Abstract
The use of tumor mutation-derived neoantigen represents a promising approach for cancer vaccines. Preclinical and early phase human clinical studies have shown the successful induction of tumor neoepitope-directed responses; however, overall clinical efficacy of neoantigen vaccines has been limited. One major obstacle of this strategy is the prevailing lack of sufficient understanding of the mechanism underlying the generation of neoantigen-specific CD8+ T cells. Here, we report a correlation between antitumor efficacy of neoantigen/toll-like receptor 3 (TLR3)/CD40 agonists vaccination and an increased frequency of circulating antigen-specific CD8+ T cells expressing CX3C chemokine receptor 1 (CX3CR1) in a preclinical model. Mechanistic studies using mixed bone marrow chimeras identified that CD40 and CD80/86, but not CD70 signaling in Batf3-dependent conventional type 1 dendritic cells (cDC1s) is required for the antitumor efficacy of neoantigen vaccine and generation of neoantigen-specific CX3CR1+ CD8+ T cells. Although CX3CR1+ CD8+ T cells exhibited robust in vitro effector function, in vivo depletion of this subset did not alter the antitumor efficacy of neoantigen/TLR3/CD40 agonists vaccination. These findings indicate that the vaccine-primed CX3CR1+ subset is dispensable for antitumor CD8+ T cell responses, but can be used as a blood-based T-cell biomarker for effective priming of CD8+ T cells as post-differentiated T cells. Taken together, our results reveal a critical role of CD40 and CD80/86 signaling in cDC1s in antitumor efficacy of neoantigen-based therapeutic vaccines, and implicate the potential utility of CX3CR1 as a circulating predictive T-cell biomarker in vaccine therapy.
Supplementary Information
The online version contains supplementary material available at 10.1007/s00262-021-02969-6.
Keywords: Vaccine therapy, Neoantigen, CX3CR1, T cells, Dendritic cells, Biomarker
Introduction
Despite objective therapeutic benefit seen in several clinical trials, the overall clinical success of the peptide-based vaccines has thus far been limited [1, 2]. This is due, at least in part, to the low immunogenicity of self-antigens, the incomplete understanding of immunological mechanisms underlying effective priming of tumor-specific T cells, and the lack of a reliable biomarker for clinical response. Somatically mutated genes within tumors can generate neoantigens, which create de novo epitopes for T cells [3]. Since neoantigens have not undergone central thymic selection, they can be deemed as highly immunogenic tumor-specific antigens [3]. Recent advances of neoantigen identification by massive parallel sequencing and computational prediction of neo-epitopes have demonstrated that individualized mutanome-based vaccinations are promising [4–7].
The chemokine receptor, CX3CR1, was recently identified as a marker of effector T-cell differentiation [8, 9]. Unlike other proliferation, co-stimulatory and co-inhibitory molecules such as Ki67, ICOS, PD-1 and CTLA-4 that are only transiently upregulated on T cells after activation, CX3CR1 is stably expressed on virus- and tumor-specific CD8+ T cells upon effective activation and differentiation via unidirectional differentiation from the CX3CR1-negative (CX3CR1−) subset during the effector phase [8, 9, 10]. Furthermore, our study and others have shown that CD8+ T cells expressing high levels of CX3CR1 exhibit decreased expression of L-selectin (CD62L) and CXCR3 [8–11], trafficking receptors necessary for entry across lymphoid organ high endothelial venules (HEV) and the tumor microvasculature, respectively [12, 13, 14], and become more prevalent in peripheral blood (PB) at the end of the primary response [9, 10]. Therefore, the CX3CR1+ subset might not contribute to the antitumor CD8+ T cell responses against established tumors due to the poor trafficking capacity compared to the CX3CR1− subset [10]; however, CX3CR1 can be useful as a blood-based marker for effective priming of CD8+ T cells as post-differentiated T cells. These observations prompted us to hypothesize that effective vaccination would be associated with the increased frequency of antigen-specific CX3CR1+ CD8+ T cells in the periphery.
Here, we used a syngeneic mouse model of colon adenocarcinoma with the neo-epitope presented in major histocompatibility complex (MHC) class I H-2Db molecules [15], and investigated the relationship between antitumor efficacy of neoantigen vaccination and the frequency of neoantigen-specific CX3CR1+ CD8+ T cells. Our results indicate that generation of circulating neoantigen-specific CX3CR1+ CD8+ T cells correlates with successful vaccination with mutated peptide and toll-like receptor 3 (TLR3)/CD40 agonists. Mechanistic studies using mixed bone marrow chimeras identified a key role of CD40 and CD80/86, but not CD70 signaling in Batf3-dependent conventional type 1 dendritic cells (cDC1s) for the generation of neoantigen-specific cytotoxic CX3CR1+ CD8+ T cells and therapeutic efficacy of neoantigen vaccine.
Materials and methods
Mice
Female C57BL/6 mice were purchased from the Jackson Laboratory. CD40−/− mice (B6.129S2-Cd40lgtm1Imx/J), CD80/86−/− mice (B6.129S4-Cd80tm1ShrCd86tm2Shr/J), and Batf3−/− mice (B6.129S(C)-Batf3tm1Kmm/J), CD2-Cre mice (C57BL/6-Tg(CD2-cre)1Lov/J), and CX3CR1–DTR mice (B6N.129P2-Cx3cr1tm3(DTR)Litt/J) on C57BL/6 background were purchased from the Jackson Laboratory, and bred in-house (Roswell Park Comprehensive Cancer Center). CD70−/− mice on C57BL/6 background have been previously described [16]. For inducible Cd2-cre/Cx3cr1+/DTR mice, we crossed CD2-Cre mice with CX3CR1–DTR mice to generate Cd2-cre/Cx3cr1+/DTR mice, allowing induction of DTR in CX3CR1+ CD8+ T cells. For depletion of CX3CR1+ CD8+ T cells, diphtheria toxin (Sigma) (250 ng/dose/mouse) was administered intraperitoneally (i.p.) every day starting from 1 day before neoantigen vaccination. All mice were 7–12 weeks old at the beginning of each experiment, maintained under specific pathogen-free, and housed in the Laboratory Animal Resources facility. All animal studies were conducted in accordance with and approved by the Institutional Animal Care and Use Committee (IACUC) at the Roswell Park Comprehensive Cancer Center.
Cells
The MC38 murine colon adenocarcinoma cell line was gift from Dr. Weiping Zou (University of Michigan). The murine B16F10 melanoma cell line was purchased from ATCC. B16F10 and MC38 cells were maintained in RPMI (Gibco) supplemented with 10% FBS (Sigma), 1% NEAA (Gibco), 2 mM GlutaMAX-1 (Gibco), 100 U/ml penicillin–streptomycin (Gibco), and 55 μM 2-mercaptoethanol (Gibco). Cells were authenticated by morphology, phenotype and growth, and routinely screened for Mycoplasma, and were maintained at 37 °C in a humidified 5% CO2 atmosphere.
In vivo vaccination and cancer immunotherapy studies
Female mice were injected subcutaneously (s.c.) with 5–8 × 105 MC38 cells into the right flank. Mice were treated s.c. with 100 μg soluble mutant Adpgk peptide (AdpgkMut) (ASMTNMELM) or AH1 (SPSYVYHQF) peptide (GenScript) with 50 μg poly(I:C) (InvivoGen) and 50 μg agonistic CD40 antibody (Ab) (clone FGK45, BioXCell) in 100 μl of PBS into the left flank twice 1 week apart. PB, spleen and tumors were harvested for immune monitoring 1 week after the second vaccination. Tumor volumes were calculated by determining the length of short (l) and long (L) diameters (volume = l2 × L/2). Experimental end points were reached when tumors exceeded 20 mm in diameter or when mice became moribund and showed signs of lateral recumbency, cachexia, lack of response to noxious stimuli, or observable weight loss.
Generation of bone marrow chimeras
To generate bone marrow chimeras, C57BL/6 mice were irradiated with 500 cGy followed by a second dose of 550 cGy 3 h apart. To obtain donor bone marrow, femurs and tibiae were harvested and the bone marrow was flushed out. For CD40−/− bone marrow chimeras, 5 × 106 bone marrow cells from CD40−/− mice were injected to irradiated C57BL/6 WT mice. For Batf3−/−/WT, Batf3−/−/CD40−/−, Batf3−/−/CD80/86−/−, and Batf3−/−/CD70−/− mixed bone marrow chimeras, 5 × 106 bone marrow cells of a 1:1 mixture were injected to irradiated C57BL/6 WT mice. After 8–12 weeks, recipients were used for the experiments.
Flow cytometry and intracellular granzyme, TNF-α and IFN-γ assay
Fluorochrome-conjugated antibodies are shown in Table S1. DAPI, LIVE/DEAD Fixable Near-IR (Thermo Fisher Scientific) staining cells were excluded from analysis. For tetramer staining, PE-labeled MHC class I (H-2Db) specific for mouse AdpgkMut 299–307 (ASMTNMELM) [15] was kindly provided by the NIH Tetramer Core Facility. Detection of intracellular granzyme A (GZMA) was performed as described before [10]. For intracellular staining of IFN-γ and TNF-α, splenocytes and tumor-infiltrating cells were cocultured with 1 μmol/L of AdpgkMut or AH1 peptide in the presence of brefeldin A (BD Biosciences) for 5 h in vitro. Samples were analyzed using an LSR II or an LSRFortessa (BD Biosciences) with FlowJo software (TreeStar).
Statistical analysis
Statistical analysis was performed using a two-tailed Student’s t-test or a Mann–Whitney U test for comparisons between two groups, a one-way ANOVA with Tukey’s multiple comparisons for comparisons more than 2 groups, or the Mantel-Cox method (log-rank test) for survival analysis using GraphPad Prism 8.02 (GraphPad Software). Correlations were assessed using the Pearson and Spearman correlation coefficients. Analyses were performed in GraphPad Prism 8.02 (GraphPad Software) and SAS v9.4 (Cary, NC) at a significance level of 0.05.
Results
Vaccination with neoantigen and TLR3/CD40 agonists elicits potent antigen-specific antitumor efficacy.
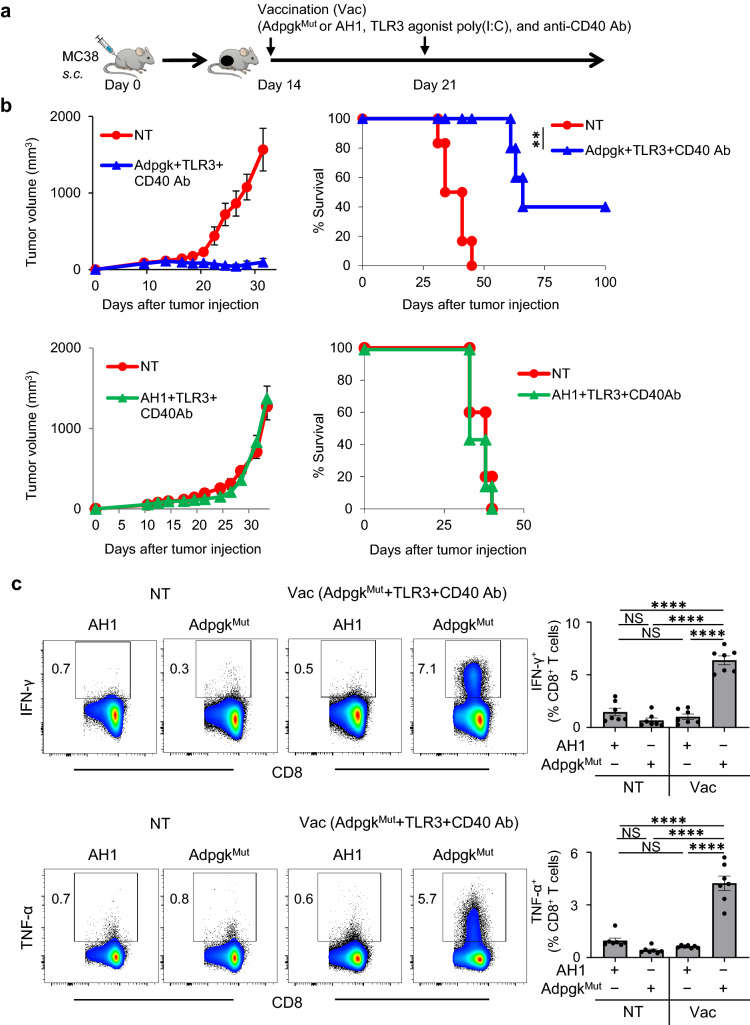
To examine the relationship between antitumor efficacy of neoantigen vaccination and the generation of neoantigen-specific CD8+ T cells, we sought to validate a preclinical model of neoantigen vaccination using MC38 colon adenocarcinoma cells that harbor a single-epitope mutation within the Adpgk protein (Supplemental Fig. 1) [15]. In this model, to maximize the therapeutic efficacy of neoantigen vaccination with AdpgkMut, dual TLR3/CD40 stimulation was used as vaccine adjuvants (Fig. 1a), which has been known to synergistically activate DCs, augment CD8+ T cell expansion, and mediate potent antitumor immunity in multiple preclinical models [16–22]. Vaccination of AdpgkMut, but not irrelevant AH1 peptide with agonistic anti-CD40 Ab, and a TLR3 agonist, poly(I:C) (referred to as AdpgkMut/TLR3/CD40 hereafter) markedly delayed the growth of established MC38 tumors and improved survival (Fig. 1b). Splenocytes and tumor-infiltrating cells from MC38 tumor-bearing mice treated with AdpgkMut/TLR3/CD40 agonists vaccination contained substantially higher frequency of CD8+ T cells that could produce IFN-γ and TNF-α against AdpgkMut but not irrelevant AH1 antigen (Fig. 1c and Supplemental Fig. 2), suggesting the generation of antigen-specific CD8+ T cells. These findings validate a preclinical model of neoantigen vaccine-based therapy to investigate the role of neoantigen-specific CD8+ T cells.
Fig. 1.
Vaccination with neoantigen and TLR3/CD40 agonists elicits potent antigen-specific antitumor efficacy. a Experimental scheme for evaluation of neoantigen/TLR3/CD40 agonists vaccination (Vac) in an MC38 tumor model. b Tumor growth and survival curves in MC38 tumor-bearing C57BL/6 mice treated with PBS (NT: non-treatment) or AdpgkMut/TLR3/CD40 agonists vaccination (upper) (n = 6 mice per group) and PBS (NT) or AH1/TLR3/CD40 agonists vaccination (lower) (n = 5–7 mice per group). c Left shows representative FACS plots showing IFN-γ (upper) or TNF-α (lower) expression gated with CD8+ T cells in splenocytes of MC38-tumor bearing mice 1 week after 2nd PBS (NT) or AdpgkMut/TLR3/CD40 agonists vaccination. Splenocytes were co-cultured with AdpgkMut or AH1 peptide in the presence of brefeldin A for 5 h before intracellular staining. Numbers denote percentage of IFN-γ+ or TNF-α+ cells. Right panel shows the frequency of the IFN-γ+ or TNF-α+ cells in CD8+ T cells in each group (n = 7 mice per group). NS not significant, **P < 0.01, ****P < 0.0001, log-rank test (b) and one-way ANOVA with Tukey’s multiple comparisons (c). Mean ± SEM
Effective neoantigen/TLR3/CD40 stimulations correlate with generation of antigen-specific effector CX3CR1+CD8+T cells.
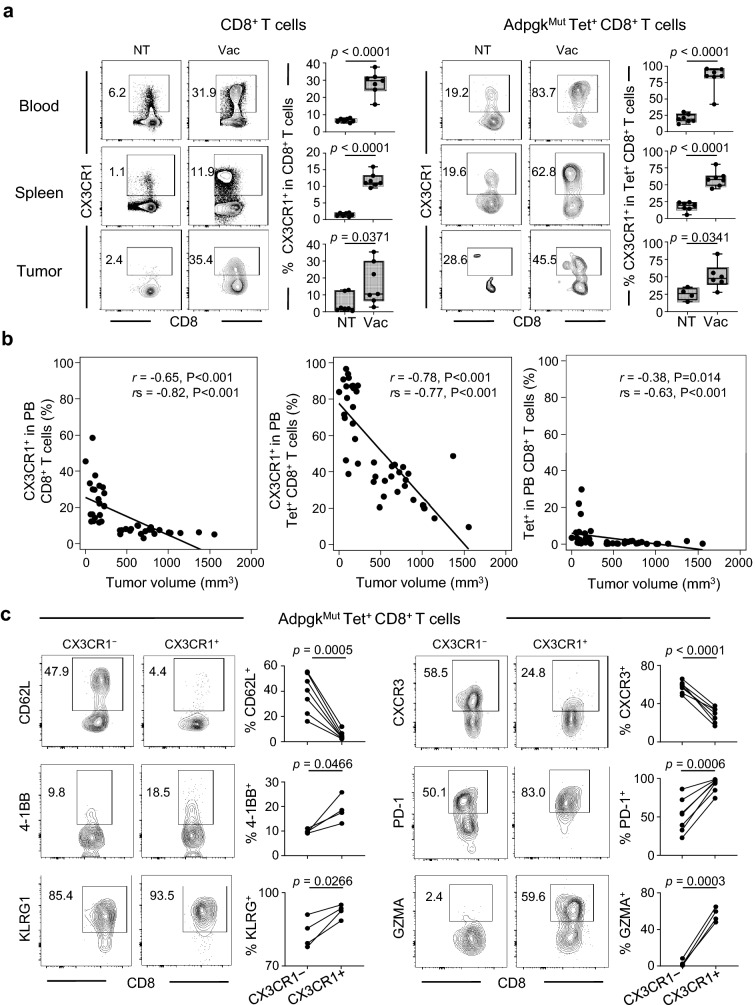
We examined whether effective neoantigen vaccination could generate CD8+ T cells expressing CX3CR1, a marker of effector T-cell differentiation [8, 9]. To this end, we collected PB, spleen and tumors 1 week after second AdpgkMut/TLR3/CD40 agonists vaccination, and assessed CX3CR1 in CD8+ and neoantigen-specific CD8+ T cells using an AdpgkMut-specific tetramer (Tet) (Supplementary Fig. 3). AdpgkMut/TLR3/CD40 agonists vaccination substantially increased the frequency of CD8+ and Tet+ CD8+ T cells expressing CX3CR1 in PB, spleen and tumors (Fig. 2a).
Fig. 2.
Effective neoantigen/TLR3/CD40 stimulations correlate with generation of antigen-specific effector CX3CR1+ CD8+ T cells. a–c, MC38 tumor-bearing C57BL/6 mice were treated with PBS (NT) or AdpgkMut/TLR3/CD40 agonists vaccination (Vac) as described in Fig. 1a. Blood, spleens and tumors were collected 1 week after 2nd Vac. a Representative FACS plots showing CX3CR1 expression in CD8+ (left) or AdpgkMut tetramer (Tet)+ CD8+ T cells (right) in PB (upper), spleen (middle) and tumors (lower). Numbers denote percentage of CX3CR1+ cells. Frequency of CX3CR1+ cells from the CD8+ or Tet+ CD8+ T cells is shown in the right panels (n = 4–7 mice per group). b Scatter plot of the frequency of PB CX3CR1+ CD8+ T cells (left), CX3CR1+ Tet+ CD8+ T cells (middle), and Tet+ CD8+ T cells (right) against tumor volume. Correlation is shown using Pearson correlation (r) and Spearman correlation coefficients (rs). c Representative FACS plots of CX3CR1− (left) or CX3CR1+ (right) cells gated with Tet+ CD8+ T cells in PB. Numbers denote percentage of each marker-positive cells. Frequency of marker-positive cells from the CX3CR1− or CX3CR1+ subset is shown in the right panels for each marker (n = 4–7 mice per group). Data shown are representative of at least two independent experiments (a–c). Two-tailed unpaired (a) and paired t-test (c). Box plots: hinges, 25th and 75th percentiles; middle line, median; whiskers, minimum to maximum value (a)
Previous clinical studies showed that functional assessment of PB T cells such as IFN-γ production and/or cytolytic activity was correlated with response in patients treated with peptide vaccines [23, 24, 25]. Therefore, we evaluated correlation between CX3CR1 expression in CD8+ T cells and capacity of producing effector cytokines, and found that the majority of CD8+ T cells producing effector cytokine against AdpgkMut were largely positive for CX3CR1 expression (Supplementary Fig. 4a). Accordingly, frequency of the CX3CR1+ subset in circulating Tet+ CD8+ T cells correlated with the frequency of CD8+ T cells secreting IFN-γ+ and TNF-α+ against AdpgkMut antigen in spleen (Supplementary Fig. 4b). Of note, tumor-infiltrating Tet+ CD8+ T cells exhibited higher capacity of producing effector cytokines than Tet− CD8+ T cells regardless of the expression of CX3CR1 (Supplementary Fig. 5a, b).
Next, we assessed relationship between tumor volume and antigen-specific CD8+ T-cell expansion and differentiation in PB from treated and untreated mice. There was a strong inverse correlation between frequency of circulating CD8+ and Tet+ CD8+ T cells expressing CX3CR1 (T-cell differentiation) and tumor volume, while the frequency of total Tet+ CD8+ T cells without CX3CR1 criteria (T-cell expansion) had a moderate inverse correlation with tumor volume (Fig. 2b). To further investigate this, we treated mice bearing MC38 tumors with different combinations of vaccination: AdpgkMut, agonistic anti-CD40 Ab, and/or poly(I:C), and examined correlation between tumor volume and the frequency of the CX3CR1+ subset in PB. AdpgkMut peptide vaccination with either anti-CD40 Ab or poly(I:C) improved survival compared to non-treatment and AdpgkMut alone (Supplementary Fig. 6a). However, combined anti-CD40 Ab and poly(I:C) stimulation synergistically enhanced AdpgkMut peptide immunization, resulting in significantly prolonged survival. We analyzed circulating T cells, and found that the frequency of CD8+ and Tet+ CD8+ T cells expressing CX3CR1 was inversely correlated with tumor volume 1 week after the second vaccination (Supplementary Fig. 6b).
Phenotypical and functional evaluation of circulating AdpgkMut-specific CX3CR1− and CX3CR1+ subsets revealed that the CX3CR1+ subset contained significantly more 4-1BB+, PD-1+, KLRG+ and GZMA+ populations (Fig. 2c), suggesting recently activated effector T cells. However, CX3CR1+ CD8+ T cells exhibited decreased expression of trafficking molecules, CD62L and CXCR3, involved in T-cell access into lymphoid tissues and tumor microenvironment (TME), respectively [12, 13, 14], in line with the higher frequency of the CX3CR1+ subset in PB in treated mice (Fig. 2a). Of note, generation of circulating CX3CR1+ CD8+ T cells was observed by AH1 peptide/TLR3/CD40 agonists vaccination in mice bearing MC38 tumors (Supplementary Fig. 7a, b) where no antitumor efficacy was identified (Fig. 1b), suggesting that an increased frequency of this subset can be an evidence of the effective activation of CD8+ T cells, but might not necessarily indicate that this subset contributes to antitumor reactivity. Therefore, we sought to determine whether CX3CR1+ CD8+ T cells were required for the antitumor efficacy of neoantigen/ TLR3/CD40 agonists vaccination.
CX3CR1+CD8+T cells are dispensable for the antitumor efficacy of neoantigen/TLR3/CD40 agonists vaccination against established tumors.
Previous preclinical studies using CX3CR1−/− mice or CX3CR1 inhibitor suggested a significance of the CX3CR1/CX3CL1 axis in T cell/NK-cell mediated antitumor immunity [26–30]. However, CX3CR1 is expressed not only on T and NK cells, but also on monocytes, DCs, monocytic myeloid-derived suppressor cells (M-MDSCs) and tumor-associated macrophages (TAMs) [31, 32], and antitumor efficacy of CX3CR1+ CD8+ T cells against established tumors remains elusive. Recently, we have reported negligible antitumor efficacy of CX3CR1hi CD8+ T cells using a mouse model where tumor antigen-specific CX3CR1hi CD8+ T cells can be selectively depleted in vivo with diphtheria toxin (DT) [10]. Furthermore, adoptive transfer of CX3CR1+ CD8+ T cells did not affect tumor growth or survival, while the CX3CR1− subset displayed potent antitumor efficacy [10].
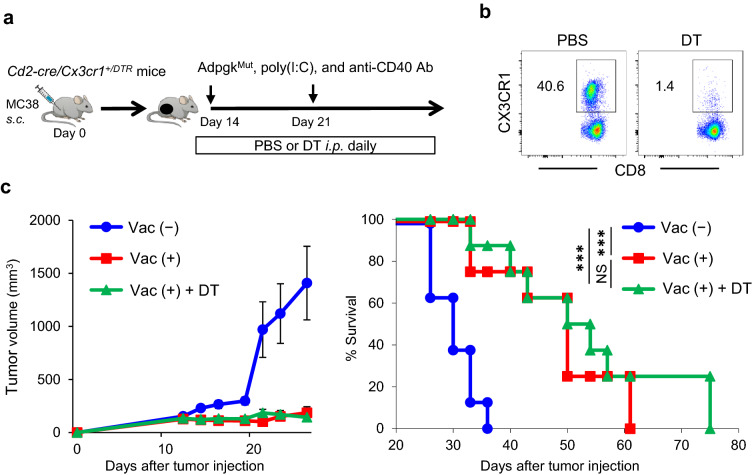
To elucidate antitumor efficacy of CX3CR1+ CD8+ T cells elicited by neoantigen/TLR3/CD40 agonists vaccination, we crossed CD2-Cre mice with CX3CR1–DTR mice, and generated Cd2-cre/Cx3cr1+/DTR mice. Expression of Cd2-cre excises the loxP-floxed stop cassette upstream of the DTR-coding region, allowing for DTR expression and CX3CR1+ CD8+ T cells can be depleted upon administration of DT, with no effect on CX3CR1− CD8+ T cells (Fig. 3a, b). MC38 tumor-bearing Cd2-cre/Cx3cr1+/DTR mice were treated with AdpgkMut/TLR3/CD40 agonists vaccination, and received phosphate-buffered saline (PBS) or DT injections every day starting 1 day before vaccination (Fig. 3b). We found that depletion of CX3CR1+ CD8+ T cells did not alter antitumor efficacy of neoantigen vaccination (Fig. 3c). This is in line with our recent study using a mouse model of adoptive T-cell therapy [10], suggesting that the expanded CX3CR1+ CD8+ T cells represent the post-differentiated in vivo effective CX3CR1− CD8+ T cell subset.
Fig. 3.
CX3CR1+ CD8+ T cells are dispensable for the antitumor efficacy of neoantigen/TLR3/CD40 agonists vaccination against established tumors. a Schematic illustration showing evaluation of in vivo antitumor efficacy of the CX3CR1+ subset using MC38 tumor-bearing Cd2-cre/Cx3cr1+/DTR mice treated with AdpgkMut/TLR3/CD40 agonists vaccination (Vac) followed by diphtheria toxin (DT) administration. DT (250 ng) was injected intraperitoneally every day from day 13. b Representative FACS plots showing selective depletion of CX3CR1+ subset in PB upon DT injection in Cd2-cre/Cx3cr1+/DTR mice treated with Vac. Expression of CX3CR1 in CD8+ T cells in PB of mice treated with PBS or DT is shown. Numbers denote percentage of the CX3CR1+ subset. c Tumor growth and survival curves of MC38 tumor-bearing Cd2-cre/Cx3cr1+/DTR mice in different treatment groups. (n = 8 mice per group). Data shown are representative from two independent experiments. NS not significant, ***P < 0.001, log-rank test (c). Mean ± SEM
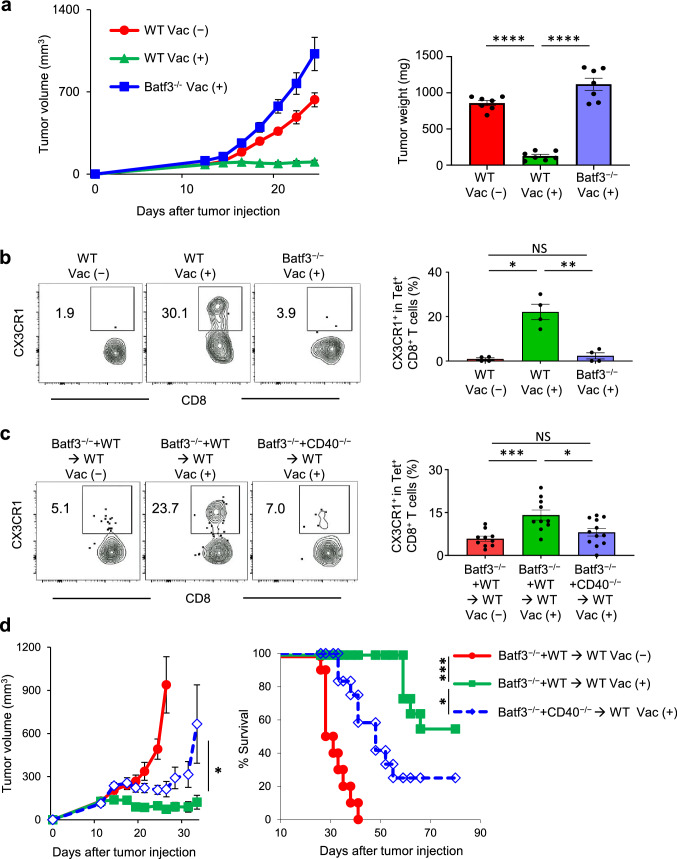
CD40 signaling is required for the antitumor efficacy of neoantigen/TLR3/CD40 agonists and generation of antigen-specific CX3CR1+ CD8+ T cells.
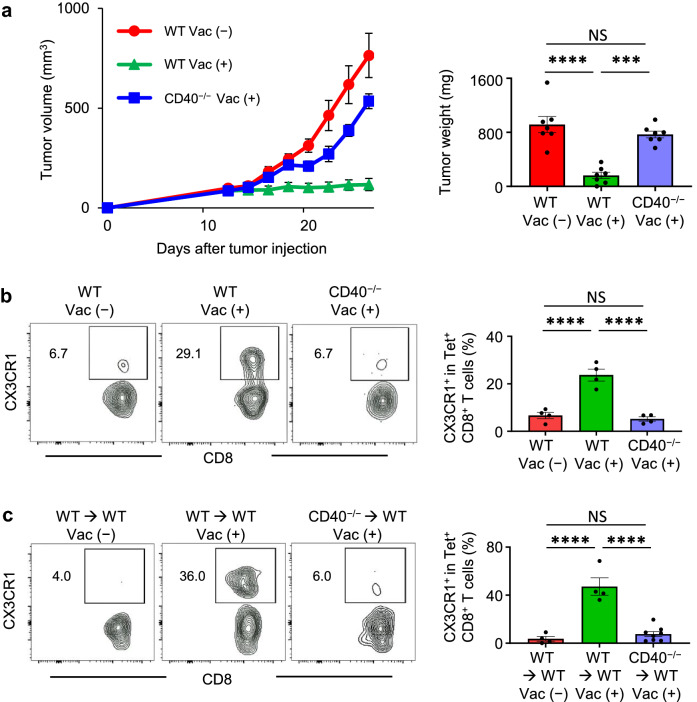
Neoantigen vaccination with a TLR3 agonist increases antigen-specific tumor-infiltrating lymphocytes in a preclinical model [33] and patients [4, 5]. CD40 signaling drives robust DC activation to generate cytotoxic CD8+ T cells [34, 35], and synergizes with the TLR3 ligand in inducing an expansion of peptide or DC vaccine-primed and adoptively transferred antigen-specific CD8+ T cells [16–19]. To delineate the role of CD40 signaling in the context of neoantigen vaccination, we examined antitumor efficacy of AdpgkMut/TLR3/CD40 agonists vaccination and generation of AdpgkMut-specific CX3CR1+ CD8+ T cells using CD40−/− mice. Antitumor efficacy of AdpgkMut/TLR3/CD40 agonists vaccination was abrogated in CD40−/− mice (Fig. 4a). The lack of increase in the frequency of AdpgkMut-specific CX3CR1+ CD8+ T cells in CD40−/− mice denoted the requirement of CD40-CD40 ligand pathway in this process (Fig. 4b), in line with recent studies showing that CD4+ T-cell help or agonistic anti-CD40 Ab facilitates generation of CX3CR1+ CD8+ T cells [26, 36]. Since CD40 molecule is not only expressed by antigen-presenting cells, but also by non-hematopoietic component such as endothelial cells [34], we generated bone marrow chimeras to further confirm the direct immunomodulatory effect of CD40 signaling in hematopoietic lineage cells. Lethally irradiated C57BL/6 mice were reconstituted with bone marrow cells from either CD40−/− or WT controls. After 8 weeks, the chimeras were implanted with MC38 tumors, followed by AdpgkMut/CD40/TLR3 stimulation. The generation of CX3CR1+CD8+ T-cell subset was substantially compromised in mice reconstituted with CD40−/− bone marrow (Fig. 4c), indicating an essential role of CD40 signaling in hematopoietic cells.
Fig. 4.
CD40 signaling is required for the antitumor efficacy of neoantigen/TLR3/CD40 agonists and generation of antigen-specific CX3CR1+ CD8+ T cells. a Left shows tumor volume curves in MC38 tumor-bearing wild type C57BL/6 mice (WT) or CD40−/− mice treated with or without AdpgkMut/TLR3/CD40 agonists vaccination (Vac) as described in Fig. 1a (n = 7 mice per group). Right shows tumor weight. Tumors were harvested 1 week after 2nd vaccination (Vac). b, c Left shows representative FACS plots showing CD8 and CX3CR1 expression gated with AdpgkMut Tet+ CD8+ T cells in PB of MC38-tumor bearing WT or CD40−/− mice (b) and bone marrow chimeric mice (WT → WT or CD40−/− → WT) (c) treated with or without Vac. Numbers denote percentage of the CX3CR1+ subset. Right shows the frequency of the CX3CR1+ subset in each group (n = 4–8 mice per group). PB was harvested 1 week after 2nd Vac. Data shown are representative of two independent experiments (a). NS: not significant, ***P < 0.001, ****P < 0.0001, one-way ANOVA with Tukey’s multiple comparisons. Mean ± SEM
A Critical role of CD40 signaling in cDC1s for the antitumor efficacy of neoantigen/TLR3/CD40 agonists vaccination and generation of antigen-specific CX3CR1+ CD8+ T cells.
Batf3-dependent conventional type 1 dendritic cells (cDC1s) specialize in cross-presentation of exogenous antigens on MHC class I molecules, and play a key role in antitumor immunity and response to immunotherapy [16, 20, 33, 37, 38, 39]. To examine whether cDC1s are relevant in our approach, we first evaluated co-stimulatory molecule expression of cDC1s by flow cytometry (Supplementary Fig. 8a). Indeed, dual TLR3/CD40 stimulation increased CD40, CD80 and CD86 expression in cDC1s in TdLN after neoantigen/TLR3/CD40 agonists vaccination (Supplementary Fig. 8b). To assess whether cDC1s are necessary for the generation of CX3CR1+ CD8+ T cells, we employed mice deficient in Batf3 transcription factor and lack cDC1s [37]. No therapeutic efficacy of vaccination was observed in Batf3−/− mice (Fig. 5a) along with a significant reduction of CX3CR1+ Tet+ CD8+ T cells (Fig. 5b), suggesting the requirement of cDC1s for generation of antigen-specific CX3CR1+ CD8+ T cells.
Fig. 5.
A critical role of CD40 signaling in cDC1s for the antitumor efficacy of neoantigen/TLR3/CD40 agonists vaccination and generation of antigen-specific CX3CR1+CD8+ T cells. a–d MC38-tumor bearing wild type C57BL/6 (WT) or Batf3−/− mice (a, b) and bone marrow chimeric mice (Batf3−/− and WT → WT or Batf3−/− and CD40−/− → WT) (c, d) were treated with or without AdpgkMut/TLR3/CD40 agonists vaccination (Vac) as described in Fig. 1a. a Tumor volume curves (left) and tumor weight (mg) in different treatment groups (n = 7 mice per group). b, c Left shows representative FACS plots showing CD8 and CX3CR1 expression gated with AdpgkMut Tet+ CD8+ T cells in PB. Right shows the frequency of the CX3CR1+ Tet+ CD8+ T cells in each group. (n = 4 (b) and 10–12 (c) mice per group). d Tumor growth and survival curves in different treatment groups (n = 10–11 mice per group). Tumors (a) and PB (b, c) were harvested 1 week after 2nd Vac. Data shown are representative of two independent experiments (a, b) and pooled from two independent experiments (c, d). *P < 0.05, **P < 0.01, ***P < 0.001, ****P < 0.0001, one-way ANOVA with Tukey’s multiple comparisons (a, b and c), Mann–Whitney U test (d for tumor volume), or log-rank test (d for survival). Mean ± SEM
cDC1s express high levels of TLR3, and a TLR3 agonist poly(I:C) has been used in personalized neoantigen vaccine trials with vaccine-related objective responses [5, 6, 7]. However, the role of CD40 signaling in cDC1s in vaccine-based immunotherapy remains elusive. To address a direct role of CD40 signaling in cDC1 lineage for CX3CR1+ CD8+ T cell development, we generated CD40−/−/Batf3−/− or wild type (WT)/Batf3−/− mixed bone marrow chimeras into irradiated C57BL/6 WT mice. CD40−/−/Batf3−/− bone marrow transplanted recipient mice (CD40−/− plus Batf3−/− mixed bone marrow chimeras) lack CD40 expression only in Batf3-dependent bone marrow-derived cells, while WT/Batf3−/− bone marrow transplanted recipient mice have an intact CD40 signaling. In CD40−/− plus Batf3−/− mixed bone marrow chimeras, CX3CR1+ CD8+ T-cell responses remained minimal compared to WT plus Batf3−/− mixed bone marrow chimeras, indicating that CD40 expression on cDC1s is pivotal for the CX3CR1+ CD8+ T-cell induction (Fig. 5c). In line with these results, mice reconstituted with CD40−/− plus Batf3−/− mixed bone marrow chimeras had substantially decreased antitumor efficacy of AdpgkMut/TLR3/CD40 agonists vaccination compared to mice with WT plus Batf3−/− mixed bone marrow chimeras (Fig. 5d).
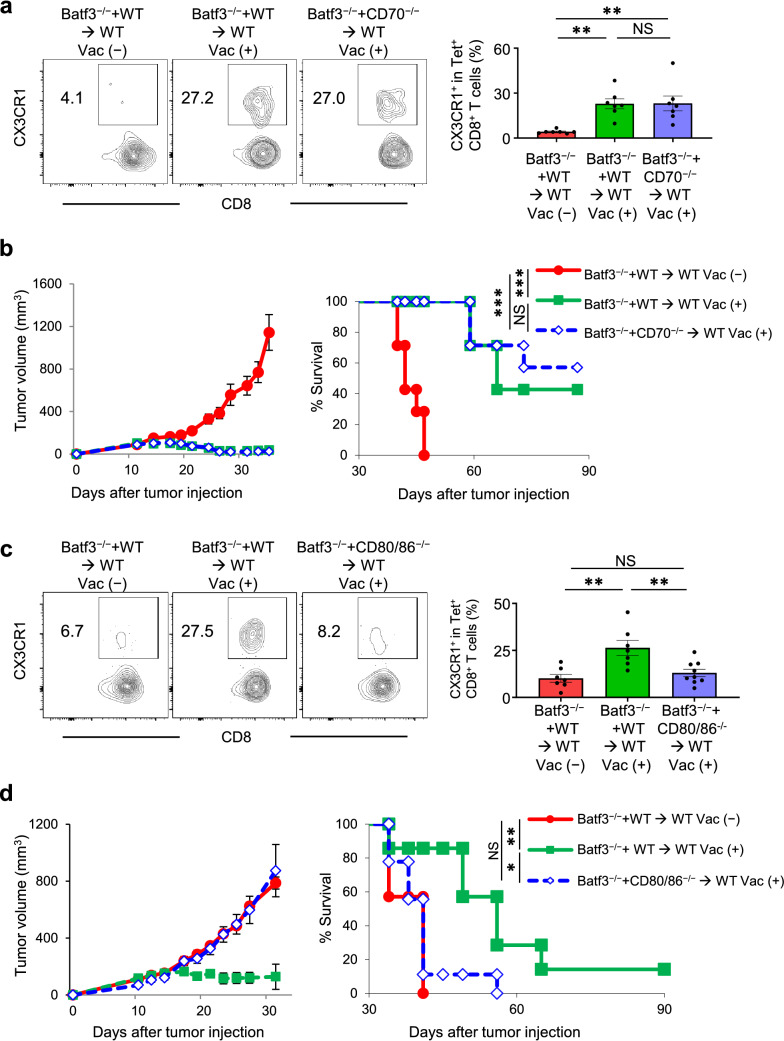
Non-redundant requirement of CD80/86 but not CD70 signaling in cDC1s for the antitumor efficacy of neoantigen/TLR3/CD40 agonists vaccination and generation of antigen-specific CX3CR1+ CD8+ T cells.
In addition to CD40, both CD70 and CD80/86 have been reported to play an important role in DC-mediated T cell activation and persistence [40]; however, the role of these molecules on cDC1s for neoantigen-specific T-cell differentiation remains elusive. To identify the potential role of CD70 and CD80/86 signaling in effective neoantigen vaccination, we first utilized CD70−/− and CD80/86−/− mice. We found that antitumor efficacy of AdpgkMut/CD40/TLR3 vaccination was abrogated in CD80/86−/− but not CD70−/− mice (Supplementary Fig. 9a). Consistent with this, generation of circulating CX3CR1+ Tet+ CD8+ T cells was markedly decreased in CD80/86−/− but not CD70−/− mice (Supplementary Fig. 9b). Next, we employed mixed bone marrow chimeric mice of CD70−/− plus Batf3−/− and CD80/86−/− plus Batf3−/− to determine the requirements of CD70 and CD80/86 signaling in cDC1s. There was no difference in the frequency of AdpgkMut-specific CX3CR1+ CD8+ T cells between WT plus Batf3−/− and CD70−/− plus Batf3−/− mixed bone marrow chimeras (Fig. 6a) with similar tumor control (Fig. 6b), suggesting a redundant role of CD70 signaling in cDC1s. In sharp contrast, generation of AdpgkMut-specific CX3CR1+ CD8+ T cells (Fig. 6c) and antitumor efficacy of AdpgkMut/TLR3/CD40 agonists vaccination (Fig. 6d) were abrogated in the CD80/86−/− plus Batf3−/− compared to the control WT plus Batf3−/− mixed bone marrow chimeras. Thus, the CD80/86-CD28 pathway in cDC1s plays a crucial role in mediating antitumor efficacy and generating antigen-specific effector CX3CR1+ CD8+ T cells in the context of neoantigen vaccination. Taken together, effective neoantigen/TLR3/CD40 agonists vaccination correlates with generation and frequency of circulating antigen-specific CX3CR1+ CD8+ T cells mediated by CD40 and CD80/86 signaling in cDC1s.
Fig. 6.
Non-redundant requirement of CD80/86 but not CD70 signaling in cDC1s for the antitumor efficacy of neoantigen/TLR3/CD40 agonists vaccination and generation of antigen-specific CX3CR1+ CD8+ T cells. a–d MC38-tumor bearing mixed bone marrow chimeric mice (Batf3−/− and WT → WT or Batf3−/− and CD70−/− → WT) (a, b) or (Batf3−/− and WT → WT or Batf3−/− and CD80/86−/− → WT) (c, d) were treated with or without AdpgkMut/TLR3/CD40 agonists vaccination (Vac) as described in Fig. 1a. a, c Left shows representative FACS plots showing CX3CR1 expression gated with AdpgkMut Tet+ CD8+ T cells in PB. Right shows the frequency of the CX3CR1+ Tet+ CD8+ T cells in each group. b, d Tumor growth and survival curves of MC38 tumor-bearing mixed bone marrow chimeric mice in different treatment groups as indicated. (n = 7 mice per group). PB was harvested 1 week after 2nd Vac (a, c). (n = 7 (a, b) and 7–9 (c, d) mice per group). Data shown are representative from two or three independent experiments. NS not significant, *P < 0.05, **P < 0.01, ***P < 0.001, one-way ANOVA with Tukey’s multiple comparisons (a, c) or log-rank test (b, d). Mean ± SEM
Discussion
In this study, we have shown potent synergistic antitumor efficacy of neoantigen/TLR3/CD40 stimulations in a preclinical model, and identified a novel link between antitumor efficacy of neoantigen vaccine and an increased frequency of circulating neoantigen-specific CX3CR1+ CD8+ T cells. Moreover, using mixed bone marrow chimeras from Batf3−/−, CD40−/−, CD70−/− and CD80/86−/− mice, we have revealed a pivotal role of CD40 and CD80/86 signaling in cDC1s for generation of neoantigen-specific CX3CR1+ CD8+ T cells and in vivo antitumor efficacy of neoantigen/TLR3/CD40 agonists vaccination.
Engagement of CD40 on DCs induces costimulatory molecules on their surface, promotes their cytokine production, facilitates the cross-presentation of antigen [34], and synergistically augments expansion of both endogenous and adoptive transferred T cells with TLR agonist [16–21]. Our data further demonstrated that among the diversity of the DC subsets cDC1s play a critical role in antitumor efficacy of TLR3/CD40 stimulation. Although poly(I:C) (TLR3 agonist) has been used in neoantigen vaccine clinical studies [5, 6], these findings underscore the implication of agonistic anti-CD40 Ab in combination with poly(I:C) for the maximal engagement of cDC1 in vaccine-based therapy.
The molecular and cellular mechanisms underlying differentiation of CX3CR1−– CX3CR1+ CD8+ T cells remain elusive. Recent studies have indicated that CD4+ T-cell help or agonistic anti-CD40 Ab facilitates generation of CX3CR1+ CD8+ T cells [26, 36]. Our results further demonstrated the non-redundant requirement of CD40 signaling in cDC1s for the generation of antigen-specific CX3CR1+ CD8+ T cells. The observation that CD70 signaling in cDC1s was dispensable for generation of the CX3CR1+ subset was unexpected given the several lines of evidence suggesting that CD40 signaling relays the CD4+ T-cell help signal by the induction of CD70 expression on DCs [16, 41]. However, not all molecules regulated by CD4+ T-cell help are mediated by CD27, and cytokine signaling may have contributed to the generation of the CX3CR1+ subset [36]. In support of this notion, IL-21 was found to modulate T-cell differentiation and generation of CX3CR1+ CD8+ T cells [26, 42]. Additional mechanistic studies are needed to further determine the signaling required for generation of the CX3CR1+ subset.
The lack of a reliable surrogate marker for clinical response remains the major limitation of vaccine clinical trials [1, 2]. Mere presence of vaccine-primed tumor-specific T cells might not correlate with effective regression of the tumor, and cannot be used as a standalone biomarker for vaccine efficacy [43]. In our study, although the frequency of PB antigen-specific total CD8+ T cells had a positive inverse correlation with tumor volume, differentiation of antigen-specific CD8+ T cells defined by CX3CR1 expression had a markedly higher inverse correlation with tumor volume. Furthermore, our findings of an increased frequency of CX3CR1+ CD8+ T cells that have the ability to produce IFN-γ, TNF-α and GZMA after successful vaccination align with the evidence from previous clinical studies showing that functional assessment of T cells such as IFN-γ production and/or cytolytic activity was correlated with response in patients treated with peptide vaccines [23, 24, 25].
An increased frequency of the CX3CR1+ subset was identified not only in Tet+ CD8+ T cells, but also in total CD8+ T cells, which had a strong inverse correlation with tumor volume. It remains unclear, however, whether this was due to an increased frequency of non-specific CX3CR1+ CD8+ T cells or an epitope-spreading phenomenon, the spread of the immune response from one antigen to another antigen expressed in the same tissue [1, 2]. Additional studies are required to determine whether AdpgkMut/TLR3/CD40 agonists vaccination-induced CX3CR1+ CD8+ T cells contain tumor-specific T cells other than AdpgkMut-specific T cells. Nevertheless, this observation suggests the potential utility of CX3CR1 as a blood-based predictive T-cell biomarker for other immunotherapies such as immune checkpoint inhibitor therapy. Indeed, our study and others revealed that changes in the frequency of PB CX3CR1+ CD8+ T cells correlate with response to immune checkpoint inhibitors in preclinical models and patients [11, 30].
Our work and others have provided some insights into the theoretical advantages of CX3CR1 as a circulating surrogate marker for vaccine efficacy. Vaccine-primed CX3CR1+ CD8+ T cells expressed low levels of CD62L and CXCR3, trafficking receptors necessary for entry across lymphoid organ HEV and the tumor microvasculature, respectively [12, 13, 14], that may have contributed to increase its frequency in PB. Furthermore, unlike other T-cell proliferation, activation or co-stimulatory/inhibitory molecules transiently expressed after activation, CX3CR1 is stably expressed on CD8+ T cells through unidirectional differentiation from CX3CR1− CD8+ T cells during the effector phase [8, 9].
Although the scope of our study is limited to CX3CR1, another future area of investigation would be to compare the utility of CX3CR1 as a circulating surrogate marker for vaccine efficacy with other molecules such as PD-1, which was reported to be expressed in circulating neoantigen-specific CD8+ T cells [44]. Notably, our study was limited by the use of a transplantable non-orthotopic mouse tumor model with high mutational burden. Thus, the utility of CX3CR1 as a circulating T-cell biomarker for vaccine therapy targeting endogenous tumor-associated antigens or tumors with lower mutational burden remains uncertain. Further studies in genetically engineered mouse models or mouse tumors harboring endogenous tumor-associated antigen are warranted to better understand the overall utility of CX3CR1 in vaccine therapy.
Our in vivo depletion study revealed that CX3CR1+ CD8+ T cells were dispensable for the antitumor efficacy of AdpgkMut/TLR3/CD40 agonists vaccination against established MC38 tumors, in agreement with our previous study using a preclinical model of adoptive T cell therapy against B16 melanoma [10]. These findings are also in parallel to the compelling evidence that terminally differentiated effector CD8+ T cells have less antitumor efficacy in vivo compared to less-differentiated T cells [45], but in contrast to previous studies demonstrating decreased antitumor immunity in CX3CR1−/− mice or with the use of the CX3CR1 antagonist [27–30, 36]. A possible explanation is that in Cd2-cre/Cx3cr1+/DTR mice, DT administration does not affect monocytes, DCs, M-MDSCs and TAMs that may express CX3CR1 [31, 32] and negatively regulate antitumor immunity. Another possibility is that CX3CR1/CX3CL1 pathway might not play a significant role in an MC38 tumor model, and trafficking of CX3CR1+ CD8+ T cells to the TME is not efficient in the setting of their decreased CXCR3 expression (12). Previous preclinical studies using mouse and xenograft tumor models revealed that augmenting CX3CR1/CX3CL1 axis enhances T cell/NK-cell mediated antitumor efficacy [27, 46, 47]. Therefore, the role of CX3CR1+ CD8+ T cells in antitumor reactivity against solid malignancies might be context-dependent, and remains to be elucidated.
In summary, our study revealed cDC1-mediated potent antitumor efficacy of neoantigen vaccine with dual TLR3/CD40 stimulation, identified a correlation between antitumor response and the frequency of antigen-specific PB CX3CR1+ CD8+ T cells, and provided the mechanistic insight into the generation of CX3CR1+ CD8+ T cells. These data suggest the maximal engagement of cDC1s with TLR3/CD40 stimulation for the development of more effective neoantigen vaccine therapy, and the potential implications of CX3CR1 as a circulating T-cell predictive biomarker for response in future clinical studies.
Supplementary Information
Below is the link to the electronic supplementary material.
Acknowledgements
We acknowledge the NIH Tetramer Core Facility (contract HHSN272201300006C) for provision of MHC class I tetramers, Dr. Weiping Zou (University of Michigan, Ann Arbor, Michigan) for MC38 cells, Dr. James J. Moon (University of Michigan, Ann Arbor, Michigan) and Dr. Toshihiro Yokoi (Roswell Park) for technical assistance, and Dr. Suzanne M. Hess and Ms. Judith Epstein (Roswell Park) for administrative assistance. This work was supported by National Cancer Institute (NCI) grant P30CA016056 involving the use of Roswell Park’s Flow and Image Cytometry, Genomic Shared, and Biostatistics and Statistical Genomics Resources. This work was supported by Roswell Park Alliance Foundation and NCI/K08CA197966 (F. Ito), NIH/R01HL135325 (X. Cao), Uehara Memorial Foundation (T. Oba), and Astellas Foundation for Research on Metabolic Disorders and the Nakatomi Foundation (T. Yamauchi).
Abbreviations
- Ab
Antibody
- Batf3
Basic leucine zipper transcription factor ATF-like 3
- cDC1
Conventional type 1 dendritic cell
- CTLA-4
Cytotoxic T-lymphocyte-associated protein 4
- CX3CR1
CX3C chemokine receptor 1
- DC
Dendritic cell
- DT
Diphtheria toxin
- DTR
Diphtheria toxin receptor
- GZMA
Granzyme A
- HEV
High endothelial venules
- ICOS
Inducible T cell co-stimulator
- IFN-γ
Interferon gamma
- i.p.
Intraperitoneally
- KLRG1
Killer-cell lectin like receptor G1
- MHC
Major histocompatibility complex
- NT
No treatment
- PB
Peripheral blood
- PBS
Phosphate-buffered saline
- PD-1
Programmed cell death protein 1
- poly(I:C)
Polyinosinic-polycytidylic acid
- s.c.
Subcutaneously
- TAM
Tumor associated macrophage
- TdLN
Tumor-draining lymph nodes
- TLR3
Toll-like receptor 3
- TME
Tumor microenvironment
- TNF-α
Tumor necrosis factor alpha
- WT
Wild-type
Authors’ contributions
T.Y. contributed development of methodology, acquisition of data, analysis and interpretation of data, writing, review, and revision of the manuscript. T.H. and T.O. contributed development of methodology, acquisition of data, analysis and interpretation of data, review, and revision of the manuscript. R.K. contributed acquisition of data, analysis and interpretation of data, review, and revision of the manuscript. K.A. contributed analysis and interpretation of data, review, and revision of the manuscript. X.C. provided CD70−/− mice, review, and revision of the manuscript. F.I. developed the concept, developed methodology, analyzed data, coordinated author activities, revised the manuscript, and provided final approval of the version to be submitted.
Funding
This work was supported by Roswell Park Comprehensive Cancer Center and its National Cancer Institute (NCI) award, P30CA016056 involving the use of Roswell Park’s Flow and Image Cytometry and Genomic Shared Resources. F.I. was supported by Roswell Park Alliance Foundation and NIH/NCI K08CA197966. X.C. was supported by NIH/R01HL135325. T.Y. was supported by Astellas Foundation for Research on Metabolic Disorders, and the Nakatomi Foundation. T.O. was supported by Uehara Memorial Foundation.
Data Availability
The datasets used and analyzed during the current study are available from the corresponding author upon reasonable request.
Declarations
Conflict of interest
The authors declare no conflict of interest.
Ethical approval
All animal studies were reviewed and approved by the Roswell Park institutional animal care and use program and facilities (protocol #1316 M and 1356 M). All aspects of animal research and husbandry were conducted in accordance with the federal Animal Welfare Act and the NIH Guide for the Care and Use of Laboratory Animals.
Informed consent
This paper has no human clinical studies that require informed consent.
Footnotes
Takayoshi Yamauchi, Toshifumi Hoki and Takaaki Oba: Co-first author.
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Butterfield LH. (2015) Cancer vaccines. In: Clinical research (ed) BMJ. 350: h988. doi: 10.1136/bmj.h988 [DOI] [PMC free article] [PubMed]
- 2.Melero I, Gaudernack G, Gerritsen W, et al. Therapeutic vaccines for cancer: an overview of clinical trials. Nat Rev Clin Oncol. 2014;11:509–524. doi: 10.1038/nrclinonc.2014.111. [DOI] [PubMed] [Google Scholar]
- 3.Schumacher TN, Schreiber RD. Neoantigens in cancer immunotherapy. Science. 2015;348:69–74. doi: 10.1126/science.aaa4971. [DOI] [PubMed] [Google Scholar]
- 4.Sahin U, Derhovanessian E, Miller M, et al. Personalized RNA mutanome vaccines mobilize poly-specific therapeutic immunity against cancer. Nature. 2017;547:222–226. doi: 10.1038/nature23003. [DOI] [PubMed] [Google Scholar]
- 5.Keskin DB, Anandappa AJ, Sun J, et al. Neoantigen vaccine generates intratumoral T cell responses in phase Ib glioblastoma trial. Nature. 2019;565:234–239. doi: 10.1038/s41586-018-0792-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ott PA, Hu Z, Keskin DB, et al. An immunogenic personal neoantigen vaccine for patients with melanoma. Nature. 2017;547:217–221. doi: 10.1038/nature22991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hilf N, Kuttruff-Coqui S, Frenzel K, et al. Actively personalized vaccination trial for newly diagnosed glioblastoma. Nature. 2019;565:240–245. doi: 10.1038/s41586-018-0810-y. [DOI] [PubMed] [Google Scholar]
- 8.Gordon CL, Lee LN, Swadling L, et al. Induction and maintenance of CX3CR1-intermediate peripheral memory CD8(+) T cells by persistent viruses and vaccines. Cell Rep. 2018;23:768–782. doi: 10.1016/j.celrep.2018.03.074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gerlach C, Moseman EA, Loughhead SM, Alvarez D, Zwijnenburg AJ, Waanders L, Garg R, de la Torre JC, von Andrian UH. The chemokine receptor CX3CR1 defines three antigen-experienced CD8 T cell subsets with distinct roles in immune surveillance and homeostasis. Immunity. 2016;45:1270–1284. doi: 10.1016/j.immuni.2016.10.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Yamauchi T, Hoki T, Oba T, Saito H, Attwood K, Sabel MS, Chang AE, Odunsi K, Ito F. CX3CR1-CD8+ T cells are critical in antitumor efficacy, but functionally suppressed in the tumor microenvironment. JCI insight. 2020;5:e133920. doi: 10.1172/jci.insight.133920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Yamauchi T, Hoki T, Oba T, et al. T-cell CX3CR1 expression as a dynamic blood-based biomarker of response to immune checkpoint inhibitors. Nat Commun. 2021;12:1402. doi: 10.1038/s41467-021-21619-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Mikucki ME, Fisher DT, Matsuzaki J, et al. Non-redundant requirement for CXCR3 signalling during tumoricidal T-cell trafficking across tumour vascular checkpoints. Nat Commun. 2015;6:7458. doi: 10.1038/ncomms8458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.von Andrian UH, Mempel TR. Homing and cellular traffic in lymph nodes. Nat Rev Immunol. 2003;3:867–878. doi: 10.1038/nri1222. [DOI] [PubMed] [Google Scholar]
- 14.Gallatin WM, Weissman IL, Butcher EC. A cell-surface molecule involved in organ-specific homing of lymphocytes. Nature. 1983;304:30–34. doi: 10.1038/304030a0. [DOI] [PubMed] [Google Scholar]
- 15.Yadav M, Jhunjhunwala S, Phung QT, et al. Predicting immunogenic tumour mutations by combining mass spectrometry and exome sequencing. Nature. 2014;515:572–576. doi: 10.1038/nature14001. [DOI] [PubMed] [Google Scholar]
- 16.Oba T, Hoki T, Yamauchi T, Keler T, Marsh HC, Cao X, Ito F. A critical role of CD40 and CD70 signaling in conventional type 1 dendritic cells in expansion and antitumor efficacy of adoptively transferred tumor-specific T cells. J Immunol. 2020;205:1867–1877. doi: 10.4049/jimmunol.2000347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Nimanong S, Ostroumov D, Wingerath J, et al. CD40 signaling drives potent cellular immune responses in heterologous cancer vaccinations. Can Res. 2017;77:1918–1926. doi: 10.1158/0008-5472.Can-16-2089. [DOI] [PubMed] [Google Scholar]
- 18.Cho HI, Celis E. Optimized peptide vaccines eliciting extensive CD8 T-cell responses with therapeutic antitumor effects. Can Res. 2009;69:9012–9019. doi: 10.1158/0008-5472.Can-09-2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ahonen CL, Doxsee CL, McGurran SM, Riter TR, Wade WF, Barth RJ, Vasilakos JP, Noelle RJ, Kedl RM. Combined TLR and CD40 triggering induces potent CD8+ T cell expansion with variable dependence on type I IFN. J Exp Med. 2004;199:775–784. doi: 10.1084/jem.20031591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Oba T, Long MD, Keler T, Marsh HC, Minderman H, Abrams SI, Liu S, Ito F. Overcoming primary and acquired resistance to anti-PD-L1 therapy by induction and activation of tumor-residing cDC1s. Nat Commun. 2020;11:5415. doi: 10.1038/s41467-020-19192-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Scarlett UK, Cubillos-Ruiz JR, Nesbeth YC, Martinez DG, Engle X, Gewirtz AT, Ahonen CL, Conejo-Garcia JR. In situ stimulation of CD40 and Toll-like receptor 3 transforms ovarian cancer-infiltrating dendritic cells from immunosuppressive to immunostimulatory cells. Can Res. 2009;69:7329–7337. doi: 10.1158/0008-5472.Can-09-0835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Khalil DN, Suek N, Campesato LF, et al. In situ vaccination with defined factors overcomes T cell exhaustion in distant tumors. J Clin Invest. 2019;129:3435–3447. doi: 10.1172/jci128562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Carbone DP, Ciernik IF, Kelley MJ, et al. Immunization with mutant p53- and K-ras-derived peptides in cancer patients: immune response and clinical outcome. J Clin Oncol. 2005;23:5099–5107. doi: 10.1200/jco.2005.03.158. [DOI] [PubMed] [Google Scholar]
- 24.Kirkwood JM, Lee S, Moschos SJ, Albertini MR, Michalak JC, Sander C, Whiteside T, Butterfield LH, Weiner L. Immunogenicity and antitumor effects of vaccination with peptide vaccine+/-granulocyte-monocyte colony-stimulating factor and/or IFN-α2b in advanced metastatic melanoma: eastern cooperative oncology group phase II trial E1696. Clin Cancer Res. 2009;15:1443–1451. doi: 10.1158/1078-0432.Ccr-08-1231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Dillon PM, Olson WC, Czarkowski A, Petroni GR, Smolkin M, Grosh WW, Chianese-Bullock KA, Deacon DH, Slingluff CL., Jr A melanoma helper peptide vaccine increases Th1 cytokine production by leukocytes in peripheral blood and immunized lymph nodes. J Immunother Cancer. 2014;2:23. doi: 10.1186/2051-1426-2-23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Zander R, Schauder D, Xin G, Nguyen C, Wu X, Zajac A, Cui W. CD4(+) T Cell help is required for the formation of a cytolytic CD8(+) T cell subset that protects against chronic infection and cancer. Immunity. 2019;51:1028–42.e4. doi: 10.1016/j.immuni.2019.10.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Xin H, Kikuchi T, Andarini S, et al. Antitumor immune response by CX3CL1 fractalkine gene transfer depends on both NK and T cells. Eur J Immunol. 2005;35:1371–1380. doi: 10.1002/eji.200526042. [DOI] [PubMed] [Google Scholar]
- 28.Zeng Y, Huebener N, Fest S, et al. Fractalkine (CX3CL1)- and interleukin-2-enriched neuroblastoma microenvironment induces eradication of metastases mediated by T cells and natural killer cells. Can Res. 2007;67:2331–2338. doi: 10.1158/0008-5472.Can-06-3041. [DOI] [PubMed] [Google Scholar]
- 29.Yu YR, Fong AM, Combadiere C, Gao JL, Murphy PM, Patel DD. Defective antitumor responses in CX3CR1-deficient mice. Int J Cancer. 2007;121:316–322. doi: 10.1002/ijc.22660. [DOI] [PubMed] [Google Scholar]
- 30.Yan Y, Cao S, Liu X, et al. CX3CR1 identifies PD-1 therapy-responsive CD8+ T cells that withstand chemotherapy during cancer chemoimmunotherapy. JCI insight. 2018;3:e97828. doi: 10.1172/jci.insight.97828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Nishimura M, Umehara H, Nakayama T, Yoneda O, Hieshima K, Kakizaki M, Dohmae N, Yoshie O, Imai T. Dual functions of fractalkine/CX3C ligand 1 in trafficking of perforin+/granzyme B+ cytotoxic effector lymphocytes that are defined by CX3CR1 expression. J Immunol. 2002;168:6173–6180. doi: 10.4049/jimmunol.168.12.6173. [DOI] [PubMed] [Google Scholar]
- 32.Bronte V, Brandau S, Chen SH, et al. Recommendations for myeloid-derived suppressor cell nomenclature and characterization standards. Nat Commun. 2016;7:12150. doi: 10.1038/ncomms12150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Sanchez-Paulete AR, Cueto FJ, Martinez-Lopez M, et al. Cancer immunotherapy with immunomodulatory anti-CD137 and anti-PD-1 monoclonal antibodies requires BATF3-dependent dendritic cells. Cancer Discov. 2016;6:71–79. doi: 10.1158/2159-8290.cd-15-0510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Elgueta R, Benson MJ, de Vries VC, Wasiuk A, Guo Y, Noelle RJ. Molecular mechanism and function of CD40/CD40L engagement in the immune system. Immunol Rev. 2009;229:152–172. doi: 10.1111/j.1600-065X.2009.00782.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Vonderheide RH. The immune revolution: a case for priming Not Checkpoint. Cancer Cell. 2018;33:563–569. doi: 10.1016/j.ccell.2018.03.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Ahrends T, Spanjaard A, Pilzecker B, Babala N, Bovens A, Xiao Y, Jacobs H, Borst J. CD4(+) T cell help confers a cytotoxic T cell effector program including coinhibitory receptor downregulation and increased tissue invasiveness. Immunity. 2017;47:848–61.e5. doi: 10.1016/j.immuni.2017.10.009. [DOI] [PubMed] [Google Scholar]
- 37.Hildner K, Edelson BT, Purtha WE, et al. Batf3 deficiency reveals a critical role for CD8α+ dendritic cells in cytotoxic T cell immunity. Science. 2008;322:1097–1100. doi: 10.1126/science.1164206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Broz ML, Binnewies M, Boldajipour B, et al. Dissecting the tumor myeloid compartment reveals rare activating antigen-presenting cells critical for T cell immunity. Cancer Cell. 2014;26:638–652. doi: 10.1016/j.ccell.2014.09.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Morrison AH, Diamond MS, Hay CA, Byrne KT, Vonderheide RH. Sufficiency of CD40 activation and immune checkpoint blockade for T cell priming and tumor immunity. Proc Natl Acad Sci U S A. 2020;117:8022–8031. doi: 10.1073/pnas.1918971117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Bak SP, Barnkob MS, Bai A, Higham EM, Wittrup KD, Chen J. Differential requirement for CD70 and CD80/CD86 in dendritic cell-mediated activation of tumor-tolerized CD8 T cells. J Immunol. 2012;189:1708–1716. doi: 10.4049/jimmunol.1201271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Borst J, Ahrends T, Babala N, Melief CJM, Kastenmuller W. CD4(+) T cell help in cancer immunology and immunotherapy. Nat Rev Immunol. 2018;18:635–647. doi: 10.1038/s41577-018-0044-0. [DOI] [PubMed] [Google Scholar]
- 42.Tian Y, Cox MA, Kahan SM, Ingram JT, Bakshi RK, Zajac AJ. A context-dependent role for IL-21 in modulating the differentiation, distribution, and abundance of effector and memory CD8 T cell subsets. J Immunol. 2016;196:2153–2166. doi: 10.4049/jimmunol.1401236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Rosenberg SA, Sherry RM, Morton KE, et al. Tumor progression can occur despite the induction of very high levels of self/tumor antigen-specific CD8+ T cells in patients with melanoma. J Immunol. 2005;175:6169–6176. doi: 10.4049/jimmunol.175.9.6169. [DOI] [PubMed] [Google Scholar]
- 44.Gros A, Parkhurst MR, Tran E, et al. Prospective identification of neoantigen-specific lymphocytes in the peripheral blood of melanoma patients. Nat Med. 2016;22:433–438. doi: 10.1038/nm.4051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Restifo NP, Dudley ME, Rosenberg SA. Adoptive immunotherapy for cancer: harnessing the T cell response. Nat Rev Immunol. 2012;12:269–281. doi: 10.1038/nri3191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Guo J, Zhang M, Wang B, Yuan Z, Guo Z, Chen T, Yu Y, Qin Z, Cao X. Fractalkine transgene induces T-cell-dependent antitumor immunity through chemoattraction and activation of dendritic cells. Int J Cancer. 2003;103:212–220. doi: 10.1002/ijc.10816. [DOI] [PubMed] [Google Scholar]
- 47.Siddiqui I, Erreni M, van Brakel M, Debets R, Allavena P. Enhanced recruitment of genetically modified CX3CR1-positive human T cells into Fractalkine/CX3CL1 expressing tumors: importance of the chemokine gradient. J Immunother Cancer. 2016;4:21. doi: 10.1186/s40425-016-0125-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The datasets used and analyzed during the current study are available from the corresponding author upon reasonable request.