Abstract
Aims
Heart failure (HF) is a leading cause of death. Early intervention is the key to reduce HF-related morbidity and mortality. This study assesses the utility of electrocardiograms (ECGs) in HF risk prediction.
Methods and results
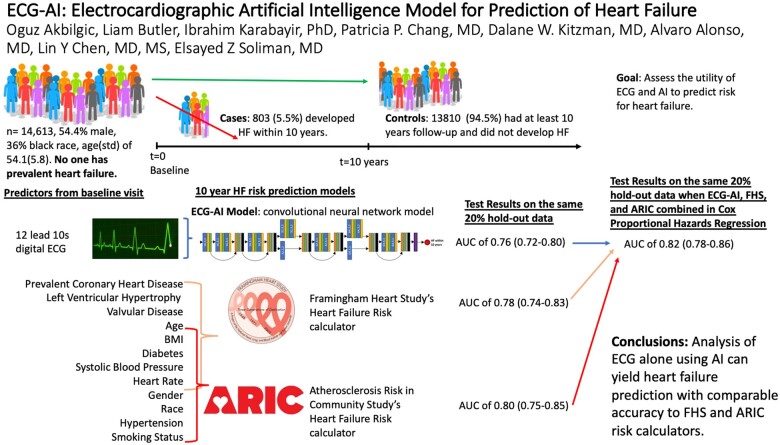
Data from the baseline visits (1987–89) of the Atherosclerosis Risk in Communities (ARIC) study was used. Incident hospitalized HF events were ascertained by ICD codes. Participants with good quality baseline ECGs were included. Participants with prevalent HF were excluded. ECG-artificial intelligence (AI) model to predict HF was created as a deep residual convolutional neural network (CNN) utilizing standard 12-lead ECG. The area under the receiver operating characteristic curve (AUC) was used to evaluate prediction models including (CNN), light gradient boosting machines (LGBM), and Cox proportional hazards regression. A total of 14 613 (45% male, 73% of white, mean age ± standard deviation of 54 ± 5) participants were eligible. A total of 803 (5.5%) participants developed HF within 10 years from baseline. Convolutional neural network utilizing solely ECG achieved an AUC of 0.756 (0.717–0.795) on the hold-out test data. ARIC and Framingham Heart Study (FHS) HF risk calculators yielded AUC of 0.802 (0.750–0.850) and 0.780 (0.740–0.830). The highest AUC of 0.818 (0.778–0.859) was obtained when ECG-AI model output, age, gender, race, body mass index, smoking status, prevalent coronary heart disease, diabetes mellitus, systolic blood pressure, and heart rate were used as predictors of HF within LGBM. The ECG-AI model output was the most important predictor of HF.
Conclusions
ECG-AI model based solely on information extracted from ECG independently predicts HF with accuracy comparable to existing FHS and ARIC risk calculators.
Keywords: Heart failure, ECG, Electrocardiogram, Deep learning, Artificial intelligence, ARIC
Graphical Abstract
Translational perspective
This study investigates whether electrocardiogram (ECG) alone, when processed via artificial intelligence, can accurately predict the risk of heart failure (HF). ECG-artificial intelligence deep learning models using only standard 10 s 12-lead ECG data from 14 613 participants from the Atherosclerosis Risk in Communities (ARIC) study cohort could predict future HF with comparable accuracy to the HF risk calculators from ARIC study and Framingham Heart Study. Artificial intelligence is capable of using ECG tracings to predict incident HF. This can also enable pre-screening of large patient populations for risk of HF remotely when adapted into smartwatches with ECG functionality.
Introduction
There are ∼6.5 million adults, with more than 550 000 yearly diagnoses, in the USA that are reported to suffer or have suffered heart failure (HF).1,2 Heart failure is a progressive complex condition that is often terminal and is a major public health concern and burden.3,4 Heart failure often results in structural or functional cardiac disorders that impair the pumping of blood between cardiac compartments and the rest of the body.5 Early signs and symptoms of HF can substantially vary between different groups of patients, which can further complicate diagnosis and treatment.5,6
Heart failure was mentioned in 13.4% of all death certificates in the USA in 2018. While there have been advances in diagnoses and management, outcomes in patients with HF are still largely variable, and risks among different subgroups can substantially change over time.2,6 Early diagnosis and treatment can significantly improve HF prognosis,7 and subsequently help reduce the health and economic burdens of HF. Although HF therapy has somewhat improved survival rates, greater efforts are needed toward early detection of cardiac disorders and prevention of HF.8 Thus, it is essential to develop HF pre-screening tools that rely on a minimal amount of data that are easy to obtain, low cost with accessibility and promise of future remote applications. At this point, better utilization of electrocardiograms (ECGs) beyond their current clinical use and interpretation has the potential to lead to the development of such HF pre-screening tools.
Several recent studies have shown the utility of artificial intelligence (AI) applied to digital ECGs (time-voltage signal) in detecting and predicting cardiovascular disease. Specifically, such AI models utilizing digital ECGs were used in prediction of atrial fibrillation,9 cardiomyopathy,10,11 and all-cause mortality.12 We hypothesize that standard 10 s 12-lead ECG alone can predict HF risk within 10 years with moderately high accuracy. We utilized data from the Atherosclerosis Risk in Communities (ARIC) study cohort to test this hypothesis.
Methods
Cohort
The ARIC is an ongoing prospective epidemiologic study conducted in four communities in the USA (Forsyth County, NC; Jackson, MS; Washington County, MD; and the northwest suburbs of Minneapolis, MN) and designed to investigate the aetiology of atherosclerosis and its clinical outcomes, and cardiovascular risk factors associated with demographics, race, gender, and time. From 1987 to 1989 (visit 1, the baseline for our analysis), a total of 15 792 participants (8710 women and 4266 of black race) were enrolled and completed a home interview and clinic visit. In this analysis, we utilized data from visit 1 and follow-up visit 2 to visit 4 (visit 2: 1990–92, visit 3: 1993–96, visit 4: 1996–98) in AI-based models while using the entire follow-up in survival analysis up to 2019.
Outcomes
Our main outcome was predicting new-onset HF events within 10 years from visit 1 baseline examination. Heart failure was defined by hospitalization and HF as a hospital discharge diagnosis [International Classification of Disease, Ninth Revision, Clinical Modification (ICD-9-CM), code 428], or in-hospital or out-of-hospital deaths attributed to HF (deaths coded as ICD-9-CM code 428 or International Classification of Disease, Tenth Revision, code 150, without a previous record of hospitalization with ICD-9-CM code 428).13
Risk factors
We used a total of 12 risk factors which were used in the ARIC HF risk calculator8 and Framingham Heart Study (FHS) HF risk calculator.3 These clinical risk factors included in the ‘ARIC’ model in this study were gender, race, age, diabetes, hypertension medication, body mass index (BMI; kg/m2), systolic blood pressure (mmHg), prevalent coronary heart disease, smoking status, and heart rate (beats per minute, b.p.m.). The clinical risk factors included in the ‘Framingham’ model were age, diabetes, BMI (kg/m2), systolic blood pressure (mmHg), prevalent coronary heart disease, heart rate (b.p.m.), left ventricular hypertrophy (LVH), and valvular disease (see Table 1) (see Supplementary material online, Section S1 for details).
Table 1.
Study cohort characteristics and risk factors
| Risk factors |
n (%) or mean (SD) |
χ 2 or T-test | |
|---|---|---|---|
| HF in 10 years (n = 13 810) | HF in 10 years (n = 803) | P-values | |
| Gender (male)a | 6179 (44.7) | 456 (57.2) | <0.001 |
| Race (Black)a | 3559 (25.8) | 289 (36.0) | <0.001 |
| Age at visit 1a,b (years) | 53.9 (5.7) | 57.2 (5.2) | <0.001 |
| BMI (kg/m2)a,b | 27.4 (5.2) | 29.5 (6.3) | <0.001 |
| Smoking statusa | <0.001 | ||
| Former | 4407 (31.9) | 284 (35.4) | |
| Current | 3485 (25.2) | 304 (37.9) | |
| Prevalent coronary heart diseaseb | 458 (3.3) | 138 (17.2) | <0.001 |
| Diabetes mellitusa,b | 1326 (9.6) | 286 (35.6) | <0.001 |
| Systolic blood pressure (mmHg)a,b | 120.5 (18.4) | 131.2 (22.9) | <0.001 |
| Hypertension medicationa | 3566 (25.8) | 420 (52.3) | <0.001 |
| Left ventricular hypertrophyb | 253 (1.9) | 50 (6.4) | <0.001 |
| Valvular diseaseb | 33 (0.2) | 9 (1.1) | <0.001 |
| Heart rate (ventricular, beats per minute)a,b | 66.4 (10.0) | 70.5 (12.3) | <0.001 |
ARIC, Atherosclerosis Risk in Communities; FHS, Framingham Heart Study; HF, heart failure; SD, standard deviation.
Variables used in ARIC risk calculator.
Variables used in FHS risk calculator.
Electrocardiogram data
Raw digital ECG data (time-voltage) for 12 leads from the baseline (visit 1) were used. A supine 12-lead ECG at 250 Hz frequency of 10 s at rest was used. The ECGs were initially obtained from the MAC PC10 personal cardiogram (Marquette Electronics, Milwaukee, WI, USA). In this study, ECG data are used as indicators for possible subclinical HF risk.
Inclusions/exclusion criteria
All ARIC participants with good quality ECG data at baseline as well as information on all relevant risk factors and HF events during the study’s long-term follow-up were eligible for inclusion in this analysis. Participants with prevalent HF (n = 739) at the baseline visit, missing HF data during follow-up, and missing or poor-quality ECGs were excluded.
Study design
We randomly split our study cohort into 80% for model building and 20% as hold-out test data. Heart failure prediction models were built using different machine learning and statistical methods with five-fold cross-validation using the 80% model building dataset. During five steps of five-fold cross-validation, we built five independent models from scratch and did not transfer any learned parameter from one model to another to avoid data leak. For each method, the model providing the highest cross-validated area under the receiver operating characteristics curve (ROC AUC) statistics were identified as the final models. The final cross-validated models were then implemented on the 20% hold-out test data. All model comparisons and evaluations were based on ROC AUC statistics obtained on the 20% hold-out test dataset. The statistical significance of the difference between the two AUC’s was compared using DeLong test.14 The models and analyses were performed using the Python programming language.
Prediction of heart failure via deep learning using raw digital electrocardiograms
We implemented convolutional neural networks (CNNs), namely the ECG-AI model, to predict HF from raw digital ECG data. We created a CNN architecture by adapting ResNet15 that receives ECG leads as 1D digital signals and outputs risk for HF (see Supplementary material online, Section S2 and Figure S1 for details).
Prediction of heart failure using existing Framingham Heart Study and Atherosclerosis Risk in Communities heart failure risk calculators
To compare our ECG-AI approach to more traditional risk calculators we used two HF risk calculators; the FHS risk calculator3 and the ARIC study risk calculator.8 Components of the ARIC and FHS risk calculators are outlined in Table 1. We implemented FHS and ARIC risk calculators on only 20% hold-out test data since we did not re-build the models.
Ensemble heart failure risk predictions
Up to this point, our analysis is based on either creating a novel CNN model to predict HF from ECGs or based on currently available FHS and ARIC HF risk calculators. However, we also investigated combinations (or ensemble) of various HF risk predictions and risk factors using a frequently used machine learning algorithm, light gradient boosting machines (LGBM).16 In this ensemble approach, we build HF prediction models on the same 80% model building data and evaluated the models on the same 20% hold-out test data for streamlined comparisons.
Time dependence analysis
We also adapted our machine and deep learning-based models for survival analysis. To achieve this, we built a Cox proportional hazards regression model by using ML- or DL-based risk predictions as independent variables of the Cox model. For a fair comparison, we then substituted t with 10 years to obtain survival probability (the risk for HF in our case) based on the 10-year risk predictions.
Results
Clinical characteristics
This analysis included 14 613 (age 54.1 + 5.8 years; 45.4% men, 36.0% blacks) with no prevalent HF at baseline. A total of 803 (5.5%; cases) developed HF within 10 years following baseline examination. The average time of diagnosis of HF from the baseline visit was 6.0 ± 2.8 years. The remaining 13 810 (94.5%) participants (controls) did not develop HF within 10 years following baseline examination. The average follow-up time for controls was 23.6 ± 7.8 years. Differences in baseline ECG abnormalities between cases and controls are presented in Supplementary material online, Table S1.
Among the 12 clinical risk factors considered, 8 variables did not have any missing data. One patient had a missing BMI and this was replaced with the study cohort average. There were 13 participants with missing smoking status and were assumed to have never smoked. There were 17 participants with missing valvular disease data, and they were considered not to have a valvular disease. Lastly, there were 331 participants with missing LVH data and were assumed not to have experienced LVH. The detailed characteristics of our study cohort in terms of clinical risk factors used were summarized in Table 1.
Heart failure prediction
We ran 11 HF prediction models using CNN or LGBM utilizing various predicting variable combinations. The AUC statistics obtained on the same 20% hold-out data are summarized in Table 2 for four of the models, while the rest of the models were presented in Supplementary material online, Table S2.
Table 2.
Heart failure prediction results
| HF risk prediction method | Model inputs (‘X’ represents inputs used in corresponding method) |
AUC (95% CI) on 20% hold-out test data | |||
|---|---|---|---|---|---|
| ECG-AI output | ECG | ARIC variablesa | FHS variablesb | ||
| CNN (ECG-AI) | X | 0.756 (0.717–0.795) | |||
| ARIC risk calculator | X | 0.802 (0.750–0.850) | |||
| FHS risk calculator | X | 0.778 (0.740–0.830) | |||
| Cox | X | X | X | 0.818 (0.777–0.858) | |
ARIC, Atherosclerosis Risk in Communities; AUC, area under the receiver operating characteristic curve; BMI, body mass index; CI, confidence interval; CNN, convolutional neural network; ECG-AI, electrocardiographic artificial intelligence; FHS, Framingham Heart Study; HF, heart failure.
ARIC variables: age, gender, race, BMI, smoking status, prevalent coronary heart disease, diabetes mellitus, systolic blood pressure, heart rate.
FHS variables: age, BMI, prevalent coronary heart disease, diabetes mellitus, systolic blood pressure, left ventricular hypertrophy, valvular disease, heart rate.
ECG-AI CNN model which only uses digital 12-lead ECG data alone as input, resulting in an AUC of 0.756 on hold-out dataset, which was not significantly different than the AUC (0.778) of the FHS risk calculator (DeLong test, P = 0.180). However, the AUC of the ECG-AI model was lower than the AUC (0.778) of the ARIC risk calculator (DeLong test, P = 0.034). In an additional analysis, we experimented with applying the same ECG-AI architecture using only lead I data. Interestingly, we obtained an AUC of 0.754 (0.709–0.798), similar to the 12-lead version.
We also built traditional Cox proportional hazards regression to model time from baseline to incident HF up to 2018 follow-up. Cox model, utilizing all ARIC and FHS risk calculator variables as well as the outcome of ECG-AI, resulted in a concordance of 0.826 (0.804–0.848). For a fair comparison with the other three models, we set t = 10 and calculated the cumulative risk for HF within 10 years and obtained an AUC of 0.821 (0.781–0.861), sensitivity of 0.711, sensitivity of 0.752, positive predictive value of 0.132, and negative predictive value of 0.980. The AUC of the Cox model was higher than both AUC of the FHS risk calculator (DeLong test, P < 0.01) and the ARIC risk calculator (DeLong test, P < 0.01). The details of the Cox model provided in Table 3 revealed that the ECG-AI outcome was the most important predictor of HF. This is also confirmed by the variable importance analysis on the LGBM model utilizing the outcome of the ECG-AI model and ARIC variables as inputs, which provided an AUC of 0.818 (see Supplementary material online, Figure S2 and Section S3).
Table 3.
Cox proportional hazards regression model modelling heart failure risk
| Covariate | Coefficient | Hazard ratio | 95% CI | P-value |
|---|---|---|---|---|
| ECG-AI outcome | 5.05 | 155.61 | 58.93–410.92 | <0.01 |
| Gender | 0.31 | 1.37 | 1.14–1.65 | <0.01 |
| Race | 0.14 | 1.15 | 0.94–1.40 | 0.176 |
| Age | 0.08 | 1.09 | 1.07–1.11 | <0.01 |
| Diabetes | 0.96 | 2.60 | 2.14–3.17 | <0.01 |
| Hypertension medication | 0.49 | 1.62 | 1.35–1.96 | <0.01 |
| BMI | 0.04 | 1.04 | 1.02–1.05 | <0.01 |
| Systolic blood pressure | 0.01 | 1.017 | 1.00–1.01 | <0.01 |
| Prevalent coronary heart disease | 0.89 | 2.44 | 1.89–3.14 | <0.01 |
| Ventricular rate | 0.02 | 1.02 | 1.02–1.03 | <0.01 |
| Left ventricular hypertrophy | 0.35 | 1.42 | 1.00–2.02 | 0.049 |
| Valvular disease | 1.35 | 3.86 | 1.98–7.53 | <0.01 |
| Smoking status | 0.56 | 1.75 | 1.56–1.96 | <0.01 |
BMI, body mass index; CI, confidence interval; ECG-AI, electrocardiographic artificial intelligence.
Subgroup analysis
Cox model yielded an AUC of 0.818 (0.781–0.858) for black, 0.816 (0.776–0.857) for white, 0.828 (0.788–0.868) for male, and 0.810 (0.769–0.851) for female participants.
Sensitivity analysis over time
Our analysis was based on ECGs recorded at baseline exams. However, we also had access to ECGs recorded over follow-up exams. We used these follow-up exams to assess the sensitivity of our model on follow-up ECGs closer to the HF events. For the 20% hold-out dataset, we run our ECG-AI and final Cox models on the ECGs collected after baseline yet still preceding HF event. For controls, we used the latest available ECG. Next, for each patient with available follow-up ECGs, we calculated as the difference between risk from original and follow-up ECG divided by the time between two ECGs. Therefore, represents the change in predicted risk per year for each patient (Table 4).
Table 4.
Response of electrocardiographic artificial intelligence and Cox proportional hazards regression models to follow-up electrocardiograms
| Mean with 95% CI as a percentage | Controls | Cases |
|---|---|---|
| ECG-AI model | 0.235 (0.178–0.291) | 1.414 (0.912–1.917) |
| Cox model | 0.061 (0.031–0.0915 | 2.568 (1.883–3.252) |
CI, confidence interval; ECG-AI, electrocardiographic artificial intelligence.
Clinical utility
We further assess the possible clinical utility of our final Cox model to identify patients at risk for HF who may benefit from cardiac imaging. Table 5 presents four different scenarios of specificity (0.70, 0.80, 0.90, 0.95) and corresponding accuracy metrics.
Table 5.
Response of electrocardiographic artificial intelligence and Cox proportional hazards regression models to follow-up electrocardiograms
| Scenarios | ECG time | TP | FP | TN | FN | Specificity | Sensitivity | Negative predictive value | Positive predictive value |
|---|---|---|---|---|---|---|---|---|---|
| 1 | Baseline | 116 | 764 | 1819 | 2 | 0.7042 | 0.9831 | 0.9990 | 0.1318 |
| Follow-up | 116 | 764 | 1819 | 2 | 0.7042 | 0.9831 | 0.9990 | 0.1318 | |
| 2 | Baseline | 108 | 515 | 2068 | 10 | 0.8006 | 0.9153 | 0.9952 | 0.1734 |
| Follow-up | 116 | 528 | 2055 | 2 | 0.7956 | 0.9831 | 0.9990 | 0.1801 | |
| 3 | Baseline | 93 | 261 | 2322 | 25 | 0.8990 | 0.7881 | 0.9893 | 0.2627 |
| Follow-up | 111 | 258 | 2325 | 7 | 0.9001 | 0.9407 | 0.9970 | 0.3008 | |
| 4 | Baseline | 77 | 127 | 2456 | 41 | 0.9508 | 0.6525 | 0.9836 | 0.3775 |
| Follow-up | 95 | 123 | 2460 | 23 | 0.9524 | 0.8051 | 0.9907 | 0.4358 |
ECG, electrocardiogram; FN, false negative; FP, fasle positive; TN, true negative; TP, true positive.
The results in Table 4 show that for scenario 1 corresponding to the specificity of 0.7, 32.5% (880 of 2701) patients would be predicted at high risk for HF, and among these high risk predicted patients, 13.2% (116 of 880) would develop HF within 10 years. For Scenario 4 corresponding to a specificity of 0.95, our model would identify 7.5% (204 of 2701) of the general population at high risk for HF where 37.7% (77 of 204) of them indeed would develop HF. Interestingly, if we would use follow-up ECGs for the same scenario, we could identify 8.1% (218 of 2701) of the patients at high risk for HF and 43.6% (95 of 218) of them indeed would develop HF.
Discussion
Heart failure prevalence is increasing globally and is more commonly experienced by older persons. This can cause both monetary and personal burden. It is not uncommon for HF to be diagnosed at a late-stage, past pharmacological intervention.7 It is therefore of high importance to predict HF at early stages and provide timely interventions. If detection and/or prediction are performed early, it can substantially reduce the overall burden. The FHS and ARIC HF risk calculators3,8 examined existing HF risk factors and proposed simple and effective HF risk calculators that would facilitate the primary prevention and early diagnosis of HF in general practice. More complex models were then developed using additional data that can add to the potential of early identification of HF. The FHS HF calculator3 uses a standard pooled logistic regression model to identify the risk of HF within 4 years, while the ARIC HF risk calculator8 uses a Cox regression model. The latter was also applied in this study. In addition to using clinical variables, this research also used 12-lead ECGs to predict HF within 10 years, aiming to obtain comparable results to that using clinical risk factors.
Several recent studies have shown the utility of AI on digital ECGs (time-voltage signals) in the detection and prediction of arrhythmias and cardiovascular disease.17,18 A range of AI models has been developed to predict the risk of abnormal heart conditions, including HF, atrial fibrillation.19–21 There has also been an effort to use machine learning models to diagnose22,23 and predict the possibility of readmission and mortality following HF using solely risk factors.22 While some recent research proposes the use of AI in the prediction of HF using both or a collection of risk factors and 12-lead ECG information, there is rarely a comparative time window, and if so, it is within a relatively short period of time, e.g. present to 5 years.24–26 Recent studies have used ECG waveform data to develop AI networks to identify specific cardiac abnormalities such as ejection fraction,27 left ventricular systolic dysfunction,28 and mitral regurgitations29 all of which are directly or indirectly related to HF. However, a key component not addressed is the time window for early identification of the possibility of HF. A meta-analysis by Grün et al.30 involving five main publications31–33 reported an almost perfect prediction of congestive HF using a 2 s ECG (ROC > 0.98). These studies, however, do not provide information on the time window considered in developing the model and how early it can detect HF. This is a very important component to achieve the best results for diagnoses and precision medicine as opposed to identifying whether a person already has developed HF. It is thus essential to develop models that consider a trade-off of accuracy with timeliness of early diagnosis.
Results obtained in this research show that existing ARIC and FHS HF risk calculators utilizing a total of 12 clinical risk factors can predict HF with AUCs of 0.80 (0.75–0.85) and 0.78 (0.74–0.83), respectively. Our ECG-AI model (model 2) utilizing solely 12-lead ECGs yielded a comparable AUC of 0.756 (0.717–0.795) and AUC of 0.780 (0.737–0.823) when combined with age and gender (Model 3 in Supplementary material online, Table S1). Also, the lead I version of ECG-AI provided a comparable accuracy to the standard 12-lead-based ECG-AI model. Although our solely ECG-based model does not improve performance over existing ARIC and FHS risk calculators, our proposed ECG-AI model may be more applicable in a clinical setting since it relies only on ECG data. Considering the widespread use and availability of ECGs, such models can facilitate future automated pre-screening tools running on cardio-servers or electronic health records (EHR). This helps identify patients who may benefit from close monitoring or cardiac imaging, such as an echocardiogram or cardiac magnetic resonance imaging (MRI). The development of these AI-based models may ease the burden on healthcare systems by reducing the number of follow-up exams. Furthermore, we speculate that the model built on solely ECG data can predict HF at similar accuracy to clinical data-based risk calculators because the clinical risk factors may subtly affect the heart’s pacemaker cells and conductive pathways. This in turn affects the action potential associated with contractile response and translated into minute changes in an ECG. An advanced CNN model could capture these ECG changes.
Our research showed that the best performing model was obtained when the CNN-based ECG-AI model output was combined with risk factors used in the ARIC and FHS risk calculators in Cox proportional hazards regression. The performance of this model was significantly higher than the performances of well-known ARIC and FHS risk calculators, where ECG-AI outcome had the largest hazards predicting HF. Furthermore, a variable importance analysis on the second-best performing model (see Supplementary material online, Figure S2), LGBM, also confirms that ECG-AI output is the most important predictor of HF. These findings imply that the information extracted from ECG via AI generates subclinical indicators more predictive of HF than the clinical risk factors in the ARIC and FHS risk calculators.
The second-best model (Model 5 in Supplementary material online, Table S1) was obtained via LGBM utilizing ECG-AI outcome and the variables of ARIC risk calculators. As detailed in Supplementary material online, Section S3, variable importance analysis showed that ECG-AI model output is the most important predictor of HF, followed by age, BMI, diabetes, and systolic blood pressure. Analysis of direction of effect showed that individuals with coronary heart disease have about 5.4% increased risk of developing HF when all other factors are unchanged. In addition, individuals with diabetes can have an increased risk of 4.8%, while those with hypertension have a 1.9% increased risk.
Previous research has also applied a novel probabilistic symbol pattern recognition approach to identify congestive HF patients using R–R intervals from ECG.34,35 Several cohort studies, including ARIC, have shown that various ECG markers are associated with incident HF.10,36–44 These findings also suggest that applying machine and deep learning approaches to ECGs can be used in developing automated HF prediction tools for early recognition of patients at risk. As a deep learning method, CNNs are applied on the classification of atrial fibrillation,45 several heart rhythms,46 left ventricular ejection fraction,47 as well as prediction of future cardiomyopathy.10 There were also efforts to show the association of known ECG characteristics with risk for HF,41 yet the digital ECGs have not been utilized. However, to the best of our knowledge, our study is the first attempt to solely utilize digital ECG data via deep learning to predict risk for HF.
Sensitivity analysis and prospective validation on additional follow-up ECG showed that both ECG-AI and the final Cox model produce significantly higher risk for ECGs closer to HF events. This may suggest that follow-up of patients at high risk via low-cost ECG can assess HF risk changes. The patients whose predicted risk exceeds a certain threshold may be followed up echocardiogram and cardiac MRI for timely diagnosis to initiate preventive therapeutics to advance patients to Stages C and D HF. As a result, such low-cost screening-based preventive strategies may improve health outcomes and reduce healthcare costs due to HF. Interestingly, our ECG-AI model performed as well using only lead I ECG compared to results obtained on 12-lead. Future work may focus on validity of our ECG-AI model on lead I ECG obtained via mobile technologies such as smartwatches.
Our study has several strengths. The performance of AI-based predictive models is severely affected by the accuracy of the outcome variable. Our study utilizes the data from one of the largest cohort studies of atherosclerosis, the ARIC, where the follow-up on HF is significantly more accurate compared to data that would be extracted from an EHR of a single institution. Also, our results show that ECG markers alone can provide HF risk prediction as accurately as established HF risk calculators relying on multiple clinical risk factors. Therefore, it can be embedded into EHR for efficient and automatic pre-screening for HF at a large scale.
Our study also has some limitations. Although the ARIC cohort is relatively representative by gathering participants from four communities in the USA, an external validation on a more representative cohort is needed to ensure generalizability for the general population. There are also limitations in understanding why ECG alone can predict HF and models utilizing many clinical risk factors. This limitation stems from the non-parametric nature of deep learning models. Further analysis is needed to uncover the black box nature of deep learning models. We do not have information on the aetiology of HF events. Hence, our results should not imply causality between ECG and HF. Another important limitation of our study is that the diagnosis of HF during follow-up included only hospitalized patients. There may be HF patients who are compensated and stable, therefore, not required hospitalization within 10 years of the baseline. Hence, despite these patients are ‘cases’, they could be coded as ‘controls’. Despite our study does not provide evidence to support that, however, a future study could focus on whether some of the false positives indeed had HF yet not require hospitalization. There are also technical limitations in implementing our ECG-AI model in clinical practice. Another limitation is the definition of HF based on ICD codes, whereby HF subtype by ejection fraction was not available. Similar prediction accuracy may be expected in cohorts where HF is diagnosed/defined in a similar way as it was in ARIC. Furthermore, future work is needed to show how well our model would predict HF with preserved ejection fraction.
To conclude, sole utilization of raw digital ECG data via deep learning results in HF prediction with moderately high accuracy, which is comparable to existing FHS risk calculator. Such ECG-based HF risk assessment can pre-screen larger patient populations by analysing existing ECGs in cardio-servers linked to EHRs. This pre-screening may help identify people who may benefit from more advanced cardiac healthcare. Furthermore, such models and technology may be adapted to smartwatches with ECG recording functionality to facilitate remote screening.
Acknowledgements
We would like to thank The Atherosclerosis Risk in Communities study consortium for providing data for this study (ARIC Proposal ID: 3678). We also thank the staff and participants of the ARIC study for their important contributions.
Funding
The Atherosclerosis Risk in Communities study has been funded in whole or in part with Federal funds from the National Heart, Lung, and Blood Institute, National Institutes of Health, Department of Health and Human Services, under Contract nos. (HHSN268201700001I, HHSN268201700002I, HHSN268201700003I, HHSN268201700005I, HHSN268201700004I). Additional funding was supported by grant K24HL148521.
Conflict of interest: none declared.
Data availability
The data used in this study may be obtained directly from Atherosclerosis Risk in Community Study via manuscript proposal mechanism.
References
- 1. Akintoye E, Briasoulis A, Egbe A, Orhurhu V, Ibrahim W, Kumar K, Alliu S, Nas H, Levine D, Weinberger J. Effect of hospital ownership on outcomes of heart failure hospitalization. Am J Cardiol 2017;120:831–837. [DOI] [PubMed] [Google Scholar]
- 2. Benjamin EJ, Muntner P, Alonso A, Bittencourt MS, Callaway CW, Carson AP, Chamberlain AM, Chang AR, Cheng S, Das SR, Delling FN, Djousse L, Elkind MSV, Ferguson JF, Fornage M, Jordan LC, Khan SS, Kissela BM, Knutson KL, Kwan TW, Lackland DT, Lewis TT, Lichtman JH, Longenecker CT, Loop MS, Lutsey PL, Martin SS, Matsushita K, Moran AE, Mussolino ME, O'Flaherty M, Pandey A, Perak AM, Rosamond WD, Roth GA, Sampson UKA, Satou GM, Schroeder EB, Shah SH, Spartano NL, Stokes A, Tirschwell DL, Tsao CW, Turakhia MP, VanWagner LB, Wilkins JT, Wong SS, Virani SS; American Heart Association Council on Epidemiology and Prevention Statistics Committee and Stroke Statistics Subcommittee. Heart disease and stroke statistics—2019 update: a report from the American Heart Association. Circulation 2019;139:e56–e528.30700139 [Google Scholar]
- 3. Kannel WB, D'Agostino RB, Silbershatz H, Belanger AJ, Wilson PW, Levy D. Profile for estimating risk of heart failure. Arch Intern Med 1999;159:1197–1204. [DOI] [PubMed] [Google Scholar]
- 4. Tripoliti EE, Papadopoulos TG, Karanasiou GS, Kalatzis FG, Goletsis Y, Bechlioulis A et al. A computational approach for the estimation of heart failure patients status using saliva biomarkers. Annu Int Conf IEEE Eng Med Biol Soc 2017;2017:3648–3651. [DOI] [PubMed] [Google Scholar]
- 5. Rosamond WD, Chang PP, Baggett C, Johnson A, Bertoni AG, Shahar E, et al. Classification of heart failure in the Atherosclerosis Risk in Communities (ARIC) study: a comparison of diagnostic criteria. Circ Heart Fail 2012;5:152–159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Rahimi K, Bennett D, Conrad N, Williams TM, Basu J, Dwight J, Woodward M, Patel A, McMurray J, MacMahon S. Risk prediction in patients with heart failure: a systematic review and analysis. JACC Heart Fail 2014;2:440–446. [DOI] [PubMed] [Google Scholar]
- 7. Yang H, Negishi K, Otahal P, Marwick TH. Clinical prediction of incident heart failure risk: a systematic review and meta-analysis. Open Heart 2015;2:e000222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Agarwal SK, Chambless LE, Ballantyne CM, Astor B, Bertoni AG, Chang PP, Folsom AR, He M, Hoogeveen RC, Ni H, Quibrera PM, Rosamond WD, Russell SD, Shahar E, Heiss G. Prediction of incident heart failure in general practice: the Atherosclerosis Risk in Communities (ARIC) study. Circ Heart Fail 2012;5:422–429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Jalali A, Lee M. Atrial fibrillation prediction with residual network using sensitivity and orthogonality constraints. IEEE J Biomed Health Inform 2020;24:407–413. [DOI] [PubMed] [Google Scholar]
- 10. Gunturkun F, Davis RL, Armstrong GT, Jefferies JL, Ness KK, Green DM, Lucas JT, Srivastava D, Hudson MM, Robison LL, Mulrooney DA, Soliman EZ, Karabayir I, Akbilgic O. Deep learning for improved prediction of late-onset cardiomyopathy among childhood cancer survivors: a report from the St. Jude Lifetime Cohort (SJLIFE). J Clin Oncol 2020;38:10545. [Google Scholar]
- 11. Gunturkun F, Akbilgic O, Davis RL, Armstrong GT, Howell RM, Jefferies JL, Ness KK, Karabayir I, Lucas Jr JT, Srivastava DK, Hudson MM, Robison LL, Soliman EZ, Mulrooney DA. Artificial intelligence assisted prediction of late onset cardiomyopathy among childhood cancer survivor. JCO J Clin Cancer Inform 2021;4:459–468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Raghunath S, Ulloa Cerna AE, Jing L, vanMaanen DP, Stough J, Hartzel DN, Leader JB, Kirchner HL, Stumpe MC, Hafez A, Nemani A, Carbonati T, Johnson KW, Young K, Good CW, Pfeifer JM, Patel AA, Delisle BP, Alsaid A, Beer D, Haggerty CM, Fornwalt BK. Prediction of mortality from 12-lead electrocardiogram voltage data using a deep neural network. Nat Med 2020;26:886–891. [DOI] [PubMed] [Google Scholar]
- 13. Rautaharju PM, Prineas RJ, Wood J, Zhang ZM, Crow R, Heiss G. Electrocardiographic predictors of new-onset heart failure in men and in women free of coronary heart disease (from the Atherosclerosis in Communities [ARIC] Study). Am J Cardiol 2007;100:1437–1441. [DOI] [PubMed] [Google Scholar]
- 14. DeLong ER, DeLong DM, Clarke-Pearson DL. Comparing the areas under two or more correlated receiver operating characteristic curves: a nonparametric approach. Biometrics 1988;44:837–845. [PubMed] [Google Scholar]
- 15. He K, Zhang X, Ren S, Sun J. Deep residual learning for image recognition. arXiv 2015;e-pring 1512.03385. [Google Scholar]
- 16. Ke G, Meng Q, Finley T, Wang T, Chen W, Ma W, Ye Q, Liu T-Y. LightGBM: a highly efficient gradient boosting decision tree. In: von Luxburg U, Guyon I, Bengio S, Wallach H, Fergus R (eds). Proceedings of the 31st International Conference on Neural Information Processing Systems. Long Beach, CA, USA: Curran Associates Inc.; 2017. p3149–3157. [Google Scholar]
- 17. Rahimi K, Bennett D, Conrad N, Williams TM, Basu J, Dwight J, Woodward M, Patel A, McMurray J, MacMahon S. Risk prediction in patients with heart failure: a systematic review and analysis. JACC Heart Fail 2014;2:440–446. [DOI] [PubMed] [Google Scholar]
- 18. Banerjee A, Chen S, Fatemifar G, Zeina M, Lumbers RT, Mielke J, Gill S, Kotecha D, Freitag DF, Denaxas F, Hemingway H. Machine learning for subtype definition and risk prediction in heart failure, acute coronary syndromes and atrial fibrillation: systematic review of validity and clinical utility. BMC Med 2021;19:1–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Hammad M, Maher A, Wang K, Jiang F, Amrani M. Detection of abnormal heart conditions based on characteristics of ECG signals. Measurement 2018;125:634–644. [Google Scholar]
- 20. Kwon J-m, Kim K-H, Jeon K-H, Kim HM, Kim MJ, Lim S-M, et al. Development and validation of deep-learning algorithm for electrocardiography-based heart failure identification. Korean Circ J 2019;49:629–639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Akbilgic O, Butler L, Karabayir I, Chang P, Kitzman D, Alonso A, Chen L , Soliman E. Artificial intelligence applied to ECG improves heart failure prediction accuracy. J Am Coll Cardiol 2021;77(18_Suppl_1):3045. [Google Scholar]
- 22. Guo A, Pasque M, Loh F, Mann DL, Payne PR. Heart failure diagnosis, readmission, and mortality prediction using machine learning and artificial intelligence models. Curr Epidemiol Rep 2020:1–8. [Google Scholar]
- 23. Choi D-J, Park JJ, Ali T, Lee S. Artificial intelligence for the diagnosis of heart failure. NPJ Dig Med 2020;3:1–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Kannel WB, D'Agostino RB, Silbershatz H, Belanger AJ, Wilson PW, Levy D. Profile for estimating risk of heart failure. Arch Intern Med 1999;159:1197–1204. [DOI] [PubMed] [Google Scholar]
- 25. Tohyama T, Funakoshi K, Kaku H, Enzan N, Ikeda M, Matsushima S, Ide T, Todaka K, Tsutsui H. Artificial intelligence-based analysis of payment system data can predict one-year mortality of hospitalized patients with heart failure. Eur Heart J 2020;41(Suppl_2). 10.1093/ehjci/ehaa946.3492. [DOI] [Google Scholar]
- 26. Nakajima K, Nakata T, Matsuo S, Doi T, Jacobson A. Machine learning model for predicting sudden cardiac death and heart failure death using 123I-metaiodobenzylguanidine. Eur Heart J Cardiovasc Imaging 2019;20(Suppl_3). 10.1093/ehjci/jez145.003. [DOI] [Google Scholar]
- 27. Verbrugge FH, Reddy YN, Attia ZI, Friedman PA, Noseworthy PA, Lopez-Jimenez F, Kapa S, Borlaug BA. Artificial intelligence predicts atrial fibrillation development from the 12-lead electrocardiogram in heart failure with preserved ejection fraction. J Card Fail 2020;26:S76. [Google Scholar]
- 28. Adedinsewo D, Carter RE, Attia Z, Johnson P, Kashou AH, Dugan JL, Albus A, Sheele JM, Bellolio F Friedman PA, Lopez-Jimenez F, Noseworthy PA. Artificial intelligence-enabled ECG algorithm to identify patients with left ventricular systolic dysfunction presenting to the emergency department with dyspnea. Circ Arrhyth Electrophysiol 2020;13:e008437. [DOI] [PubMed] [Google Scholar]
- 29. Kwon J-m, Kim K-H, Akkus Z, Jeon K-H, Park J, Oh B-H. Artificial intelligence for detecting mitral regurgitation using electrocardiography. J Electrocardiol 2020;59:151–157. [DOI] [PubMed] [Google Scholar]
- 30. Grün D, Rudolph F, Gumpfer N, Hannig J, Elsner LK, Von Jeinsen B, Hamm CW, Rieth A, Guckert M, Till Keller Till. Identifying heart failure in ECG data with artificial intelligence—a meta-analysis. Front Dig Health 2020;2:67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Sudarshan VK, Acharya UR, Oh SL, Adam M, Tan JH, Chua CK, Chua KP, Tan RS. Automated diagnosis of congestive heart failure using dual tree complex wavelet transform and statistical features extracted from 2 s of ECG signals. Comput Biol Med 2017;83:48–58. [DOI] [PubMed] [Google Scholar]
- 32. Acharya UR, Fujita H, Oh SL, Hagiwara Y, Tan JH, Adam M, et al. Deep convolutional neural network for the automated diagnosis of congestive heart failure using ECG signals. Appl Intell 2019;49:16–27. [Google Scholar]
- 33. Lih OS, Jahmunah V, San TR, Ciaccio EJ, Yamakawa T, Tanabe M, et al. Comprehensive electrocardiographic diagnosis based on deep learning. Artif Intell Med 2020;103:101789. [DOI] [PubMed] [Google Scholar]
- 34. Akbilgic O, Howe JA. Symbolic pattern recognition for sequential data. Seq Anal 2017;36:528–540. [Google Scholar]
- 35. Mahajan R, Viangteeravat T, Akbilgic O. Improved detection of congestive heart failure via probabilistic symbolic pattern recognition and heart rate variability metrics. Int J Med Inform 2017;108:55–63. [DOI] [PubMed] [Google Scholar]
- 36. Rautaharju PM, Zhang ZM, Haisty WK Jr, Prineas RJ, Kucharska-Newton AM, Rosamond WD, Soliman EZ. Electrocardiographic predictors of incident heart failure in men and women free from manifest cardiovascular disease (from the Atherosclerosis Risk in Communities [ARIC] study). Am J Cardiol 2013;112:843–849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Zhang ZM, Rautaharju PM, Soliman EZ, Manson JE, Martin LW, Perez M, Vitolins M, Prineas RJ. Different patterns of bundle-branch blocks and the risk of incident heart failure in the Women's Health Initiative (WHI) study. Circ Heart Fail 2013;6:655–661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Zhang ZM, Rautaharju PM, Prineas RJ, Loehr L, Rosamond W, Soliman EZ. Usefulness of electrocardiographic QRS/T angles with versus without bundle branch blocks to predict heart failure (from the Atherosclerosis Risk in Communities Study). Am J Cardiol 2014;114:412–418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Zhang ZM, Rautaharju PM, Prineas RJ, Loehr L, Rosamond W, Soliman EZ. Ventricular conduction defects and the risk of incident heart failure in the Atherosclerosis Risk in Communities (ARIC) study. J Card Fail 2015;21:307–312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Almahmoud MF, O'Neal WT, Qureshi W, Soliman EZ. Electrocardiographic versus echocardiographic left ventricular hypertrophy in prediction of congestive heart failure in the elderly. Clin Cardiol 2015;38:365–370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. O'Neal WT, Mazur M, Bertoni AG, Bluemke DA, Al-Mallah MH, Lima JAC, Kitzman D, Soliman EZ. Electrocardiographic predictors of heart failure with reduced versus preserved ejection fraction: the multi-ethnic study of atherosclerosis. J Am Heart Assoc 2017;6:e006023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. O'Neal WT, Sandesara PB, Samman-Tahhan A, Kelli HM, Hammadah M, Soliman EZ. Heart rate and the risk of adverse outcomes in patients with heart failure with preserved ejection fraction. Eur J Prev Cardiol 2017;24:1212–1219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Qureshi WT, Zhang ZM, Chang PP, Rosamond WD, Kitzman DW, Wagenknecht LE, Soliman EZ. Silent myocardial infarction and long-term risk of heart failure: the ARIC study. J Am Coll Cardiol 2018;71:1–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Ilkhanoff L, Liu K, Ning H, Nazarian S, Bluemke DA, Soliman EZ, Lloyd-Jones DM. Association of QRS duration with left ventricular structure and function and risk of heart failure in middle-aged and older adults: the Multi-Ethnic Study of Atherosclerosis (MESA). Eur J Heart Fail 2012;14:1285–1292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Kamaleswaran R, Mahajan R, Akbilgic O. A robust deep convolutional neural network for the classification of abnormal cardiac rhythm using single lead electrocardiograms of variable length. Physiol Meas 2018;39:035006. [DOI] [PubMed] [Google Scholar]
- 46. Zhu H, Cheng C, Yin H, Li X, Zuo P, Ding J, Lin F, Wang J, Zhou B, Li Y, Hu S, Xiong Y, Wang B, Wan G, Yang X, Yuan Y. Automatic multilabel electrocardiogram diagnosis of heart rhythm or conduction abnormalities with deep learning: a cohort study. Lancet Digit Health 2020;2:e348–e357. [DOI] [PubMed] [Google Scholar]
- 47. Adedinsewo D, Carter RE, Attia Z, Johnson P, Kashou AH, Dugan JL, Albus M , Sheele JM, Bellolio F, Friedman PA, Lopez-Jimenez F, Noseworthy PA. Artificial intelligence-enabled ECG algorithm to identify patients with left ventricular systolic dysfunction presenting to the emergency department with dyspnea. Circ Arrhythm Electrophysiol 2020;13:e008437. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data used in this study may be obtained directly from Atherosclerosis Risk in Community Study via manuscript proposal mechanism.