Abstract
Objectives:
Data regarding the longitudinal relationship of global longitudinal strain (GLS) and echocardiographic parameters are lacking in peripartum cardiomyopathy (PPCM). We evaluated GLS and its correlation with change (Δ) in left ventricular ejection fraction (LVEF).
Methods:
We retrospectively identified women age ≥16 years hospitalized at Montefiore Medical Center in Bronx, NY from 1999–2015 with International Statistical Classification of Diseases and Related Health Problems, 9th revision codes for PPCM or an occurrence of unexplained heart failure during or up to 5 months postpartum. N = 195 charts were reviewed for inclusion/exclusion criteria, n = 53 patients met criteria for PPCM, and of those, n = 13 had a baseline and follow-up echocardiogram suitable for GLS analysis.
Results:
Of those eligible for strain analysis, the mean age was 30 ± 6 years, 46.2% identified as Black and 38.5% as Hispanic/Latina. Baseline LVEF was 30 (25, 35)%, GLS was −13.2 (−14, −7.6)%. At a mean follow-up time of 1.2 ± 0.7 years, 11/13 had persistently mild −15.6 (−16.3, −12.7)%, and 2/13 severely abnormal GLS −7.05 (−7.1, −7.0)%. There was no correlation between baseline GLS and ΔLVEF (r = .014, P = .965).
Conclusions:
Global longitudinal strain is a sensitive method to identify subclinical myocardial dysfunction. In this series of women with PPCM, GLS remained persistently abnormal over time, even if LVEF improved. Future studies should examine the implication of persistently abnormal GLS in PPCM.
Keywords: cardiomyopathy, echocardiogram, heart failure, pregnancy
1 |. INTRODUCTION
Peripartum cardiomyopathy (PPCM) is a rare cause of myocardial dysfunction which occurs in late pregnancy and the postpartum period, defined as a left ventricular ejection fraction (LVEF) <45% in the absence of any other identifiable cause.1–3 Absent a definitive molecular marker, the diagnosis of PPCM is one of exclusion based on clinical and echocardiographic features. Risk factors for PPCM include African descent, age, pregnancy-related hypertension, multiparity, obesity, chronic hypertension, and prolonged used of tocolytics.4 The incidence of PPCM is estimated as high as 1:100 live births in Haiti and Nigeria, and 1:1000–4000 live births in the United States, but differences in definition, overlapping, and misdiagnoses may impact these figures.5 Incidence is reported to be 16-fold higher in individuals of Afro-Caribbean race-ethnicity than whites.6 It has been purported that genetic predisosition, in addition to hormonal changes such as excess prolactin, hemodynamic changes of pregnancy, nutritional deficiencies, and autoimmunity, may play a role in its pathophysiology.7 Clinically, PPCM is undistinguishable from heart failure caused by other etiologies. Disease-specific therapy is poorly understood given the paucity of randomized controlled trials. The prognosis is variable, with 50% of patients demonstrating recovery of LVEF after 12 months.8
Two prior studies of PPCM, one cross-sectional and one longitudinal, have examined myocardial signatures of cardiomyopathy by echocardiography using strain measures. Strain is a dimensionless index of cardiac movement in multidimensional planes indicating the change in length between 2 points.9 Global longitudinal strain (GLS) is highly reproducible, provides a broader range of interpretation than LVEF, independently predicts mortality in heart failure of reduced EF, and is superior in detecting subtle LV dysfunction.9 Strain parameters were studied cross-sectionally in 47 women presenting to a single center with PPCM, 95.6% of which were African American.10 Nearly 3/4 of patients in the study had complete or partial LVEF recovery. Findings indicated that baseline GLS was not associated with all-cause mortality, rehospitalization, or lack of LVEF recovery, but the study lacked repeated measures over time.10 Strain was studied prospectively in 89 women included across 30 centers in the Investigations of Pregnancy Associated Cardiomyopathy (IPAC) consortium.11 Over 1 year of follow-up, GLS was associated with the composite outcome of death, heart transplant, left ventricular assist device (LVAD) implant, or persistently decreased LVEF <50%. GLS was independently associated with adverse outcomes despite adjustment for LVEF, age, race, or New York Heart Association functional heart failure class. In this study, GLS appeared to remain persistently abnormal even at follow-up, but lacked data on change in end-systolic and end-diastolic volumes over time and data on race-ethnic minorities outside of Black/African Americans. Given the paucity of data regarding GLS in women with PPCM, particularly in Hispanic/Latinas, we retrospectively evaluated repeated measures of echocardiographic GLS in women presenting with PPCM to our center, with the hypothesis that GLS might remain persistently abnormal despite LVEF recovery.
2 |. MATERIALS AND METHODS
2.1 |. Subjects and study design
We retrospectively queried the electronic medical record (EMR) for women age ≥16 years hospitalized at Montefiore Medical Center (MMC) in Bronx, NY from 1999–2015 using Looking Glass Clinical Analytics (LGCA, Streamline Health), a search engine for the MMC electronic medical record.12 We queried International Statistical Classification of Diseases and Related Health Problems, 9th revision diagnosis and procedure codes (ICD-9) for PPCM or heart failure or Current Procedural Terminology (CPT) for heart transplantation or left ventricular assist device placement during pregnancy or up to 5 months after delivery (674.53, 674.54, 428.0, 428.1, 428.21, 428.22, 428.23, 428.40, 428.41, 428.43, 428.9, 37.5, 37.51, 37.62, 37.65). Demographic characteristics were obtained using LGCA. Additional clinical information and history during the index hospitalization were abstracted by direct review of records by trained student and physician abstractors. Inclusion criteria for the overall study were peripartum LVEF ≤45% and idiopathic, nonischemic cardiomyopathy and exclusion criteria were missing LVEF, missing documentation relating low EF to the peripartum period, congenital heart disease, valvular disease, coronary artery disease, septicemia, cocaine/alcohol abuse, chemotherapy, chest irradiation, eclampsia, autoimmune disease, supraventricular arrhythmia, and human immunodeficiency virus (HIV). We designed the study to mirror the IPAC definition of PPCM, but did include 1 individual who was diagnosed in the first trimester of pregnancy given that women can present with it intrapartum.13 We additionally excluded those with eclampsia, autoimmune disease, supraventricular arrhythmia, and HIV as confounding causes of cardiomyopathy.3 Inclusion criteria for the strain imaging study were as above and additionally, a baseline and at least 1 follow-up echocardiogram in the MMC system. Echocardiograms closest to 6 months and 12 months of follow-up were preferentially evaluated for strain imaging. Exclusion criteria were no follow-up echocardiogram and an echocardiogram that could not be interpreted for GLS. Study data were collected and managed with REDCap.14 The study was approved by the Institutional Review Board of the Albert Einstein College of Medicine.
2.2 |. Demographic and clinical variables
Gravity and parity, race and ethnicity were self-reported. Body mass index (BMI) was calculated as weight (kg) divided by height squared (m2). Body surface area was calculated by the method of DuBois and DuBois.15 Chronic hypertension (HTN) was defined by history or past treatment with antihypertensive medication. Gestational hypertension was defined by history of treatment with antihypertensive medication in pregnancy alone. Chronic diabetes was defined by history or past use of antidiabetic medication. Gestational diabetes was defined by history or use of antidiabetic medication in pregnancy alone. Prior history of PPCM was by history or evaluated by direct record review from a trained abstractor using the above inclusion and exclusion criteria.
2.3 |. Echocardiographic evaluation and deformation measurements
Left ventricular ejection fraction was calculated using Simpson’s method of disks and verified by visual inspection by echocardiographers for clinical purposes. Septal and LV posterior wall thickness, LV systolic and diastolic diameters, LV mass and left atrial (LA) volume were measured from 2D images. Philips QLAB 10.2 software (New York, NY) was used for strain analysis of echocardiograms obtained after 2009 and TomTec (Chicago, IL) to generate Figure 2.16 Echocardiographic assessment of LV myocardial deformation included regional and GLS. End-systolic and end-diastolic volume (ESV and EDV, respectively) were measured. All measurements were taken from the first echocardiographic study performed at presentation and studies closest to 6 and 12 months of follow-up. For each study, the regional longitudinal systolic strain in the apical 2-, 3-, and 4-chamber views was obtained. The automatic tracking in systole was adjusted manually, and GLS was calculated by the software as an average of the 18 segments. Strain measurements were performed blinded to the clinical characteristics of the patients.
FIGURE 2.
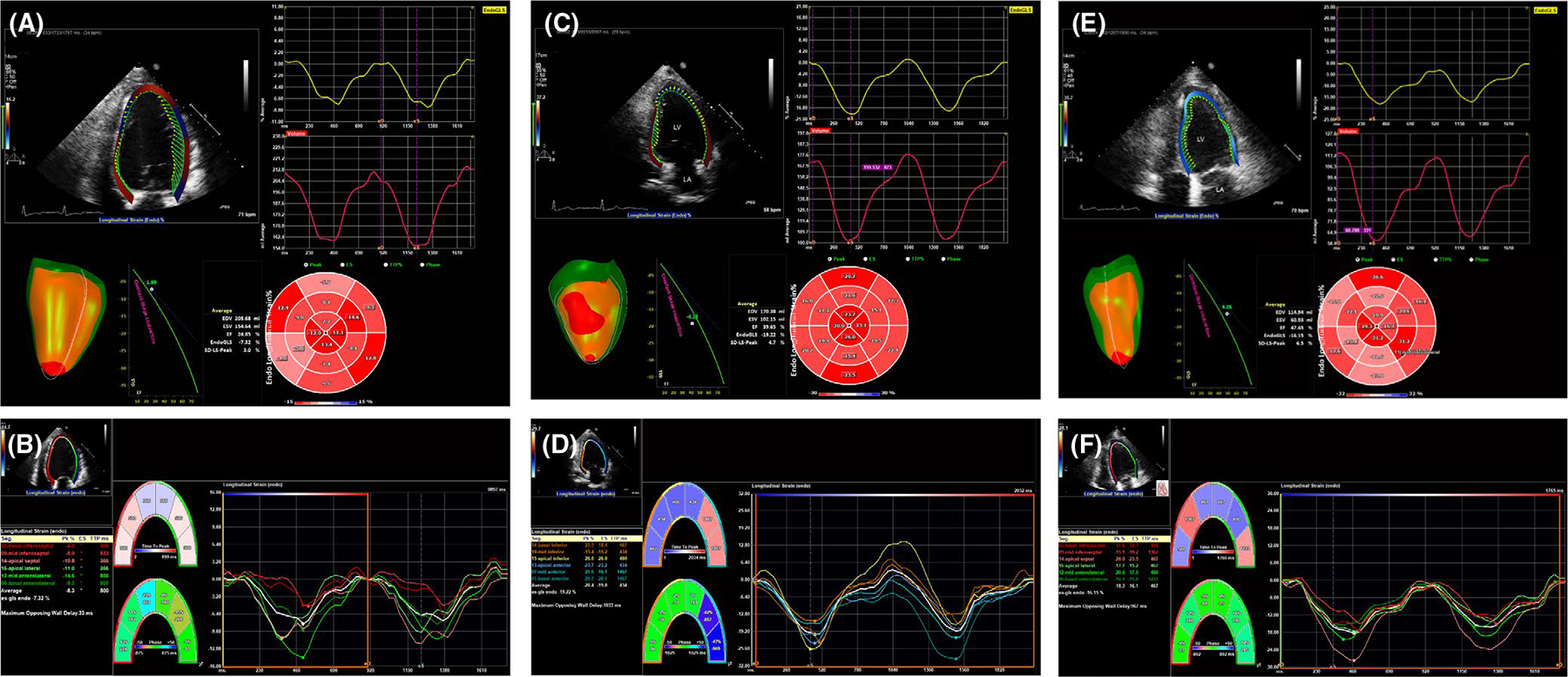
(A) Representative GLS and bull’s eye plots depicted by region of the left ventricle and their corresponding waveforms in a single subject with recovered left ventricular ejection fraction (LVEF), but persistent abnormal strain pattern: (A, B) baseline, LVEF 20%, (C,D) approximately 4 mo later, LVEF 40%–45% and (E, F) approximately 12 mo of follow-up, LVEF 50%–55%. GLS = global longitudinal strain
2.4 |. Statistical analysis
Categorical variables were expressed as frequency (n, %) and continuous variables as mean ±standard deviation or median and interquartile range. Wilcoxon signed-rank, Kruskal–Wallis, Mann–Whitney tests, and Spearman’s correlations were calculated for skewed data and t test for normally distributed data. Analyses were performed with STATA 14.1(College Station, TX) and GraphPad Prism 8 (La Jolla, CA). A two-tailed P < .05 defined statistical significance.
3 |. RESULTS
9th revision diagnosis and procedure codes search yielded n = 195 inpatients with a potential diagnosis of PPCM admitted from 1999–2015, and their charts were reviewed for inclusion/exclusion criteria (Figure 1). Approximately 1/4 were excluded for LVEF >45%, nearly 1/3 for LVEF ≤45% with an alternative diagnosis, and 1/6 lacked documentation of LVEF. Review of records for alternative diagnoses revealed diverse causes for low LVEF in peripartum women presenting to our center. PPCM was the diagnosis in nearly 1/2 with LVEF <45%. Patients who met entry criteria for PPCM were included in the Montefiore PPCM study (n = 53). Patients having at least two echocardiograms of a native heart with quality suitable for strain analysis were included in the strain imaging study (n = 13).
FIGURE 1.
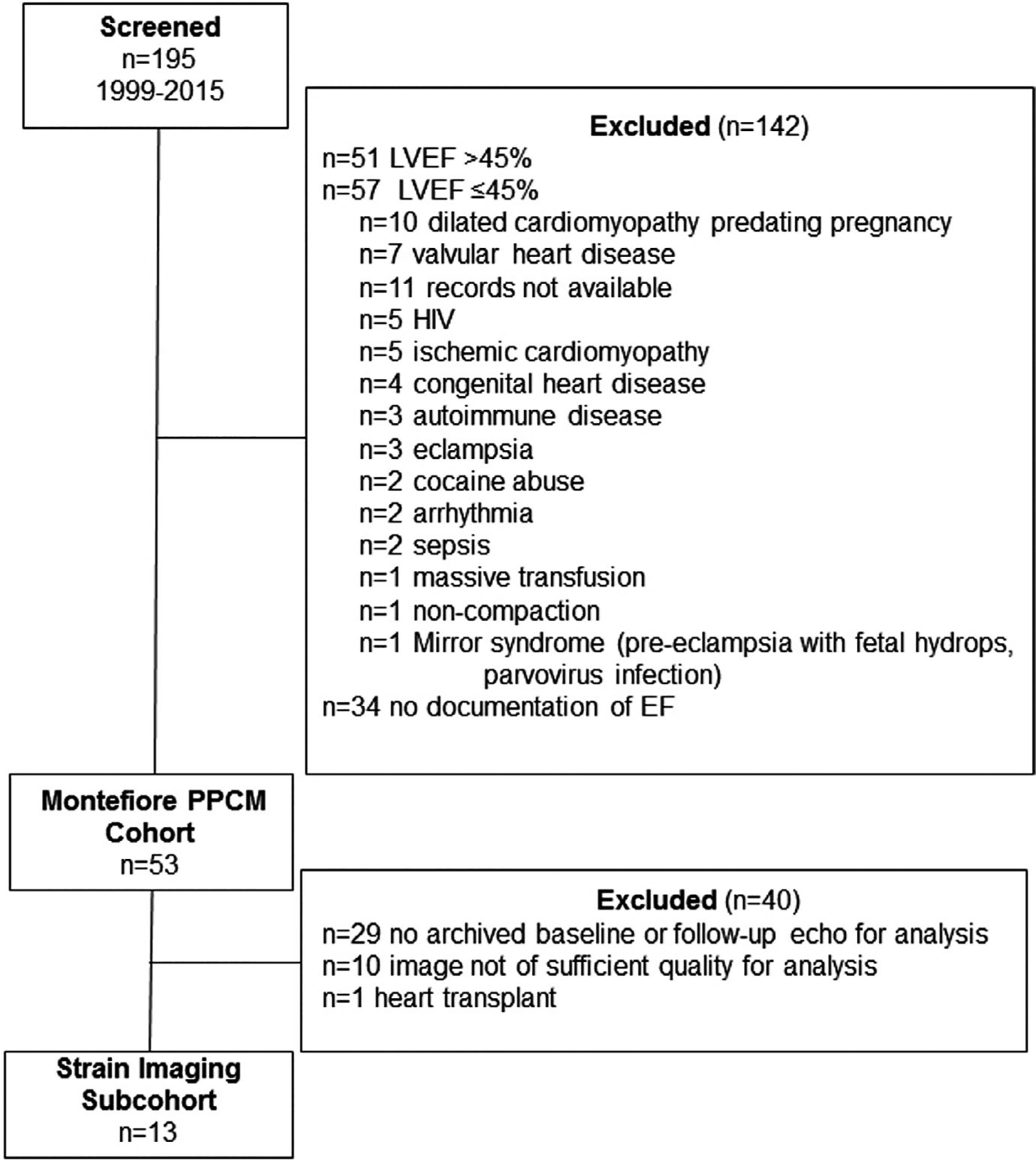
Flowchart of patients screened for inclusion in the strain imaging study
Demographics and general clinical characteristics are described in Table 1. Clinical characteristics in the strain study were representative of the entire PPCM cohort. The mean age of women in the strain imaging study was 30 ± 6 years. The majority self-identified as Black/African American or Hispanic/Latina ethnicity (Black or white race). The women were multigravida; nearly 1/2 were obese and had either chronic or gestational hypertension. Over 1/3 had antepartum preeclampsia. Women in the strain subset were diagnosed with PPCM at a median of 6.4 (IQR 0.7, 16.7) weeks after delivery, except for 1 who was diagnosed at 13 weeks intrapartum. The baseline LVEF was moderately decreased, baseline GLS was mildly reduced, LV systolic and diastolic diameters, LV mass index, as well as ESV and EDV indices were increased (Table 2). Representative strain analysis and bull’s eye plot from an individual with persistent strain abnormality despite LVEF recovery is shown in Figure 2. GLS at last follow-up was improved in all except 2 individuals (Figure 3A). LVEF, LV systolic and diastolic diameters, LV mass index, and ESV and EDV indices improved at follow-up (Table 2 and Figure 3B,C,D). Six individuals had baseline heart rates >100 beats per minute which precluded accurate assessment of diastolic measures, and most had insufficient tricuspid regurgitation which precluded accurate assessment of pulmonary arterial pressures.
TABLE 1.
Baseline characteristics of women with PPCM (n = 53) and strain imaging subset (n = 13)
| Covariate | PPCM (n = 53) | Strain imaging n = 13) |
|---|---|---|
| Age, y | 31 ± 7 | 30 ± 6 |
| Race n, % | ||
| Black/African American | 32, 60.4 | 6, 46.2 |
| Hispanic/Latina | 15, 28.3 | 5, 38.5 |
| Non-Hispanic White | 2, 3.8 | 1, 7.7 |
| Other | 2, 3.8 | 1, 7.7 |
| Southeast Asian | 1, 1.9 | 0, 0 |
| Not reported | 1, 1.9 | 0, 0 |
| Gravity | 3 (2.5) | 3 (1.5) |
| Parity | 2 (1.3) | 1 (0.3) |
| Body mass index ≥30 n, % | 30, 60.0 | 6, 46.2 |
| Chronic hypertension n, % | 13, 24.5 | 2, 15.4 |
| Gestational hypertension n,% | 11, 20.8 | 4, 30.8 |
| Prior preeclampsia n, % | 9, 17.0 | 2, 15.4 |
| Antepartum preeclampsia n,% | 10, 18.9 | 5, 38.5 |
| Chronic diabetes n, % | 3, 5.7 | 0, 0 |
| Gestational diabetes n,% | 8, 15.1 | 2, 15.4 |
| Prior history of PPCM n, % | 7, 13.2 | 1, 7.7 |
| Echocardiography parameters | ||
| LVEF baseline, % | 30 (20, 35) | 30 (25, 35) |
| LVEF at last follow-up, % | 45 (35, 51)a | 45 (35, 55) |
Abbreviations: LVEF = left ventricular ejection fraction; PPCM = peripartum cardiomyopathy.
Missing in 12.
TABLE 2.
Echocardiographic variables in the strain imaging subset (n = 13)
| Baseline | Last follow-up | P value | |
|---|---|---|---|
| Septal thickness, mm | 6.8 (6.2, 8.2) | 7.7 (6.9, 9.3) | .529 |
| LV posterior wall thickness, mm | 7.7 (7.4, 8.7) | 8.2 (7.6, 8.4) | .944 |
| LV diastolic diameter, mm | 58 (51, 64) | 49 (45, 54) | .028 |
| LV systolic diameter, mm | 44 (42, 56) | 36 (34, 40) | .046 |
| EDV index, mL/m2 | 68.5 (64.0, 122.6) | 65.3 (60.0, 95.5) | .016 |
| ESV index, mL/m2 | 47.3 (36.3, 85.3) | 40.0 (34.4, 63.4) | .016 |
| LVEF, % | 30 (25, 35) | 45 (35, 55) | <.001 |
| LV mass index, g/m2 | 89.7 (66.0, 110.4) | 63.6 (56.3, 83.8) | .017 |
| LA volume index, mL/m2 | 31.9 (29.9, 38.3) | 28.6 (23.6, 30.3) | .311 |
| GLS, % | −13.2 (−14, −7.6) | −13.3 (−16.1, −12.3) | .033 |
| SBP, mm Hg | 117 ± 19 | 111 ± 14 | .399 |
| HR, bpm | 92 (77–105) | 75 (67–81) | .023 |
Note: Data are presented as median and interquartile range or mean and standard deviation.
Abbreviations: EDV = end-diastolic volume; ESV = end-systolic volume; GLS = global longitudinal strain; HR = heart rate; LA = left atrium; LV = left ventricular; LVEF = left ventricular ejection fraction; SBP = systolic blood pressure.
FIGURE 3.
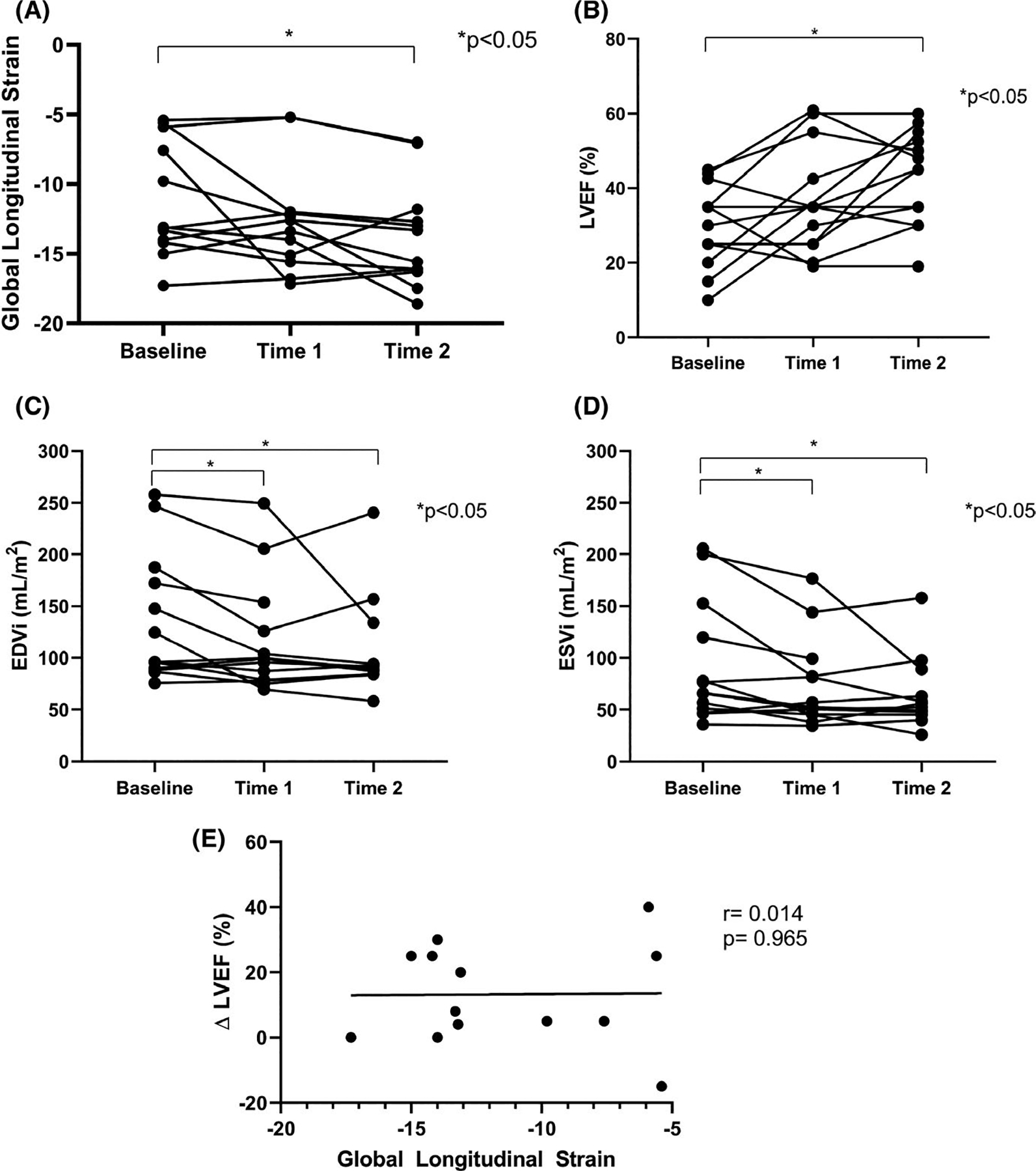
(A) GLS at baseline, follow-up time 1 and time 2. There was a statistically significant improvement in GLS over time, but most values were indicative of persistently mild to severely reduced GLS. (B) LVEF at baseline, time 1 and time 2. There were statistically significant increases in LVEF over time. (C) EDVi at baseline, time 1 and time 2. There were statistically significant decreases in EDVi over time. (D) ESVi at baseline, time 1 and time 2. There were statistically significant decreases in ESVi over time. (E) GLS vs change (Δ) in LVEF between baseline and time 2 (2.3 ± 3.1 y). There was no major correlation between the two measures. Time 1 = 0.7 ± 1.3 y. Time 2 = 2.3 ± 3.1 y. Several participants had identical LVEF values which overlap in the figure at each time point. One participant had measures of LVEF at baseline and time 2. EDVi = end-diastolic volume index; ESVi = end-systolic volume index; GLS = global longitudinal strain; LVEF = left ventricular ejection fraction
Two individuals underwent left ventricular assist device implantation. One recovered to LVEF >50% but had a persistently severe GLS −7.0%. The second recovered to a moderately depressed LVEF 30% with a persistently mild GLS −12.7%. Baseline GLS was not different by race-ethnicity (Black −13.65, 95% CI (−14, −9.8) vs Hispanic/Latina −7.6, 95% CI (−13.2, −5.9) vs white −13.1 vs other −15.0, P =.524), nor was the GLS at last follow-up (Black −12.8, 95% CI (−16.1, −11.8) vs Hispanic/Latina −13, 95% CI (−16.1, −12.7), vs white −18.6 vs other −15.6, P =.432).
Median GLS at baseline was −13.2 (−14, −7.6) % and −13.3 (−16.1, −12.3) % at last follow-up (P =.033), indicating statistically significant, but not clinically significant improvement in strain. Restricting analysis to the 8 women with a baseline GLS above −10% eliminated the statistically significant improvement in strain at last follow-up (baseline GLS −14.0 (−14.6,−13.3) % vs last follow-up, −15.9, (−16.8, −13.2) %, P =.484); thus, improvement in GLS was driven by those individuals with the most abnormal values. Despite improvement of echocardiographic LVEF to ≥50% in 4/13 at 1 year, GLS was persistently abnormal in all cases. Baseline GLS was not correlated with change in LVEF (Figure 3E).
4 |. DISCUSSION
Global longitudinal strain is an emerging measure applicable to a wide range of left ventricular heart disease, is more reliable than LVEF, particularly when evaluating serial measures, has high precision, is more reproducible than traditional measures such as E, A, and e’, and provides additional prognostic value.9,17 Worsened strain is an early marker of cardiac dysfunction prior to clinical heart failure which has become particularly useful in evaluating cardiotoxicity of chemotherapy, and its applicability to other subclinical cardiomyopathies is of great interest given the relative ease and low cost of integrating strain measures into the workflow of usual echocardiographic imaging.9 Having GLS cut points in pregnancy would be useful in discerning pathologic vs normal symptoms of pregnancy and ruling out PPCM or other pathologic changes.18 We have incomplete understanding of normal GLS in pregnancy as there are no established reference ranges.
A previous study from our center of 74 healthy pregnant women indicated that values of GLS were unchanged in pregnancy and only the time to peak strain rate for diastolic global radial strain rate and diastolic global longitudinal strain rate were increased in the 2nd and 3rd trimester.19,20 In a separate single-center study of 68 women, GLS and LVEF significantly decreased in late pregnancy but rebounded to baseline after delivery.21 Thus, there is controversy in how GLS changes even in healthy pregnancy. Change in strain may be different by trimester, single vs multiple gestations, maternal age, and by other co-morbidities in pregnancy, like diabetes, in which GLS is reduced.22 Identifying women at risk or predicting the prognosis of established PPCM would be useful in disease management.
In our study, we found many causes of peripartum acute congestive heart failure or pulmonary edema in mostly race-ethnic minority women living in an urban community. Of those with LVEF ≤45%, almost half were due to PPCM. Despite improvement in LVEF, EDV, and ESV indices over time, GLS remained mildly abnormal in the subset having images suitable for strain analysis. Baseline GLS did not correlate with the absolute change in LVEF. GLS, EDV, and ESV indices have been described as more sensitive measures of myocardial change than LVEF, and this may be particularly relevant in PPCM.9
Due to the small sample size, we did not relate change in GLS to clinical outcomes. For perspective, a previous multi-center study in the IPAC consortium enlisted 30 centers to enroll 90 cases, while we identified 53 cases over 15 years in a single center. The IPAC study, the only prospective study of GLS in PPCM to date, showed that GLS improved prediction of recovery above and beyond LVEF at 1 year of follow-up, and women also had persistent mildly abnormal GLS.11 Our findings confirm and extend the IPAC study conclusions in a different cohort, only the second study of which we are aware, to investigate serial echocardiographic measurements of women with PPCM. We add new data on change in ESV and EDV indices over time and information about Hispanic/Latina women, albeit, a small number.
While alterations of strain patterns in patients with PPCM have been previously described, the persistence of abnormal GLS despite normalization of LVEF and volumes is a more recent finding, the implication of which is unclear. A potential explanation for persistently abnormal GLS is that there is diffuse microscopic fibrosis as a response to edema or injury; however, a cardiac MRI study of patients with PPCM failed to show evidence of scarring.23 Alternatively, women susceptible to developing PPCM could have preexisting alterations in strain. Prospective studies of peripartum cardiomyopathy with a standardized echocardiography protocol and repeated measures would be best for future studies of regional strain. How GLS relates to subsequent risk of recurrent PPCM and lifetime risk of clinical heart failure requires additional investigation.
5 |. LIMITATIONS
The retrospective design of this study is an inherent limitation and only a subset of patients had repeat echocardiograms adequate for strain analysis. While the subset was comparable to the main cohort, we cannot exclude selection or survival bias. In a previous study, Black women were more likely to have persistently abnormal GLS than whites; however, the high proportion of race-ethnic minorities in our sample precluded comparisons with white women. Hispanic/Latina was self-reported as a race category and, thus, is inclusive of Black and white individuals. We used the IPAC definition for PPCM, but additionally excluded some individuals with HIV, eclampsia, arrhythmia, and autoimmune disease as other potential causes of cardiomyopathy, which may have omitted some true cases.
6 |. CONCLUSIONS
Global longitudinal strain is a sensitive method to identify myocardial dysfunction. In this series of mostly race-ethnic minority women with peripartum cardiomyopathy, GLS was not correlated with change in LVEF. At mean follow-up of 1.2 ± 0.7 years, GLS remained persistently abnormal, despite LVEF recovery. Future studies should investigate the long-term clinical significance of persistently abnormal GLS in women with PPCM.
Funding information
AEB recognizes support from the Empire Clinical Research Investigator Program, American Heart Association Mentored and Clinical Population Research Award 17MCPRP33630098, National Institutes of Health (NIH)/National Center for Advancing Translational Science Einstein-Montefiore CTSA Grant UL1TR001073, and K23 HL146982 NIH National Heart, Lung and Blood Institute
Footnotes
CONFLICT OF INTEREST
AEB has contracted research with Abbott, Inc and CSL-Behring for which Montefiore Medical Center receives compensation.
DATA AVAILABILITY STATEMENT
Upon request.
REFERENCES
- 1.Bhattacharyya A, Basra SS, Sen P, et al. Peripartum cardiomyopathy: a review. Tex Heart Inst J. 2012;39:8–16. [PMC free article] [PubMed] [Google Scholar]
- 2.Sliwa K, Hilfiker-Kleiner D, Petrie MC, et al. Current state of knowledge on aetiology, diagnosis, management, and therapy of peripartum cardiomyopathy: a position statement from the heart failure association of the European society of cardiology working group on peripartum cardiomyopathy. Eur J Heart Fail. 2010;12:767–778. [DOI] [PubMed] [Google Scholar]
- 3.McNamara DM, Elkayam U, Alharethi R, et al. Clinical outcomes for peripartum cardiomyopathy in north america: results of the ipac study (investigations of pregnancy-associated cardiomyopathy). J Am Coll Cardiol. 2015;66:905–914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Sliwa K, Mebazaa A, Hilfiker-Kleiner D, et al. Clinical characteristics of patients from the worldwide registry on peripartum cardiomyopathy (PPCM). Eur J Heart Fail. 2017;19:1131–1141. [DOI] [PubMed] [Google Scholar]
- 5.Arany Z Understanding peripartum cardiomyopathy. Annu Rev Med. 2018;69:165–176. [DOI] [PubMed] [Google Scholar]
- 6.Irizarry OC, Levine LD, Lewey J, et al. Comparison of clinical characteristics and outcomes of peripartum cardiomyopathy between African American and Non-African American Women. JAMA Cardiol. 2017;2:1256–1260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Arany Z, Elkayam U. Peripartum cardiomyopathy. Circulation. 2016;133:1397–1409. [DOI] [PubMed] [Google Scholar]
- 8.Bauersachs J, Arrigo M, Hilfiker-Kleiner D, et al. Current management of patients with severe acute peripartum cardiomyopathy: practical guidance from the heart failure association of the european society of cardiology study group on peripartum cardiomyopathy. Eur J Heart Fail. 2016;18:1096–1105. [DOI] [PubMed] [Google Scholar]
- 9.Potter E, Marwick TH. Assessment of left ventricular function by echocardiography: the case for routinely adding global longitudinal strain to ejection fraction. JACC Cardiovasc Imaging. 2018;11:260–274. [DOI] [PubMed] [Google Scholar]
- 10.Briasoulis A, Mocanu M, Marinescu K, et al. Longitudinal systolic strain profiles and outcomes in peripartum cardiomyopathy. Echocardiography. 2016;33:1354–1360. [DOI] [PubMed] [Google Scholar]
- 11.Davis EM, Ewald G, Givertz MM, et al. Maternal obesity affects cardiac remodeling and recovery in women with peripartum cardiomyopathy. Am J Perinatol. 2018;36(05):476–483. [DOI] [PubMed] [Google Scholar]
- 12.Bellin E, Fletcher DD, Geberer N, et al. Democratizing information creation from health care data for quality improvement, research, and education-the montefiore medical center experience. Acad Med. 2010;85:1362–1368. [DOI] [PubMed] [Google Scholar]
- 13.Sliwa K, Petrie MC, van der Meer P, et al. Clinical presentation, management, and 6-month outcomes in women with peripartum cardiomyopathy: an ESC EORP registry. Eur Heart J. 2020;41:3787–3797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Harris PA, Taylor R, Thielke R, et al. Research electronic data capture (RedCap)–a metadata-driven methodology and workflow process for providing translational research informatics support. J Biomed Inform. 2009;42:377–381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Dubois DDE. A formula to estimate the approximate surface area if height and weight be known. Arch Intern Med. 1916;17:863–871. [Google Scholar]
- 16.Menting ME, McGhie JS, Koopman LP, et al. Normal myocardial strain values using 2d speckle tracking echocardiography in healthy adults aged 20 to 72 years. Echocardiography. 2016;33:1665–1675. [DOI] [PubMed] [Google Scholar]
- 17.Farsalinos KE, Daraban AM, Ünlü S, et al. Head-to-head comparison of global longitudinal strain measurements among nine different vendors: the EACVI/ASE inter-vendor comparison study. J Am Soc Echocardiogr. 2015;28:1171–1181, e1172. [DOI] [PubMed] [Google Scholar]
- 18.Ajmi H, Abid D, Milouchi S, et al. Interest of speckle tracking in the detection of cardiac involvement in pregnant women with hyper-tensive disorder. Pregnancy Hypertens. 2018;11:136–141. [DOI] [PubMed] [Google Scholar]
- 19.Ando T, Kaur R, Holmes AA, et al. Physiological adaptation of the left ventricle during the second and third trimesters of a healthy pregnancy: a speckle tracking echocardiography study. Am J Cardiovasc Dis. 2015;5:119–126. [PMC free article] [PubMed] [Google Scholar]
- 20.Tso GJLJ, Shaban NM, Lui GK, Trivedi HA, Cohen MNBP, Taub CC. Normal echocardiographic measurements in uncomplicated pregnancy, a single center experience. J Cardiovasc Dis. 2014;5:3–8. [Google Scholar]
- 21.Cong J, Fan T, Yang X, et al. Structural and functional changes in maternal left ventricle during pregnancy: a three-dimensional speckle-tracking echocardiography study. Cardiovasc Ultrasound. 2015;13:6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Meah VL, Backx K, Cockcroft JR, et al. Left ventricular mechanics in late second trimester of healthy pregnancy. Ultrasound Obstet Gynecol. 2019;54:350–358. [DOI] [PubMed] [Google Scholar]
- 23.Schelbert EB, Elkayam U, Cooper LT, et al. Myocardial damage detected by late gadolinium enhancement cardiac magnetic resonance is uncommon in peripartum cardiomyopathy. J Am Heart Assoc. 2017;6. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Upon request.


