Abstract
Aims
Cardiovascular disease is a major threat to maternal health, with cardiomyopathy being among the most common acquired cardiovascular diseases during pregnancy and the postpartum period. The aim of our study was to evaluate the effectiveness of an electrocardiogram (ECG)-based deep learning model in identifying cardiomyopathy during pregnancy and the postpartum period.
Methods and results
We used an ECG-based deep learning model to detect cardiomyopathy in a cohort of women who were pregnant or in the postpartum period seen at Mayo Clinic. Model performance was evaluated using the area under the receiver operating characteristic curve (AUC), accuracy, sensitivity, and specificity. We compared the diagnostic probabilities of the deep learning model with natriuretic peptides and a multivariable model consisting of demographic and clinical parameters. The study cohort included 1807 women; 7%, 10%, and 13% had left ventricular ejection fraction (LVEF) of 35% or less, <45%, and <50%, respectively. The ECG-based deep learning model identified cardiomyopathy with AUCs of 0.92 (LVEF ≤ 35%), 0.89 (LVEF < 45%), and 0.87 (LVEF < 50%). For LVEF of 35% or less, AUC was higher in Black (0.95) and Hispanic (0.98) women compared to White (0.91). Natriuretic peptides and the multivariable model had AUCs of 0.85 to 0.86 and 0.72, respectively.
Conclusions
An ECG-based deep learning model effectively identifies cardiomyopathy during pregnancy and the postpartum period and outperforms natriuretic peptides and traditional clinical parameters with the potential to become a powerful initial screening tool for cardiomyopathy in the obstetric care setting.
Keywords: Artificial intelligence, Cardiomyopathy, ECG, Heart failure, Peripartum, Pregnancy
Graphical Abstract
Detecting cardiomyopathies in pregnant and postpartum women. AUC, area under the receiver operating characteristic curve; ECG, electrocardiogram.
Introduction
Maternal morbidity and mortality in the USA exceeds other developed countries,1,2 with cardiovascular disease as the leading cause of mortality during pregnancy and the postpartum period.3,4 Cardiovascular disease has been identified as a major threat to motherhood and women’s health in the USA5 and UK,6 accounting for approximately 33% of all pregnancy-related deaths7 and complicating up to 160 000 pregnancies in the USA each year.5 Cardiomyopathy is among the most common acquired cardiovascular diseases during pregnancy and the postpartum period; however, diagnosis is challenging due to overlap between pathologic cardiovascular symptoms and those seen with normal pregnancy.8 A review of maternal deaths in California from 2002 to 2006 found that a delay in diagnosis, despite the presence of clinical warning signs, was responsible for approximately half of all deaths attributed to cardiomyopathy in the peripartum period.8 Peripartum cardiomyopathy (PPCM) is a well-described cause of left ventricular systolic dysfunction (LVSD) and heart failure in this patient population.9 However, other forms of cardiomyopathy with LVSD may present prior to, during, or just after pregnancy with associated severe adverse effects.10 Current guidelines from the American College of Cardiology and the American College of Obstetrics and Gynecology (ACOG) do not recommend routine screening for cardiomyopathy in pregnant women and those in the postpartum period. A recently developed electrocardiogram (ECG)-based deep learning model has been demonstrated to be effective in identifying cardiomyopathies in clinical and non-clinical settings.11–13 Currently, there is no information on its value in detecting pregnancy-related cardiomyopathies. This study aims to evaluate the effectiveness of an ECG-based deep learning model for diagnosing cardiomyopathies during pregnancy and the postpartum period.
Methods
Study population
We identified adult women aged 18–49 years evaluated at Mayo Clinic (Phoenix, AZ, USA; Jacksonville, FL, USA; and Rochester, MN, USA) and Mayo Clinic Health System hospitals who were pregnant or in the postpartum period using pregnancy-related International Classification of Diseases (ICD) 9 and 10 diagnosis codes, as well as the Hospital International Classification of Disease Adapted (HICDA) codes, a modification of ICD-8.14,15 The pregnancy-related diagnosis codes are available through the Rochester Epidemiology Project, Data Exploration Portal.15 Women were included if they had a diagnosis date between 1 January 2000 and 31 December 2020, and had at least 1 standard 12‐lead ECG performed within 300 days following their diagnosis date and an echocardiogram (performed at the discretion of the managing physician) within 30 days following the ECG. This time interval was selected to include all cardiomyopathies present or diagnosed during pregnancy and up to 1 year postpartum regardless of aetiology. This broad time period was chosen because the Centers for Disease Control defines pregnancy-related mortality as deaths occurring during or within 1 year of pregnancy.16 Given cardiomyopathy is the leading cause of pregnancy-related deaths, we intended to capture any cardiomyopathy occurring in pregnancy and through 12 months postpartum.
For patients with multiple echocardiograms, the earliest available within the study period was selected. For patients with multiple ECGs during that time frame, the closest ECG to the echocardiogram was selected. All ECGs were acquired at a sampling rate of 500 Hz using a GE Marquette ECG machine (GE Healthcare) and stored using the MUSE ECG data management system (GE Healthcare). Echocardiographic data were obtained from a Mayo Clinic custom database (Echo Image Management System). Left ventricular ejection fraction (LVEF) was calculated based on standards recommended by the American Society of Echocardiography.17 LVEF values used were selected in order of priority: two-dimensional biplane method of disks summation (modified Simpson method), two-dimensional linear, M-mode, and visual estimation.
Exclusion
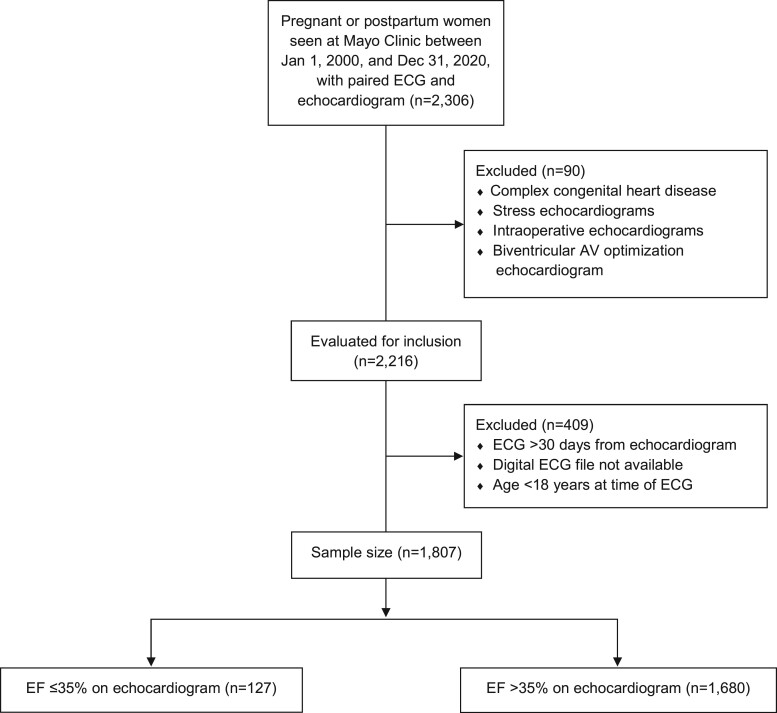
We identified women with congenital heart disease15 and excluded those with complex congenital heart disease based on echocardiogram reports and prespecified HICDA and ICD diagnosis codes (Supplementary material online, Table S1). We also excluded echocardiograms performed intraoperatively, during stress testing, or cardiac resynchronization therapy atrioventricular optimization procedures. Our final study cohort included 1807 patients (Figure 1). The study was approved by the Mayo Clinic Institutional Review Board, including a waiver of informed consent.
Figure 1.
Study population flow diagram. AV, atrioventricular; ECG, electrocardiogram; EF, ejection fraction.
Covariates
Data on demographic, clinical, and laboratory variables, including natriuretic peptides, were extracted from the electronic health record. Additional clinical diagnosis/comorbid conditions were extracted if present at any time prior through 30 days following the index ECG except for pre-eclampsia/eclampsia. Pre-eclampsia/eclampsia was identified within 100 days prior and up to 30 days following the echocardiogram in order to capture its diagnosis within the index pregnancy and not subsequent pregnancies. Our goal was to compare the performance of the ECG-deep learning model in predicting cardiomyopathy at predetermined LVEF cut points compared to natriuretic peptides and a combination of clinical and demographic variables.
Outcomes
Our primary outcome was the identification of women with LVSD defined as LVEF of 35% or less. Our secondary outcomes included two additional LVEF cut points: LVEF <45% and LVEF <50%. All cut points were selected due to their clinical relevance. Patients with an LVEF of 35% or less are considered to be at the highest risk for sudden cardiac death from ventricular arrhythmias.18 Patients in the peripartum period with an LVEF <45% without other aetiologies identified for systolic dysfunction are considered to have PPCM, a clinically distinct condition unique to pregnant women and those in the postpartum period.9 Identification of individuals with an LVEF <50% is important as they often meet clinical criteria for medical interventions to manage cardiomyopathy,18 and the most recent universal definition of heart failure classifies this as reduced ejection fraction.19 For diagnostic accuracy assessment the criterion standard test was LVEF, as measured on a two‐dimensional echocardiogram.
Deep learning model
We employed a previously developed and validated ECG-based deep learning model for the identification of LVEF of 35% or less11,20 with no additional training or optimization. This model employed a convolutional neural network trained with Keras with a Tensorflow (Google LLC) and specifics of the model derivation have been previously published.11,20 The patient population used in the initial development of the model included 97 829 patients who had an ECG and echocardiogram performed within a 2-week interval.11 Patient data used in training the original model were excluded from this analysis.
Statistical analysis
Model performance was evaluated using the area under the receiver operating characteristic curve (AUC), estimated by modelling the prediction probabilities for LVSD generated by the deep learning model to categorized echocardiographic LVEF assessment. The categorization of LVEF at 35% or less was of primary interest since it matched the original model development; however, less severe degrees of LVSD were considered by categorizing LVEF at <45% and <50% for secondary analyses of the model’s performance. Measures of diagnostic performance (i.e. accuracy, sensitivity, specificity, positive predictive value, and negative predictive value) were calculated using the previously determined threshold of 0.256 or less, which indicates a positive screen. Ninety-five percent exact confidence intervals (CIs) were calculated for measures of diagnostic performance with large sample approximation of the DeLong method,21 with optimization by Sun and Xu.22 Stratified analyses were also conducted by race, ethnicity, age, and hypertension status.
To further evaluate the utility of the deep learning model through benchmarking performance, additional exploratory analyses were conducted. This deep learning model’s performance was compared to B-type natriuretic peptide (BNP) or N-terminal pro-BNP (NT-proBNP) among patients who had these measured within 30 days prior to their echocardiogram. AUC values for BNP and NT-proBNP were evaluated alone and in combination with the deep learning model. Although natriuretic peptide levels may increase up to two-fold in normal pregnancy, values have been noted to remain within normal range.5 AUC was also evaluated for a multivariable model including demographic, clinical, and laboratory variables. All 95% CIs and P-values reported are two-sided. A P-value of <0.05 was considered statistically significant. Statistical analysis was conducted using R, version 3.6.2.23
Results
Study population characteristics
A total of 1807 women were included in the study. Overall, the median (interquartile range) age of patients was 30.5 (26.4–35.1) years; most were White [1428 (79.0%)]. One hundred and twenty-seven (7.0%), 184 (10.2%), and 230 (12.7%) had LVEF of 35% or less, <45%, and <50%, respectively. Two hundred and sixty (14.4%) had natriuretic peptide levels measured within the prespecified 30-day window prior to selected echocardiogram. Additional demographic and clinical characteristics are shown in Table 1.
Table 1.
Demographic and clinical characteristics of study populationa
| Characteristics | LVEF >35% (n = 1680) | LVEF ≤35% (n = 127) | Overall (N = 1807) | P-valueb |
|---|---|---|---|---|
| Age, years, median (IQR) | 30.5 (26.4–35.0) | 31.3 (26.5–36.7) | 30.5 (26.4–35.1) | 0.29 |
| Weight, BMI, kg/m2 | 0.53 | |||
| Normal weight (18.5–24.9) | 254 (27.2) | 11 (20.8) | 265 (26.8) | |
| Underweight (<18.5) | 14 (1.5) | 1 (1.9) | 15 (1.5) | |
| Overweight (25.0–29.9) | 249 (26.6) | 12 (22.6) | 261 (26.4) | |
| Obese (≥30) | 418 (44.7) | 29 (54.7) | 447 (45.2) | |
| Race | ||||
| White | 1350 (80.4) | 78 (61.4) | 1428 (79.0) | <0.001 |
| Black | 139 (8.3) | 34 (26.8) | 173 (9.6) | |
| Other/unknown | 191 (11.4) | 15 (11.8) | 206 (11.4) | |
| Ethnicity | 0.01 | |||
| Hispanic or Latino | 81 (5.2) | 12 (10.8) | 94 (5.7) | |
| Not Hispanic or Latino | 1469 (94.7) | 99 (89.2) | 1568 (94.3) | |
| Family history of heart disease | ||||
| All relatives | 440 (45.5) | 21 (44.7) | 461 (45.5) | 0.91 |
| First degree relatives | 215 (22.3) | 12 (25.5) | 227 (22.4) | 0.60 |
| Time between ECG and echocardiogram, days, median (IQR) | 1.0 (0.0–6.0) | 0.0 (0.0–1.0) | 1.0 (0.0–5.0) | <0.001 |
| Serum creatinine, mg/dL, median (IQR)c | 0.7 (0.6–0.8) | 0.9 (0.7–1.1) | 0.7 (0.6–0.9) | <0.001 |
| Serum haemoglobin, g/dL, median (IQR)c | 11.8 (10.4–12.9) | 11.7 (9.6–13.1) | 11.8 (10.4–13.0) | 0.86 |
| NT-ProBNP, pg/mL, median (IQR)c | 229.2 (77.2–899.7) | 3264.0 (1035.0–5644.8) | 430.0 (93.0–1554.0) | <0.001 |
| BNP, pg/mL, median (IQR)c | 128.0 (25.0–341.0) | 864.0 (463.2–1054.0) | 145.0 (29.0–401.5) | <0.001 |
| Aortic aneurysm | 27 (1.6) | 1 (0.8) | 28 (1.5) | 0.47 |
| Cardiac arrhythmia | 776 (46.2) | 48 (37.8) | 824 (45.6) | 0.067 |
| Chronic pulmonary disease | 96 (5.7) | 10 (7.9) | 106 (5.9) | 0.32 |
| Diabetes | 239 (14.2) | 19 (15.0) | 258 (14.3) | 0.82 |
| Cerebrovascular disease | 80 (4.8) | 7 (5.5) | 87 (4.8) | 0.70 |
| Congenital heart disease | 117 (7.0) | 3 (2.4) | 120 (6.6) | 0.045 |
| Chronic kidney disease | 50 (3.0) | 4 (3.1) | 54 (3.0) | 0.91 |
| Hypertension | 401 (23.9) | 44 (34.6) | 445 (24.6) | 0.007 |
| Ischaemic heart disease | 52 (3.1) | 16 (12.6) | 68 (3.8) | <0.001 |
| Preeclampsia/eclampsia | 147 (8.8) | 16 (12.6) | 163 (9.0) | 0.14 |
| Superficial/deep vein thrombosis | 122 (7.3) | 12 (9.4) | 134 (7.4) | 0.36 |
| Valvular heart disease | 288 (17.1) | 35 (27.6) | 323 (17.9) | 0.003 |
The bold face values are considered statistically significant given they are less than 0.05. BMI, body mass index; BNP, B-type natriuretic peptide; ECG, electrocardiogram; IQR, interquartile range; LVEF, left ventricular ejection fraction; NT-ProBNP, N-terminal Pro B-type natriuretic peptide.
Data summarized as no. (%) unless otherwise indicated. Percentages may not total 100 due to rounding and missing/unknown data not displayed. Weight (missing n = 819), Ethnicity (missing n = 145), serum creatinine (missing n = 460), serum haemoglobin (missing n = 369), NT-proBNP (missing n = 1598), BNP (missing n = 1756).
P-values presented are from Fisher’s exact test for count data and Wilcoxon rank sum tests for numeric variables.
Effective sample size for laboratory values: serum creatinine = 1347; haemoglobin = 1438; NT-proBNP = 209; BNP = 51.
Primary outcome: detection of cardiomyopathy at LVEF of 35% or less
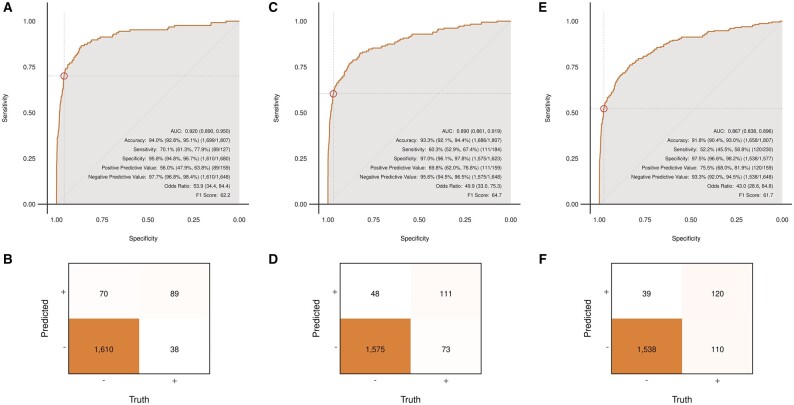
Detection of an LVEF of 35% or less using the deep learning model achieved an AUC of 0.920 (95% CI 0.880–0.950) with an accuracy of 94.0% (95% CI 92.8–95.1). Sensitivity, specificity, positive predictive value, and negative predictive value were 70.1%, 95.8%, 56.0%, and 97.7%, respectively (Figure 2A and B). Among those with a false-positive screen (n = 70), 22 (31.4%) had an LVEF <45%, and 31 (44.3%) had an LVEF <50%. The median (interquartile range) LVEF for patients with a false-positive screen was 51.5% (43.0–59.8%), which is below the ejection fraction range (54–74%) classified as normal for women based on the American Society of Echocardiography guidelines.17
Figure 2.
(A–F) Receiver operating characteristic curves and confusion matrices for identification of cardiomyopathy among pregnant and postpartum women at pre-specified ejection fraction values. (A and B) Ejection fraction ≤35%; (C and D) ejection fraction <45%; and (E and F) ejection fraction <50%. AUC, area under the receiver operating characteristic curve.
Secondary outcomes: detection of cardiomyopathy at LVEF <45% and <50%
For LVEF <45%, the deep learning model achieved an AUC of 0.890 (95% CI 0.861–0.919), with an accuracy of 93.3% (95% CI 92.1–94.4). Sensitivity, specificity, positive predictive value, and negative predictive values were 60.3%, 97.0%, 69.8%, and 95.6%, respectively (Figure 2C and D). For LVEF <50%, the deep learning model achieved an AUC of 0.867 (95% CI 0.838–0.896), with an accuracy of 91.8% (95% CI 90.4–93.0). Sensitivity, specificity, positive predictive value, and negative predictive value were 52.2%, 97.5%, 75.5%, and 93.3%, respectively (Figure 2E and F).
Subpopulations
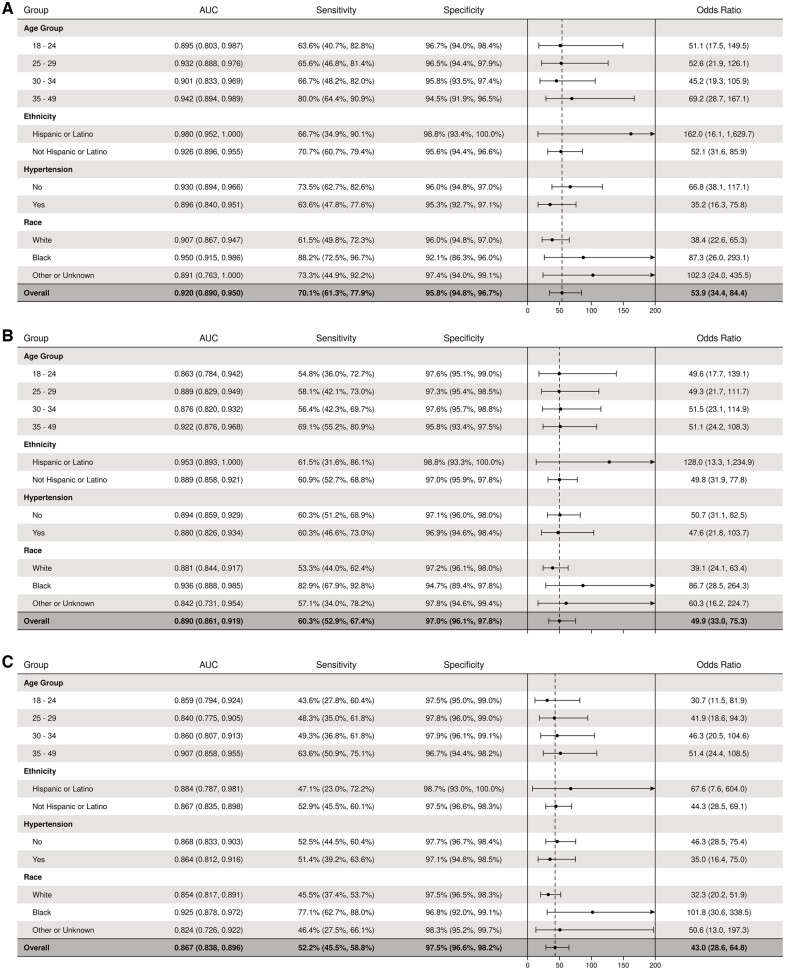
We evaluated the effectiveness of the deep learning model at all LVEF cut points by ethnicity, race, age group, and hypertension status (including hypertensive disease of pregnancy) (Figure 3). AUCs for LVEF of 35% or less, <45%, and <50% were higher among Hispanic women (0.98, 0.95, and 0.88, respectively), Black women (0.95, 0.94, and 0.93), women older than 35 years (0.94, 0.92, and 0.91), and women without hypertension (0.93, 0.89, and 0.87). AUC values otherwise remained stable in all subgroups for all prespecified LVEF cut points.
Figure 3.
Forest plots showing deep learning model performance for identification of left ventricular systolic dysfunction stratified by subgroups. (A) Ejection fraction ≤35%; (B) ejection fraction <45%; and (C) ejection fraction <50%. AUC, area under the receiver operating characteristic curve.
Exploratory analysis: multivariable model, natriuretic peptides, optimal cut points
A multivariable logistic regression model in a subset of the study population without missing values (n = 1248) included age, race, ethnicity, and clinical parameters (serum creatinine, congenital heart disease, hypertension, ischaemic heart disease, and valvular heart disease). These variables were selected based on a significant association with the left ventricular dysfunction in Table 1. Age was also included (although not significant in Table 1) due to prior literature demonstrating a higher risk of cardiomyopathy with advancing maternal age. This model was able to identify LVEFs of 35% or less with an AUC of 0.72 (Table 2). The associations seen in Table 2 are consistent with current literature demonstrating higher odds of cardiomyopathy among Black (odds ratio 3.58; 95% CI 2.14–5.89) and Hispanic (odds ratio 2.29; 95% CI 1.04–4.68) women, as well as women with ischaemic heart disease (odds ratio 3.83; 95% CI 1.81–7.71). It is important to note that the deep learning model does not require a detailed knowledge of the patient’s medical history and clearly outperforms this model.
Table 2.
Multivariable model evaluating demographic and clinical parameters for predicting cardiomyopathy (LVEF ≤35%)a
| Characteristics | Odds ratio | 95% CI | P-value |
|---|---|---|---|
| Ethnicity | |||
| Not Hispanic or Latino | Ref | Ref | 1.00 |
| Hispanic or Latino | 2.29 | 1.04–4.68 | 0.029 |
| Race | |||
| White | Ref | Ref | 1.00 |
| Black | 3.58 | 2.14–5.89 | <0.001 |
| Other or unknown | 1.00 | 0.44–2.06 | >0.90 |
| Age, years | |||
| 18–24 | Ref | Ref | 1.00 |
| 25–29 | 1.22 | 0.64–2.38 | 0.50 |
| 30–34 | 1.07 | 0.56–2.08 | 0.80 |
| 35–49 | 1.08 | 0.57–2.09 | 0.80 |
| Serum creatinine | 1.23 | 1.04–1.45 | 0.01 |
| Congenital heart disease | 0.35 | 0.08–1.03 | 0.09 |
| Hypertension | 1.12 | 0.70–1.75 | 0.60 |
| Ischaemic heart disease | 3.83 | 1.81–7.71 | <0.001 |
| Valvular heart disease | 1.68 | 0.98–2.78 | 0.05 |
AUC, area under the receiver operating characteristic curve; LVEF, left ventricular ejection fraction; Ref, reference.
Model AUC = 0.72.
Among the 14% of our study population who had BNP or NT-proBNP values measured within 30 days prior to their echocardiogram, NT-proBNP and BNP identified LVSD (LVEF ≤35%) with AUCs of 0.85 (95% CI 0.79–0.91) and 0.86 (95% CI 0.69–1.00), respectively. The deep learning model had an AUC of 0.90 in this subset and outperformed natriuretic peptides at all prespecified LVEF cut points, demonstrating an advantage of the deep learning model beyond its non-invasive nature. The addition of NT-proBNP to the deep learning model did not add a statistically significant incremental value with improvement in AUC from 0.90 to 0.94 (P = 0.10; DeLong test for correlated AUCs) for LVEF of 35% or less (Figure 4).
Figure 4.
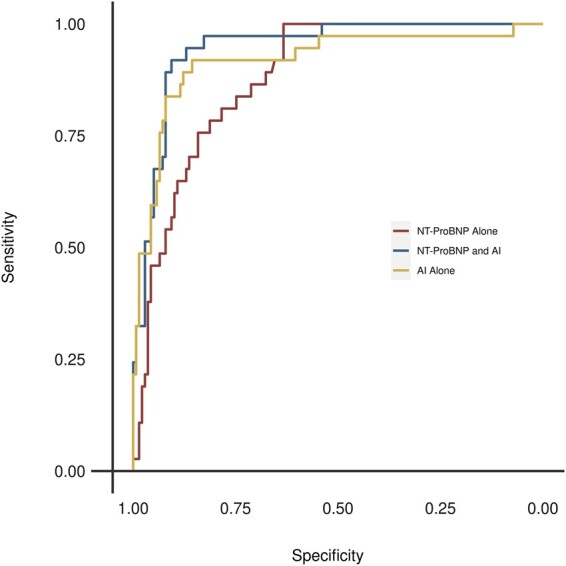
Receiver operating characteristic curves for identification of left ventricular ejection fraction <35% among pregnant and postpartum women who had NT-ProBNP Measured. AI, artificial intelligence; NT-ProBNP, N-terminal pro B-type natriuretic peptide.
We evaluated multiple cut points for the deep learning model’s prediction probabilities to fully assess the change in diagnostic probabilities that might be optimal for a screening test (Supplementary material online, Table S2) where a higher sensitivity and negative predictive value may be beneficial. Given the associated risk of a false-positive test is obtaining an echocardiogram, a non-invasive test, this is an acceptable risk. At a threshold value of 0.10 or greater, sensitivity, specificity, and positive and negative predictive values of the deep learning model were 77%, 92%, 41%, and 98.2%, respectively. Changing this threshold value to 0.45 or greater, sensitivity, specificity, and positive and negative predictive values were 59%, 97%, 61%, and 97%, respectively.
Discussion
This study provides preliminary evidence that an ECG-based deep learning model can be an effective instrument to screen for LVSD among pregnant women and those in the postpartum period and lays the groundwork to support its application in routine obstetric care. We also demonstrate that the deep learning model outperforms current practice using natriuretic peptides, demographic, and clinical parameters for predicting cardiomyopathy. This study also shows the effectiveness of the deep learning model in populations with disproportionately high maternal mortality rates (Black and Hispanic); however, larger studies with more diverse cohorts are needed to confirm these findings.
Cardiovascular disease is the leading cause of maternal deaths in the USA and is now estimated to be responsible for 33% of all pregnancy-related deaths based on data from the Centers for Disease Control and Prevention Pregnancy Mortality Surveillance System from 2007 to 20167 and 23% of indirect maternal deaths in the UK based on a 2016 to 2018 study.6 As such, early identification of cardiovascular disease in the pregnant and postpartum period is essential and provides a unique opportunity for timely initiation of appropriate medical management18 and monitoring, with the ultimate goal of reducing associated maternal morbidity and mortality. Notable disparities in maternal outcomes also exist, with higher rates of morbidity and mortality among non-White women of lower socioeconomic status and those over 40 years of age.5,24 Failure to identify cardiovascular disease symptoms and delays in diagnosis are believed to be contributing to this critical health inequity.5 Cardiomyopathy occurring in pregnancy and the postpartum period is difficult to diagnose due to the similarity in symptoms seen with normal pregnancy.8 The field of cardio-obstetrics is now an emerging multidisciplinary specialty due to recognized interactions between pregnancy and cardiovascular disease.4,25–27
The 2019 ACOG guidelines,5 2018 European Society of Cardiology guidelines,10 and 2020 scientific statement from the American Heart Association4 endorse obtaining additional testing (including ECG and echocardiography) in pregnant women and those in the postpartum period with history or physical examination features suggestive of cardiovascular disease. However, it remains unclear which patients with symptoms (sometimes indistinguishable from normal pregnancy-related symptoms) will benefit from additional testing. These guidelines also acknowledge that diagnosing cardiomyopathy in this population is challenging, making them an ideal group for targeted screening. A 12-lead ECG is available in most clinical settings and can be performed rapidly with minimal training. In addition, an ECG is inexpensive compared to echocardiography, making it an attractive option as a screening tool. Although echocardiography remains the criterion-standard test to evaluate LVSD,17 it requires additional expertise to perform and interpret and may not be available in standard obstetric settings.28
The American College of Cardiology and American Heart Association heart failure guidelines endorse the use of natriuretic peptides for screening the general population for heart failure29; however, evidence of their utility in the pregnant and postpartum population remains limited. Some studies with relatively small samples have evaluated the use of natriuretic peptides in the pregnant and postpartum population and demonstrated its effectiveness at the standard cut points, but also noted that certain pregnancy-related changes and conditions, such as pre-eclampsia, can affect natriuretic peptide levels, necessitating the use of a different cut point.30,31 In addition, pre-eclampsia is known to be a risk factor for PPCM.9 This suggests the misclassification rate with natriuretic peptides in this population might be higher than the range (14–29%) reported in the general population.32 In addition to the pregnant state and associated conditions, other factors such as body mass index, comorbidities (e.g. renal failure, pulmonary hypertension, infections, arrhythmias), and use of angiotensin receptor neprilysin inhibitor29,33–38 can alter natriuretic peptide levels. Compared to the limitations associated with using natriuretic peptides, the diagnostic accuracy of the ECG-based deep learning model has been demonstrated to be robust in detecting LVSD across different age groups, sex, and race/ethnicity,11,39 highlighting its potential as a much more effective screening tool.
Pregnancy-related cardiomyopathies
PPCM is a unique phenotype defined as LVEF <45% occurring in the last month of pregnancy or within 5 months postpartum without previously known structural heart disease and in the absence of an alternate explanation.40,41 Although this condition is considered rare, with a reported incidence of approximately 1 in 1000 live births nationally, there has been an upward trend of occurrence from 8.5 to 11.8 per 10 000 live births from 2004 to 2011.42 It has also been suggested that many cases may be missed and the true incidence rate remains unknown.9 PPCM is strongly associated with age, with more than 50% of cases occurring in women older than 30 years and a 10-fold increase in odds among women older than 40 years compared to women younger than 20 years.41,42 PPCM is also known to disproportionately affect Black women, who tend to present with more advanced disease and more impaired LVSD, with a corresponding lower rate of LVEF recovery and a higher risk of death.42–45 Some studies have demonstrated a three- to four-fold increase to as much as a 16-fold higher risk of PPCM in Black women compared to White women.46–48 In addition, studies have shown Black women have a 10-fold higher risk of death related to cardiovascular disease (pregnancy-related mortality rate of 16.3 deaths per 100 000 live births) compared with other racial and ethnic groups.49 The clinical presentation and outcomes of PPCM is also known to vary by region and the largest study of PPCM published found almost 70% of women had an ejection fraction ≤35% at diagnosis.50 We demonstrate that the deep learning model performs well across multiple subgroups and its performance is highest among higher-risk women (Black, Hispanic, and ≥35 years of age) at all LVEF cut points. This screening tool can potentially aid early and appropriate diagnosis in Black and Hispanic women, narrowing the disparities gap.
Other forms of cardiomyopathy have also been reported to occur during prepartum, peripartum, and postpartum periods, including dilated cardiomyopathy and acquired cardiomyopathy due to myocarditis, hypertension, valvular heart disease, ischaemia, stress, or toxins.4 Regardless of the aetiology of the cardiomyopathy in pregnancy and the postpartum period, the associated risk of adverse effects remains high. Unfortunately, the incidence and prevalence of any cardiomyopathy with LVSD among pregnant or reproductive age women in the USA remains unknown. Some international studies in predominantly Black populations have reported an incidence of 1 per 299 livebirths51 and prevalence rates of 6–10% in the peripartum period.52 Large scale prospective studies and registries are needed to address this important question. In addition, the ECG-based deep learning model is easily scalable and could potentially be employed for screening in large population-based studies.
This study addresses multiple key recommendations from the American Heart Association and ACOG Presidential Advisory, including enhancement of screening for cardiovascular disease in women, leveraging technology to improve cardiovascular health in women, and bridging collaborations between the disciplines of cardiology and obstetrics and gynaecology.53
Limitations
Since patients were identified for inclusion based on HICDA and ICD diagnosis codes, it is possible that some patients may have been missed or inappropriately included. In addition, we retrospectively evaluated and only included patients who had both an ECG and echocardiogram performed which likely excludes a large portion of patients seen in routine obstetric care and can affect estimated diagnostic probabilities due to selection bias. The study sample included few minority women and assessment of body mass index was obtained with weights recorded on echocardiography reports, which were performed during pregnancy for some patients, and as such, may not accurately reflect pre-pregnancy weight. Lastly, the sample size for the multivariable model and natriuretic peptides analysis was restricted relative to the overall sample size on account of missing laboratory data (obtained through retrospective chart review) for many of the patients. No imputation was performed, and results need to be evaluated as hypothesis generating.
Strengths
A major strength of this study is the effectiveness and robustness of the deep learning model in a unique patient population of women who were pregnant or in the postpartum period who would likely greatly benefit from screening. This study further adds to the literature regarding use of artificial intelligence (AI)-based tools in clinical practice and bridges the gap between cardiovascular medicine and obstetrics.
Conclusion and future directions
An ECG-based deep learning model can effectively identify LVSD among women who are pregnant or in the postpartum period. The use of this model for screening purposes in routine obstetric practice can facilitate early identification of high-risk patients. Further studies are needed to confirm the feasibility and value of this model in diverse patient populations, especially among Black women who are reported to have the highest risk, as well as its association with clinical outcomes. Efforts are currently underway to validate the AI-ECG in a separate patient population external to the current institution where this model was derived. Of note, the AI-ECG was recently evaluated in a pragmatic clinical trial and was found to increase the diagnosis of low ejection fraction in the primary care setting.54 Subsequent plans include prospective studies in pregnant and postpartum women to evaluate the effectiveness of the AI-ECG and its impact on clinical outcomes. With the advent of wearables and smart devices, there is a potential opportunity to obtain personalized ECGs in nonclinical settings. If models using smartphone compatible portable ECG device technology are successful, they can be rapidly deployed across the entire world regardless of an elaborate healthcare infrastructure, with the potential for early disease detection and prevention of adverse outcomes for the mother and her unborn child.
Supplementary material
Supplementary material is available at European Heart Journal – Digital Health online.
Supplementary Material
Acknowledgements
We would like to thank the Mayo Clinic Florida Digital Innovation Laboratory Team Program and the Mayo Clinic Rochester Cardiovascular Data Science Team for providing statistical guidance and support for the successful completion of this project.
Funding
This study was supported by the Mayo Clinic Women’s Health Research Center and the Mayo Clinic Building Interdisciplinary Research Careers in Women’s Health (BIRCWH) Program funded by the National Institutes of Health (K12 HD065987). The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Conflict of interest: Mayo Clinic has licensed the underlying technology to EKO devices, a maker of digital stethoscopes with embedded electrocardiogram electrodes and to Anumana. Mayo Clinic may receive financial benefit from the use of this technology, but at no point will Mayo Clinic benefit financially from its use for the care of patients at Mayo Clinic. I.Z.A., F.L.-J., and P.A.F. may also receive financial benefit from this agreement.
Data availability
The data underlying this article will be shared on reasonable request to the corresponding author. All requests for raw and analyzed data and related materials, excluding programming code, will be reviewed by the Mayo Clinic legal department and Mayo Clinic Ventures to verify whether the request is subject to any intellectual property or confidentiality obligations. Requests for patient-related data not included in the paper will not be considered. Any data and materials that can be shared will be released via a Material Transfer Agreement.
References
- 1.GBD 2015 Maternal Mortality Collaborators. Global, regional, and national levels of maternal mortality, 1990-2015: a systematic analysis for the Global Burden of Disease Study 2015. Lancet 2016;388:1775–1812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Moaddab A, Dildy GA, Brown HL, Bateni ZH, Belfort MA, Sangi-Haghpeykar H, Clark SL. Health care disparity and pregnancy-related mortality in the United States, 2005-2014. Obstet Gynecol 2018;131:707–712. [DOI] [PubMed] [Google Scholar]
- 3. Creanga AA, Syverson C, Seed K, Callaghan WM. Pregnancy-related mortality in the United States, 2011-2013. Obstet Gynecol 2017;130:366–373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Mehta LS, Warnes CA, Bradley E, Burton T, Economy K, Mehran R, Safdar B, Sharma G, Wood M, Valente AM, Volgman AS; American Heart Association Council on Clinical Cardiology; Council on Arteriosclerosis, Thrombosis and Vascular Biology; Council on Cardiovascular and Stroke Nursing; and Stroke Council. Cardiovascular considerations in caring for pregnant patients: a scientific statement from the American Heart Association. Circulation 2020;141:e884–e903. [DOI] [PubMed] [Google Scholar]
- 5.American College of Obstetricians and Gynecologists' Presidential Task Force on Pregnancy and Heart Disease and Committee on Practice Bulletins—Obstetrics. ACOG Practice Bulletin No. 212: pregnancy and heart disease. Obstet Gynecol 2019;133:e320–e356. [DOI] [PubMed] [Google Scholar]
- 6. Knight M, Bunch K, Tuffnell D, Shakespeare J, Kotnis R, Kenyon S, Kurinczuk J. Saving Lives, Improving Mothers’ Care-Lessons learned to inform maternity care from the UK and Ireland Confidential Enquiries into Maternal Deaths and Morbidity 2016-18. In. University of Oxford: Oxford: National Perinatal Epidemiology Unit. University of Oxford; 2020. https://www.npeu.ox.ac.uk/assets/downloads/mbrrace-uk/reports/maternal-report-2020/MBRRACE-UK_Maternal_Report_Dec_2020_v10_ONLINE_VERSION_1404.pdf.
- 7. Petersen EE, Davis NL, Goodman D, Cox S, Syverson C, Seed K, Shapiro-Mendoza C, Callaghan WM, Barfield W. Racial/ethnic disparities in pregnancy-related deaths—United States, 2007-2016. MMWR Morb Mortal Wkly Rep 2019;68:762–765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Hameed AB, Lawton ES, McCain CL, Morton CH, Mitchell C, Main EK, Foster E. Pregnancy-related cardiovascular deaths in California: beyond peripartum cardiomyopathy. Am J Obstet Gynecol 2015;213:379.e1–10. [DOI] [PubMed] [Google Scholar]
- 9. Davis MB, Arany Z, McNamara DM, Goland S, Elkayam U. Peripartum cardiomyopathy: JACC state-of-the-art review. J Am Coll Cardiol 2020;75:207–221. [DOI] [PubMed] [Google Scholar]
- 10. Regitz-Zagrosek V, Roos-Hesselink JW, Bauersachs J, Blomstrom-Lundqvist C, Cifkova R, De Bonis M, Iung B, Johnson MR, Kintscher U, Kranke P, Lang IM, Morais J, Pieper PG, Presbitero P, Price S, Rosano GMC, Seeland U, Simoncini T, Swan L, Warnes CA; ESC Scientific Document Group. 2018 ESC Guidelines for the management of cardiovascular diseases during pregnancy. Eur Heart J 2018;39:3165–3241. [DOI] [PubMed] [Google Scholar]
- 11. Attia ZI, Kapa S, Lopez-Jimenez F, McKie PM, Ladewig DJ, Satam G, Pellikka PA, Enriquez-Sarano M, Noseworthy PA, Munger TM, Asirvatham SJ, Scott CG, Carter RE, Friedman PA. Screening for cardiac contractile dysfunction using an artificial intelligence-enabled electrocardiogram. Nat Med 2019;25:70–74. [DOI] [PubMed] [Google Scholar]
- 12. Adedinsewo D, Carter RE, Attia Z, Johnson P, Kashou AH, Dugan JL, Albus M, Sheele JM, Bellolio F, Friedman PA, Lopez-Jimenez F, Noseworthy PA. Artificial intelligence-enabled ECG algorithm to identify patients with left ventricular systolic dysfunction presenting to the emergency department with dyspnea. Circ Arrhythm Electrophysiol 2020;13:e008437. [DOI] [PubMed] [Google Scholar]
- 13. Attia IZ, Tseng AS, Benavente ED, Inojosa JM, Clark TG, Malyutina S, Kapa S, Schirmer H, Kudryavtsev AV, Noseworthy PA, Carter RE, Ryabikov A, Perel P, Friedman PA, Leon DA, Lopez-Jimenez F. External validation of a deep learning electrocardiogram algorithm to detect ventricular dysfunction. Int J Cardiol 2021;329:130–135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. St Sauver J, Buntrock J, Rademacher D. Comparison of Mayo Clinic coding systems. In: Technical Report. 2010. https://www.mayo.edu/research/documents/biostat-83pdf/doc-10026715.
- 15. St Sauver JL, Grossardt BR, Finney Rutten LJ, Roger VL, Majerus M, Jensen DW, Brue SM, Bock-Goodner CM, Rocca WA. Rochester epidemiology project data exploration portal. Prev Chronic Dis 2018;15:E42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Centers for Disease Control and Prevention: Division of Reproductive Health—National Center for Chronic Disease Prevention and Health Promotion. Pregnancy Mortality Surveillance System. https://www.cdc.gov/reproductivehealth/maternal-mortality/pregnancy-mortality-surveillance-system.htm (3 August, 2021).
- 17. Lang RM, Badano LP, Mor-Avi V, Afilalo J, Armstrong A, Ernande L, Flachskampf FA, Foster E, Goldstein SA, Kuznetsova T, Lancellotti P, Muraru D, Picard MH, Rietzschel ER, Rudski L, Spencer KT, Tsang W, Voigt JU. Recommendations for cardiac chamber quantification by echocardiography in adults: an update from the American Society of Echocardiography and the European Association of Cardiovascular Imaging. J Am Soc Echocardiogr 2015;28:1–39.e14. [DOI] [PubMed] [Google Scholar]
- 18. Yancy CW, Jessup M, Bozkurt B, Butler J, Casey DE Jr, Drazner MH, Fonarow GC, Geraci SA, Horwich T, Januzzi JL, Johnson MR, Kasper EK, Levy WC, Masoudi FA, McBride PE, McMurray JJV, Mitchell JE, Peterson PN, Riegel B, Sam F, Stevenson LW, Tang WHW, Tsai EJ, Wilkoff BL; American College of Cardiology Foundation; American Heart Association Task Force on Practice Guidelines. 2013 ACCF/AHA Guideline for the management of heart failure: a report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines. J Am Coll Cardiol 2013;62:e147–e239. [DOI] [PubMed] [Google Scholar]
- 19. Bozkurt B, Coats AJ, Tsutsui H, Abdelhamid M, Adamopoulos S, Albert N, Anker SD, Atherton J, Bohm M, Butler J, Drazner MH, Felker GM, Filippatos G, Fonarow GC, Fiuzat M, Gomez-Mesa JE, Heidenreich P, Imamura T, Januzzi J, Jankowska EA, Khazanie P, Kinugawa K, Lam CSP, Matsue Y, Metra M, Ohtani T, Francesco Piepoli M, Ponikowski P, Rosano GMC, Sakata Y, Seferovi CP, Starling RC, Teerlink JR, Vardeny O, Yamamoto K, Yancy C, Zhang J, Zieroth S. Universal definition and classification of heart failure: a report of the Heart Failure Society of America, Heart Failure Association of the European Society of Cardiology, Japanese Heart Failure Society and Writing Committee of the Universal Definition of Heart Failure. J Card Fail 2021;27:387–413. [DOI] [PubMed] [Google Scholar]
- 20. Attia ZI, Kapa S, Yao X, Lopez-Jimenez F, Mohan TL, Pellikka PA, Carter RE, Shah ND, Friedman PA, Noseworthy PA. Prospective validation of a deep learning electrocardiogram algorithm for the detection of left ventricular systolic dysfunction. J Cardiovasc Electrophysiol 2019;30:668–674. [DOI] [PubMed] [Google Scholar]
- 21. DeLong ER, DeLong DM, Clarke-Pearson DL. Comparing the areas under two or more correlated receiver operating characteristic curves: a nonparametric approach. Biometrics 1988;44:837–845. [PubMed] [Google Scholar]
- 22. Sun X, Xu W. Fast implementation of DeLong’s algorithm for comparing the areas under correlated receiver operating characteristic curves. IEEE Signal Process Lett 2014;21:1389–1393. [Google Scholar]
- 23.R Core Team (2019). R: A Language and Environment for Statistical Computing. Vienna, Austria: R Foundation for Statistical Computing.
- 24. Burgess APH, Dongarwar D, Spigel Z, Salihu HM, Moaddab A, Clark SL, Fox K. Pregnancy-related mortality in the United States, 2003-2016: age, race, and place of death. Am J Obstet Gynecol 2020;222:489.e1–489.e8. [DOI] [PubMed] [Google Scholar]
- 25. Davis MB, Walsh MN. Cardio-obstetrics. Circ Cardiovasc Qual Outcomes 2019;12:e005417. [DOI] [PubMed] [Google Scholar]
- 26. Sharma G, Lindley K, Grodzinsky A. Cardio-obstetrics: developing a niche in maternal cardiovascular health. J Am Coll Cardiol 2020;75:1355–1359. [DOI] [PubMed] [Google Scholar]
- 27. Sharma G, Zakaria S, Michos ED, Bhatt AB, Lundberg GP, Florio KL, Vaught AJ, Ouyang P, Mehta L. Improving cardiovascular workforce competencies in cardio-obstetrics: current challenges and future directions. J Am Heart Assoc 2020;9:e015569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Gil Martinez P, Mesado Martinez D, Curbelo Garcia J, Cadinanos Loidi J. Amino-terminal pro-B-type natriuretic peptide, inferior vena cava ultrasound, and biolectrical impedance analysis for the diagnosis of acute decompensated CHF. Am J Emerg Med 2016;34:1817–1822. [DOI] [PubMed] [Google Scholar]
- 29. Yancy CW, Jessup M, Bozkurt B, Butler J, Casey DE Jr, Colvin MM, Drazner MH, Filippatos GS, Fonarow GC, Givertz MM, Hollenberg SM, Lindenfeld J, Masoudi FA, McBride PE, Peterson PN, Stevenson LW, Westlake C. 2017 ACC/AHA/HFSA focused update of the 2013 ACCF/AHA guideline for the management of heart failure: a report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines and the Heart Failure Society of America. J Am Coll Cardiol 2017;70:776–803. [DOI] [PubMed] [Google Scholar]
- 30. Malhame I, Hurlburt H, Larson L, Poppas A, Nau C, Bourjeily G, Mehta N. Sensitivity and specificity of B-type natriuretic peptide in diagnosing heart failure in pregnancy. Obstet Gynecol 2019;134:440–449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Afshani N, Moustaqim-Barrette A, Biccard BM, Rodseth RN, Dyer RA. Utility of B-type natriuretic peptides in preeclampsia: a systematic review. Int J Obstet Anesth 2013;22:96–103. [DOI] [PubMed] [Google Scholar]
- 32. Martindale JL, Wakai A, Collins SP, Levy PD, Diercks D, Hiestand BC, Fermann GJ, Desouza I, Sinert R. Diagnosing acute heart failure in the emergency department: a systematic review and meta‐analysis. Acad Emerg Med 2016;23:223–242. [DOI] [PubMed] [Google Scholar]
- 33. Homsak E, Ekart R. Hemodiafiltration affects NT-proBNP but not ST2 serum concentration in end-stage renal disease patients. Clin Biochem 2016;49:1159–1163. [DOI] [PubMed] [Google Scholar]
- 34. Santos-Araujo C, Leite-Moreira A, Pestana M. Clinical value of natriuretic peptides in chronic kidney disease. Nefrologia 2015;35:227–233. [DOI] [PubMed] [Google Scholar]
- 35. Kim BJ, Hwang SJ, Sung KC, Kim BS, Kang JH, Lee MH, Park JR. Assessment of factors affecting plasma BNP levels in patients with chronic atrial fibrillation and preserved left ventricular systolic function. Int J Cardiol 2007;118:145–150. [DOI] [PubMed] [Google Scholar]
- 36. Tagore R, Ling LH, Yang H, Daw HY, Chan YH, Sethi SK. Natriuretic peptides in chronic kidney disease. Clin J Am Soc Nephrol 2008;3:1644–1651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Kadri AN, Kaw R, Al-Khadra Y, Abuamsha H, Ravakhah K, Hernandez AV, Tang WHW. The role of B-type natriuretic peptide in diagnosing acute decompensated heart failure in chronic kidney disease patients. Arch Med Sci 2018;14:1003–1009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Madamanchi C, Alhosaini H, Sumida A, Runge MS. Obesity and natriuretic peptides, BNP and NT-proBNP: mechanisms and diagnostic implications for heart failure. Int J Cardiol 2014;176:611–617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Noseworthy PA, Attia ZI, Brewer LC, Hayes SN, Yao X, Kapa S, Friedman PA, Lopez-Jimenez F. Assessing and mitigating bias in medical artificial intelligence: the effects of race and ethnicity on a deep learning model for ECG analysis. Circ Arrhythm Electrophysiol 2020;13:e007988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Honigberg MC, Givertz MM. Arrhythmias in peripartum cardiomyopathy. Card Electrophysiol Clin 2015;7:309–317. [DOI] [PubMed] [Google Scholar]
- 41. Honigberg MC, Givertz MM. Peripartum cardiomyopathy. Bmj 2019;364:k5287. [DOI] [PubMed] [Google Scholar]
- 42. Arany Z, Elkayam U. Peripartum cardiomyopathy. Circulation 2016;133:1397–1409. [DOI] [PubMed] [Google Scholar]
- 43. McNamara DM, Elkayam U, Alharethi R, Damp J, Hsich E, Ewald G, Modi K, Alexis JD, Ramani GV, Semigran MJ, Haythe J, Markham DW, Marek J, Gorcsan J 3rd, Wu WC, Lin Y, Halder I, Pisarcik J, Cooper LT, Fett JD. Clinical outcomes for peripartum cardiomyopathy in North America: results of the IPAC study (Investigations of Pregnancy-Associated Cardiomyopathy). J Am Coll Cardiol 2015;66:905–914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Goland S, Modi K, Hatamizadeh P, Elkayam U. Differences in clinical profile of African-American women with peripartum cardiomyopathy in the United States. J Card Fail 2013;19:214–218. [DOI] [PubMed] [Google Scholar]
- 45. Irizarry OC, Levine LD, Lewey J, Boyer T, Riis V, Elovitz MA, Arany Z. Comparison of clinical characteristics and outcomes of peripartum cardiomyopathy between African American and Non-African American women. JAMA Cardiol 2017;2:1256–1260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Brar SS, Khan SS, Sandhu GK, Jorgensen MB, Parikh N, Hsu JW, Shen AY. Incidence, mortality, and racial differences in peripartum cardiomyopathy. Am J Cardiol 2007;100:302–304. [DOI] [PubMed] [Google Scholar]
- 47. Harper MA, Meyer RE, Berg CJ. Peripartum cardiomyopathy: population-based birth prevalence and 7-year mortality. Obstet Gynecol 2012;120:1013–1019. [DOI] [PubMed] [Google Scholar]
- 48. Gentry MB, Dias JK, Luis A, Patel R, Thornton J, Reed GL. African-American women have a higher risk for developing peripartum cardiomyopathy. J Am Coll Cardiol 2010;55:654–659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Main EK, McCain CL, Morton CH, Holtby S, Lawton ES. Pregnancy-related mortality in California: causes, characteristics, and improvement opportunities. Obstet Gynecol 2015;125:938–947. [DOI] [PubMed] [Google Scholar]
- 50. Sliwa K, Petrie MC, van der Meer P, Mebazaa A, Hilfiker-Kleiner D, Jackson AM, Maggioni AP, Laroche C, Regitz-Zagrosek V, Schaufelberger M. Clinical presentation, management, and 6-month outcomes in women with peripartum cardiomyopathy: an ESC EORP registry. Eur Heart J 2020;41:3787–3797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Sliwa K, Bohm M. Incidence and prevalence of pregnancy-related heart disease. Cardiovasc Res 2014;101:554–560. [DOI] [PubMed] [Google Scholar]
- 52. Karaye KM, Mohammed IY, Sa'idu H, Ishaq NA, Balarabe SA, Tukur J, Adedeji T, Makinde ON, Adebayo RA. Prevalence of left ventricular dysfunction and relationship with serum selenium in apparently healthy pregnant women: results from the PEACE Registry. In: International Cardiovascular Forum Journal. 2020: Abstract 20.
- 53. Brown HL, Warner JJ, Gianos E, Gulati M, Hill AJ, Hollier LM, Rosen SE, Rosser ML, Wenger NK; American Heart Association and the American College of Obstetricians and Gynecologists. Promoting risk identification and reduction of cardiovascular disease in women through collaboration with obstetricians and gynecologists: a presidential advisory from the American Heart Association and the American College of Obstetricians and Gynecologists. Circulation 2018;137:e843–e852. [DOI] [PubMed] [Google Scholar]
- 54. Yao X, Rushlow DR, Inselman JW, McCoy RG, Thacher TD, Behnken EM, Bernard ME, Rosas SL, Akfaly A, Misra A, Molling PE, Krien JS, Foss RM, Barry BA, Siontis KC, Kapa S, Pellikka PA, Lopez-Jimenez F, Attia ZI, Shah ND, Friedman PA, Noseworthy PA. Artificial intelligence-enabled electrocardiograms for identification of patients with low ejection fraction: a pragmatic, randomized clinical trial. Nat Med 2021;27:815–819. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data underlying this article will be shared on reasonable request to the corresponding author. All requests for raw and analyzed data and related materials, excluding programming code, will be reviewed by the Mayo Clinic legal department and Mayo Clinic Ventures to verify whether the request is subject to any intellectual property or confidentiality obligations. Requests for patient-related data not included in the paper will not be considered. Any data and materials that can be shared will be released via a Material Transfer Agreement.