Abstract
Despite the increased use of hemp fiber, negligible attention has been given to upgrade the hemp hurd, which constitutes up to 70 wt % of the hemp stalk and is currently considered a low-value byproduct. In this work, valorization of hemp hurd was performed by reductive catalytic fractionation (RCF) in the presence of a metal catalyst. We found an unexpectedly high yield of monophenolic compounds (38.3 wt %) corresponding to above 95% of the theoretical maximum yield. The high yield is explained by both a thin cell wall and high S-lignin content. In addition, organosolv pulping was performed to generate a pulp that was bleached to produce dissolving-grade pulp suitable for textile fiber production (viscosity, 898 mL/g; ISO-brightness, 90.2%) and nanocellulose. Thus, we have demonstrated a novel value chain from a low-value side stream of hemp fiber manufacture that has the potential to increase textile fiber production with 100% yield and also give bio-oil for green chemicals.
Keywords: Hemp hurd, Lignin, Biomass valorization, Reductive catalytic fractionation, Organosolv pulping, Dissolving pulp, Nanocellulose, Monophenolic compounds
Short abstract
Hemp hurd, a byproduct from hemp fiber production, was successfully valorized by reductive catalytic fractionation to give high yields of monophenolic compounds and a pulp suitable for textiles or nanocellulose.
Introduction
Natural fibers are massively cultivated from plants, especially cotton, which is widely grown as a global economic crop mainly for textile manufacture.1 Cotton fields cover 34.5 million (M) hectares (ha) of arable land worldwide with an average yield of 2.14 tons ha–1 yr–1 seed cotton, corresponding to a global average annual production of cotton lint of 0.76 tons ha–1 yr–1.2 Even though cotton is a bio-based fiber, its sustainability is questionable.3 This is due to the high maintenance cost of growing cotton, which demands large amounts of pesticides and chemical fertilizers derived from fossil oil.4,5 In addition, around 3 tons of water is consumed for each ton of cotton produced.6 Measures have been made to tackle these problems such as genetic modification, site selection, and nonchemical control strategies. However, cotton production is still debated.7−9 This has led to an effort to replace cotton with bast fiber crops such as flax and hemp that do not require the same maintenance.10,11
Hemp (Cannabis Sativa) is a herbaceous crop that can be grown in most climates, while cotton is limited to cultivation in subtropical climate zones. Hemp has recently gained attention for natural fiber production due to both agricultural and sustainability reasons. This includes resistance to drought and pests, prevention of soil erosion, and less water demand in comparison to other fiber crops. It can supply both phytochemical and lignocellulosic biomass applications.12 From a phytochemical perspective, hemp produces various chemicals such as phenolic compounds, terpenes, and cannabinoids which can be utilized in the pharmaceutical industry.13 The hemp stem composes of approximately 30 wt % of outer bast fiber and 70 wt % of the inner core (woody part also known as hurd or shiv).14 The two fractions must be separated to be further utilized. Hemp fiber extraction starts with retting, where pectin is digested by natural microorganisms and the inner core is separated from the fiber. A mechanical process is then applied to break down the stem using fluted rolls followed by scutching, which separates the fiber from the hurd core. The final step is hackling, also called decortication, to comb the fiber and remove unwanted particles.15 The hemp fiber is predominately utilized for the textile industry, insulation material, and production of bioplastics in the automotive industry.16,17 However, the hemp hurd, which constitutes 70 wt % of the hemp stalks, is considered a byproduct from textile fiber production and is only used for low-value applications, such as animal bedding due to its high absorption capacity, and as a concrete additive. Due to the supply exceeding the demand, the excessive waste of hemp hurd is currently disposed by combustion and landfill accumulation.18 There are few examples of research that utilize hemp hurd. In most cases, the main focus has been on material applications such as antibacterial, biocomposite, and activated carbon materials.19−21
Reductive catalytic fractionation (RCF)22−27 is a promising strategy that integrates lignin depolymerization with biomass fractionation using heterogeneous catalysis. It produces lignin-derived aromatic monomers and oligomers along with carbohydrate pulps and sugars. In general, RCF can be performed in batch and flow-through systems in the presence of a metal catalyst, hydrogen donor, and a mixture of aqueous and organic solvents.
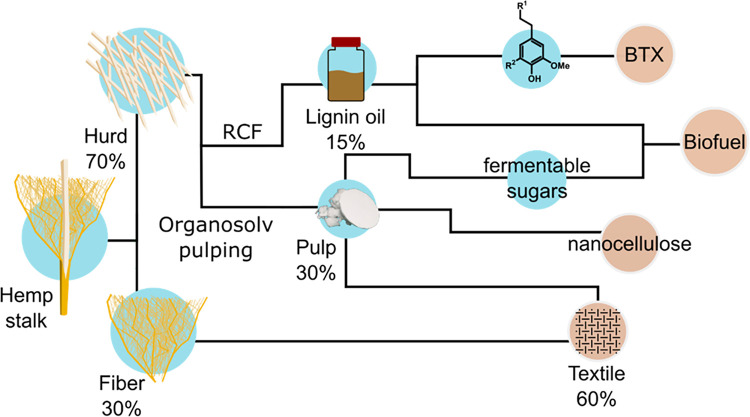
In this work, valorization of hemp hurd by organosolv pulping has been performed in a batch system to yield a high-value pulp suitable for textile fibers and nanocellulose.28,29 By the application of RCF, a lignin oil enriched in monophenolic compounds was generated that could be used for bulk chemical production such as BTX (Scheme 1).30−32 In recent findings using flow-through systems, pulp can be obtained without the contamination of the catalyst together with the lignin oil.33−35 However, for this preliminary study of this herbaceous type biomass, a flow-through methodology was not our focus. However, this will be of interest in future studies. Instead, we performed organosolv pulping and were able to increase the overall yield of fiber for textiles production by 100%.
Scheme 1. Hemp Hurd Valorized by RCF to Yield a Lignin Oil and Organosolv Pulping to Yield Textile Fiber.
Results and Discussion
RCF of Hemp Hurd
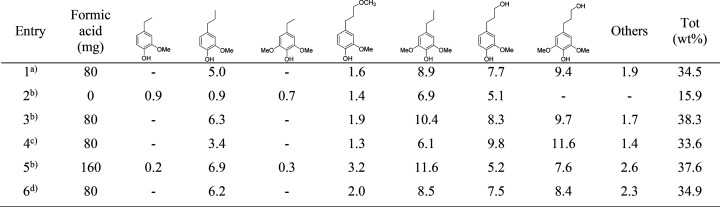
RCF was performed in a steel pressure reactor using a transition-metal catalyst (Pd/C) and formic acid as the hydrogen donor. The solvent system comprised MeOH/H2O (7/3 v/v), and p-toluenesulfonic acid (p-TSA) 1.1 g/L was added as a hydrolysis catalyst. The reaction conditions were optimized by varying the reaction time and the amounts of formic acid. The outcome of the reactions was detected both qualitatively and quantitatively by GC-MS and GC-FID on comparison to synthesized monophenolic compounds and quantified using an internal standard. The distribution of monophenolic compounds from GC-MS after RCF is shown in Table 1. When the reaction time was increased, the yield of monophenolic compounds increased to 38.3 wt % in 4 h (entry 3). When the reaction time was increased to 8 h (entry 6), the yield of monophenolic compounds decreased, which could possibly be explained by hydrodearomatization, as it is one of the major side reactions that causes a low aromatic monomer yield.30,36−38 However, hydrodearomatized products were not detected by GC, probably due to the volatility of the compounds and low yields of dearomatized products (38.3% vs 34.9%). The influence of p-TSA was investigated. We found that the yield of monophenolic compounds decreased from 38.3 to 33.6 wt % in the absence of p-TSA (entry 4). This shows that solvolysis is promoted by the acid catalyst. Without formic acid, the yield of monophenolic compounds was significantly decreased to 15.9 wt % showed in Entry 2, due to the deficiency of hydrogen donors to facilitate hydrogenation/hydrogenolysis for the generation of stable monophenolic compounds. Higher concentrations of formic acid did not improve the results (Entry 5).
Table 1. RCF Optimization and Monophenolic Yield Quantified by GC-FID.
Reaction conditions: 3 h, p-TSA (1.1 g/L).
Reaction conditions: 4 h, p-TSA (1.1 g/L).
Reaction conditions: 4 h, no p-TSA involved.
Reaction conditions: 8 h, p-TSA (1.1 g/L).
RCF of hemp hurd shows an unexpectedly high monomer yield (38.3 wt %). This corresponds to above 95% of the theoretical maximum yield determined by thioacidolysis (39.7%) (see the Supporting Information). We propose that the density and particle size of biomass potentially affect lignin extraction. Recent reports have emphasized the effect of density and particle size of biomass toward lignin extraction.39,40 Thus, the morphology of hemp hurd was investigated by SEM (scanning electron microscopy), as shown in Figure 1. A honeycomb-like cell wall structure was observed. The average cell wall thickness was measured to be 1.95 μm (standard deviation 0.17), which is slightly thinner than that of other herbaceous crops (∼2.00–3.00 μm).41−43 This might explain the observed high yield of monophenolic compounds. This is rationalized by an enhancement of the intrinsic kinetics of the solvent-based fractionation, where a thinner cell wall promotes mass transfer through diffusion during solvolytic lignin extraction.39 Moreover, 80% delignification can be achieved after organosolv and no lignin monomer was observed (Figures S8 and S9) due to lignin recondensation.
Figure 1.
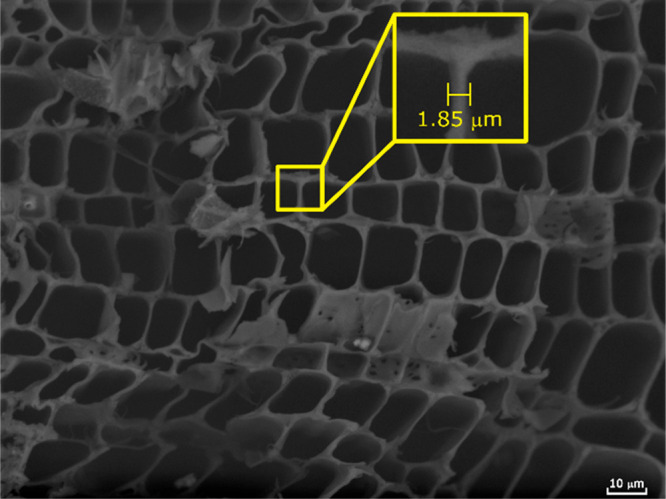
SEM image of the cross-section of hemp hurd wood chips.
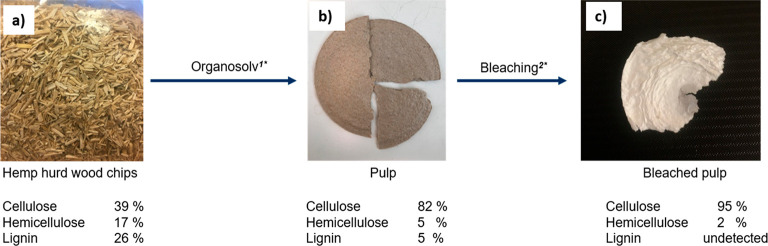
To obtain a high-quality pulp, the RCF conditions described above could not be applied due to catalyst contamination of the pulp. In this study, a separate organosolv pulping process was performed on the hemp hurd (Figure 2a) without metal catalyst to evaluate the pulp for fiber production. It should be noted that, after organosolv pulping, the cellulose fraction was isolated as a pulp (Figure 2b).44 Such pulps can be used in the commercial production of cellulose derivatives such as cellulose acetate, cellulose nitrate, and viscose.28 To meet the requirements for these applications, a pulp with both a high viscosity and brightness is required. Thus, chlorite bleaching was applied to remove the remaining lignin residues to give the white pulp shown in Figure 2c with a high viscosity (898 mL/g) and high ISO-brightness (90.2%), which meets the requirements for viscose production.
Figure 2.
Pulping process from hemp hurd wood chips to bleached pulp with lignocellulosic composition: (a) raw material before RCF; (b) obtained pulp after organosolv pulping; (c) obtained bleached pulp after chlorite bleaching. Reaction conditions: (1*) 0.8 g of hemp hurd stick, EtOH/H2O (65/35) 10 mL, 100 μL of 1% HCl, 175 °C, 3 h; (2*) 6.0 g of pulp, 300 mL of 1.7% NaClO2 solution, 300 mL of 2.7% NaOH and 7.5% of AcOH solution, 80 °C, 2 h.
Nanocellulose
Nanocellulose is a general class of materials that can be subdivided into three groups: cellulose nanocrystals (CNC), cellulose nanofibers (CNF), and bacterial cellulose (BC).45 The main difference, which also leads to morphological variations among these three groups, is the production method of each one of them. Generally, CNC is derived from chemical treatment of cellulose sources (acid hydrolysis or oxidation), CNF is obtained from the delamination of cellulose pulps with mechanical treatment (using high-pressure homogenizers, microfluidizers, or grinders), and BC is produced by bacteria.46,47 Due to its high aspect ratio and fiber entanglement, CNF has been used as fillers and reinforcement in nanocomposites and/or hybrids in order to not only improve the mechanical properties (i.e., tensile strength, and Young’s modulus) but also enhance the thermal stability of other biopolymers (i.e., chitosan) or lower the swelling degree and water solubility of synthetic polymers: for instance, poly(vinyl alcohol) films.47 Furthermore, CNF has been used for the preparation of edible films with possible application in food packaging,48 membranes for water filtration and catalytic hydrogenation of dyes,49 aerogels for thermal insulation and fire retardancy,50 and coatings to provide antifouling properties to PVA filters.51 In this study, CNF was obtained in the form of a translucent colloidal dispersion, as shown in Figure 3. AFM (atomic force microscopy) images confirmed the nanoscale morphology with diameters in the range of 5–10 nm of the produced CNF (Figure 4).
Figure 3.

Colloidal dispersion of hemp hurd CNF.
Figure 4.
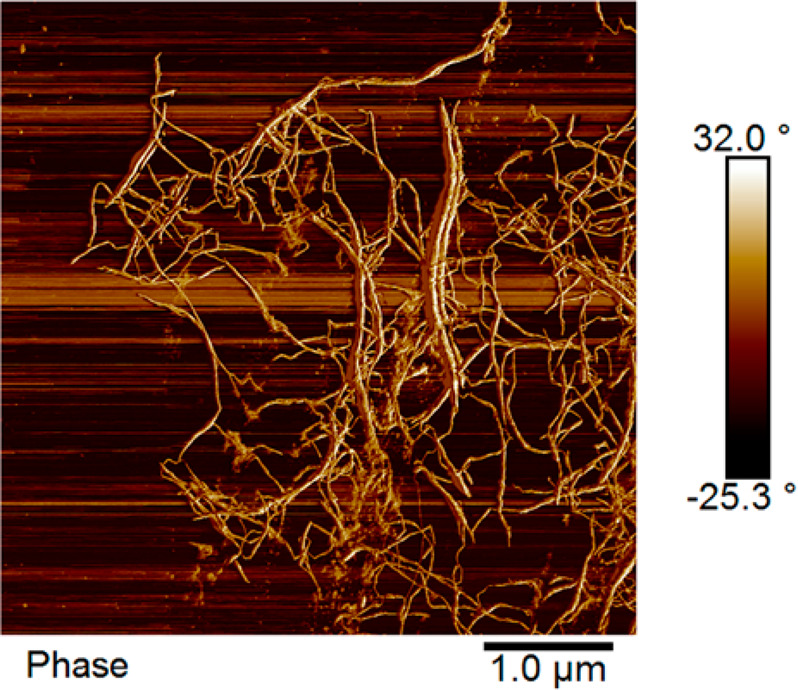
AFM image of hemp hurd CNF.
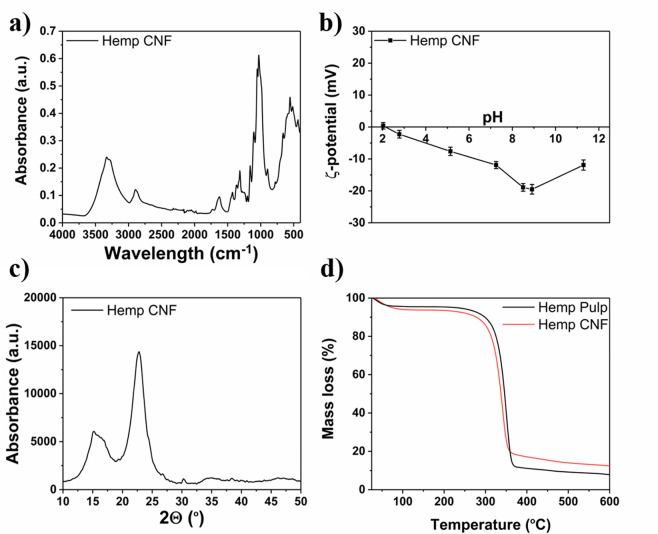
From the FTIR spectrum (Figure 5a) of the produced CNF, characteristic peaks of a stretching vibration corresponding to O–H bonds of the hydroxyl groups (3330 cm–1), a stretching vibration corresponding to the C–H bonds (2896 cm–1), and the stretching vibration of the C=O bonds of the carbonyl groups (1620 cm–1) can be observed.52,53 The presence of carbonyl groups is explained by the residual hemicellulose in the pulp after bleaching (approximately 2 wt %, as mentioned earlier), which is also confirmed by the surface charge from a conductometric titration, which showed a density of 43 mmol/kg, and the ζ-potential values, which are negative throughout a pH range between 2 and 12 (Figure 5b).54 The XRD diffractogram (Figure 5c) shows the characteristic peaks at 16 and 22° corresponding to the (110) and (200) Miller indices, respectively. The peak of the former index appears to be broader, which is expected in the case of CNF.39 The crystallinity index (CrI) of the produced CNF was calculated from the obtained diffractogram to be 79%, using the empirical method reported by Segal et al.55 The TGA spectra (Figure 5d) show an onset of thermal degradation at 328 °C for the hemp pulp and at 317 °C for the hemp CNF. In the case of CNF, a severe reduction in the molecular weight of the polymer chains in combination with a higher number of terminal points of the chains explain the earlier degradation onset.56 At the end of the degradation process, the hemp pulp showed an 87 wt % change in mass while the hemp CNF showed an 83 wt % change in mass (after the amount of water/humidity in the samples was considered). This difference and, hence, the production of more char from the CNF have been previously reported and attributed to the smaller size of the individual particles.53
Figure 5.
Characterization of hemp hurd CNF: (a) FTIR, (b) ζ-potential, (c) XRD, and (d) TGA of the produced CNF.
Overall, the morphology, surface chemistry, and physical properties of the produced CNF agree with those of the conventional CNF; hence, the bleached pulp obtained in this work is an excellent candidate for the production of high-quality CNF.
Conclusions
Valorization of hemp hurd, a byproduct from textile fiber production, was performed by RCF. The RCF was conducted in the presence of a transition-metal catalyst (Pd/C) and formic acid in MeOH/H2O with the addition of p-TSA to yield 38.8 wt % of monophenolic compounds. This corresponds to above 95% of the theoretical maximum yield. We propose that the high yield can be explained by the high S/G ratio and the thin cell wall of the hemp hurd. Organosolv pulping followed by chlorite bleaching was performed to obtain pulp in 50 wt % yield that meets the requirement for viscose production. Thus, this increases the overall yield of textile fiber production from hemp to 100%. This pulp was also successfully used to produce nanocellulose in high yields. This research discloses a new value chain of using hemp hurd, a byproduct from hemp fiber production where both the lignin and cellulose fractions have been valorized.
Experimental Section
Feedstock Analysis
The full feedstock analysis including extraction, two-step acid hydrolysis, HSQC, nitrobenzene oxidation, and thioacidolysis can be found in the Supporting Information
RCF of Hemp Hurd
Raw hemp hurd (0.2 g), Pd/C 5% (20 mg), and 4 mL of MeOH/H2O (7/3) containing 1.1 g/L of p-TSA were placed in a 7 mL steel reactor, followed by addition of 80 mg of formic acid. The reaction was conducted at 200 °C. The lignin oil was extracted with DCM, washed with water, and dried over anhydrous Na2SO4. The catalyst was filtered through Celite. The collected organic phase was filtered and concentrated under reduced pressure. The crude product was dissolved in 10 mL of acetonitrile, tetracosane as an internal standard was added for GC-MS/FID analysis, and the yield of monophenolic compounds was determined to be 38.3 wt %.
Organosolv Pulping of Hemp Hurd
The dissolving-grade pulp was prepared by placing 0.8 g of hemp hurd, 10 mL of the solvent mixture EtOH/H2O (65/35), and 100 μL of 1% H2SO4 in a 20 mL stainless steel reactor. The reaction mixture was heated at 175 °C for 3 h. After the reaction, the reactor was cooled to room temperature and the pulp was separated by filtration. The wood pulp was transferred into a 2 L beaker that contained 600 mL of distilled water and disintegrated with a T-25 digital ULTRA-TURRAX Homogenizer with 20.4 × 1000 rpm for 15 min. The 0.4 g of the solid pulp was obtained after filtration (50 wt % from the original hemp hurd). The obtained solid pulp was then bleached with 6.0 g pulp/600 mL of bleaching solution (300 mL of 1.7% NaClO2 + 300 mL of 2.7% NaOH + 7.5% AcOH). The bleaching was conducted at 80 °C for 2 h. After completion, the reaction mixture was cooled to room temperature and the bleached pulp was separated by filtration with negligible loss in weight and dried overnight for further analysis.
Supporting Information Available
The Supporting Information is available free of charge at https://pubs.acs.org/doi/10.1021/acssuschemeng.1c06607.
Detailed experimental procedures, analytical data, and spectra (PDF)
Author Contributions
S.M., N.L., and J.S.M.S. performed the experimental work on characterization of biomass and RCF and upgrading of cellulose to dissolving pulp and enzymatic hydrolysis; D.G. and A.P.M. performed the characterization of pulp and nanocellulose production and evaluation; P.O. and V.P. contributed to understanding the value chain and generation of hemp hurd; all authors contributed to writing the manuscript.
This project has received funding from the Bio Based Industries Joint Undertaking under the European Union’s Horizon 2020 research and innovation program under grant agreement No. 744349 (SSUCHY project) and the Swedish Foundation for Strategic Environmental Research (Mistra:project Mistra SafeChem, project no. 2018/11)
The authors declare the following competing financial interest(s): J.S.M.S. is founder of RenFuel, a company that valorizes lignin. No other authors have any conflict of interests.
Supplementary Material
References
- Günaydin G. K.; Avinc O.; Palamutcu S.; Yavas A.; Soydan A. S.. Naturally Colored Organic Cotton and Naturally Colored Cotton Fiber Production. In Organic Cotton: Is it a Sustainable Solution?; Gardetti M. A., Muthu S. S., Eds.; Springer: Singapore, 2019; Textile Science and Clothing Technology; pp 81–99. 10.1007/978-981-10-8782-0_4. [DOI] [Google Scholar]
- Khan M. A.; Wahid A.; Ahmad M.; Tahir M. T.; Ahmed M.; Ahmad S.; Hasanuzzaman M.. World Cotton Production and Consumption: An Overview. In Cotton Production and Uses: Agronomy, Crop Protection, and Postharvest Technologies; Ahmad S., Hasanuzzaman M., Eds.; Springer: Singapore, 2020; pp 1–7. 10.1007/978-981-15-1472-2_1. [DOI] [Google Scholar]
- Li B.; Tian Q.; Wang X.; Han B.; Liu L.; Kong X.; Si A.; Wang J.; Lin Z.; Zhang X.; Yu Y.; Yang X. Phenotypic Plasticity and Genetic Variation of Cotton Yield and Its Related Traits under Water-Limited Conditions. Crop J. 2020, 8 (6), 966–976. 10.1016/j.cj.2020.02.003. [DOI] [Google Scholar]
- Bachmann F. Potential and Limitations of Organic and Fair Trade Cotton for Improving Livelihoods of Smallholders: Evidence from Central Asia. Renew. Agric. Food Syst. 2012, 27 (2), 138–147. 10.1017/S1742170511000202. [DOI] [Google Scholar]
- Yilmaz I.; Akcaoz H.; Ozkan B. An Analysis of Energy Use and Input Costs for Cotton Production in Turkey. Renewable Energy 2005, 30 (2), 145–155. 10.1016/j.renene.2004.06.001. [DOI] [Google Scholar]
- Mekonnen M. M.; Hoekstra A. Y. The Green, Blue and Grey Water Footprint of Crops and Derived Crop Products. Hydrol. Earth Syst. Sci. 2011, 15 (5), 1577–1600. 10.5194/hess-15-1577-2011. [DOI] [Google Scholar]
- Wossink A.; Denaux Z. S. Environmental and Cost Efficiency of Pesticide Use in Transgenic and Conventional Cotton Production. Agric. Syst. 2006, 90 (1), 312–328. 10.1016/j.agsy.2006.01.004. [DOI] [Google Scholar]
- Morse S.; Bennett R. M.; Ismael Y. Genetically Modified Insect Resistance in Cotton: Some Farm Level Economic Impacts in India. Crop Prot. 2005, 24 (5), 433–440. 10.1016/j.cropro.2004.09.008. [DOI] [Google Scholar]
- Shun-xiang R. E. N.; Zhen-zhong W.; Bao-li Q. I. U.; Yuan X. The Pest Status of Bemisia Tabaci in China and Non-Chemical Control Strategies*. Insect Sci. 2001, 8 (3), 279–288. 10.1111/j.1744-7917.2001.tb00453.x. [DOI] [Google Scholar]
- Duque Schumacher A. G.; Pequito S.; Pazour J. Industrial Hemp Fiber: A Sustainable and Economical Alternative to Cotton. J. Cleaner Prod. 2020, 268, 122180. 10.1016/j.jclepro.2020.122180. [DOI] [Google Scholar]
- Ebskamp M. J. M. Engineering Flax and Hemp for an Alternative to Cotton. Trends Biotechnol. 2002, 20 (6), 229–230. 10.1016/S0167-7799(02)01953-4. [DOI] [PubMed] [Google Scholar]
- Ingrao C.; Lo Giudice A.; Bacenetti J.; Tricase C.; Dotelli G.; Fiala M.; Siracusa V.; Mbohwa C. Energy and Environmental Assessment of Industrial Hemp for Building Applications: A Review. Renewable Sustainable Energy Rev. 2015, 51, 29–42. 10.1016/j.rser.2015.06.002. [DOI] [Google Scholar]
- Montserrat-de la Paz S.; Marín-Aguilar F.; García-Giménez M. D.; Fernández-Arche M. A. Hemp (Cannabis Sativa L.) Seed Oil: Analytical and Phytochemical Characterization of the Unsaponifiable Fraction. J. Agric. Food Chem. 2014, 62 (5), 1105–1110. 10.1021/jf404278q. [DOI] [PubMed] [Google Scholar]
- Cranshaw W.; Schreiner M.; Britt K.; Kuhar T. P.; McPartland J.; Grant J. Developing Insect Pest Management Systems for Hemp in the United States: A Work in Progress. J. Integr. Pest Manag. 2019, 10 (1), 1. 10.1093/jipm/pmz023. [DOI] [Google Scholar]
- Musio S.; Müssig J.; Amaducci S. Optimizing Hemp Fiber Production for High Performance Composite Applications. Front. Plant Sci. 2018, 9, 1. 10.3389/fpls.2018.01702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shahzad A. Impact and Fatigue Properties of Hemp–Glass Fiber Hybrid Biocomposites. J. Reinf. Plast. Compos. 2011, 30 (16), 1389–1398. 10.1177/0731684411425975. [DOI] [Google Scholar]
- Murugu Nachippan N.; Alphonse M.; Bupesh Raja V. K.; Shasidhar S.; Varun Teja G.; Harinath Reddy R. Experimental Investigation of Hemp Fiber Hybrid Composite Material for Automotive Application. Mater. Today Proc. 2021, 44, 3666–3672. 10.1016/j.matpr.2020.10.798. [DOI] [Google Scholar]
- González-García S.; Hospido A.; Feijoo G.; Moreira M. T. Life Cycle Assessment of Raw Materials for Non-Wood Pulp Mills: Hemp and Flax. Resour. Conserv. Recycl. 2010, 54 (11), 923–930. 10.1016/j.resconrec.2010.01.011. [DOI] [Google Scholar]
- Khan B. A.; Wang J.; Warner P.; Wang H. Antibacterial Properties of Hemp Hurd Powder against E. Coli. J. Appl. Polym. Sci. 2015, 132 (10), 41588. 10.1002/app.41588. [DOI] [Google Scholar]
- Xiao X.; Chevali V. S.; Song P.; He D.; Wang H. Polylactide/Hemp Hurd Biocomposites as Sustainable 3D Printing Feedstock. Compos. Sci. Technol. 2019, 184, 107887. 10.1016/j.compscitech.2019.107887. [DOI] [Google Scholar]
- Liu S.; Ge L.; Gao S.; Zhuang L.; Zhu Z.; Wang H. Activated Carbon Derived from Bio-Waste Hemp Hurd and Retted Hemp Hurd for CO2 Adsorption. Compos. Commun. 2017, 5, 27–30. 10.1016/j.coco.2017.06.002. [DOI] [Google Scholar]
- Anderson E. M.; Stone M. L.; Katahira R.; Reed M.; Beckham G. T.; Román-Leshkov Y. Flowthrough Reductive Catalytic Fractionation of Biomass. Joule 2017, 1 (3), 613–622. 10.1016/j.joule.2017.10.004. [DOI] [Google Scholar]
- Sun Z.; Fridrich B.; de Santi A.; Elangovan S.; Barta K. Bright Side of Lignin Depolymerization: Toward New Platform Chemicals. Chem. Rev. 2018, 118 (2), 614–678. 10.1021/acs.chemrev.7b00588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schutyser W.; Renders T.; Van den Bosch S.; Koelewijn S.-F.; Beckham G. T.; Sels B. F. Chemicals from Lignin: An Interplay of Lignocellulose Fractionation, Depolymerisation, and Upgrading. Chem. Soc. Rev. 2018, 47 (3), 852–908. 10.1039/C7CS00566K. [DOI] [PubMed] [Google Scholar]
- Rinaldi R.; Jastrzebski R.; Clough M. T.; Ralph J.; Kennema M.; Bruijnincx P. C. A.; Weckhuysen B. M. Paving the Way for Lignin Valorisation: Recent Advances in Bioengineering, Biorefining and Catalysis. Angew. Chem., Int. Ed. 2016, 55 (29), 8164–8215. 10.1002/anie.201510351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van den Bosch S.; Schutyser W.; Vanholme R.; Driessen T.; Koelewijn S.-F.; Renders T.; De Meester B.; Huijgen W. J. J.; Dehaen W.; Courtin C. M.; Lagrain B.; Boerjan W.; Sels B. F. Reductive Lignocellulose Fractionation into Soluble Lignin-Derived Phenolic Monomers and Dimers and Processable Carbohydrate Pulps. Energy Environ. Sci. 2015, 8 (6), 1748–1763. 10.1039/C5EE00204D. [DOI] [Google Scholar]
- Abu-Omar M. M.; Barta K.; Beckham G. T.; Luterbacher J. S.; Ralph J.; Rinaldi R.; Román-Leshkov Y.; Samec J. S. M.; Sels B. F.; Wang F. Guidelines for Performing Lignin-First Biorefining. Energy Environ. Sci. 2021, 14, 262. 10.1039/D0EE02870C. [DOI] [Google Scholar]
- Oprea M.; Voicu S. I. Recent Advances in Composites Based on Cellulose Derivatives for Biomedical Applications. Carbohydr. Polym. 2020, 247, 116683. 10.1016/j.carbpol.2020.116683. [DOI] [PubMed] [Google Scholar]
- Sarkanen K. V. The chemistry of delignification in pulp bleaching. Pure Appl. Chem. 1962, 5 (1–2), 219–232. 10.1351/pac196205010219. [DOI] [Google Scholar]
- Galkin M. V.; Samec J. S. M. Lignin Valorization through Catalytic Lignocellulose Fractionation: A Fundamental Platform for the Future Biorefinery. ChemSusChem 2016, 9 (13), 1544–1558. 10.1002/cssc.201600237. [DOI] [PubMed] [Google Scholar]
- Kumaniaev I.; Subbotina E.; Galkin M. V.; Srifa P.; Monti S.; Mongkolpichayarak I.; Tungasmita D. N.; Samec J. S. M. A Combination of Experimental and Computational Methods to Study the Reactions during a Lignin-First Approach. Pure Appl. Chem. 2020, 92 (4), 631–639. 10.1515/pac-2019-1002. [DOI] [Google Scholar]
- Fan M.; Deng S.; Wang T.; Li Q. Production of BTX through Catalytic Depolymerization of Lignin. Chin. J. Chem. Phys. 2014, 27 (2), 221–226. 10.1063/1674-0068/27/02/221-226. [DOI] [Google Scholar]
- Anderson E. M.; Stone M. L.; Hülsey M. J.; Beckham G. T.; Román-Leshkov Y. Kinetic Studies of Lignin Solvolysis and Reduction by Reductive Catalytic Fractionation Decoupled in Flow-Through Reactors. ACS Sustainable Chem. Eng. 2018, 6 (6), 7951–7959. 10.1021/acssuschemeng.8b01256. [DOI] [Google Scholar]
- Lan W.; Du Y. P.; Sun S.; Behaghel de Bueren J.; Heroguel F.; Luterbacher J. S. Continuous Hydrogenolysis of Acetal-Stabilized Lignin in Flow. Green Chem. 2021, 23 (1), 320–327. 10.1039/D0GC02928A. [DOI] [Google Scholar]
- Kumaniaev I.; Subbotina E.; Savmarker J.; Larhed M.; Galkin M. V.; Samec J. S. M. Lignin Depolymerization to Monophenolic Compounds in a Flow-through System. Green Chem. 2017, 19 (24), 5767–5771. 10.1039/C7GC02731A. [DOI] [Google Scholar]
- Liao Y.; Koelewijn S.-F.; Van den Bossche G.; Van Aelst J.; Van den Bosch S.; Renders T.; Navare K.; Nicolaï T.; Van Aelst K.; Maesen M.; Matsushima H.; Thevelein J. M.; Van Acker K.; Lagrain B.; Verboekend D.; Sels B. F. A Sustainable Wood Biorefinery for Low–Carbon Footprint Chemicals Production. Science 2020, 367 (6484), 1385–1390. 10.1126/science.aau1567. [DOI] [PubMed] [Google Scholar]
- Sun Z.; Bottari G.; Afanasenko A.; Stuart M. C. A.; Deuss P. J.; Fridrich B.; Barta K. Complete Lignocellulose Conversion with Integrated Catalyst Recycling Yielding Valuable Aromatics and Fuels. Nat. Catal. 2018, 1 (1), 82–92. 10.1038/s41929-017-0007-z. [DOI] [Google Scholar]
- Di Francesco D.; Subbotina E.; Rautiainen S.; Samec J. S. M. Ductile Pd-Catalysed Hydrodearomatization of Phenol-Containing Bio-Oils Into Either Ketones or Alcohols Using PMHS and H2O as Hydrogen Source. Adv. Synth. Catal. 2018, 360 (20), 3924–3929. 10.1002/adsc.201800614. [DOI] [Google Scholar]
- Thornburg N. E.; Pecha M. B.; Brandner D. G.; Reed M. L.; Vermaas J. V.; Michener W. E.; Katahira R.; Vinzant T. B.; Foust T. D.; Donohoe B. S.; Román-Leshkov Y.; Ciesielski P. N.; Beckham G. T. Mesoscale Reaction–Diffusion Phenomena Governing Lignin-First Biomass Fractionation. ChemSusChem 2020, 13, 4495–4509. 10.1002/cssc.202000558. [DOI] [PubMed] [Google Scholar]
- Pinkert A.; Goeke D. F.; Marsh K. N.; Pang S. Extracting Wood Lignin without Dissolving or Degrading Cellulose: Investigations on the Use of Food Additive -Derived Ionic Liquids. Green Chem. 2011, 13 (11), 3124–3136. 10.1039/C1GC15671C. [DOI] [Google Scholar]
- Xia Q.; Chen C.; Yao Y.; He S.; Wang X.; Li J.; Gao J.; Gan W.; Jiang B.; Cui M.; Hu L. In Situ Lignin Modification toward Photonic Wood. Adv. Mater. 2021, 33 (8), 2001588. 10.1002/adma.202001588. [DOI] [PubMed] [Google Scholar]
- Jiang Y.; Lawrence M.; Ansell M. P.; Hussain A. Cell Wall Microstructure, Pore Size Distribution and Absolute Density of Hemp Shiv. R. Soc. Open Sci. 2018, 5 (4), 171945. 10.1098/rsos.171945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mongioví C.; Lacalamita D.; Morin-Crini N.; Gabrion X.; Ivanovska A.; Sala F.; Placet V.; Rizzi V.; Gubitosa J.; Mesto E.; Ribeiro A. R. L.; Fini P.; Vietro N. D.; Schingaro E.; Kostić M.; Cosentino C.; Cosma P.; Bradu C.; Chanet G.; Crini G. Use of Chènevotte, a Valuable Co-Product of Industrial Hemp Fiber, as Adsorbent for Pollutant Removal. Part I: Chemical, Microscopic, Spectroscopic and Thermogravimetric Characterization of Raw and Modified Samples. Molecules 2021, 26 (15), 4574. 10.3390/molecules26154574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jafari V.; Sixta H.; van Heiningen A. Kinetics of Oxygen Delignification of High-Kappa Pulp in a Continuous Flow-Through Reactor. Ind. Eng. Chem. Res. 2014, 53 (20), 8385–8394. 10.1021/ie403336x. [DOI] [Google Scholar]
- Trache D.; Tarchoun A. F.; Derradji M.; Hamidon T. S.; Masruchin N.; Brosse N.; Hussin M. H. Nanocellulose: From Fundamentals to Advanced Applications. Front. Chem. 2020, 8, 1. 10.3389/fchem.2020.00392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klemm D.; Cranston E. D.; Fischer D.; Gama M.; Kedzior S. A.; Kralisch D.; Kramer F.; Kondo T.; Lindström T.; Nietzsche S.; Petzold-Welcke K.; Rauchfuß F. Nanocellulose as a Natural Source for Groundbreaking Applications in Materials Science: Today’s State. Mater. Today 2018, 21 (7), 720–748. 10.1016/j.mattod.2018.02.001. [DOI] [Google Scholar]
- Klemm D.; Kramer F.; Moritz S.; Lindström T.; Ankerfors M.; Gray D.; Dorris A. Nanocelluloses: A New Family of Nature-Based Materials. Angew. Chem., Int. Ed. 2011, 50 (24), 5438–5466. 10.1002/anie.201001273. [DOI] [PubMed] [Google Scholar]
- Valencia L.; Nomena E. M.; Mathew A. P.; Velikov K. P. Biobased Cellulose Nanofibril–Oil Composite Films for Active Edible Barriers. ACS Appl. Mater. Interfaces 2019, 11 (17), 16040–16047. 10.1021/acsami.9b02649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Georgouvelas D.; Abdelhamid H. N.; Li J.; Edlund U.; Mathew A. P. All-Cellulose Functional Membranes for Water Treatment: Adsorption of Metal Ions and Catalytic Decolorization of Dyes. Carbohydr. Polym. 2021, 264, 118044. 10.1016/j.carbpol.2021.118044. [DOI] [PubMed] [Google Scholar]
- Zhou S.; Apostolopoulou-Kalkavoura V.; Tavares da Costa M. V.; Bergström L.; Strømme M.; Xu C. Elastic Aerogels of Cellulose Nanofibers@Metal–Organic Frameworks for Thermal Insulation and Fire Retardancy. Nano-Micro Lett. 2020, 12 (1), 9. 10.1007/s40820-019-0343-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aguilar-Sanchez A.; Jalvo B.; Mautner A.; Rissanen V.; Kontturi K. S.; Abdelhamid H. N.; Tammelin T.; Mathew A. P. Charged Ultrafiltration Membranes Based on TEMPO-Oxidized Cellulose Nanofibrils/Poly(Vinyl Alcohol) Antifouling Coating. RSC Adv. 2021, 11 (12), 6859–6868. 10.1039/D0RA10220B. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Foster E. J.; Moon R. J.; Agarwal U. P.; Bortner M. J.; Bras J.; Camarero-Espinosa S.; Chan K. J.; Clift M. J. D.; Cranston E. D.; Eichhorn S. J.; Fox D. M.; Hamad W. Y.; Heux L.; Jean B.; Korey M.; Nieh W.; Ong K. J.; Reid M. S.; Renneckar S.; Roberts R.; Shatkin J. A.; Simonsen J.; Stinson-Bagby K.; Wanasekara N.; Youngblood J. Current Characterization Methods for Cellulose Nanomaterials. Chem. Soc. Rev. 2018, 47 (8), 2609–2679. 10.1039/C6CS00895J. [DOI] [PubMed] [Google Scholar]
- Soni B.; Hassan E. B.; Mahmoud B. Chemical Isolation and Characterization of Different Cellulose Nanofibers from Cotton Stalks. Carbohydr. Polym. 2015, 134, 581–589. 10.1016/j.carbpol.2015.08.031. [DOI] [PubMed] [Google Scholar]
- Pääkkö M.; Ankerfors M.; Kosonen H.; Nykänen A.; Ahola S.; Österberg M.; Ruokolainen J.; Laine J.; Larsson P. T.; Ikkala O.; Lindström T. Enzymatic Hydrolysis Combined with Mechanical Shearing and High-Pressure Homogenization for Nanoscale Cellulose Fibrils and Strong Gels. Biomacromolecules 2007, 8 (6), 1934–1941. 10.1021/bm061215p. [DOI] [PubMed] [Google Scholar]
- Segal L.; Creely J. J.; Martin A. E.; Conrad C. M. An Empirical Method for Estimating the Degree of Crystallinity of Native Cellulose Using the X-Ray Diffractometer. Text. Res. J. 1959, 29 (10), 786–794. 10.1177/004051755902901003. [DOI] [Google Scholar]
- Kumar A.; Negi Y. S.; Choudhary V.; Bhardwaj N. K. Characterization of Cellulose Nanocrystals Produced by Acid-Hydrolysis from Sugarcane Bagasse as Agro-Waste. J. Mater. Phys. Chem. 2014, 2 (1), 1–8. 10.12691/jmpc-2-1-1. [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.