Abstract
Background
The increasing number of infections caused by antibiotic resistant strains of Pseudomonas aeruginosa posed a very serious challenge for clinical practice. This standing is driving scientists to develop new antibiotics against these microorganisms.
Methods
In this study, we measured the MIC/MBC values and estimated the ability of tested molecules to prevent bacterial biofilm formation to explore the effectiveness of β-lactam antibiotics ceftolozane/tazobactam, ceftazidime/avibactam, meropenem/vaborbactam, and ceragenins CSA-13, CSA-44, and CSA-131 against 150 clinical isolates of Pseudomonas aeruginosa that were divided into five groups, based on their antibiotic resistance profiles to beta-lactams. Selected strains of microorganisms from each group were also subjected to prolonged incubations (20 passages) with ceragenins to probe the development of resistance towards those molecules. Cytotoxicity of tested ceragenins was evaluated using human red blood cell (RBCs) hemolysis and microscopy observations of human lung epithelial A549 cells after ceragenin treatment. Poloxamer 407 (pluronic F-127) at concentrations ranging from 0.5% to 5% was tested as a potential drug delivery substrate to reduce ceragenin toxicity.
Results
Collected data proved that ceragenins at low concentrations are highly active against clinical strains of Pseudomonas aeruginosa regardless of their resistance mechanisms to conventional antibiotics. Ceragenins also show low potential for resistance development, high antibiofilm activity, and controlled toxicity when used together with poloxamer 407.
Conclusion
This data strongly supports the need for further study directed to develop this group of molecules as new antibiotics to fighting infections caused by antibiotic resistant strains of Pseudomonas aeruginosa.
Keywords: ceragenin, antibacterial agents, Pseudomonas aeruginosa, antibiotic resistant bacteria, new antibiotics
Introduction
The increasing number of infections caused by antibiotic-resistant microorganisms is a significant public health problem. Resistance can affect different groups of pathogens, but Gram-negative bacteria including Pseudomonas aeruginosa (PA) are more likely to be resistant to many classes of antibiotics. The most important resistance mechanisms in Gram-negative rods are; the production of enzymes that inactivate antibiotics, changes in the cell wall porin channels, and the presence of efflux pumps that remove antibiotics from the bacterial cells. Multidrug resistance significantly limits treatment options for patients with infections caused by these pathogens.1 PA is often associated with severe nosocomial infections such as ventilator-associated pneumonia (VAP), blood stream infections, urinary tract infections associated with catheterization or surgery, and burn wound infections. Infections are especially common with immunocompromised and critically ill patients.2 PA is also associated with chronic respiratory infections in patients suffering from cystic fibrosis,3 chronic obstructive pulmonary disease, and bronchiectasis. Community acquired infections in immunocompetent patients are less frequent and less severe. They are mainly otitis externa (swimmer’s ear) and hot tub folliculitis.4
Most P. aeruginosa infections are associated with biofilm formation and the production of various virulence factors.5 Their expression is regulated by the quorum sensing process and differs in acute and chronic infections.6 It is worth noting that, in general, there is no association between the determinants of virulence and the PA multidrug resistance.7
The emergence and the dissemination of multidrug (MDR) and extensively drug-resistant (XDR) PA strains in hospital settings makes the treatment of infections caused by this microorganism one of the most challenging. In some cases, the only therapeutic options are polymyxins: colistin and polymyxin B. They are typically not used as monotheraphy but in combination with antipseudomonal antibiotics (one or more) depending on susceptibility. Apart from colistin, other antimicrobials displaying activity against PA include classical antipseudomonal beta-lactams (BL) (ceftazidime, cefepime, aztreonam, piperacillin/tazobactam, carbapenems), aminoglycosides, quinolones, and fosfomycin.8
Recently, new β-lactams have been introduced into clinical practice. One of them is ceftolozane/tazobactam (C/T) – the combination of a novel cephalosporin with the “old” β-lactamase inhibitor (BLI) – tazobactam in a 2:1 ratio. The addition of inhibitor broadens the spectrum of the antibiotic to include ESBL (extended-spectrum β-lactamase)-producing organisms. C/T is intended for treatment of infections caused by multidrug-resistant Gram-negative rods. Unfortunately C/T is not active against carbapenemase-producing organisms.9 Thanks to its excellent activity against PA, C/T is often a good choice for use against PA strains resistant to carbapenems provided the resistance is not due to carbapenemase production.10
The second new drug is ceftazidime/avibactam (C/A). This is a combination of ceftazidime with a novel synthetic non-β-lactam/β-lactamase inhibitor.11 The addition of avibactam broadens the spectrum of ceftazidime with Gram-negative rods producing β-lactamases such as AmpC (cefalosporynase AmpC), ESBLs, carbapenemases KPC (Klebsiella pneumoniae carbapenemase), and some OXA (oxacillinase klase D) enzymes but not the metalo–β-lactamases.12 These new drugs should not be used against bacteria other than MDR. They appear to be a good carbapenem-sparing alternative for treatment of infections caused by ESBL producers. Reducing carbapenem consumption means reduced selection of carbapenemase-producing strains and a lower impact on the gut microbiota.13
Another new two-component therapeutic is meropenem/vaborbactam (M/V) consisting of carbapenem and a novel boronic acid-based beta-lactamase inhibitor. Vaborbactam inhibits successfully many Ambler class A enzymes (eg, CTX-M, TEM, SHV, KPC) and Ambler class C beta-lactamases (eg, AmpC). Vaborbactam alone has no antimicrobial activity but in combination with meropenem appears to be a good option in the treatment of infections caused by Gram-negative resistant strains, especially KPC-producing Enterobacteriaceae.14
Although several new antibiotics have been introduced into clinical practice in recent years, the development of novel therapeutic strategies are still highly important, especially against multidrug-resistant strains. Ceragenins (CSAs) are promising new antimicrobial compounds with a unique mechanism of action. They were designed as small-molecule, non-peptide mimics of antimicrobial peptides (AMPs). Ceragenins replicate the physicochemical properties of AMPs (eg, the positive charge and amphipathic nature of the molecule allowing for membrane insertion) and they are less toxic and more stable under physiological conditions. CSAs are insensitive to proteolytic enzymes, and their production on a large scale is simpler and less expensive than peptide substance15,16
Due to multiple positive charges, ceragenins interact electrostatically with negatively charged cell membranes causing changes in the organization of the membrane lipid bilayer, resulting in the loss of selective permeability and cell death. Additionally, ceragenins have the ability to bind bacterial endotoxins (eg, LPS and lipoteichoic acid), which translates into anti-inflammatory effects.17,18 Because its mechanism of action is similar to AMPs that are naturally present in the human body, the probability of development of resistance is relatively small.16 Similarly to AMPs, ceragenins display broad antibacterial,19 antifungal,20 and antiprotozoal21 activity. They also act against some viruses.22 A valuable feature of ceragenins is their activity against MDR strains and the ability to inhibit the growth of microorganisms in the form of a biofilm.23 Due to their amphiphilic character, CSAs associate well with micelles, including those prepared from poloxamer .24 This association can reduce the potential cytotoxic activity of these compounds. CSA-13 is the most studied ceragenin. Others, such as CSA-44, CSA-131, CSA-138, and CSA-142,25 have been designed and tested in order to optimize antimicrobial activity and to increase the safety of use.
The primary aim of the present study was to assess the activity of selected ceragenins CSA-13, CSA-131, and CSA-44 in the background of the activity of three new conventional beta–lactam antibiotics (ceftolozane/tazobactam, ceftazidime/avibactam, meropenem/vaborbactam) against clinical strains of PA with different patterns of resistance.
Materials and Methods
Bacterial Strains
A group of 150 PA clinical isolates (each isolate from one subject) were tested in this study. All strains came from the collection gathered in the Department of Clinical Microbiology of the Świętokrzyskie Oncology Center in Kielce (Poland). Strains were isolated from samples collected from oncological patients (96 men, 64%, and 54 women, 36%, aged 25–88 years), admitted to the Center in the years 2009–2020. Samples were taken mainly from patients with clinical signs of infection and sent to the laboratory for routine microbiological diagnostics. Only 13 strains came from colonized people without infection, mainly patients of the Hematology Clinic and the Intensive Care Unit. The study was approved by the Ethical Committee for Human Studies of the Jan Kochanowski University in Kielce (No. 23/2019), and the guidelines outlined in the Declaration of Helsinki were followed. For this study informed consent was waived by Ethical Committee for Human Studies of the Jan Kochanowski University in Kielce (No. 23/2019) since the bacteria strains came from the hospital collection. The source of clinical strains is presented in Table 1.
Table 1.
Source of Clinical Pseudomonas aeruginosa Strains (n=150)
| Source | Number of Strains |
|---|---|
| Blood | 37 |
| Urine | 17 |
| Skin and soft tissues, including surgical wounds | 41 |
| Lower respiratory tract | 34 |
| Upper respiratory tract | 8 |
| Others (screening tests) | 13 |
Samples were cultured accordingly to the relevant laboratory procedures. Pseudomonas-like colonies were isolated from MacConkey agar (Thermofisher Scientific) and identified by VITEK 2 Compact (bioMérieux). The antimicrobial susceptibility testing was performed by disc diffusion method (only for colistin the MIC (minimal inhibitory concentration) was determined using broth microdilution test) in accordance with the current EUCAST (European Committee on Antimicrobial Susceptibility Testing) recommendations.26 Strains characterized by patterns of resistance to the basic panel of clinically used antibiotics, and in the case of multidrug-resistant strains, production of carbapemases, were stored in the MAST CRYOBANK system (Mast Diagnostics) at −70°C. For further studies the stored strains were revived on TSA agar (Thermofisher Scientific) in aerobic conditions. As a control stain Pseudomonas aeruginosa ATCC 25285 was used.
Susceptibility Testing
For the disc diffusion method, the following disks containing antibiotics were used: piperacillin (30 μg), ceftazidime (10 μg), piperacillin/tazobactam (30/6 μg), imipenem (10 μg), meropenem (10 μg), cefepime (30 μg), aztreonam (30 μg), ticarcillin/clavulanic acid (80/5 μg), ciprofloxacin (5 μg), levofloxacin (5 μg), gentamicin (10 μg), amikacin (30 μg), and tobramycin (10 μg) (all from Thermofisher Scientific). Each strain that was not susceptible to meropenem and/or imipenem and resistant to ticarcillin/clavulanic acid was tested for carbapenemase (MBL or KPC) production. Two phenotypic tests were used: the DDST (double-disk synergy tested with imipenem (10 μg), ceftazidime (30 μg), and disc with EDTA) for MBL detection27 and test with meropenem (meropenem and boronic acid, 10 μg of each) for KPC detection. All procedures were performed according to the EUCAST recommendations.28 The strains detected as carbapenemase-positive in phenotypic tests were reanalyzed using molecular Xpert Carba-R commercial tests (Cepheid) detecting the following carbapenemase gene families: blaKPC, blaNDM, blaVIM, blaOXA-48, and blaIMP. The MICs for colistin was determined by the commercial broth microdilution test (Merlin) and for three new antibiotics: ceftolozane/tazobactam, ceftazidime/avibactam, and meropenem/vaborbactam by commercial gradient tests (Liofilchem MIC Test Strips) in accordance with the producer’s instructions.
To determine MICs for ceragenins CSA-13, CSA-131, and CSA-44, broth microdilution assays were adopted and performed in accordance with the guidelines of EUCAST.29 For the broth microdilution assay, 96-well microtiter plates were used. In general, serial 2-fold dilutions (from 256 µg/mL to 0.5 µg/mL) of the tested ceragenin were prepared in Mueller-Hinton Broth (Thermofisher Scientific). The appropriate inoculum of each isolate was prepared from fresh 18–24 hour culture on solid medium then added to the wells to a final concentration of 5×105 CFU/mL per well. The incubation was performed at 35±1°C for 18±2 hours in aerobic conditions. The MIC values were read macroscopically as the lowest concentrations of the antimicrobial agent that inhibited the microbial growth. MICs for three new antibiotics: ceftolozane/tazobactam, ceftazidime/avibactam, and meropenem/vaborbactam, and three ceragenins: CSA-13, CSA-131, and CSA-44, were determined for all 150 PA clinical strains. This experiment was also performed under the same conditions with the use of poloxamer at concentrations of 0.5, 1, 2, and 5%. After the determination of the MIC concentration, the MBC (minimal bactericidal concentration) values were determined by planting samples from wells on LB agar and incubating at 37°C in aerobic condition for the next 18±2 hours. The MBC values were read as the concentrations at which no bacterial growth was observed on the solid medium. The MBCs evaluation was performed for CSA-13, CSA-131, and CSA-44 for all tested clinical strains. The ceragenins used in experiments were synthetized following previously described procedures.30
Serial Passage
To evaluate the probability of resistance development to ceragenins, a serial passage experiment with one representative strain from each group and a reference PA ATCC 27853 strain was performed. Before starting this set of experiments, the MIC values were assessed. Then, bacteria growing in a well with defined concentration of ceragenin CSA-13, -44, and -131 (just below the MIC value) were transferred to fresh MH broth to reach a final concentration of 0.5 MFa.
From such a suspension another determination of the MIC value was performed. This procedure was repeated 20-times. The incubation conditions were always the same (37°C and 18–24 hours).31,32
Prevention of Biofilm Formation
One representative strain for each of the five study groups of PA and one reference strain (ATCC 27853) were used to evaluate the antibiofilm activity of CSA-13, CSA-131, and CSA-44. A fluorimetric method was used to assess the ability of ceragenins to prevent PA biofilm formation. The tested strains were incubated in microtiter plates with ceragenins in the concentration range 1–50 µg/mL. After 24, 48, and 72 hours the contents of the plates were washed with PBS to remove planktonic cells, and then incubated with resazurin (0.2 mg/mL) for 1 hour (Sigma-Aldrich). After this time, fluorescence (Eex/Eem 520/590) was measured with Tecan Spark plates reader (Spark Control Magellan V2.2, Austria).23 This experiment was performed in triplicate.
Hemolytic Activity of Ceragenins
Human red blood cells (RBCs) were used to determine the hemolytic activity of ceragenins. Blood was taken from healthy volunteers, who provided informed consent.
RBCs suspended in PBS (hematocrit ~5%) were added to solutions of ceragenins (1–50 µg/mL) and incubated for 1, 6, and 12 hours at 37°C. After incubation, samples were centrifuged at 2,500 g for 10 minutes. The amount of released hemoglobin was measured colorimetrically at λ=540 nm with a Tecan Spark plates reader (Spark Control Magellan V2.2, Austria). As a positive control, 1% Triton X-100 was used (100% of released hemoglobin). The hemolytic activity of ceragenins was calculated considering positive control as 100% of hemolysis.23 In the second set of experiments the ability of Pluronic F-127 to reduce the toxicity of ceragenins was assessed. For this purpose, ceragenins were incubated with RBCs in the presence of Pluronic F-127 at concentrations 0.5, 1, 2, and 5%. These assays were performed in triplicate as described previously.33
Cytotoxicity of Ceragenins Towards Human Lung Epithelial Cells
Using MTT assay with 3-[4,5-dimethylthiazol-2-yl]-2,5-diphenyltetrazolium bromide) we assessed the cytotoxic activity of ceragenins towards human lung adenocarcinoma A549 (ATCC® CCL-185™) cells. The cells were cultured in Dulbecco’s DMEM Eagle’s Medium (Sigma Aldrich ATCC® 30–2002™) enriched with 10% FBS (fetal bovine serum) (Sigma Aldrich) and PSA antibiotic kit (penicillin 100 U/mL, streptomycin 100 μg/mL, amphotericin B 250 ng/mL) (Sigma Aldrich). The culture was maintained at 37°C, with an atmosphere containing 5% CO2. In the first step, 1×104 of A549 cells were incubated in the medium mentioned above for 24 hours in a 96-well plate. The next day, the cells were washed with PBS and ceragenins CSA-13, CSA-44, and CSA-131 were added at concentrations of 1, 2, 5, 10, 20, and 50 µg/mL. After 24 hours of incubation, the supernatant was discarded, the plates were washed twice with PBS, and MTT was added to wells at a final concentration of 0.5 mg/mL and incubated for the next 4 hours. The precipitated formazan was dissolved in DMSO (dimethyl sulfoxide) and the absorbance was read at 540 nm (Varioskan Lux, Thermo Fisher Scientific, Waltham, MA). A549 cell viability was calculated as a percentage compared to control. At the same time, an experiment to assessing the cytotoxicity of ceragenins in the presence of Pluronic F-127 at the concentration of 1, 2, and 5% (v/w) per well was performed. Results were compared to the control (the absorbance from wells with A549 cells in medium without ceragenins and without pluronic – 100% viablity). Morphological changes of A549 cells were also assessed using a light microscope with 20x magnification lens.34,35
Statistical Analysis
Graph Pad Prism version 8 (San Diego, CA) was used for statistical analyses. Data are presented as mean ± standard deviation (SD) of three-to-six experiments. Two-tailed Student’s test was used to determine the significance of differences, and a p-value of ≤0.05 was considered statistically significant.
Results
Patterns of Resistance of the Tested Strains
After initial susceptibility testing, all strains were divided into five groups based on the patterns of resistance to the tested β-lactams (piperacillin, piperacillin/tazobactam, ticarcillin/clavulanic acid, ceftazidime, cefepime, aztreonam, imipenem, meropenem). In general, most strains (II, III, and IV groups containing a total of 120 isolates) displayed different mechanisms of resistance to karbapenems (CRPA; carbapenem resistant PA). The smallest amount among them were MBL - producing PA (group IV, n=13). Molecular tests showed that all strains belonging to this group produced VIM-type metalobetalactamase (MBL). KPC carbapenemase producing strains have not been detected in either phenotypic nor molecular tests. All strains from groups III and IV were classified as XDR and from group V as MDR using standardized definitions created by international experts.36
They describe MDR as strains non-susceptible to more than one antibiotic in three or more antimicrobial classes and XDR as non-susceptibility to more than one antibiotic in all but two or fewer classes. There were no pandrug-resistant (PDR) strains – non-susceptibile to all antibiotics in our collection (Table 2).
Table 2.
Characteristics of Group of Pseudomonas aeruginosa Strains (n=150)
| Group I (n=22) | Strains susceptible to all tested β-lactams |
| Group II (n=40) | Strains (resistant/not susceptible) to karbapenems and susceptible to other β-lactams |
| Group III (n=67) | Strains resistant to all tested β-lactams, MBL-negative; XDR strains |
| Group IV (n=13) | Strains resistant to all tested β-lactams, MBL-positive; XDR strains |
| Group V (n=8) | Strains susceptible to karbapenems with variable pattern of resistance to other β-lactams; MDR strains |
MIC values for new β-lactams vary between the groups of strains. All strains from group I were susceptible to the examined new β-lactams. In group II (strains resistant/not susceptible to karbapenems and susceptible to other β-lactams) the ranges of MICs values are wide; most isolates are susceptible to these three drugs. For the MDR but MBL-negative PA (group III) among three tested antibiotics meropenem/waborbactam displays the weakest antimicrobial activity. Strains from group IV (MBL producers) are all resistant to the new combinations of β-lactams with inhibitors and the MICs are the highest. Strains from group V are all susceptible to C/T, C/A, and M/V. As for colistin, only single strains showed resistance to this drug. They came from groups I and II. In total, 98% of strains displayed susceptibility to colistin. The EUCAST breakpoints for ceftolozane/tazobactam, ceftazidime/avibactam, meropenem/vaborbactam, and colistin are as follows: 4, 8, 8, and 2 mg/L.37
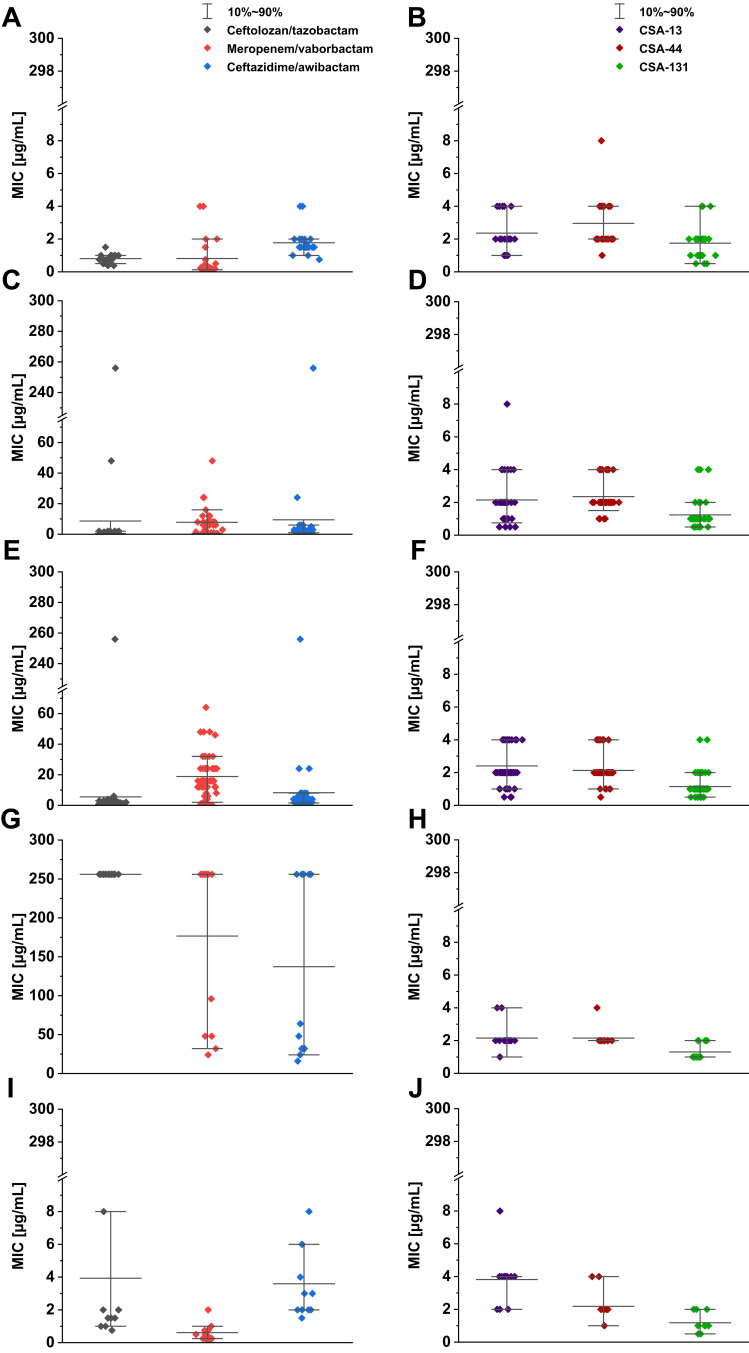
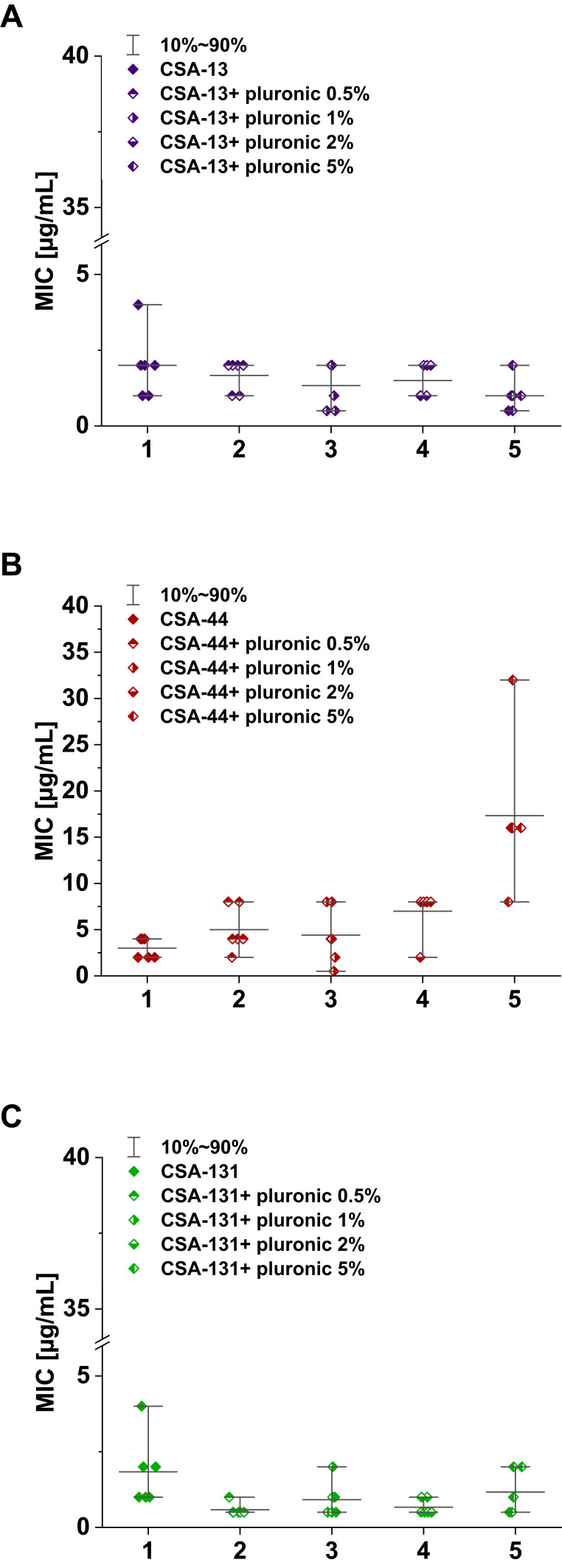
MIC range, MIC50, MIC90, and susceptible rate of ceftolozane/tazobactam, ceftazidime/awibactam, meropenem/vaborbactam, and colistin against PA strains are presented in Table 3, and the MICs distribution in Figure 1. MIC range, MIC50, MIC90, and susceptible rate of C/T, C/A, M/V, and colistin against CRPA strains (group II, III, IV) are summarized in Table 4. The ranges of MIC values for each ceragenin against PA strains with and without resistance mechanisms to examined β-lactams are similar between groups and comparable to the reference strain ATCC 27853. Among tested ceragenins the greatest activity was observed for CSA-131. MIC and MBC values of ceragenins: CSA-13, CSA-44, and CSA-131 against all 150 PA strains are presented in Table 5 and the MIC distribution in Figure 1. The MICs of ceragenins with pluronic F127 are shown in Figure 2. With respect to representative strains of each group tested, we found that the antibacterial activity ceragenins in most tested conditions was better and only slightly reduced for ceragenin CSA-44.
Table 3.
MIC Range, MIC50, MIC90, and Susceptible Rate of C/T, C/A, M/V, and Colistin Against Pseudomonas aeruginosa Strains (n=150)
| Group of Strains/ Control Strain | Antibiotic | MIC Range (µg/mL) | MIC50 (µg/mL) | MIC90 (µg/mL) | MBC Range (µg/mL) | S |
|---|---|---|---|---|---|---|
| All clinical P. aeruginosa (n=150) |
Ceftolozane/tazobactam | 0.25–>256 | 2 | 64 | ND | 87% |
| Ceftazidime/awibactam | 1–>256 | 4 | 32 | ND | 88% | |
| Meropenem/vaborbactam | 0.125–256 | 8 | 64 | ND | 51% | |
| Colistin | 0.125–8 | 1 | 2 | ND | 98% | |
| Group I (n=22) |
Ceftolozane/tazobactam | 0.5–2 | 1 | 1 | ND | 100% |
| Ceftazidime/awibactam | 1–4 | 2 | 2 | ND | 100% | |
| Meropenem/vaborbactam | 0.125–4 | 0.25 | 2 | ND | 100% | |
| Colistin | 0.5–8 | 1 | 2 | ND | 91% | |
| Group II (n=40) |
Ceftolozane/tazobactam | 0.25–>256 | 1 | 2 | ND | 95% |
| Ceftazidime/awibactam | 1–256 | 2 | 8 | ND | 95% | |
| Meropenem/vaborbactam | 0.25–64 | 8 | 16 | ND | 78% | |
| Colistin | 0.125–4 | 1 | 2 | ND | 98% | |
| Group III (n=67) |
Ceftolozane/tazobactam | 0.5–>256 | 2 | 4 | ND | 93% |
| Ceftazidime/awibactam | 1–>256 | 4 | 8 | ND | 96% | |
| Meropenem/vaborbactam | 0.125–64 | 16 | 32 | ND | 22% | |
| Colistin | 0.25–2 | 1 | 2 | ND | 100% | |
| Group IV (n=13) |
Ceftolozane/tazobactam | 256–>256 | 256 | >256 | ND | 0% |
| Ceftazidime/awibactam | 16–>256 | 64 | 256 | ND | 0% | |
| Meropenem/vaborbactam | 32–256 | 256 | 256 | ND | 0% | |
| Colistin | 0.5–2 | 1 | 2 | ND | 100% | |
| Group V (n=8) |
Ceftolozane/tazobactam | 1–8 | 2 | 2 | ND | 100% |
| Ceftazidime/awibactam | 2–8 | 2 | 8 | ND | 100% | |
| Meropenem/vaborbactam | 0.125–1 | 0.25 | 1 | ND | 100% | |
| Colistin | 0.5–2 | 1 | 2 | ND | 100% | |
| P. aeruginosa ATCC 27853 | Ceftolozane/tazobactam | 0.75 | – | – | ND | – |
| Ceftazidime/awibactam | 0.75 | – | – | ND | – | |
| Meropenem/vaborbactam | 0.25 | – | – | ND | – | |
| Colistin | 0.25 | – | – | ND | – |
Abbreviations: ND, not determined; S, susceptible strains.
Figure 1.
Range of the minimum inhibitory concentrations (MICs) of ceftolozane-tazobactam, meropenem-vaborbactam and ceftazidime-avibactam recorded for P. aeruginosa strains (A - group I, C- group II, E - group III, G - group IV, I - group V) and CSAs (CSA-13, CSA-44, CSA-131) (B - group I, D - group II, F - group III, H - group IV, J - group V). Compared for the reference strain of P. aeruginosa ATCC 27853 MIC value for ceftolozane-tazobactam was 0.75 μg/mL, ceftazidime-avibactam – 0.75 μg/mL, meropenem-vaborbactam – 0.25 μg/mL, CSA-13 – 2 μg/mL, CSA-44 - 2 μg/mL and CSA-131 - 1 μg/mL.
Table 4.
Summarized MIC Range, MIC50, MIC90, and Susceptible Rate of C/T, C/A, and M/V Against CRPA Strains (n=120)
| Group of Strains | Antibiotic | MIC range (µg/mL) | MIC50 (µg/mL) | MIC90 (µg/mL) | MBC range (µg/mL) | S |
|---|---|---|---|---|---|---|
| Group II, III, IV (n=120) | Ceftolozane/tazobactam | 0.25–>256 | 2 | 256 | ND | 83% |
| Ceftazidime/awibactam | 1–>256 | 4 | 32 | ND | 85% | |
| Meropenem/vaborbactam | 0.125–256 | 16 | 64 | ND | 38% | |
| Colistin | 0.125–4 | 1 | 2 | ND | 99% |
Abbreviations: ND, not determined, S, susceptible strains.
Table 5.
MIC Range, MIC50, MIC90, MBC Range, MBC50, and MBC90 of Ceragenins Against Pseudomonas aeruginosa Strains (n=150)
| Group of Clinical Strains/Control Strain | Ceragenin | MIC range (µg/mL) | MIC50 (µg/mL) | MIC90 (µg/mL) | MBC range (µg/mL) | MBC50 (µg/mL) | MBC90 (µg/mL) |
|---|---|---|---|---|---|---|---|
| All clinical P. aeruginosa (n=150) |
CSA13 | 0.5–8 | 2 | 4 | 0.5–8 | 4 | 8 |
| CSA44 | 0.5–8 | 2 | 4 | 0.5–8 | 4 | 8 | |
| CSA131 | 0.5–4 | 1 | 2 | 0.5–8 | 2 | 4 | |
| Group I (n=22) |
CSA13 | 1–4 | 2 | 4 | 1–8 | 4 | 8 |
| CSA44 | 1–8 | 2 | 4 | 1–8 | 4 | 8 | |
| CSA131 | 0.5–4 | 2 | 4 | 1–8 | 2 | 4 | |
| Group II (n=40) |
CSA13 | 0.5–8 | 2 | 4 | 0.5–8 | 4 | 8 |
| CSA44 | 1–4 | 2 | 4 | 1–8 | 2 | 8 | |
| CSA131 | 0.5–4 | 1 | 2 | 0.5–8 | 2 | 4 | |
| Group III (n=67) |
CSA13 | 0.5–4 | 2 | 4 | 0.5–8 | 4 | 8 |
| CSA44 | 0.5–4 | 2 | 2 | 0.5–8 | 4 | 8 | |
| CSA131 | 0.5–4 | 1 | 4 | 0.5–8 | 2 | 4 | |
| Group IV (n=13) |
CSA13 | 1–4 | 2 | 4 | 1–8 | 4 | 4 |
| CSA44 | 2–4 | 2 | 2 | 2–4 | 2 | 4 | |
| CSA131 | 1–2 | 1 | 2 | 1–4 | 2 | 2 | |
| Group V (n=8) |
CSA13 | 2–8 | 4 | 8 | 2–8 | 4 | 8 |
| CSA44 | 2–4 | 2 | 4 | 2–8 | 4 | 8 | |
| CSA131 | 0.5–2 | 1 | 2 | 1–2 | 1 | 2 | |
| Pseudomonas aeruginosa ATCC 27853 | CSA13 | 2 | – | – | 2 | – | – |
| CSA44 | 2 | – | – | 2 | – | – | |
| CSA131 | 1 | – | – | 1 | – | – |
Figure 2.
Range of the minimum inhibitory concentrations (MIC) of CSA ((A) CSA-13, (B) group CSA-44, (C) group CSA-131) in combination with pluronic F127 0.5, 1, 2, and 5% in doses of 1–50 μg/mL.
The MIC range, MIC50, MIC90 MBC range, MBC50, and MBC90 of CSA-13, CSA-44, and CSA-131 against CRPA strains (groups II, III, and IV) are summarized in Table 6.
Table 6.
Summarized MIC Range, MIC50, MIC90 MBC Range, MBC50, and MBC90 of CSA13, CSA44, and CSA131 Against CRPA Strains (n=120)
| Group of Clinical Strains/Control Strain | Antimicrobial Agent | MICs (µg/mL) Range | MIC50 (µg/mL) | MIC90 (µg/mL) | MBCs (µg/mL) Range | MBC50 (µg/mL) | MBC90 (µg/mL) |
|---|---|---|---|---|---|---|---|
| Group II, III, IV (n=120) | CSA13 | 0.5–8 | 2 | 4 | 0.5–8 | 4 | 8 |
| CSA44 | 0.5–4 | 2 | 4 | 0.5–8 | 2 | 8 | |
| CSA131 | 0.5–4 | 1 | 2 | 0.5–8 | 2 | 4 |
Serial Passage
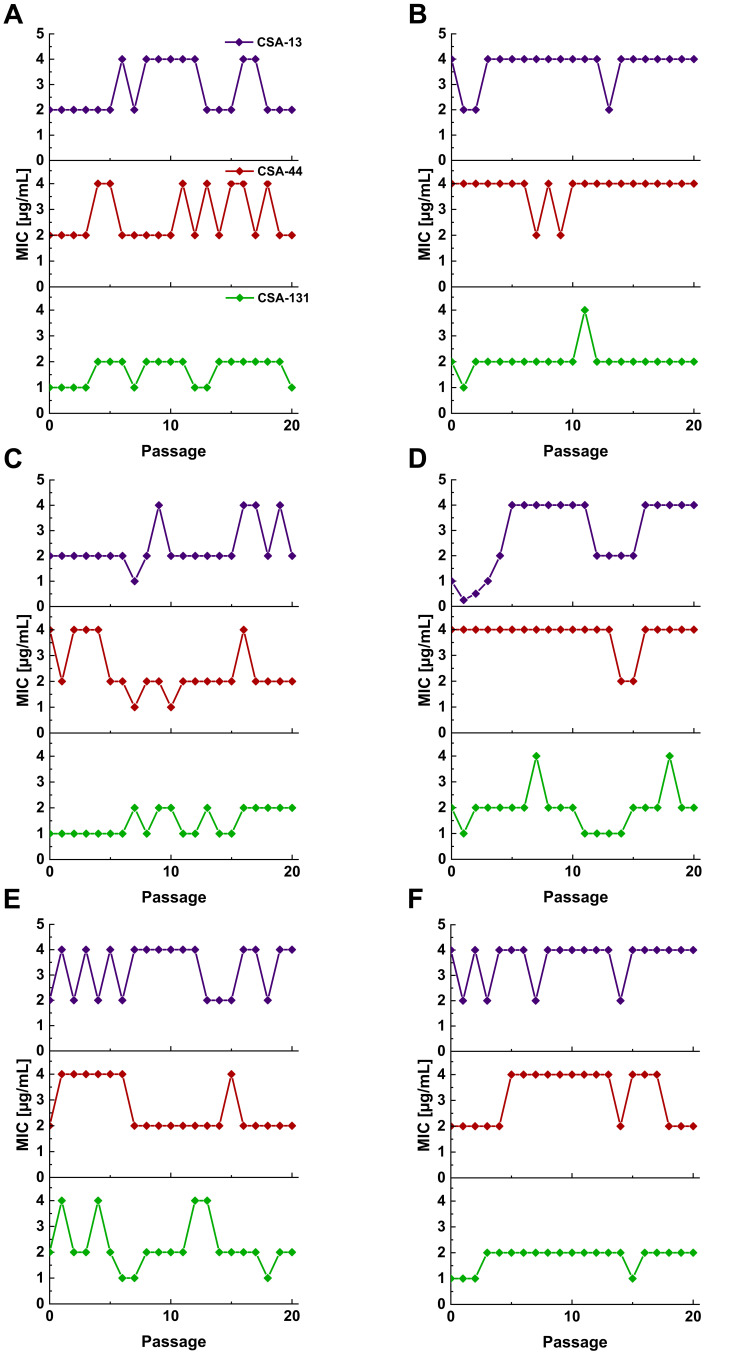
In addition to ceragenin activity against PA strains with different patterns of resistance to β-lactams, our studies provide evidence that prolonged incubations of PA with ceragenins is very unlikely to induce development of resistance towards those molecules. After 20 passages, no significant differences in the MICs were observed (a variation over a 2-fold dilution is acceptable for this method).38 Ceragenins MICs against selected PA strains over 20 passages are presented in over 20 passages are presented in Figure 3.
Figure 3.
MICs values for P. aeruginosa ATCC 27853 (A), and representative strains from group I (B), group II (C), group III (D), group IV (E), and group V (F) of CSA-13, CSA-44, and CSA-131 during serial passages.
Ceragenins’ Activity to Prevent Biofilm Formation
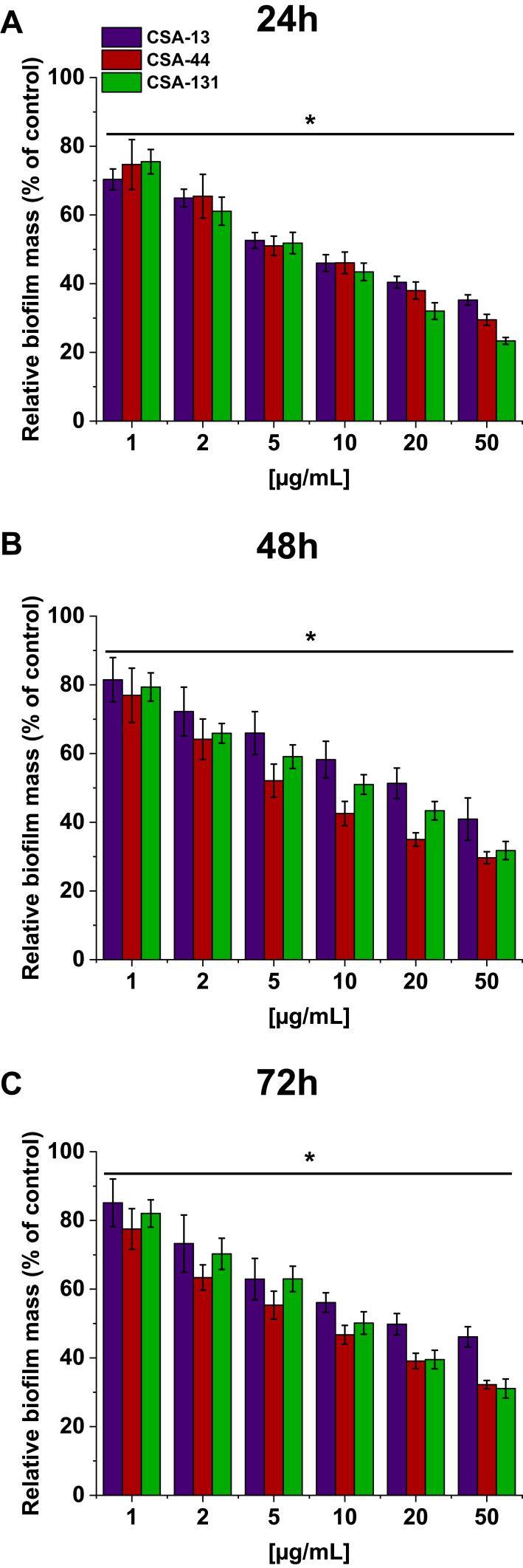
In addition to their bactericidal activity, ceragenins effectively prevent PA biofilm formation. Figure 4 shows the relative biofilm mass of PA cultured without (the control) and in the presence of increasing concentrations of ceragenins. CSA-44 shows the strongest ability in prevention of biofilm formation. This is visible mainly for measurements performed after 48 and 72 hours. Figure 4 shows the average values for five strains, one from each group.
Figure 4.
Relative biofilm mass of P. aeruginosa during treatment with CSA-13, CSA-44, and CSA-131. Formation of biofilm in the presence of CSAs ranging from 1–50 μg/mL was assessed using the resazurin-based fluorimetric method after 24 (A), 48 (B), and 72 (C) hours incubation. Results show mean±SD from three measurements. * indicates statistical significance.
Hemolytic Activity of Ceragenins and Effect of Pluronic F-127
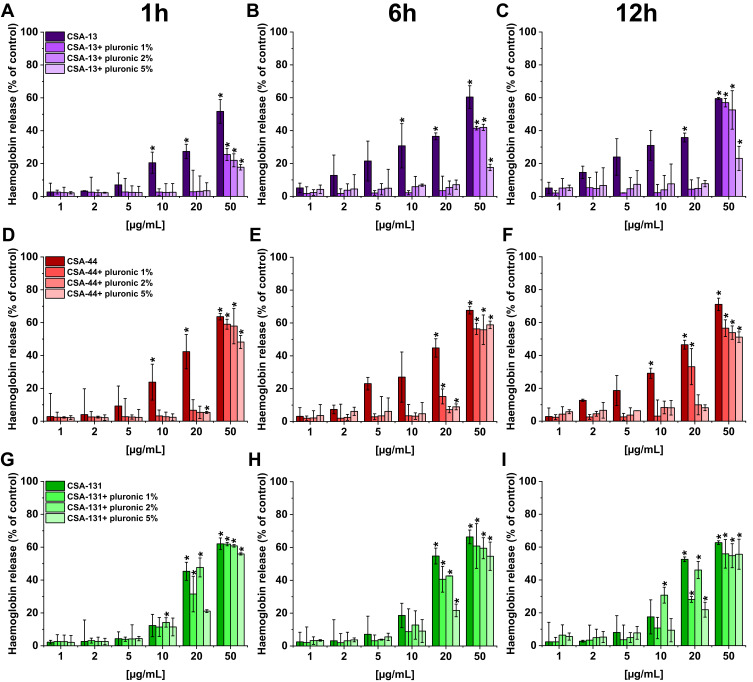
In order to demonstrate the potential toxic effect of ceragenins on human cells, tests with human erythocytes were performed. Our studies indicate that all ceragenins caused low hemolysis after 1 hour incubation (CSA-131 also after 6 and 12 hours) when used at concentrations ranging from 1–5 µg/mL (hemoglobin release <10% of control) (Figure 5). The use of Pluronic F-127 reduced the hemolytic effect of ceragenins. For CSA-13 and CSA-44 the statistically significant influence is visible for the higher doses, independently of pluronic concentration (1, 2, or 5%). At a concentration of 50 µg/mL, only the highest Pluronic F-127 concentration (5%) reduced RBC hemolysis to 20% of control. In the case of CSA-131, the effect of Pluronic F-127 is less visible. In general, these results show that the addition of Pluronic F-127 might be used to limit RBC hemolysis.
Figure 5.
Hemoglobin release from human red blood cells (RBCs) incubated in the presence of CSA-13 (A–C), CSA-44 (D–F), and CSA-131 (G–I) alone and with 1%, 2%, and 5% pluronic at doses of 1–50 μg/mL after 1 hour (A, D, G), 6 hours (B, E, H), and 12 hours (C, F, I). Results show mean±SD, n=3; * indicates statistical significance.
Cytotoxicity of Ceragenins Against Lung Adenocarcinoma A549 Cells
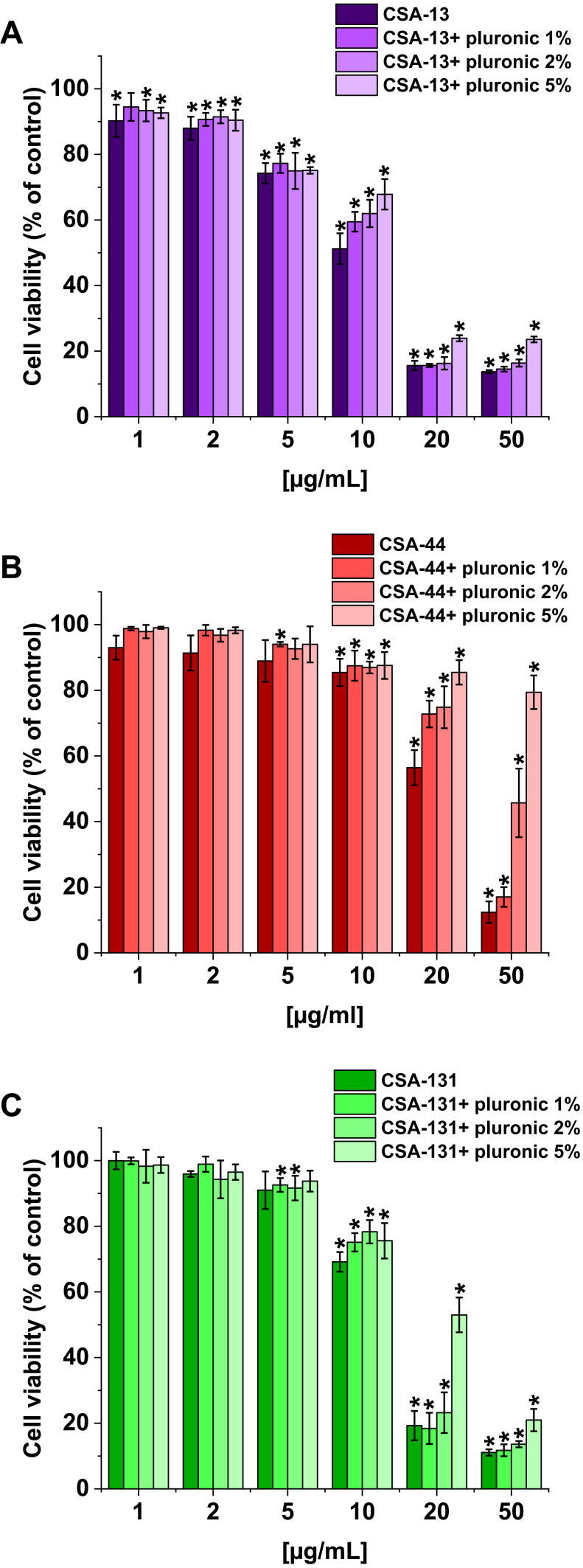
The second experiment to evaluate the cytotoxicity of ceragenins was performed with human lung adenocarcinoma A549 cells. The viability of A549 cells incubated with ceragenins CSA-13, CSA-44, and CSA-131 added at concentrations 1, 2, 5, 10, 20, and 50 µg/mL with and without Pluronic F-127 was examined. CSA-44 and CSA-131 show the greatest effect on cell viability when used in doses of 20 and 50 µg/mL. For these doses, the greatest effect of poloxamer is also visible. When used in doses of 1, 2, and 5 µg/mL they have little effect on cell viability. CSA-13 shows cytotoxicity also at slightly lower doses. In addition, with CSA-13, the pluronic effect is less significant. The results are presented in Figure 6. The morphological changes of A549 cells under the influence of ceragenins and the inhibiting impact of pluronic are also presented as representative microscopic images in Supplementary Figures 1–3.
Figure 6.
Viability of lung adenocarcinoma cells (A549) upon treatment with CSA alone and with 1%, 2%, and 5% pluronic at doses of 1–50 μg/mL. CSA-13 (A), CSA-44 (B), and CSA-131 (C). Results show: mean±SD, n=3; * indicates statistical significance.
Discussion
The resistance of PA to antibiotics significantly reduces the options for effective treatment of infections caused by this microorganism and encourages the search for new therapeutic pathways. Despite the emergence of new antimicrobial drugs, there are still no effective treatments for some infections. In our study we assessed the activity of three new β-lactams: ceftolozane/tazobactam (C/T), ceftazidime/avibactam (C/A), and meropenem/vaborbactam (M/V), and three ceragenins (CSA-13, CSA-44, and CSA-131) against 150 non-repetitive clinical isolates with different resistant patterns collected from oncological patients. To our knowledge, this is the first study performed in an experimental setting that evaluates simultaneously the activity of selected novel, conventional antibiotic and ceragenins compounds as promising antibacterial agents towards PA strains. We referred activities of tested drugs to the colistin, which is an important, sometimes the last choice antibiotic for treatment of infection caused by multidrug-resistant PA. In our study, most strains (98%) were susceptible to colistin. We detected resistance to colistin in a few strains displaying susceptibility to all β-lactams and in strains resistant/not susceptible to carbapenems and susceptible to other β-lactams. Strains with other resistant patterns, even MBL-positive were susceptible to colistin. The percentage of strains susceptible to colistin in our study was slightly lower than those obtained in the global surveillance study analyzing 8,010 isolates of PA and 38,266 isolates of Enterobacterales collected from 2012 to 2014 from various countries located in different geographic regions. They displayed a high (99.7%) susceptibility to colistin in PA strains which was lower (83.2%) in Enterobacterales. Similar to our research, all MBL-positive PA were susceptible to colistin.39 Unfortunately, the increasing use of colistin in infections caused by multidrug-resistant nonfermentative Gram-negative bacteria as well as against CRE (carbapenemase-resistant Enterobacterales) leads to the emergence of resistant strains.40 Even if PA is susceptible to colistin, therapeutic outcomes are sometimes unsatisfactory. This may be due to the use of too low doses and the development of resistance during treatment.41 In infections caused by MDR PA, colistin is used in combination with carbapenems, fosfomycin, and aminoglycosides. The introduction of novel antibiotics active against MDR Gram-negative rods into the medical market broadens the possibilities of treating infections caused by these microorganisms.42 The important advantages of new drugs such as C/T, C/A, or M/W are predictable pharmacokinetics, rapid tissue distribution, good tolerability and safety profile, as well as the possibility of being an alternative to carbapenems. The most disadvantages are no activity against MBL-producers, the high costs of therapy, the lack of an oral formulation, and the risk of superinfection with fungi or more resistant bacteria.43
Ceftolozane/tazobactam and ceftazidime/avibactam have been available for several years from its approval in the US and in Europe. Meropenem/vaborbactam was introduced into the medical market a few years later. Among the three novel antibiotics examined in our study, M/V displays the worst activity against PA. The susceptibility rate for the entire collection is 51% and 38% for CRPA strains. M/V displays, as was expected, no activity against MBL-producers and poor activity against MBL-negative strains resistant to all tested β-lactams. The susceptibilities of our isolates are lower than those obtained by Carvalhaes et al. In their large study conducted in the US, 89.5% of total PA strains collected in 2014 were susceptible to M/V versus 76.4% susceptible to meropenem alone. For MDR strains, the percentage of M/V sensitivity versus meropenem alone was 59.0% versus 21.1%, and for XDR strains, the percentage of M/V sensitivity versus meropenem alone was 48.6% versus 10%.44
In contrast to these data, Castanheira et al45 show the same activity both for meropenem and M/V against Pseudomonas aeruginosa strains (about 80% of PA strains in this collection were susceptible to these antibiotics). Our study and those cited above show that the results largely depend on the composition of the collection, in particular on the percentage of carbapenemase producing strains. Because vaborbactam is a good inhibitor of serine KPC carbapenemase, M/V has a distinct advantage over meropenem alone, mainly in the case of KPC-producing bacteria. Therefore, the best use for these drugs is the treatment of infections caused by KPC-producers.
In contrast to M/V, in our study, the other two antibiotics, C/T and C/A, display much better activity (87% and 88%, respectively) against all our PA collection. For CRPA strains the activity of C/T and C/A are slightly lower (83% and 85%). The activity of those antibiotics against PA has also been compared in several other studies.46–51 In most of them, 72–94% of strains described as meropenem-resistant or carbapenem-resistant or resistant to at least one of the β-lactams, were susceptible to C/T and 62–92% to C/A. Our results concerning CRPA are within the above ranges, but there are also studies showing that the percentage of susceptibility may be much lower. The example is the study by Ahmed et al52 where 26.7% of tested PA isolates were MBL-producers so the activity of C/T and C/A was 40% and 48%, respectively. In general, the susceptibility of PA to the novel β-lactams varies between studies and is sometimes difficult to compare. Similar to the M/V evaluation studies, this is mainly attributed to the selection of the tested strains (resistance patterns, participation of MDR and XDR strains, especially those producing carbapenemases, the time of the strains collecting, etc.). For example, if tested collection of PA strains contain KPC-producers, a clear advantage of C/A over C/T is visible.53
Although in our studies the percentage of susceptible strains to C/T and C/A are almost the same, the MIC50 values for C/T are 2-fold lower than for C/A, indicating that C/T could be a better agent for the treatment of PA infection. Our results are consistent with those obtained in some other studies where similar susceptibility rates for C/A and C/T were observed and MICs for ceftolozan/tazobactam were from 2–4-fold lower than for ceftazidim/avibactam.46,50 These findings confirm the suggestions of some authors42,54 that, although both antibiotics could be used, it is C/T that should be the potent, the main drug in the combination therapy with other antibiotics (colistin, fosfomycin, aminoglycoside) in infections caused by MDR/XDR PA strains. Such a choice is supported by the fact that this drug is not affected by the main resistance mechanisms in PA (AmpC overproduction, loss of the porin OprD, overexpression of efflux pumps) and has high affinity for the penicillin-binding proteins. So, the reasonable place for this new antibiotic in the hospital antibiotic policy is using C/T or C/A for the treatment of resistant PA infections and C/A and M/W for infections caused by KPC-producing strains.
As ours and many other studies show, the tested antibiotics do not solve all problems with PA resistance. The greatest challenges are MBL carbapenemase producing strains. None of the inhibitors used in the composition of these drugs can hydrolyze the MBL enzyme.53,55,56 In our study MICs values for 13 MBL-positive strains were very high. All MBLs were identified as VIM type, which is the most frequent MBL enzyme in European MBL-positive strains.39 Despite the undoubted progress in the development of new antibiotics, the current therapies of infections caused by MDR bacteria, including PA, are still limited.
New potential antimicrobial drugs could be derived from products of natural origin or their mimics. The examples are plant essential oils, including those from Melaleuca alternifolia, Thymus vulgaris,57 and from Lavanda sumianand Lavanda grosso58 as well as water extract from exotic fruit Borojoa patinoi Cuatrecasas59 which show activity against multidrug-resistant PA strains.
Ceragenins, non-peptide mimics of AMPs, also seem to be a promising option. Although CSA-13 – a member from the first generation of ceragenins, is the most frequently investigated, the representatives of next generation, eg, CSA-142, CSA-192,60 and CSA-138, CSA-131, and CSA-4461 have also been studied. In our study, we assessed the activity of CSA-13, CSA-44, and CSA-131 against clinical PA strains and we compared them with the activity of three novel conventional β-lactam antibiotics. The results show the good activity of these compounds against various clinical PA strains both susceptible to conventional antibiotics as well as against MDR and XDR strains, even MBL-producers preserving susceptibility only to colistin.
In contrast to conventional antibiotics, the ranges of MICs values for each ceragenin against PA strains with and without resistance mechanisms to β-lactams are similar between groups and comparable to the reference strain ATCC 27853. MBCs equal or 2-times higher than MICs values suggest a bactericidal effect against the tested bacteria. Our results are consistent with those obtained by Vila-Farrés et al,61 who tested the activity of several ceragenins, including CSA-44, CSA-131, and CSA-138, against Acinetobacter baumannii and PA clinical isolates including colistin-resistant strains. Both in their work and in ours, CSA-131 showed the best activity. The MIC50 and MIC90 values for our entire collection of strains were 1 mg/L and 2 mg/L, respectively. Another study evaluating the in vitro effect of ceragenins against 20 clinical CRPA strains also showed that CSA-131 is the most active ceragenin against PA, although MIC50 and MIC90 values were higher than in our study and in the study cited above.62 The consistent level of antibacterial activity of ceragenins is independent of the mechanisms of resistance to conventional antibiotics.
Apart from high antimicrobial potency, ceragenins have some additional advantages over other antibiotics. One of them – minimal potential for resistance development – is particularly important in the era of increasing resistance to antibiotics, even those recently introduced into therapy. Ceragenins, as compounds mimicking natural antibacterial peptides, seemmuch more stable than conventional antibiotics against the bacterial resistance mechanisms. In our experinental setting its stability was observed during 20 passages, that mostly confirmed the previous study of Pollard et al,32 where some strains of Staphylococcus aureus, PA, and A. baumannii were subjected to 30 passages with CSA-13 or selected antibiotics (ciprofloxacin and colistin for Gram-negative bacteria and vancomycin for S. aureus). More precisely, for Gram-negative bacteria a modest decrease of susceptibility to CSA-13 and high resistance to conventional antibiotics was detected. S. aureus developed resistance to vancomycin and ciprofloxacin but MIC values for CSA-13 were below 1 mg/L after 30 passages. Our study shows that after 20 passages the MICs values of CSA-13, CSA-44, and CSA-131 did not increase and resistance to these compounds did not develop. Although there are similarities between linear AMPs, ceragenins, and colistin (membrane insertion, mechanism of action, and the lipid A modifications as a possible mechanism of resistance), no or only very little cross-resistance has been detected between ceragenins/antimicrobial peptides and colistin. Ceragenins CSA-131 and CSA-44 remain active against Gram-negative bacteria highly resistant to colistin.31
Another promising feature of ceragenins is its antibiofilm activity which was documented in our previous studies towards aerobic63 and anaerobic bacteria64 as well as fungi.20 The current study also shows the activity of CSAs in the prevention of PA biofilm formation. Its impact is dose- and time-dependent. The results are in agreement with an experiment carried out with a highly virulent PA Liverpool epidemic LESB58 strain capable of producing large amounts of biofilm and long-term survival in respiratory epithelial cells. CSA-13 and CSA-131 inhibit biofilm formation by this bacteria even in experimental settings containing purulent sputum.65 In our study ceragenins display antibiofilm activity against the strains which are susceptible as well as resistant to conventional antibiotics. It is worth noting that ceragenins can not only prevent the biofilm formation but also reduce the amount of established, mature biofilm.66 The possibility of practical use of ceragenins as potential compounds preventing the biofilm formation is supported by further in vitro studies. Latorre et al67 presented a significant reduction of PA, Escherichia coli and S. aureus biofilm formation on endotracheal tubes coated with CSA-131. Gu et al showed that CSAs covalently attached to contact lenses mimic the functions of the endogenous antimicrobial peptides and prevent bacterial colonization on this abiotic surface.68 Ceragenins mimic not only the antibiofilm activity of AMPs but also immunomodulatory stimulation and wound healing promotion. The work by Olekson et al shows that they have potential application as antimicrobial drugs as well as therapeutics supporting wound healing.34 The cited examples show the possibilities for topical application of ceragenins. Several in vivo models show the efficacy of ceragenins in preventing local infections. Examples include: experiments with orthopedic implants coated by CSA-13 preventing perioperative device-related infection69–71 and trials with tracheostomy tubes coated with hydrogel containing CSA-131, where the controlled release of ceragenin effectively prevented the tube from being colonized by bacteria and fungi with good tolerance by the host organism.72
A limitation for systemic administration of ceragenins is the risk of toxicity to the host cells, especially if high doses of antibacterials are used. We conducted several experiments to assess the potential cytotoxicity of ceragenins. In the experiment performed with red blood cells, we showed that the hemolytic activity of CSAs was observed mainly at their higher doses. The use of Pluronic F-127 reduced the haemolytic effect of ceragenins without inhibiting the antimicrobial activity. These results are consistent with the results obtained by others.33 Similarly, in the experiment with A549 human lung adenocarcinoma cells, ceragenins used at higher doses had a toxic effect on cell viability, which was inhibited by Pluronic. Overall, these results show that the addition of Pluronic F-127 reduces toxicity towards host cells without interference with CSAs antibacterial activity. Hashemi et al24 came to similar conclusions. They conducted in vitro and ex vivo experiments showing the effect of poloxamer on the toxicity of ceragenins to porcine explants of tracheal and lung tissue. Thanks to the encapsulation of ceragenin in Pluronic micelles, it was possible to use high doses of these compounds, necessary for the elimination of biofilm, without disturbing the function of the respiratory epithelium. Systemic applications undoubtedly require further research, but substances such as poloxamer are a promising way to solve the problem of ceragenins’ toxicity.
Conclusion
Both the natural and acquired resistance of PA to antibiotics significantly reduces the possibilities of effective treatment of infections caused by this microorganism. Although the novel combination β-lactam/β-lactamase inhibitors expand the arsenal of antipseudomonal drugs, they do not show activity against all strains such as MBL-producers. Moreover, resistance to these novel antibiotics has already emerged. In contrast, ceragenins displayed promising properties such as: activity against microorganisms, regardless of their resistance mechanisms to conventional antibiotics, and low potential for resistance development. As synthetic molecules, the structures of ceragenins can be modified to achieve better properties including greater stability in blood and lower toxicity.
Funding Statement
This work was financially supported by grants from the National Science Centre, Poland UMO-2018/31/B/NZ6/02476 (to RB) and by a program of the Ministry of Science and Higher Education under the project name “Regional Initiative of Excellence in 2019–2022”, project number 024/RID/2018/19 (financing amount: 11,999,000.00 PLN). Part of the study was conducted with the use of equipment purchased by the Medical University of Białystok as part of the RPOWP 2007–2013 funding, Priority I, Axis 1.1, contract No. UDA- RPPD.01.01.00-20-001/15-00 dated 26.06.2015. The funders had no role in the study design, data collection and analysis, decision to publish, or preparation of the manuscript.
Disclosure
Dr Paul B. Savage reports personal fees from N8 Medical, outside the submitted work. The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
References
- 1.Karam G, Chastre J, Wilcox MH, Vincent JL. Antibiotic strategies in the era of multidrug resistance. Crit Care. 2016;20(1):136. doi: 10.1186/s13054-016-1320-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Pachori P, Gothalwal R, Gandhi P. Emergence of antibiotic resistance. Genes Dis. 2019;6(2):109–119. doi: 10.1016/j.gendis.2019.04.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bucki R, Durnaś B, Wątek M, et al. Targeting polyelectrolyte networks in purulent body fluids to modulate bactericidal properties of some antibiotics. Infect Drug Resist. 2018;11:77–86. doi: 10.2147/IDR.S145337 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Juan C, Peña C, Oliver A. Host and pathogen biomarkers for severe pseudomonas aeruginosa infections. J Infect Dis. 2017;215(suppl_1):S44–S51. doi: 10.1093/infdis/jiw299 [DOI] [PubMed] [Google Scholar]
- 5.Gajdács M, Kárpáti K, Stájer A, et al. Insights on carbapenem-resistant Pseudomonas aeruginosa: phenotypic characterization of relevant isolates. Acta Biol Szegediensis. 2021;65:105–112. doi: 10.14232/abs.2021.1.105-112 [DOI] [Google Scholar]
- 6.Banzhaf M, Resendis-Antonio O, Zepeda-Mendoza ML. Uncovering the dynamic mechanisms of the pseudomonas aeruginosa quorum sensing and virulence networks using boolean modelling. IEEE Trans Nanobioscience. 2020;19(3):394–402. doi: 10.1109/TNB.2020.2977820 [DOI] [PubMed] [Google Scholar]
- 7.Gajdács M, Baráth Z, Kárpáti K, et al. No correlation between biofilm formation, virulence factors, and antibiotic resistance in. Antibiotics. 2021;10(9). doi: 10.3390/antibiotics10091134 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Horcajada JP, Montero M, Oliver A, et al. Epidemiology and treatment of multidrug-resistant and extensively drug-resistant pseudomonas aeruginosa infections. Clin Microbiol Rev. 2019;32(4). doi: 10.1128/CMR.00031-19 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hong MC, Hsu DI, Bounthavong M. Ceftolozane/tazobactam: a novel antipseudomonal cephalosporin and β-lactamase-inhibitor combination. Infect Drug Resist. 2013;6:215–223. doi: 10.2147/IDR.S36140 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Giacobbe DR, Bassetti M, De Rosa FG, et al. Ceftolozane/tazobactam: place in therapy. Expert Rev Anti Infect Ther. 2018;16(4):307–320. doi: 10.1080/14787210.2018.1447381 [DOI] [PubMed] [Google Scholar]
- 11.Sharma R, Park TE, Moy S. Ceftazidime-Avibactam: a Novel Cephalosporin/β-Lactamase Inhibitor Combination for the Treatment of Resistant Gram-negative Organisms. Clin Ther. 2016;38(3):431–444. doi: 10.1016/j.clinthera.2016.01.018 [DOI] [PubMed] [Google Scholar]
- 12.Zasowski EJ, Rybak JM, Rybak MJ. The β-Lactams Strike Back: ceftazidime-Avibactam. Pharmacotherapy. 2015;35(8):755–770. doi: 10.1002/phar.1622 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gentile I, Maraolo AE, Borgia G. What is the role of the new β-lactam/β-lactamase inhibitors ceftolozane/tazobactam and ceftazidime/avibactam? Expert Rev Anti Infect Ther. 2016;14(10):875–878. doi: 10.1080/14787210.2016.1233060 [DOI] [PubMed] [Google Scholar]
- 14.Petty LA, Henig O, Patel TS, Pogue JM, Kaye KS. Overview of meropenem-vaborbactam and newer antimicrobial agents for the treatment of carbapenem-resistant. Infect Drug Resist. 2018;11:1461–1472. doi: 10.2147/IDR.S150447 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Surel UNK, Marzec M, Savege PB, Bucki R. Ceragenins-a new weapon to fight multidrug resistant bacterial infections. Med Studies. 2014;207–213. doi: 10.5114/ms.2014.45428 [DOI] [Google Scholar]
- 16.Hashemi M, Holden B, Durnas B, Bucki R, Savage P. Ceragenins as mimics of endogenous antimicrobial peptides. J Antimicrob Agents. 2017;3(1000141):2472–1212.1000141. doi: 10.4172/2472-1212.1000141 [DOI] [Google Scholar]
- 17.Epand RF, Pollard JE, Wright JO, Savage PB, Epand RM. Depolarization, bacterial membrane composition, and the antimicrobial action of ceragenins. Antimicrob Agents Chemother. 2010;54(9):3708–3713. doi: 10.1128/AAC.00380-10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hashemi MM, Holden BS, Savage PB. Ceragenins as non-peptide mimics of endogenous antimicrobial peptides. Fighting Antimicrobial Resist. 2018;1:139–169. [Google Scholar]
- 19.Leszczynska K, Namiot D, Byfield FJ, et al. Antibacterial activity of the human host defence peptide LL-37 and selected synthetic cationic lipids against bacteria associated with oral and upper respiratory tract infections. J Antimicrob Chemother. 2013;68(3):610–618. doi: 10.1093/jac/dks434 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Durnaś B, Wnorowska U, Pogoda K, et al. Candidacidal Activity of Selected Ceragenins and Human Cathelicidin LL-37 in Experimental Settings Mimicking Infection Sites. PLoS One. 2016;11(6):e0157242. doi: 10.1371/journal.pone.0157242 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lara D, Feng Y, Bader J, Savage PB, Maldonado RA. Anti-trypanosomatid activity of ceragenins. J Parasitol. 2010;96(3):638–642. doi: 10.1645/GE-2329.1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Howell MD, Streib JE, Kim BE, et al. Ceragenins: a class of antiviral compounds to treat orthopox infections. J Invest Dermatol. 2009;129(11):2668–2675. doi: 10.1038/jid.2009.120 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Chmielewska SJ, Skłodowski K, Piktel E, et al. NDM-1 Carbapenemase-Producing Enterobacteriaceae are Highly Susceptible to Ceragenins CSA-13, CSA-44, and CSA-131. Infect Drug Resist. 2020;13:3277–3294. doi: 10.2147/IDR.S261579 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hashemi MM, Holden BS, Taylor MF, et al. Antibacterial and Antifungal Activities of Poloxamer Micelles Containing Ceragenin CSA-131 on Ciliated Tissues. Molecules. 2018;23(3):596. doi: 10.3390/molecules23030596 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ozbek-Celik B, Damar-Celik D, Mataraci-Kara E, Bozkurt-Guzel C, Savage PB. Comparative in vitro activities of first and second-generation ceragenins alone and in combination with antibiotics against multidrug-resistant. Antibiotics. 2019;8(3). doi: 10.3390/antibiotics8030130 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.EUCAST. Antimicrobial susceptibility testing (AST) of bacteria. Disk diffusion methodology. Detailed description of the EUCAST disk diffusion test. Version 9.0, 2021, https://www.eucast.org/ast_of_bacteria/disk_diffusion_methodology/
- 27.Lee K, Lim YS, Yong D, Yum JH, Chong Y. Evaluation of the Hodge test and the imipenem-EDTA double-disk synergy test for differentiating metallo-beta-lactamase-producing isolates of Pseudomonas spp. and Acinetobacter spp. J Clin Microbiol. 2003;41(10):4623–4629. doi: 10.1128/jcm.41.10.4623-4629.2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Doi Y, Potoski BA, Adams-Haduch JM, Sidjabat HE, Pasculle AW, Paterson DL. Simple disk-based method for detection of Klebsiella pneumoniae carbapenemase-type beta-lactamase by use of a boronic acid compound. J Clin Microbiol. 2008;46(12):4083–4086. doi: 10.1128/JCM.01408-08 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.EUCAST. Antimicrobial susceptibility testing (AST) of bacteria. MIC determination of non-fastidious and fastidious organisms. Version 3.0, 2021. https://www.eucast.org/fileadmin/src/media/PDFs/EUCAST_files/MIC_testing/Reading_guide_BMD_v_3.0_2021.pdf
- 30.Ding B, Guan Q, Walsh JP, et al. Correlation of the antibacterial activities of cationic peptide antibiotics and cationic steroid antibiotics. J Med Chem. 2002;45(3):663–669. doi: 10.1021/jm0105070 [DOI] [PubMed] [Google Scholar]
- 31.Hashemi MM, Rovig J, Weber S, Hilton B, Forouzan MM, Savage PB. Susceptibility of colistin-resistant, gram-negative bacteria to antimicrobial peptides and ceragenins. Antimicrob Agents Chemother. 2017;61(8). doi: 10.1128/AAC.00292-17 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Pollard JE, Snarr J, Chaudhary V, et al. In vitro evaluation of the potential for resistance development to ceragenin CSA-13. J Antimicrob Chemother. 2012;67(11):2665–2672. doi: 10.1093/jac/dks276 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Leszczyńska K, Namiot A, Cruz K, et al. Potential of ceragenin CSA-13 and its mixture with pluronic F-127 as treatment of topical bacterial infections. J Appl Microbiol. 2011;110(1):229–238. doi: 10.1111/j.1365-2672.2010.04874.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Olekson MA, You T, Savage PB, Leung KP. Antimicrobial ceragenins inhibit biofilms and affect mammalian cell viability and migration. FEBS Open Bio. 2017;7(7):953–967. doi: 10.1002/2211-5463.12235 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Piktel E, Markiewicz KH, Wilczewska AZ, et al. Quantification of Synergistic Effects of Ceragenin CSA-131 combined with iron oxide magnetic nanoparticles against cancer cells. Int J Nanomedicine. 2020;15:4573–4589. doi: 10.2147/IJN.S255170 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Magiorakos AP, Srinivasan A, Carey RB, et al. Multidrug-resistant, extensively drug-resistant and pandrug-resistant bacteria: an international expert proposal for interim standard definitions for acquired resistance. Clin Microbiol Infect. 2012;18(3):268–281. doi: 10.1111/j.1469-0691.2011.03570.x [DOI] [PubMed] [Google Scholar]
- 37.EUCAST. Breakpoint tables for interpretation of MICs and zone diameters. Version 11.0. 2021. https://www.eucast.org/eucast_news/news_singleview/?tx_ttnews%5Btt_news%5D=406&cHash=9a92d976312c67c9b935f1f75bb5b71a
- 38.Mouton JW, Muller AE, Canton R, Giske C, Kahlmeter G, Turnidge J. MIC-based dose adjustment: facts and fables-authors’ response. J Antimicrob Chemother. 2018;73(9):2585–2586. doi: 10.1093/jac/dky195 [DOI] [PubMed] [Google Scholar]
- 39.Kazmierczak KM, Rabine S, Hackel M, et al. Multiyear, Multinational Survey of the Incidence and Global Distribution of Metallo-β-Lactamase-Producing Enterobacteriaceae and Pseudomonas aeruginosa. Antimicrob Agents Chemother. 2016;60(2):1067–1078. doi: 10.1128/AAC.02379-15 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Dalmolin TV, de Lima-morales D, Barth AL. Plasmid-mediated colistin resistance: what do we know? J Infect. 2018;1(2). doi: 10.29245/2689-9981/2018/2.1109 [DOI] [Google Scholar]
- 41.Almutairy R, Aljrarri W, Noor A, et al. Impact of Colistin Dosing on the Incidence of Nephrotoxicity in a Tertiary Care Hospital in Saudi Arabia. Antibiotics. 2020;9(8). doi: 10.3390/antibiotics9080485 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Karaiskos I, Lagou S, Pontikis K, Rapti V, Poulakou G. The “Old” and the “New” Antibiotics for MDR Gram-Negative Pathogens: for Whom, When, and How. Front Public Health. 2019;7:151. doi: 10.3389/fpubh.2019.00151 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Bassetti M, Vena A, Croxatto A, Righi E, Guery B. How to manage Pseudomonas aeruginosa infections. Drugs Context. 2018;7:212527. doi: 10.7573/dic.212527 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Carvalhaes CG, Shortridge D, Sader HS, Castanheira M. Activity of Meropenem-Vaborbactam against Bacterial Isolates Causing Pneumonia in Patients in U.S. Hospitals during 2014 to 2018. Antimicrob Agents Chemother. 2020;64(3). doi: 10.1128/AAC.02177-19 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Castanheira M, Huband MD, Mendes RE, Flamm RK. Meropenem-Vaborbactam Tested against Contemporary Gram-Negative Isolates Collected Worldwide during 2014, Including Carbapenem-Resistant, KPC-Producing, Multidrug-Resistant, and Extensively Drug-Resistant Enterobacteriaceae. Antimicrob Agents Chemother. 2017;61(9). doi: 10.1128/AAC.00567-17 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Buehrle DJ, Shields RK, Chen L, et al. Evaluation of the in vitro activity of ceftazidime-avibactam and ceftolozane-tazobactam against meropenem-resistant pseudomonas aeruginosa isolates. Antimicrob Agents Chemother. 2016;60(5):3227–3231. doi: 10.1128/AAC.02969-15 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Alatoom A, Elsayed H, Lawlor K, et al. Comparison of antimicrobial activity between ceftolozane-tazobactam and ceftazidime-avibactam against multidrug-resistant isolates of Escherichia coli, Klebsiella pneumoniae, and Pseudomonas aeruginosa. Int J Infect Dis. 2017;62:39–43. doi: 10.1016/j.ijid.2017.06.007 [DOI] [PubMed] [Google Scholar]
- 48.Grupper M, Sutherland C, Nicolau DP. Multicenter Evaluation of Ceftazidime-Avibactam and Ceftolozane-Tazobactam Inhibitory Activity against Meropenem-Nonsusceptible Pseudomonas aeruginosa from Blood, Respiratory Tract, and Wounds. Antimicrob Agents Chemother. 2017;61(10). doi: 10.1128/AAC.00875-17 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Humphries RM, Hindler JA, Wong-Beringer A, Miller SA. Activity of ceftolozane-tazobactam and ceftazidime-avibactam against beta-lactam-resistant pseudomonas aeruginosa isolates. Antimicrob Agents Chemother. 2017;61(12). doi: 10.1128/AAC.01858-17 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Mirza HC, Hortaç E, Koçak AA, et al. In vitro activity of ceftolozane-tazobactam and ceftazidime-avibactam against clinical isolates of meropenem-non-susceptible Pseudomonas aeruginosa: a two-centre study. J Glob Antimicrob Resist. 2020;20:334–338. doi: 10.1016/j.jgar.2019.09.016 [DOI] [PubMed] [Google Scholar]
- 51.Hirsch EB, Brigman HV, Zucchi PC, et al. Ceftolozane-tazobactam and ceftazidime-avibactam activity against β-lactam-resistant Pseudomonas aeruginosa and extended-spectrum β-lactamase-producing Enterobacterales clinical isolates from U.S. medical centres. J Glob Antimicrob Resist. 2020;22:689–694. doi: 10.1016/j.jgar.2020.04.017 [DOI] [PubMed] [Google Scholar]
- 52.Sid Ahmed MA, Khan FA, Sultan AA, et al. β-lactamase-mediated resistance in MDR-Pseudomonas aeruginosa from Qatar. Antimicrob Resist Infect Control. 2020;9(1):170. doi: 10.1186/s13756-020-00838-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Evans SR, Tran TTT, Hujer AM, et al. Rapid Molecular Diagnostics to Inform Empiric Use of Ceftazidime/Avibactam and Ceftolozane/Tazobactam Against Pseudomonas aeruginosa: PRIMERS IV. Clin Infect Dis. 2019;68(11):1823–1830. doi: 10.1093/cid/ciy801 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Montravers P, Bassetti M. The ideal patient profile for new beta-lactam/beta-lactamase inhibitors. Curr Opin Infect Dis. 2018;31(6):587–593. doi: 10.1097/QCO.0000000000000490 [DOI] [PubMed] [Google Scholar]
- 55.Giani T, Arena F, Pollini S, et al. Italian nationwide survey on Pseudomonas aeruginosa from invasive infections: activity of ceftolozane/tazobactam and comparators, and molecular epidemiology of carbapenemase producers. J Antimicrob Chemother. 2018;73(3):664–671. doi: 10.1093/jac/dkx453 [DOI] [PubMed] [Google Scholar]
- 56.Patel TS, Pogue JM, Mills JP, Kaye KS. Meropenem-vaborbactam: a new weapon in the war against infections due to resistant Gram-negative bacteria. Future Microbiol. 2018;13:971–983. doi: 10.2217/fmb-2018-0054 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Amorese V, Donadu M, Usai D, et al. In vitro activity of essential oils against Pseudomonas aeruginosa isolated from infected hip implants. J Infect Dev Ctries. 2018;12(11):996–1001. doi: 10.3855/jidc.10988 [DOI] [PubMed] [Google Scholar]
- 58.Donadu M, Usai D, Pinna A, et al. In vitro activity of hybrid lavender essential oils against multidrug resistant strains of Pseudomonas aeruginosa. J Infect Dev Ctries. 2018;12(1):9–14. doi: 10.3855/jidc.9920 [DOI] [PubMed] [Google Scholar]
- 59.Chaves-López C, Usai D, Donadu MG, et al. Potential of Borojoa patinoi Cuatrecasas water extract to inhibit nosocomial antibiotic resistant bacteria and cancer cell proliferation in vitro. Food Funct. 2018;9(5):2725–2734. doi: 10.1039/c7fo01542a [DOI] [PubMed] [Google Scholar]
- 60.Bozkurt Guzel C, Oyardi O, Savage P. Comparative in vitro antimicrobial activities of CSA-142 and CSA-192, second-generation ceragenins, with CSA-13 against various microorganisms. J Chemother. 2018;30(6–8):332–337. doi: 10.1080/1120009X.2018.1534567 [DOI] [PubMed] [Google Scholar]
- 61.Vila-Farrés X, Callarisa AE, Gu X, Savage PB, Giralt E, Vila J. CSA-131, a ceragenin active against colistin-resistant Acinetobacter baumannii and Pseudomonas aeruginosa clinical isolates. Int J Antimicrob Agents. 2015;46(5):568–571. doi: 10.1016/j.ijantimicag.2015.08.003 [DOI] [PubMed] [Google Scholar]
- 62.Bozkurt Güzel C, Avci NM, Savage P. Activities of the Cationic Steroid Antibiotics CSA-13, CSA-131, CSA-138, CSA-142, and CSA-192 Against Carbapenem-resistant. Turk J Pharm Sci. 2020;17(1):63–67. doi: 10.4274/tjps.galenos.2018.26566 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Wnorowska U, Piktel E, Durnaś B, Fiedoruk K, Savage PB, Bucki R. Use of ceragenins as a potential treatment for urinary tract infections. BMC Infect Dis. 2019;19(1):369. doi: 10.1186/s12879-019-3994-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Durnaś B, Piktel E, Wątek M, et al. Anaerobic bacteria growth in the presence of cathelicidin LL-37 and selected ceragenins delivered as magnetic nanoparticles cargo. BMC Microbiol. 2017;17(1):167. doi: 10.1186/s12866-017-1075-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Wnorowska U, Niemirowicz K, Myint M, et al. Bactericidal activities of cathelicidin LL-37 and select cationic lipids against the hypervirulent Pseudomonas aeruginosa strain LESB58. Antimicrob Agents Chemother. 2015;59(7):3808–3815. doi: 10.1128/AAC.00421-15 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Nagant C, Pitts B, Stewart PS, Feng Y, Savage PB, Dehaye JP. Study of the effect of antimicrobial peptide mimic, CSA-13, on an established biofilm formed by Pseudomonas aeruginosa. Microbiologyopen. 2013;2(2):318–325. doi: 10.1002/mbo3.77 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Latorre MC, Pérez-Granda MJ, Savage PB, et al. Endotracheal tubes coated with a broad-spectrum antibacterial ceragenin reduce bacterial biofilm in an in vitro bench top model. J Antimicrob Chemother. 2021;76(5):1168–1173. doi: 10.1093/jac/dkab019 [DOI] [PubMed] [Google Scholar]
- 68.Gu X, Jennings JD, Snarr J, Chaudhary V, Pollard JE, Savage PB. Optimization of ceragenins for prevention of bacterial colonization of hydrogel contact lenses. Invest Ophthalmol Vis Sci. 2013;54(9):6217–6223. doi: 10.1167/iovs.13-12664 [DOI] [PubMed] [Google Scholar]
- 69.Sinclair KD, Pham TX, Williams DL, Farnsworth RW, Loc-Carrillo CM, Bloebaum RD. Model development for determining the efficacy of a combination coating for the prevention of perioperative device related infections: a pilot study. J Biomed Mater Res B Appl Biomater. 2013;101(7):1143–1153. doi: 10.1002/jbm.b.32924 [DOI] [PubMed] [Google Scholar]
- 70.Williams DL, Haymond BS, Beck JP, et al. In vivo efficacy of a silicone‒cationic steroid antimicrobial coating to prevent implant-related infection. Biomaterials. 2012;33(33):8641–8656. doi: 10.1016/j.biomaterials.2012.08.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Schindeler A, Yu NY, Cheng TL, et al. Local delivery of the cationic steroid antibiotic CSA-90 enables osseous union in a rat open fracture model of Staphylococcus aureus infection. J Bone Joint Surg Am. 2015;97(4):302–309. doi: 10.2106/JBJS.N.00840 [DOI] [PubMed] [Google Scholar]
- 72.Hashemi MM, Rovig J, Bateman J, et al. Preclinical testing of a broad-spectrum antimicrobial endotracheal tube coated with an innate immune synthetic mimic. J Antimicrob Chemother. 2018;73(1):143–150. doi: 10.1093/jac/dkx347 [DOI] [PMC free article] [PubMed] [Google Scholar]