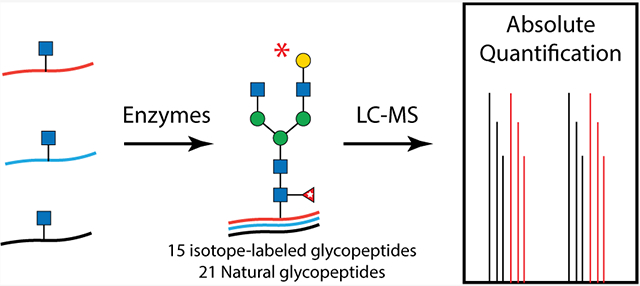
Abstract
Absolute glycoproteomics quantification has drawn tremendous attention owing to its prospects in biomarker discovery and clinical implementation but is impeded by a general lack of suitable heavy isotope-labeled glycopeptide standards. In this study, we devised a facile chemoenzymatic strategy to synthesize a total of 36 human IgG glycopeptides attached with well-defined glycoforms, including 15 isotope-labeled ones with a mass increment of 6 Da to their native counterparts. Spiking of these standards into human sera enabled simplified, robust, and precise absolute quantification of IgG glycopeptides in a subclass-specific fashion. Additionally, the implementation of the absolute quantification approach revealed subclass-dependent alteration of serum IgG galactosylation and sialylation in colon cancer samples.
Graphical Abstract
INTRODUCTION
Glycosylation impacts the structures and functions of proteins, thereby playing vital roles in many physiological processes, including cell signaling, immune responses, and host–pathogen interactions,1 with significant revelations in connection to numerous pathological processes.2,3 The strong correlation between glycosylation perturbations and human diseases has prompted intensive interests in glycomics studies toward biomarker discovery via relative and absolute quantification,4 with the recent focus on individual proteins, mostly immunoglobulin G (IgG).
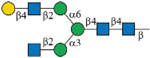
IgG is one of the most abundant proteins in human serum that occupies a central role in humoral immune responses, including neutralization, opsonization, and complement activation.5,6 Human IgG can be further divided into four subclasses (IgG1–4) according to the heavy chain variations,7 each subclass has a unique profile with respect to occurrences, antigen bindings, and functions.6 Regardless, all human IgG molecules carry a conserved N-glycosylation site of asparagine (Asn) 297 within the Fc region, which is predominantly occupied by core-fucosylated biantennary N-glycans, with varied levels of galactosylation, α2–6 sialyation, and bisected N-acetylglucosamine (GlcNAc) (Figure 1A).8,9 It is well recognized that the fine structures of Fc glycans have a profound impact on the functions of IgG,10 particularly, regulating IgG effector functions by modulating the binding between Fc and its receptors. Furthermore, altered IgG glycosylation is frequently observed under various pathological scenarios. For example, different types of cancers, viral infections, and autoimmune diseases have been shown to exhibit low overall sialylation and galactosylation on serum Fc N-glycans.3,10 A recent report on weaker associations of IgG2 galactosylation and sialylation compared to those of IgG1 and IgG4 suggested a subclass-specific correlation manner.11 Therefore, site-specific absolute quantification of Fc glycans is of great importance to IgG glyco-biomarker discovery and clinical implementations.
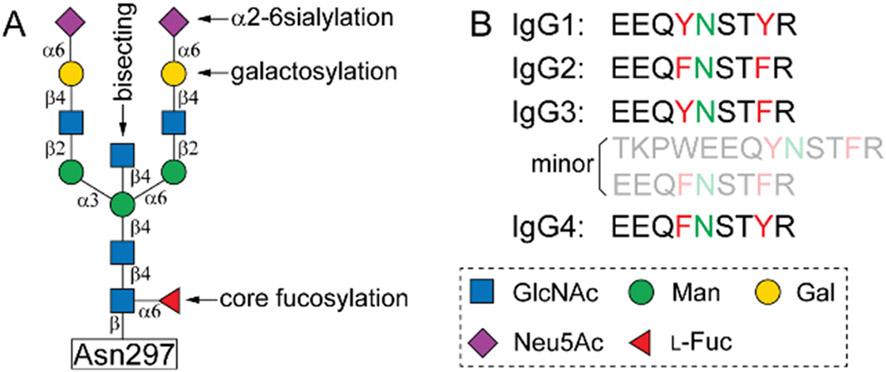
Figure 1.
Structure of a full-length biantennary complex N-glycan attached to Asn297 (A), and tryptic peptide sequences of human IgG subclasses containing the site (B).
Currently, the quantification of IgG glycosylation mainly relies on relative quantitative strategies. This is typically measured by normalizing the signal strength of particular glycoforms to that of the total glycoforms. However, clinical applications of glycosylation as biomarkers go beyond glycoform ratios. It would require more robust and precise methods, such as absolute quantification, which can determine absolute concentrations of target glycoforms. Isotope dilution mass spectrometry (MS) has become routine in metabolomics, proteomics, and recently glycomics12,13 However, the general lack of isotope-labeled glycopeptide standards remains the major impediment to absolute glycoproteomics quantification. As a result, validated strategies such as metabolic incorporation14 and isotopic tagging15-19 are limited to relative quantification and typically involve multiple enrichment steps. Recently, two reports achieved accurate quantification of target glycopeptides by using isotope-labeled synthetic standards, despite the lack of glycoform diversity of the standards due to synthetic challenges.20,21 Herein, we describe an efficient strategy for subclass-specific absolute quantification of IgG glycosylation with the synthesis of 13C-labeled human IgG tryptic glycopeptides attached with diverse complex N-glycans. Spiking of these synthetic 13C-labeled glycopeptides into human sera enabled robust and precise absolute quantification of IgG glycopeptides in a subclass-specific fashion.
EXPERIMENTAL SECTION
Chemoenzymatic Synthesis of 13C-Labeled IgG Glycopeptides.
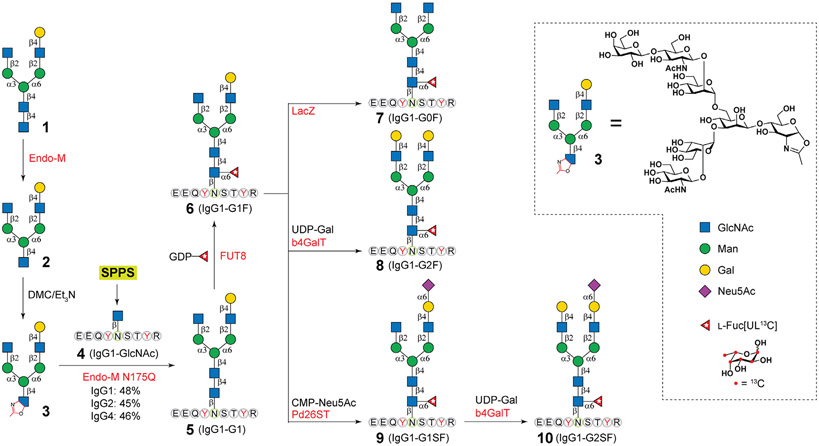
Asymmetric N-glycan 1 was prepared as previously described,22,23 then digested by using Endo-M to provide 2 (Figure 2). The treatment of 2 with chloro-1,3-dimethylimidazolinium chloride/triethylamine (DMC/TEA) provided 3. The IgG 1 tryptic peptide with a GlcNAc residue (4) was synthesized by using Wang resin through the Fmoc-strategy on a CEM Liberty Blue Peptide Synthesizer (CEM) and then purified by high pressure liquid chromatography (HPLC) (Supporting Information). To synthesize 5, 3 (20 mg) and 4 (10 mg) were dissolved in 100 mM MES buffer (pH 7.0), then an adequate amount of Endo-M-N175Q was added to the mixture, and incubated at 30 °C for 10 mins. The glycopeptide 5 was then purified by HPLC and subject to FUT8-catalyzed core-fucosylation to generate compound 6. Further treatment of 6 by β-galactosidase (LacZ) afforded 7. The β1–4 galactosylation of 6 by β1–4 galactosyltransferase (β4GalT) provided 8, and the α2–6 sialylation of 6 with α2–6 sialyltransferase gave 9. The additional installment of a Gal residue to 9 by β4GalT provided 10. Detailed procedures for these glycosylation reactions, product purification, and separation are provided in the Supporting Information.
Figure 2.
Chemoenzymatic synthesis of stable isotope-labeled human IgG1 Fc tryptic glycopeptides. Endo-M, endo-β-N-acetylglucosaminidase from Mucor hiemalis; Endo-M N175Q, Endo-M mutant devoid of hydrolysis activity; FUT8, human α1–6 fUcosyltransferase; LacZ, β-galactosidase from E. coli; b4GalT, β1–4 galactosyltransferase from bovine; Pd26ST, α2–6 sialyltransferase from Photobacterium damsela. SPPS, solid-phase peptide synthesis.
Nano LC–MS/MS Analysis.
Nano RP HPLC–MS experiments were performed on an LTQ-Orbitrap Elite mass spectrometer (Thermo Fisher) equipped with an EASY-spray source and nano-LC UltiMate 3000 high-performance liquid chromatography system (Thermo Fisher). The analysis was performed on an EASY-Spray PepMap C18 column (75 μm × 50 cm, 2 μm) with a linear gradient from 3 to 40% mobile phase B for 120 mins at a flow rate of 300 nL/min (mobile phase A: 1.95% ACN, 97.95% H2O, and 0.1% FA; mobile phase B: 79.95% ACN, 19.95% H2O, and 0.1% FA). The mass spectrometer was operated in data-dependent mode.
For the quantification of IgG glycopeptides, a selected-scan survey MS experiment (m/z range from 375 to 2000; automatic gain control target, 1,000,000 ions; and maximum ion accumulation time, 50 ms) was acquired by an Orbitrap mass spectrometer. Under parallel reaction monitoring (PRM) mode, selected ions were fragmented by higher-energy collision dissociation (HCD) under 30% collision energy; scan speed was 12 Hz at a resolution setting of 15,000. The PRM result was analyzed using Thermo Xcalibur qual browser software. The peak area of glycopeptides was integrated and used for quantitation. The PRM transitions are provided in Table S1.
Limit of Detection and Limit of Quantification.
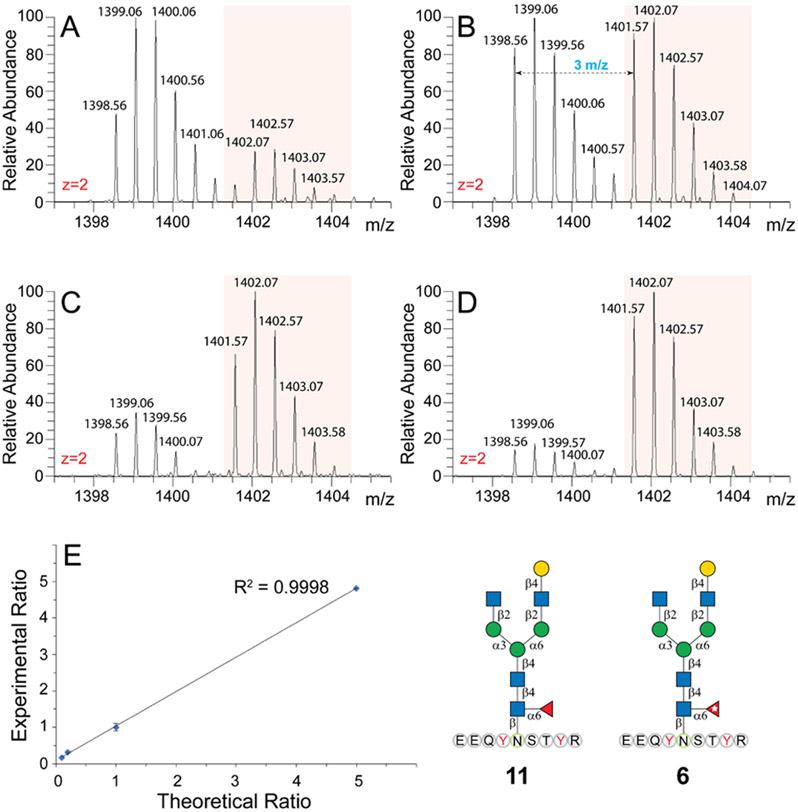
Native glycopeptide IgG1-G1F (11) and its isotope-labeled counterpart IgG1-G1F* (6) were mixed with 5:1, 1:1, 1:5, and 1:10 ratios and analyzed by LC–MS/MS as described above. The intensities of glycopeptides were measured by the PRM peak area at m/z 1398.5530 and 1401.5631, which represent [M + 2H]2+ peaks of these glycopeptides.
Absolute Quantification of Human Serum IgG Fc Glycopeptides.
Healthy human serum and colon cancer patients’ sera (3.5 uL) were dissolved in 8 M urea followed by addition of 10 mM DTT and brief boiling for 10 min. Then, the mixture was incubated at 37 °C for 30 mins. Subsequently, the mixture was incubated with 20 mM IAA for 40 min at room temperature, which was then quenched by the addition of 20 mM DTT. The synthetic stable isotope-labeled glycopeptides were then spiked into the denatured mixture and diluted with 50 mM ammonia bicarbonate (ABC) to make the urea concentration less than 1 M. The spiked samples were further digested by trypsin (1:50) at 37 °C for 12 h and desalted by HyperSep™ C18 cartridges (Sigma-Aldrich). The cartridges were preactivated with ACN and then pre-equilibrated with 0.1% TFA solution. Glycopeptides were eluted by 80% ACN with 0.1% TFA and concentrated for LC–MS/MS analysis.
For the quantification of sialylated IgG3/4 glycopeptides, hydrophilic interaction liquid chromatography (HILIC) enrichment was performed after desalting as previously described.24 Briefly, iSPE HILIC cartridges were pretreated with 300 μL of ACN, 400 μL of 0.1% TFA, and 400 μL 80% ACN/0.1% TFA. Samples that dissolved in 80% ACN/0.1% TFA were then loaded onto the cartridges, and flowthroughs were reloaded twice. After washing with 900 μL 80% ACN/ 0.1% TFA, the glycopeptides were eluted using 1 mL of 0.1% TFA, which was then concentrated and loaded on LC–MS/MS for analysis.
RESULTS AND DISCUSSION
Chemoenzymatic Synthesis of 13C-Labeled IgG Glycopeptides.
Many chemical and chemoenzymatic strategies had been developed to access homogenous glycopeptides,25-27 including the advanced endo-β-N-acetylglucosaminidase (ENGase)-catalyzed transglycosylation.26,28 However, nearly all reports focused on the variation of the peptide moiety, whereas attached N-glycoforms have been limited to high-mannose and symmetric complex types. Glycopeptides with asymmetric N-glycoforms have never been synthesized. Recently, we and others reported a litany of chemoenzymatic strategies to synthesize and diversify N-glycans,23,29-31 yielding hundreds of complex glycoforms. With the advancement of this strategy, we envisioned that IgG Fc peptides (Figure 1B) attached with 1 could serve as general “Cores” for enzymatic extensions to afford diverse glycopeptides. As depicted in Figure 2, to obtain such “Cores”, compound 1 (85 mg) that was prepared as previously described22,23 was first subjected to Endo-M digestion to remove the reducing end GlcNAc, affording 71 mg of compound 2 with a yield of 97%. The purity and structure of 2 were confirmed by HPLC, HR-MS, 1H, 13C, and 2D nuclear magnetic resonance (NMR) (Supporting Information). Oxazoline 3 was then prepared by treating 2 with DMC/TEA32 followed by brief G15 purification under base conditions (5 mM NaOH). Coupling of 3 and IgG1-GlcNAc (4, refer Supporting Information for synthetic details) catalyzed by Endo-M N175Q was found to be fast and efficient, which is in agreement with previous reports.32-35 A donor/acceptor mole ratio of 2:1 enabled a satisfactory yield of 48% within 10 min. A higher donor/acceptor ratio may further increase yields32-35 but was not adopted due to limited quantities of 3.
Stable isotopes were conventionally incorporated into peptides by solid-phase peptide synthesis (SPPS) owning to the affordability of amino acid isotopologues.20,21 Alternatively, we conceived enzymatic isotope incorporation. As illustrated in Figure 2A, 13C-labeled L-fucose (Fuc*, 13C6H12O5, 99 atom % 13C, >99.6% CP) was introduced onto compound 5 by human α1–6 fucosyltransferase (FUT8) in the presence of guanosine 5′-diphosphate 13C-fucose (GDP-Fuc*). After overnight incubation, a new m/z peak of 2802.157 corresponding to isotope-labeled glycopeptide IgG1-G1F* (6) was observed in the MALDI-TOF MS spectrum (Figure S1) concurrent with the disappearing of the peak for 5. A parallel reaction superseding GDP-Fuc* with GDP-Fuc revealed a product m/z peak with 6.0063 Da decrement, corresponding to native IgG1-G1F (11). Compared to SPPS, the enzymatic approach for isotope incorporation is highly efficient and requires much less isotopologues. The nascent compound 6 (8 mg, 94% yield) purified by HPLC (Supporting Information) was then readily diversified into four more glycopeptides with excellent yields catalyzed by a litany of robust glyco-enzymes (Figure 2). For example, the treatments with β-galactosidase from E. coli (LacZ) and β1–4 galactosyltransferase from bovine (β4GalT) yielded glycopeptides with symmetric glycoforms, G0F* (7) and G2F* (8), respectively. Sialylated glycopeptide 9 was obtained by α2–6 sialylation and β1–4 galactosylation using α2–6 sialyltransferase from Photobacterium damsela (Pd26ST), and 10 was synthesized from 9 by β4GalT.
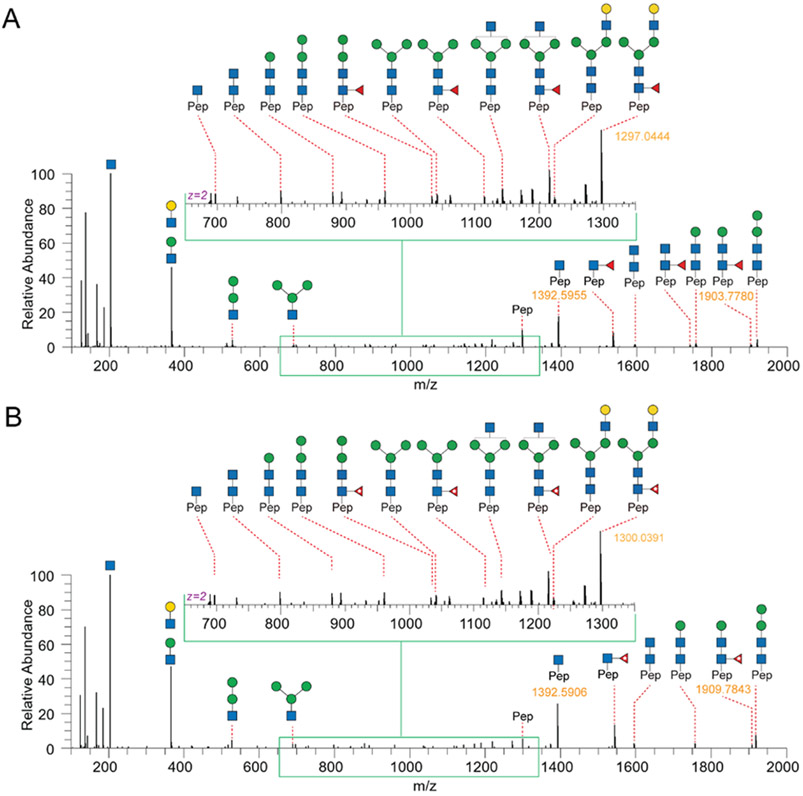
As shown in Figure 1B, each subclass of human IgG contains a unique Fc peptide sequence except for minor allotypes of IgG3.36 It is thus possible to take advantage of the amino acid variations for subclass-specific IgG glycoproteomics analysis. In addition, since the Fc tryptic peptide of IgG3 is isobaric to that of IgG4, it is possible to quantify the two scarce subclasses together. We thus synthesized IgG2 and IgG4 Fc glycopeptides and their isotopologues as described for IgG1 (Supporting Information). Collectively, 15 isotope-labeled IgG Fc glycopeptides (6–10, 18–22, and 30–34) and 21 native ones were successfully synthesized (Table 1). The structure and connectivity of each glycopeptide were ensured by the regio- and stereoselectivity of involved enzymes and later confirmed by NMR and LC–MS/MS (Supporting Information). As an example, Figure 3 showed HCD-MS/MS analysis of a pair of glycopeptide isotopologues, compounds 11 and 6. The purities of all synthesized glycopeptides and 13C-labeled counterparts are determined by HPLC to be >98% (Supporting Information). The quantity of synthetic glycopeptides was determined by standard curves that were established by corresponding peptides (Figure S2).
Table 1.
Homogenous IgG Fc Glycopeptides Prepared in this Study and their Yields
| Glycoforms | IgG1 EEQYNSTYR |
IgG2 EEQFNSTFR |
IgG4 EEQFNSTYR |
|---|---|---|---|
| Native human IgG Fc glycopeptides (Yields) | |||
|
|
4 | 16 | 28 |
|
5 (48%) | 17 (45%) | 29 (46%) |
|
11 (91%) | 23 (95%) | 35 (92%) |
|
12 (89%) | 24 (90%) | 36 (88%) |
|
13 (96%) | 25 (91%) | 37 (90%) |
|
14 (91%) | 26 (94%) | 38 (93%) |
|
15 (93%) | 27 (91%) | 39 (89%) |
| 13C-labeled human IgG Fc glycopeptides (Yields) | |||
|
6 (94%) | 18 (95%) | 30 (92%) |
|
7 (91%) | 19 (87%) | 31 (90%) |
|
8 (93%) | 20 (94%) | 32 (95%) |
|
9 (92%) | 21 (95%) | 33 (91%) |
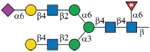
|
10 (89%) | 22 (91%) | 34 (90%) |
Figure 3.
HCD-MS/MS analysis of synthesized human IgG Fc tryptic glycopeptide 11 (A) and its isotopologue 6 with a mass increment of 6.0063 Da (B).
Absolute Quantification of IgG Fc Glycopeptides in Human Serum.
Because isotopic peaks from native glycopeptides may influence the first monoisotopic peak of 13C-enriched ones, especially when the analyte intensity is substantially higher than its isotopologue, it is necessary to ensure adequate amounts of 13C-labeled glycopeptides are spiked for absolute quantification. Figure 4A-D shows the spectra for [M + 2H]2+ of IgG1-G1F (11) and its isotopologue (6) mixed with 5:1, 1:1, 1:5, and 1:10 ratios. The peak area ratios of extracted ion chromatograms of 11 (1398.56–1401.07) and 6 (1401.57–1404.07) precisely match the synthetic mix ratio. An R-square value of 0.9998 for linear fitting between the theoretical mix ratio and experimental peak area ratio revealed perfect linearity in a mixing range of 5:1 to 1:10. To confirm that the 13C isotope label has no influence on retention times, the 15 13C-labeled glycopeptides and their isotopologues were mixed and subject to LC–MS/MS analysis under PRM mode (Table S1). Extracted ion chromatograms show that retention times of native glycopeptides and their isotopologues are identical (Figure S3). Furthermore, substantial differences in retention times were observed among the three subclasses of IgG glycopeptides, which could effectively reduce the interference caused by glycopeptide coelution.
Figure 4.
MS spectra of mixing native Fc glycopeptide 11 with its isotope-labeled counterpart 6 in the ratios of (A) 5:1, (B) 1:1, (C) 1:5, and(D) 1:10. Panel (E) illustrates the linearity of experimental ratios vs theoretical ratios.
Absolute glycoproteomics quantification of IgG was simply achieved by direct spiking of isotope-labeled glycopeptide standards for mass spectrometry analysis. To minimize isotope loss,37 the 15 13C-labeled standards in appropriate amounts (Table S2) were spiked into pooled human serum (Sigma) after reduction and alkylation but before trypsin digestion and desalting with C18 cartridges. Samples mixed with isotope-labeled standards were directly analyzed by LC–MS under PRM mode. All neutral glycopeptides as well as acidic glycopeptides (sialylated ones) of IgG1 and IgG2 can be detected and quantified (Table 2), while sialylated ones of IgG3/4 were not detectable in complex mixtures, presumably due to low abundances and/or low ionization efficiencies.38 Thus, HILIC enrichment was performed to quantify sialylated IgG3/4 glycopeptides (Supporting Information). The determined quantitation of IgG1 glycopeptides are consistent with a previous report,39 and calculated proportions of the five glycoforms in each subclass (60% of IgG 1, 30% of IgG2, and 10% of IgG3/4) fall into previously reported ranges.40 In addition, the subsequent intraday repeatability (injecting prepared samples in triplicate on day 1 and day 30) and interday repeatability (compare results from day 1 and day 30) experiments revealed a high reproducibility of this absolute quantification method. For example, the relative standard deviations (RSDs) in the intraday repeatability test fall into a low range from 0.78 to 11.85%, except for low abundant IgG1-G1SF and IgG3/4P-G2SF for 19.37 and 23.03%, respectively. Similarly, the RSD of interday repeatability ranges from 2.94 to 10.53% with the only exception of low abundant glycopeptide IgG3/4P-G1F for 23.23%. These results demonstrated the applicability of isotope dilution MS for absolute quantification of serum IgG glycopeptides.
Table 2.
Absolute Quantification of IgG Glycopeptides of Platelet-Poor Human Plasmaa
| day 1 (n = 3) | day 30 (n = 3) | interday | ||||
|---|---|---|---|---|---|---|
| IgG Glyco-peptides | conc nmol/mL | RSD % | conc nmol/mL | RSD % | RSD % | |
| IgG1 | G0F | 15.42 ± 0.39 | 2.54 | 17.90 ± 1.17 | 6.53 | 10.53 |
| G1F | 19.99 ± 0.16 | 0.78 | 21.13 ± 1.47 | 6.96 | 3.91 | |
| G2F | 12.76 ± 0.83 | 6.48 | 13.31 ± 0.44 | 3.29 | 2.94 | |
| G1SF | 0.87 ± 0.17 | 19.37 | 0.78 ± 0.03 | 3.51 | 8.29 | |
| G2SF | 11.05 ± 0.14 | 1.29 | 11.80 ± 1.25 | 10.63 | 4.65 | |
| IgG2 | G0F | 10.28 ± 0.23 | 2.24 | 10.85 ± 0.45 | 4.15 | 3.80 |
| G1F | 9.73 ± 0.57 | 5.82 | 10.38 ± 0.19 | 1.87 | 4.54 | |
| G2F | 4.15 ± 0.17 | 4.02 | 4.48 ± 0.07 | 1.58 | 5.50 | |
| G1SF | 3.35 ± 0.67 | 20.12 | 3.55 ± 0.43 | 12.25 | 4.21 | |
| G2SF | 2.31 ± 0.24 | 10.39 | 2.42 ± 0.23 | 9.34 | 3.33 | |
| IgG3/4 | G0F | 2.07 ± 0.15 | 7.39 | 2.32 ± 0.15 | 6.64 | 8.18 |
| G1F | 2.90 ± 0.13 | 4.31 | 4.04 ± 0.48 | 11.85 | 23.23 | |
| G2F | 1.85 ± 0.07 | 3.62 | 1.68 ± 0.16 | 9.80 | 6.59 | |
| G1SF | ND | NA | ND | NA | NA | |
| G2SF | 1.82 ± 0.42 | 23.03 | ND | NA | NA | |
ND, not detectable. NA, not applicable.
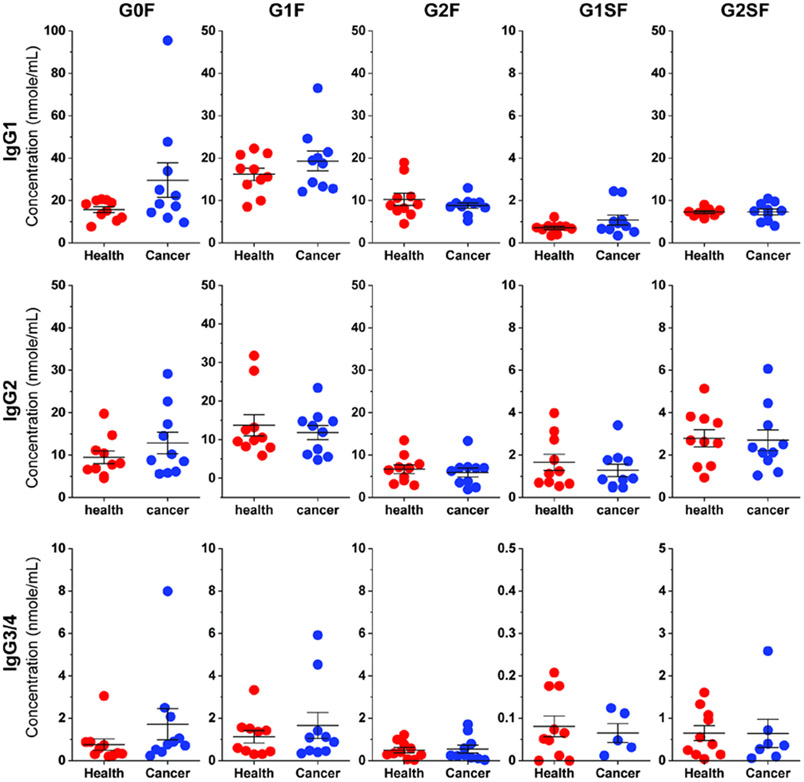
Quantification of IgG Glycopeptides in Colon Cancer Sera Revealed Subclass-Specific Glycosylation Alteration.
Having validated the absolute quantification method, we then implemented it to quantify IgG glycopeptides in colon cancer sera. Compared to healthy controls, it is observed that the quantity of top abundant glycoforms G0F and G1F in IgG1 of cancer sera increased along with the decrease of G2F (Figure 5 and Table S4), which is in agreement with previous reports on overall decreased galactosylation in cancer sera.3,10 On the other hand, while the concentration of G0F in IgG2 increased, that of G1F and G2F in the subclass remained at the same level. The Gal-ratio of G0/(G1 + G2 × 2) was measured to indicate the degree of IgG galactosylation, and increased values were reported in pathogenesis groups compared to control groups.41-43 With absolute quantities of IgG tryptic glycopeptides, such ratios can be easily calculated in a subclass-specific manner. For example, a significantly higher Gal-ratio (G0F/(G1F + G2F × 2)) in IgG1 of the colon cancer group (0.80) was determined compared to that of normal controls (0.43). In contrast, the Gal-ratio in IgG2 has a moderate increase (0.35 in the control group and 0.54 in the colon cancer group). Such a subclass-specific correlation manner may stem from their different binding profiles toward protein antigens (IgG1) or bacterial polysaccharides (IgG2).6
Figure 5.
Dot plot of IgG glycopeptide concentrations in healthy and colon cancer serum samples.
Altered IgG sialylation was also observed in human diseases. For example, increased IgG sialylation is associated with multiple myeloma, whereas decreased IgG sialylation correlates with colorectal cancer.44 Our results showed decreased concentrations of G1SF and G2SF in IgG2 and IgG3/4 of colon cancer sera but increased amounts in IgG1 (Figure 5). Nevertheless, the overall proportion of sialylated glycans (G1SF and G2SF) in each IgG subclass of colon cancer sera decreased compared to that in controls, which is in agreement with previous reports.44 Collectively, absolute quantification of IgG glycopeptides using isotope-labeled standards provides a robust strategy to study altered sera IgG glycosylation and may be readily applied in a large cohort of serum specimens for biomarker discovery.
CONCLUSIONS
Absolute glycoproteomics quantification of serum IgG is of great importance for biomarker discovery and clinical implementation but has long been impeded by the lack of suitable isotope-labeled glycopeptide standards. We developed a general strategy to access glycopeptides attached with diverse glycoforms and fulfilled the preparation of 36 IgG Fc glycopeptides, with or without stable isotope labels. Simple spiking of the standards streamlined absolute quantification of serum IgG Fc glycopeptides in a subclass-specific manner. The strategy can be readily adopted to generate diverse isotope-labeled glycopeptides, and the prepared glycopeptides represent ideal standards for isotope dilution mass spectrometry to study altered serum IgG glycosylation in pathogenesis processes.
Supplementary Material
ACKNOWLEDGMENTS
This work was supported by the National Heart, Lung, and Blood Institute (U54HL142019 to L.L.). We acknowledge Dr. Xi Chen from University of California, Davis for providing the PD26ST plasmid.
Footnotes
Supporting Information
The Supporting Information is available free of charge at https://pubs.acs.org/doi/10.1021/acs.analchem.0c04462.
Detailed synthetic procedures and reaction conditions, NMR spectroscopy, and mass spectrometry data for synthesized compounds (PDF)
The authors declare no competing financial interest.
Contributor Information
Shuaishuai Wang, Department of Chemistry, Georgia State University, Atlanta, Georgia 30303, United States.
Ding Liu, Department of Chemistry, Georgia State University, Atlanta, Georgia 30303, United States.
Jingyao Qu, State Key Laboratory of Microbial Technology, Shandong University, Qingdao 266237, Shandong, China.
He Zhu, Department of Chemistry, Georgia State University, Atlanta, Georgia 30303, United States.
Congcong Chen, Department of Chemistry, Georgia State University, Atlanta, Georgia 30303, United States.
Christopher Gibbons, Department of Chemistry, Georgia State University, Atlanta, Georgia 30303, United States.
Harmon Greenway, Department of Chemistry, Georgia State University, Atlanta, Georgia 30303, United States.
Peng Wang, Department of Chemistry, Georgia State University, Atlanta, Georgia 30303, United States.
Roni J. Bollag, Department of Pathology, Augusta University, Augusta, Georgia 30912, United States.
Kebin Liu, Department of Biochemistry and Molecular Biology, Augusta University, Augusta, Georgia 30912, United States.
Lei Li, Department of Chemistry, Georgia State University, Atlanta, Georgia 30303, United States.
REFERENCES
- (1).Varki A; Gagneux P In Essentials of Glycobiology, rd; Varki A; Cummings RD; Esko JD; Stanley P; Hart GW; Aebi M; Darvill AG; Kinoshita T; Packer NH; Prestegard JH; Schnaar RL; Seeberger PH, Eds.: Cold Spring Harbor (NY), 2015, pp. 77–88. [PubMed] [Google Scholar]
- (2).Abou-Abbass H; Abou-El-Hassan H; Bahmad H; Zibara K; Zebian A; Youssef R; Ismail J; Zhu R; Zhou S; Dong X; Nasser M; Bahmad M; Darwish H; Mechref Y; Kobeissy F Electrophoresis 2016, 37, 1549–1561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (3).Reily C; Stewart TJ; Renfrow MB; Novak J Nat. Rev. Nephrol 2019, 15, 346–366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (4).Delafield DG; Li L Mol. Cell. Proteomics 2020, No. 002095. [Google Scholar]
- (5).Lu LL; Suscovich TJ; Fortune SM; Alter G Nat. Rev. Immunol 2018, 18, 46–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (6).Vidarsson G; Dekkers G; Rispens T Front Immunol. 2014, 5, 520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (7).Schur PH Monogr. Allergy 1988, 23, 1–11. [PubMed] [Google Scholar]
- (8).Zauner G; Selman MH; Bondt A; Rombouts Y; Blank D; Deelder AM; Wuhrer M Mol. Cell. Proteomics 2013, 12, 856–865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (9).Hong Q; Lebrilla CB; Miyamoto S; Ruhaak LR Anal. Chem 2013, 85, 8585–8593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (10).Cobb BA Glycobiology 2020, 30, 202–213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (11).Plomp R; Ruhaak LR; Uh HW; Reiding KR; Selman M; Houwing-Duistermaat JJ; Slagboom PE; Beekman M; Wuhrer M Sci. Rep 2017, 7, 12325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (12).Echeverria B; Etxebarria J; Ruiz N; Hernandez A; Calvo J; Haberger M; Reusch D; Reichardt NC Anal Chem 2015, 87, 11460–11467. [DOI] [PubMed] [Google Scholar]
- (13).Wang Z; Arnold K; Xu Y; Pagadala V; Su G; Myatt H; Linhardt RJ; Liu J Cummun. Biol 2020, 3, 425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (14).Woo CM; Iavarone AT; Spiciarich DR; Palaniappan KK; Bertozzi CR Nat. Methods 2015, 12, 561–567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (15).Liu Z; Cao J; He Y; Qiao L; Xu C; Lu H; Yang P J. Proteome Res 2010, 9, 227–236. [DOI] [PubMed] [Google Scholar]
- (16).Kurogochi M; Amano J Molecules 2014, 19, 9944–9961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (17).Wang J; Zhou C; Zhang W; Yao J; Lu H; Dong Q; Zhou H; Qin L Proteome Sci. 2014, 12, 4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (18).Pabst M; Benesova I; Fagerer SR; Jacobsen M; Eyer K; Schmidt G; Steinhoff R; Krismer J; Wahl F; Preisler J; Zenobi R J. Proteome Res 2015, 15, 326–331. [DOI] [PubMed] [Google Scholar]
- (19).Kim JY; Oh D; Kim SK; Kang D; Moon MH Anal. Chem 2014, 86, 7650–7657. [DOI] [PubMed] [Google Scholar]
- (20).Roy R; Ang E; Komatsu E; Domalaon R; Bosseboeuf A; Harb J; Hermouet S; Krokhin O; Schweizer F; Perreault H J. Am. Soc. Mass Spectrom 2018, 29, 1086–1098. [DOI] [PubMed] [Google Scholar]
- (21).Nilsson J; Brinkmalm G; Ramadan S; Gilborne L; Noborn F; Blennow K; Wallin A; Svensson J; Abo-Riya MA; Huang X; Larson G Sci. Rep 2019, 9, 5522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (22).Chen C; Zhang Y; Xue M; Liu XW; Li Y; Chen X; Wang PG; Wang F; Cao H Chem. Commun 2015, 51, 7689–7692. [DOI] [PubMed] [Google Scholar]
- (23).Calderon AD; Zhou J; Guan W; Wu Z; Guo Y; Bai J; Li Q; Wang PG; Fang J; Li L Org. Biomol. Chem 2017, 15, 7258–7262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (24).Zhu H; Wang S; Liu D; Ding L; Chen C; Liu Y; Wu Z; Bollag R; Liu K; Alexander WM Anal. Chem 2020, 92, 6297–6303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (25).Unverzagt C; Kajihara Y Chem. Soc. Rev 2013, 42, 4408–4420. [DOI] [PubMed] [Google Scholar]
- (26).Li C; Wang LX Chem. Rev 2018, 118, 8359–8413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (27).Gamblin DP; Scanlan EM; Davis BG Chem. Rev 2009, 109, 131–163. [DOI] [PubMed] [Google Scholar]
- (28).Fairbanks AJ Chem. Soc. Rev 2017, 46, 5128–5146. [DOI] [PubMed] [Google Scholar]
- (29).Li L; Liu Y; Ma C; Qu J; Calderon AD; Wu B; Wei N; Wang X; Guo Y; Xiao Z; Song J; Sugiarto G; Li Y; Yu H; Chen X; Wang PG Chem. Sci 2015, 6, 5652–5661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (30).Liu L; Prudden AR; Capicciotti CJ; Bosman GP; Yang JY; Chapla DG; Moremen KW; Boons GJ Nat. Chem 2019, 11, 161–169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (31).Wang Z; Chinoy ZS; Ambre SG; Peng W; McBride R; de Vries RP; Glushka J; Paulson JC; Boons GJ Science 2013, 341, 379–383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (32).Ravi Kumar HV; Naruchi K; Miyoshi R; Hinou H; Nishimura S Org. Lett 2013, 15, 6278–6281. [DOI] [PubMed] [Google Scholar]
- (33).Umekawa M; Higashiyama T; Koga Y; Tanaka T; Noguchi M; Kobayashi A; Shoda S; Huang W; Wang LX; Ashida H; Yamamoto K Biochim. Biophys. Acta 2010, 1800, 1203–1209. [DOI] [PubMed] [Google Scholar]
- (34).Huang W; Giddens J; Fan SQ; Toonstra C; Wang LX J. Am. Chem. Soc 2012, 134, 12308–12318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (35).Wu Z; Jiang K; Zhu H; Ma C; Yu Z; Li L; Guan W; Liu Y; Zhu H; Chen Y; Li S; Li J; Cheng J; Zhang L; Wang PG Bioconjugate Chem. 2016, 27, 1972–1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (36).de Haan N; Falck D; Wuhrer M Glycobiology 2020, 30, 226–240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (37).Zhou S; Tello N; Harvey A; Boyes B; Orlando R; Mechref Y Electrophoresis 2016, 37, 1489–1497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (38).Yu A; Zhao J; Peng W; Banazadeh A; Williamson SD; Goli M; Huang Y; Mechref Y Electrophoresis 2018, 39, 3104–3122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (39).Cao C; Yu L; Fu D; Yuan J; Liang X Anal. Chim. Acta 2020, 1102, 130–139. [DOI] [PubMed] [Google Scholar]
- (40).Hamilton RG Clin. Chem 1987, 33, 1707–1725. [PubMed] [Google Scholar]
- (41).Qian Y; Wang Y; Zhang X; Zhou L; Zhang Z; Xu J; Ruan Y; Ren S; Xu C; Gu J J. Proteome Res 2013, 12, 4046–4055. [DOI] [PubMed] [Google Scholar]
- (42).Ren S; Zhang Z; Xu C; Guo L; Lu R; Sun Y; Guo J; Qin R; Qin W; Gu J Cell Res. 2016, 26, 963–966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (43).Qin W; Pei H; Qin R; Zhao R; Han J; Zhang Z; Dong K; Ren S; Gu J J. Cancer 2018, 9, 906–913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (44).Zhang Z; Wuhrer M; Holst S Glycoconjugate J. 2018, 35, 139–160. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.