Abstract
Background
Bed rest or restriction of activity, with or without hospitalisation, have been advocated for women with hypertension during pregnancy to improve pregnancy outcome. However, benefits need to be demonstrated before such interventions can be recommended since restricted activity may be disruptive to women's lives, expensive, and increase the risk of thromboembolism.
Objectives
To assess the effects on the mother and the baby of different degrees of bed rest, compared with each other, and with routine activity, in hospital or at home, for primary treatment of hypertension during pregnancy.
Search methods
We searched the Cochrane Pregnancy and Childbirth Group's Trials Register (January 2010).
Selection criteria
Randomised trials evaluating bed rest for women with hypertension in pregnancy were selected.
Data collection and analysis
Two review authors assessed trials for inclusion independently, and extracted data. Data were entered into RevMan software and double‐checked.
Main results
Four small trials (449 women) were included. Three were of good quality. Two trials (145 women) compared strict bed rest with some rest, in hospital, for women with proteinuric hypertension. There was insufficient evidence to demonstrate any differences between the groups for reported outcomes. Two trials (304 women) compared some bed rest in hospital with routine activity at home for non‐proteinuric hypertension. There was reduced risk of severe hypertension (one trial, 218 women; relative risk (RR) 0.58, 95% confidence interval (CI) 0.38 to 0.89) and a borderline reduction in risk of preterm birth (one trial, 218 women; RR 0.53, 95% CI 0.29 to 0.99) with some rest compared to normal activity. More women in the bed rest group opted not to have the same management in future pregnancies, if the choice were given (one trial, 86 women; RR 3.00, 95% CI 1.43 to 6.31). There were no significant differences for any other outcomes.
Authors' conclusions
Few randomised trials have evaluated rest for women with hypertension during pregnancy, and important information on side‐effects and cost implication is missing from available trials. Although one small trial suggests that some bed rest may be associated with reduced risk of severe hypertension and preterm birth, these findings need to be confirmed in larger trials. At present, there is insufficient evidence to provide clear guidance for clinical practice. Therefore, bed rest should not be recommended routinely for hypertension in pregnancy, especially since more women appear to prefer unrestricted activity, if the choice were given.
Plain language summary
Bed rest with or without hospitalisation for hypertension during pregnancy
Not enough evidence to say if bed rest in pregnancy helps women and their babies when women have high blood pressure.
High blood pressure in pregnant women can contribute to babies being small, being born too soon and having considerable health problems. Women with high blood pressure are often advised to rest in bed either at home or in hospital. It is suggested that this might help to reduce the mother's blood pressure and so provide benefits for the baby. However, there may be adverse effects; for example, some women may find it stressful, it may contribute to blood clots in the legs and can put a burden on the woman's family. Although one small trial suggested that there may be some possible benefits, there are insufficient data to be confident. Moreover, trials did not address possible adverse effects of bed rest. More women seemed to prefer normal activity at home rather than resting in hospital, if a choice were given. Further research is needed.
Background
Raised blood pressure during pregnancy is one of the commonest medical complications, occurring in 6% to 8% of all pregnancies (ACOG 1996). There are controversies about the definition of hypertensive disorders during pregnancy, and several classifications have been suggested (ASSHP 1993; Davey 1988; NHBPEP 2000; North 1999; Roberts 1993). These are discussed in more detail in Abalos 2007. However, most include the four main categories (a) chronic hypertension; (b) pre‐eclampsia, characterised by hypertension with proteinuria; (c) pre‐eclampsia superimposed on chronic hypertension; and (d) pregnancy‐induced hypertension or gestational hypertension, transient hypertension without proteinuria. Hypertension is usually defined as blood pressure of at least 140 mmHg systolic or 90 mmHg diastolic, or both, and proteinuria is defined as at least 0.3 g protein in a 24‐hour collection, or 30 mg/dL or more (1+ on dipstick) in a random urine sample (NHBPEP 2000). An important issue in classification of hypertensive disorders of pregnancy is the ability to differentiate hypertensive disorders present before pregnancy from those that are pregnancy‐specific, of which the more ominous is pre‐eclampsia. The impact of the two groups of conditions on the mother and fetus is different, as is their management.
About a quarter of the cases of hypertension during pregnancy are due to chronic hypertension (Lindheimer 1999). Women with uncomplicated mild to moderate hypertension often have uneventful pregnancies, but nearly 20% of women with chronic hypertension develop superimposed pre‐eclampsia; development of pre‐eclampsia or severe hypertension is responsible for most of the morbidity in these pregnancies (Sibai 1998). Pre‐eclampsia, either presenting 'de novo' or superimposed on chronic hypertension or renal disease is the disorder most likely to endanger both mother and fetus (Lindheimer 1999). The cardinal features of pre‐eclampsia are hypertension and proteinuria, but women may also have abnormalities of liver function or coagulation, or both, thrombocytopenia (low platelet counts) or eclampsia (the rare occurrence of seizures superimposed on pre‐eclampsia). Potential consequences for the fetus are intrauterine growth restriction, preterm birth, or death. For women with gestational hypertension the outcome is usually good, although hypertension frequently recurs in subsequent pregnancies (Chesley 1978).
The potential worsening from mild/moderate to severe disease and the difficulty in distinguishing between pre‐eclampsia, chronic, secondary and gestational hypertension, and combinations of these entities gives strong support for close supervision of all hypertensive pregnant women (NHBPEP 2000; Saudan 1998; Walker 2000).
Systolic blood pressure has been demonstrated to be higher in ambulating women, which is the logic behind the use of bed rest for hypertension during pregnancy. Bed rest, either in hospital or at home, has a long history in the treatment of mild pregnancy‐induced hypertension and pre‐eclampsia, and has drawn the attention of different authors. Since 1952, when Hamlin reported the virtual disappearance of eclampsia with a policy of vigorous antenatal education and supervision (including early admission to hospital and numerous interventions besides bed rest) (Hamlin 1952), the notion that bed rest might improve the outcome of hypertensive pregnancies became engrained in the mind of practitioners and health service planners. Hospitalisation was then strongly recommended, not only for closer supervision to monitor the mother and fetus for signs of deterioration, but also for bed rest. Thus, hospitalisation to enhance compliance with bed rest became a common practice for decades. It is not known whether bed rest is beneficial, regardless of whether combined with hospitalisation. Over the last ten years, domiciliary or day care facilities have been used in Europe to monitor pregnancies with hypertension or mild pre‐eclampsia, thus avoiding hospitalisation. However, use of such facilities appears to occur infrequently in many other settings, especially in developing countries.
The extent to which bed rest is prescribed in pregnancy is difficult to determine, either in hospital or in outpatient clinics, as it is not clear whether the advice to rest in bed is recorded as frequently as it is advised (Goldenberg 1994). Using the 1988 US National Infant Mortality Survey (Sanderson 1991) Goldenberg et al found that 4.8% of pregnant women were advised by their physicians to rest in bed for at least one week because of hypertension (Goldenberg 1994). This represents nearly 200,000 women of the four million annual United States births. A great number of women also reduce their work load or stop working. This could be stressful for the woman and disruptive for her family (Kramer 1986; Maloni 1993), and could also have important consequences in terms of costs for both the families and the health services.
Even though, for most women with hypertension, the period of bed rest is likely to be relatively short, concerns about its safety have been raised. There may be an increase in the likelihood of adverse events such as thrombosis, muscle atrophy, bone demineralisation and calcium depletion (Maloni 1993).
Bed rest has been prescribed both as a primary therapy, and as an adjunct to other treatments for hypertension during pregnancy, such as antihypertensive drugs. There is a need to evaluate bed rest, in hospital or at home, to determine whether its use improves pregnancy outcome sufficiently to warrant such a recommendation. If bed rest is found to be clearly effective in improving outcome, then the costs, disruptions in normal living and maternal stress should be evaluated further in risk and cost‐benefit analyses.
A number of other interventions for women with hypertension during pregnancy are covered by other Cochrane reviews. These include salt restriction (Duley 2005), antiplatelet agents (Duley 2007), abdominal decompression (Hofmeyr 1996), antihypertensive drug treatments for mild to moderate hypertension (Abalos 2007), and drug regimens for severe hypertension (Duley 2006). There is also a separate review assessing the effect of oral beta blockers in mild to moderate hypertension during pregnancy (Magee 2003).
The aim of this review was to assess the relative benefits, risks, and side‐effects for the woman and baby of different degrees of bed rest compared with each other or with routine activity, with or without hospitalisation, as a primary therapy for the treatment of hypertension during pregnancy. If bed rest is beneficial overall, a secondary aim was to assess the comparative effects of bed rest in hospital and at home.
Objectives
To determine the possible benefits, risks, and side‐effects of different degrees of bed rest compared with each other, and with routine activity, in hospital or at home, as primary treatment for raised blood pressure during pregnancy. A secondary aim was to compare the effects of bed rest in hospital with bed rest at home.
Methods
Criteria for considering studies for this review
Types of studies
Randomised controlled trials evaluating bed rest as a primary treatment for women with hypertension in pregnancy. Trials with quasi‐randomised designs were excluded.
Types of participants
Pregnant women with raised blood pressure (defined, whenever possible, as systolic blood pressure greater than or equal to 140 mmHg or diastolic blood pressure greater than or equal to 90 mmHg, or both). Women were included regardless of whether they had proteinuria or previous antihypertensive treatment and irrespective of whether they had a singleton or multiple pregnancy.
Types of interventions
Interventions considered for this review included: (1) any comparisons, in hospital or at home, between:
strict bed rest: when the woman is confined to bed, and only allowed up to go to the toilet;
some rest: when the woman is encouraged to restrict activity, whether in bed or not, but is allowed some voluntary activity; and
routine activity: unrestricted activity.
(2) comparisons of any rest in hospital versus any rest at home.
We excluded studies evaluating bed rest as adjunctive therapy to other interventions for hypertension in pregnancy.
Types of outcome measures
For the woman
Main outcomes
(1) Severe hypertension: defined, whenever possible, as either systolic blood pressure greater than or equal to 160 mmHg, or diastolic blood pressure greater than or equal to 110 mmHg. Trials where the definition of severe hypertension is not clear will also be included and clearly documented. (2) Pre‐eclampsia: defined, whenever possible, as new onset proteinuria (greater than or equal to 1+ or greater than or equal to 300 mg/24 hour) after 20 weeks' gestation in pregnant women with hypertension.
Other outcomes
(3) Death: during pregnancy, childbirth, or up to 42 days after end of pregnancy. (4) Severe pre‐eclampsia: the following are features of severe disease: severe hypertension, severe proteinuria (usually greater than or equal to 3 g protein in 24 hours, or 3+ on dipstick), reduced urinary volume (less than or equal to 400 to 500 ml in 24 hours), neurological disturbances such as headache, visual disturbances, and exaggerated tendon reflexes, upper abdominal pain, pulmonary oedema (fluid in the lungs), impaired liver function tests, high serum creatinine, low platelets, intrauterine growth restriction or reduced liquor volume. Trials where the definition of severe pre‐eclampsia is not clear will be included. (5) Severe maternal morbidity such as eclampsia, liver or renal failure, haemolysis, elevated liver enzymes, low platelets syndrome, disseminated intravascular coagulation), and cerebrovascular accident (stroke). (6) Use of antihypertensive drugs to control blood pressure. (7) Placental abruption. (8) Elective delivery: including elective caesarean sections and induction of labour. (9) Caesarean section. (10) Use of hospital resources: antenatal hospital admission and length of stay greater than seven days, visit to day care unit. (11) Side‐effects such as thromboembolism, muscle atrophy, bone demineralisation, and calcium depletion.
For the baby
Main outcomes
(1) Death: fetal deaths including miscarriage (fetal losses before viability, usually taken as 20 or 24 weeks), stillbirth (death in utero after 20 or 24 weeks' gestation or however defined), perinatal death (stillbirth and death in the first seven days of life), and neonatal death (death in the first 28 days after birth). (2) Small‐for‐gestational age: low birthweight for gestational age, below the third, fifth or 10th percentile, using the most severe reported. (3) Preterm birth: all births less than or equal to 37 completed weeks, and more severe prematurity, defined as less than 32 or less than 34 weeks.
Other outcomes
(4) Apgar score at five minutes: low (less than seven) and very low (less than four). (5) Endotracheal intubation. (6) Admission to neonatal intensive care nursery. (7) Respiratory distress syndrome.
Satisfaction outcomes
Woman's views of the intervention and satisfaction with care.
Care provider's satisfaction with care.
Economic cost outcomes
Providers' costs.
Users' (women/families) costs.
Search methods for identification of studies
Electronic searches
We searched the Cochrane Pregnancy and Childbirth Group's Trials Register by contacting the Trials Search Co‐ordinator (January 2010).
The Cochrane Pregnancy and Childbirth Group’s Trials Register is maintained by the Trials Search Co‐ordinator and contains trials identified from:
quarterly searches of the Cochrane Central Register of Controlled Trials (CENTRAL);
weekly searches of MEDLINE;
handsearches of 30 journals and the proceedings of major conferences;
weekly current awareness alerts for a further 44 journals plus monthly BioMed Central email alerts.
Details of the search strategies for CENTRAL and MEDLINE, the list of handsearched journals and conference proceedings, and the list of journals reviewed via the current awareness service can be found in the ‘Specialized Register’ section within the editorial information about the Cochrane Pregnancy and Childbirth Group.
Trials identified through the searching activities described above are each assigned to a review topic (or topics). The Trials Search Co‐ordinator searches the register for each review using the topic list rather than keywords.
For details of the additional searching we undertook for the initial version of the review, using the search strategy listed in Meher 2005, seeAppendix 1.
We did not apply any language restrictions.
Data collection and analysis
Selection of studies
Assessment of the trials for inclusion was performed independently by two authors. Authors were not blinded to the authors, source of the articles, and results. Differences in opinion were resolved by discussion.
Assessment of study quality
Methodological quality was assessed with the following criteria. (1) How selection bias at entry to the trial was avoided: how the random assignment was generated and how allocation concealment was ensured. A quality score for concealment of allocation was assigned to each trial, using the following criteria: (A) adequate concealment of allocation, such as telephone randomisation, consecutively numbered sealed opaque envelopes; (B) unclear whether adequate concealment of allocation; (C) inadequate concealment of allocation such as open random‐number tables, sealed envelopes that are not numbered and opaque.
Trials with quasi‐random design were excluded.
(2) How selection bias after entry to the trial was avoided: what was the withdrawal rate and whether the analysis was done on an intention‐to‐treat basis. For withdrawals, studies were classified as follows: (A) less than 5% of participants excluded from analysis; (B) 5% to 10% of participants excluded from analysis; (C) more than 10% and up to 20% of participants excluded from analysis.
Trials were excluded if it was not possible to analyse data on an intention‐to‐treat basis or 20% or more participants were excluded, or both.
(3) How assessment bias was avoided: how the investigator, the participant or the person assessing the outcome, or both, was blinded to the allocated group. In case of no blinding at all, how objective was the endpoint measured. Blinding was assessed in the following way: (a) blinding of participant (yes/no/unclear or not specified); (b) blinding of caregiver (yes/no/unclear or not specified); (c) blinding of outcome assessment (yes/no/unclear or not specified).
Data extraction and entry
Data were extracted by two authors, and discrepancies were resolved through discussion. Data were entered onto the Review Manager software (RevMan 2003), and checked for accuracy.
Statistical analyses
Statistical analyses were carried out using the Review Manager software (RevMan 2003). All outcomes were dichotomous data, so results are presented as summary relative risk with 95% confidence intervals. The I2 statistic was used to assess heterogeneity between trials, where relevant. As significant heterogeneity (I2 greater than 50%) was not detected, results were pooled using a fixed‐effect model.
Subgroup analyses
A prespecified subgroup was based on the type of hypertensive disease at trial entry: gestational hypertension; hypertension with proteinuria; and chronic hypertension. This analysis was not done because of the small numbers in the review. It will be included in future updates when sufficient data are available.
Results
Description of studies
Details for each trial can be found in the 'Characteristics of included studies' table and the 'Characteristics of excluded studies' table.
This review includes four small trials involving a total of 449 women. Two were conducted in Zimbabwe (Crowther 1986; Crowther 1992), one in the UK (Mathews 1982), and one in Hong Kong (Leung 1998). Two were multicentre (Crowther 1992; Mathews 1982).
Participants
All participants were women with a singleton pregnancy, between 26 and 38 weeks' gestation at trial entry. Two trials recruited both primigravidae and multigravidae women (Crowther 1992; Leung 1998), and the other two did not report on parity.
The women had diastolic blood pressure between 90 to 110 mmHg. One trial also specified systolic pressure of at least 140 mmHg. Two studies included women with chronic or gestational hypertension without proteinuria (Crowther 1992; Leung 1998). The other two included women with unspecified proteinuric hypertension (Crowther 1986; Mathews 1982). No trials reported on whether women were using antihypertensive therapy at trial entry, and only one reported on use of antihypertensives as an outcome measure (Leung 1998).
Interventions
Two trials compared strict bed rest in hospital with some rest in hospital (Crowther 1986; Mathews 1982). Two trials compared some bed rest in hospital with normal activity at home (Crowther 1992; Leung 1998).
Outcomes
Definitions of outcomes used by trialists were consistent with those specified in this review.
One trial reported on women' s views of the intervention and satisfaction with care (Leung 1998). The other outcomes from this study have not been reported because more than 20% participants were excluded from analysis for all other outcomes.
No trials reported on side‐effects of bed rest or costs to health care.
Excluded studies
Seven studies were excluded from the review. Four were excluded because all comparison groups had bed rest. Three of these studies compared an antihypertensive drug plus bed rest with bed rest alone (Cameron 1985; Catalano 1997; Sibai 1992). The fourth was a three‐arm study in which bed rest alone was compared with both compliance enhancement training and a bio‐behavioural intervention (Somers 1989). Two studies were excluded because the participants were pregnant women with normal blood pressure (Herrera 1993; Spinapolice 1983). One trial was excluded because women were able to opt out of the trial after randomisation if they were not happy with the allocated treatment, and they were excluded from the analysis. Although the number of women who opted out is not known even after contacting trialists, it seems likely this was greater than 20% (Mathews 1977).
Risk of bias in included studies
Details for each trial are in the 'Characteristics of included studies' table.
Three trials were of good quality (Crowther 1986; Crowther 1992; Leung 1998) and one was of uncertain quality (Mathews 1982).
Allocation concealment
Allocation concealment was adequate in three trials (Crowther 1986; Crowther 1992; Leung 1998) but unclear in the fourth (Mathews 1982).
Completeness of follow up
Two trials stated that there were no losses to follow up (Crowther 1986; Crowther 1992). One trial (Mathews 1982) excluded 13% of women from the analysis (equal in both groups). Reasons for exclusion included women not complying with their allocated treatment. The fourth trial (Leung 1998), after recruiting women on a single reading of hypertension, excluded a large number of women (25.6%) mainly because blood pressure was no longer raised in these women after randomisation or because the women went into labour prior to confirmation of the diagnosis of hypertension (13 from bed rest group and 8 from control group). However, only 4.4% women were excluded from analysis for data on women's views of the intervention.
Blinding
One trial stated that only the outcome assessor for fetal outcomes was blinded (Crowther 1992). The other three trial reports did not mention blinding. Given the intervention under evaluation, blinding of the participants to this intervention is not possible. Although it would be possible to blind outcome assessment, it is unlikely that this was done because this would be quite a major undertaking, and it is reasonable to assume that it would have been reported had it been done.
Effects of interventions
Four small trials (449 women) were included.
Comparison one: strict bed rest in hospital versus some rest in hospital
Two trials (145 women) evaluated strict bed rest in hospital versus some rest in hospital.
Severe hypertension
One trial (105 women) reported the risk of severe hypertension for strict bed rest in hospital compared to some rest in hospital (relative risk (RR) 1.18, 95% confidence interval (CI) 0.93 to 1.49).
Death of baby: miscarriage, stillbirth, perinatal death, or neonatal death
Two trials (145 women) reported on stillbirth, perinatal death, and neonatal death. Even taking all deaths together there were insufficient data for any reliable conclusions about the possible differential effect (RR 1.07, 95% CI 0.52 to 2.19).
Preterm birth
One trial (105 women) reported the risk of preterm birth for strict bed rest in hospital compared to some rest in hospital (RR 0.98, 95% CI 0.71 to 1.35).
Other outcomes
There were no statistically significant differences for any other outcomes when comparing strict bed rest in hospital with some rest in hospital: two trials (145 women) reported on severe pre‐eclampsia (RR 1.50, 95% CI 0.98 to 2.30), and one trial (105 women) reported on eclampsia (RR 0.33, 95% CI 0.01 to 7.85), elective delivery (RR 1.08, 95% CI 0.87 to 1.34), placental abruption (RR 4.91, 95% CI 0.24 to 99.82), endotracheal intubation (RR 0.65, 95% CI 0.11 to 3.76) and admission of the baby to neonatal intensive care nursery (RR 0.75, 95% CI 0.49 to 1.17).
Comparison two: some rest in hospital versus routine activity at home
Two trials (304 women) evaluated some rest in hospital versus routine activity at home in women with non‐proteinuric hypertension.
Severe hypertension
There was a reduction in the risk of severe hypertension (one trial, 218 women; RR 0.58, 95% CI 0.38 to 0.89) with some rest in hospital compared to normal activity at home.
Pre‐eclampsia
In one trial (218 women), the RR for developing pre‐eclampsia was 0.98 (95% CI 0.80 to 1.20).
Death of baby: miscarriage, stillbirth, perinatal death, or neonatal death
In one trial (218 women) reporting on stillbirth, perinatal death, and neonatal death, there were insufficient data for any reliable conclusions even when all deaths were taken together (RR 1.96, 95% CI 0.18 to 21.34).
Preterm birth
In one trial (218 women), the RR for preterm birth was 0.53 (95% CI 0.29 to 0.99) and for very preterm birth (less than 34 weeks' gestation) 0.49 (95% CI 0.09 to 2.62) with some rest in hospital compared to normal activity at home.
Small‐for‐gestational age
The RR of small‐for‐gestational‐age babies was 0.98 (95% CI 0.51 to 1.91; one trial (218 women)).
Other outcomes
Women's views
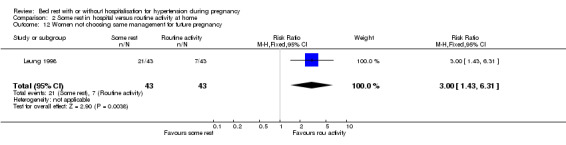
One trial (88 women) reported on women's views. Although both groups seemed to be equally satisfied with their care (RR 0.98, 95% CI 0.93 to 1.02), more women in the bed rest group opted not to have the same management in future pregnancies, if the choice were given (RR 3.00, 95% CI 1.43 to 6.31).
There were no statistically significant differences for any other reported outcomes including elective delivery (RR 0.98, 95% CI 0.70 to 1.37), caesarean section (RR 1.41, 95% CI 0.79 to 2.52), and admission to neonatal intensive care unit (RR 0.82, 95% CI 0.37 to 1.81).
Discussion
Bed rest or restriction of normal activity either at home or in hospital have long been advocated for women with high blood pressure during pregnancy. Despite this, only four small adequately controlled trials have evaluated the potential effects of these interventions. The evidence available from these trials is insufficient to draw reliable conclusions for clinical practice.
Two trials compared strict bed rest in hospital with some rest in hospital for pregnant women with proteinuric hypertension. We have no evidence that there is any difference in outcome between the two interventions. One trial was of good quality (Crowther 1986), and the other was of uncertain quality (Mathews 1982). The findings between trials were mostly consistent, except for perinatal mortality where the point effect of the good quality trial showed worse outcome with strict bed rest and the point effect of the other trial showed benefit from strict bed rest. However, the confidence intervals for both trials were wide and crossed the line of no effect for this outcome, implying that available evidence is insufficient, and the true answer may lie anywhere. Two trials compared some rest in hospital with routine activity at home for pregnant women with non‐proteinuric hypertension, and were of good quality (Crowther 1992; Leung 1998). In one trial (218 women), there was a 42% reduction in the risk of severe hypertension for women who rested in hospital. This finding must be interpreted with caution, as the number of women in the trial was small, so results are more susceptible to the play of chance. The confidence interval is also wide, indicating that the true effect may be anywhere between an 11% reduction in risk to a 62% reduction. Women allocated routine activity at home were monitored with weekly blood pressure measurements in antenatal clinics. It is not reported whether the diagnosis of severe hypertension was based on persistent elevation of blood pressure, or a single episode, and whether antihypertensive medication was required. The clinical significance of this outcome is therefore unclear. It may, at least in part, be related to 'white coat hypertension', where the stress of coming to a hospital leads to transient elevation in blood pressure. This same trial also showed a borderline statistically significant reduction in the risk of preterm birth (RR 0.53, 95% CI 0.29 to 0.99) for women with some bed rest in hospital. However, there was no apparent effect on very preterm birth. As above, the numbers are small, and the confidence interval is wide. It is therefore difficult to draw any firm conclusions about the possible differential effects on preterm birth. While statistically significant results are present in this review, they need to be confirmed, and then clinically significant benefits still need to be demonstrated.
Questions remain about the quantification of rest and activity in the trials. We have made an attempt to quantify 'rest' by dividing it into 'strict' and 'some' based on definitions used by trialists, but the hours of bed rest each day are not reported. Standardising definitions for 'routine' or unrestricted activity is even more difficult. One woman's routine activity may be strenuous work all day, whereas another woman's routine activity may be light work with frequent restful breaks. Although it can be difficult to address these issues in trials, better descriptions of what constitutes rest and baseline level of activity would add to the validity and applicability of results obtained from this review.
The potential effects of bed rest may be related to the type of hypertensive disease a woman has. Although the effects of bed rest on women with proteinuric hypertension (first comparison) are unclear, women with non‐proteinuric hypertension (second comparison) seemed to benefit from some rest, with a reduced risk of severe hypertension. Further trials are needed to assess the influence of type of hypertensive disease on effects of bed rest.
All three trials evaluated the effects of bed rest in hospital. It is arguable whether this rest is actually 'restful' for all women, or does the added stress of hospitalisation in certain women undermine any benefits of the prescribed bed rest. Although no trials specifically reported on women's views of bed rest with regards to the disruption to their lives, or stress associated with hospitalisation, in one trial, less women in the bed rest group opted to have the same treatment in future pregnancies, possibly as a result of these factors.
Bed rest in hospital or at home has financial implication for women and their families, and for healthcare services, but included trials did not report on these costs.
Prolonged bed rest may be associated with complications such as thrombosis, muscle atrophy or bone demineralization. Bed rest may be prescribed for a variable duration in pregnancy, but it is likely that the risk of adverse effects would be higher with lengthier periods of immobility. However, included trials did not report on adverse effects. Greater awareness of the hazards of prolonged periods of immobility may well make interventions such as strict bed rest obsolete for hypertension in pregnancy. What merits further evaluation is whether some rest or restriction of activity, either at home or in hospital, is beneficial.
Bed rest in hospital allows increased surveillance of the woman, and it is proposed that timely access to medical care in hospital may improve pregnancy outcome. At present we have little evidence to support or refute this argument, but if such benefits do exist, they must be weighed against the numbers needed to treat to avert any adverse outcome. On the other hand, being in hospital may predispose to detection bias, and provoke a more interventionalist approach. Moreover, where increased surveillance is required, day care units, where available, are now becoming widely recognised as alternatives to hospital admission for management of complicated pregnancies (Kröner 2001).
Given the paucity of trials, and the absence of relevant and important information from available trials, no reliable conclusions can be made from this review to guide clinical practice regarding bed rest for management of women with hypertension in pregnancy. No conclusive, clinically significant benefits of bed rest have been demonstrated, the possibility of adverse effects remains, and women appear to prefer normal activity and outpatient care in comparison to bed rest with hospitalisation as a care plan.
Authors' conclusions
Implications for practice.
Women with hypertension in pregnancy are often advised to rest in bed, either at home or in the hospital, to prevent progression of hypertension and improve pregnancy outcome. This intervention has been evaluated in few well‐controlled trials, and there is insufficient evidence to provide clear guidance for clinical practice. Although one small trial suggests that some bed rest may be associated with a reduced risk of severe hypertension and preterm birth, these findings need to be confirmed in other larger trials. Moreover, it appears that more women prefer outpatient management with unrestricted activity in comparison to hospitalisation and bed rest if the choice were given. Evidence currently available from randomised trials does not support routine recommendation of bed rest for hypertension in pregnancy.
Implications for research.
Large well‐controlled randomised trials are needed to evaluate the benefits and risks of rest in hospital and at home for women with proteinuric and non‐proteinuric hypertension in pregnancy. Trials need to report not only on pregnancy outcome, but also on the potential side‐effects of bed rest, women's views of the intervention and the costs involved, as these factors are also important determinants in formulating recommendations for clinical practice.
What's new
| Date | Event | Description |
|---|---|---|
| 18 January 2010 | New search has been performed | Search updated. No new trials identified |
History
Protocol first published: Issue 1, 2002 Review first published: Issue 4, 2005
| Date | Event | Description |
|---|---|---|
| 10 November 2008 | Amended | Contact details amended |
| 11 February 2008 | Amended | Converted to new review format. |
| 25 October 2007 | New search has been performed | Search updated. No new trial reports identified. |
| 26 May 2005 | New citation required and conclusions have changed | Substantive amendment |
Acknowledgements
Our thanks to Lelia Duley for her helpful comments on the review.
As part of the pre‐publication editorial process, this review has been commented on by four peers (an editor and three referees who are external to the editorial team), one or more members of the Pregnancy and Childbirth Group's international panel of consumers and the Group's Statistical Adviser.
Appendices
Appendix 1. Additional searching for initial version of review
| Sources searched | Search strategy |
| The Cochrane Central Register of Controlled Trials (The Cochrane Library 2005, Issue 1) and EMBASE (January 2002 to December 2004). | We used the search strategy listed in the generic protocol (see Meher 2005 in 'Additional references' ). |
Data and analyses
Comparison 1. Strict bed rest in hospital versus some rest in hospital.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Severe hypertension | 1 | 105 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.18 [0.93, 1.49] |
| 2 Severe pre‐eclampsia | 2 | 145 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.50 [0.98, 2.30] |
| 3 Eclampsia | 1 | 105 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.33 [0.01, 7.85] |
| 4 Elective delivery including elective caesarean section or induction of labour, or both | 1 | 105 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.08 [0.87, 1.34] |
| 5 Placental abruption | 1 | 105 | Risk Ratio (M‐H, Fixed, 95% CI) | 4.91 [0.24, 99.82] |
| 6 Death of baby, including miscarriage, stillbirth, perinatal death, and neonatal death | 2 | 145 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.07 [0.52, 2.19] |
| 7 Death of baby by timing of death | 2 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 7.1 Stillbirth | 2 | 145 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.11 [0.45, 2.75] |
| 7.2 Perinatal death | 2 | 145 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.07 [0.52, 2.19] |
| 7.3 Neonatal death | 2 | 145 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.99 [0.28, 3.50] |
| 8 Preterm birth | 1 | 105 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.98 [0.71, 1.35] |
| 9 Endotracheal intubation | 1 | 105 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.65 [0.11, 3.76] |
| 10 Admission to neonatal intensive care nursery | 1 | 105 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.75 [0.49, 1.17] |
1.1. Analysis.
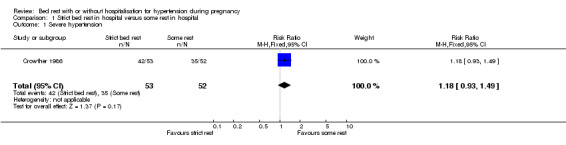
Comparison 1 Strict bed rest in hospital versus some rest in hospital, Outcome 1 Severe hypertension.
1.2. Analysis.
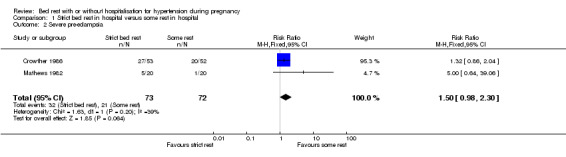
Comparison 1 Strict bed rest in hospital versus some rest in hospital, Outcome 2 Severe pre‐eclampsia.
1.3. Analysis.
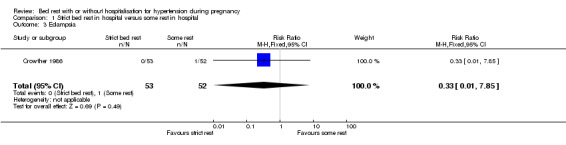
Comparison 1 Strict bed rest in hospital versus some rest in hospital, Outcome 3 Eclampsia.
1.4. Analysis.
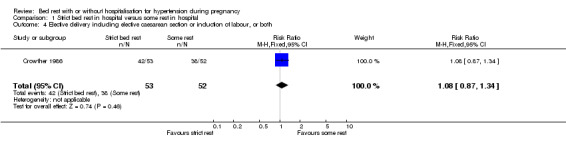
Comparison 1 Strict bed rest in hospital versus some rest in hospital, Outcome 4 Elective delivery including elective caesarean section or induction of labour, or both.
1.5. Analysis.
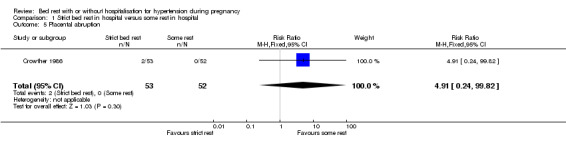
Comparison 1 Strict bed rest in hospital versus some rest in hospital, Outcome 5 Placental abruption.
1.6. Analysis.
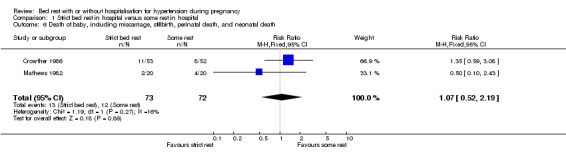
Comparison 1 Strict bed rest in hospital versus some rest in hospital, Outcome 6 Death of baby, including miscarriage, stillbirth, perinatal death, and neonatal death.
1.7. Analysis.
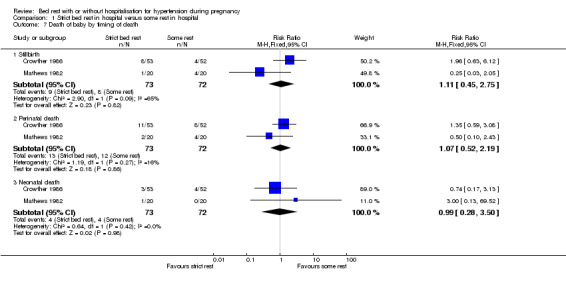
Comparison 1 Strict bed rest in hospital versus some rest in hospital, Outcome 7 Death of baby by timing of death.
1.8. Analysis.
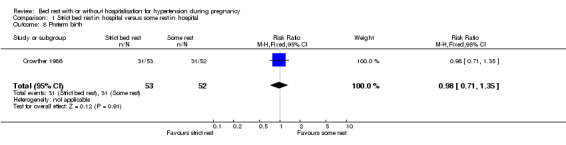
Comparison 1 Strict bed rest in hospital versus some rest in hospital, Outcome 8 Preterm birth.
1.9. Analysis.
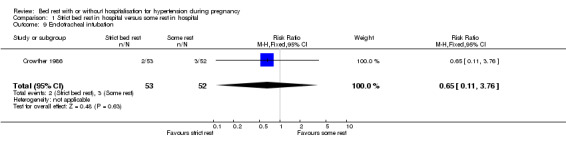
Comparison 1 Strict bed rest in hospital versus some rest in hospital, Outcome 9 Endotracheal intubation.
1.10. Analysis.
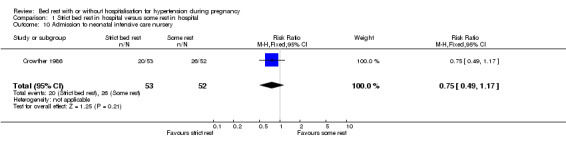
Comparison 1 Strict bed rest in hospital versus some rest in hospital, Outcome 10 Admission to neonatal intensive care nursery.
Comparison 2. Some rest in hospital versus routine activity at home.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Severe hypertension | 1 | 218 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.58 [0.38, 0.89] |
| 2 Pre‐eclampsia | 1 | 218 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.98 [0.80, 1.20] |
| 3 Elective delivery, including elective caesarean section or induction of labour, or both | 1 | 218 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.98 [0.70, 1.37] |
| 4 Caesarean section | 1 | 218 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.41 [0.79, 2.52] |
| 5 Death of baby | 1 | 218 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.96 [0.18, 21.34] |
| 6 Death of baby by timing of death | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 6.1 Stillbirth | 1 | 218 | Risk Ratio (M‐H, Fixed, 95% CI) | 4.91 [0.24, 101.10] |
| 6.2 Perinatal death | 1 | 218 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.96 [0.18, 21.34] |
| 6.3 Neonatal death | 1 | 218 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.33 [0.01, 7.95] |
| 7 Small‐for‐gestational age | 1 | 218 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.98 [0.51, 1.91] |
| 8 Preterm birth | 1 | 218 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.53 [0.29, 0.99] |
| 9 Preterm birth (subgroup by gestational age) | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 9.1 Birth < 37 weeks | 1 | 218 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.53 [0.29, 0.99] |
| 9.2 Birth < 34 weeks | 1 | 218 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.49 [0.09, 2.62] |
| 10 Admission to neonatal intensive care unit | 1 | 218 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.82 [0.37, 1.81] |
| 11 Women satisfied with management | 1 | 86 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.98 [0.92, 1.04] |
| 12 Women not choosing same management for future pregnancy | 1 | 86 | Risk Ratio (M‐H, Fixed, 95% CI) | 3.0 [1.43, 6.31] |
2.1. Analysis.
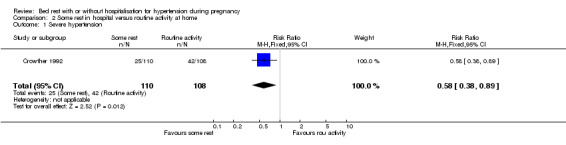
Comparison 2 Some rest in hospital versus routine activity at home, Outcome 1 Severe hypertension.
2.2. Analysis.
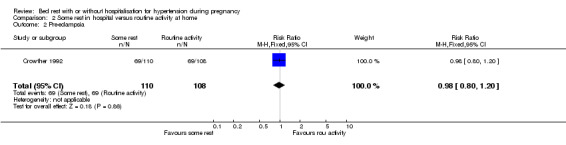
Comparison 2 Some rest in hospital versus routine activity at home, Outcome 2 Pre‐eclampsia.
2.3. Analysis.
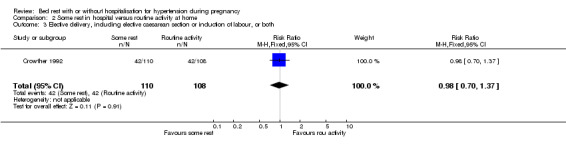
Comparison 2 Some rest in hospital versus routine activity at home, Outcome 3 Elective delivery, including elective caesarean section or induction of labour, or both.
2.4. Analysis.
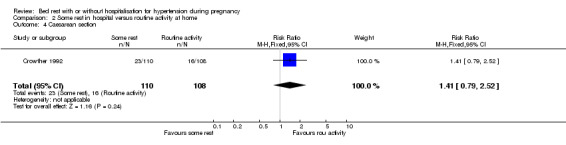
Comparison 2 Some rest in hospital versus routine activity at home, Outcome 4 Caesarean section.
2.5. Analysis.
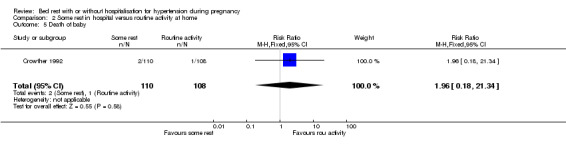
Comparison 2 Some rest in hospital versus routine activity at home, Outcome 5 Death of baby.
2.6. Analysis.
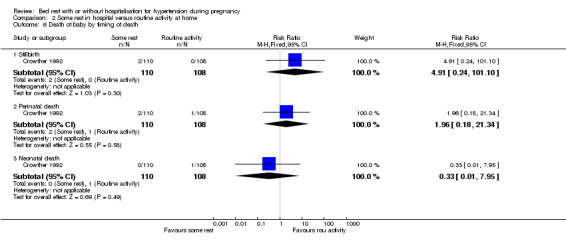
Comparison 2 Some rest in hospital versus routine activity at home, Outcome 6 Death of baby by timing of death.
2.7. Analysis.
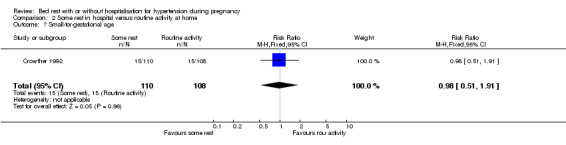
Comparison 2 Some rest in hospital versus routine activity at home, Outcome 7 Small‐for‐gestational age.
2.8. Analysis.
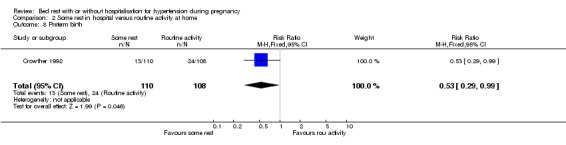
Comparison 2 Some rest in hospital versus routine activity at home, Outcome 8 Preterm birth.
2.9. Analysis.
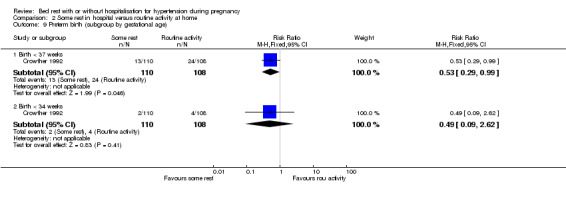
Comparison 2 Some rest in hospital versus routine activity at home, Outcome 9 Preterm birth (subgroup by gestational age).
2.10. Analysis.
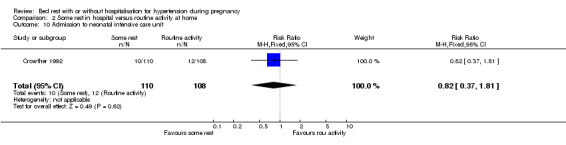
Comparison 2 Some rest in hospital versus routine activity at home, Outcome 10 Admission to neonatal intensive care unit.
2.11. Analysis.
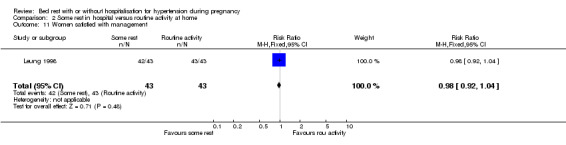
Comparison 2 Some rest in hospital versus routine activity at home, Outcome 11 Women satisfied with management.
2.12. Analysis.
Comparison 2 Some rest in hospital versus routine activity at home, Outcome 12 Women not choosing same management for future pregnancy.
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Crowther 1986.
| Methods | Randomisation: block randomisation using variable blocks and random number tables. Allocation concealment: consecutively numbered opaque, sealed envelopes. Follow up: no loss of participants (A). Blinding: none. | |
| Participants | 105 women with a singleton pregnancy at 28‐38 weeks, with proteinuric hypertension (diastolic BP 90‐109 mmHg and proteinuria 1+ or more). No other complications of pregnancy. | |
| Interventions | Exp: admission to hospital for strict bed rest until delivery. Ambulation only to toilet. Controls: allowed to move around the hospital ward as desired. | |
| Outcomes | Women: severe hypertension (diastolic BP > 109 mmHg); increase in proteinuria; fulminating pre‐eclampsia; eclampsia; placental abruption; IOL. Baby: preterm delivery; low birthweight; very low birthweight; passage of meconium; Apgar score; perinatal death; early neonatal death; stillbirth; endotracheal intubation; admission to special care nursery. | |
| Notes | Setting: Zimbabwe. 1 centre. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Low risk | A ‐ Adequate |
Crowther 1992.
| Methods | Randomisation: block randomisation, stratified into 3 groups: primiparous, multiparous without chronic hypertension, and multiparous with chronic hypertension. Allocation concealment: consecutively numbered, opaque, sealed envelopes. Follow up: no loss of participants (A). Blinding: for fetal outcomes only. | |
| Participants | 218 primigravidae and multigravidae women with a singleton pregnancy at 28‐38 weeks, with non‐proteinuric hypertension (BP at least 140/90 mmHg). Excluded if diastolic BP >/= 110 mmHg, symptomatic, caesarean section scar, or APH during the pregnancy. | |
| Interventions | Exp: admission to hospital for rest. Allowed to move around the ward voluntarily. 4 hourly BP check and daily urinalysis. Controls: normal activity at home with no restrictions. Daily self analysis of urine for protein. Reviewed weekly for BP, weight, bloods. | |
| Outcomes | Woman: severe hypertension (>/= 160/110 mmHg); proteinuria; caesarean section; IOL. Baby: birthweight (mean); birthweight (< 2500 g); small‐for‐gestational age (< 10%); admission to intensive care nursery; length of stay in hospital; perinatal death; Apgar score at 1 and 5 minutes. | |
| Notes | Setting: Zimbabwe. 1 maternity hospital and 13 peripheral clinics. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Low risk | A ‐ Adequate |
Leung 1998.
| Methods | Randomisation: women were "allocated randomly" to 2 groups. No further information. Allocation concealment: consecutively numbered, opaque, sealed envelopes. Follow up: 25.6% excluded from analysis. 2 defaulted follow up and delivered somewhere else. Others were excluded either because hypertension was absent in these women postrandomisation or because the women went into labour prior to confirmation of the diagnosis of hypertension (13 from bed rest group and 8 from control group). However, only 4.4% women were excluded from analysis for data on women's views of the intervention (2 woman in each group). Blinding: none. | |
| Participants | 90 primigravidae and multigravidae women with a singleton pregnancy at 28‐38 weeks, with non‐proteinuric hypertension (diastolic BP 90‐100 mmHg) after 5 minutes rest. Excluded if proteinuria >/= 1+ or symptoms of severe PE. | |
| Interventions | Exp: admission to antenatal ward in hospital. Advised to rest in bed as much as possible. Controls: normal activity at home with no restrictions. Daily self analysis of urine for protein. Reviewed weekly in day care centre (day ward with 12 beds) or outpatient clinic for BP, fetal monitoring, urinalysis, bloods. | |
| Outcomes | Women: hypertension (dBP > 90 x 2), severe hypertension (dBP >/= 110 mmHg x 2); proteinuria; mode of delivery; IOL; use of antihypertensive medication; women's views and preferences (questionnaire). Baby: birthweight (mean); small‐for‐gestational age (not defined); admission to intensive care nursery; length of stay in hospital; stillbirth/neonatal death; Apgar score at 1 and 5 minutes. | |
| Notes | Setting: Hong Kong. 1 centre. The only outcome we have reported from this paper is women's views and satisfaction with care as > 20% women have been excluded from analysis for all other outcomes. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Low risk | A ‐ Adequate |
Mathews 1982.
| Methods | Randomisation: volunteers allocated "at random" to either of the 2 groups. No further information. Allocation concealment: sealed envelopes. Follow up: 13% participants excluded from analysis for various reasons (3 participants in each group) (C). Blinding: none. | |
| Participants | 40 women with a singleton pregnancy at 26 to over 36 weeks, with proteinuric hypertension (diastolic BP 90 to 109 mmHg, and proteinuria more than a trace), and asymptomatic. | |
| Interventions | Exp: admission to hospital for strict bed rest. Controls: allowed to move around the ward in hospital. A pedometer was attached to both groups of women as a crude measure of whole body activity. | |
| Outcomes | Women: plasma urea and urate; serum human placental lactogen and oestriol; development of premonitory symptoms of eclampsia; hypertension; proteinuria; mode of delivery. Baby: perinatal death (stillbirths and neonatal death), gestation at delivery, birthweight, small‐for‐gestational age, sex of baby. | |
| Notes | Setting: UK. 2 centres. Missing data: complete data on all 40 participants available only for 2 outcomes in the published report: perinatal death and development of premonitory symptoms. For other outcomes, data reported for only 10 'high‐risk' participants. Trialist contacted for missing data for other outcomes but data were not available. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Unclear risk | B ‐ Unclear |
APH: antepartum haemorrhage BP: blood pressure Exp: experiment IOL: induction of labour PE: pre‐eclampsia dBP: diastolic blood pressure
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Cameron 1985 | Comparison of labetalol with bed rest versus bed rest alone, in hospital. Abstract only. Methods: "randomised". No further information. Participants: 85 pregnant women with hypertension. Intervention: as above. Outcomes: blood pressure, proteinuria, biochemical and haematological tests, tests of placental function, adverse effects of therapy on mother and baby. |
| Catalano 1997 | Comparison of nifedipine with bed rest versus bed rest alone, in hospital for women with mild pre‐eclampsia. Methods: "randomly allocated". No further information. Participants: 100 women at 26‐36 weeks' gestation, with mild pre‐eclampsia (not defined in translated summary). Intervention: as above. Outcomes: blood pressure; prolongation of pregnancy; days of hospitalisation; preterm birth; mean birthweight; small‐for‐gestational age. |
| Herrera 1993 | Participants: normotensive women. Methods: randomisation by computer‐generated list. Allocation concealment by closed envelopes. Participants: 74 primigravida women at 28‐29 weeks' gestation with normal blood pressure and a positive roll‐over test. Intervention: bed rest at home and nutritional supplements (soy protein, linoleic acid, calcium) versus no bed rest at home and placebo (iron tablets). Outcomes: pregnancy‐induced hypertension; pre‐eclampsia; caesarean section; gestational age at birth; mean birthweight. |
| Mathews 1977 | Participants excluded after randomisation if they opted out of the study because they were not happy with allocated treatment. Trialist contacted to determine the number of participants excluded after randomisation, but this information was not available. Methods: participants "allocated at random" to 1 of 4 groups using previously prepared cards contained in envelopes. No further information. Participants: 135 women with singleton pregnancy between 28 to over 38 weeks, with non‐proteinuric hypertension (diastolic BP between 90 and 109 mmHg), and asymptomatic. Intervention: (4 groups) bed rest in hospital with or without sedation (phenobarbitone 15 mg 3 times daily) versus normal activity at home with or without sedation. Outcomes: severe hypertension (BP > 109 mmHg); proteinuria; eclampsia; induction of labour; operative vaginal delivery; caesarean section; biochemical parameters; stillbirths and neonatal death; fetal distress during labour; Apgar score; small for gestation; preterm birth. |
| Sibai 1992 | Comparison of nifedipine with bed rest versus bed rest alone, in hospital. Methods: randomised controlled trial. Participants: 200 women at 26‐36 weeks' gestation with proteinuric and non‐proteinuric hypertension. Interventions: as above. Outcomes: blood pressure; haematological and biochemical parameters; days in hospital; prolongation of pregnancy; preterm delivery; gestation at birth; mean birthweight; small‐for‐gestational age; placental weight; cord blood gas. |
| Somers 1989 | 3‐arm trial in which all 3 groups of participants had bed rest varying from 15.8 to 18 hours per day along with additional interventions. Methods: "randomly" assigned. No further information. Participants: 45 women at 30‐36 weeks with hypertension, all prescribed restricted physical activity for hypertension. Interventions: 3 arms: bed rest at home versus bed rest and compliance enhancement training at home verus bed rest, compliance enhancement training, and bio‐behavioral interventions at home. Outcomes: mean arterial pressure; compliance with bed rest. |
| Spinapolice 1983 | Participants normotensive pregnant women. Methods: "random allocation". No further information available. Participants: 32 nulliparous women at 28‐32 weeks' gestation with a normal blood pressure and positive roll‐over test. Intervention: bed rest for 4 to 6 hours at home versus normal activity at home. Outcomes: gestational hypertension; pre‐eclampsia; gestation at delivery; birthweight; Apgar scores. |
BP: blood pressure
Differences between protocol and review
The original published protocol was updated while drafting the 2007 updated review. The differences include: (1) a new contact author (S Meher) and additional sources of support were added. (2) the 'Objectives' were reworded and additional comparisons were included. This was done to accommodate for variation of definitions of 'bed rest' and 'normal activity' used by trialists. One trialist's bed rest in hospital (rest in bed but allowed to move around the ward) was another trialist's normal activity in hospital (this trialist defined bed rest as strict bed rest where the participant only got up to go to the toilet). To avoid pooling interventions which crossed arms in different trials, we divided bed rest into different degrees and created new comparisons of different degrees of bed rest. Moreover, the original protocol did not allow for comparisons of bed rest and normal activity across different settings in the same trial, for example, bed rest in hospital versus normal activity at home. We felt that these were important comparisons that needed to be included in the review.
Contributions of authors
Guillermo Carroli wrote the initial version of the protocol. Edgardo Abalos contributed to the final version of the protocol. The protocol was modified by Shireen Meher and Edgardo Abalos following its publication due to the discovery of unexpected but relevant comparisons and definitions of bed rest used by trialists.
Shireen Meher and Edgardo Abalos decided on eligible trials and did the data extraction. Data were entered by Shireen Meher, and double checked by Edgardo Abalos. Shireen Meher drafted the review, and the final report was prepared with contributions from Edgardo Abalos.
Sources of support
Internal sources
Centro Rosarino de Estudios Perinatales‐CREP‐Rosario, Argentina.
The University of Liverpool, UK.
External sources
Human Reproduction Programme‐HRP‐World Health Organization. Geneva, Switzerland.
Health Technology Assessment, UK.
Declarations of interest
None known.
New search for studies and content updated (no change to conclusions)
References
References to studies included in this review
Crowther 1986 {published and unpublished data}
- Crowther CA. Strict bed rest vs ambulation in the management of patients with proteinuric hypertension in pregnancy. Personal communication 2004.
Crowther 1992 {published data only}
- Crowther CA, Bouwmeester AM, Ashurst HM. Does admission to hospital for bed rest prevent disease progression or improve fetal outcome in pregnancy complicated by non‐proteinuric hypertension?. British Journal of Obstetrics and Gynaecology 1992;99:13‐7. [DOI] [PubMed] [Google Scholar]
Leung 1998 {published data only}
- Leung KY, Sum TK, Tse CY, Law KM, Chan MYM. Is in‐patient management of diastolic blood pressure between 90 and 100 mm Hg during pregnancy necessary?. Hong Kong Medical Journal 1998;4:211‐7. [PubMed] [Google Scholar]
Mathews 1982 {published data only}
- Mathews DD, Agarwal V, Shuttleworth TP. A randomized controlled trial of complete bed rest vs ambulation in the management of proteinuric hypertension during pregnancy. British Journal of Obstetrics and Gynaecology 1982;89:128‐31. [DOI] [PubMed] [Google Scholar]
- Mathews DD, Agarwal V, Shuttleworth TP. The effect of rest and ambulation on plasma urea and urate levels in pregnant women with proteinuric hypertension. British Journal of Obstetrics and Gynaecology 1980;87:1095‐8. [DOI] [PubMed] [Google Scholar]
References to studies excluded from this review
Cameron 1985 {published data only}
- Cameron AD, Walker JJ, Bonduelle M, Calder AA. A randomised trial of the antihypertensive agent, labetalol, against bed rest in pregnancy hypertension. Archives of Gynecology 1985;237 Suppl:295. [Google Scholar]
Catalano 1997 {published data only}
- Catalano D, Ercolano S, Pollio F, Ascione L, Russo C, De S, et al. Evaluation of the monotherapy with nifedipine in the management of preeclampsia [Valutazione della monoterapia con nifedipina nel management della gestosi EPH]. Giornale Italiano Di Ostetricia e Ginecologia 1997;19:373‐5. [Google Scholar]
Herrera 1993 {published data only}
- Herrera JA. Nutritional factors and rest reduce pregnancy‐induced hypertension and pre‐eclampsia in positive roll‐over test primigravidas. International Journal of Gynecology & Obstetrics 1993;41:31‐5. [DOI] [PubMed] [Google Scholar]
Mathews 1977 {published and unpublished data}
- Mathews DD. A randomized controlled trial of bed rest and sedation or normal activity and non‐sedation in the management of non‐albuminuric hypertension in late pregnancy. British Journal of Obstetrics and Gynaecology 1977;84:108‐14. [DOI] [PubMed] [Google Scholar]
Sibai 1992 {published data only}
- Barton JR, Mercer BM, Sibai BM. The effect of nifedipine on urinary excretion of calcium in preeclampsia. American Journal of Perinatology 1997;14(10):609‐12. [DOI] [PubMed] [Google Scholar]
- Sibai BM, Barton JR, Akl S, Sarinoglu C, Mercer BM. A randomized prospective comparison of nifedipine and bed rest versus bed rest alone in the management of preeclampsia remote from term. American Journal of Obstetrics and Gynecology 1992;167(4 Pt 1):879‐84. [DOI] [PubMed] [Google Scholar]
- Sibai BM, Barton JR, Akl S, Sarinoglu C, Mercer BM. A randomized prospective comparison of nifedipine and bed rest vs bed rest alone in the management of preeclampsia remote from term. American Journal of Obstetrics and Gynecology 1992;166:280. [DOI] [PubMed] [Google Scholar]
Somers 1989 {published data only}
- Somers PJ, Gevirtz RN, Jasin SE, Chin HG. The efficacy of biobehavioral and compliance interventions in the adjunctive treatment of mild pregnancy‐induced hypertension. Biofeedback & Self Regulation 1989;14:309‐18. [DOI] [PubMed] [Google Scholar]
Spinapolice 1983 {published data only}
- Spinapolice RX, Feld S, Harrigan JT. Effective prevention of gestational hypertension in nulliparous women at high risk as identified by the rollover test. American Journal of Obstetrics and Gynecology 1983;146:166‐8. [DOI] [PubMed] [Google Scholar]
Additional references
Abalos 2007
- Abalos E, Duley L, Steyn DW, Henderson‐Smart DJ. Antihypertensive drug therapy for mild to moderate hypertension during pregnancy. Cochrane Database of Systematic Reviews 2007, Issue 1. [Art. No.: CD002252. DOI: 10.1002/14651858.CD002252.pub2] [DOI] [PubMed] [Google Scholar]
ACOG 1996
- American College of Obstetricians and Gynecologists. Hypertension in Pregnancy. ACOG Technical Bulletin 1996;219:1‐8. [Google Scholar]
ASSHP 1993
- Anonymous. Australian Society for the Study of Hypertension in Pregnancy: Consensus statement: management of hypertension in pregnancy: executive summary. Medical Journal of Australia 1993;158:700‐2. [PubMed] [Google Scholar]
Chesley 1978
- Chesley LC. Hypertensive Disorders in Pregnancy. New York: Appleton‐Century‐Crofts, 1978. [Google Scholar]
Davey 1988
- Davey DA, MacGilivray I. The classification and definition of the hypertensive disorders of pregnancy. American Journal of Obstetrics and Gynecology 1988;158:892‐8. [DOI] [PubMed] [Google Scholar]
Duley 2005
- Duley L, Henderson‐Smart D, Meher S. Altered dietary salt for preventing pre‐eclampsia, and its complications. Cochrane Database of Systematic Reviews 2005, Issue 4. [Art. No.: CD005548. DOI: 10.1002/14651858.CD005548.] [DOI] [PMC free article] [PubMed] [Google Scholar]
Duley 2006
- Duley L, Henderson‐Smart DJ, Meher S. Drugs for treatment of very high blood pressure during pregnancy. Cochrane Database of Systematic Reviews 2006, Issue 3. [Art. No.: CD001449. DOI: 10.1002/14651858.CD001449.pub2] [DOI] [PubMed] [Google Scholar]
Duley 2007
- Duley L, Henderson‐Smart DJ, Meher S, King JF. Antiplatelet agents for preventing pre‐eclampsia and its complications. Cochrane Database of Systematic Reviews 2007, Issue 2. [Art. No.: CD004659. DOI: 10.1002/14651858.CD004659.pub2] [DOI] [PubMed] [Google Scholar]
Goldenberg 1994
- Goldenberg R, Cliver S, Bronstein J, Cutter G, Andrews W, Mennemeyer S. Bed rest in pregnancy. Obstetrics & Gynecology 1994;84:131‐6. [PubMed] [Google Scholar]
Hamlin 1952
- Hamlin RHJ. The prevention of eclampsia and pre‐eclampsia. Lancet 1952;i:64‐8. [DOI] [PubMed] [Google Scholar]
Hofmeyr 1996
- Hofmeyr GJ. Abdominal decompression for suspected fetal compromise/pre‐eclampsia. Cochrane Database of Systematic Reviews 1996, Issue 1. [Art. No.: CD000004. DOI: 10.1002/14651858.CD000004] [DOI] [PubMed] [Google Scholar]
Kramer 1986
- Kramer P, Constan D, Krzeminiski J, Brondi D, Martin C. Hospitalisation on the high risk maternity unit. General Hospital Psychiatry 1986;8:33‐9. [DOI] [PubMed] [Google Scholar]
Kröner 2001
- Kröner C, Turnbull D, Wilkinson C. Antenatal day care units versus hospital admission for women with complicated pregnancy. Cochrane Database of Systematic Reviews 2001, Issue 4. [Art. No.: CD001803. DOI: 10.1002/14651858.CD001803] [DOI] [PubMed] [Google Scholar]
Lindheimer 1999
- Lindheimer MD, Roberts JM, Cunningham FG. Hypertensive Disorders in Pregnancy. 2nd Edition. Stamford, Connecticut: Appleton & Lange, 1999. [Google Scholar]
Magee 2003
- Magee LA, Duley L. Oral beta‐blockers for mild to moderate hypertension during pregnancy. Cochrane Database of Systematic Reviews 2003, Issue 3. [Art. No.: CD002863. DOI: 10.1002/14651858.CD002863] [DOI] [PubMed] [Google Scholar]
Maloni 1993
- Maloni JA, Chance B, Zhang C, Cohen AW, Betts D, Gange SJ. Physical and psychosocial side effects of antepartum hospital bed rest. Nursing Research 1993;42:197‐203. [PubMed] [Google Scholar]
Meher 2005
- Meher S, Duley L, Prevention of Pre‐eclampsia Cochrane Review authors. Interventions for preventing pre‐eclampsia and its consequences: generic protocol. Cochrane Database of Systematic Reviews 2005, Issue 2. [Art. No.: CD005301. DOI: 10.1002/14651858.CD005301] [Google Scholar]
NHBPEP 2000
- Anonymous. Report of the National High Blood Pressure Education Program Working Group on High Blood Pressure in Pregnancy. American Journal of Obstetrics and Gynecology 2000;183(1):S1‐S22. [PubMed] [Google Scholar]
North 1999
- North RA, Taylor RS, Schellenberg JC. Evaluation of the definition of pre‐eclampsia. British Journal of Obstetrics and Gynaecology 1999;106:767‐73. [DOI] [PubMed] [Google Scholar]
RevMan 2003 [Computer program]
- The Cochrane Collaboration. Review Manager (RevMan). Version 4.2 for Windows. Oxford, England: The Cochrane Collaboration, 2003.
Roberts 1993
- Roberts JM, Redman CWG. Pre‐eclampsia: more than pregnancy‐induced hypertension. Lancet 1993;341:1447‐51. [DOI] [PubMed] [Google Scholar]
Sanderson 1991
- Sanderson M, Placek PJ, Keppel KG. The 1998 national maternal and infant health survey: design, content, and data availability. Birth 1991;18:26‐32. [DOI] [PubMed] [Google Scholar]
Saudan 1998
- Saudan P, Brown M, Buddle M, Jones M. Does gestational hypertension become pre‐eclampsia?. British Journal of Obstetric and Gynaecology 1998;105:1177‐84. [DOI] [PubMed] [Google Scholar]
Sibai 1998
- Sibai BM, Lindheimer MD, Hauth J, Caritis S, Dorsten P, Klebanoff M, et al. Risk factors for preeclampsia, abruptio placentae, and adverse neonatal outcomes among women with chronic hypertension. New England Journal of Medicine 1998;339:667‐71. [DOI] [PubMed] [Google Scholar]
Walker 2000
- Walker J. Pre‐eclampsia. Lancet 2000;356:1260‐5. [DOI] [PubMed] [Google Scholar]


