Abstract
Background:
Few studies have jointly estimated incidence of MCI, conversion to probable dementia, and mortality in a nationally representatie sample.
Methods:
We used data from six waves of the National Health and Aging Trends Study (2011-2016). Multivariable-adjusted multi-state survival models (MSMs) were used to model incidence upon accounting for misclassification.
Results:
A total of 6,078 eligible NHATS participants were included (average age: 77.49 ± 7.79 years; 58.42% females; 68.99% non-Hispanic white). The incidence of MCI was estimated to be 41.0 [35.5, 47.3]/1,000 person-years (PY). Participants converted to probable dementia at a high rate of 241.3 [189.6, 307.0]/1,000 PY, though a small number also reverted from MCI to cognitively normal. Education was associated with lower incidence of MCI and conversion to probable dementia, but increased mortality in those with MCI. There were also substantial racial and ethnic disparities in the incidence of MCI and dementia.
Conclusions:
Our results underscore the relatively common incidence of and conversions between MCI and dementia in community-dwelling older Americans and uncover the beneficial impact of education to withstand cognitive impairment before death.
Keywords: dementia, cognitive dysfunction, cognition, multistate modeling
Introduction
Longer life expectancies and reduced mortality rates in the United States (U.S.) have led to significant population aging, reduced quality of life and increased mortality from cognitive decline. 1 The onset of MCI and dementia emerge from cognitive decline processes that are complex and dynamic, with a large amount of within-individual and between-individual differences. 2 As adults age, many people experience subtle changes in cognitive abilities that do not affect their independence in daily living activities, but some experience a level of cognitive impairment that may be more severe than expected and indicate onset of possible mild cognitive impairment (MCI). 3 A person experiencing MCI may remain mildly impaired, progress to severe dementia, or even revert to normal cognition. 4
Population-based studies are critical when estimating expectations about the incidence of MCI and conversion rates to dementia, and to provide vital information for public health policy. 5 However, previous estimates of incidence and conversion rates primarily come from Cox proportional survival models and ratio estimates, which generally concentrate on time to the outcome of one cognitive event at a time but cannot examine the potential for reversion. 6,7 Fragmented estimates can cause loss of information when individuals pass through intermediate conditions such as MCI when experiencing the onset of dementia. 8,9 These models also lack a joint consideration for the relationship between rates to and from cognitively normal, MCI, probable dementia, and death. Additionally, misclassification of cognitive status is a fairly common problem that occurs when individuals are assigned to inappropriate categories. Diagnoses of Alzheimer’s disease and other subtypes of dementia are often misclassified in both clinical and population screening settings. 10,11 For example, MCI may be reasonably misclassified as dementia or as cognitively normal depending on the classification schema and premorbid levels of cognitive function. Misclassifications of respondents’ actual cognitive statuses decrease the accuracy of cognitive decline estimates, which are important to address when modeling incidence rates. Thus, there is a need to account for misclassification in cognitive aging studies.
In response to the limitations outlined above, we aimed to estimate the joint course of incidence, progression, and regression of cognitive decline in a representative study of cognitive decline accounting for misclassification probabilities by using a multi-state survival model (MSM). Recent advances have applied MSMs to describe the series of changes in cognitive function, 12,13 which are built on the Markov process that permits the distribution of a future state determined by the current state of disease. 14 The MSM model also allowed us to estimate reversion from MCI to normal cognition. Furthermore, as an extension, a matrix of misclassification probability can fit an MSM to estimate the probability of misclassifications. 14 We utilized MSMs accounting for misclassification for time-to-event analyzes in one cohesive model, including estimates of MCI incidence, conversion from MCI to dementia, reversion from MCI to cognitively normal, and risks of death in a sample of the National Health and Aging Trends Study (NHATS). The results of our study can contribute to joint and precise estimates of the course of U.S. citizen’ s cognitive course as they age.
Methods
Sample
Six waves from National Health and Aging Trends Study (NHATS) were utilized to examine the incidence and conversion rates of MCI and dementia across the 2011-2016 period. NHATS is a nationally representative cohort of adults aged 65 years or older and is publicly available online (https://www.nhats.org/). 15,16 It uses a 3-stage sampling design from the national insurance of Medicare enrollment file and targeted sample sizes by age group and race; older ages and blacks were oversampled to improve the precision of estimates. 17 The NHATS allowed proxy respondents to provide information for respondents with severe cognitive, speech, or hearing problems. The NHATS collected information from individuals, as well as proxy respondents, that living in communities and residential care settings, but not in nursing homes. The first NHATS cohort was enrolled in 2011 and replenished in 2015 to maintain representation for the older Medicare population. 18 Our analytic sample only targeted the first cohort for a longer period of observation.
Respondents who lacked two valid cognitive assessment data were excluded. In total, 6,078 participants were included in our analyzes.
Measures
Cognitive assessment
The NHATS data provided a wide range of cognitive information. Respondents took cognitive tests in 3 domains, including memory testing (immediate and delayed 10-word recall); orientation testing (providing the date, month, year, and day of the week, and naming the President and Vice President); and executive function testing (clock drawing test). Apart from self-reported dementia diagnoses, NHATS also recorded dementia diagnoses and a score of dementia screening interview (AD8) reported by proxy respondents that assessed memory, orientation, judgment, and function of our sample respondents. 19,20 In sum, for self-respondents, a report of dementia diagnosis and cognitive tests were available; for proxy respondents, a report of dementia diagnosis and an AD8 score were available, and for those could be asked cognitive tests judged by his/her proxy, cognitive tests were also available.
We classified NHATS respondents into 3 groups—cognitively normal, MCI, and probable dementia, based upon a protocol developed by NHATS investigators with reasonably good sensitivity and specificity. 21,22 Probable dementia was determined by 1) a self-report or proxy-report of Alzheimer’s disease or dementia diagnosis; or 2) AD8 scores ≥2; or 3) a score of ≤1.5 standard deviations below the mean in at least two to three (orientation, memory, and executive functioning) cognitive functioning domains tested. MCI was defined by a score of ≤1.5 standard deviations below the mean in at least one of the three cognitive tests, as the core clinical criteria of the National Institute on Aging-Alzheimer’s Association framework refers to impairment in at least one cognitive domain. 23 Respondents that met neither the standard for dementia nor the standard for MCI were classified as cognitively normal.
Demographics
Demographics were collected from the baseline round in 2011. Advanced age and sex are the two most widely recognized risk factors for cognitive decline. Race/ethnicity is also found to be related to the risk of cognitive decline and progression to dementia. 24 Education plays a beneficial role in both cognitive assessment and decline processes. 25 Thus, we controlled for covariates of age, gender (male, female), education (less than high school diploma, high school diploma, college degree and beyond), and race/ethnicity (Non-Hispanic white, Non-Hispanic black, Hispanic, other minorities).
Detailed data on the date of interviews and the date of death were incorporated as time variables in our MSMs. The follow-up date (month/year) was reported; each time interval between the first survey and follow-up was measured in months. Missing values of the survey month were replaced using the modal month in that wave. Death dates were retrieved from the sensitive demographic files reported by informants; missing values of the month of death were estimated to be six months after the last follow-up.
Statistical Analyzes
MSMs were used to estimate time-to-event transitions across cognitive statuses, which allowed for more than two states and more transitions extended to the standard survival model. We defined a four-state MSM model governed by a transition intensity matrix, allowing transitions between consecutive states in cognitive decline (successively progressing from cognitively normal to, MCI to, probable dementia to death), reversion from MCI to cognitively normal, and transition to death from any state. Transitions from probable dementia back to MCI or to cognitively normal were not allowed. The few respondents with MCI were allowed to revert to cognitively normal before the going on to develop dementia. 26
To simultaneously estimate transition rates and probabilities of misclassification, we used extended MSMs fit with a matrix of misclassification probabilities. In this way, screening (observed) cognitive statuses of respondents were acknowledged to be subject to error, as they were not always precisely the same as the true (latent) states. In our extended MSMs, the observed cognitive states conditionally depend on the underlying true states which follow the Markov process. The misclassification probabilities for all the defined disease transitions were estimated. 27 Demographic characteristics of the sample at baseline were summarized and included in our adjusted MSMs.
We conducted analyzes using the MSM package in RStudio. 28 All statistical tests used two-sided alpha = 0.05 to identify statistically significant coefficients and 95% confidence intervals (95% CI) were used.
Results
Sample Characteristics
Baseline sample characteristics are shown in Table 1. The sample included 6,078 participants that were predominantly females (n = 3,551, 58.42%) and non-Hispanic whites (n = 4,193, 68.99%), with participants averaging 77.49 years old, the majority of who were educated with a high-school degree (n = 3,088, 51.27%), followed by an average of 4.72 ± 1.61 years.
Table 1.
Baseline Demographic Characteristics of Eligible Respondents.
| Characteristic | N (%) |
|---|---|
| Female gender | 3,551 (58.42) |
| Education | |
| Less than a high school diploma | 1,585 (26.32) |
| High school diploma or equivalent | 3,088 (51.27) |
| College degree or beyond | 1,350 (22.41) |
| Race/ethnicity | |
| Non-Hispanic white | 4,193 (68.99) |
| Non-Hispanic black | 1,316 (21.65) |
| Hispanic | 353 (5.81) |
| Other minorities | 216 (3.55) |
| Mean (SD), N | |
| Age at baseline | 77.49 (7.79), 6,078 |
| Follow-up time [year] | 4.72 (1.61), 6,078 |
| Total | 6,078 |
Results From Unadjusted Models
General incidence rates
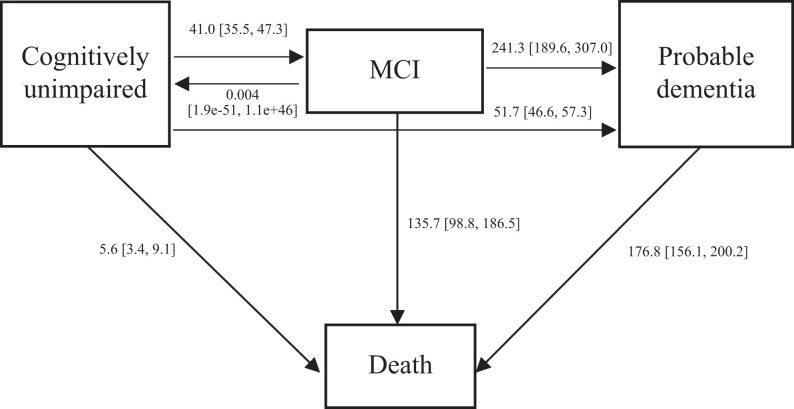
For all the participants, the crude incidence rate of MCI was estimated to be 41.0 [35.5, 47.3]/1,000 person-years (PY), while the crude incidence rate of probable dementia was 51.7 [46.6, 57.3]/1,000 PY. Respondents who were classified as having MCI were at high risk of advancing to probable dementia (= 241.3 [189.6, 307.0]/1,000 PY). A very small number of participants reverted from MCI to a normal cognitive status (0.004 PY; 95% CI: [1.9e-51, 1.1e+46]). Mortality was fairly uncommon in cognitively normal older adults of 5.6 [3.4, 9.1]/1,000 PY, but relatively high in respondents with MCI and probable dementia, who had mortality rates of 135.7 [98.8, 186.5]/1,000 PY and 176.8 [156.1, 200.2]/1,000 PY, respectively. Compared to cognitively normal respondents, respondents with MCI were 24.23 times more likely to pass away, while respondents with probable dementia were 31.57 times more likely. Cognitively normal respondents were 7.32 times as likely to develop MCI and 9.23 times to probable dementia as to die without MCI or probable dementia (Figure 1). All the estimates presented have accounted for misclassification.
Figure 1.
Annual cognitive transition rate for 65 years old and above. Notes: We built a 4-state survival model (MSM) for the cognitive functioning of respondents, including cognitively normal state, mild cognitive impairment (MCI) state, probable dementia state, and death state. The arrows indicate possible instantaneous state-to-state transitions. Respondents can transit between the consecutive state in cognitive decline (successively progressing from cognitively normal to, MCI to, probable dementia to death), revert from MCI to cognitively normal, develop probable dementia from cognitively normal, and transit to death from any state. Numbers expressed as transition rate /1,000 person-year with [95% Confidence Interval].
Age-specific incidence rates
We also computed age-specific incidence rates accounting for misclassification for 5-year age intervals. For the youngest bracket aged between 65 and 70 years, crude MCI incidence was only 9.6 [5.6, 16.3]/1,000 PY, but increased dramatically to 91.2 [70.4, 118.1]/1,000 PY for the oldest-old group aged 85 years and older. Similarly, crude probable dementia incidence for the youngest bracket increased from 11.5 [7.5, 17.7]/1,000 PY to 170.4 [149.6, 194.0]/1,000 PY for the oldest. Consistently, MCI respondents across age groups were at high risk of advancing to probable dementia. Compared to cognitively normal older adults, mortality was relatively high among respondents with MCI and probable dementia. Other than the age group between 70 and 75 years old, a very small number of participants reverted from MCI to a cognitively normal status (Table 2).
Table 2.
Age-Specific Annual Cognitive Transition Rates.
| Previous status | Latter status | 65-70 years old | 70-75 years old | 75-80 years old | 80-85 years old | 85 years and older |
|---|---|---|---|---|---|---|
| Cognitively normal | MCI | 9.6 [5.6, 16.3] | 24.8 [16.4, 37.6] | 42.0 [32.0, 55.1] | 47.1 [32.4, 68.3] | 91.2 [70.4, 118.1] |
| MCI | Cognitively normal | 0.004 [3e-103, 5e+91] | 97.6 [19.7, 484.2] | 0.001 [3e-129, 7e+116] | 0.003 [1e-96, 9e+84] | 0.01 [6e-110, 2e+99] |
| MCI | Probable dementia | 221.5 [85.7, 572.7] | 141.7 [64.1, 313.2] | 225.8 [137.7, 370.4] | 199.3 [106.0, 375.2] | 339.9 [221.5, 521.8] |
| Cognitively normal | Probable dementia | 11.5 [7.5, 17.7] | 18.7 [13.2, 26.6] | 35.4 [27.8, 45.1] | 73.4 [59.3, 90.8] | 170.4 [149.6, 194.0] |
| Cognitively normal | Death | 7.8 [5.3, 11.4] | 7.9 [5.0, 12.5] | 6.2 [2.8, 14.0] | 9.6 [4.0, 23.1] | 2e-7 [1e-111, 3e+97] |
| MCI | Death | 53.1 [9.0, 312.3] | 44.8 [10.6, 188.5] | 93.5 [40.4, 216.3] | 187.7 [104.8, 336.3] | 177.6 [109.5, 288.1] |
| Probable dementia | Death | 54.7 [23.3, 128.5] | 110.6 [65.3, 187.4] | 99.0 [66.0, 148.4] | 151.2 [114.8, 199.2] | 253.1 [217.7, 294.3] |
Notes:
Numbers expressed as transition rate /1,000 person-year with [95% Confidence Interval].
Results From Adjusted Models
In adjusted models (Table 3), a 5-year advanced baseline age was associated with an increased incidence risk of MCI (adjusted Hazard ratio; aHR = 2.100 [1.874, 2.353]), increased incidence of probable dementia (aHR = 2.386 [2.146, 2.652]), and increased mortality risk of probable dementia respondents (aHR = 1.441 [1.266, 1.639]). Compared to females, males had a higher risk of developing MCI (aHR = 1.387 [1.091, 1.762]) and an increased probability of death with MCI (aHR = 1.881 [1.129, 3.134]).
Table 3.
Hazard Ratios (95% Confidence Interval) for the Effect of Baseline Age, Gender, Education, and Race/Ethnicity on Cognitive Decline Process.
| Transition | Baseline age [95% CIs] 1 | Gender [95% CIs] 2 | Less than a high school siploma 3 [95% CIs] | College graduate 3 [95% CIs] | Non-Hispanic black 4 [95% CIs] | Hispanic 4 [95% CIs] | Other minorities 4 [95% CIs] |
|---|---|---|---|---|---|---|---|
| Cognitively normal to MCI | 2.100 [1.874, 2.353] | 1.387 [1.091, 1.762] | 3.802 [2.847, 5.076] | 0.688 [0.495, 0.956] | 2.156 [1.647, 2.822] | 2.948 [1.921, 4.525] | 2.511 [1.513, 4.167] |
| Cognitively normal to probable dementia | 2.386 [2.146, 2.652] | 0.926 [0.753, 1.138] | 2.051 [1.652, 2.548] | 0.683 [0.511, 0.913] | 1.766 [1.417, 2.202] | 2.164 [1.545, 3.031] | 1.254 [0.612, 2.566] |
| Cognitively normal to death | 0.723 [0.500, 1.045] | 1.135 [0.616, 2.092] | 1.649 [0.777, 3.501] | 0.620 [0.298, 1.290] | 0.545 [0.190, 1.562] | 4e-5 [1e-110, 2e+101] | 0.500 [0.015, 17.035] |
| MCI to cognitively normal | 2.224 [0.167, 2.959] | 1.537 [0.897, 2.633] | 95.189 [0.012, 8e+5] | 0.001 [1e-106, 3e+99] | 1.257 [0.645, 2.450] | 2.993 [1.308, 6.850] | 0.382 [0.048, 3.052] |
| MCI to probable dementia | 0.968 [0.778, 1.205] | 0.910 [0.547, 1.513] | 0.779 [0.440, 1.382] | 1.049 [0.581, 1.894] | 0.448 [0.219, 0.918] | 0.713 [0.283, 1.795] | 0.759 [0.280, 2.053] |
| MCI to death | 0.978 [0.797, 1.200] | 1.881 [1.129, 3.134] | 0.374 [0.202, 0.693] | 0.960 [0.505, 1.826] | 1.035 [0.554, 1.935] | 0.607 [0.219, 1.682] | 1.071 [0.368, 3.118] |
| Probable dementia to death | 1.441 [1.266, 1.639] | 1.100 [0.858, 1.412] | 0.842 [0.654, 1.083] | 0.738 [0.506, 1.075] | 0.838 [0.636, 1.105] | 0.544 [0.339, 0.874] | 0.171 [0.033, 0.880] |
Notes:
1Entered as 5-year age groups.
2Reference group: Female respondents.
3Reference group: Respondents had a high school diploma or equivalent.
4Reference group: Non-Hispanic white respondents.
Bold face indicates statistically significant results.
Compared to respondents who had a high school diploma or equivalent, respondents who had less than a high school diploma had an increased risk of MCI incidence (aHR = 3.802 [2.847, 5.076]) and an increased risk of probable dementia (aHR = 2.051 [1.652, 2.548]), but with a decreased death risk with MCI (aHR = 0.374 [0.202, 0.693]). Comparatively, college graduates had decreased risks of both MCI (aHR = 0.688 [0.495, 0.956]) and probable dementia (aHR = 0.683 [0.511, 0.913]).
Compared to non-Hispanic whites, non-Hispanic blacks were more likely to develop MCI (aHR = 2.156 [1.647, 2.822]) and probable dementia (aHR = 1.766 [1.417, 2.202]); Hispanics were also more likely to develop MCI (aHR = 2.948 [1.921, 4.525]) and probable dementia (aHR = 2.164 [1.545, 3.031]); while other minorities were more likely to develop MCI (aHR = 2.511 [1.513, 4.167]). There were also reduced mortality risks of death for Hispanic respondents with probable dementia (aHR = 0.544 [0.339, 0.874]) and respondents of other minorities with probable dementia (aHR = 0.171 [0.033, 0.880]). In terms of the reversion from MCI to cognitively normal, Hispanic MCI respondents had a higher probability of transitioning from MCI to cognitively normal, in contrast to non-Hispanic whites (aHR = 2.993 [1.308, 6.850]).
Misclassification probabilities
In our unadjusted model, respondents with MCI were very likely to be misclassified as cognitively normal respondents (10.4% [7.0%, 15.3%]), or as having probable dementia (15.4% [10.2%, 22.6%]). Comparing unadjusted models to adjusted models, the misclassifications of treating cognitively normal respondents as MCI decreased from 8.2% [7.7%, 8.6%] to 7.2% [6.8%, 7.7%], and the probability of misclassifying cognitively normal respondents as having probable dementia respondents decreased from 4.5% [4.2%, 4.8%] to 4.2% [3.9%, 4.5%]. For MCI respondents misclassified as probable dementia, the misclassification probability decreased from 15.4% [10.2%, 22.6%] to 12.0% [8.3%, 17.3%]. Meanwhile, the probability of misclassifying MCI as cognitively normal increased from 10.4% [7.0%, 15.3%] to 16.2% [10.7%, 23.8%], misclassifying probable dementia as cognitively normal increased from 0.5% [0.2%, 1.2%] to 0.6% [0.3%, 1.3%], and misclassifying probable dementia as MCI increased from 1.0% [0.3%, 3.3%] to 2.0% [1.0%, 3.9%] (Table 4).
Table 4.
Probabilities of Misclassification in MSM.
| Observed status | Actual status estimated | Unadjusted annual transitional probability (%) 1 | Adjusted annual transitional probability (%) 2 |
|---|---|---|---|
| MCI | Cognitively normal | 8.2 [7.7, 8.6] | 7.2 [6.8, 7.7] |
| Probable dementia | Cognitively normal | 4.5 [4.2, 4.8] | 4.2 [3.9, 4.5] |
| Cognitively normal | MCI | 10.4 [7.0, 15.3] | 16.2 [10.7, 23.8] |
| Probable dementia | MCI | 15.4 [10.2, 22.6] | 12.0 [8.3, 17.3] |
| Cognitively normal | Probable dementia | 0.5 [0.2, 1.2] | 0.6 [0.3, 1.3] |
| MCI | Probable dementia | 1.0 [0.3, 3.3] | 2.0 [1.0, 3.9] |
Notes:
1Unadjusted MSM.
2Adjusted MSM accounting for 5-year baseline age, gender, education, and race/ethnicity.
Discussion
This study aimed to examine incidence rates for MCI and conversion to dementia in a large nationally representative prospective cohort of older American residents. We estimated the joint courses of incidence, progression, and regression of cognitive decline in a longitudinal, representative study of MCI and dementia by using MSMs accounting for misclassification probabilities. Our results demonstrated that incidence of MCI and probable dementia were relatively common in community-dwelling older Americans. We estimated the incidence of MCI was 41.0/1,000 PY and probable dementia was 51.7/1,000 PY among cognitively normal respondents aged 65 years and older. Results were consistent with ranges of meta-analysis and systematic reviews worldwide estimating the incidence of MCI (22.5, 60.1) /1,000 PY 29 and dementia (33.08, 84.42) /1,000 PY. 30 For age-specific incidence rates, previous research has reported incidence rates for MCI with 95% CI /1,000 person-years (PY) that ranged 22.5 (5.1-51.4) for ages 75-79 years, 40.9 (7.7-97.5) for ages 80-84 years, and 60.1 (6.7-159.0) for ages 85 years and above. 29
MCI incidence estimates were high when compared to other studies. For example, one study examining incidence of MCI reported that 8.8% of participants experienced the onset of MCI in the Health and Retirement Study (HRS) in 2012. 31 Explanations for incongruent results might include: heterogeneity resulting from the intervals between survey years, differences in the gender and race proportions between samples, differences in inclusion and cognitive status diagnosis criteria, and differences in the methodology used to analyze transitions. The novelty of this study derives from its correction of these issues in traditional age- and sex-standardized analyzes for incidence rates and in our avoidance of the problems of arbitrary observation times and potential misclassifications in our MSM models. Indeed, we addressed a major knowledge gap concerning the most recent cognitive trajectory in the U.S. with improved accuracy. To help address the most recent controversy on whether the population trends in dementia risk declines over time, for example, we think this method could be used across a range of cohorts to determine whether trends have changed broadly or if reductions stem from reductions in misclassification rates.
Our results highlight relatively large probabilities of misclassification and some individuals who appear to be misclassified as cognitively normal when longitudinal data disagrees with baseline scores that are indicative of cognitive impairment. There are also many individuals who are misclassified as mildly impaired but may already have dementia. Adjusted MSMs accounting for covariates has reduced misclassifications compared to unadjusted MSMs in general. Covariates included in MSMs primarily decreased misclassification error in respondents with normal cognition or those with MCI. Overall, misclassification seemed fairly common and are under-examined processes that warrant further development.
A large proportion of the elderly population appears to be afflicted by MCI worldwide. 32 Despite existing knowledge of the prevalence of MCI, there are barely estimates of incidence rate. Our research revealed increasing risks of incidence of MCI as people age. For the reversion from MCI to cognitively normal states, reversion rates for age groups in this study ranged substantially. Explanations may include that the reversion rate is closely associated with misclassification, and weaknesses in definition and diagnostic tools resulted in a high false-positive rate. 33 Misclassification considered in our MSMs may help reduce reversion rate estimates. In general, we have little knowledge about the estimates of the oldest-old and the mechanism behind the reversion of MCI.
Results also show that both MCI and probable dementia were associated with increased risks of mortality, which is supported by previous evidence that cognitive decline shortens life expectancy. 34 We add to the current literature by providing a better understanding of the relationship between cognitive trajectories and older age, gender, race/ethnicity, and education in the risks of death. Our research contributes to our understanding of cognitive aging and the social determinants that govern the risk of MCI and dementia leading to death in older age. Notably, we considered not only the progression to dementia but also reversion from MCI to cognitively normal to describe the cognitive trajectory of the general population. Among the large number of studies of the incidence of MCI, few also incorporated reversions from dementia. Compared to proportion rates of reverting to normal cognition from previous reviews, 35 our estimates of reversion probability are relatively low, which may be attributed to the strict criteria of dementia in NHATS. Longitudinal research is essential for distinguishing different outcomes among MCI patients.
Results reinforced prior research suggesting that social characteristics are associated with future cognitive trajectories. Advanced baseline age is associated with increased risks of dementia and MCI incidence, consistent with previous studies demonstrating that the risk of dementia steadily increases with age. 36 Our results also revealed that respondents who had less than a high school diploma had increased risk of MCI and probable dementia, but with a decreased risk of mortality among those with MCI, while college graduates had decreased risks of both MCI and probable dementia. These reveal strong protective effects against cognitive impairment but accelerated cognitive decline once the onset of cognitive impairment begins. These findings correspond with the cognitive reserve theory, 37 which states that education helps to delay the onset of cognitive impairment but accelerates the progression of cognitive impairment and decreases the likelihood of reversion to cognitively normal once the process of impairment begins.
We further showed that minority groups have increased risks of cognitive decline compared to Non-Hispanic whites. Cognitively normal non-Hispanic blacks and Hispanics face higher risks of developing MCI and dementia, while other minorities face increased MCI incidence. Consistent with previous evidence of racial/ethnic disparities in aging, 38 elderly African Americans and Caribbean Hispanics are thought to suffer from a higher incidence of dementia. 24 Yet, we also found that Hispanics and other minorities had reduced mortality from dementia and that Hispanic MCI respondents were more likely to revert to cognitively normal from MCI when compared to non-Hispanic Whites. This work potentially suggests that the etiology and experience of MCI may be different in minority populations than in the White population. Further research is needed to determine the role of cognitive reserve in this population and the factors that may influence minorities’ aging brains. Future studies should, for example, examine interactions between socioeconomic status and race/ethnicity to determine if having a lower socioeconomic status accelerates the cognitive decline of minorities. With the demographic shift toward a larger elderly U.S. population in the near future, the postponement of cognitive impairment and subsequent shifts in the risk of dementia and accelerated aging in racial and ethnic minorities hide much more severe risks. Overall, disadvantaged socioeconomic status and lower level of education among minorities further aggravate the future aging crisis.
Strengths and Limitations
Our study is novel both in its use of a large longitudinal sample of older minorities in America and its use of MSM, a recently developed extension of the classical survival model based on Markov modeling. A sample of Medicare beneficiaries over 65 years old supports our findings. We used an advanced method to model respondents’ transitions across cognitive statuses and death. To effectively measure the changes in cognition, we used a battery of cognitive assessments combined with proxy reported information to screen for probable dementia and MCI in a nationally representative population. This method demonstrated showed reliable specificity and sensitivity. While previous studies likely suffer from cutoff biases of neuropsychological tests, our research used MSM models to correct this bias and resolve practice effects by extending a misclassification matrix. There are a few limitations in our research worth noting. First, our analyzes did not account for the complex survey design due to the limitation of the R package we used, diminishing generalizability by over-weighting certain populations. Notably, we were unable to account for the population sampling frame, which oversampled some individuals. Second, NHATS participants were screening-detected rather than clinically diagnosed for MCI and dementia. Although our MSM accounted for misclassification and informant reporting metrics were used to help indicate dementia diagnoses, misclassifications are virtually unavoidable. Third, while successfully modeled, our four-state MSMs for cognitive state contained a direct conversion from cognitively normal to probable dementia, indicating that some individuals experienced rapid transitions that we failed to observe cross through the MCI domain. These individuals are highly likely to have dementia from cerebrovascular disease, as exhibited by more rapid declines in cognition. 39 Future studies may use more frequent follow-ups to improve the tracking of transitions and should allow for these transitions to be separately modeled when risk factors for specific diseases causing dementia are being examined. Fourth, some other factors may bias the observed cognitive status, such as depression, physical function, time-related hearing decline, and cardiovascular disease, which should be included in further research. 40 We attempted to incorporate a brief screening instrument for depression in NHATS, but the validity of the brief 2-question questionnaire was limited. This, coupled with the growing concern of reverse causality that depression in old age may be an indicator of neurodegenerative disease, 41 we decided not to incorporate depression into our final models. Finally, more research is needed to confirm these results and examine the representativeness of these analyzes from this cohort.
Conclusion
There is relatively limited evidence of the overall burden of MCI in the population, and estimates of the conversion of MCI to dementia are often derived from clinical studies where estimates may be subject to selection bias. Very little, to date, is known about the misclassification rates among those with MCI and dementia in the population. This study jointly estimated the incidence of MCI and conversion to dementia in a large nationally representative longitudinal cohort. Our estimates of the incidence and conversion rates of MCI and dementia, as well as the number of older adults at risk, contribute to a better understanding of the cognitive transitional trajectory of the general U.S. population and show protective factors, such as education, and replicate prior risk factors, including increased age and racial/ethnic minority status.
Acknowledgments
We thank Amber Luo for helpful proofreading and editing.
Abbreviations
- 95% CI
95% confidence intervals
- AD8
dementia screening interview
- aHR
adjusted Hazard Ratio
- HRS
Health and Retirement Study
- MCI
mild cognitive impairment
- MSM
multi-state survival model
- NHATS
National Health and Aging Trends Study
- PY
person-years
- The U.S.
the United States
Authors’ Note: YZ analyzed data and led the development of the manuscript. GN critically edited the manuscript. SC conceived the study, oversaw the development of the manuscript, and critically edited the manuscript. All authors approved the analysis and the final version of the manuscript.
The authors declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The authors disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: Funding for this analysis was provided by the National Institute on Aging (NIH/NIA RF1 AG058595). The National Health and Aging Trends Study (NHATS) is sponsored by the National Institute on Aging (NIA U01AG032947) through a cooperative agreement with the Johns Hopkins Bloomberg School of Public Health.
ORCID iDs: Yun Zhang  https://orcid.org/0000-0002-9837-1365
https://orcid.org/0000-0002-9837-1365
Sean Clouston  https://orcid.org/0000-0002-6124-0329
https://orcid.org/0000-0002-6124-0329
References
- 1. Onandia-Hinchado I, Diaz-Orueta U. Health related quality of life and cognitive decline in older populations: preliminary results from NeuroDemeNPsia study. J Appl Gerontol. 2020;39(6):618–626. [DOI] [PubMed] [Google Scholar]
- 2. Clouston SA, Richmond LL, Scott SB, et al. Pattern recognition to objectively differentiate the etiology of cognitive decline: analysis of the impact of stroke and Alzheimer’s disease. Neuroepidemiology. 2020;54(6):446–453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Blazer DG, Yaffe K, Liverman CT. Cognitive Aging: Progress in Understanding and Opportunities for Action. National Academies Press; 2015. [PubMed] [Google Scholar]
- 4. Pandya SY, Clem MA, Silva LM, Woon FL. Does mild cognitive impairment always lead to dementia? A review. J Neurol Sci. 2016;369:57–62. [DOI] [PubMed] [Google Scholar]
- 5. Gao S, Burney HN, Callahan CM, Purnell CE, Hendrie HC. Incidence of dementia and Alzheimer disease over time: a meta-analysis. J Am Geriatr Soc. 2019;67(7):1361–1369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Prince M, Ali G-C, Guerchet M, Prina AM, Albanese E, Wu Y-T. Recent global trends in the prevalence and incidence of dementia, and survival with dementia. Alzheimers Res Ther. 2016;8(1):23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Ward A, Tardiff S, Dye C, Arrighi HM. Rate of conversion from prodromal Alzheimer’s disease to Alzheimer’s dementia: a systematic review of the literature. Dement Geriatr Cogn Dis Extra. 2013;3(1):320–332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Andersen PK, Keiding N. Multi-state models for event history analysis. Stat Methods Med Res. 2002;11(2):91–115. [DOI] [PubMed] [Google Scholar]
- 9. de Wreede LC, Fiocco M, Putter H. The mstate package for estimation and prediction in non- and semi-parametric multi-state and competing risks models. Comput Methods Programs Biomed. 2010;99(3):261–274. [DOI] [PubMed] [Google Scholar]
- 10. Bradford A, Kunik ME, Schulz P, Williams SP, Singh H. Missed and delayed diagnosis of dementia in primary care: prevalence and contributing factors. Alzheimer Dis Assoc Disord. 2009;23(4):306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Hwang AB, Boes S, Nyffeler T, Schuepfer G. Validity of screening instruments for the detection of dementia and mild cognitive impairment in hospital inpatients: a systematic review of diagnostic accuracy studies. PLoS One. 2019;14(7):e0219569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Robitaille A, van den Hout A, Machado RJ, et al. Transitions across cognitive states and death among older adults in relation to education: a multistate survival model using data from six longitudinal studies. Alzheimers Dement. 2018;14(4):462–472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Vermunt L, Sikkes SA, Van Den Hout A, et al. Duration of preclinical, prodromal, and dementia stages of Alzheimer’s disease in relation to age, sex, and APOE genotype. Alzheimers Dement. 2019;15(7):888–898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Jackson CH, Sharples LD, Thompson SG, Duffy SW, Couto E. Multistate Markov models for disease progression with classification error. J R Stat Soc, Ser D Stat. 2003;52(2):193–209. [Google Scholar]
- 15. Patel KV, Guralnik JM, Dansie EJ, Turk DC. Prevalence and impact of pain among older adults in the United States: findings from the 2011 National Health and Aging Trends Study. Pain. 2013;154(12):2649–2657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Kasper JD, Freedman VA. National Health and Aging Trends Study Round 1 User Guide: Final Release. Johns Hopkins University School of Public Health; 2012. [Google Scholar]
- 17. Freedman VA, Kasper JD. Cohort profile: the National Health and aging trends study (NHATS). Int J Epidemiol. 2019;48(4):1044–1045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Montaquila J, Freedman V, Edwards B, Kasper J. Round 1 sample design and selection. NHATS technical paper# 1. Johns Hopkins University School of Public Health. 2012. [Google Scholar]
- 19. Hendry K, Lees RA, McShane R, Noel-Storr AH, Stott DJ, Quinn TJ. AD-8 for diagnosis of dementia across a variety of healthcare settings. Cochrane Database Syst Rev. 2014;5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Hendry K, Green C, McShane R, et al. AD-8 for detection of dementia across a variety of healthcare settings. Cochrane Database Syst Rev. 2019;3(3):CD011121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Morris JC. Revised criteria for mild cognitive impairment may compromise the diagnosis of Alzheimer disease dementia. Arch Neurol. 2012;69(6):700–708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Kasper JD, Freedman VA, Spillman BC. Classification of persons by dementia status in the National Health and Aging Trends Study. Technical Paper. 2013;5:1–4. [Google Scholar]
- 23. Albert MS, DeKosky ST, Dickson D, et al. The diagnosis of mild cognitive impairment due to Alzheimer’s disease: recommendations from the National Institute on aging—Alzheimer’s association workgroups on diagnostic guidelines for Alzheimer’s disease. Alzheimers Dement. 2011;7(3):270–279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Mehta KM, Yeo GW. Systematic review of dementia prevalence and incidence in United States race/ethnic populations. Alzheimers Dement. 2017;13(1):72–83. [DOI] [PubMed] [Google Scholar]
- 25. Clouston SA, Smith DM, Mukherjee S, et al. Education and cognitive decline: an integrative analysis of global longitudinal studies of cognitive aging. J Gerontol B Psychol Sci Soc Sci. 2020;75(7):e151–e160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Roberts R, Knopman DS. Classification and epidemiology of MCI. Clin Geriatr Med. 2013;29(4):753–772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Polisetti H. Hidden Markov Chain Analysis: Impact of Misclassification on Effect of Covariates in Disease Progression and Regression. University of South Florida; 2016. [Google Scholar]
- 28. Jackson CH. Multi-state models for panel data: the msm package for R. J Stat Softw. 2011;38(8):1–29. [Google Scholar]
- 29. Gillis C, Mirzaei F, Potashman M, Ikram MA, Maserejian N. The incidence of mild cognitive impairment: a systematic review and data synthesis. Alzheimers Dement (Amst). 2019;11:248–256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Fiest KM, Jette N, Roberts JI, et al. The prevalence and incidence of dementia: a systematic review and meta-analysis. Can J Neurol Sci. 2016;43(S1):S3–S50. [DOI] [PubMed] [Google Scholar]
- 31. Langa KM, Larson EB, Crimmins EM, et al. A comparison of the prevalence of dementia in the United States in 2000 and 2012. JAMA Intern Med. 2017;177(1):51–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Hu C, Yu D, Sun X, Zhang M, Wang L, Qin H. The prevalence and progression of mild cognitive impairment among clinic and community populations: a systematic review and meta-analysis. Int Psychogeriatr. 2017;29(10):1595. [DOI] [PubMed] [Google Scholar]
- 33. Kochan NA, Slavin MJ, Brodaty H, et al. Effect of different impairment criteria on prevalence of “objective” mild cognitive impairment in a community sample. Am J Geriatr Psychiatry. 2010;18(8):711–722. [DOI] [PubMed] [Google Scholar]
- 34. Wilson R, Segawa E, Hizel L, Boyle P, Bennett D. Terminal dedifferentiation of cognitive abilities. Neurology. 2012;78(15):1116–1122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Thomas KR, Edmonds EC, Eppig JS, et al. MCI-to-normal reversion using neuropsychological criteria in the Alzheimer’s disease neuroimaging initiative. Alzheimers Dement. 2019;15(10):1322–1332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Hersi M, Irvine B, Gupta P, Gomes J, Birkett N, Krewski D. Risk factors associated with the onset and progression of Alzheimer’s disease: a systematic review of the evidence. Neurotoxicology. 2017;61:143–187. [DOI] [PubMed] [Google Scholar]
- 37. Stern Y. What is cognitive reserve? Theory and research application of the reserve concept. J Int Neuropsychol Soc. 2002;8(3):448–460. [PubMed] [Google Scholar]
- 38. RTI Press. Racial and Ethnic Disparities Among Individuals With Alzheimer’s Disease in the United States: A Literature Review. Research Triangle Park; 2014. [Google Scholar]
- 39. Clouston SA, Zhang Y, Smith DM. Pattern recognition to identify stroke in the cognitive profile: secondary analyses of a prospective cohort study. Cerebrovasc Dis. 2019;9(3):114–122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Plassman BL, Williams JW, Jr, Burke JR, Holsinger T, Benjamin S. Systematic review: factors associated with risk for and possible prevention of cognitive decline in later life. Ann Intern Med. 2010;153(3):182–193. [DOI] [PubMed] [Google Scholar]
- 41. Singh-Manoux A, Marmot MG, Glymour M, Sabia S, Kivimäki M, Dugravot A. Does cognitive reserve shape cognitive decline? Ann Neurol. 2011;70(2):296–304. [DOI] [PMC free article] [PubMed] [Google Scholar]