Abstract
Background
Hypertensive disorders during pregnancy are important causes of maternal mortality and morbidity worldwide. The long‐term outcome of surviving mothers will depend largely on whether intracranial haemorrhage or renal failure developed. Low‐dose dopamine is used for the prevention and treatment of acute renal failure, but its role in the management of pregnant women with severe pre‐eclampsia is unclear.
Objectives
To assess the effects of low‐dose dopamine used for oliguria in severe pre‐eclampsia on mothers and their children.
Search methods
We searched the Cochrane Pregnancy and Childbirth Group's Trials Register (May 2009).
Selection criteria
Randomised trials comparing low‐dose dopamine (dosages not higher than 5 microgram/kg/minute) with either placebo or no dopamine in women with severe pre‐eclampsia and acute renal failure, or who are considered to be at risk of acute renal failure.
Data collection and analysis
The two review authors assessed trial quality and data independently.
Main results
Only one randomised placebo controlled trial of six hours' duration, including 40 postpartum women, was found. This study showed a significant increase in urinary output over six hours in women receiving dopamine. It is unclear if this was of any benefit to the women.
Authors' conclusions
It is unclear whether low‐dose dopamine therapy for pre‐eclamptic women with oliguria is worthwhile. It should not be used other than in prospective trials.
Plain language summary
Low‐dose dopamine for women with severe pre‐eclampsia
No data on the use of low‐dose dopamine in women with severe pre‐eclampsia who have very low urine output.
Pre‐eclampsia is a condition in pregnancy involving high blood pressure and protein in the urine. Most women with mild pre‐eclampsia give birth without problems. However, severe pre‐eclampsia can cause major problems with the functioning of the liver, kidneys and blood clotting. Some women also have very low urine output, which causes further complications. Drugs which help to increase urine output may possibly help with this problem. Low‐dose dopamine has been suggested as one such drug, but the review found no trials of low‐dose dopamine for women in pregnancy who have severe pre‐eclampsia complicated by low urine output. It is suggested that this drug should first be tested in non‐pregnant women with very low urine output before it is considered for trials with pregnant women, because of the potential for severe adverse effects if the dose is exceeded.
Background
Hypertensive disorders during pregnancy remain the most important causes of maternal mortality in developed and developing countries (Anthony 1996; HMSO 1994). It has been estimated that as many as 50,000 mothers may die of hypertension in pregnancy annually. Most current classification systems of hypertensive disease in pregnancy are based on the presence of hypertension and proteinuria. In essence, hypertensive pregnant women are classified into three groups, depending on their underlying condition. They may have chronic (usually essential) hypertension, which is often known to have existed prior to pregnancy. Gestational hypertension develops during pregnancy. Pre‐eclampsia is defined as the development of significant proteinuria in a woman with underlying hypertension during pregnancy. It usually occurs during the second half of pregnancy and complicates 2% to 8% of pregnancies (WHO 1998). The development of pre‐eclampsia is associated with poorer maternal and perinatal outcomes. However, pre‐eclampsia is a multisystem disorder that affects essentially every organ and system in the body. Hypertension and proteinuria represent only two facets of a complex pathophysiological process characterised by widespread vascular endothelial damage and dysfunction in many systems. In a recent report from a developing country, 131 maternal deaths due to hypertensive disorders of pregnancy were analysed (Pattinson 1998). Seventy‐five deaths (57%) were associated with eclampsia. The major final cause of death was intracranial haemorrhage, while other causes included liver rupture and associated postpartum haemorrhage. Pulmonary oedema, kidney failure and disseminated intravascular coagulation were noted as contributory causes. Mothers can also survive these problems but remain disabled subsequently. Their long‐term outcome will depend largely on whether intracranial haemorrhage or renal failure developed (Redman 1994).
While acute renal failure can be a potentially fatal condition, decreased urine output (oliguria) in women with pre‐eclampsia is not necessarily indicative of renal failure and the cause should be determined before any treatment is considered. Oliguria may be a normal response to poor fluid intake, the stress of surgery or lowered blood pressure after antihypertensive drugs (Redman 1994). It will most commonly be the result of decreased intravascular volume, but in a small number of women, excessive contraction of the renal blood vessels associated with pre‐eclampsia may be the cause of oliguria. Acute renal failure requiring dialysis is an extremely rare complication in the industrialised world (incidence 0.01%), but in the developing world as many as 25% of referrals for dialysis are pregnancy related. While not all of these women would have had hypertension or pre‐eclampsia, the latter remains the major cause of renal dysfunction in pregnancy (Lindheimer 2000).
The recommended first‐line therapy is a single‐fluid challenge (Dildy 1994), although this approach has not been validated in randomised trials. Either 500 ml crystalloid (Clark 1986) or 300 ml colloid solution (Belfort 1989) has been recommended. However, repeated dosages in the face of persistent oliguria may lead to pulmonary oedema, a serious lung complication with an increased maternal mortality. On the other hand, withholding fluid may cause acute renal problems (tubular necrosis) or even long‐term renal damage.
Prolonged oliguria associated with vasospasm may lead to permanent renal damage. Dopamine is widely used in a variety of clinical settings for the prevention and treatment of acute renal failure in non‐pregnant women (Burton 1999; Cuthbertson 1997). Its use is based on physiological evidence suggesting that selective renal vasodilation occurs with low‐dose infusions. The efficacy of dopamine to prevent complications such as death or prevention of subsequent dialysis has recently been questioned (Chertow 1996). Much emphasis has also been placed on potential side‐effects (Denton 1997; Thompson 1994). These include arrhythmias and impaired oxygen supply to the heart, gut and peripheral organs. Other possible unfavourable effects include increased urine output in a patient with decreased intravascular blood flow, pulmonary hypertension and other lung problems and decreased motility of the gut. Dopamine can also cause immune dysfunction and reduced secretion of various hormones such as growth hormone, prolactin and dehydroepiandrosterone. The incidence of these possible adverse effects is unknown and will only become available after adequately‐sized prospective randomised controlled trials have been done. Until recently, no large randomised controlled trials were available. However, in a multicentre double‐blind placebo‐controlled randomised trial with 328 non‐pregnant participants, the authors concluded that the administration of low‐dose dopamine by continuous intravenous infusion to critically ill patients at risk of renal failure did not confer clinically significant protection from renal dysfunction (Bellomo 2000). Arrhythmias were common in this trial in both the dopamine and placebo groups (53/161 versus 54/163).
The purpose of this review is to determine whether, in the rare instance of a woman with severe pre‐eclampsia who is unresponsive to conventional methods, low‐dose dopamine may provide an adjunctive therapy to restore urine output, especially after delivery. However, increased urinary output would only be worthwhile if it was associated with clinically important improvements in the outcome of the woman or her baby, such as improved maternal and perinatal survival, or a decrease in the need for dialysis.
Readers may wish to refer to the following Cochrane systematic generic protocol for further information: 'Interventions for preventing pre‐eclampsia and its consequences' (Meher 2005).
Objectives
To assess whether low‐dose dopamine given to women with severe pre‐eclampsia and acute renal failure, or who are considered to be at risk of acute renal failure, influences maternal and perinatal mortality and morbidity.
Methods
Criteria for considering studies for this review
Types of studies
All randomised trials comparing low‐dose dopamine with either placebo or no dopamine in women with severe pre‐eclampsia and acute renal failure, or who are considered to be at risk of acute renal failure. We will exclude quasi‐randomised trials.
Types of participants
All women with severe pre‐eclampsia who were considered by the authors to either have acute renal failure or who were considered to be at risk of acute renal failure during the antenatal or immediate postpartum period (within 48 hours after delivery).
Types of interventions
Comparison of dopamine with placebo or no treatment. Low‐dose dopamine is defined as dosages not higher than 5 microgram/kg/minute.
Types of outcome measures
For the women: death, eclampsia, dialysis, mode of delivery, changes in urine output after administration of dopamine and drug‐related adverse events. Adverse events may include effects on blood circulation such as disturbances of heart rate, the workload of the heart, and changes in blood volume. We also planned to consider data on long‐term follow up of women.
For the children: stillbirth, neonatal death, gestational age at birth, complications related to prematurity (such as use of mechanical ventilation), necrotising enterocolitis and bleeding episodes (such as periventricular haemorrhage), admission to neonatal intensive care unit, length of stay in hospital, as well as long‐term outcomes such as neurodevelopmental outcome at follow up. Due to the severity of the maternal disease in women where dopamine treatment is considered, expectant management is unlikely and outcomes such as gestational age at delivery and complications of prematurity reflect the time of onset of disease rather than effects of therapy.
Search methods for identification of studies
Electronic searches
We searched the Cochrane Pregnancy and Childbirth Group’s Trials Register by contacting the Trials Search Co‐ordinator (May 2009).
The Cochrane Pregnancy and Childbirth Group’s Trials Register is maintained by the Trials Search Co‐ordinator and contains trials identified from:
quarterly searches of the Cochrane Central Register of Controlled Trials (CENTRAL);
weekly searches of MEDLINE;
handsearches of 30 journals and the proceedings of major conferences;
weekly current awareness alerts for a further 44 journals plus monthly BioMed Central email alerts.
Details of the search strategies for CENTRAL and MEDLINE, the list of handsearched journals and conference proceedings, and the list of journals reviewed via the current awareness service can be found in the ‘Specialized Register’ section within the editorial information about the Cochrane Pregnancy and Childbirth Group.
Trials identified through the searching activities described above are each assigned to a review topic (or topics). The Trials Search Co‐ordinator searches the register for each review using the topic list rather than keywords.
We did not apply any language restrictions.
Searching other resources
We planned to contact authors for more information if data supplied in the publication were insufficient.
Data collection and analysis
The authors extracted data to assess eligibility and to describe trial characteristics. Wilhelm Steyn and Petrus Steyn extracted the data from the relevant study independently. Any discrepancies would have been discussed and resolved by consensus, if required. We would have sought unpublished data from the researchers. We performed statistical analyses using the Review Manager software (RevMan 2003), with results presented as mean difference.
We planned to assess the validity of each trial to be included according to the criteria in the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2005), with a grade allocated to each on the basis of allocation concealment A (adequate), B (unclear), or C (inadequate). We planned to contact trial authors for further details if the method of allocation concealment was unclear.
Assessment of methodological quality of included studies
The authors assessed the quality of the included trial independently, using the criteria outlined in the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2005). Methods used for generation of the randomisation sequence is described. We assessed the included study for quality of the concealment of allocation, completeness of follow up and blinding.
(1) Allocation concealment: a quality score for concealment of allocation was assigned, using the following criteria: (A) adequate concealment of allocation, such as telephone randomisation, consecutively‐numbered, sealed opaque envelopes; (B) unclear whether concealment of allocation was adequate;such as list or table used, sealed envelopes that are not numbered or opaque, or study does not report any concealment approach; (C) inadequate concealment of allocation such as open random‐number tables, use of case record numbers, dates of birth or days of the week.
Where the method of allocation concealment was unclear, if possible, we would have attempted to contact authors to provide further details.
(2) We assessed completeness of follow up: (A) less than 3% of participants excluded from the analysis; (B) 3% to 9.9% of participants excluded from the analysis; (C) 10% to 19.9% of participants excluded from the analysis. Excluded: if not possible to enter data based on intention to treat, or 20% or more participants were excluded from the analysis of that outcome, or both. (3) We assessed blinding using the following criteria: (A) blinding of participants (yes/no/unclear or unspecified); (B) blinding of caregiver (yes/no/unclear or unspecified); (C) blinding of outcome assessment (yes/no/unclear or unspecified).
If necessary, we would have performed sensitivity analysis to assess the influence of differences in allocation concealment, completeness of follow up or blinding of assessment of outcome between different studies.
Results
Description of studies
We found one study meeting the inclusion criteria (Mantel 1997). This was a double‐blind randomised controlled trial of high quality, but it lasted only six hours. Forty women were included after delivery (see 'Characteristics of included studies' table for more information). We did not include another study, also of high quality, because the use of low‐dose dopamine in 80 postpartum women was compared with frusemide and not with placebo (Keiseb 2002).
Risk of bias in included studies
The included study (Mantel 1997) was of high quality. No participants were excluded from analysis. Blinding was such that neither participant or investigator knew or were likely to guess the allocated outcome.
Effects of interventions
The authors reported a significant increase in urinary output of women receiving low‐dose dopamine compared to the placebo group over the six hours (mean difference 291.56, 95% confidence interval 31.75 to 551.37, median 57 ml/hour versus 15 ml/hour).The authors reported serious complications that occurred, but did not distinguish between those before and during the study. We contacted the authors, but no further information on the outcomes as defined was available.
Discussion
There are limited data available on the use of low‐dose dopamine in women with severe pre‐eclampsia. Low‐dose dopamine influences renal haemodynamics by vasodilatation through stimulation of dopamine receptors, while higher dosages cause systemic vasoconstriction (Mantel 2001). There is unfortunately an unpredictable overlap of dose responses, creating the potential for serious adverse effects. The only randomised controlled trial in pregnancy showed a significant improvement in urine output in 20 postpartum pre‐eclamptic women with oliguria, compared to 20 women who received placebo. However, the study duration was only six hours and no other data were given on renal function or any long‐term effects. The significance of this finding is questionable, as urine output may not be a good substitute marker of renal function. In another study, the use of low‐dose dopamine was compared with furosemide in 80 postpartum patients (Keiseb 2002). Twenty of the women who had been randomised improved their urine output within four hours and did not require medication. The two regimens were equally effective in improving urine output, while there was no statistically significant difference in women who eventually required haemodialysis. The authors did not include a placebo group in their study. Appropriate data in the non‐obstetric literature are also lacking. The use of low‐dose dopamine in women with severe pre‐eclampsia and acute renal failure, or who are considered to be at risk of acute renal failure, should therefore be restricted to prospective randomised controlled trials.
Authors' conclusions
Implications for practice.
There are currently few data supporting the use of low‐dose dopamine in women with severe pre‐eclampsia. Its use should be discouraged due to potentially severe adverse effects.
Implications for research.
While there may be a place for prospective randomised controlled trials to assess the role of low‐dose dopamine in women with oliguria due to renal failure in severe pre‐eclampsia, the appropriateness of such a trial should be seriously considered given the lack of evidence of benefit in non‐obstetric patients and the potentially serious side‐effects. If further trials are conducted, it is important that all clinically appropriate outcomes are reported.
What's new
| Date | Event | Description |
|---|---|---|
| 31 May 2009 | New search has been performed | Search updated. No new trials identified. |
History
Protocol first published: Issue 1, 2002 Review first published: Issue 1, 2007
| Date | Event | Description |
|---|---|---|
| 11 September 2008 | Amended | Converted to new review format. |
Acknowledgements
Rebecca Smyth.
As part of the pre‐publication editorial process, this review has been commented on by three peers (an editor and two referees who are external to the editorial team), one or more members of the Pregnancy and Childbirth Group's international panel of consumers and the Group's Statistical Adviser.
Data and analyses
Comparison 1. Dopamine versus placebo.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Urinary output | 1 | 40 | Mean Difference (IV, Fixed, 95% CI) | 291.56 [31.75, 551.37] |
1.1. Analysis.
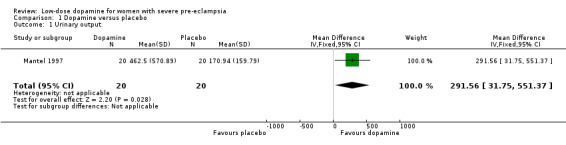
Comparison 1 Dopamine versus placebo, Outcome 1 Urinary output.
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Mantel 1997.
| Methods | A double‐blind, randomised controlled trial. Sequentially‐numbered, opaque, sealed envelopes. | |
| Participants | 40 postpartum pre‐eclamptic women with oliguria (< 30 ml urine per hour over 2 hours) which did not respond to an intravenous fluid challenge of 300 ml crystalloid fluid given over 30 minutes. 28 women had gestational proteinuric hypertension, while the remaining 12 women were excluded if there was clinical evidence of pulmonary oedema, if a diuretic had been used in the previous 24 hours or if oliguria was due to persistent haemorrhage. | |
| Interventions | Dopamine 1‐5 microgram/kg per minute by intravenous infusion or sterile water by intravenous infusion. | |
| Outcomes | Hourly blood pressure, pulse, antihypertensive treatment, intravenous fluid administration and urine output for the 6 hours prior to and during the trial. | |
| Notes | Maternal complications are reported as having occurred before and during the trial. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Low risk | A ‐ Adequate |
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Keiseb 2002 | No placebo group. |
Contributions of authors
Wilhelm Steyn planned the review and wrote the protocol, while Petrus Steyn commented on the final version of the protocol. Both review authors assessed the available studies independently. Wilhelm Steyn wrote the review and Petrus Steyn commented on the drafts.
Declarations of interest
None known.
New search for studies and content updated (no change to conclusions)
References
References to studies included in this review
Mantel 1997 {published data only}
- Mantel GD, Makin J. A double blind randomised controlled trial of the use of low dose dopamine in post partum pre‐eclamptic or eclamptic women with oliguria. 10th World Congress of the International Society for the Study of Hypertension in Pregnancy; 1996 August 4‐8; Seattle, Washington, USA. 1996:125.
- Mantel GD, Makin J. A double blind randomised controlled trial of the use of low dose dopamine in post partum pre‐eclamptic or eclamptic women with oliguria. Fifteenth Conference on Priorities in Perinatal Care in Southern Africa; 1996 March 5‐8; Goudini Spa, South Africa. 1996.
- Mantel GD, Makin J. Low dose dopamine in postpartum pre‐eclamptic women with oliguria: a double‐blind, placebo controlled, randomised trial. British Journal of Obstetrics and Gynaecology 1997;104(10):1180‐3. [DOI] [PubMed] [Google Scholar]
References to studies excluded from this review
Keiseb 2002 {published data only}
- Keiseb J, Moodley J, Connolly CA. Comparison of the efficacy of continuous furosemide and low‐dose dopamine infusion in preeclampsia/eclampsia related oliguria in the immediate postpartum period. Hypertension in Pregnancy 2002;21(3):225‐34. [DOI] [PubMed] [Google Scholar]
Additional references
Anthony 1996
- Anthony J. Pre‐eclampsia and maternal mortality ‐ a universal problem. South African Medical Journal 1996;86(5):513. [PubMed] [Google Scholar]
Belfort 1989
- Belfort M, Uys P, Dommisse J, Davey DA. Haemodynamic changes in gestational proteinuric hypertension: the effects of rapid volume expansion and vasodilator therapy. British Journal of Obstetrics and Gynaecology 1989;96(6):634‐41. [DOI] [PubMed] [Google Scholar]
Bellomo 2000
- Bellomo R, Chapman M, Finfer S, Hickling K, Myburgh J. Low‐dose dopamine in patients with early renal dysfunction: a placebo‐controlled randomised trial. Lancet 2000;356(9248):2139‐43. [DOI] [PubMed] [Google Scholar]
Burton 1999
- Burton CJ, Tomson CRV. Can the use of low‐dose dopamine for treatment of acute renal failure be justified?. Postgraduate Medical Journal 1999;75(883):269‐74. [DOI] [PMC free article] [PubMed] [Google Scholar]
Chertow 1996
- Chertow GM, Sayegh MH, Allgren RL, Lazarus JM. Is the administration of dopamine associated with adverse or favorable outcomes in acute renal failure?. American Journal of Medicine 1966;101(1):49‐53. [DOI] [PubMed] [Google Scholar]
Clark 1986
- Clark SL, Greenspoon JS, Aldahl D, Phelan JP. Severe preeclampsia with persistent oliguria: management of hemodynamic subsets. American Journal of Obstetrics and Gynecology 1986;154(3):490‐4. [DOI] [PubMed] [Google Scholar]
Cuthbertson 1997
- Cuthbertson BH, Noble DW. Dopamine in oliguria. BMJ 1997;314(7082):690‐1. [DOI] [PMC free article] [PubMed] [Google Scholar]
Denton 1997
- Denton R, Slater R. Just how benign is renal dopamine?. European Journal of Anaesthesiology 1997;14(4):347‐9. [DOI] [PubMed] [Google Scholar]
Dildy 1994
- Dildy GA, Phelan JP, Cotton DB. Complications of pregnancy induced hypertension. In: Clark SL, Cotton DB, Hankins GDV, Phelan JP editor(s). Critical care obstetrics. Oxford: Blackwell Science, 1994:251‐88. [Google Scholar]
Higgins 2005
- Higgins JPT, Green S, editors. Cochrane Handbook for Systematic Reviews of Interventions 4.2.5 [updated May 2005]. In: The Cochrane Library, Issue 3, 2005. Chichester, UK: John Wiley & Sons, Ltd.
HMSO 1994
- HMSO. Report on confidential enquiries into maternal deaths in the United Kingdom 1988‐1990. London: Department of Health Welsh Office, Scottish Office Home and Health Department, Department of Health and Social Security, Northern Ireland HMSO, 1994. [Google Scholar]
Lindheimer 2000
- Lindheimer MD, Grunfeld J‐P, Davison JM. Renal disorders. In: Barron WM, Lindheimer MD editor(s). Medical disorders during pregnancy. St Louis: Mosby, 2000:39‐70. [Google Scholar]
Mantel 2001
- Mantel GD. Care of the critically ill parturient: oliguria and renal failure. Best Practice & Research. Clinical Obstetrics and Gynaecology 2001;15(4):563‐81. [DOI] [PubMed] [Google Scholar]
Meher 2005
- Meher S, Duley L, Prevention of Pre‐eclampsia Cochrane Review Authors. Interventions for preventing pre‐eclampsia and its consequences: generic protocol. Cochrane Database of Systematic Reviews 2005, Issue 2. [DOI: 10.1002/14651858.CD005301] [DOI] [Google Scholar]
Pattinson 1998
- Pattinson B. Saving mothers: report on confidential enquiries into maternal deaths in South Africa 1998. Pretoria: Department of Health, 1998. [Google Scholar]
Redman 1994
- Redman CWG. Hypertension in pregnancy. In: Swales JD editor(s). Textbook of hypertension. Oxford: Blackwell Scientific Publications, 1994:767‐84. [Google Scholar]
RevMan 2003 [Computer program]
- The Nordic Cochrane Centre, The Cochrane Collaboration. Review Manager (RevMan). Version 4.2 for Windows. Copenhagen: The Nordic Cochrane Centre, The Cochrane Collaboration, 2003.
Thompson 1994
- Thompson BT, Cockrill BA. Renal‐dose dopamine: a siren song?. Lancet 1994;344(8914):7‐8. [DOI] [PubMed] [Google Scholar]
WHO 1998
- World Health Organization International Collaborative Study of Hypertensive Disorders of Pregnancy. Geographic variation in the incidence of hypertension in pregnancy. American Journal of Obstetrics and Gynecology 1988;158(1):80‐3. [PubMed] [Google Scholar]


