Abstract
Purpose
To examine (1) the trend and associated factors of Oncotype DX (ODX) use among hormone receptor-positive (HR+) breast cancer (BC) patients in 2004–2015; (2) the trend of reported chemotherapy by Recurrence Score (RS); and (3) the survival differences associated with ODX use.
Methods
ODX data from Genomic Health Inc. were linked with 17 SEER registries data. HR + BC cases with lymph node negative (N0) or 1–3 positive LNs (N1) from 2004–2015 were analyzed. The Cochrane-Armitage trend test, logistic regression, Kaplan–Meier survival curve, and stratified Cox model were performed. Survival analysis was restricted to HR+/HER2− patients from 2010 to 2014, matched on propensity score.
Results
ODX use increased substantially from 2004 to 2015 (N0: 2.0% to 42.7%; N1: 0.3% to 27.9%). Non-Hispanic black and Medicaid insured patients had lower odds of receiving ODX. N0 patients with moderately differentiated or 2.1–5.0 cm tumor and N1 patients with well-differentiated or < 2.0 cm tumor had higher odds of using ODX. The reported chemotherapy use decreased significantly with low and intermediate RS, and increased for high RS among N0 patients. ODX use was associated with better breast cancer-specific survival [hazard ratio (95% CI) N0 1.96 (1.60–2.41), N1 1.90 (1.42–2.54)] and overall survival [N0 2.06 (1.83–2.31), N1 1.72 (1.42–2.09)], especially in the first 36 months.
Conclusion
ODX use has increased significantly since 2004, nonetheless disparities remain, especially for racial/ethnic minorities and Medicaid insured patients. Administering chemotherapy based on ODX results has been improved among N0 patients. Patients receiving ODX had better survival than those not.
Keywords: Oncotype DX, Breast cancer, Trend, Survival
Introduction
Over the past few decades, precision medicine has made substantial progress in breast cancer treatment. Estrogen receptor (ER), progesterone receptor (PR), and human epidermal growth factor receptor 2 (HER2) have been used to classify breast cancer into subtypes which can benefit from different treatment. The most common subtype is hormone receptor positive (HR+), i.e., ER+ and/or PR+, and HER2−, which has the best prognosis and used to be treated uniformly with adjuvant endocrine and chemotherapy. It then has been found that not every patient with this subtype has the same recurrence risk or receives the equal benefit from chemotherapy. Compared to the traditional clinicopathologic factors such as grade or tumor size, certain gene expression profiling tools provide higher precision in differentiating patients within this subgroup.
The Oncotype DX Recurrence Score assay® (ODX) is a 21-gene expression assay which was originally developed to quantify the risk of distant recurrence among ER+ and HER2− breast cancer patients with negative axillary lymph node (N0) [1, 2]. It then has been validated to identify N0 patients who can benefit from chemotherapy [3]. Since 2008, National Comprehensive Cancer Network (NCCN) guidelines recommend ODX for ER+ /PR+ and HER2− N0 patients [4]. Chemotherapy was required only if ODX indicates a high Recurrence Score (RS). Subsequently, similar predictive effect of ODX has been found among patients with 1–3 positive ipsilateral axillary lymph nodes (N1) [5, 6] and NCCN guidelines were revised to extend ODX to N1 patients beginning in 2015.
With the increasing recommendation of ODX in clinical practice, several studies examined the use of ODX among breast cancer patients using hospital-based datasets in a relatively short time period [7–10]. It is important to understand how the trend of ODX utilization changes and how ODX utilization influences the use of chemotherapy over time, using data from population-based cancer registries. More importantly, despite the widespread use of ODX, no population-based study investigated whether the utilization of ODX influences the survival of HR+ and HER2− breast cancer patients. This study aimed to (1) examine the trend of ODX utilization for HR+ and N0 or N1 breast cancer cases between 2004 and 2015, as well as the factors associated with ODX use; (2) investigate the trend of having reported chemotherapy in patients with low, intermediate, high RS, and those not receiving ODX; and (3) compare breast cancer-specific survival (BCSS) and overall survival (OS) between patients who had test done and those who did not.
Methods
Data source and study population
The Surveillance, Epidemiology, and End Results (SEER) Program of the National Cancer Institute (NCI) is a population-based cancer surveillance program, collecting cancer incidence, and survival data [11]. Genomic Health Inc. (Redwood City, CA) is the sole ODX testing provider in the US. Data on ODX testing and results from Genomic Health Inc. were linked with 2004–2015 breast cancer incidence data from 17 SEER registries. The patients were followed up through the end of 2015. Details of the data linkage have been described elsewhere [12].
For objective 1, the following eligibility criteria were applied: (1) female patients diagnosed with American Joint Committee on Cancer (AJCC) stage I-III breast cancer between 2004 and 2015; (2) with ER+ /PR+; (3) received mastectomy or lumpectomy; (4) N0 with > 0.5 cm tumor or N1 with any known tumor size; and (5) cases that are not with tubular or mucinous histology (histology ICD-O-3: 8211, 8480, 8481) and not identified from death certificate or autopsy. For objective 2, proportion of chemotherapy was examined among patients having low RS, intermediate RS, high RS, and not receiving ODX over the time: N0 patients in 2004–2015 or N1 patients in 2007–2015 (very small case count of N1 patients having intermediate or high RS in 2004–2006). For objective 3, additional eligibility criteria applied on the analytic dataset for objective 1: (1) HR+ and HER2− patients diagnosed between 2010 and 2014 (data on HER2 status were available only for cases diagnosed in 2010 and after); (2) breast cancer as the only tumor; (3) patients having RS available or not receiving ODX (patients who received ODX but RS not available were excluded because they cannot receive enough survival benefit from the test receipt); (4) cases with tumor size > 5.0 cm or unknown grade excluded due to small case count. Detailed information and rationales of patient selection were described in Supplementary Fig. 1.
Variables
Patients who received ODX included those who had ODX ordered but did not receive test results. Patients receiving ODX within 12 months after cancer diagnosis were considered as receiving the test. RS was categorized based on its original cutoff points (low: < 18, intermediate: 18–30, and high: ≥ 31) and TAILORx cutoff points (low: < 11, intermediate: 11–25, and high: ≥ 26). Survival outcomes included BCSS and OS (in months). For BCSS, patients who died from causes other than breast cancer or alive at the end of follow-up were censored; patients who died from unknown reason were coded as missing (excluded from the analysis of BCSS). All analyses were stratified by lymph node status: N0 and N1.
Other variables included age at diagnosis (< 50, 50–59, 60–69, 70–79, ≥ 80), race/ethnicity (non-Hispanic white (NHW), non-Hispanic black (NHB), non-Hispanic American Indian/Alaska Native (NHAI/AN), non-Hispanic Asian or Pacific Islander (NHAPI), Hispanics, non-Hispanic unknown race), marital status (married, single, other, unknown), insurance type (insured, Medicaid, insured but insurance type not specified, uninsured, unknown), tumor grade (well differentiated, moderately differentiated, poorly differentiated/undifferentiated, unknown), tumor size (0.5–2.0 cm for N0 or ≤ 2.0 cm for N1, 2.1–5.0 cm, > 5.0 cm), surgery and reported radiation (mastectomy with no/unknown radiation, mastectomy plus radiation, lumpectomy with no/unknown radiation, lumpectomy plus radiation), reported chemotherapy (yes, no/unknown), and SEER state/area. Insurance type was available since 2007 thus included in the analyses of data from 2007 and after [13]. Surgery type and reported radiation were combined into a new variable because of the interaction between these two variables on ODX use. As 4 California cancer registries and 3 Georgia cancer registries were combined into state California and state Georgia, respectively, variable SEER state/area included 10 states (California, Connecticut, Georgia, Hawaii, Iowa, Kentucky, Louisiana, New Jersey, New Mexico, and Utah) and 2 metropolitan areas (Detroit and Seattle).
Statistical analysis
Chi-square test was applied to compare the distribution of covariates between patients who received ODX and those who did not. Cochrane-Armitage trend test was conducted for trend analysis [14]. Because large sample size could easily result in highly significant estimates and this study had a very large sample size when all years data were combined together, to identify factors significantly associated with the test order, multivariable logistic regression was conducted in the data from each year separately.
To examine the survival benefit from ODX use in the overall patient population and in the subpopulations defined by tumor characteristics, patients were first stratified by tumor characteristics and diagnosis year. Within each stratum, propensity score was calculated using logistic regressions with logit function [15] based on age at diagnosis, race/ethnicity, marital status, insurance, surgery, and reported radiation. Among cases with propensity score 0.1–0.9 within each stratum, those having RS were 1:1 matched with those not receiving ODX based on propensity score [15]. Then the matched patients from each stratum were combined together. Details of propensity score matching were described in Supplementary Fig. 1c. Kaplan–Meier survival curves and stratified Cox proportional hazards models were performed in the combined groups. Because the proportional hazard assumption was violated for some models for N0 patients, heaviside function of Cox model was conducted [16]. Therefore, in addition to one hazard ratio (HR) for the entire follow-up time, two additional HRs were reported for each Cox model: one for follow-up time 0–36 months and one for 37–72 months.
Results
Patient characteristics and trend of Oncotype DX use
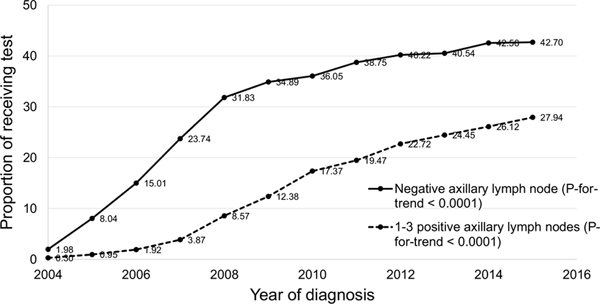
Of 288,684 N0 and 101,712 N1 eligible cases diagnosed between 2004 and 2015, 31.5% and 14.8% received ODX, respectively (Table 1). The majority of patients were NHW, married, insured, or having moderately differentiated tumor. Among N0 patients, those at younger age, married, having moderately differentiated tumors were more likely to receive ODX (P < 0.0001). Among N1 patients, those aged 60–69, married, having well-differentiated or ≤ 2.0 cm tumors had higher proportion of test order (P < 0.0001). From 2004 to 2015, the proportion of ODX use increased steadily from 2.0% to 42.7% among N0 patients and from 0.3 to 27.9% among N1 patients (P-for-trend < 0.0001 for each) (Fig. 1). The proportion also increased gradually among patient subpopulations defined by sociodemographic or tumor characteristics (Supplementary Tables 1 and 2).
Table 1.
Patient characteristics and the proportion of receiving Oncotype DX test by patient characteristics, hormone receptor-positive breast cancer patients from 17 SEER registries, 2004–2015 (N = 390,396)
| N0 (N = 288,684) |
N1 (N = 101,712) |
|||
|---|---|---|---|---|
| % | % of patients with ODX | % | % of patients with ODX | |
|
| ||||
| All | 31.47 | 14.76 | ||
| Age | P < 0.0001 | P < 0.0001 | ||
| < 50 | 17.41 | 41.16 | 27.77 | 10.92 |
| 50–59 | 22.64 | 42.10 | 26.24 | 15.75 |
| 60–69 | 27.48 | 37.03 | 24.02 | 19.50 |
| 70–79 | 20.64 | 20.05 | 14.63 | 17.52 |
| ≥ 80 | 11.83 | 3.86 | 7.34 | 4.75 |
| Race/ethnicity | P < 0.0001 | P < 0.0001 | ||
| NH white | 75.11 | 31.32 | 70.03 | 15.63 |
| NH black | 7.69 | 32.05 | 10.03 | 12.28 |
| NH American Indian/Alaska Native | 0.39 | 31.66 | 0.44 | 14.77 |
| NH Asian or Pacific Islander | 7.73 | 32.55 | 7.89 | 13.69 |
| Hispanic (all races) | 8.69 | 31.08 | 11.23 | 12.31 |
| NH unknown race | 0.38 | 35.50 | 0.38 | 16.15 |
| Marital status | P < 0.0001 | P < 0.0001 | ||
| Married | 55.64 | 35.40 | 57.25 | 15.74 |
| Single | 12.48 | 33.43 | 14.25 | 14.03 |
| Other | 27.52 | 22.64 | 24.47 | 12.91 |
| Unknown | 4.36 | 31.44 | 4.03 | 14.80 |
| Insurance type (2007–2015) | P < 0.0001 | P < 0.0001 | ||
| Insured | 75.01 | 38.17 | 71.99 | 19.42 |
| Medicaid | 8.48 | 33.34 | 11.63 | 13.49 |
| Insured but insurance type not specified | 13.74 | 34.76 | 13.14 | 18.76 |
| Uninsured | 1.08 | 39.48 | 1.67 | 15.27 |
| Unknown | 1.69 | 38.25 | 1.57 | 19.12 |
| Tumor grade | P < 0.0001 | P < 0.0001 | ||
| Well differentiated | 28.14 | 29.43 | 16.85 | 22.50 |
| Moderately differentiated | 48.53 | 34.48 | 49.37 | 16.53 |
| Poorly differentiated/undifferentiated | 20.46 | 28.15 | 30.48 | 8.26 |
| Unknown | 2.86 | 24.14 | 3.30 | 8.91 |
| Tumor size, cm | P < 0.0001 | P < 0.0001 | ||
| 0.6–2.0 for N0 or ≤ 2.0 for N1 | 74.33 | 32.21 | 47.29 | 19.45 |
| 2.1–5.0 | 23.29 | 30.59 | 44.00 | 11.54 |
| > 5.0 | 2.38 | 16.84 | 8.71 | 5.60 |
| Surgery & reported radiation | P < 0.0001 | P < 0.0001 | ||
| Mastectomy, no/unknown radiation | 32.28 | 30.39 | 36.43 | 13.16 |
| Mastectomy, radiation | 2.76 | 24.94 | 18.47 | 8.63 |
| Lumpectomy, no/unknown radiation | 16.79 | 20.86 | 12.65 | 13.03 |
| Lumpectomy, radiation | 48.17 | 36.27 | 32.44 | 20.73 |
| Reported chemotherapy | P < 0.0001 | P < 0.0001 | ||
| Yes | 23.73 | 28.89 | 63.20 | 8.12 |
| No/unknown | 76.27 | 32.27 | 36.80 | 26.17 |
| State/area | P < 0.0001 | P < 0.0001 | ||
| California | 41.17 | 26.00 | 40.97 | 13.23 |
| Connecticut | 5.59 | 34.85 | 4.81 | 16.18 |
| Georgia | 10.18 | 38.38 | 10.84 | 15.98 |
| Hawaii | 1.92 | 38.04 | 1.71 | 18.34 |
| Iowa | 4.02 | 27.20 | 3.80 | 13.43 |
| Kentucky | 5.37 | 30.29 | 4.96 | 13.21 |
| Louisiana | 4.46 | 34.31 | 4.84 | 15.36 |
| Detroit | 5.02 | 39.25 | 5.24 | 17.10 |
| New Jersey | 11.26 | 41.52 | 11.41 | 17.98 |
| New Mexico | 2.00 | 41.27 | 2.09 | 21.05 |
| Utah | 2.20 | 27.31 | 2.66 | 9.33 |
| Seattle | 6.81 | 27.24 | 6.68 | 14.59 |
| Recurrence score (original cutoff points)a | – | – | ||
| Low (< 18) | 56.52 | – | 59.36 | – |
| Intermediate (18–30) | 35.74 | – | 34.08 | – |
| High (≥ 31) | 7.74 | – | 6.56 | – |
| Recurrence score (TAILOXr cutoff points)a | – | – | ||
| Low (< 11) | 20.52 | – | 21.72 | – |
| Intermediate (11–25) | 64.29 | – | 64.98 | – |
| High (≥ 26) | 15.19 | – | 13.30 | – |
N0 with negative axillary lymph node, N1 with 1–3 positive ipsilateral axillary lymph nodes, ODX Oncotype DX, NH non-Hispanic
Among patients who had Recurrence Score
Fig. 1.
Trend of receiving Oncotype DX test among female hormone receptor-positive breast cancer patients, 17 SEER registries, 2004–2015. (ODX Oncotype DX, N0 with negative lymph node, N1 with 1–3 positive lymph nodes)
Factors associated with Oncotype DX use
Table 2 shows the adjusted odds ratio (aOR) of ODX use in 2015 for sociodemographic and clinical factors. Regardless of lymph node status, patients aged 80 years or older, NHB, with other or unknown marital status, having Medicaid insurance, or having poorly differentiated/undifferentiated or sized > 5.0 cm tumors were less likely to use ODX than their counterparts. Compared to patients aged 60–69 years, patients younger than 50 had 32% higher odds of test order if with N0 tumor, but 27% lower odds of test order if with N1 tumor. N0 patients with moderately differentiated or 2.1–5.0 cm tumors had 31% or 15% higher odds of test order than their counterparts with well-differentiated or 0.6–2.0 cm tumors. In contrast, among N1 patients, well-differentiated or ≤ 2.0 cm tumors were associated with higher odds of test order. Compared to patients receiving mastectomy with no/unknown radiation therapy, patients receiving lumpectomy plus radiation had higher odds of test use regardless of lymph node status. New Jersey had the highest odds and Utah had the lowest odds of test order among 12 states/areas for both N0 and N1 patients.
Table 2.
Adjusted odds ratio (95% confidence interval) for receiving Oncotype DX test among patients diagnosed in 2015, 17 SEER registries
| N0 |
N1 |
|||
|---|---|---|---|---|
| aOR | 95% CI | aOR | 95% CI | |
|
| ||||
| Age | ||||
| < 50 | 1.32 | 1.22, 1.43 | 0.73 | 0.64, 0.84 |
| 50–59 | 1.35 | 1.26, 1.44 | 0.96 | 0.84, 1.09 |
| 60–69 | Ref | Ref | ||
| 70–79 | 0.49 | 0.46, 0.53 | 1.03 | 0.89, 1.19 |
| ≥ 80 | 0.08 | 0.07, 0.10 | 0.25 | 0.19, 0.33 |
| Race/ethnicity | ||||
| NH white | Ref | Ref | ||
| NH black | 0.90 | 0.82, 0.99 | 0.71 | 0.60, 0.85 |
| NH American Indian/Alaska Native | 1.02 | 0.69, 1.51 | 1.25 | 0.62, 2.52 |
| NH Asian or Pacific Islander | 1.05 | 0.96, 1.16 | 0.96 | 0.80, 1.15 |
| Hispanic (all races) | 0.97 | 0.89, 1.06 | 0.73 | 0.62, 0.86 |
| NH unknown race | 0.95 | 0.67, 1.34 | 0.96 | 0.54, 1.72 |
| Marital status | ||||
| Married | Ref | Ref | ||
| Single | 0.84 | 0.78, 0.91 | 0.98 | 0.85, 1.14 |
| Other | 0.90 | 0.85, 0.96 | 0.86 | 0.76, 0.98 |
| Unknown | 0.77 | 0.68, 0.87 | 0.72 | 0.56, 0.94 |
| Insurance type | ||||
| Insured | Ref | Ref | ||
| Medicaid | 0.76 | 0.69, 0.83 | 0.65 | 0.55, 0.77 |
| Insured but insurance type not specified | 0.94 | 0.87, 1.01 | 1.00 | 0.87, 1.16 |
| Uninsured | 0.73 | 0.56, 0.95 | 1.18 | 0.76, 1.82 |
| Unknown | 0.95 | 0.77, 1.17 | 1.24 | 0.83, 1.87 |
| Tumor grade | ||||
| Well differentiated | Ref | Ref | ||
| Moderately differentiated | 1.31 | 1.24, 1.39 | 0.73 | 0.65, 0.83 |
| Poorly differentiated/undifferentiated | 0.80 | 0.74, 0.87 | 0.31 | 0.27, 0.37 |
| Unknown | 0.58 | 0.46, 0.73 | 0.24 | 0.16, 0.37 |
| Tumor size, cm | ||||
| 0.6–2.0 for N0 or ≤ 2.0 for N1 | Ref | Ref | ||
| 2.1–5.0 | 1.15 | 1.09, 1.23 | 0.64 | 0.57, 0.70 |
| > 5.0 | 0.55 | 0.46, 0.66 | 0.26 | 0.21, 0.33 |
| Surgery & reported radiation | ||||
| Mastectomy, no/unknown radiation | Ref | Ref | ||
| Mastectomy, radiation | 0.88 | 0.73, 1.05 | 0.74 | 0.64, 0.86 |
| Lumpectomy, no/unknown radiation | 0.77 | 0.71, 0.84 | 0.79 | 0.67, 0.93 |
| Lumpectomy, radiation | 1.38 | 1.30, 1.46 | 1.52 | 1.35, 1.71 |
| State/area | ||||
| California | Ref | Ref | ||
| Connecticut | 1.86 | 1.66, 2.09 | 1.38 | 1.09, 1.74 |
| Georgia | 1.45 | 1.32, 1.58 | 1.00 | 0.85, 1.19 |
| Hawaii | 2.11 | 1.75, 2.56 | 1.28 | 0.88, 1.86 |
| Iowa | 1.26 | 1.10, 1.44 | 0.87 | 0.67, 1.12 |
| Kentucky | 1.05 | 0.94, 1.18 | 0.86 | 0.68, 1.08 |
| Louisiana | 1.57 | 1.39, 1.78 | 0.86 | 0.68, 1.10 |
| Detroit | 2.18 | 1.93, 2.46 | 1.23 | 0.97, 1.55 |
| New Jersey | 2.64 | 2.41, 2.88 | 1.77 | 1.51, 2.07 |
| New Mexico | 2.26 | 1.88, 2.70 | 1.14 | 0.81, 1.60 |
| Utah | 0.46 | 0.38, 0.55 | 0.45 | 0.32, 0.63 |
| Seattle | 0.98 | 0.88, 1.09 | 1.02 | 0.83, 1.25 |
N0 with negative axillary lymph node, N1 with 1–3 positive ipsilateral axillary lymph nodes, aOR adjusted odds ratio, CI confidence interval, ref reference group, NH non-Hispanic
Association of the sociodemographic and clinical factors with test order was also examined for each year from 2004 to 2014. The results were not shown, because similar associations were observed over time. It is worth noting that racial/ethnic disparities in ODX order were narrowed over time, especially among N0 patients (Supplementary Fig. 2). Compared to N0 NHW patients, the aOR (95% confidence interval [CI]) increased from 0.50 (0.29–0.83) in 2004 to 0.90 (0.82–0.99) in 2015 for NHB, from 0.48 (0.30–0.77) to 1.05 (0.96–1.16) for NHAPI, and from 0.40 (0.24–0.65) to 0.97 (0.89–1.06) for Hispanics. Patterns were similar for N1 patients, and aOR remained significant for NHB (aOR 0.71, 95% CI 0.60–0.85) and Hispanic patients (aOR 0.73, 95% CI 0.62–0.86) in 2015.
Trend of reported chemotherapy by recurrence score
Using original RS categorization, from 2004 to 2015, the proportion of chemotherapy use decreased from 13.3 to 2.0% in N0 patients with low RS, from 37.8 to 29.3% in intermediate RS patients, and increased from 66.7 to 76.1% in high RS patients (P-for-trend < 0.0001 for each), while the proportion remained stable in N0 patients not using ODX (25.7% to 25.4%, P-for-trend = 0.2) (Fig. 2a). As the proportion among N1 patients not receiving ODX increased over time (65.8% to 72.3%, P-for-trend < 0.0001), it decreased for N1 patients with low RS (28.9% to 16.2%, P-for-trend < 0.0001) and no significant change was observed for intermediate RS and high RS patients (Fig. 2b). The patterns remained similar when using TAILORx categorization for N0 patients (Fig. 2c): decreasing from 5.8% to 1.6% for RS < 11, from 23.6 to 10.2% for RS 11–25, and increasing from 61.8% to 66.9% for RS ≥ 26 (P-for-trend < 0.0001 for each). Among N1 patients, the decrease in chemotherapy use was significant for both TAILORx low and intermediate categories (P-for-trend < 0.0001 for each) but the increase in high-risk patients were not significant (Fig. 2d).
Fig. 2.
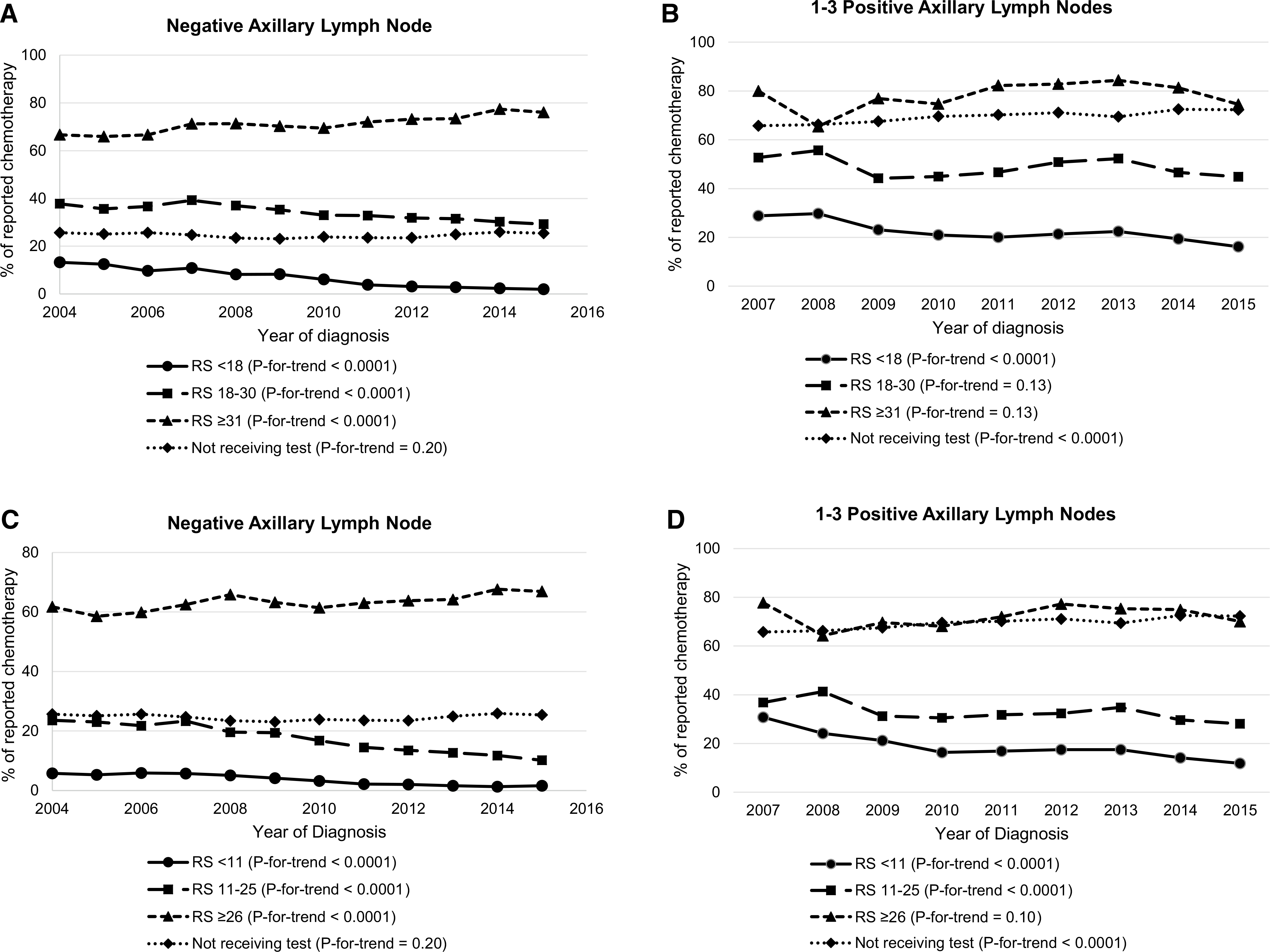
Trend of having reported chemotherapy by Recurrence Score among female hormone receptor-positive breast cancer patients, 17 SEER registries, 2004–2015. a Patients with negative axillary lymph node by Recurrence Score original categories; b Patients with 1–3 positive axillary lymph nodes by Recurrence Score original categories; c Patients with negative axillary lymph node by TAILORx categories; d Patients with 1–3 positive axillary lymph nodes by TAILORx categories
Survival difference associated with Oncotype DX use
After matching by propensity score, 50,468 N0 and 13,554 N1 HR+ and HER2− patients diagnosed in 2010–2014 were selected for survival analysis. The median follow-up time was 38 (range 0–71) months for N0 and 35 (range 0–71) months for N1 patients. The frequency of tumor size and grade was exactly same in two matched groups; and other patient characteristics were also evenly distributed by ODX use status. Overall, regardless of lymph node status, patients who did not receive ODX had worse BCSS (N0: HR 1.92, 95% CI 1.56–2.35; N1: HR 1.86, 95% CI 1.39–2.49) and OS (N0: HR 2.03, 95% CI 1.81–2.28; N1: HR 1.71, 95% CI 1.41–2.08) than those who had test, and the survival difference was more prominent in the first 36 months of follow-up (Table 3). The Kaplan–Meier survival curves showed significant difference (Log Rank P < 0.0001 for each, Fig. 3). After stratified by tumor size and grade, the survival difference was significant in most strata (Supplementary Fig. 3).
Table 3.
Hazard ratio (95% confidence interval) of breast cancer-specific death and any cause death for HR+/HER2− breast cancer patients who did not receive Oncotype DX test compared to those who received the test, 2010–2014, 17 SEER registries
| Breast cancer-specific survival |
Overall survival |
||||
|---|---|---|---|---|---|
| HR | 95% CI | HR | 95% CI | ||
|
| |||||
| N0 | 0–72 mon | 1.92 | 1.56, 2.35 | 2.03 | 1.81, 2.28 |
| 0–36 mon | 2.25 | 1.73, 2.93 | 2.25 | 1.95, 2.59 | |
| 37–72 mon | 1.47 | 1.07, 2.04 | 1.67 | 1.37, 2.03 | |
| N1 | 0–72 mon | 1.86 | 1.39, 2.49 | 1.71 | 1.41, 2.08 |
| 0–36 mon | 2.13 | 1.45, 3.12 | 1.89 | 1.49, 2.40 | |
| 37–72 mon | 1.54 | 0.98, 2.41 | 1.40 | 1.00, 1.96 | |
Within the stratum defined by diagnosis year, lymph node status, tumor size, and tumor grade, patients who received Oncotype DX test and those who did not were matched on propensity score which was calculated based on age, race/ethnicity, marital status, insurance, surgery, and radiation therapy
HR hormone receptor, HER2 human epidermal growth factor receptor 2, N0 with negative axillary lymph node, N1 with 1–3 positive ipsilateral axillary lymph nodes, HR hazard ratio, CI confidence interval
Fig. 3.
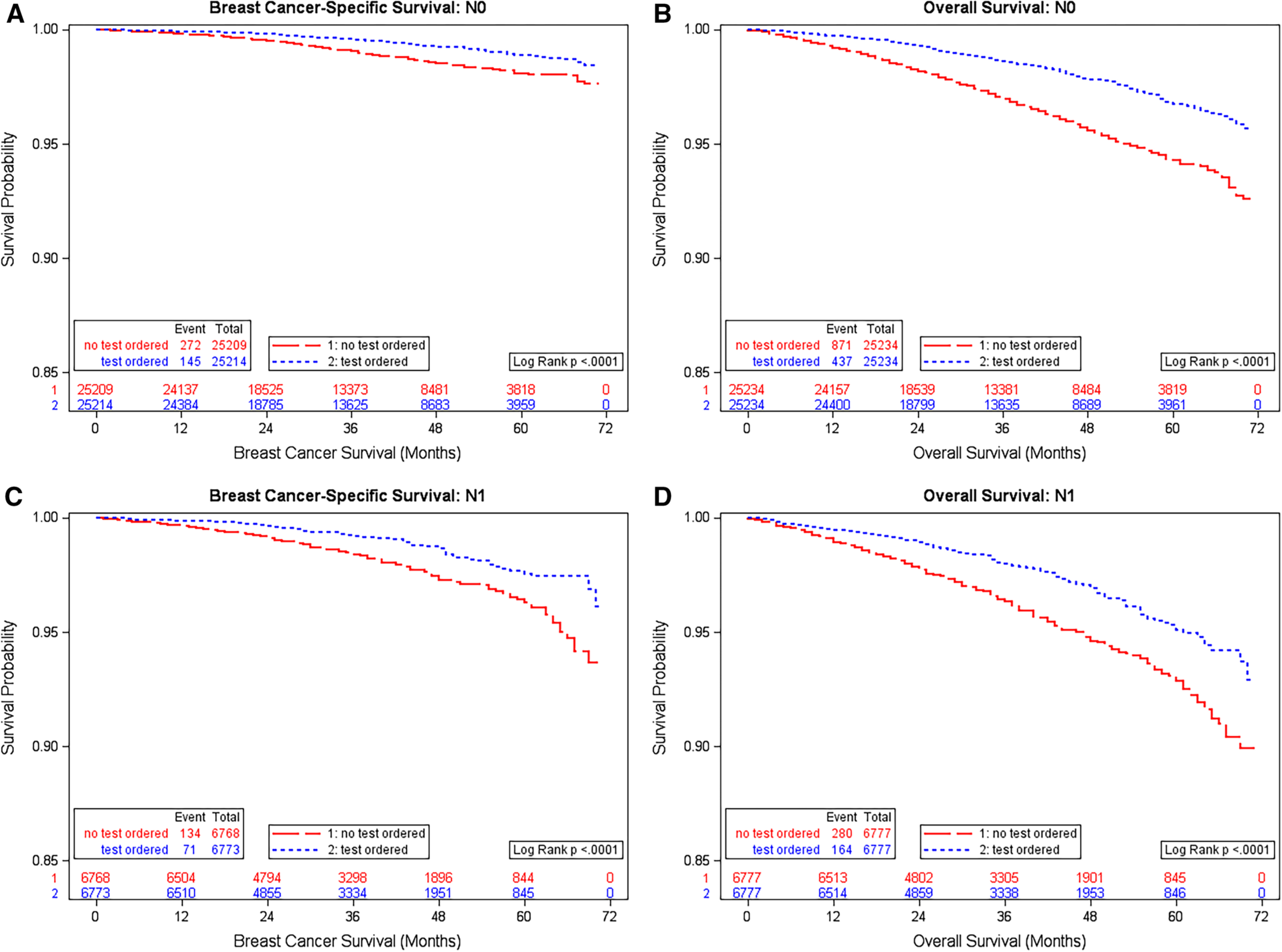
Kaplan–Meier plot of breast cancer-specific survival and overall survival by Oncotype DX test order status among HR+ /HER2− breast cancer patients, 17 SEER registries, 2010–2014. a Breast cancer-specific survival, with negative axillary lymph node; b Overall survival, with negative axillary lymph node; c Breast cancer-specific survival, with 1–3 positive axillary lymph nodes; d Overall survival, with 1–3 positive axillary lymph nodes
Discussion
Using population-based SEER cancer registry data linked with test results from the sole ODX provider, we found that the proportion of ODX use increased steadily over time in the past decade. Aged 80 years or older, NHB, unmarried, or Medicaid insured patients were less likely to receive ODX. Moderately differentiated or 2.1–5.0 cm tumors in N0 patients and well-differentiated or ≤ 2.0 cm tumors in N1 patients were associated with higher test order. The proportion of reported chemotherapy decreased for N0 patients with low to intermediate RS, but increased with high RS. Patients who used ODX had better BCSS and OS than those not using the test.
To our knowledge, this study is the largest and the most contemporary population-based study to examine the trend of ODX utilization among N0 and N1 patients separately using data from 17 SEER cancer registries. ODX is one of the first commercially available genomic tests for breast cancer in the U.S. The clinical guidelines regarding ODX use has evolved. American Society of Clinical Oncology (ASCO) initially published guidelines recommending the use of ODX to N0 breast cancer patients with ER+ in 2007 [17], and updated to ER+ /PR+ and HER2− N0 patients in 2016 [18]. NCCN guidelines adopted ODX since 2008, recommending it to patients who received surgery for tumors with ER+ /PR+, HER2−, N0, tumor size > 1 cm or 0.6–1.0 cm but moderately or poorly differentiated or with unfavorable features, histology of ductal, lobular, mixed, or metaplastic [4]. In 2015, NCCN guidelines expanded the ODX use to N0 tumors sized 0.6–1.0 cm with any grade and N1 tumors [4]. In our study, among the patients who are eligible to receive ODX based on the most recent NCCN guidelines (except HER2 status due to the data unavailable), we found that the proportion of ODX use increased substantially for both N0 and N1 patients. The proportions we reported were similar to the findings from a national random sample of breast cancer patients selected from SEER registries (NCI SEER Patterns of Care data) (2.7% in 2004 and 8.0% in 2005 of N0 patients) [19], and another study using data from National Cancer Data Base (NCDB) (15.0% in 2010 to 24.5% in 2013 among N1 patients) [10], but different from other studies due to various study populations. The first study using data from 17 medical centers, predominantly academic cancer centers, reported that 20.4% of stage I–III ER+ /PR+ breast cancer patients received ODX from 2006 to 2008 [7]. In addition to not separating N0 vs. N1 patients, another reason contributing to the findings different from our study is that this study did not restrict to patients who were eligible for ODX. The N0 tumors with small size (≤ 0.5 cm) and the tumors with more than 3 positive lymph nodes are not recommended for ODX and the inclusion of them could decrease the proportion of overall utilization. Another study reported lower ODX use among Medicare beneficiaries [8], which is consistent with the evidence that older patients were less likely to use ODX, particularly among N0 patients [9, 19, 20]. Additional studies using data from NCDB [21–23] or single state cancer registry [9, 24] showed different results depends on the selection of study participants.
Racial/ethnic disparity in access to health care always remains a public health concern, especially when a novel medical technique is available. Except a few studies with smaller sample size [19, 25] or including only Medicare patients [8], most of previous research reported significant racial/ethnic disparities in ODX use [7, 9, 10, 20, 22–24]. Our study, for the first time, found the changing pattern of racial/ethnic difference in ODX utilization from 2004 to 2015, where the disparities were more prominent in the first few years and have been narrowed over time, in particular among N0 patients. Policy intervention such as Medicare coverage could be a possible explanation of the increasing access to ODX for minorities. As several major private insurance companies started to cover ODX testing between 2005 and 2008, Centers for Medicare and Medicaid Services (CMS) granted Medicare coverage to N0 patients since 2006 and to N1 patients since 2012 [26]. Genomic Health Inc. also offers financial assistance to low-income patients [27]. Patients with Medicaid coverage had lower ODX use and minorities are more likely to be covered by Medicaid, racial/ethnic disparities could be mitigated with the inclusion of ODX testing in Medicaid coverage policy.
Our study found that N0 patients with moderately differentiated or medium sized tumor or N1 patients with well-differentiated or small-sized tumor had higher proportion of ODX order, which is consistent with previous findings [8, 25]. As ODX is an assay aiming chemotherapy decision making, for tumors with a high clinicopathologic risk, such as N1 tumors with large tumor size or high grade, or tumors with low clinicopathologic risk, such as N0 tumors with small tumor size or low-grade, physicians could think they have enough information for chemotherapy decision thus ODX is not necessary. In addition to clinicopathologic factors, we also found geographic variation in ODX order, which warrants further investigation.
Our analysis on chemotherapy was exploratory, because SEER data tended to underreport chemotherapy utilization [28]. However, assuming the extent of underreport was consistent across each RS group, we observed improved adherence to ODX results in chemotherapy utilization over the study period. A meta-analysis of 3,104 N0 patients pooled from 14 studies reported that 5.8%, 37.4%, and 83.4% of patients with low, intermediate, and high RS received chemotherapy, respectively [29], which was slightly higher than our findings. The proportion of chemotherapy recommendation increasing with RS has also been demonstrated among N1 patients diagnosed in 2010–2013 [10]. With the expanding use of ODX, administering chemotherapy based on RS, as well as the high proportion of low RS [21], it is not surprising that several studies demonstrated a remarkable decline in chemotherapy receipt among all ER+ breast cancer patients over our study period [7, 10, 21]. Research has also shown oncologists incline to revoke their chemotherapy recommendation for N1 tumors after receiving ODX results, especially when patients have concern about chemotherapy toxicity [30]. We would expect a continuing decline in the utilization of chemotherapy among HR+ patients in the next few years, especially among N1 patients with low RS, because ODX has been officially recommended to N1 patients in 2015.
Our study, to our knowledge, is the first to examine the survival difference associated with ODX use. We found that with the adjustment of sociodemographic and clinical factors, ODX use was associated with better BCSS and OS, although the survival benefit was not consistent across the groups defined by clinicopathologic factors. As a biomarker used for precision medicine, ODX test results could guide physicians to provide better and more appropriate treatment. In particular, ODX results can avoid toxicity from unnecessary chemotherapy or prompt necessary chemotherapy which could be easily omitted due to favorable clinicopathologic characteristics. For example, among N1 patients with large tumor size or high grade who were given chemotherapy traditionally, ODX could prevent unnecessary chemotherapy if ODX shows a low RS; in contrast, for N0 patients with favorable clinicopathologic features, ODX could distinguish the tumors with high RS for which the chemotherapy is necessary but easily omitted. We observed survival effect is more notable in the first 36 months which is harmonized with previous research that RS’ prediction of survival benefit from chemotherapy is better in the first few years and no additional prediction beyond 5 years [5].
The major strength of our study is the quality and representativeness of our dataset. The large-scale population-based sample allowed the evaluation of the trend over time, stratification by tumor clinicopathologic features, and greater generalizability. In addition, the data linkage with Genomic Health Inc. database ensured the completeness and ascertainment of ODX data. However, our study is also subjected to several limitations. First, due to the lack of HER2 data, the proportion of ODX use among eligible patients could be slightly underestimated, because patients with HER2+ tumors do not need ODX. Second, the chemotherapy is tended to be underreported in SEER data. This is also the reason we did not conduct multivariable analysis on the variable of reported chemotherapy. Third, as the first study examining survival benefit associated with ODX use, our study is limited by the lack of comorbidity information, although the similar pattern on BCSS and OS could minimize the confounding effect from comorbidity. Lastly, due to the lack of information on other genomic test, small proportion of patients who did not use ODX could use other genomic test. One study using NCDB data found that ODX represents about 95% of genomic tests used by breast cancer patients [23]. In addition, NCCN guidelines started recommending other genomic test since 2016.
In conclusions, our findings suggest the increasing use of ODX since 2004, but disparities remain, especially for racial/ethnic minorities with N1 tumors and patients with Medicaid insurance. Utilization of chemotherapy has been increasingly adhered to ODX results over time. Among eligible patients, those who used test had better BCSS and OS, and the survival benefit was more prominent in the first 36 months of follow-up.
Supplementary Material
Funding
This study was supported by National Cancer Institute Surveillance, Epidemiology, and End Results program (NCI SEER HHSN261201300016I), the Centers for Disease Control and Prevention National Program of Cancer Registries (CDC NPCR U58DP003915), National Institutes of Health/National Institute on Minority Health and Health Disparities (NIH/NIMHD R15MD012387), and Clemson University internal funding.
Footnotes
Compliance with ethical standards
Conflict of interest The authors declare that they have no conflict of interest.
Electronic supplementary material The online version of this article (https://doi.org/10.1007/s10549-020-05557-x) contains supplementary material, which is available to authorized users.
References
- 1.Paik S, Shak S, Tang G, Kim C, Baker J, Cronin M, Baehner FL, Walker MG, Watson D, Park T, Hiller W, Fisher ER, Wickerham DL, Bryant J, Wolmark N (2004) A multigene assay to predict recurrence of tamoxifen-treated, node-negative breast cancer. N Engl J Med 351(27):2817–2826. 10.1056/NEJMoa041588 [DOI] [PubMed] [Google Scholar]
- 2.Sparano JA, Gray RJ, Makower DF, Pritchard KI, Albain KS, Hayes DF, Geyer CE Jr, Dees EC, Perez EA, Olson JA Jr, Zujewski J, Lively T, Badve SS, Saphner TJ, Wagner LI, Whelan TJ, Ellis MJ, Paik S, Wood WC, Ravdin P, Keane MM, Gomez Moreno HL, Reddy PS, Goggins TF, Mayer IA, Brufsky AM, Toppmeyer DL, Kaklamani VG, Atkins JN, Berenberg JL, Sledge GW (2015) Prospective validation of a 21-gene expression assay in breast cancer. N Engl J Med 373(21):2005–2014. 10.1056/NEJMoa1510764 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Paik S, Tang G, Shak S, Kim C, Baker J, Kim W, Cronin M, Baehner FL, Watson D, Bryant J, Costantino JP, Geyer CE Jr, Wickerham DL, Wolmark N (2006) Gene expression and benefit of chemotherapy in women with node-negative, estrogen receptor-positive breast cancer. J Clin Oncol 24(23):3726–3734. 10.1200/jco.2005.04.7985 [DOI] [PubMed] [Google Scholar]
- 4.NCCN Clinical Practice Guideline in Oncology (NCCN Guideline®) for Breast Cancer. © National Comprehensive Cancer Network, Inc.
- 5.Albain KS, Barlow WE, Shak S, Hortobagyi GN, Livingston RB, Yeh IT, Ravdin P, Bugarini R, Baehner FL, Davidson NE, Sledge GW, Winer EP, Hudis C, Ingle JN, Perez EA, Pritchard KI, Shepherd L, Gralow JR, Yoshizawa C, Allred DC, Osborne CK, Hayes DF (2010) Prognostic and predictive value of the 21-gene recurrence score assay in postmenopausal women with node-positive, oestrogen-receptor-positive breast cancer on chemotherapy: a retrospective analysis of a randomised trial. Lancet Oncol 11(1):55–65. 10.1016/s1470-2045(09)70314-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Dowsett M, Cuzick J, Wale C, Forbes J, Mallon EA, Salter J, Quinn E, Dunbier A, Baum M, Buzdar A, Howell A, Bugarini R, Baehner FL, Shak S (2010) Prediction of risk of distant recurrence using the 21-gene recurrence score in node-negative and node-positive postmenopausal patients with breast cancer treated with anastrozole or tamoxifen: a TransATAC study. J Clin Oncol 28(11):1829–1834. 10.1200/jco.2009.24.4798 [DOI] [PubMed] [Google Scholar]
- 7.Hassett MJ, Silver SM, Hughes ME, Blayney DW, Edge SB, Herman JG, Hudis CA, Marcom PK, Pettinga JE, Share D, Theriault R, Wong YN, Vandergrift JL, Niland JC, Weeks JC (2012) Adoption of gene expression profile testing and association with use of chemotherapy among women with breast cancer. J Clin Oncol 30(18):2218–2226. 10.1200/jco.2011.38.5740 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Dinan MA, Mi X, Reed SD, Hirsch BR, Lyman GH, Curtis LH (2015) Initial trends in the use of the 21-gene recurrence score assay for patients with breast cancer in the medicare population, 2005–2009. JAMA Oncol 1(2):158–166. 10.1001/jamaoncol.2015.43 [DOI] [PubMed] [Google Scholar]
- 9.Cress RD, Chen YS, Morris CR, Chew H, Kizer KW (2016) Underutilization of gene expression profiling for early-stage breast cancer in California. Cancer Causes Control 27(6):721–727. 10.1007/s10552-016-0743-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Peethambaram PP, Hoskin TL, Day CN, Goetz MP, Habermann EB, Boughey JC (2017) Use of 21-gene recurrence score assay to individualize adjuvant chemotherapy recommendations in ER+/HER2− node positive breast cancer—a National Cancer Database study. NPJ Breast Cancer 3:41. 10.1038/s41523-017-0044-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Surveillance, Epidemiology, and End Results Program (2019) https://seer.cancer.gov/about/overview.html. Accessed 15 Oct 2019
- 12.Petkov VI, Miller DP, Howlader N, Gliner N, Howe W, Schussler N, Cronin K, Baehner FL, Cress R, Deapen D, Glaser SL, Hernandez BY, Lynch CF, Mueller L, Schwartz AG, Schwartz SM, Stroup A, Sweeney C, Tucker TC, Ward KC, Wiggins C, Wu XC, Penberthy L, Shak S (2016) Breast-cancer-specific mortality in patients treated based on the 21-gene assay: a SEER population-based study. NPJ Breast Cancer 2:16017. 10.1038/npjbcancer.2016.17 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.National Cancer Institute. Surveillance, Epidemiology, and End Results Program (2019) https://seer.cancer.gov/seerstat/variables/seer/insurance-recode/. Accessed 15 Oct 2019
- 14.Agresti A (2002) Categorical dta analysis, 2ed. Wiley, New York [Google Scholar]
- 15.Coca-Perraillon M (2019) Local and global optimal propensity score matching. https://support.sas.com/resources/papers/proceedings/proceedings/forum2007/185-2007.pdf. Accessed 15 Oct 2019 [Google Scholar]
- 16.Kleinbaum DG, Klein M (2012) Survival analysis, 3rd edn. Springer, New York [Google Scholar]
- 17.Harris L, Fritsche H, Mennel R, Norton L, Ravdin P, Taube S, Somerfield MR, Hayes DF, Bast RC Jr (2007) American Society of Clinical Oncology 2007 update of recommendations for the use of tumor markers in breast cancer. J Clin Oncol 25(33):5287–5312. 10.1200/jco.2007.14.2364 [DOI] [PubMed] [Google Scholar]
- 18.Harris LN, Ismaila N, McShane LM, Andre F, Collyar DE, Gonzalez-Angulo AM, Hammond EH, Kuderer NM, Liu MC, Mennel RG, Van Poznak C, Bast RC, Hayes DF (2016) Use of biomarkers to guide decisions on adjuvant systemic therapy for women with early-stage invasive breast cancer: american society of clinical oncology clinical practice guideline. J Clin Oncol 34(10):1134–1150. 10.1200/jco.2015.65.2289 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Enewold L, Geiger AM, Zujewski J, Harlan LC (2015) Oncotype Dx assay and breast cancer in the United States: usage and concordance with chemotherapy. Breast Cancer Res Treat 151(1):149–156. 10.1007/s10549-015-3366-7 [DOI] [PubMed] [Google Scholar]
- 20.Press DJ, Ibraheem A, Dolan ME, Goss KH, Conzen S, Huo D (2018) Racial disparities in omission of oncotype DX but no racial disparities in chemotherapy receipt following completed oncotype DX test results. Breast Cancer Res Treat 168(1):207–220. 10.1007/s10549-017-4587-8 [DOI] [PubMed] [Google Scholar]
- 21.Parsons BM, Landercasper J, Smith AL, Go RS, Borgert AJ, Dietrich LL (2016) 21-Gene recurrence score decreases receipt of chemotherapy in ER+ early-stage breast cancer: an analysis of the NCDB 2010–2013. Breast Cancer Res Treat 159(2):315–326. 10.1007/s10549-016-3926-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Jasem J, Amini A, Rabinovitch R, Borges VF, Elias A, Fisher CM, Kabos P (2016) 21-Gene recurrence score assay as a predictor of adjuvant chemotherapy administration for early-stage breast cancer: an analysis of use, therapeutic implications, and disparity profile. J Clin Oncol 34(17):1995–2002. 10.1200/jco.2015.65.0887 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Bhutiani N, Egger ME, Ajkay N, Scoggins CR, Martin RC 2nd, McMasters KM (2018) Multigene signature panels and breast cancer therapy: patterns of use and impact on clinical decision making. J Am Coll Surg 226(4):406–412.e401. 10.1016/j.jamcollsurg.2017.12.043 [DOI] [PubMed] [Google Scholar]
- 24.Ricks-Santi LJ, McDonald JT (2017) Low utility of Oncotype DX(R) in the clinic. Cancer Med 6(3):501–507. 10.1002/cam4.837 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Roberts MC, Weinberger M, Dusetzina SB, Dinan MA, Reeder-Hayes KE, Carey LA, Troester MA, Wheeler SB (2016) Racial variation in the uptake of Oncotype DX testing for early-stage breast cancer. J Clin Oncol 34(2):130–138. 10.1200/jco.2015.63.2489 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Trosman JR, Van Bebber SL, Phillips KA (2010) Coverage policy development for personalized medicine: private payer perspectives on developing policy for the 21-gene assay. J Oncol Pract 6(5):238–242. 10.1200/jop.000075 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Genomic Health (2019) https://www.oncotypeiq.com/en-US/breast-cancer/patients-and-caregivers/stage-0-dcis/insurance-coverageand-financial-assistance. Accessed 15 Oct 2019
- 28.Noone AM, Lund JL, Mariotto A, Cronin K, McNeel T, Deapen D, Warren JL (2016) Comparison of SEER treatment data with medicare claims. Med Care 54(9):e55–64. 10.1097/mlr.0000000000000073 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Carlson JJ, Roth JA (2013) The impact of the Oncotype Dx breast cancer assay in clinical practice: a systematic review and meta-analysis. Breast Cancer Res Treat 141(1):13–22. 10.1007/s10549-013-2666-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kurian AW, Bondarenko I, Jagsi R, Friese CR, McLeod MC, Hawley ST, Hamilton AS, Ward KC, Hofer TP, Katz SJ (2018) Recent trends in chemotherapy use and oncologists’ treatment recommendations for early-stage breast cancer. J Natl Cancer Inst 110(5):493–500. 10.1093/jnci/djx239 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.