimulation (ICMS) of area 1 of somatosensory cortex and were experienced as originating from the participant’s own palm and fingers (Fig. S1). This result demonstrates that a neural interface that mimics principles of sensorimotor control can be intuitively used by a person with significant motor impairments.
Fig. 1:
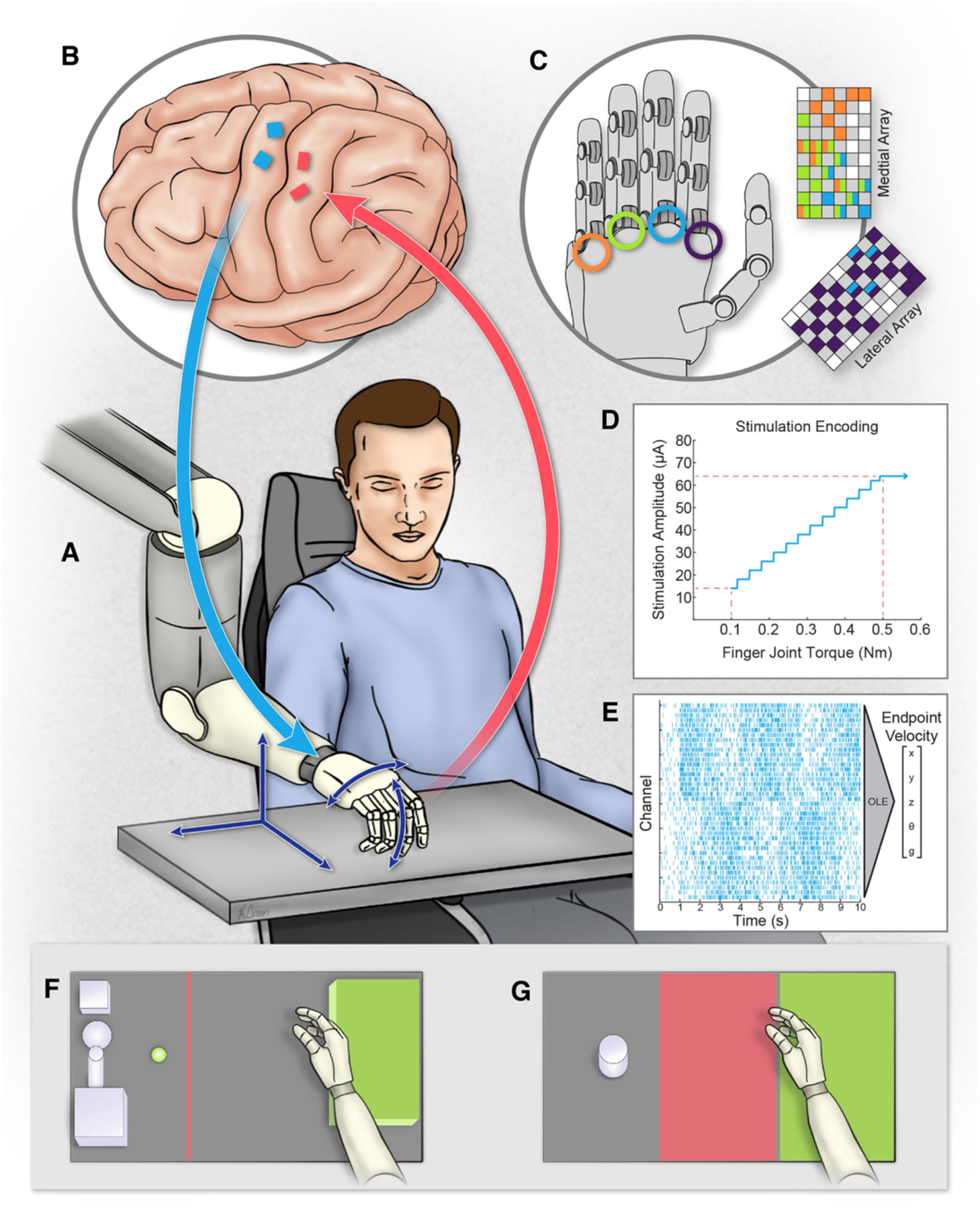
Overview of the bidirectional BCI system. (A) The participant used the intracortical BCI to control a robotic prosthesis in real time, controlling all five dimensions (dark blue arrows) continuously from the start to the end of the trial. (B) Four microelectrode arrays were implanted in the left hemisphere. Arrays in motor cortex (blue) recorded neural activity to control the prosthesis. Arrays in somatosensory cortex (red) delivered stimulation pulses, evoking sensory percepts referred to the hand. (C) Torque measurements from the robotic hand controlled stimulation of individual electrodes. Colored grids (adapted from Flesher et al. 2016) represent electrodes and locations on the hand where stimulation evoked a percept. Index finger torque was used to drive stimulation of the index finger sensation and middle finger torque was used to drive stimulation of electrodes associated with the middle, ring, and pinky finger. (D) Stimulation current amplitude was modulated by torque using a linear transformation. (E) Example raster plot of neural data recorded from motor cortex and decoded into endpoint velocities using an optimal linear estimator. (F) Overhead view of the Action Research Arm Test (ARAT). Different objects (not all shown) were positioned at the presentation location (green dot), grasped, and then placed on the platform (green box) as quickly as possible. (G) Overhead view of the object transfer task showing the object (gray), transit (red) and target (green) zones. (Image Credit: Kenzie Green)
The male participant in this study was 28 years old at the time of device implant and had tetraplegia due to a C5 motor/C6 sensory ASIA B spinal cord injury sustained 10 years prior to providing informed consent (19). This study was conducted under an Investigational Device Exemption from the U.S. Food and Drug Administration and is registered at ClinicalTrials.gov (NCT01894802). Two microelectrode arrays with 88 wired electrodes were implanted in the hand and arm region of motor cortex (Fig. 1B) to decode movement intent (Fig. 1E, see supplemental materials). Two additional microelectrode arrays with 32 wired electrodes were implanted in area 1 (Fig. 1B), which is a cutaneous region of somatosensory cortex (21). Stimulation evoked sensations in the palm and fingers of the participant’s right hand, which he described as having cutaneous qualities (Fig. 1C,D, Fig. S1) (19). We used an observation-based paradigm (6) to train a new 5 degree-of-freedom (DoF) velocity decoder each day (3 DoF hand endpoint, wrist rotation and hand grasp). Stimulation was never delivered during calibration (Fig. S3) and the participant had an unobstructed view of the robotic hand for all trials. Prior to these experiments, the participant had practiced the tasks for approximately two years.
We compared performance with and without ICMS on a modified version of the ARAT (11, 22) (Fig. 1F and Movies S1–S3). The ARAT variant we used involved picking up one of eight objects and placing each one on a platform as quickly as possible. We also included a ninth task from the ARAT set: picking up a cup of water, pouring its contents into another cup, and setting the cup back down, upright, on the table. Each object was attempted three times in each session. The trials were timed, scored from 0–3 (see supplementary material), and the highest scores for each object were summed together to produce the ARAT score; the maximum score was 27. In the first four sessions with ICMS-induced sensations driven by robotic touch, he achieved a median ARAT score of 21. This was significantly higher than the median score of 17 achieved during the next four sessions without ICMS-evoked tactile sensations (p = 0.029, Wilcoxon rank-sum test, Table 1, Fig. 2A) as well as 23 sessions conducted during the 23 months prior to these experiments (p = 0.005, Wilcoxon rank-sum test, Table 1, Fig. 2A). The scores in the four experimental sessions without ICMS were no different than the prior 23 sessions (p = 0.65, Wilcoxon rank-sum test). Previous ARAT sessions included four exploratory trials with ICMS-driven tactile feedback (Fig. 2A), however, stimulation parameters were variable and trials with and without ICMS were intermixed, rather than occurring in a blocked design (Fig. S3), making the feedback unreliable.
Table 1:
Performance metrics for each task per experiment day. ARAT scores were computed as the sum of the best score per object, with a maximum score of 27. Each of the nine objects was attempted 3 times, so that the maximum number of trials attempted per session was 27. The median and inter-quartile (IQR) trial time for successful ARAT trials are shown for each session. The median and IQR trials times for all successful trials were calculated by pooling trial times across all four sessions per feedback condition and calculating the median and IQR from the aggregate distribution. The total number of object transfers is the sum of all five two-minute trials per day.
| Session | ARAT Score (out of 27) | ARAT Trials Completed (out of 27) | Median and IQR trial time for Successful ARAT Trials (s) | Object Transfer (transfers per day) | |
|---|---|---|---|---|---|
| With ICMS Feedback | 1 | 21 | 19 | 11.9 (6.6 – 27.7) | 97 |
| 2 | 21 | 22 | 12.0 (5.6 – 38.9) | 74 | |
| 3 | 21 | 21 | 8.8 (6.0 – 17.2) | 93 | |
| 4 | 20 | 19 | 8.1 (4.6 – 11.9) | 88 | |
|
|
|||||
| Summary | Median: 21 | Median: 20 | Median of all trials: 10.2 (5.5 – 18.1) | Total: 352 | |
|
| |||||
| Without ICMS Feedback | 1 | 19 | 23 | 14.0 (11.1 – 30.9) | 88 |
| 2 | 16 | 19 | 27.6 (18.8 – 37.2) | 55 | |
| 3 | 17 | 23 | 18.7 (12.3 – 41.7) | 74 | |
| 4 | 17 | 13 | 40.5 (15.5 – 48.4) | 98 | |
|
|
|||||
| Summary | Median: 17 | Median: 21 | Median of all trials: 20.9 (13.1 – 40.5) | Total: 315 | |
Fig. 2:
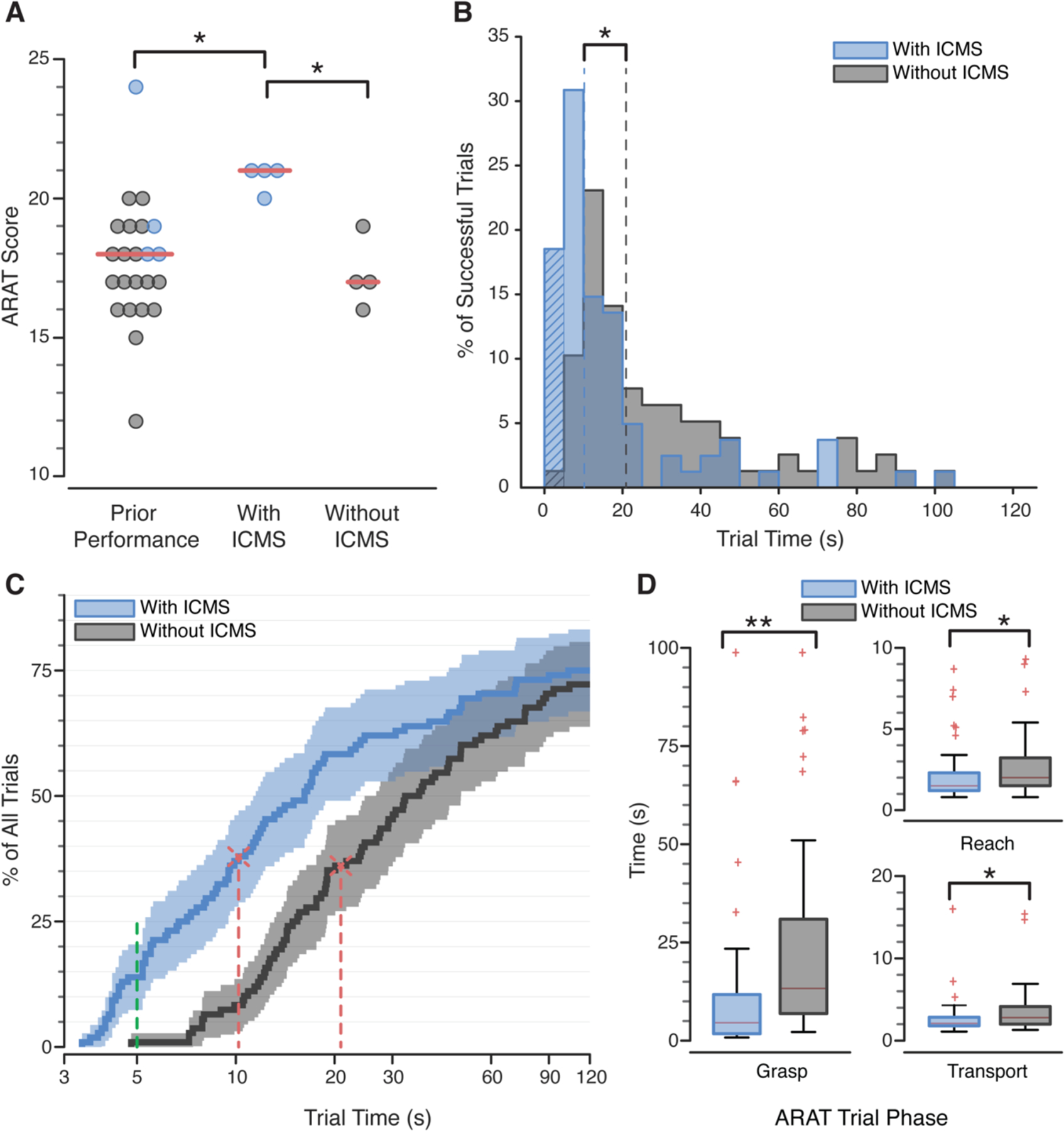
Effect of ICMS on ARAT performance. (A) ARAT scores when ICMS feedback was provided were significantly better than prior ARAT scores (*p = 0.005), which occasionally employed ICMS feedback (blue dots), and to data from the current experiment without ICMS feedback (*p = 0.029). Red lines indicate median scores. (B) Histogram of successful trial times completed with (blue) and without (gray) ICMS tactile feedback. Median trial times (dotted lines) were significantly faster with ICMS (*p < 0.0001). Hatched bars represent trials completed in under five seconds. (C) Empirical cumulative distribution of individual trial times, including failed trials, shown on a log-normalized axis. Vertical red lines indicate when 50% of successful trials were completed. Data to the left of the vertical green line represent trials completed in under five seconds. Shading indicates the 95% confidence bounds, calculated with Greenwood’s formula. (D) Amount of time spent in each phase of the ARAT task. Red lines are medians, box outlines are interquartile ranges, and whiskers are the range of the data excluding outliers (red ‘+’). All task phases were faster when ICMS feedback was provided (*p<0.001, **p<0.0001, Table S2). (A-D) Significance was assessed with a Wilcoxon rank-sum test.
ARAT scores improved because individual trials were completed much faster when ICMS feedback was delivered (Fig. 2B,C, Movie S2). In the ARAT scoring system, successfully transferring an object in less than five seconds (a score of three) is considered able-bodied performance (22). Without tactile sensations, this score was achieved only once during all 108 trials. When tactile sensations were provided, a score of three was achieved 15 times. Further, 14% of the trials with tactile feedback were completed more quickly than the very fastest trial without ICMS (Fig. 2C). Times for successfully completed trials decreased by 51.2%, from a median of 20.9 s to 10.2 s, when tactile feedback was provided (p < 0.0001, Wilcoxon rank-sum test, Table 1, Fig. 2B,C and Movie S3). These faster completion times were the cause of the 3.5-point improvement in the ARAT score that occurred when ICMS was provided and can be interpreted to mean that ICMS-induced tactile sensations allowed 3.5 more objects, out of 9 possible objects, to be transported to the platform in a normal time (< 5 seconds). While the performance gains were not the same for every object, completion times improved significantly for more than half of the ARAT objects, and the median completion times were lower in all cases (Fig. S4, Table S1). The objects with the largest improvements were rated by the participant as being the easiest to manipulate within the constraints of the robotic hand. Some of the smaller objects were difficult to pick up given the grasping kinematics of the fingers; the tips of the fingers often made contact with the object rather than the pads of the distal phalanx, making grasp less stable. Further, the participant only had control over one grasp dimension that opened and closed all of the fingers as a group. There was no change in the total number of successfully completed trials with or without ICMS (p = 0.83, Wilcoxon rank-sum, Table 1).
The median time spent attempting to grasp an object – defined as the period of time between object contact and object liftoff – decreased by 66% from 13.3 s without ICMS to 4.6 s with ICMS (p < 0.0001, Wilcoxon rank-sum test, Table S2, Fig. 2D) and accounted for 88% of the total improvement. The median time spent in the reaching and transport phases (see supplementary material) both decreased by 25% with ICMS. Once the participant had successfully grasped an object, he rarely dropped it. This occurred just four times with ICMS and five times without. All the drops resulted from an unstable grasp rather than the participant opening the hand.
We also tested the effect of providing ICMS-induced tactile feedback on functional performance using an object transfer task (Fig. 1G, Movie S4). In this task, the participant was asked to pick up an object from the left side of the workspace (object zone), carry it across the table (transit zone), and drop it on the right side (target zone) as many times as possible in two minutes. Four sessions without ICMS and four sessions with ICMS were conducted (Fig. S3) and this task was repeated five times per session. The time spent in the object zone (Fig. 1G) decreased by 30.3%, from 3.3 ± 1.2 s per transfer without ICMS to 2.3 ± 0.4 s per transfer with ICMS (p = 0.002, t-test, Fig. 3A). With ICMS, the participant moved the prosthetic hand significantly less in the object zone (32.4 ± 5.9 cm/transfer with ICMS, 44.2 ± 13.1 cm/transfer without ICMS, p = 0.0007, t-test, Fig. 3B) and spent less time in the immediate vicinity of the object (Fig. 3C). The time spent in the target zone also decreased while the time spent in the transit zone was unaffected (Fig. 3A, Table S3). Overall, 352 transfers were completed with ICMS while 315 transfers were completed without ICMS (Table 1) and the number of transfers increased from 15.8 ± 3.8 transfers per two-minute trial to 17.8 ± 2.4 transfers per trial with ICMS, though this difference was not statistically significant (p = 0.050, t-test).
Fig. 3:
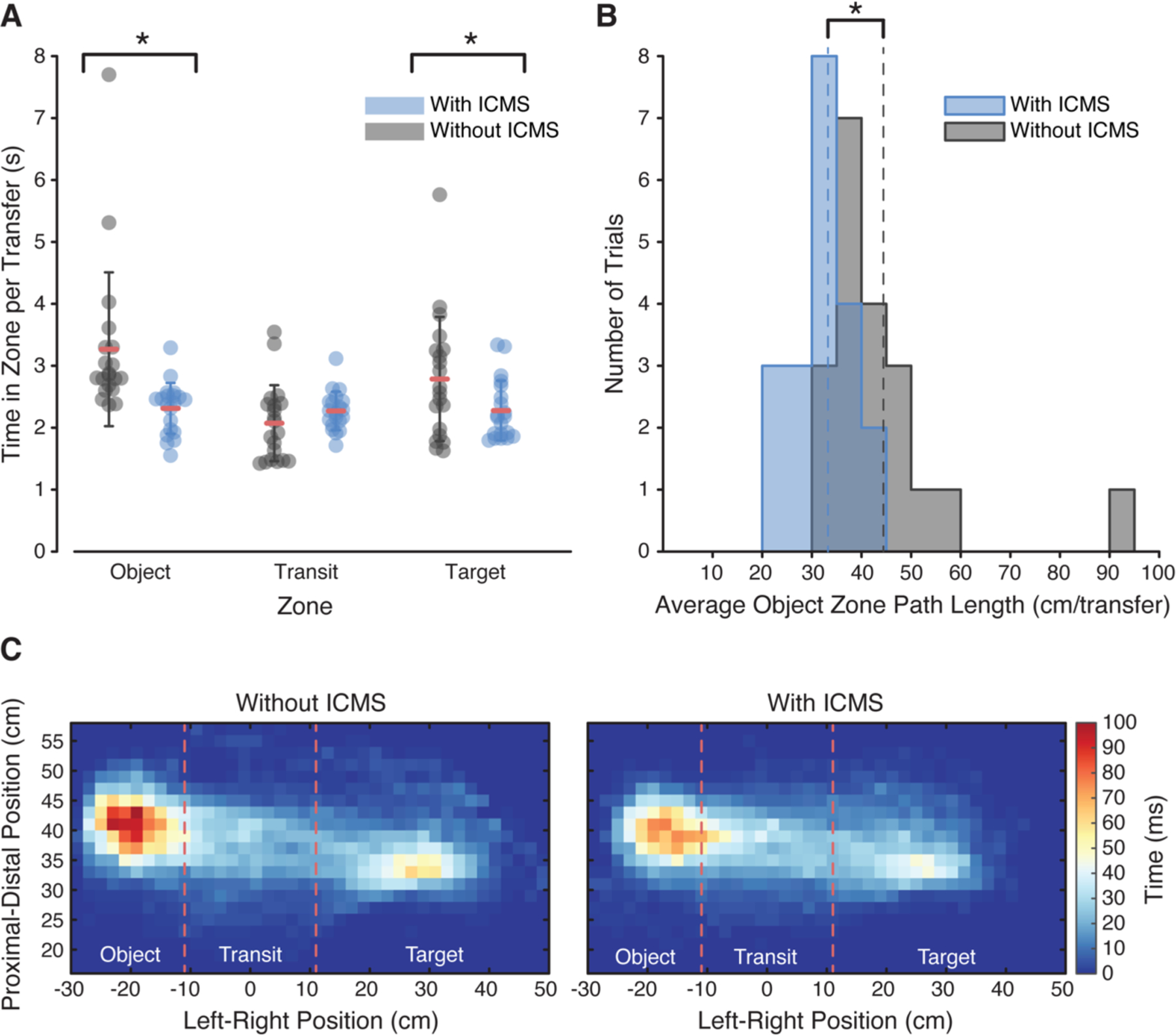
Effect of ICMS on object transfer performance. (A) Amount of time spent in each task zone, per transfer, by feedback condition (n = 20 trials per feedback condition). Data for all trials are shown with the mean value indicated by the red lines and the whiskers indicating one standard deviation. The amount of time spent in the object and target zones decreased significantly with ICMS feedback (*p = 0.002 and 0.048, t-test, respectively). (B) Distribution of average path lengths in the object zone per trial for the two feedback conditions, computed as the total path length divided by the number of transfers. Mean path length decreased with ICMS feedback (*p = 0.0007, t-test). (C) Spatial map of the average amount of time spent in each location in the workspace per transfer. Each individual square represents a 2 x 2 cm region of the workspace. The color indicates the average amount of time spent in each location per transfer. Without stimulation, more time was spent near the object in the object zone as shown by the darker red colors in the object zone. Red lines indicate zone boundaries.
Because differences in neural decoder performance could lead to differences in the ability to control the arm, it is possible that the improvements on days with artificial tactile feedback were due simply to the decoder. We therefore used a random target sequence task (6) each day to measure how well the participant could independently control each DoF. This task was always performed without ICMS (Fig. S3). On days when functional tasks were completed with ICMS, fewer target sequence trials were successfully completed (Table S4). The median endpoint translation velocity of the robot during the sequence task was also slightly slower (Table S4), suggesting that the decoder performance itself–and thus the participant’s ability to control the robotic arm–was not biased towards better performance on days with ICMS.
Creating artificial tactile feedback using a bidirectional BCI substantially improved functional performance during reaching and grasping tasks compared to a motor-only BCI with visual feedback. In contrast to many studies where the effects of artificial sensations on task performance are measured without visual or auditory feedback (14, 15, 23–27), our aim was to determine whether a bidirectional BCI would improve performance on tasks that were already possible with existing sensory modalities, namely vision. This bidirectional BCI taps into cortical sensorimotor systems that remain intact after injury, allowing a person with chronic tetraplegia to, in some cases, perform functional tasks at able-bodied speeds.
This better performance was driven primarily by reducing the time spent attempting to grasp objects. Indeed, in the ARAT task, the grasp time was cut by more than half with ICMS, accounting for 88% of the overall time reduction. Without tactile sensations, the participant spent more time placing the hand in a position that would ensure a stable grasp. Small performance gains also occurred during the reaching phase when ICMS was not delivered. This improvement suggests that the participant approached the task with increased confidence and speed when he knew that ICMS-evoked tactile sensations would signal object contact. Similar effects were observed during the object transfer task; shorter path lengths near the object indicate fewer corrective movements and increased confidence.
As with any single-subject study, it is not guaranteed that these findings will generalize to future experiments and participants. However, there are several reasons to believe that these results indicate the potential of restoring somatosensory percepts using ICMS in a bidirectional BCI. First, using the same fundamental neural decoding and control methods, two participants have achieved similar scores on functional tasks with vision alone (5, 6, 28). With the current participant, these scores were only exceeded when ICMS-evoked tactile feedback was provided (Fig. 2A), suggesting that without artificial tactile feedback, control is impaired, much as it is when tactile sensations are absent in people with otherwise normal motor control capabilities (13, 29). Second, performance improvements were driven primarily by reductions in the time taken to successfully grasp an object (Fig. 2D, Fig. 3A). State transitions, such as object contact (30) during grasping, are uniquely encoded by tactile feedback in the intact nervous system. That the percepts signaled these state transitions with high temporal accuracy, and enabled the participant to grasp objects more quickly, suggests that ICMS delivered to area 1 of somatosensory cortex improves task performance in a way that is congruent to the way natural cutaneous feedback improves grasp performance. In a similar way, behaviorally relevant state transitions also occur during object release (30), potentially explaining the slightly improved times during the transport phase in the ARAT task and the time spent in the target zone in the object transfer task. Finally, when ICMS-induced percepts were provided after two years of consistent ARAT scores, performance improved significantly, and when they were removed, performance returned to pre-ICMS levels (Fig. 2A). These observations suggest that the improvements were primarily due to the addition of reliable sensory information, rather than the result of additional practice. This immediate performance improvement also demonstrates that ICMS in somatosensory cortex was not akin to sensory substitution cues that could have been provided by electrical or mechanical stimulation of intact skin or audio or visual cues, as the relationship between these cues and behavior must be learned (31). This learning requirement and other factors related to attentional load have limited the impact of sensory substitution in real-world scenarios (32). The immediate improvements that we observed with ICMS in area 1 also demonstrates the benefits of providing intuitive feedback. In contrast, ICMS feedback in a relevant area of cortex, but in an unintuitive way, requires considerable learning time (thousands of trials) to use effectively (33).
In this study, we chose one sensory encoding scheme that leveraged two capabilities of ICMS – variable intensity and multiple focal percepts (19) – to provide proportional feedback that evoked sensations localized to individual fingers (Fig. S1, Fig. S2). Future work should examine how the stimulation encoding design (e.g., proportional vs. on-off) may impact performance across a wide range of tasks. For example, the participant rarely dropped any of the objects once they were successfully grasped. However, many of the objects were rigid and there was no penalty for grasping the objects too firmly. Tasks that involve fragile objects or more precise control of hand posture and grasp force could be more dependent on specific sensory encoding schemes.
Ultimately, ICMS-induced tactile percepts improved task performance to levels never previously observed, decreased the time spent reaching and grasping in ways that were analogous to the role of natural tactile sensations during grasp state transitions, and do not appear to be the result of practice. That artificial tactile sensations substantially improved performance demonstrates that engineered approaches that mimic known sensorimotor circuits–albeit imperfectly at present–will have a major impact on the future performance of BCIs. This is particularly significant for individuals with conditions such as SCI where the peripheral nervous system is no longer intact.
Supplementary Material
Acknowledgements
We thank N. Copeland for his continuing and extraordinary commitment to this study as well as insightful discussions with the study team; Debbie Harrington for regulatory management of the study; Ahmed Jorge for help with data collection; Peter Gibson and Ben Clarkson for video data processing; the University of Pittsburgh Clinical and Translational Science Institute and the Office of Investigator-Sponsored Investigational New Drugs and Investigational Device Exemption support for assistance with protocol development and regulatory reporting and compliance; the volunteer members of the Data Safety and Monitoring Board for their continued monitoring of this study; H. Jourdan for financial and organizational support; Blackrock Microsystems (Salt Lake City, UT, USA), especially Robert Franklin, for technical support related to this project; and Krishna Shenoy for helpful discussions and insights regarding data interpretation.
Funding:
This material is based upon work supported by the Defense Advanced Research Projects Agency (DARPA) and Space and Naval Warfare Systems Center Pacific (SSC Pacific) under Contract No. N66001-16-C-4051 and the Revolutionizing Prosthetics program (Contract No. N66001-10-C-4056). SNF was supported by an NSF Graduate Research Fellowship under grant number DGE-1247842. The views, opinions, and/or findings contained in this article are those of the authors and should not be interpreted as representing the official views or policies of DARPA, SSC Pacific, or the US Government.
Footnotes
Competing interests: The authors declare no competing interests.
Data and materials availability: All data are available in the manuscript or the supplementary materials.
References and Notes
- 1.“National Spinal Cord Injury Statistical Center, Facts and Figures at a Glance” (Birmingham, AL, 2018). [Google Scholar]
- 2.Anderson KD, J. Neurotrauma 21, 1371–83 (2004). [DOI] [PubMed] [Google Scholar]
- 3.Collinger JL et al. , J. Rehabil. Res. Dev 50, 145–60 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hochberg LR et al. , Nature 485, 372–5 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Wodlinger B et al. , J. Neural Eng 12, 016011 (2015). [DOI] [PubMed] [Google Scholar]
- 6.Collinger JL et al. , Lancet (London, England) 381, 557–64 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bouton CE et al. , Nature 533, 247–50 (2016). [DOI] [PubMed] [Google Scholar]
- 8.Ajiboye AB et al. , Lancet (London, England) 389, 1821–1830 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hochberg LR et al. , Nature 442, 164–71 (2006). [DOI] [PubMed] [Google Scholar]
- 10.Pandarinath C et al. , Elife 6, e18554 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lyle RC, Int. J. Rehabil. Res 4, 483–92 (1981). [DOI] [PubMed] [Google Scholar]
- 12.Monzée J, Lamarre Y, Smith AM, J. Neurophysiol 89, 672–83 (2003). [DOI] [PubMed] [Google Scholar]
- 13.Rothwell JC et al. , Brain 105 (Pt 3), 515–42 (1982). [DOI] [PubMed] [Google Scholar]
- 14.Raspopovic S et al. , Sci. Transl. Med 6, 222ra19 (2014). [DOI] [PubMed] [Google Scholar]
- 15.Wendelken S et al. , J. Neuroeng. Rehabil 14, 121 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Dhillon GS, Lawrence SM, Hutchinson DT, Horch KW, J. Hand Surg. Am 29, 605–15; discussion 616–8 (2004). [DOI] [PubMed] [Google Scholar]
- 17.Graczyk EL, Resnik L, Schiefer MA, Schmitt MS, Tyler DJ, Sci. Rep 8, 9866 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Penfield W, Boldrey E, Brain 60, 389–443 (1937). [Google Scholar]
- 19.Flesher SN et al. , Sci. Transl. Med 8, 361ra141 (2016). [DOI] [PubMed] [Google Scholar]
- 20.Salas MA et al. , Elife 7 (2018), doi: 10.7554/eLife.32904. [DOI] [Google Scholar]
- 21.Delhaye BP, Long KH, Bensmaia SJ, Compr. Physiol 8, 1575–1602 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Yozbatiran N, Der-Yeghiaian L, Cramer SC, Neurorehabil. Neural Repair 22, 78–90 (2008). [DOI] [PubMed] [Google Scholar]
- 23.Schiefer M, Tan D, Sidek SM, Tyler DJ, J. Neural Eng 13, 016001 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Graczyk EL et al. , Sci. Transl. Med 8, 362ra142 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Klaes C et al. , J. Neural Eng 11, 056024 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.O’Doherty JE et al. , Nature 479, 228–31 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lee B et al. , Front. Syst. Neurosci 12, 24 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Downey JE et al. , Sci. Rep 7, 16947 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Johansson RS, Riso R, Häger C, Bäckström L, Exp. Brain Res 89, 181–191 (1992). [DOI] [PubMed] [Google Scholar]
- 30.Johansson RS, Flanagan JR, Nat. Rev. Neurosci 10, 345–59 (2009). [DOI] [PubMed] [Google Scholar]
- 31.Stronks HC, Nau AC, Ibbotson MR, Barnes N, Brain Res 1624, 140–152 (2015). [DOI] [PubMed] [Google Scholar]
- 32.Loomis JM, Klatzky RL, Giudice NA, in Assistive Technology for Blindness and Low Vision (2012).
- 33.Dadarlat MC, O’Doherty JE, Sabes PN, Nat. Neurosci 18, 138–44 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


