Abstract
Background
β-lactam antibiotics with dissimilar R-group side chains are associated with low cross-reactivity. Despite this, patients with β-lactam allergies are often treated with non-β-lactam alternative antibiotics. An institutional β-lactam side chain–based cross-reactivity chart was developed and implemented to guide in antibiotic selection for patients with β-lactam allergies.
Methods
This single-center, retrospective cohort study analyzed the impact of the implementation of the cross-reactivity chart for patients with pneumonia. Study time periods were defined as January 2013 to October 2014 prior to implementation of the chart (historical cohort) and January 2017 to October 2018 (intervention cohort) following implementation. The primary outcome was the incidence of β-lactam utilization between time periods. Propensity-weighted scoring and interrupted time-series analyses compared outcomes.
Results
A total of 341 and 623 patient encounters were included in the historical and intervention cohorts, respectively. There was a significantly greater use of β-lactams in the intervention cohort (70.4% vs 89.3%; P < .001) and decreased use of alternative therapy (58.1% vs 36%; P < .001). There was no difference in overall allergic reactions between cohorts (2.4% vs 1.6%; P = .738) or in reactions caused by β-lactams (1.3% vs 0.9%; P = .703). Inpatient mortality increased (0% vs 6.4%; P < .001); however, no deaths were due to allergic reactions. Healthcare facility–onset Clostridioides difficile infections decreased between cohorts (1.2% vs 0.2%; P = .032).
Conclusions
Implementation of a β-lactam side chain–based cross-reactivity chart and enhanced allergy assessment was associated with increased use of β-lactams in patients with pneumonia without increasing allergic reactions.
Keywords: allergy, antimicrobial stewardship, β-lactam side chain, pneumonia
Implementation of an internally developed, cross-reactivity chart for antibiotic selection in patients with documented β-lactam allergies, in combination with enhanced allergy assessment, significantly improved the prescribing of β-lactam antibiotic therapy in patients with pneumonia without increasing allergic reactions.
Clinically significant immunologically mediated cross-reactivity among β-lactams is associated with R-group side chain homology, and β-lactam antibiotics with dissimilar side chains are thought to be associated with lower rates of cross-reactivity [1–13]. Expert-recommended comprehensive allergy assessments typically incorporate the administration of structurally dissimilar β-lactam antibiotics [2, 7, 8]. Despite this, many patients with any β-lactam allergies are treated with non-β-lactam alternative therapies (eg, fluoroquinolones) that are associated with known risks including clinical failures and increased adverse events (eg, Clostridioides difficile infection [CDI]) [2, 10, 14–18].
Recommendations for antibiotic treatment of pneumonias include regimens containing either β-lactam or non-β-lactam alternative therapy [19, 20]. Preference is not specified; however, antimicrobial stewardship programs (ASPs) often utilize first-line β-lactam use to avoid risks of alternative therapy [2, 10, 14–18]. The presence of a β-lactam allergy has been associated with 21% lower use of β-lactams and increased use of non-β-lactam alternative antibiotics for the treatment of pneumonia [21].
Strategies to increase β-lactam use in patients with β-lactam allergies include pathways that incorporate patient assessment through historical review, direct oral challenges, penicillin skin testing (PST), and specialist evaluation [2, 22, 23]. Several strategies may present challenges for institutions limited by resources, legal authority, or the time-limiting nature of the intervention in relation to the volume of patients with pneumonia [2, 24]. For example, the availability of an allergist for inpatient consultations and inpatient PST in the United States in community hospitals has been reported as 14% and 18%, respectively [25]. In addition, the reliability of cephalosporin skin testing is debated, and a positive PST does not rule out use of all β-lactams [10, 26]. These challenges underscore the necessity for strategies able to optimize prescribing on a wide scale and in settings with limited resources.
A previous analysis at our institution showed significant improvements in β-lactam prescribing for surgical prophylaxis with no increase in incidence of allergic reactions following introduction of an antibiotic side chain–based cross-reactivity chart and enhanced allergy assessment processes [27]. We describe the impact of our approach for the management of patients with β-lactam allergies on antibiotic use in patients with pneumonia.
METHODS
Development and Integration
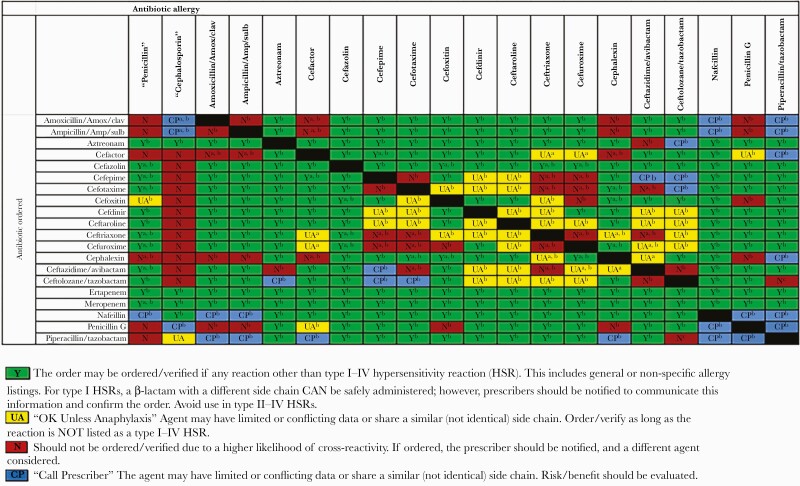
A multidisciplinary team at our hospital implemented an institutional approach for antibiotic stewardship of patients with β-lactam allergies in October 2014. Strategies included enhanced allergy assessment and the use of an internally developed, literature-based, antibiotic side chain–based cross-reactivity chart to guide prescribing (Figure 1). The chart provides guidance based on a patient’s allergy type and severity and the likelihood of reaction based on antibiotic side chains. No allergy-related changes were made in the electronic medical record for allergy documentation or to guide antibiotic ordering at the time of prescribing. Pharmacists actively intervened to assess for previous tolerance of β-lactams, ensure compliance with chart recommendations, and communicate with prescribers when warranted. Consultation with allergy/immunology specialists was available for assessment and interventions including PST, oral challenges, and desensitization when requested. In general, consultations were used sparingly and typically in cases with limited treatment options when the resistance profile of an organism and/or the patient allergy profiles made prescribing structurally dissimilar antibiotics challenging.
Figure 1.
Institutional β-lactam cross-reactivity chart.
Patient Consent Statement
The design of the work and waiver of patient consent has been approved by the St Joseph Mercy Health System Institutional Review Board.
Study Design
This single-center, retrospective, cohort study at a 548-bed community teaching hospital analyzed the impact of the implementation of allergy assessment processes with a cross-reactivity chart in adult patients with documented β-lactam allergies and pneumonia. Study time periods were defined as January 2013 to October 2014 prior to implementation (historical cohort) and January 2017 to October 2018 following implementation (intervention cohort). Patients ≥18 years of age who received an antibiotic during their inpatient stay with discharge coding indicating pneumonia were identified through our institution’s data warehouse. Pneumonia and concomitant disease states were identified by the Agency for Healthcare Research and Quality’s Clinical Classifications Software coding (Supplementary Table 1) [28]. Patients were excluded if they did not have a documented β-lactam allergy at their time of encounter. Patients with multiple admissions meeting inclusion criteria were counted as distinct encounters.
The primary outcome was the incidence of β-lactam use per patient encounter between time periods. Study outcomes also included the incidence of allergic reactions, 30-day readmission, all-cause mortality at discharge and 30-days postdischarge, healthcare facility–onset CDI (HO-CDI), hospital and antibiotic costs, antimicrobial utilization and duration of therapy, and incidence of β-lactam use per encounter by documented allergy severity between time periods. Additional analyses investigated β-lactam use by first and subsequent pneumonia encounters, and aggregate antibiotic days of therapy (DOTs) per 1000 patient-days by antibiotic and antibiotic class between time periods.
Preexisting allergies were grouped for analysis as drug intolerance, mild reactions, type I hypersensitivity reactions (HSRs), unknown allergies, and type II–IV HSRs (Supplementary Table 2) [29]. Patients with multiple β-lactam allergies of varying severity and antibiotic type were categorized according to the most severe reaction listed.
Use per patient encounter of β-lactam and other antibiotics was defined as any receipt of any dose during an encounter. Classification of antibiotic groupings is further defined in Supplementary Table 3 [30]. Patient-specific antibiotic DOTs and receipt of diphenhydramine, epinephrine, and hydrocortisone were obtained from institutional data warehouse reporting. Antibiotic duration per encounter was defined as the sum of unique calendar day DOTs during an encounter. The daily wholesale acquisition cost of antibiotics received was adjusted to 2021 dollars and multiplied by the respective antibiotic DOTs to determine antibiotic cost [31]. Antibiotic DOTs per 1000 patient-days were also analyzed in the aggregate by calendar quarter between time periods. Patient-days unique to the study population were calculated from encounter-specific LOS determined from admission dates in the respective admission calendar quarter. DOTs avoided was determined by multiplying difference in DOTs per patient day between cohorts by the patient-days in the intervention cohort.
Allergic reactions were confirmed through manual chart review and allergy-related International Classification of Diseases, Ninth and Tenth Revision, Clinical Modification (ICD-9-CM, ICD-10-CM) coding (Supplementary Table 4) [32–35]. Chart review to assess allergic reactions was performed in the following cases: patients with ICD-9/10-CM coding indicating a reaction; patients who received a β-lactam with any preexisting documented β-lactam type I–IV HSR; patients with inpatient or 30-day mortality; patients who had a new β-lactam allergy added to their record within 1 year of their encounter; patients who received an antibiotic from the same class as their documented β-lactam allergy (eg, cephalosporin receipt with any documented cephalosporin allergy); and patients who received diphenhydramine, epinephrine, or hydrocortisone at any point during their encounter. Potential reactions identified through electronic queries or manual review were reviewed by board-certified allergy/immunology physicians to attribute specific antibiotic or other drug-related cause based on timing, likelihood of reactions from antibiotics received, severity and type of reaction, and receipt of additional medications.
HO-CDI was determined through Centers for Disease Control and Prevention’s National Healthcare Safety Network reporting by trained infection and prevention personnel [36]. HO-CDI cases were reviewed to confirm onset post–receipt of antibiotics. Thirty-day mortality and 30-day readmissions were determined through a review of an institutional database that analyzed national and statewide death indexes and statewide health-system reporting of mortality and readmission. The cost of inpatient care was unavailable for patients after May 2018 due to changes in data warehouse reporting and excluded if unavailable.
Statistical Analysis
Descriptive statistics were presented for all study variables separately by study period. Differences in demographics, comorbidities, coinfections, and initiation of antibiotics within 48 hours of admission were tested between the 2 study periods to determine if the 2 periods differ significantly on potential confounders using unadjusted t tests or χ2 tests. The treatment effect was then estimated using propensity score–weighted versions of t tests and χ2 tests. The propensity score weights were calculated to adjust for potential confounding demographic and clinical characteristics including additional infectious disease diagnoses, intensive care unit (ICU) stay during encounter, hospital and ICU length of stay, malignancy, asthma, chronic obstructive pulmonary disease, acute and chronic renal failure, hemodialysis, chronic kidney disease, Charlson Comorbidity Index, an internal scoring tool for patient mortality, and allergy severity type [37]. Outcomes were assessed both prior to and after propensity score weighting. Propensity score weighting was not used in comparisons of variables with overlapping patients. Interrupted time series (ITS) analyses were performed on key outcomes of interest to analyze trends between time periods. Analyses were performed in R version 3.6.0 and SPSS IBM SPSS Statistics, version 27 (IBM, Armonk, New York) software, and significance was defined as a P value ≤ .05.
Post hoc analyses for exploratory purposes analyzed demographic and clinical characteristics between cohorts in patients who experienced 30-day mortality using unadjusted t test, Fisher exact test, or χ2 test.
RESULTS
A total of 6795 patient encounters with discharge coding indicating pneumonia were identified. Of these, 5831 (85.8%) were excluded due to a lack of β-lactam allergy (Supplementary Figure 1). Nine hundred sixty-four patient encounters were included in the final dataset with 341 in the historical cohort and 623 in the intervention cohort, respectively (Table 1). Baseline demographic and clinical variables were balanced after propensity score weighting with the exceptions of patients with concomitant mycoses infections, infective arthritis and osteomyelitis, influenza, and malignancy. There were no differences in specific antibiotic allergies, and variations in several specific antibiotic allergy severity types between cohorts (Supplementary Table 5).
Table 1.
Demographics and Clinical Characteristics by Cohort
| Variable | Historical Cohort (n = 341) | Intervention Cohort (n = 623) | P Value | PS-Weighted P Value |
|---|---|---|---|---|
| Categorical variables | ||||
| Sex | .385 | .381 | ||
| Female | 226 (66.3) | 394 (63.2) | ||
| Male | 115 (33.7) | 229 (36.8) | ||
| Race | .152 | .267 | ||
| White | 310 (90.9) | 545 (87.5) | ||
| Other | 31 (9.1) | 78 (12.5) | ||
| Concomitant infection | ||||
| Septicemia | 122 (35.8) | 247 (39.7) | .266 | .696 |
| Urinary tract infection | 40 (11.7) | 75 (12) | .97 | .792 |
| Bacterial infection; unspecified site | 15 (4.4) | 55 (8.8) | .016 | .09 |
| Peri-, endo-, and myocarditis; cardiomyopathy | 16 (4.7) | 30 (4.8) | >.999 | .82 |
| Aspiration pneumonitis | 16 (4.7) | 24 (3.9) | .648 | .519 |
| Mycoses | 7 (2.1) | 35 (5.6) | .015 | .035 |
| Influenza | 5 (1.5) | 29 (4.7) | .017 | .035 |
| Skin and subcutaneous soft tissue infection | 6 (1.8) | 17 (2.7) | .47 | .186 |
| Intestinal infection | 4 (1.2) | 15 (2.4) | .282 | .112 |
| Viral infection | 3 (0.9) | 17 (2.7) | .091 | .249 |
| Peritonitis and intestinal abscess | 0 (0) | 6 (1) | .095 | .075 |
| Infective arthritis and osteomyelitis | 0 (0) | 12 (1.9) | .011 | .011 |
| Diabetes mellitus | 118 (34.6) | 222 (35.6) | .803 | .425 |
| Cardiovascular disease | 163 (47.8) | 372 (59.7) | <.001 | .714 |
| Malignancy | 75 (22) | 17 (2.7) | <.001 | .002 |
| Asthma | 48 (14.1) | 89 (14.3) | >.999 | .964 |
| COPD | 119 (34.9) | 293 (47) | <.001 | .216 |
| Acute and unspecified renal impairment | 83 (24.3) | 175 (28.1) | .238 | .525 |
| Chronic renal impairment | 86 (25.2) | 206 (33.1) | .014 | .745 |
| Hemodialysis | 72 (21.1) | 226 (36.3) | <.001 | .069 |
| ICU stay | 42 (12.3) | 77 (12.4) | >.999 | .973 |
| Receipt of antimicrobial within 48 hours of admission | 333 (97.7) | 608 (97.6) | >.999 | .831 |
| Allergy type | ||||
| Drug intolerance | 19 (5.6) | 45 (7.2) | .396 | .304 |
| Mild reaction | 69 (20.2) | 148 (23.8) | .242 | .796 |
| Type I HSR | 120 (35.2) | 262 (42.1) | .044 | .485 |
| Unknown allergy | 130 (38.1) | 161 (25.8) | <.001 | .133 |
| Type II–IV HSR | 3 (0.9) | 7 (1.1) | >.999 | .784 |
| Continuous variables | ||||
| Age, y, mean (SD) | 68.2 (15.7) | 70.5 (15.4) | .034 | .69 |
| Charlson Comorbidity Index, mean (SD) | 1.8 (1.3) | 2.3 (1.4) | <.001 | .124 |
| Length of stay, days, median (IQR) | 5 (3–8) | 5 (3–8) | .831 | .85 |
| ICU length of stay, days, median (IQR)a | 7 (2–14) | 5 (2–9) | .218 | .451 |
| Internal mortality indexb [37] | 2.9 (1) | 2.5 (1) | <.001 | .072 |
Data are presented as No. (%) unless otherwise specified.
Abbreviations: COPD, chronic obstructive pulmonary disease; HSR, hypersensitivity reaction; ICU, intensive care unit; IQR, interquartile range; PS, propensity score; SD, standard deviation.
Analysis performed on subset of patients with an ICU stay (historical cohort, n = 42; intervention cohort, n = 77).
Analysis performed on subset of patients with available data (historical cohort, n = 289; intervention cohort, n = 602).
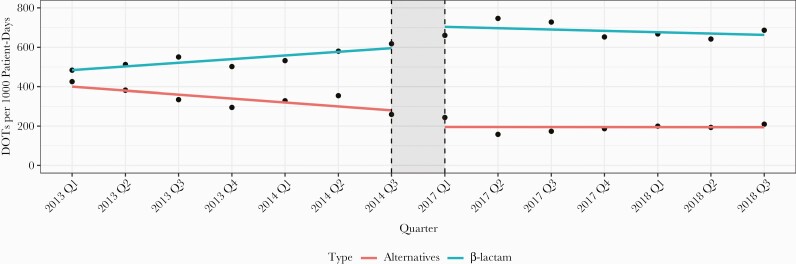
β-lactam use per patient encounter significantly increased in the intervention cohort (70.4% vs 89.3%; P < .001) (Table 2). β-lactam use increased in patients with documented mild reactions (78.3% vs 92.6%; P = .005) and those with type I HSRs (56.7% vs 85.9%; P < .001). There was no difference in total DOTs, duration, and antibiotic cost per encounter (Table 3). ITS analyses indicated there was a significant level increase (P = .011) and a flattening in slope of β-lactam utilization (P = .02). There was a significant level decrease (P = .024) and no change in the slope of alternative antibiotics utilization (P = .053) (Figure 2). β-lactam use improved in both first encounters (69% vs 88.2%; P < .001) and for patients previously admitted with pneumonia during the study time periods (80.5% vs 93.8%; P = .027) (Supplementary Table 6).
Table 2.
Outcomes by Cohort (Unadjusted and Propensity Score Weighted)
| Variable | Historical Cohort (n = 341) | Intervention Cohort (n = 623) | P Value | PS-Weighted P Value |
|---|---|---|---|---|
| Categorical outcomes | ||||
| Received β-lactam | 240 (70.4) | 556 (89.3) | <.001 | <.001 |
| Allergy type | ||||
| Drug intolerance | n = 19 | n = 45 | ||
| Received β-lactam | 17 (89.5) | 41 (91.1) | >.999 | |
| Mild reaction | n = 69 | n = 148 | ||
| Received β-lactam | 54 (78.3) | 137 (92.6) | .005 | |
| Type I HSR | n = 120 | n = 262 | ||
| Received β-lactam | 68 (56.7) | 225 (85.9) | <.001 | |
| Unknown allergy | n = 130 | n = 161 | ||
| Received β-lactam | 100 (76.9) | 148 (91.9) | <.001 | |
| Type II–IV HSR | n = 3 | n = 7 | ||
| Received β-lactam | 1 (33.3) | 5 (71.4) | .5 | |
| Readmission | 50 (14.7) | 102 (16.4) | .546 | .806 |
| In-hospital mortality | 0 (0) | 40 (6.4) | <.001 | <.001 |
| Received β-lactam | … | 37 (92.5) | ||
| Received alternative therapy | … | 34 (85) | ||
| 30-day mortality | 8 (2.3) | 89 (14.3) | <.001 | <.001 |
| Received β-lactama | 7 (87.5) | 80 (89.9) | >.999 | |
| Received alternative therapya | 8 (100) | 79 (88.8) | >.999 | |
| HO-CDI | 4 (1.2) | 1 (0.2) | .056 | .032 |
| Continuous outcomes, median (IQR) | ||||
| Inpatient costs, $b | 7921 (4611–14 600) | 7454 (4624–13 431) | .524 | .303 |
| Antibiotic days of therapy | 8 (5–13) | 8 (5–12) | .98 | .9 |
| Antibiotic duration, days | 5 (3–8) | 5 (3–7) | .426 | .62 |
| Antibiotic cost per patient-day, $ | 68 (28–143) | 76 (36–153) | .029 | .1 |
Data are presented as No. (%) unless otherwise specified.
Abbreviations: HO-CDI, healthcare facility–onset Clostridioides difficile infection; HSR, hypersensitivity reaction; PS, propensity score.
Data are analyzed in patients with 30-day mortality (historical cohort, n = 8; intervention cohort, n = 89).
Data are analyzed in patients with available data (historical cohort, n = 341; intervention cohort, n = 529).
Table 3.
Antimicrobial Utilization by Cohort
| Variablea | Historical Cohort (n = 341) | Intervention Cohort (n = 623) | P Value |
|---|---|---|---|
| β-lactam antibiotics | |||
| DOTs per encounter | 4 (3–7) | 4 (3–6) | .627 |
| Duration per encounter | 4 (3–7) | 4 (3–6) | .492 |
| Cefepime, ceftriaxone, piperacillin-tazobactam | |||
| Use per encounterb,c | 236 (69.2) | 541 (86.8) | <.001 |
| DOTs per encounter | 4 (3–7) | 4 (3–6) | .220 |
| Duration per encounter | 4 (2–7) | 4 (2–6) | .143 |
| Cephalosporins | |||
| Use per encounterb,c | 280 (82.1) | 483 (77.5) | .111 |
| DOTs per encounter | 4 (3–7) | 4 (3–6) | .260 |
| Duration per encounter | 4 (3–7) | 4 (3–6) | .181 |
| Cefepime | |||
| Use per encounterb | 131 (38.4) | 345 (55.4) | <.001 |
| DOTs per encounter | 4 (3–6) | 4 (2–5) | .012 |
| Duration per encounter | 4 (3–6) | 4 (2–5) | .012 |
| Ceftriaxone | |||
| Use per encounterb | 137 (40.2) | 274 (44) | .283 |
| DOTs per encounter | 3 (2–6) | 3 (2–5) | .423 |
| Duration per encounter | 3 (2–6) | 3 (2–5) | .423 |
| Other cephalosporins | |||
| Use per encounterb,c | 12 (3.5) | 43 (6.9) | .043 |
| DOTs per encounter | 2 (1–4) | 2 (1–2) | .727 |
| Duration per encounter | 2 (1–4) | 2 (1–2) | .661 |
| Piperacillin-tazobactam | |||
| Use per encounterb | 10 (2.9) | 48 (7.7) | .005 |
| DOTs per encounter | 2 (1–3) | 3 (1–5) | .282 |
| Duration per encounter | 2 (1–3) | 3 (1–5) | .282 |
| Antimicrobials other than β-lactams | |||
| Use per encounterb,c | 101 (29.6) | 67 (10.8) | <.001 |
| DOTs per encounter | 5 (3–8) | 5 (3–7) | .008 |
| Duration per encounter | 4 (3–7) | 4 (3–6) | .044 |
| Alternative pneumonia antibiotics | |||
| Use per encounterb,c | 198 (58.1) | 224 (36) | <.001 |
| DOTs per encounter | 3 (2–5) | 2 (1–5) | .027 |
| Duration per encounter | 3 (1–5) | 2 (1–4) | .023 |
| Fluoroquinolones | |||
| Use per encounterb,c | 166 (48.7) | 164 (26.3) | <.001 |
| DOTs per encounter | 3 (1–4) | 2 (1–3) | .002 |
| Duration per encounter | 3 (1–4) | 2 (1–3) | .003 |
| Carbapenems | |||
| Use per encounterb,c | 10 (2.9) | 33 (5.3) | .124 |
| DOTs per encounter | 3 (1–5) | 4 (3–8) | .068 |
| Duration per encounter | 3 (1–5) | 4 (3–8) | .070 |
| Aztreonam | |||
| Use per encounterb | 16 (4.7) | 19 (3) | .261 |
| DOTs per encounter | 2 (1–7) | 2 (1–3) | .526 |
| Duration per encounter | 2 (1–7) | 2 (1–3) | .526 |
| Aminoglycosides | |||
| Use per encounterb,c | 20 (5.9) | 19 (3) | .051 |
| DOTs per encounter | 1 (1–2) | 1 (1–1) | .200 |
| Duration per encounter | 1 (1–2) | 1 (1–1) | .200 |
| Clindamycin | |||
| Use per encounterb | 33 (9.7) | 42 (6.7) | .133 |
| DOTs per encounter | 1 (1–3) | 1 (1–3) | .883 |
| Duration per encounter | 1 (1–3) | 1 (1–3) | .883 |
| Gram-positive coverage antibiotics | |||
| Use per encounterb,c | 185 (54.3) | 296 (47.5) | .053 |
| DOTs per encounter | 3 (1–4) | 2 (1–3) | <.001 |
| Duration per encounter | 3 (1–4) | 2 (1–3) | <.001 |
| Vancomycin, intravenous | |||
| Use per encounterb | 184 (54) | 290 (46.5) | .033 |
| DOTs per encounter | 2 (1–3) | 2 (1–3) | .274 |
| Duration per encounter | 2 (1–3) | 2 (1–3) | .274 |
| Linezolid | |||
| Use per encounterb | 7 (2.1) | 8 (1.3) | .516 |
| DOTs per patient | 5 (1–7) | 4 (1–5) | .513 |
| Duration per encounter | 5 (1–7) | 4 (1–5) | .513 |
| Atypical coverage antibiotics | |||
| Use per encounterb,c | 157 (46) | 324 (52) | .088 |
| DOTs per encounter | 3 (1–2) | 4 (2–5) | .428 |
| Duration per encounter | 3 (2–5) | 4 (2–5) | .423 |
Data are presented as median (interquartile range) unless otherwise specified.
Abbreviations: DOTs, days of therapy; PS, propensity score.
Data reported in patients who received therapy.
Data are No. (%).
Patients receiving multiple antibiotics in category are counted once.
Figure 2.
Interrupted time-series analysis of β-lactam and alternative antibiotic days of therapy (DOTs) per 1000 patient-days, by calendar quarter.
β-lactams for the treatment of pneumonia increased (69.2% vs 86.8%; P < .001) without change in duration or DOTs. Within this grouping, cefepime use per encounter increased (38.4% vs 55.4%; P < .001) and was associated with shorter durations. Cefepime was used more frequently in patients with documented type I and unknown reactions (Supplementary Table 7). The use of piperacillin-tazobactam and other cephalosporins increased without changes in duration. Ceftriaxone use and duration was unchanged.
The use of alternative pneumonia antibiotics per encounter significantly decreased (58.1% vs 36%; P < .001), as did alternative antibiotic DOTs and duration. Decreases in this category were driven by a reduction in fluoroquinolone use per encounter (48.7% vs 26.3%; P < .001), DOTs (3 vs 2; P = .002), and duration (3 days vs 2 days; P = .003), leading to predicted avoidance of 568 fluoroquinolone DOTs. The use of vancomycin per encounter (54% vs 46.5%; P = .033), and gram-positive pneumonia antibiotic DOTs per encounter and duration per encounter decreased between cohorts.
There was no change in the incidence of allergic reactions overall (2.4% vs 1.6%; P = .738), nor in type I–IV reactions (0.9% vs 0.6%; P = .458) (Table 4). In the subset of patients who received β-lactams and in whom reactions were attributed to β-lactams, there was no change in total reactions (1.3% vs 0.9%; P = .703) or type I–IV reactions (1.3% vs 0.2%; P = .084). There was no change in allergic reactions attributed to alternative therapy (1.8% vs 0.9%; P = .221). In the intervention cohort, 1 patient (0.2%) experienced a type I–IV HSR attributed to β-lactam therapy, compared with 3 patients (0.5%) secondary to alternative therapy.
Table 4.
Allergic Reactions by Cohort
| Variable | Historical Cohort | Intervention Cohort | P Value | PS-Weighted P Value |
|---|---|---|---|---|
| Study cohort | n = 341 | n = 623 | ||
| Any allergic reaction | 8 (2.4) | 10 (1.6) | .573 | .738 |
| Drug intolerance | 3 (0.9) | 0 (0) | .044 | .025 |
| Mild reactions | 2 (0.6) | 6 (1) | .719 | .329 |
| Type I–IV combined reactions | 3 (0.9) | 4 (0.6) | .703 | .458 |
| Type I HSR | 1 (0.3) | 1 (0.2) | >.999 | .521 |
| Type II–IV HSR | 2 (0.6) | 3 (0.5) | >.999 | .616 |
| Allergic reaction (excluding drug intolerance reactions) | 5 (1.5) | 10 (1.6) | >.999 | .781 |
| Received β-lactam | n = 240 | n = 556 | ||
| Reaction secondary to β-lactam usea,b | 3 (1.3) | 5 (0.9) | .703 | |
| Drug intoleranceb | 0 (0) | 0 (0) | ||
| Mild reactionsb | 0 (0) | 4 (0.7) | .322 | |
| Type I–IV combined reactions | 3 (1.3) | 1 (0.2) | .084 | |
| Type I HSRb | 1 (0.4) | 0 (0) | .302 | |
| Type II–IV HSRb | 2 (0.8) | 1 (0.2) | .218 | |
| Received alternative therapy | n = 335 | n = 586 | ||
| Reaction secondary to alternative usea,c | 6 (1.8) | 5 (0.9) | .221 | |
| Drug intolerancec | 3 (0.9) | 0 (0) | .048 | |
| Mild reactionsc | 2 (0.6) | 2 (0.3) | .625 | |
| Type I–IV combined reactions | 1 (0.3) | 3 (0.5) | >.999 | |
| Type I HSRc | 0 (0) | 1 (0.2) | >.999 | |
| Type II–IV HSRc | 1 (0.3) | 2 (0.3) | >.999 | |
| Allergic reactions secondary to alternative use (excluding drug intolerance reactions)a,c | 3 (0.9) | 5 (0.9) | >.999 |
Data are presented as No. (%) unless otherwise specified.
Abbreviations: HSR, hypersensitivity reaction; PS, propensity score.
One allergic reaction was thought to be either from β-lactam or alternate therapy and included in both categories (historical cohort, n = 1).
Data were analyzed in patients who received β-lactam antibiotics (historical cohort, n = 240; intervention cohort, n = 556).
Data were analyzed in patients who received alternative antibiotics (historical cohort, n = 335; intervention cohort, n = 586).
Five of 556 patients (0.9%) who received a β-lactam in the intervention cohort experienced an HSR attributed to a β-lactam. Patients 1 and 2 were given cefepime with a documented mild penicillin allergy. Patient 3 was given ceftriaxone with mild cephalexin and penicillin allergies. Patient 4 was given piperacillin-tazobactam with a preexisting mild cephalexin allergy and ceftriaxone type I HSR. Patient 5 was given amoxicillin-clavulanate with a mild penicillin allergy. Antibiotic selection was consistent with cross-reactivity chart recommendations for patients 1–3. Patient 4 received therapy where the chart recommends a risk benefit assessment for β-lactam use. Nonconsistent antibiotic selection occurred in patient 5.
HO-CDI decreased between cohorts (1.2% vs 0.2%; P = .032). There was a significant increase in both inpatient mortality (0% vs 6.4%; P < .001) and 30-day mortality (2.4% vs 14.3%; P < .001). There was no difference in β-lactam or alternative therapy use between cohorts in patients who experienced mortality at 30 days. No inpatient deaths were attributed to an allergic reaction. A single patient within the mortality cohort experienced a reaction, which resolved 10 days prior to death. There were no changes in 30-day readmission or hospital cost.
Post hoc analyses of patients who experienced 30-day mortality showed that patients in the historical cohort had higher malignancy (50% vs 4.5%; P = .001) and diabetes (75% vs 31.5%; P = .021). No other significant differences were noted.
DISCUSSION
Results of our analysis demonstrated an increase in β-lactam utilization in patients with documented β-lactam allergies and pneumonia following implementation of a side chain–based cross-reactivity chart and enhanced allergy assessment to guide antibiotic prescribing and ASP surveillance and intervention. There were no increases in β-lactam allergic reactions, including type I–IV HSRs, despite increased use.
These results are similar to previously reported results from our institution, which detailed significant improvements in β-lactam selection for surgical prophylaxis [27]. Analyses across similar time periods show increased prescribing resulting in 85% and 89% β-lactam use for surgical prophylaxis and pneumonia, respectively. Importantly, this increased β-lactam use has not resulted in increases in allergic reactions in either population. A total of 1094 surgical prophylaxis patients with documented β-lactam allergies received β-lactam antibiotics (98% cefazolin) resulting in 3 (0.3%) reactions. Cefazolin does not share a side chain with other β-lactams, and use of later-generation cephalosporin- or penicillin-based regimens used for the treatment of pneumonia inherently carry more cross-reactivity risk than cefazolin due to increased side chain similarities [11–13]. Despite this, our results also showed low allergic reaction rates in intervention cohort patients receiving β-lactams (0.9%).
This rate is comparable to reports of allergy incidence in individuals exposed to different classes of antibiotics. Macy and Poon reported the incidence of new allergies in outpatients exposed to various classes of antibiotics including penicillins (1.3%), cephalosporins (0.9%), and fluoroquinolones (0.8%) [38]. In another large database study, 1.1% of patients with a history of a penicillin allergy had a new cephalosporin allergy reported within 30 days of receipt of a cephalosporin course compared to 0.4% of those without an antibiotic allergy, or 0.7% with cephalosporin allergies [18]. Patients in our analysis were as likely to develop an allergic reaction from alternative therapy as they were from β-lactam treatment. Results further contribute to the body of literature supporting the safety profile of structurally dissimilar prescribing.
To our knowledge, this is the only report of the use of a cross-reactivity chart combined with enhanced allergy assessment in patients with pneumonia. On a grander scale, despite the availability of and dissemination of high-quality cross-reactivity charts, reports of strategies incorporating structurally dissimilar antibiotic prescribing are limited. Allergy management pathways to date that incorporate recommendations based on a cross-reactivity foundation have utilized computerized guideline applications or medical record alerts. For example, a computerized guideline application with clinical decision support incorporating PST and specialist evaluation along with recommendations for later-generation cephalosporin use in patients with mild reactions, and with or without test dosing in patients with type I HSRs, increased prescribing of penicillins and cephalosporins from 38% to 50% in patients with penicillin allergies [39]. Simply removing the electronic medical record warning to avoid cephalosporin use in patients with penicillin allergies increased cephalosporin use from 18% to 27% without differences in anaphylaxis or new allergies at a large, integrated health system [40]. We believe incorporation of active interventions through pharmacist and ASP efforts contributed to improvements. We have described results in surgery and pneumonia populations; however, our evaluation process is for all antibiotic indications in β-lactam allergic patients. Active strategies incorporating side chain–based allergy assessment and active pharmacist review may be an easily implemented, effective, and deployable strategy for ASPs to incorporate into their existing allergy stewardship strategies with the potential for wide-scale influential change.
Our analysis also noted a significant reduction in HO-CDI in the intervention cohort. Patients with penicillin allergies have been shown to have a 23%–26% increased incidence of CDI [18]. A United Kingdom study reported 35% of the increased risk was due to receipt of β-lactam alternatives including fluoroquinolones, clindamycin, and macrolides. Fluoroquinolones alone accounted for 16% of the increased risk [16]. The reduction in HO-CDI was a secondary outcome, likely multifactorial, and influenced by additional institutional initiatives to reduce CDI; however, decreased use and exposure to fluoroquinolones, may have contributed to improvements in this important metric.
There was a significant increase in 30-day mortality and in-hospital mortality in the intervention cohort, though no inpatient deaths were attributed to allergic reactions. Post hoc analyses confirmed the validity of patient identification queries and mortality reporting. In general, individuals in the intervention group were older, had higher Carlson Comorbidity Index scores, and higher risk for mortality as indicated by an internal mortality index [37]. With propensity score weighting, these differences were not significant. In patients who died by 30 days, the only differences between groups were higher malignancy and diabetes in the historical cohort and there was no difference in β-lactam or alternative use between time periods. We are unaware of mortality differences associated with increased β-lactam use or decreased alternative therapy use in patients with pneumonia. The finding that no patients died in the hospital in the historical cohort is noteworthy. Similar β-lactam and alternative use in patients who died suggests this finding is unrelated to antibiotic selection; however, further research is warranted.
This study had several limitations including the single-center, retrospective nature of the analysis. Documented allergies and reactions were limited to those present in the medical record at the time of encounter. Allergy clarification and history may have occurred following patient discussions, which further guided prescribing. Our analysis of allergic reactions may have been impacted if reactions were not properly coded or documented, not identified by investigators during manual chart review, or occurred postdischarge without a new β-lactam allergy charted in the medical record within 1 year. Alternative antibiotic allergic reactions may have been undercounted since postdischarge queries for the addition of new allergies were limited to β-lactam allergies. Potential unaccounted confounders such as risk factors for multidrug-resistant infections, culture and susceptibility variations, and changes in the mix of hospital-associated and community-acquired pneumonia cases were not assessed. Variations in any of these metrics could influence antibiotic selection and our lack of inclusion is an acknowledged limitation.
Antibiotic use could have occurred any time during the encounter and the entirety of use may not have been exclusively for the treatment of pneumonia. For example, a patient may have received various courses of antibiotics depending on concomitant infections. The comparison cohorts were well matched following propensity score weighting for concomitant infections, and commonly used pneumonia antibiotics were reported as a categorical grouping; however, this lack of pneumonia-specific sophistication is a limitation.
Our ASP continued to evolve throughout the extended timeframe of this study. Strategies included enhanced ASP surveillance and intervention in patients at the highest risk of mortality, participation in a statewide initiative to improve antibiotic prescribing in pneumonia and urinary tract infections, regularly updated institutional guidelines for common infectious diseases that often encouraged structurally dissimilar prescribing, and active syndrome-specific interventions targeting pneumonia and other common infectious diseases [41–43]. In general, these strategies emphasized appropriate antibiotic selection and duration. For example, a pneumonia care bundle was implemented, which alerted pharmacists to prescribed antibiotics for the treatment of pneumonia through an alert built in commercially available pharmacy surveillance software. Active surveillance, communication, and intervention by pharmacists were expected to ensure that a suite of predefined quality measures was met. Structurally dissimilar antibiotic selection was implied, and treatment duration was emphasized. These overlapping ASP strategies encouraged active scrutiny of antibiotic use and likely contributed to results. Further investigations comparing effective strategies to optimize antibiotic use in patients with β-lactam allergies are encouraged.
We describe results of a real-world implementation of a cross-reactivity chart to guide structurally dissimilar antibiotic prescribing in patients with pneumonia. Adoption was aided by targeted active pharmacist intervention, ASP oversight, and multidisciplinary buy-in to promote β-lactam use. Initiatives to improve structurally dissimilar β-lactam prescribing may present a resource-friendly, deployable approach that can work in combination with the suite of available allergy management strategies.
In conclusion, implementation of a β-lactam side chain–based cross-reactivity chart combined with enhanced allergy assessment was associated with increased utilization of β-lactams and reduced non-B-lactam alternative therapy use in patients with pneumonia without increasing allergic reactions.
Supplementary Data
Supplementary materials are available at Open Forum Infectious Diseases online. Consisting of data provided by the authors to benefit the reader, the posted materials are not copyedited and are the sole responsibility of the authors, so questions or comments should be addressed to the corresponding author.
Notes
Acknowledgments. The authors wish to thank Kishore Anam, Kara Brockhaus, PharmD; Paul Brumley, PharmD; Alyssa Divens, PharmD; Cheryl Morrin; Austin Pytlowany, PharmD; and Michelle Schultz, PharmD, for their assistance with this project at various points throughout the development, implementation, and review.
Financial support. This work was funded by an institutional research grant from the St Joseph Mercy Health System Research Committee (grant number E-20-931).
Potential conflicts of interest. All authors: No reported conflicts of interest.
All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
References
- 1. Campagna JD, Bond MC, Schabelman E, Hayes BD.. The use of cephalosporins in penicillin-allergic patients: a literature review. J Emerg Med 2012; 42:612–20. [DOI] [PubMed] [Google Scholar]
- 2. Shenoy ES, Macy E, Rowe T, Blumenthal KG.. Evaluation and management of penicillin allergy: a review. JAMA 2019; 321:188–99. [DOI] [PubMed] [Google Scholar]
- 3. DePestel DD, Benninger MS, Danziger L, et al. Cephalosporin use in treatment of patients with penicillin allergies. J Am Pharm Assoc 2008; 48:530–40. [DOI] [PubMed] [Google Scholar]
- 4. Zagursky RJ, Pichichero ME.. Cross-reactivity in β-lactam allergy. J Allergy Clin Immunol Pract 2018; 6:73–81. [DOI] [PubMed] [Google Scholar]
- 5. Picard M, Robitaille G, Karam F, et al. Cross-reactivity to cephalosporins and carbapenems in penicillin-allergic patients: two systematic reviews and meta-analyses. J Allergy Clin Immunol Pract 2019; 7:2722–38.e5. [DOI] [PubMed] [Google Scholar]
- 6. Trubiano JA, Stone CA, Grayson ML, et al. The 3 Cs of antibiotic allergy-classification, cross-reactivity, and collaboration. J Allergy Clin Immunol Pract 2017; 5:1532–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Blumenthal KG, Shenoy ES, Wolfson AR, et al. Addressing inpatient beta-lactam allergies: a multihospital implementation. J Allergy Clin Immunol Pract 2017; 5:616–25.e7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Blumenthal KG, Shenoy ES, Varughese CA, et al. Impact of a clinical guideline for prescribing antibiotics to inpatients reporting penicillin or cephalosporin allergy. Ann Allergy Asthma Immunol 2015; 115:294–300.e2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Goodman EJ, Morgan MJ, Johnson PA, et al. Cephalosporins can be given to penicillin-allergic patients who do not exhibit an anaphylactic response. J Clin Anesth 2001; 13:561–4. [DOI] [PubMed] [Google Scholar]
- 10. Macy E, Blumenthal KG.. Are cephalosporins safe for use in penicillin allergy without prior allergy evaluation? J Allergy Clin Immunol Pract 2018; 6:82–9. [DOI] [PubMed] [Google Scholar]
- 11. Pichichero ME. A review of evidence supporting the American Academy of Pediatrics recommendation for prescribing cephalosporin antibiotics for penicillin-allergic patients. Pediatrics 2005; 115:1048–57. [DOI] [PubMed] [Google Scholar]
- 12. Pichichero ME. Cephalosporins can be prescribed safely for penicillin-allergic patients. J Fam Pract 2006; 55:106–12. [PubMed] [Google Scholar]
- 13. Pichichero ME. Use of selected cephalosporins in penicillin-allergic patients: a paradigm shift. Diag Microbiol Infect Dis 2007; 57:13S–8S. [DOI] [PubMed] [Google Scholar]
- 14. MacFadden DR, LaDelfa A, Leen J, et al. Impact of reported beta-lactam allergy on inpatient outcomes: a multicenter prospective cohort study. Clin Infect Dis 2016; 63:904–10. [DOI] [PubMed] [Google Scholar]
- 15. Jeffres MN, Narayanan PP, Shuster JE, Schramm GE.. Consequences of avoiding β-lactams in patients with β-lactam allergies. J Allergy Clin Immunol 2016; 137:1148–53. [DOI] [PubMed] [Google Scholar]
- 16. Blumenthal KG, Lu N, Zhang Y, et al. Risk of meticillin resistant Staphylococcus aureus and Clostridium difficile in patients with a documented penicillin allergy: population based matched cohort study. BMJ 2018; 361:k2400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Pépin J, Saheb N, Coulombe MA, et al. Emergence of fluoroquinolones as the predominant risk factor for Clostridium difficile–associated diarrhea: a cohort study during an epidemic in Quebec. Clin Infect Dis 2005; 41:1254–60. [DOI] [PubMed] [Google Scholar]
- 18. Macy E, Contreras R.. Health care use and serious infection prevalence associated with penicillin “allergy” in hospitalized patients: a cohort study. J Allergy Clin Immunol 2014; 133:790–6. [DOI] [PubMed] [Google Scholar]
- 19. Metlay JP, Waterer GW, Long AC, et al. Diagnosis and treatment of adults with community-acquired pneumonia. An official clinical practice guideline of the American Thoracic Society and Infectious Diseases Society of America. Am J Respir Crit Care Med 2019; 200:e45–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Kalil AC, Metersky ML, Klompas M, et al. Management of adults with hospital-acquired and ventilator-associated pneumonia: 2016 clinical practice guidelines by the Infectious Diseases Society of America and the American Thoracic Society. Clin Infect Dis 2016; 63:e61–111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Mancini CM, Wimmer M, Schultz LT, et al. Association of penicillin or cephalosporin allergy documentation and antibiotic use in hospitalized patients with pneumonia. J Allergy Clin Immunol Pract 2021; 9:3060–8.e1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Wolfson AR, Huebner EM, Blumenthal KG.. Acute care beta-lactam allergy pathways: approaches and outcomes. Ann Allergy Asthma Immunol 2019; 123:16–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Barlam TF, Cosgrove SE, Abbo LM, et al. Implementing an antibiotic stewardship program: guidelines by the Infectious Diseases Society of America and the Society for Healthcare Epidemiology of America. Clin Infect Dis 2016; 62:e51–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Bland CM, Bookstaver PB, Griffith NC, et al. A practical guide for pharmacists to successfully implement penicillin allergy skin testing. Am J Health Syst Pharm 2019; 76:136–47. [DOI] [PubMed] [Google Scholar]
- 25. Mancini CM, Fu X, Zhang Y, et al. Penicillin allergy evaluation access: a national survey. Clin Infect Dis 2020; 71:2972–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Yoon SY, Park SY, Kim S, et al. Validation of the cephalosporin intradermal skin test for predicting immediate hypersensitivity: a prospective study with drug challenge. Allergy 2013; 68:938–44. [DOI] [PubMed] [Google Scholar]
- 27. Collins CD, Scheidel C, Anam K, et al. Impact of an antibiotic side-chain-based cross-reactivity chart combined with enhanced allergy assessment processes for surgical prophylaxis antimicrobials in patients with β-lactam allergies. Clin Infect Dis 2021; 72:1404–12. [DOI] [PubMed] [Google Scholar]
- 28. Healthcare Cost and Utilization Project. Tools and software. 2019. https://www.hcup-us.ahrq.gov/. Accessed 14 June 2021.
- 29. Gell PGH, Coombs RA.. The Classification of Allergic Reactions Underlying Disease. 1st ed. Oxford, UK: Blackwell; 1963:97. [Google Scholar]
- 30. van Santen KL, Edwards JR, Webb AK, et al. The standardized antimicrobial administration ratio: a new metric for measuring and comparing antibiotic use. Clin Infect Dis 2018; 67:179–85. [DOI] [PubMed] [Google Scholar]
- 31. American Academy of Pediatrics. Red Book Online. Greenwood Village, CO: Truven Health Analytics. Accessed 20 April 2021. [Google Scholar]
- 32. Saff RR, Li Y, Santhanakrishnan N, et al. Identification of inpatient allergic drug reactions using ICD-9-CM codes. J Allergy Clin Immunol Pract 2019; 7:259–64.e1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Saff RR, Camargo CA Jr, Clark S, et al. Utility of ICD-9-CM codes for identification of allergic drug reactions. J Allergy Clin Immunol Pract 2016; 4:114–9.e1. [DOI] [PubMed] [Google Scholar]
- 34. Banerji A, Rudders S, Clark S, et al. Retrospective study of drug-induced anaphylaxis treated in the emergency department or hospital: patient characteristics, management, and 1-year follow-up. J Allergy Clin Immunol Pract 2014; 2:46–51. [DOI] [PubMed] [Google Scholar]
- 35. Liang EH, Chen LH, Macy E.. Adverse reactions associated with penicillins, carbapenems, monobactams, and clindamycin: a retrospective population-based study. J Allergy Clin Pract 2020; 8:1302–13.e2. [DOI] [PubMed] [Google Scholar]
- 36. Centers for Disease Control and Prevention. Multidrug-resistant organism and Clostridioides difficile infection (MDRO/CDI) module. 2021. https://www.cdc.gov/nhsn/PDFs/pscManual/12pscMDRO_CDADcurrent.pdf. Accessed 15 June 2021.
- 37. Cowen ME, Strawderman RL, Czerwinski JL, et al. Mortality predictions on admission as a context for organizing care activities. J Hosp Med 2013; 8:229–35. [DOI] [PubMed] [Google Scholar]
- 38. Macy E, Poon K-YT.. Self-reported antibiotic allergy incidence and prevalence: age and sex effects. Am J Med 2009; 122:778.e1–7. [DOI] [PubMed] [Google Scholar]
- 39. Blumenthal KG, Wickner PG, Hurwitz S, et al. Tackling inpatient penicillin allergies: assessing tools for antimicrobial stewardship. J Allergy Clin Immunol 2017; 140:154–61.e6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Macy E, McCormick TA, Adams JL, et al. Association between removal of a warning against cephalosporin use in patients with penicillin allergy and antibiotic prescribing. JAMA Netw Open 2021; 4:e218367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Collins CD, Kollmeyer S, Scheidel C, et al. Impact of a mortality prediction rule for organizing and guiding antimicrobial stewardship program activities. Open Forum Infect Dis. 2021; 8:ofab056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Vaughn VM, Flanders SA, Snyder A, et al. Excess antibiotic treatment duration and adverse events in patients hospitalized with pneumonia: a multihospital cohort study. Ann Intern Med 2019; 171:153–63. [DOI] [PubMed] [Google Scholar]
- 43. Collins CD, Kabara JJ, Michienzi SM, Malani AN.. Impact of an antimicrobial stewardship care bundle to improve the management of patients with suspected or confirmed urinary tract infection. Infect Control Hosp Epidemiol 2016; 37:1499–501. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.