Abstract
Fetal supraventricular tachycardia management is challenging, with consequences for both the fetus and the mother. If left untreated, fetal hydrops may ensue, at which point delivery and treatment of the arrhythmia is preferred. However, if the fetus is not at term nor near-term, significant doses of antiarrhythmics may be needed to achieve adequate transplacental bioavailability. Although digoxin has classically been the mainstay of treatment, the use of flecainide or sotalol as monotherapy or in combination with digoxin is being studied. Interdisciplinary team management and shared decision-making between the physician and patient are key to achieving successful outcomes. Adult cardiologists, particularly inpatient consultation services or through burgeoning cardio-obstetrics programs, may, in some practice settings, be asked to evaluate or co-manage pregnant women with fetal arrhythmia.
Keywords: tachycardia, pregnancy, fetal, arrhythmia
Fetal supraventricular tachycardia (SVT) is an uncommon phenomenon that can lead to fetal hydrops and intrauterine demise. The diagnosis and treatment of this pathology has been a source of ongoing research, with a specific focus on identifying the most efficacious, first-line transplacental anti-arrhythmic drug. In 2014, the American Heart Association released a comprehensive report outlining current diagnostic and treatment modalities for fetal cardiac disease, including fetal arrhythmias.1 Pregnant women are prescribed antiarrhythmic drugs to achieve therapeutic transplacental levels and are monitored for toxicity. There are a wide variety of management paradigms for pregnancies complicated by fetal tachycardia, and physician involvement may span maternal-fetal medicine (MFM), obstetrics, and fetal and adult cardiology. Cardio-obstetric programs are becoming more widespread and adult cardiologists may be consulted for evaluation or co-management of pregnant women with fetal arrhythmias. While fetal cardiologists often treat both the mother and fetus alongside physicians and obstetricians without adult cardiology involvement, there may be practice settings where adult cardiologists can provide additional support on the maternal side, as adult cardiologists are familiar with dosing and the side effects of anti-arrhythmic drugs.
Admission to an MFM antepartum service is often recommended with cardiology co-management. Maternal telemetry is required at initiation of treatment to monitor for arrhythmias, heart block, and prolongation of the QT interval. Fetal and maternal well-being are closely monitored during the treatment course, since both are at risk for complications.2 In this review, we aim to summarize the current evidence and highlight how adult cardiologists can support their MFM, obstetrician, and fetal cardiology colleagues in the care of pregnant women undergoing transplacental treatment of fetal SVT.
EPIDEMIOLOGY
Fetal arrhythmias are reported in up to 3% of pregnancies, of which two-thirds are due to SVTs.2 Persistent SVT eventually leads to impaired diastolic filling and a reduced compliance of the fetal myocardium. In turn, this causes elevated atrial and central venous filling pressures. Severe congestive heart failure of the fetus and subsequent fetal hydrops can ensue. Fetal hydrops is the result of high output cardiac failure and is defined as an abnormal accumulation of fluid in two or more fetal compartments, including ascites, pleural effusion, pericardial effusion, and skin edema. In some patients it may also be associated with polyhydramnios (an amniotic fluid index greater than 25 cm) and placental edema.3 Associated placental edema causes impaired transport of oxygen and fetal hypoxia.4 Absorption of drugs through the placenta also becomes unreliable at this stage, necessitating higher anti-arrhythmic doses administered to the pregnant woman.
The fetuses at highest risk of developing heart failure are those with incessant SVT, those with earlier onset of SVT (<32 weeks) and those with structural heart disease, which occurs in up to 10% of SVTs.4 Untreated disease can prompt early or pre-term delivery, and in the case of hydrops, fetal mortality can be as high as 50%.4 Even in treated cases, mortality rates can reach 35% and cause significant perinatal morbidity such as cerebrovascular ischemia and thromboembolism. Anti-arrhythmic medications are generally well tolerated in pregnant women, although serious adverse effects can occur because the maternal serum level may be above the usual therapeutic range in order to achieve transplacental bioavailability.5
DIAGNOSIS
Fetal tachyarrhythmias must be distinguished from tachycardia due to maternal or fetal conditions such as fetal hypoxia, infection, or hyperthyroidism, as differentiating the underlying etiology guides the treatment plan. Fetal electrocardiography (ECG) has limitations, including a low signal-to-noise ratio, unreliable reproducibility, and time intensive setup.1 Magneto-cardiography, under research, uses surface sensors to record electrical activity in a magnetically-shielded environment and subtracts maternal noise on the ECG to favor fetal electrical signals.1 However, this technology is still experimental.
Since surface ECGs are not useful for interpretation of fetal conduction, the echocardiographer can extrapolate conduction through the atrioventricular (AV) node by ultrasound (Figure 1).1 A fetal echocardiogram is currently the gold standard for diagnosing arrhythmias using the M-Mode or time motion display.2 Various ultrasound modalities are useful in this regard, including 2D echo, M-mode, and pulsed wave Doppler. M-mode evaluates the heart rate, relationship of atrial and ventricular contraction, and systolic function. Pulsed wave Doppler also allows measurements of fetal heart rate and the relationship of atrial and ventricular contractions with simultaneous inflow and outflow or venous and arterial sampling. Normal sinus rhythm generally ranges from 110–160 beats per minute (bpm) while SVTs range from 200–300 bpm. Heart rates in this range suggest pathology such as reentrant SVT or atrial flutter. In the case of atrial flutter, atrial rates range from 250–500 bpm. M-mode echocardiography allows for evaluation of the ratio of atrial to ventricular contraction to guide diagnosis. Fetal SVT is characterized by a tachycardia with 1:1 AV conduction, and is the most common cause of sustained fetal tachyarrhythmia, with heart rates commonly reaching more than 220 bpm. The mechanism of the underlying arrhythmia guides therapy. Re-entry tachycardia is commonly diagnosed between 24–32 weeks of gestation and often secondary to an accessory conduction pathway.6 The relationship between fetal ventricular and atrial contractions – the V-A interval – is useful for tachycardia categorization. If the V-A interval is shorter than the A-V interval (so-called short V-A tachycardia), the mechanism is most likely a non-decremental AV accessory pathway. Long V-A tachycardias are less common, and are due to either sinus tachycardia, ectopic atrial tachycardia, or a permanent junctional reciprocating tachycardia (Figure 2). The hallmark of fetal atrial flutter is more atrial than ventricular contractions on echocardiogram.
Figure 1:
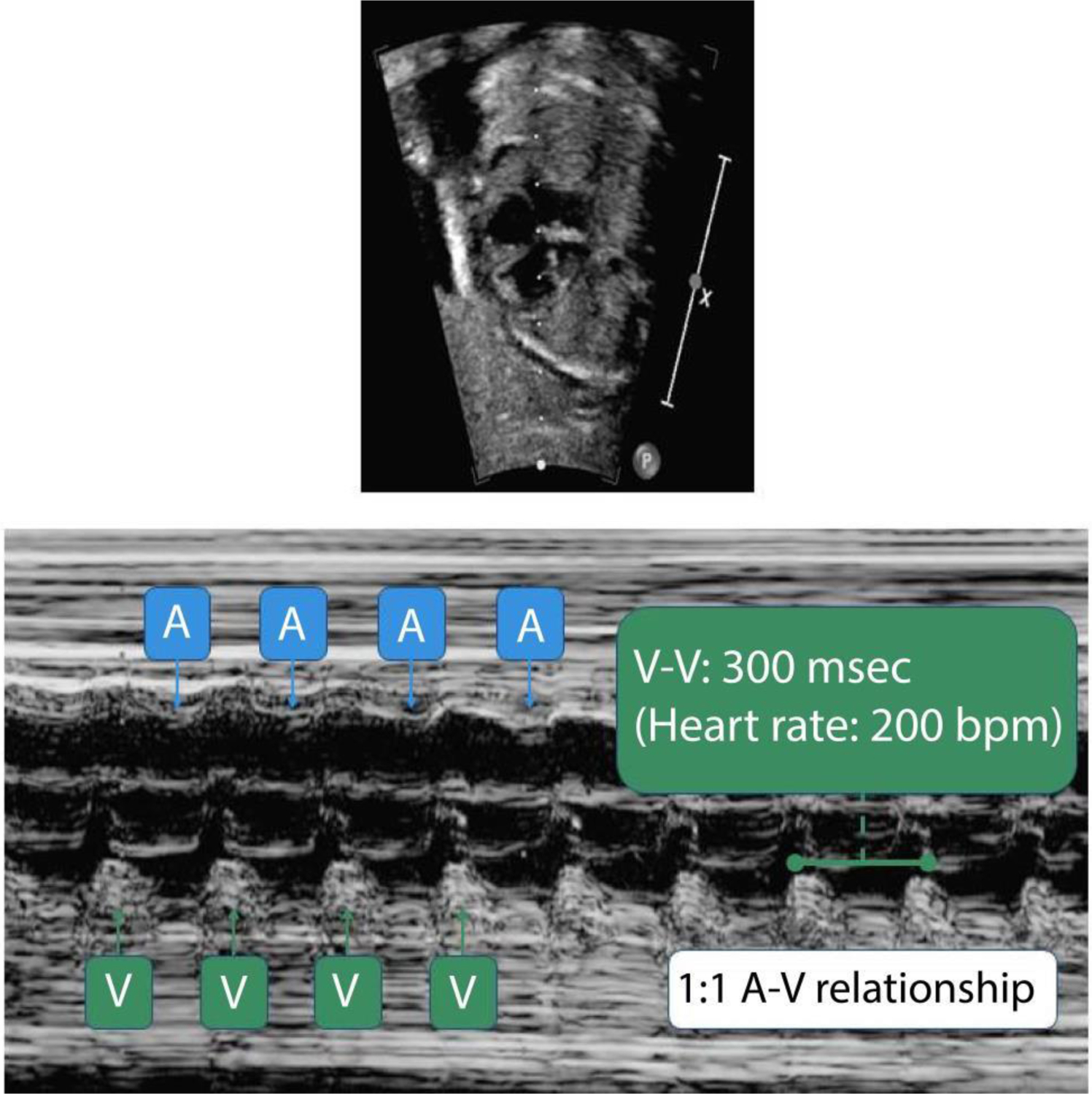
Fetal echocardiogram demonstrating a rate of 200 beats per minute (bpm) on the pulse wave Doppler with severe biventricular dysfunction. Normal fetal heart rate ranges from 110–160 bpm, while supraventricular tachycardia from 200–300 bpm. msec, milliseconds.
Figure 2:
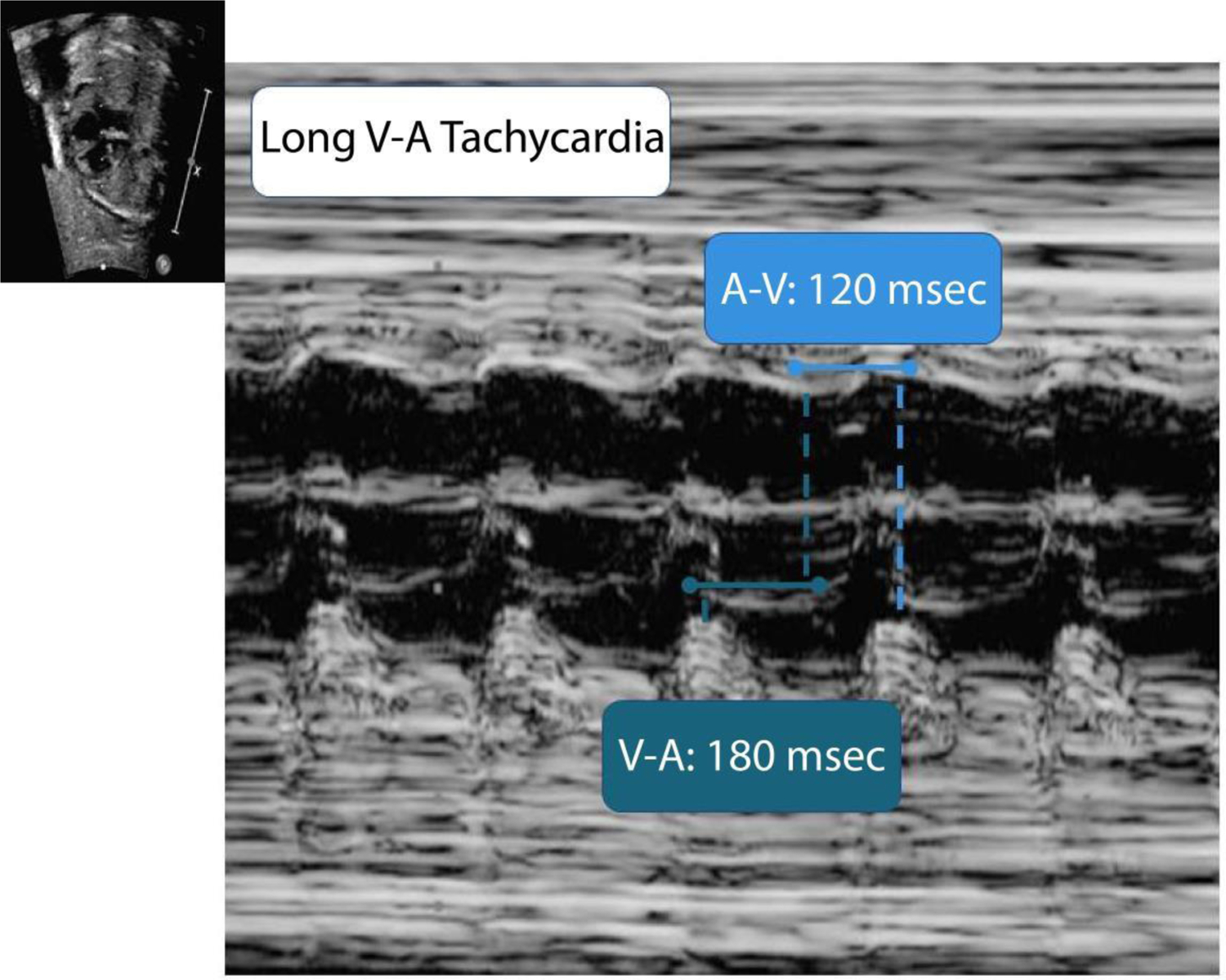
Fetal echocardiogram demonstrating a long ventriculo-atrial (V-A) interval tachycardia. More atrial than ventricular contractions indicates fetal atrial flutter. Msec, milliseconds.
However, the fetal echocardiogram is limited in that it evaluates rhythm abnormalities based on mechanical findings rather than electrical signals, and it may not capture transient, intermittent arrhythmias. Additionally, the R-P relationship observed in fetal SVT is only a guide and starting point in elucidating the arrhythmia mechanism— it does not represent a definitive diagnosis.
TREATMENT
The treatment goal for fetal arrhythmias is to prevent or reverse fetal hydrops and ventricular dysfunction, and ultimately, to prevent fetal demise. Achieving complete restoration of normal sinus rhythm is not necessary to avoid fetal hydrops, and rate control is the priority.1 Ideally, fetuses at or near term should be delivered if fetal hydrops is diagnosed. If delivery is not feasible, transplacental pharmacologic therapy administered to the mother via either the oral or intravenous route is recommended. However, it should be noted that transplacental therapy poses special challenges given the incomplete transfer of agents across the placenta. This is due to a combination of shifting maternal circulating volumes throughout each trimester, increased maternal drug metabolism, increased maternal renal clearance as gestational age progresses, and potential placental edema, thus, medication doses have to be adjusted.4 Potentially maternal toxic doses of medications are required to reach post-placental therapeutic levels. Assessing, mitigating and monitoring maternal risk for toxicity is a niche for adult cardiologists who may be involved in these cases. Direct fetal treatment, bypassing the maternal circulation via intramuscular buttock, thigh, or intracordal routes, have been reported to restore sinus rhythm successfully and may be reasonable in cases of severe hydrops and incessant SVT, but these options have also been associated with fetal death.7 Fetal toxicity, bradycardia, and fetal cardiac arrest are among the adverse outcomes associated with direct fetal treatment.7
The ideal first-line treatment for SVT is a subject of current research. Digoxin has traditionally been favored given its clearly established side effect profile, long history and practicality of use.1 Newer data have posited flecainide as first-line therapy.1, 7–9 A meta-analysis of retrospective observational studies reported that flecainide may be no different than digoxin in efficacy, and shows no difference in the rate of maternal side effects or fetal demise.10, 11 Other options include sotalol and amiodarone. For hydropic fetuses, an evolving observation is that digoxin may be less effective, and therapy with antiarrhythmics such as flecainide and/or sotalol may be preferred.12, 13
The Fetal Atrial Flutter and Supraventricular Tachycardia (FAST) Therapy trial is a prospective multicenter, randomized, controlled trial designed to investigate fetal SVT therapy.14 The trial aims to enroll 600 patients, with the primary aim of determining the probability of a normal pregnancy outcome after treatment started with digoxin or sotalol (atrial flutter without hydrops); digoxin or flecainide (SVT without hydrops); or digoxin plus sotalol or digoxin plus flecainide (SVT with hydrops). Lastly, the role of maternal hyperglycemia in the treatment of the hydropic fetus with SVT is under investigation. Hyperglycemia is hypothesized to support the health of the fetal myocardium, although it poses risks for fetal development, like macrosomia, and if prolonged, fetal demise.4, 15 Prolonged maternal hyperglycemia can inflict a maternal risk of diabetes and result in end organ damage to maternal kidneys eyes, and cardiovascular system, thus, a hemoglobin A1c <6% is generally recommended for all pregnancies.
Digoxin
Digoxin is a well-established anti-arrhythmic commonly used in adult clinical cardiology practice. The mechanism of action of digoxin in SVTs is primarily via enhanced vagal tone inhibiting atrioventricular nodal conduction, increasing the effective refractory period, shortening the atrial effective refractory period, and decreasing conduction velocity (Figure 3). The suggested digoxin loading dose is 1 mg orally or intravenously in divided doses over a 24-h period, with a maintenance dose of 0.25 mg by mouth twice a day to achieve a therapeutic range of 0.7–2 ng/mL. The serum digoxin level should be monitored at least 6 hours after a dose adjustment.9 Maternal metabolism of drugs may be enhanced and the volume of distribution changes throughout pregnancy, necessitating regular follow-up of blood levels and ECGs. Digoxin is renally excreted, and in patients with kidney disease, a lower loading dose with more frequent serum level and ECG monitoring may be warranted, particularly in those with a history of autoimmune, diabetic or hypertensive kidney disease. Potential side effects seen at these higher doses include visual changes (alteration in color vision, scotoma, or blindness), gastrointestinal effects (anorexia, nausea, vomiting, abdominal pain), or mental/cognitive disturbances (weakness, confusion, disorientation, or delirium). Symptoms are usually seen at toxic levels greater than 3 ng/mL. ECG changes such as sinus bradycardia, first-degree block and Mobitz I second-degree block were reported in 18.2% of women with a digoxin level greater than 2 ng/mL in a retrospective case series.8 These abnormalities reversed after withdrawing therapy. ECG changes, like premature ventricular complexes, atrial tachycardia, accelerated junctional tachycardia, or ventricular tachycardia, may portend sudden cardiac death or ventricular fibrillation, and the goal of frequent monitoring is to recognize impending signs and symptoms of maternal toxicity.
Figure 3:
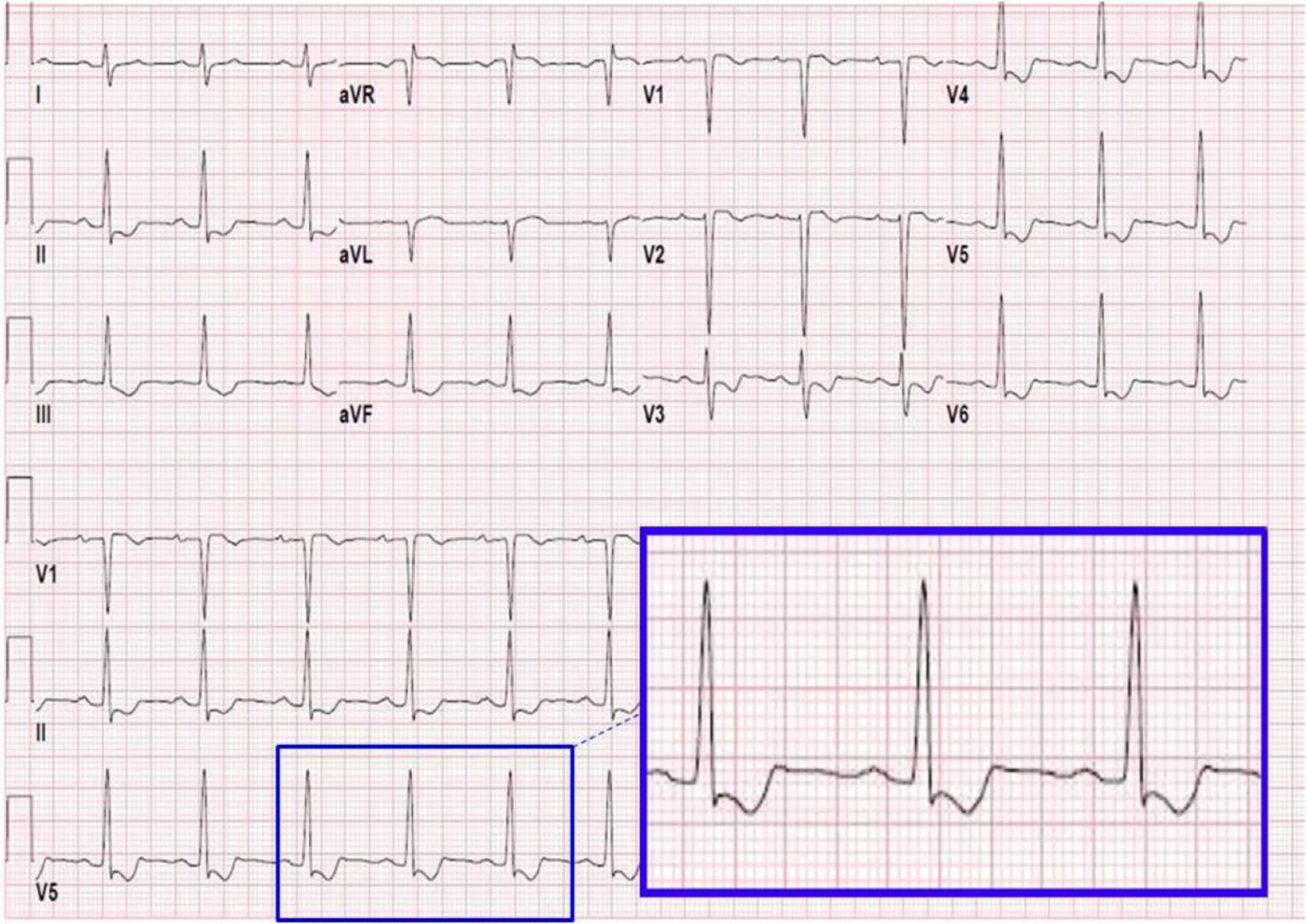
Evidence of digoxin effect with “slurred” S waves on the maternal electrocardiogram (inset). Frequent ECG and symptom monitoring are required in transplacental treatment of fetal supraventricular tachycardia due to potential for maternal toxicity with anti-arrhythmics.
Flecainide
Flecainide is a Class Ic antiarrhythmic which blocks the fast inward sodium channels during the action potential, prolonging the refractory period of the AV node. This blockade also shortens the duration of action potentials through the Purkinje fibers. Two meta-analyses have shown flecainide to be effective in achieving fetal sinus rhythm.1, 7 In a retrospective observational study, 81.2% of fetuses treated with flecainide as a first-line therapy (flecainide or digoxin with flecainide) converted to sinus rhythm with a median time to conversion of 3 days (Figure 4).13 A retrospective study showed that 15 out of 17 fetuses treated with flecainide converted to sinus rhythm.16 A flecainide dose of 100 mg orally three times a day has been most commonly used. If trough levels are available, the dose is adjusted to attain a therapeutic level of 0.2–1 μg/mL, and the daily dose should not exceed 600 mg. Side effects include dizziness, vision changes, nausea, constipation and abdominal pain. Toxicity is suggested when there is a new bundle branch block or QTc prolongation to >0.48 s. Its use is contraindicated in patients with coronary artery disease, myocardial infarction, cardiogenic shock, bifascicular or second/third degree AV block, and left ventricular dysfunction. Thus, a detailed clinical history, baseline ECG and echocardiogram should be obtained, in addition to any further cardiac testing, to rule out existing maternal heart failure or coronary artery disease. Flecainide dosing must be adjusted in patients with kidney disease, may have slower elimination in patients with hepatic impairment, and women with abnormal baseline chemistries may benefit from more frequent monitoring.
Figure 4:
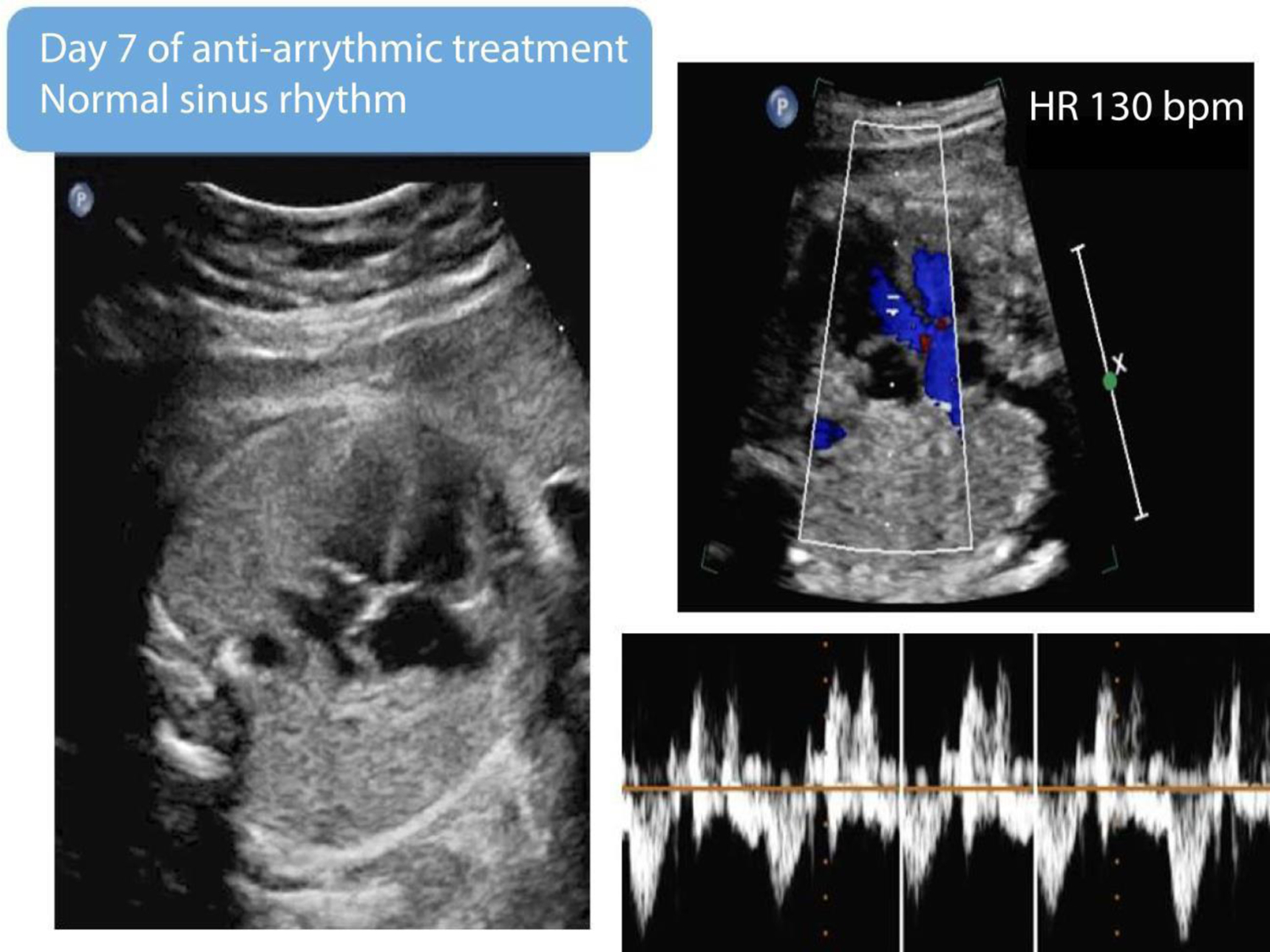
After transplacental therapy with digoxin and flecainide, a repeat fetal echocardiogram demonstrated a rate of 130 beats per minute (bpm) with 1:1 atrioventricular conduction, consistent with normal sinus rhythm.
Sotalol
Sotalol is a class III antiarrhythmic with beta-blocker properties. The main mechanism of action is blockade of the rapid component of the delayed rectifier potassium current, which occurs during phase 3 of the action potential. The effect of sotalol on the action potential is associated with reverse use dependency, which is defined as an inverse correlation between the heart rate and the QT interval. Sotalol’s antiarrhythmic action is more pronounced at lower heart rates, causing the QT interval to be more prolonged as the heart rate slows and explaining the association between bradycardia and antiarrhythmic drug-induced torsades-de-pointes (TdP). MFM and obstetric physicians should be aware of potential drug-drug interactions which prolong the QT, including certain anti-depressants, anesthetics, antibiotics and the common induction agent, oxytocin. Fetal and adult cardiologists can help the team avoid QT prolonging drugs. Sotalol is renally excreted and dosing should be adjusted in patients with advanced kidney disease.
Sotalol has been evaluated as a first-line drug in fetal SVT with hydrops at a starting dose of 80 to 160 mg orally twice daily, which can be up-titrated slowly. Adverse effects are nausea, vomiting, dizziness and lightheadedness. Signs of sotalol toxicity include new bundle branch block or QTc prolongation >0.48 s. Like flecainide, sotalol can have a pro-arrhythmic potential and should be used judiciously in patients with preexisting arrhythmias or QTc prolongation. Caution should be taken given its narrow therapeutic window, as the incidence of TdP is dose dependent. A large case series found that digoxin and flecainide were superior in conversion of fetal reentrant SVT, while sotalol was superior for fetuses with atrial flutter.17 Conversion to sinus rhythm at day 10 occurred in 63% of treated fetuses in SVT, but only in 41% of treated atrial flutter cases.17 In cases of atrial flutter, sotalol had superior conversion rates. An 85% response rate was demonstrated when sotalol was used alone or combined with digoxin in fetuses diagnosed with SVT or atrial flutter.18 Sotalol should be used cautiously in women who are relatively bradycardic.
MATERNAL CONSIDERATION
Prior to initiating therapy, a thorough history, physical examination, and ECG should be obtained from the pregnant woman. Special interest should focus on concurrent medications, a family history of cardiac disease or sudden cardiac death, a history of long QT syndrome, ECG evidence of prolonged QTc or a history of drug-induced TdP. A baseline maternal echocardiogram is useful to rule out heart disease, especially if flecainide is being considered. Initiating anti-arrhythmic therapy with inpatient telemetry is recommended for the first 24–48 hours to monitor for dysrhythmia and QTc prolongation. Of note, 78% of mothers starting anti-arrhythmic therapy may experience adverse events which include nausea, vomiting, ECG changes, and elevated brain natriuretic peptide concentrations.19
Laboratory tests should be examined to rule out secondary causes of fetal arrhythmia and evaluate maternal organ function. A metabolic panel including evaluation of calcium, magnesium, potassium, and thyroid-stimulating hormone should be examined for any abnormalities which may explain arrhythmia or exacerbate QT abnormalities.1 Monitoring of the blood glucose level should be followed to rule out gestational diabetes. Currently, this is a two step process: an initial glucose challenge test with 50 grams of glucose, followed by a 1-hour blood serum glucose analysis. Values > 140 mg/dL are considered screen positive and result in further investigation with a 3-hour glucose tolerance test of 100 grams of glucose. Two out of four positive values meet the criteria for a diagnosis of gestational diabetes: fasting >95mg/dL, 1 hour >180 mg/dL, 2 hour >155 mg/dL and 3 hour >140 mg/dL.
Once target therapeutic levels and fetal heart rates are achieved, weekly fetal echocardiograms are recommended to evaluate for treatment failure, since recurrence rates between 8–15% are reported.17 Home fetal Doppler monitors may be considered in cases with high suspicion of treatment failure, allowing for the mother to more rapidly detect fetal tachycardia (heart rate > 160 bpm).6 Prior to discharge, mothers should be counseled on pharmacologic side effects. Maternal ECGs and serum drug levels should be obtained approximately every two weeks. Home blood pressure monitoring may be useful because the signs and symptoms of preeclampsia overlap with anti-arrhythmic drug toxicity, including maternal headache, visual changes, nausea, vomiting, and it may be difficult to differentiate the two. If signs/symptoms develop, patients may also be assessed for preeclampsia with laboratory chemistries (serum creatinine, liver function tests, uric acid, and platelet levels), blood pressure monitoring, and physical exam.
Avoiding preeclampsia is especially important for mothers who receive treatment for fetal hydrops, since renal insufficiency induced by preeclampsia with severe features can impair drug clearance for many anti-arrhythmics. Aspirin therapy has been shown to be effective in reducing preeclampsia risk due to salutary effects on placental health, particularly when initiated before 28 weeks, and is recommended in higher risk women with chronic medical conditions, such as those with diabetes, chronic hypertension and renal disease.20, 21 Adult cardiologists should be aware of this unique indication for aspirin in women, based on risk factors for preeclampsia as outlined by the American College of Obstetrics & Gynecology. Therapeutic strategies and patient monitoring should be undertaken with interdisciplinary input, particularly with respect to delivery planning. MFM specialists, and fetal and adult cardiologists can be helpful in his regard, and patient preferences must be considered.1, 15
CONCLUSION
Fetal SVT is an uncommon phenomenon that can lead to fetal hydrops and intrauterine demise. Delivery is imperative for full-term, hydropic infants, and then management of the arrhythmia can be accomplished postpartum. Otherwise, transplacental therapy via maternal administration of antiarrhythmic therapy is a consideration. Digoxin, flecainide, or sotalol, including combination therapy for incessant SVT have been used, and trials are ongoing to determine which drug(s) should be first-line therapy. Given incomplete transplacental transfer of these medications, higher doses must be administered to the pregnant woman, necessitating close monitoring with serial ECGs or telemetry for cardiotoxicity. Fortunately, the most frequent maternal adverse effects are minor gastrointestinal symptoms, resolving after dose reduction. A multi-disciplinary, team-based strategy is essential for achieving safe and successful maternal and fetal outcomes. Adult cardiologists, particularly in settings where they support MFM, obstetricians and fetal cardiologists in the care of high-risk women, should be aware of maternal considerations in the treatment of fetal SVT.
Figure 5:
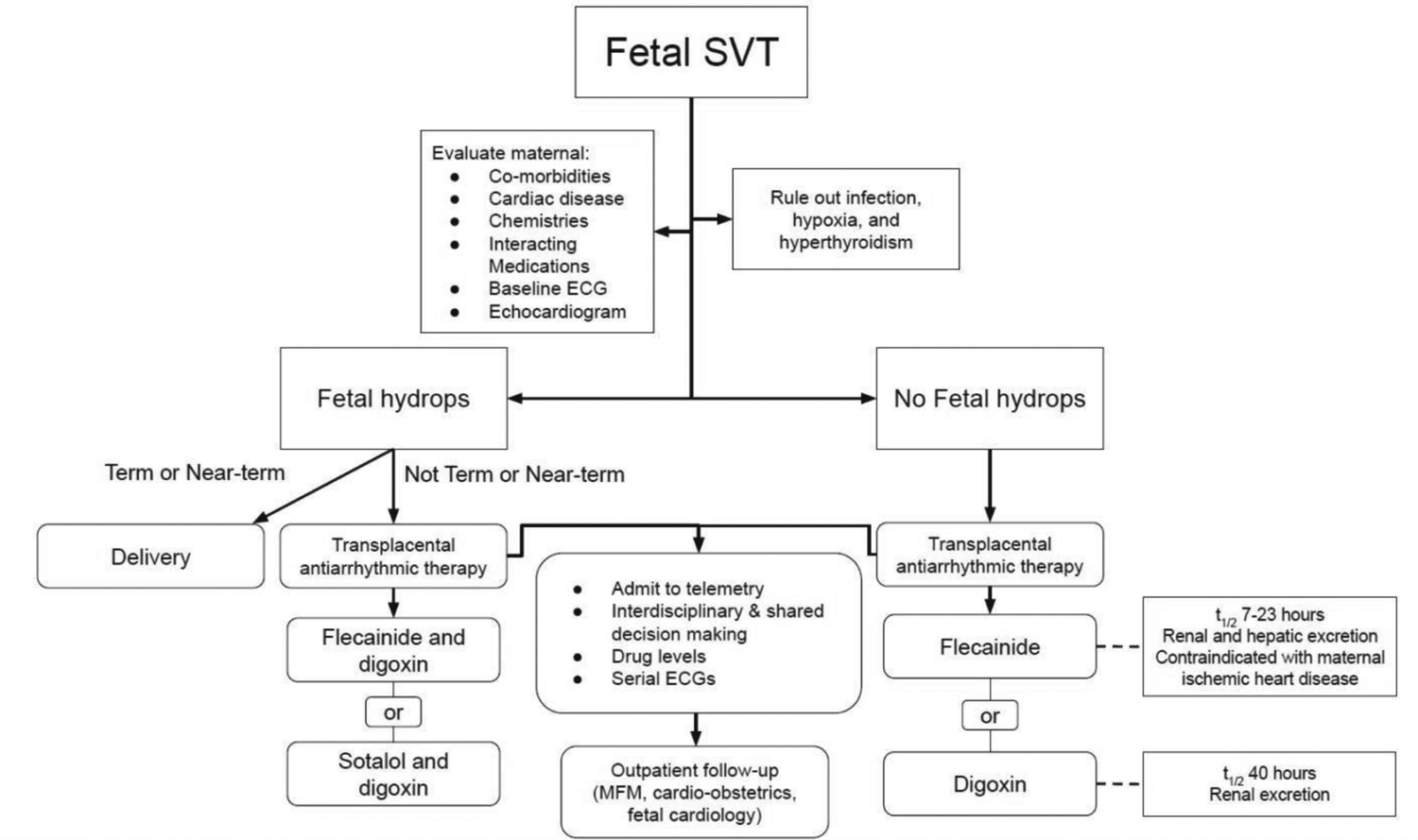
Overview of maternal evaluation and potential treatment algorithm when caring for pregnant patients with fetal SVT. SVT, supraventricular tachycardia; ECG, electrocardiogram; MFM, maternal-fetal medicine.
Funding:
AEB acknowledges support from an American Heart Association Mentored and Clinical Population Research Award 17MCPRP33630098 and National Institutes of Health, NHLBI grant K23HL146982.
Footnotes
Disclosures: AEB served as site principal investigator for multi-center trials sponsored by Abbott, AstraZeneca, Sanofi-Aventis, CSL-Behring, for which her institution received compensation and received an honorarium from ClearView Healthcare Partners, LLC.
The authors have no conflict of interest.
REFERENCES
- 1.Donofrio MT, Moon-Grady AJ, Hornberger LK, et al. Diagnosis and treatment of fetal cardiac disease: a scientific statement from the American Heart Association. Circulation 2014;129:2183–242. [DOI] [PubMed] [Google Scholar]
- 2.Detterich JA, Pruetz J, Sklansky MS. Color M-mode sonography for evaluation of fetal arrhythmias. J Ultrasound Med 2012;31:1681–8. [DOI] [PubMed] [Google Scholar]
- 3.Resnik R ed. Creasy and Resnik’s Maternal-Fetal Medicine: Principles and Practice. 8th Edition ed. Elsevier: ——, 2019. [Google Scholar]
- 4.Hornberger LK, Sahn DJ. Rhythm abnormalities of the fetus. Heart, 2007;93:1294–300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Simpson LL. Fetal supraventricular tachycardias: diagnosis and management. Semin Perinatol 2000;24:360–72. [DOI] [PubMed] [Google Scholar]
- 6.Wacker-Gussmann A, Strasburger JF, Cuneo BF, et al. Diagnosis and treatment of fetal arrhythmia. Am J Perinatol, 2014;31:617–28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hansmann M, Gembruch U, Bald R, et al. Fetal tachyarrhythmias: transplacental and direct treatment of the fetus-a report of 60 cases. Ultrasound Obstet Gynecol 1991;1:162–8. [DOI] [PubMed] [Google Scholar]
- 8.Moatassim S, Touleimat S, Hazelzet T, et al. Maternal complications induced by digoxin treatment of fetal tachycardia: A retrospective series of 18 cases. J Gynecol Obstet Hum Reprod 2018;47:35–38. [DOI] [PubMed] [Google Scholar]
- 9.Malhame I, Gandhi C, Tarabulsi G, et al. Maternal monitoring and safety considerations during antiarrhythmic treatment for fetal supraventricular tachycardia. Obstet Med 2019;12:66–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Alsaied T, Baskar S, Fares M, et al. First-line antiarrhythmic transplacental treatment for fetal tachyarrhythmia: A systematic review and meta-Analysis. J Am Heart Assoc 2017;6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hill GD, Kovach JR, Saudek DE, et al. Transplacental treatment of fetal tachycardia: A systematic review and meta-analysis. Prenat Diagn 2017;37:1076–1083. [DOI] [PubMed] [Google Scholar]
- 12.Jaeggi E, Fouron JC, Fournier A, et al. Ventriculo-atrial time interval measured on M mode echocardiography: a determining element in diagnosis, treatment, and prognosis of fetal supraventricular tachycardia. Heart 1998;79:582–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Strizek B, Berg C, Gottschalk I, et al. High-dose flecainide is the most effective treatment of fetal supraventricular tachycardia. Heart Rhythm 2016;13:1283–8. [DOI] [PubMed] [Google Scholar]
- 14.Jaeggi E FAST Therapy Trial of Fetal Tachyarrhythmia. 2019. [access March 2020]; Available from: https://clinicaltrials.gov/ct2/show/NCT02624765.
- 15.Wolfe DS, Hameed AB, Taub CC, et al. Addressing maternal mortality: the pregnant cardiac patient. Am J Obstet Gynecol 2019;220:167 e1–167 e8. [DOI] [PubMed] [Google Scholar]
- 16.Ekiz A, Kaya B, Bornaun H, et al. Flecainide as first-line treatment for fetal supraventricular tachycardia. J Matern Fetal Neonatal Med 2018;31:407–412. [DOI] [PubMed] [Google Scholar]
- 17.Jaeggi ET, Carvalho JS, De Groot E, et al. Comparison of transplacental treatment of fetal supraventricular tachyarrhythmias with digoxin, flecainide, and sotalol: results of a nonrandomized multicenter study. Circulation 2011;124:1747–54. [DOI] [PubMed] [Google Scholar]
- 18.Shah A, Moon-Grady A, Bhogal N, et al. Effectiveness of sotalol as first-line therapy for fetal supraventricular tachyarrhythmias. Am J Cardiol 2012;109:1614–8. [DOI] [PubMed] [Google Scholar]
- 19.Miyoshi T, Maeno Y, Hamasaki T, et al. Antenatal therapy for fetal supraventricular tachyarrhythmias: multicenter trial. J Am Coll Cardiol 2019;74:874–885. [DOI] [PubMed] [Google Scholar]
- 20.Rolnik DL, Wright D, Poon LC, et al. Aspirin versus placebo in pregnancies at high risk for preterm preeclampsia. N Engl J Med 2017;377:613–622. [DOI] [PubMed] [Google Scholar]
- 21.ACOG Committee Opinion No. 743 Summary: Low-dose aspirin use during pregnancy. Obstet Gynecol 2018;132:254–256. [DOI] [PubMed] [Google Scholar]


