Abstract
Background
Selection of intracorporeal anastomosis (IA) or extracorporeal anastomosis (EA) in laparoscopic right colectomy (LRC) remains controversial. This meta-analysis aimed to evaluate the effectiveness and safety of IA compared with EA in LRC patients.
Methods
Literature was searched systematically for randomized controlled trials (RCTs) that compared IA with EA in LRC patients until May 2021. The eligible studies for risk of bias were assessed using the Cochrane Risk of Bias Tool. Data were extracted and analysed for the following outcomes of interest: operative time, length of incision, nodal harvest, bowel function recovery, postoperative pain, postoperative complications (wound infection, anastomotic leak, ileus, obstruction, reoperation), death at 30 days, duration of hospital stay and 30-day readmission.
Results
Five RCTs, including a total of 559 patients, were eligible for meta-analysis. All of the trials reported adequate random sequence generation and allocation concealment. There were significantly better outcomes in the IA group than in the EA group in time to first flatus (mean difference (MD) −0.71 (95 per cent c.i. −1.12 to −0.31), P = 0.0005), time to first passage of stool (MD −0.53 (95 per cent c.i. −0.69 to −0.37), P < 0.00001), visual analogue scale of pain on postoperative day (POD) 3 (MD −0.76 (95 per cent c.i. −1.23 to −0.28), P = 0.002), POD 4 (MD −0.83 (95 per cent c.i. −1.46 to −0.20), P = 0.01), POD 5 (MD −0.60 (95 per cent c.i. −0.95 to −0.25), P = 0.0007), length of incision (MD −1.52 (95 per cent c.i. −2.30 to −0.74), P = 0.0001) and wound infection (relative risk 0.46 (95 per cent c.i. 0.23 to 0.91), P = 0.02). However, there were no statistically significant differences between the two groups in duration of hospital stay (P = 0.47), operative time (P = 0.07), number of lymph nodes harvested (P = 0.70), anastomotic leak (P = 0.88), postoperative ileus (P = 0.48), bleeding (P = 0.15), bowel obstruction (P = 0.24), reoperation (P = 0.34), readmission within 30 days (P = 0.26), and death (P = 0.70).
Conclusion
Compared with EA, IA shows a faster recovery of bowel function with fewer wound infections.
Electronic databases were searched systematically for randomized controlled trials (RCTs) that compared intracorporeal anastomosis (IA) with extracorporeal anastomosis (EA) in patients who underwent laparoscopic right colectomy. Compared with EA, IA shows a faster recovery of bowel function with fewer postoperative complications. This meta-analysis of RCTs generated a high level of evidence supporting the choice to perform IA.
Introduction
The advantages of laparoscopic right colectomy (LRC) for colon carcinoma compared with open right colectomy (ORC) have been confirmed by several trials1–3. LRC is superior to ORC in early recovery and short-term complications and equivalent in oncological outcomes4,5. For the ileocolic anastomosis of LRC, extracorporeal anastomosis (EA) is used more frequently than intracorporeal anastomosis (IA), due to technical facilities and the shorter surgical time compared with IA6,7. However, EA requires greater mobilization and exteriorization of the bowel through the abdominal incision for further steps, which may lead to tissue injury to the mesentery and affect the recovery of bowel function7. IA was reported to have a longer operative time than EA in several retrospective studies, whereas a faster recovery after surgery was demonstrated because of the shorter incision and less traction and mobilization of the mesentery8–10. In recent years, IA has gained more and more attention because of the development of intracorporeal devices and suturing techniques (linear stapler and barbed suture, among others). However, the selection of IA versus EA remains controversial among surgeons, mainly depending on their expertise and personal preference. Aiming to provide a robust guideline for surgeons, a meta-analysis of RCTs was performed to evaluate the effectiveness and safety of IA compared with EA in LRC patients.
Methods
This study was conducted according to the recommendations of the PRISMA statement11.
Literature search
A systematic literature search was performed up to 30 May 2021, using the terms ‘laparoscopic right colectomy/laparoscopic right hemicolectomy/laparoscopic right colon resection’, ‘intracorporeal anastomosis/anastomoses’, and ‘extracorporeal anastomosis/anastomoses’ in the following databases: MEDLINE, EMBASE, the Cochrane Library, the Clinical Trials Database (ClinicalTrials.gov http://clinicaltrials.gov/, World Health Organization International Clinical Trials Registry http://apps.who.int/trialsearch/, ISRCTN Register http://www.isrctn.com/ and Chinese Clinical Trial Registry http://www.chictr.org.cn/index.aspx), China National Knowledge Infrastructure (CNKI, https://www.cnki.net/) and Wanfang Med Online (http://med.wanfangdata.com.cn/). The reference lists of the identified relevant articles, conference proceedings and ongoing trial databases were further screened for potentially relevant studies. There was no language restriction while screening for the relevant studies.
The titles and abstracts of all of the identified articles were screened, and the trials were included for analysis according to the following criteria: RCTs that compared IA with EA; patients with diseases that needed to be treated with LRC; and outcomes included effectiveness or postoperative complications. Studies were excluded if they were retrospective, had no randomization or had no control arm.
Data extraction and quality assessment
Two investigators independently extracted the following data from all the included trials: patient characteristics, study design, patient inclusion and exclusion criteria, surgery process, intraoperative results and postoperative outcomes. Details of randomization, allocation concealment, blinding, number of patients allocated to each arm, and procedures of IAs and EAs were recorded. If the important data were not reported, the authors were contacted as early as possible. The same reviewers assessed the methodological quality of each trial. A third reviewer was consulted if there were any discrepancies, and consensus was reached by discussion. The quality of each included study was determined using the Cochrane Collaboration's tool for assessing the risk of bias, a value of low risk, high risk or unclear was assigned to the seven domains: random sequence generation, allocation concealment, blinding of participants and personnel, blinding of outcome assessment, incomplete outcome data, selective reporting and other bias12.
Data analysis and outcomes of interest
Data were extracted and analysed for the following primary outcomes: postoperative recovery (time to first flatus, time to first passage of stool, duration of hospital stay, postoperative pain); and operative data (length of incision, operative time and number of lymph nodes harvested).
Secondary outcomes (complications) were: anastomotic leak, wound infection, postoperative ileus, bleeding (gastrointestinal or anastomotic bleeding), bowel obstruction, reoperation, readmission within 30 days and death at 30 days.
A sensitivity analysis was also performed for eliminating the potential clinical heterogeneities of different types of ileocolic anastomosis. A subgroup analysis based on the peristaltic orientation of anastomosis was also performed.
Statistical analysis
Statistical analyses were performed with the recommendations of the Cochrane Collaboration Guidelines12. Review Manager software (RevMan, version 5.4.1 for Windows) was used to perform this meta-analysis13. Dichotomous outcomes were presented as relative risk (RR) and continuous outcomes were presented as mean difference (m.d.); 95 per cent confidence intervals were quantified for all the analyses. Data expressed as median with range were converted to mean with standard deviation for continuous outcomes using methods as described before14. Heterogeneity was assessed with Cochran's χ2 test and the I2 test. Statistically significant heterogeneity was considered when P was <0.100 and the I2 test value was >50 per cent15. The fixed effects model was used if there was no significant statistical heterogeneity (P > 0.100 and I2 < 50 per cent). If heterogeneity existed, the random effects model was applied16. Sensitivity analyses were performed with trials of performing side-to-side and stapled ileocolic anastomosis. Subgroup analysis was performed by stratifying the trials based on the peristaltic orientation of anastomosis (antiperistaltic anastomosis and isoperistaltic anastomosis).
Results
An initial screening resulted in the identification of a total of 1836 potentially relevant studies. Further analysis revealed that only five RCTs with 559 patients met all the inclusion criteria and these underwent a full analysis (Fig. 1)17–21. The characteristics of the included trials are shown in Table 1. In these five RCTs, IA was performed in 281 of 559 patients (50.3 per cent), while EA was performed in 278 patients (49.7 per cent). Most of the included patients were diagnosed with malignant tumours of the right colon (534 patients, 95.5 per cent). The sites of the tumour were as follows: 259 in the caecum (46.3 per cent), 171 in the ascending colon (30.6 per cent) and 129 in the colon liver flexure (23.1 per cent). For the type of ileocolic anastomosis, four trials reported that side-to-side (476 of 499 patients, 95.4 per cent), stapled (465 of 499 patients, 93.2 per cent), and anti-peristaltic (279 of 499 patients, 55.9 per cent) were the preferred choices17–19,21. Enterotomies were all closed with two layers of absorbable sutures. In the IA group, four trials reported that the incisions for specimen extraction were Pfannenstiel incision (160 of 251 patients, 63.7 per cent), midline (54 of 251 patients, 21.5 per cent), transverse (20 of 251 patients, 8.0 per cent) and others (17 of 251 patients, 6.8 per cent); in the EA group, the ratios of above-mentioned incisions were 0.8 per cent (2 of 248 patients), 43.1 per cent (107 of 248 patients), 48.0 per cent (119 of 248 patients) and 8.1 per cent (20 of 248 patients) respectively17–19,21. The length of the incision was significantly shorter in the IA group than in the EA group. The mesenteric defects were all closed in two trials17,21. These studies17,21 plus another study19, applied several recommendations of the enhanced recovery after surgery (ERAS) protocol22, such as no perioperative mechanical bowel preparation, postoperative analgesia, early resumption of diet, removal of the urinary catheter on postoperative day (POD) 1 and early mobilization on POD 1; the full ERAS protocol was implemented in the other two trials18,20.
Fig. 1.
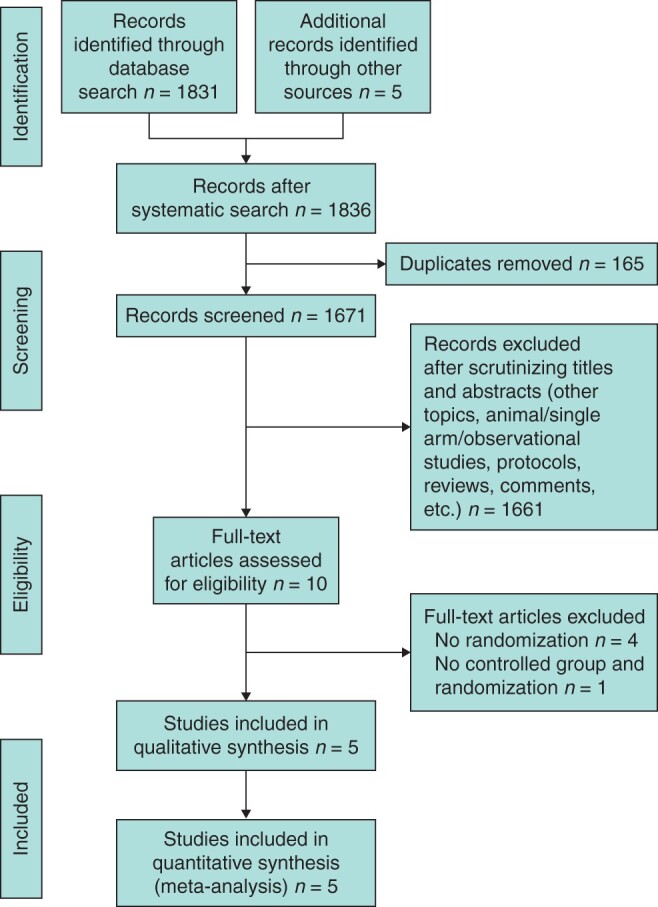
Flow diagram of literature search
Table 1.
General characteristics of the included trials
| Study | Anastomosis | No. of patients | Age (years) | Male N (%) | BMI, kg/m2 | Center | ERAS (complete/partial) |
|---|---|---|---|---|---|---|---|
| Allaix 201917 | IA | 70 | 70 (65–77)† | 39 (55.7) | 24 (22.4–28.7)† | Single-centre | Partial |
| EA | 70 | 72 (65–77)† | 41 (58.6) | 25.2 (22.9–29.2)† | |||
| Bollo 202018 | IA | 69 | 72.7 (10.4)* | 34 (49.3) | 27.4 (5.4)* | Single-centre | Complete |
| EA | 70 | 70.9 (11.7)* | 39 (55.7) | 26.3 (4.7)* | |||
| Ferrer 202119 | IA | 82 | 70.51 (9.88)* | 43 (52.4) | 28.25 (4.69)* | Multi-centre | Partial |
| EA | 78 | 68.65 (12.51)* | 39 (50.0) | 28.67 (4.54)* | |||
| Mari 201820 | IA | 30 | 66 (42–83)† | 19 (63.3) | 24.3 (5.9)* | Single-centre | Complete |
| EA | 30 | 72 (39–87)† | 16 (53.3) | 26.1 (3.3)* | |||
| Vignali 201621 | IA | 30 | 67.4 (1.8)* | 16 (53.3) | 24.6 (4.3)* | Single-centre | Partial |
| EA | 30 | 64.7 (2.9)* | 14 (46.7) | 24.8 (3.4)* |
| Study | Anastomosis | No. of patients | Tumour |
Tumour location |
|||
|---|---|---|---|---|---|---|---|
| Malignant | Benign | Caecum | Ascending colon | Colon liver flexure | |||
| Allaix 201917 | IA | 70 | 54 | 16 | 39 | 18 | 13 |
| EA | 70 | 61 | 9 | 41 | 12 | 17 | |
| Bollo 202018 | IA | 69 | 69 | 0 | 29 | 21 | 19 |
| EA | 70 | 70 | 0 | 27 | 12 | 31 | |
| Ferrer 202119 | IA | 82 | 82 | 0 | 35 | 27 | 20 |
| EA | 78 | 78 | 0 | 33 | 31 | 14 | |
| Mari 201820 | IA | 30 | 30 | 0 | 12 | 16 | 2 |
| EA | 30 | 30 | 0 | 14 | 14 | 2 | |
| Vignali 201621 | IA | 30 | 30 | 0 | 14 | 9 | 7 |
| EA | 30 | 30 | 0 | 15 | 11 | 4 | |
| Study | Anastomosis | No. of patients | Type of ileocolic anastomosis |
|||||
|---|---|---|---|---|---|---|---|---|
| Side-to-side | End-to-end | End-to-side | Stapled | Hand-sewn | Orientation | |||
| Allaix 201917 | IA | 70 | 70 | 0 | 0 | 70 | 0 | Anti-peristaltic |
| EA | 70 | 47 | 1 | 22 | 36 | 34 | ||
| Bollo 202018 | IA | 69 | 69 | 0 | 0 | 69 | 0 | Anti-peristaltic |
| EA | 70 | 70 | 0 | 0 | 70 | 0 | ||
| Ferrer 202119 | IA | 82 | 82 | 0 | 0 | 82 | 0 | Iso-peristaltic |
| EA | 78 | 78 | 0 | 0 | 78 | 0 | ||
| Mari 201820 | IA | 30 | NA | NA | NA | NA | NA | NA |
| EA | 30 | NA | NA | NA | NA | NA | ||
| Vignali 201621 | IA | 30 | 30 | 0 | 0 | 30 | 0 | Iso-peristaltic |
| EA | 30 | 30 | 0 | 0 | 30 | 0 | ||
*Mean (s.d.),
†Median (range).
ERAS, enhanced recovery after surgery; NA, not available.
Risk of bias in included trials
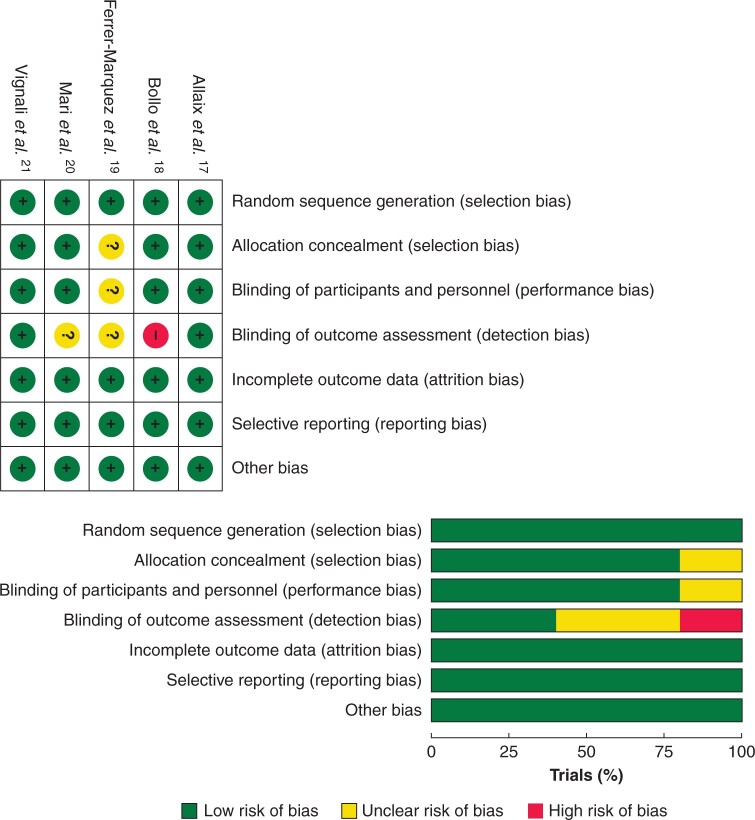
The Cochrane Risk of Bias Tool was used to assess the potential risk of bias in the included trials (Fig. 2). All of the trials reported adequate random sequence generation and allocation concealment. All of the patients were blinded to their treatment in four trials17,18,20,21, one trial did not report blinding of patients19, and two trials blinded the outcome assessors17,21. Surgeon blinding would have been inappropriate in all of the included trials. However, the randomization envelope was only opened to the surgeons at the beginning of the procedure or anastomosis. Attrition bias and reporting bias are both at low risk.
Fig. 2.
Risk of bias summary and graph
Each risk of bias item for the included trials is presented as a percentage across all the included trials.
Primary outcomes
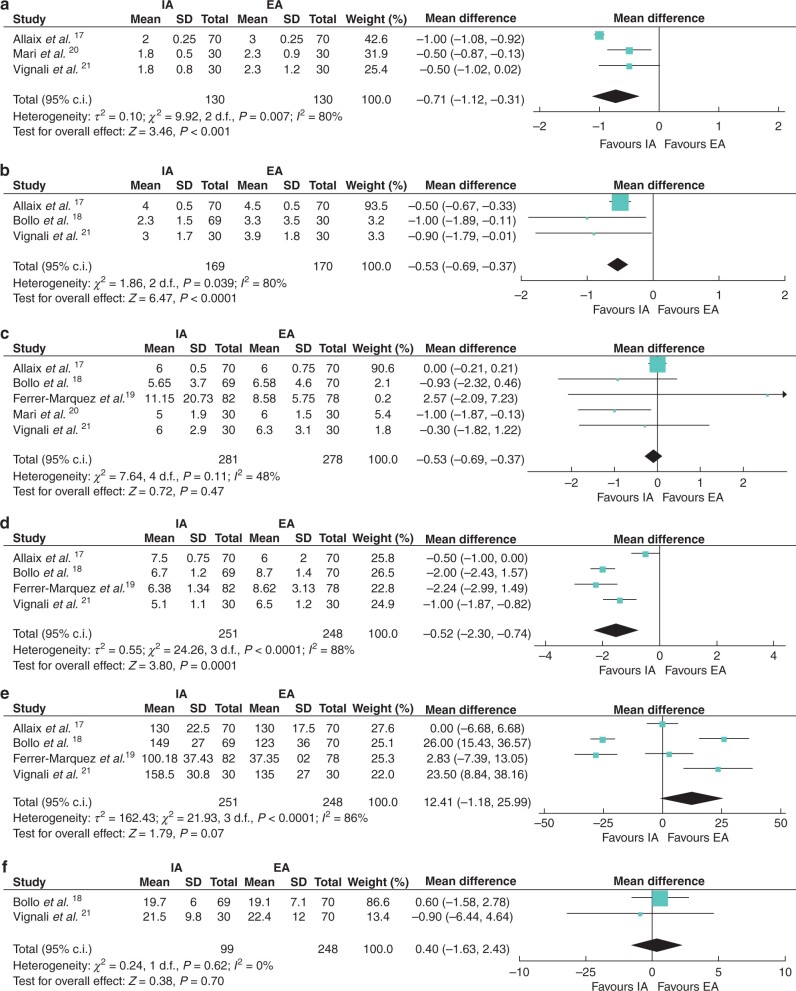
In the IA group, the time of the first flatus was significantly shorter than in the EA group (m.d. −0.71 (95 per cent c.i. −1.12 to −0.31), P = 0.0005). Three trials reported this outcome17,20,21, with heterogeneity (I2 = 80 per cent) (Fig. 3a).
Fig. 3.
Forest plots of primary outcomes (postoperative recovery and operative data)
a Time to first flatus; b time to first passage of stool; c duration of hospital stay; d length of incision; e operative time; and f lymph nodes harvested. IA, intracorporeal anastomosis; EA, extracorporeal anastomosis.
In the IA group, the first passage of stool was significantly faster than in the EA group (m.d. −0.53 (95 per cent c.i. −0.69 to −0.37), P < 0.00001). Three trials reported this outcome17,18,21, with no heterogeneity (I2 = 0 per cent) (Fig. 3b).
Duration of hospital stay was not significantly different between the two groups (m.d. −0.07 (95 per cent c.i. −0.27 to 0.13), P = 0.47). Five trials reported the data, and significant heterogeneity was not observed between them (I2 = 48 per cent) (Fig. 3c).
Postoperative pain was evaluated in three trials using a visual analogue scale (VAS) ranging from 0 to 10 (0 = no pain, 10 = maximal pain)17,18,21. The results of meta-analysis showed that patients in the IA group had a lower VAS score than those in the EA group on POD 3 (m.d. −0.76 (95 per cent c.i. −1.23 to −0.28), P = 0.002), POD 4 (m.d. −0.83 (95 per cent c.i. −1.46 to −0.20), P = 0.01) and POD 5 (m.d. −0.60 (95 per cent c.i. −0.95 to −0.25), P = 0.0007); however, no significant difference was found between two groups on POD 1 (m.d. −0.05 (95 per cent c.i. −0.27 to 0.17), P = 0.66) and POD 2 (m.d. −0.60 (95 per cent c.i. −1.44 to 0.25), P = 0.17) (Fig. S1).
The incision length was significantly shorter in the IA group than in the EA group (m.d. −1.52 (95 per cent c.i. −2.30 to −0.74), P = 0.0001). Four trials reported the data17–19,21 and heterogeneity was observed between them (I2 = 88 per cent) (Fig. 3d).
The operative time of the two groups was not significantly different (m.d. 12.41 (95 per cent c.i. −1.18 to 25.99), P = 0.07). Four trials reported the data17–19,21 and heterogeneity was observed between them (I2 = 86 per cent) (Fig. 3e).
The number of lymph nodes harvested was similar between the IA and EA groups (m.d. 0.40 (95 per cent c.i. −1.63 to 2.43), P = 0.70). Two trials, including only patients with malignant tumours, reported the data18,21, with no heterogeneity (I2 = 0 per cent) (Fig. 3f).
Secondary outcomes
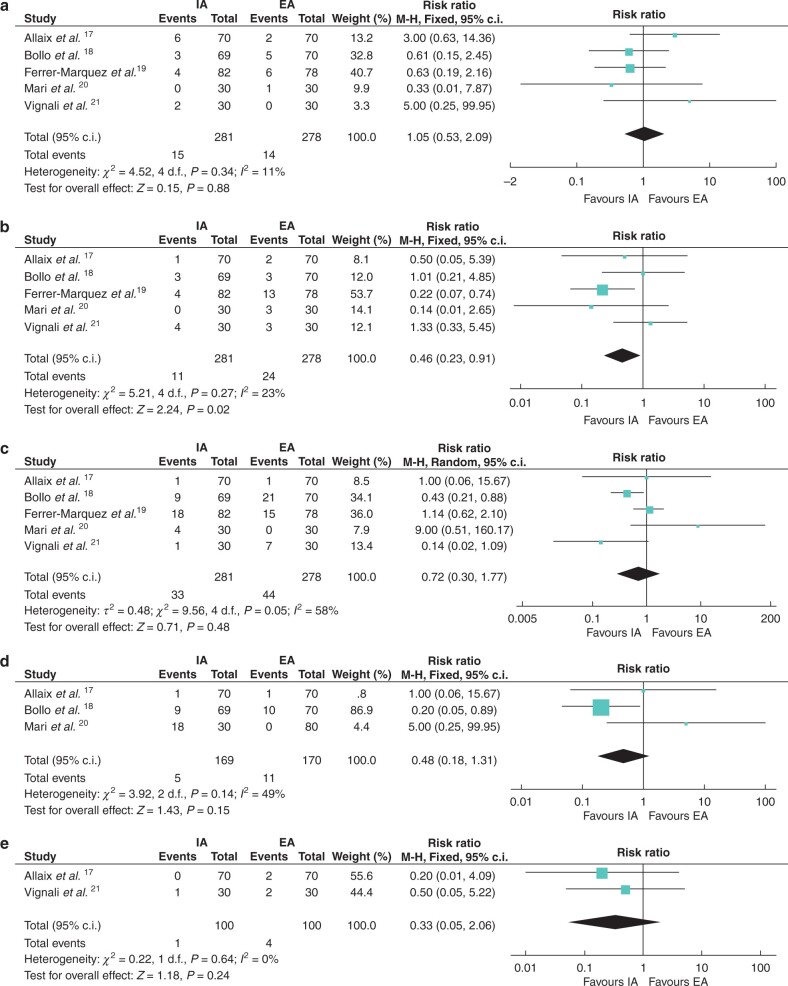
A total of 15 (5.3 per cent) patients in the IA group and 14 (5.0 per cent) patients in the EA group experienced anastomotic leak (RR 1.05 (95 per cent c.i. 0.53 to 2.09), P = 0.88). Five trials reported the data, and no significant heterogeneity was seen between them (I2 = 11 per cent) (Fig. 4a). Eleven (3.9 per cent) patients in the IA group and 24 (8.6 per cent) patients in the EA group experienced wound infection (RR 0.46 (95 per cent c.i. 0.23 to 0.91), P = 0.02). Five trials reported the data, with no significant heterogeneity (I2 = 23 per cent) (Fig. 4b).
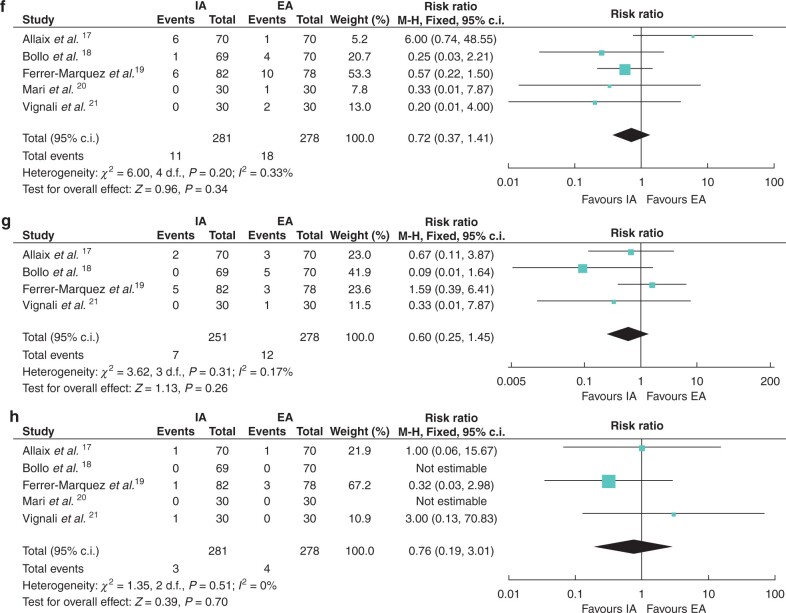
Fig. 4.
Forest plots of secondary outcomes (complications)
a Anastomotic leak; b wound infection; c postoperative ileus; d bleeding; e bowel obstruction; f reoperation; g readmission within 30 days; and h death. IA, intracorporeal anastomosis; EA, extracorporeal anastomosis.
Fig. 4.
(continued)
The incidence of postoperative ileus was similar between the IA group (33 patients, 11.7 per cent) and the EA group (44 patients, 15.8 per cent) (RR 0.72 (95 per cent c.i. 0.30 to 1.77), P = 0.48). Five trials reported this outcome, with heterogeneity (I2 = 58 per cent) (Fig. 4c).
Five (3.0 per cent) patients in the IA group and 11 (6.5 per cent) patients in the EA group experienced bleeding (RR 0.48 (95 per cent c.i. 0.18 to 1.31), P = 0.15). Three trials reported the data17,18,20, with no significant heterogeneity (I2 = 49 per cent) (Fig. 4d).
One (1.0 per cent) patient in the IA group and four (4 per cent) patients in the EA group experienced bowel obstruction (RR 0.33 (95 per cent c.i. 0.05 to 2.06), P = 0.24). Two trials reported the data17,21, with no heterogeneity (I2 = 0 per cent) (Fig. 4e).
Thirteen (4.6 per cent) patients in the IA group and 18 (6.5 per cent) patients in the EA group experienced reoperation (RR 0.72 (95 per cent c.i. 0.37 to 1.41), P = 0.34). Five trials reported the data, with no significant heterogeneity (I2 = 33 per cent) (Fig. 4f).
A total of seven (2.8 per cent) patients in the IA group and 12 (4.8 per cent) patients in the EA group experienced readmission within 30 days (RR 0.60 (95 per cent c.i. 0.25 to 1.45), P = 0.26). Four trials reported the data17–19,21 and no significant heterogeneity was seen between them (I2 = 17 per cent) (Fig. 4g).
Three (1.1 per cent) patients in the IA group and four (1.4 per cent) patients in the EA group died in the postoperative period (RR 0.76 (95 per cent c.i. 0.19 to 3.01), P = 0.70). Five trials reported the data, with no heterogeneity (I2 = 0 per cent) (Fig. 4h).
Sensitivity analysis
One trial that did not report the type of ileocolic anastomosis20 and one trial that included the mixed type of ileocolic anastomosis17 were excluded from the sensitivity analysis. The other three trials, all performing side-to-side and stapled ileocolic anastomosis, were analysed. The analyses showed that the time to first passage of stool (m.d. −0.95 (95 per cent c.i. −1.58 to −0.32), P = 0.003), VAS scores of POD 2 (m.d. −1.04 (95 per cent c.i. −1.60 to −0.47), P = 0.003) and 4 (m.d. −0.51 (95 per cent c.i. −0.96 to −0.05), P = 0.03), length of incision (m.d. −1.86 (95 per cent c.i. −2.32 to −1.41), P < 0.00001) and bleeding (RR 0.20 (95 per cent c.i. 0.05 to 0.89), P = 0.03) favoured the IA group, while the operative time (m.d. 17.12 (95 per cent c.i. 1.47 to 32.78), P = 0.03) favoured the EA group. There was no significant difference in the other outcomes (Table S1).
Subgroup analysis
This analysis was focused on the peristaltic orientation of the anastomosis (antiperistaltic anastomosis, two RCTs, 279 patients17,18; and isoperistaltic anastomosis, two RCTs, 200 patients19,21). In the subgroup of antiperistaltic anastomosis, time to first flatus (m.d. −1.00 (95 per cent c.i. −1.08 to −0.92), P < 0.00001), time to first passage of stool (m.d. −0.50 (95 per cent c.i. −0.67 to −0.33), P < 0.00001), VAS scores on POD 3 (m.d. −0.74 (95 per cent c.i. −1.40 to −0.08), P = 0.03), POD 4 (m.d. −1.24 (95 per cent c.i. −1.33 to −1.16), P < 0.00001) and POD 5 (m.d. −0.75 (95 per cent c.i. −0.83 to −0.67), P < 0.00001), postoperative ileus (RR 0.46 (95 per cent c.i. 0.23 to 0.91), P = 0.03) and bleeding (RR 0.28 (95 per cent c.i. 0.08 to 0.96), P = 0.04) were all in favour of the IA group. In the subgroup of isoperistaltic anastomosis, time to first passage of stool (m.d. −0.90 (95 per cent c.i. −1.79 to −0.01), P = 0.048) and length of incision (m.d. −1.78 (95 per cent c.i. −2.61 to −0.96), P < 0.0001) were both in favour of the IA group. There was no significant difference in the other outcomes (Table S2).
Discussion
Laparoscopic colectomy is increasingly becoming a standard treatment for benign and malignant colonic disease in many centres around the world23,24. In LRC, IA and EA are the two main anastomotic techniques for restoration of bowel continuity. Several meta-analyses have been published comparing IA versus EA on short-term outcomes, morbidity and death in patients undergoing LRC24–28. Based on these initial conclusions, IA appears to be safe in terms of postoperative complications and is potentially more effective in recovery after surgery. However, most of the included studies were retrospective in the former meta-analyses, which made the level of evidence lower than in the meta-analysis of RCTs29. This may be one reason why surgeons do not perform IA routinely. Another reason is the technical difficulty of performing the laparoscopic hand-sewn suture27. However, new suturing techniques, such as barbed sutures, facilitate laparoscopic suturing because it is unnecessary to tie a knot30. The use of barbed sutures in laparoscopic colectomy for enterotomy closure is associated with a shorter operative time31. Several new RCTs of ileocolic anastomosis in LRC were published from 2016 to 2021, which provided a high level of evidence for the issue of IA versus EA17–21.
In this meta-analysis involving 559 patients from five RCTs, patients treated with IA had faster recovery of flatus and defaecation, less postoperative pain, shorter length of the incision and fewer wound infections than patients treated using EA. The data demonstrated that IA was associated with a faster recovery of bowel function than EA, which is consistent with findings reported by others27,28. Although technically demanding and requiring advanced laparoscopic skills, IA does not require bowel exteriorization and reduces intestinal manipulation, leading to less traction on and fewer tissue injuries to the mesentery7,32. A past study demonstrated that tissue injury clearly had a major role in the surgical stress response (SSR), which may affect the recovery of bowel function, and elevated SSR in EA patients was shown by apparently higher levels of IL-6, C-reactive protein and white blood cells than in IA patients20. Notably, it was difficult to exteriorize the transverse colon or terminal ileum in obese patients via a small abdominal incision owning to a short and thickened bowel mesentery20,33,34. To achieve EA in patients with high BMI, surgeons may need to perform excessive traction on the colon and terminal ileum, which leads easily to tissue injury to the mesentery. Conversely, IA was not affected by BMI, owing to its avoidance of mesenteric traction to externalize the bowel for anastomosis. An increased inflammatory response in postsurgical intestinal muscularis has also been demonstrated from excessive bowel handling in vivo, leading to an increase in or exacerbation of postoperative ileus35. In this meta-analysis of the antiperistaltic anastomosis subgroup, fewer cases of postoperative ileus were observed in IA patients than in EA patients. Obviously, the less the manipulation, the lower the incidence of postoperative ileus18. In addition, it has been reported that IA may reduce the likelihood of intestinal twisting because of a better view with laparoscopic visualization for performing the anastomosis, following a lower operative conversion rate33,34,36,37. However, high operative conversion rates with increased morbidity were reported in laparoscopically assisted colectomy with EA38,39.
For IA, a small incision is enough to extract the specimen only. In the included trials, the abdominal incision length was significantly reduced in the IA group. This was also found in a meta-analysis of observational studies28. A midline incision was used for 43.1 per cent of patients in the EA group, which was associated with the highest risk of incisional hernia, as previously reported24. In the IA group, Pfannenstiel incision (63.7 per cent) was used in more than half of the patients; the incidence of incisional hernias and postoperative pain could be less in this group because of rapid wall suturing (fewer muscle layers) with reduced operative time and shorter length of incision40,41. A further potential advantage of Pfannenstiel incision is the cosmetic effect due to its invisibility. Owning to short follow-up, incisional hernia was not reported in any of the five included trials. Several other studies have shown a higher incidence of incisional hernia in the EA group than in the IA group9,24,40. Notably, IA with a Pfannenstiel incision could reduce the rate of incisional hernias compared with EA with a vertical midline incision42.
All of the included patients routinely received postoperative multimodal analgesics based on ERAS recommendations for about 48 hours22, and similar VAS pain scores were seen on PODs 1 and 2 between the IA and EA groups. However, lower VAS scores were shown on PODs 3, 4 and 5 of the IA group. Undoubtedly, a shorter incision was an important factor associated with less postoperative pain. The incidence of wound infection in the EA group was higher than in the IA group. Except for longer incisions, another potential reason may be wound contamination while performing EA through the incision24. Theoretically, intra-abdominal infection of the IA group is expected to be high because of the possibility of faecal spillage when performing ileocolic anastomosis. However, a similar rate of abdominal abscess was observed between the IA and EA groups8,19, which suggests that no significant intraperitoneal spillage occurred in the IA group before the enterotomies were closed. Some measures may be beneficial for preventing intra-abdominal infection, such as using atraumatic bulldogs to block spillage43, administering prophylactic antibiotics44, irrigating the abdominal cavity locally after anastomosis45 and ensuring adequate nutritional support46.
The anastomotic leak rate is an important measure to determine the success of each anastomotic technique6. IA was considered to have a greater likelihood of anastomotic leak due to the technical difficulty26, whereas higher odds of the anastomotic leak were observed in the EA group previously reported24. One potential reason may be the shortage of anastomotic blood supply following mesenteric injuries caused by traction on the bowel ends through the extraction site incision33. Another reason is that hand-sewn anastomosis, which was reported to be associated with anastomotic leak more than with stapled anastomosis47, was used more often in the EA group than in the IA group24. However, based on the present meta-analysis, LRCs with IA or EA were both safe, with no significant differences in the rate of anastomotic leak between the two groups. It should be added that stapled anastomosis was also used mostly in the EA group (214 of 248 patients, 86.3 per cent). The extensive experience of participant surgeons in laparoscopic colorectal surgery eliminated the impact of technical difficulty to some extent in the IA group. For an experienced laparoscopic surgeon, it has been reported that the learning curve of ileocolonic intracorporeal anastomosis was short, and the total operating time of the IA method was shorter than that of the EA method after a minimal learning curve period of about 18 cases37. Even for high-risk patients with obesity and high ASA grade, the surgical outcomes of total intracorporeal laparoscopic colectomy are equal to those of low-risk patients except for a longer operative time34. In this meta-analysis, similar operative time was demonstrated between the IA and EA groups. However, sensitivity analysis for patients undergoing side-to-side ileocolic anastomosis with a stapler showed a longer operative time in the IA group. Intracorporeal suturing to close the enterocolostomy is undoubtedly the most challenging procedure for surgeons, whereas the use of barbed suture facilitated the procedure and resulted in a similar surgical time between IA and EA19.
In this meta-analysis, both the IA and EA groups obtained good oncological radicality owing to a similar number of lymph nodes harvested. Furthermore, it was demonstrated that the lengths of the distal and proximal margins were similar between the two groups17,48. Therefore, in the short term, the IA method is oncologically equivalent to the EA method; that is, the extent of tumour resection (length of resection and number of lymph nodes) is related to the short-term therapeutic effects. No significant difference in long-term outcomes was found in overall survival and disease-free survival at 3 years8 and 5.7 years49 of follow-up between the two groups.
There were several limitations to this meta-analysis. The ERAS protocol was not performed completely in all the included trials. Different experiences among surgeons probably led to variable operative time. Also, the ratio of the type of surgical incision was not the same between IA and EA groups. These factors may affect the recovery process of patients and surgical outcomes. Finally, the number of the included patients was small. Large RCTs comparing IA with EA are necessary in the future.
Supplementary Material
Acknowledgments
No funding supported this work. N.S. and H.-Y.Z. contributed equally to this work.
Supplementary material
Supplementary material is available at BJS Open online.
Disclosure. The authors declare no conflicts of interest.
Contributor Information
Hongyu Zhang, Department of Gastrointestinal Surgery, The First Affiliated Hospital of Zhengzhou University, Zhengzhou, China.
Nan Sun, Department of Plastic Surgery, The First Affiliated Hospital of Zhengzhou University, Zhengzhou, China.
Yang Fu, Department of Gastrointestinal Surgery, The First Affiliated Hospital of Zhengzhou University, Zhengzhou, China.
Chunlin Zhao, Department of Gastrointestinal Surgery, The First Affiliated Hospital of Zhengzhou University, Zhengzhou, China.
References
- 1. Arezzo A, Passera R, Ferri V, Gonella F, Cirocchi R, Morino M. Laparoscopic right colectomy reduces short-term mortality and morbidity. Results of a systematic review and meta-analysis. Int J Colorectal Dis 2015;30:1457–1472. [DOI] [PubMed] [Google Scholar]
- 2. Buunen M, Veldkamp R, Hop WC, Kuhry E, Jeekel J et al. ; Colon Cancer Laparoscopic or Open Resection Study Group. Survival after laparoscopic surgery versus open surgery for colon cancer: long-term outcome of a randomised clinical trial. Lancet Oncol 2009;10:44–52. [DOI] [PubMed] [Google Scholar]
- 3. Yamamoto S, Inomata M, Katayama H, Mizusawa J, Etoh T, Konishi F et al. ; Japan Clinical Oncology Group Colorectal Cancer Study Group. Short-term surgical outcomes from a randomized controlled trial to evaluate laparoscopic and open D3 dissection for stage II/III colon cancer: Japan Clinical Oncology Group Study JCOG 0404. Ann Surg 2014;260:23–30. [DOI] [PubMed] [Google Scholar]
- 4. Tjandra JJ, Chan MK. Systematic review on the short-term outcome of laparoscopic resection for colon and rectosigmoid cancer. Colorect Dis 2006;8:375–388. [DOI] [PubMed] [Google Scholar]
- 5. Kuhry E, Schwenk WF, Gaupset R, Romild U, Bonjer HJ. Long-term results of laparoscopic colorectal cancer resection. Cochrane Database Syst Rev 2008; (2)CD003432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Carnuccio P, Jimeno J, Pares D. Laparoscopic right colectomy: a systematic review and meta-analysis of observational studies comparing two types of anastomosis. Tech Coloproctol 2014;18:5–12. [DOI] [PubMed] [Google Scholar]
- 7. Jian-Cheng T, Shu-Sheng W, Bo Z, Jian F, Liang Z. Total laparoscopic right hemicolectomy with 3-step stapled intracorporeal isoperistaltic ileocolic anastomosis for colon cancer: an evaluation of short-term outcomes. Medicine (Baltimore) 2016;95:e5538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Lee KH, Ho J, Akmal Y, Nelson R, Pigazzi A. Short- and long-term outcomes of intracorporeal versus extracorporeal ileocolic anastomosis in laparoscopic right hemicolectomy for colon cancer. Surg Endosc 2013;27:1986–1990. [DOI] [PubMed] [Google Scholar]
- 9. Shapiro R, Keler U, Segev L, Sarna S, Hatib K, Hazzan D. Laparoscopic right hemicolectomy with intracorporeal anastomosis: short- and long-term benefits in comparison with extracorporeal anastomosis. Surg Endosc 2016;30:3823–3829. [DOI] [PubMed] [Google Scholar]
- 10. Anania G, Santini M, Scagliarini L, Marzetti A, Vedana L, Marino S et al. A totally mini-invasive approach for colorectal laparoscopic surgery. World J Gastroenterol 2012;18:3869–3874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Moher D, Liberati A, Tetzlaff J, Altman DG; PRISMA Group. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. Int J Surg 2010;8:336–341. [DOI] [PubMed] [Google Scholar]
- 12. Higgins JPT, Thomas J, Chandler J, Cumpston M, Li T., Page MJ (eds). Cochrane Handbook for Systematic Reviews of Interventions, 2nd edn. Chichester, UK: John Wiley & Sons, 2019. [Google Scholar]
- 13. Review Manager (RevMan) [Computer program]. Version 5.4.1. The Cochrane Collaboration, 2020. https://training.cochrane.org/online-learning/core-software-cochrane-reviews/revman.
- 14. Hozo SP, Djulbegovic B, Hozo I. Estimating the mean and variance from the median, range, and the size of a sample. BMC Med Res Methodol 2005;5:13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Higgins JP, Thompson SG. Quantifying heterogeneity in a meta-analysis. Stat Med 2002;21:1539–1558. [DOI] [PubMed] [Google Scholar]
- 16. DerSimonian R, Laird N. Meta-analysis in clinical trials. Control Clin Trials 1986;7:177–188. [DOI] [PubMed] [Google Scholar]
- 17. Allaix ME, Degiuli M, Bonino MA, Arezzo A, Mistrangelo M, Passera R et al. Intracorporeal or extracorporeal ileocolic anastomosis after laparoscopic right colectomy: a double-blinded randomized controlled trial. Ann Surg 2019;270:762–767. [DOI] [PubMed] [Google Scholar]
- 18. Bollo J, Turrado V, Rabal A, Carrillo E, Gich I, Martinez MC et al. Randomized clinical trial of intracorporeal versus extracorporeal anastomosis in laparoscopic right colectomy (IEA trial). Br J Surg 2020;107:364–372. [DOI] [PubMed] [Google Scholar]
- 19. Ferrer-Marquez M, Rubio-Gil F, Torres-Fernandez R, Moya-Forcen P, Belda-Lozano R, Arroyo-Sebastian A, et al. Intracorporeal versus extracorporeal anastomosis in patients undergoing laparoscopic right hemicolectomy: a multicenter randomized clinical trial (The IVEA-study). Surg Laparosc Endosc Percutan Tech 2021;31:408–413. [DOI] [PubMed] [Google Scholar]
- 20. Mari GM, Crippa J, Costanzi ATM, Pellegrino R, Siracusa C, Berardi V et al. Intracorporeal anastomosis reduces surgical stress response in laparoscopic right hemicolectomy: a prospective randomized trial. Surg Laparosc Endosc Percutan Tech 2018;28:77–81. [DOI] [PubMed] [Google Scholar]
- 21. Vignali A, Bissolati M, De Nardi P, Di Palo S, Staudacher C. Extracorporeal vs. intracorporeal ileocolic stapled anastomoses in laparoscopic right colectomy: an interim analysis of a randomized clinical trial. J Laparoendosc Adv Surg Tech A 2016;26:343–348. [DOI] [PubMed] [Google Scholar]
- 22. Gustafsson UO, Scott MJ, Schwenk W, Demartines N, Roulin D, Francis N et al. ; International Association for Surgical Metabolism and Nutrition (IASMEN); Enhanced Recovery After Surgery Society for Perioperative Care; European Society for Clinical Nutrition and Metabolism. Guidelines for perioperative care in elective colonic surgery: Enhanced Recovery After Surgery (ERAS(R)) Society recommendations. World J Surg 2013;37:259–284. [DOI] [PubMed] [Google Scholar]
- 23. Juo YY, Hyder O, Haider AH, Camp M, Lidor A, Ahuja N. Is minimally invasive colon resection better than traditional approaches? First comprehensive national examination with propensity score matching. JAMA Surg 2014;149:177–184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Emile SH, Elfeki H, Shalaby M, Sakr A, Bassuni M, Christensen P et al. Intracorporeal versus extracorporeal anastomosis in minimally invasive right colectomy: an updated systematic review and meta-analysis. Tech Coloproctol 2019;23:1023–1035. [DOI] [PubMed] [Google Scholar]
- 25. Cirocchi R, Trastulli S, Farinella E, Guarino S, Desiderio J, Boselli C et al. Intracorporeal versus extracorporeal anastomosis during laparoscopic right hemicolectomy – systematic review and meta-analysis. Surg Oncol 2013;22:1–13. [DOI] [PubMed] [Google Scholar]
- 26. Wu Q, Jin C, Hu T, Wei M, Wang Z. Intracorporeal versus extracorporeal anastomosis in laparoscopic right colectomy: a systematic review and meta-analysis. J Laparoendosc Adv Surg Tech A 2017;27:348–357. [DOI] [PubMed] [Google Scholar]
- 27. Milone M, Elmore U, Vignali A, Gennarelli N, Manigrasso M, Burati M et al. Recovery after intracorporeal anastomosis in laparoscopic right hemicolectomy: a systematic review and meta-analysis. Langenbecks Arch Surg 2018;403:1–10. [DOI] [PubMed] [Google Scholar]
- 28. Aiolfi A, Bona D, Guerrazzi G, Bonitta G, Rausa E, Panizzo V et al. Intracorporeal versus extracorporeal anastomosis in laparoscopic right colectomy: an updated systematic review and cumulative meta-analysis. J Laparoendosc Adv Surg Tech A 2020;30:402–412. [DOI] [PubMed] [Google Scholar]
- 29. Delgado-Rodriguez M, Sillero-Arenas M. Systematic review and meta-analysis. Med Intensiva (Engl Ed) 2018;42:444–453. [DOI] [PubMed] [Google Scholar]
- 30. Manigrasso M, Velotti N, Calculli F, Aprea G, Di Lauro K, Araimo E et al. Barbed suture and gastrointestinal surgery. A retrospective analysis. Open Med (Wars) 2019;14:503–508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Hamamoto H, Okuda J, Izuhara K, Ishii M, Osumi W, Masubuchi S et al. Closure of enterotomy after side-to-side ileocolic anastomosis with two barbed sutures in totally laparoscopic colectomy for right-sided colon cancer. Surg Today 2021;51:457–461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Grams J, Tong W, Greenstein AJ, Salky B. Comparison of intracorporeal versus extracorporeal anastomosis in laparoscopic-assisted hemicolectomy. Surg Endosc 2010;24:1886–1891. [DOI] [PubMed] [Google Scholar]
- 33. Blumberg D. Laparoscopic colectomy performed using a completely intracorporeal technique is associated with similar outcome in obese and thin patients. Surg Laparosc Endosc Percutan Tech 2009;19:57–61. [DOI] [PubMed] [Google Scholar]
- 34. Iorio T, Blumberg D. Totally intracorporeal laparoscopic colectomy (TILC) is associated with similar surgical outcomes in high and low operative risk patients. Surg Laparosc Endosc Percutan Tech 2013;23:154–158. [DOI] [PubMed] [Google Scholar]
- 35. Schwarz NT, Beer-Stolz D, Simmons RL, Bauer AJ. Pathogenesis of paralytic ileus: intestinal manipulation opens a transient pathway between the intestinal lumen and the leukocytic infiltrate of the jejunal muscularis. Ann Surg 2002;235:31–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Hellan M, Anderson C, Pigazzi A. Extracorporeal versus intracorporeal anastomosis for laparoscopic right hemicolectomy. JSLS 2009;13:312–317. [PMC free article] [PubMed] [Google Scholar]
- 37. Marchesi F, Pinna F, Percalli L, Cecchini S, Ricco M, Costi R et al. Totally laparoscopic right colectomy: theoretical and practical advantages over the laparo-assisted approach. J Laparoendosc Adv Surg Tech A 2013;23:418–424. [DOI] [PubMed] [Google Scholar]
- 38. Nelson H., Sargent DJ, Wieand HS, Fleshman J, Anvari M, Stryker SJ et al. ; Clinical Outcomes of Surgical Therapy Study Group. A comparison of laparoscopically assisted and open colectomy for colon cancer. N Engl J Med 2004;350:2050–2059. [DOI] [PubMed] [Google Scholar]
- 39. Belizon A, Sardinha CT, Sher ME. Converted laparoscopic colectomy: what are the consequences? Surg Endosc 2006;20:947–951. [DOI] [PubMed] [Google Scholar]
- 40. Vergis AS, Steigerwald SN, Bhojani FD, Sullivan PA, Hardy KM. Laparoscopic right hemicolectomy with intracorporeal versus extracorporeal anastamosis: a comparison of short-term outcomes. Can J Surg 2015;58:63–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Ricci C, Casadei R, Alagna V, Zani E, Taffurelli G, Pacilio CA et al. A critical and comprehensive systematic review and meta-analysis of studies comparing intracorporeal and extracorporeal anastomosis in laparoscopic right hemicolectomy. Langenbecks Arch Surg 2017;402:417–427. [DOI] [PubMed] [Google Scholar]
- 42. Widmar M, Aggarwal P, Keskin M, Strombom PD, Patil S, Smith JJ et al. Intracorporeal anastomoses in minimally invasive right colectomies are associated with fewer incisional hernias and shorter length of stay. Dis Colon Rectum 2020;63:685–692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Chang K, Fakhoury M, Barnajian M, Tarta C, Bergamaschi R. Laparoscopic right colon resection with intracorporeal anastomosis. Surg Endosc 2013;27:1730–1736. [DOI] [PubMed] [Google Scholar]
- 44. Ahn BK, Lee KH. Single-dose antibiotic prophylaxis is effective enough in colorectal surgery. ANZ J Surg 2013;83:641–645. [DOI] [PubMed] [Google Scholar]
- 45. Platell C, Papadimitriou JM, Hall JC. The influence of lavage on peritonitis. J Am Coll Surg 2000;191:672–680. [DOI] [PubMed] [Google Scholar]
- 46. Yamamoto T, Nakahigashi M, Shimoyama T, Umegae S. Does preoperative enteral nutrition reduce the incidence of surgical complications in patients with Crohn's disease? A case-matched study. Colorectal Dis 2020;22:554–561. [DOI] [PubMed] [Google Scholar]
- 47. Choy PY, Bissett IP, Docherty JG, Parry BR, Fitzgerald MA. Stapled versus handsewn methods for ileocolic anastomoses. Cochrane Database Syst Rev 2011; (3)CD004320. [DOI] [PubMed] [Google Scholar]
- 48. Roscio F, Bertoglio C, De Luca A, Frattini P, Scandroglio I. Totally laparoscopic versus laparoscopic assisted right colectomy for cancer. Int J Surg 2012;10:290–295. [DOI] [PubMed] [Google Scholar]
- 49. Hanna MH, Hwang GS, Phelan MJ, Bui TL, Carmichael JC, Mills SD et al. Laparoscopic right hemicolectomy: short- and long-term outcomes of intracorporeal versus extracorporeal anastomosis. Surg Endosc 2016;30:3933–3942. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.