Abstract
Background:
We lack specific treatments for traumatic brain injury (TBI), which remains the leading cause of trauma-related morbidity and mortality. Treatment with valproic acid (VPA) improves outcomes in models of severe TBI with concurrent hemorrhage. However, it is unknown if VPA will have similar benefits after isolated nonlethal TBI, which is the more common clinical scenario. The goal of this study was to evaluate the effect of VPA treatment in a preclinical isolated TBI swine model on neurologic outcomes and brain lesion size and to perform detailed pharmacokinetic (PK) analyses for a future clinical trial.
Methods:
Yorkshire swine (n=10; 5/cohort) were subjected to TBI (8 mm controlled cortical impact). An hour later, we randomized them to receive VPA (150 mg/kg) or saline placebo (control). Neurologic severity scores (NSS) were assessed daily (0=normal- 36=comatose), brain lesion size was measured on postinjury (PID) 3, and serial blood samples were collected for PK studies.
Results:
Physiologic parameters and laboratory values were similar in both groups. VPA treated animals demonstrated significantly better NSS on PID 1 (control=9.2±4.4; VPA=0±0; p=0.001). VPA treated animals had significantly smaller brain lesion sizes (mean volume in mm3: Control=3130±2166; VPA=764 ±208: p=0.02). PK data confirmed adequate plasma and tissue levels of VPA.
Conclusion:
In this clinically relevant model of isolated TBI, a single dose of VPA attenuates neurological impairment and decreases brain lesion size.
Keywords: Neuroprotection, Traumatic Brain Injury, Valproic Acid, Pre-clinical swine model
Introduction
Traumatic brain injury (TBI) annually is the cause of over 3 million emergency department visits, hospitalizations, and deaths.1,2 This results in a tremendous loss in productivity and financial burden for society.3 Our research group has demonstrated that treatment with Valproic Acid (VPA), a well-established neuroleptic agent, has the potential to improve neurologic outcomes when used as treatment in pre-clinical animal studies.4–7 However, the swine models of TBI in previous studies were designed to replicate combat trauma, and included concomitant hemorrhagic shock with or without additional extracranial injuries. While that data was vital in showing VPA’s efficacy in ‘worst case’ clinical scenarios, it is unknown how applicable those results are to the far more common clinical presentation of an isolated TBI in the civilian setting.8
VPA is a histone deacetylase inhibitor (HDACi) that increases acetylation of proteins such as histones, inducing conformational changes in chromatin structure and thereby augmenting the expression of key genes and proteins. Intravenously administered VPA circulates in two forms; bound to plasma proteins or unbound (free VPA). Early administration of a single dose of VPA has been shown to decrease brain injury lesion size and improve neurologic recovery after combined TBI and hemorrhagic shock (HS).4–7 This work also established two key findings: 1) there is a higher concentration of free VPA after severe injury compared to the uninjured controls and 2) there is an exposure-response relationship between the concentration of free VPA and days to neurologic recovery.7 It is currently unknown whether VPA treatment would enhance neurologic recovery or what pharmacokinetic changes will take place in TBI models that do not include hemorrhagic shock.
The aim of this study is to address this knowledge gap and assess pharmacokinetic changes of VPA’s impact after isolated TBI. We hypothesized that administration of VPA would improve neurologic recovery and decrease the brain lesion size in swine subjected to a nonlethal, isolated TBI.
Methods
Animal Protocol
In conducting research using animals, the investigators adhered to the Animal Welfare Act Regulations and other Federal statutes relating to animals and experiments involving animals and the principles set forth in the current version of the Guide for Care and Use of Laboratory Animals, National Research Council. The experimental protocol was approved by the Institutional Animal Care and Use Committee. Female Yorkshire swine weighing 36 kg to 46 kg were used for the experiments (Michigan State University, East Lansing, MI). After induction with Telazol (2-8 mg/kg) (Pfizer, New York, NY), animals were kept under general anesthesia with inhaled isoflurane (1-3%) and mechanically ventilated for the duration of the operation. A 20-gauge peripheral intravenous (IV) catheter was placed in the ear for VPA administration. A unilateral femoral cutdown was performed and a 5-Fr, 11-cm arterial catheter (Super Sheath, Boston Scientific Corporation, Marlborough, MA) was placed for arterial pressure monitoring. Traumatic brain injury was produced by controlled cortical impact as previously described while the animals were in prone position.11 Briefly, the head was first fixed in a stereotactic frame. . A scalp flap was raised, and a circular craniotomy (21 mm diameter) was made to expose the dura anterolateral to the bregma. A controlled cortical impact device inflicted the TBI on the exposed dura using a 15 mm diameter impactor, at 4m/s velocity, with 100 millisecond dwell time and a depth of 8 mm. One hour after TBI, animals were randomized to a normal saline vehicle (control group; n=5) or 150 mg/kg VPA (Virbec, Fort Worth, TX) delivered intravenously via a peripheral ear vein over 1 hour (VPA group; n=5). The primary operator was blinded to which group each animal was randomized to until after all the data were collected. After the 1-hour administration of either normal saline or VPA, the removed bone piece was replaced and the scalp incision was repaired, the femoral catheter was removed, and the animals were extubated for a 3-day observation period. Postoperatively animals were singly housed and had access to food and water ad lib. Pain was controlled with a fentanyl patch (75 μg/hr).
Neuroseverity Scoring
A validated scoring tool for neurologic injury was used to evaluate both severity of injury and recovery trajectory. The neuroseverity score (NSS)4,6,12 was assigned based on nine domains: level of consciousness, standing position, head position, gait, motor function (hind limbs/forelimbs), utterance, feeding/drinking, and behavior. It is a 36 point-scale with zero indicating no injury and 32 indicating severe injury (scoring rubric can be found in supplemental Data 1). Neuroseverity scoring was performed by two blinded observers daily for 3 days following the TBI. To avoid the confounding effects of general anesthesia, NSS assessment was not done until 24 hours after emergence from anesthesia on post-injury day (PID) 1.
Brain Lesion Size Measurement
Animals were euthanized at the end of the 3-day observation period. Brains were harvested and sliced into 5-mm coronal sections using a sectioning block (University of Michigan Medical Innovation Center, Ann Arbor, MI). Sections were then stained with 2% 2,3,5-triphenyltetrazolium chloride (TTC; SigmaChemical Co., St. Louis, MO) to distinguish viable from non-viable tissue. Brain lesion size (mm3) were calculated using ImageJ analysis software (National Institutes of Health, Bethesda, MD).9,10
Pharmacokinetic and Pharmacodynamic Assay and Analysis
Blood samples used for pharmacokinetic analysis were collected from VPA-treated swine at the end of the 1-hour infusion (normal saline or VPA). Both total and free plasma concentrations were assayed by liquid chromatography–mass spectrometry, and use of deuterated internal standard (valproic acid-d6). The limit of quantification was 50 ng/mL. Details of pharmacokinetic sample preparation, assays can be found in Supplemental Data 2. The VPA maximum concentrations (Cmax) were defined as the measurement at the end of infusion.
Statistical Analysis
Data are represented as group mean ± standard deviation (SD) values, unless otherwise specified. All analyses were performed using GraphPad Prism version 8.4 (GraphPad Software, San Diego, CA). Continuously variables (NSS and hemodynamic parameters) were analyzed using a repeated measures analysis of variance (ANOVA). Brown-Forsythe test was performed to ensure equal variance between the groups when performing ANOVA. If variance was not equal between the groups, nonparametric Kruskal-Wallis testing was performed. Statistical significance was defined as p < 0.05. Brain lesion size was analyzed using a Mann-Whitney U test. A p-value of <0.05 was chosen to determine statistical significance. Statistical outliers were determined using the Grubbs’ test with a significance level of 0.05.
The primary endpoint was NSS on PID1. Power analysis with α = 0.05 and b = 0.80, β mean 20 in control group and mean 9 in VPA treated groups, with SD of 6 based on prior experiments5,7,12 calculated the sample size needed for hypothesis testing was n=5 in each group. Secondary outcomes were subsequent PIDs’ NSS and brain lesion size.
Results
Survival
A total of 10 animals were subjected to injuries as per protocol. All of the animals survived to the predesignated end point (3 days post injury) without any post-operative complications.
Physiologic and Laboratory Parameters
There was no significant difference in physiologic parameters between the animals in the control or treatment groups (Figure 1). Both groups had brief increases in heart rate and mean arterial blood pressure following TBI but this trended down prior to treatment. Arterial blood gas parameters are in Supplemental Table 1. Overall, there were no significant differences in pH, sodium, potassium, or hemoglobin levels between the groups. Animal treated with VPA had statistically significant higher levels of lactate at the time of extubation (Control: 1.3 mmol ± 0.27, VPA 150 mg/kg: 2.7 mmol ± 0.28; p= <0.0001) (Figure 1). This trend is consistent with our prior work (4,5,6,7,11). The lactic acidosis that was observed following VPA administration has been demonstrated to be unrelated to tissue perfusion and hemodynamic parameters and resolves spontaneously without intervention.
Figure 1.
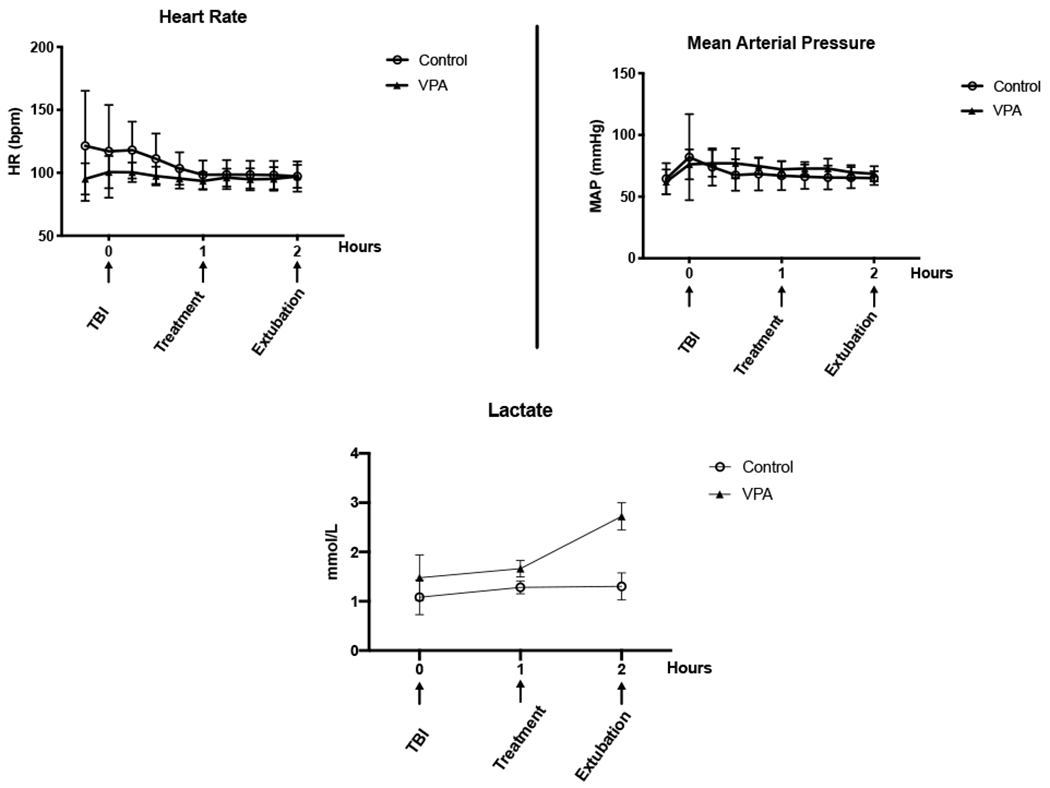
– Hemodynamic and Physiologic Response to Injury. Intraoperative measurements of (A) heart rate [HR; beats per minute (bmp)] (B) mean arterial pressure [MAP; mm Hg], and (C) lactate [mmol/L]. The X-axis represents time in 1-hour increments starting at instrumentation with arrows indicating significant events in the experiment. Error bars denote standard deviation. * statistical significance at that particular timepoint.
Neurologic Recovery
The control group exhibited the most severe neurologic injury on PID1, and most animals in that group returned to normal by PID3. No animal in the VPA treated group exhibited any evidence of neurological injury based on NSS. The VPA treated group had a significantly lower NSS score on PID 1 (mean score: control = 9.2 ± 4.5 versus VPA = 0 ± 0 p = 0.04). Figure 2 demonstrates the NSS scores were higher for the control group for PID 2 and PID 3 as well but those did not reach statistical significance. Two-way ANOVA of NSS showed a significant difference between the two experiment groups (p=0.01) and that PID was also a significant factor (p < 0.0001).
Figure 2.
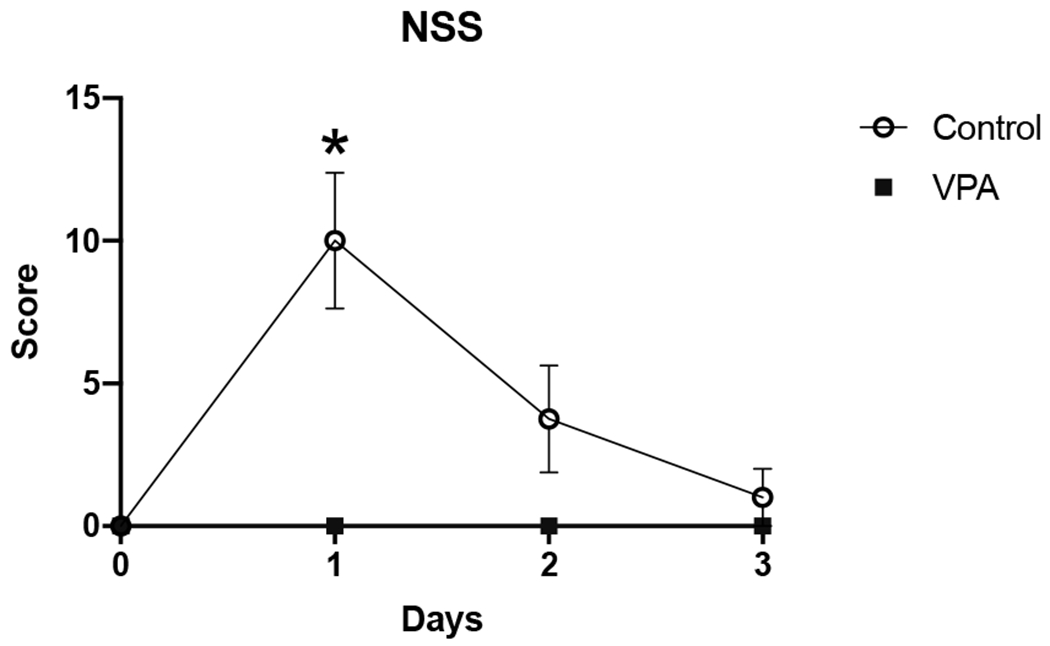
– Neuroseverity Score (NSS). Neurologic impairment of animals as measured by two blinded observers. Score is plotted on the y-axis from 0 to 36 with 36 being severe neurologic impairment/coma. Over the 3-day PID observation period, VPA treated animals consistently had lower NSS scores. Data presented as group mean ± Standard deviation (SD). Asterisks indicate time points of statistically significant difference between the control and treatment group.
Brain Lesion Size
Brain lesion size was measured on PID 3 (Figure 3) and VPA treated animals were found to have significantly smaller brain lesion sizes (mean volume in mm3: Control=3130 ± 2166; VPA = 764 ± 208: p=0.02). There was one animal in the treatment group with a lesion size > 3 times the others and was identified to be an outlier by Grubbs’ test (p value < 0.05), and was thus removed from the analysis.
Figure 3.
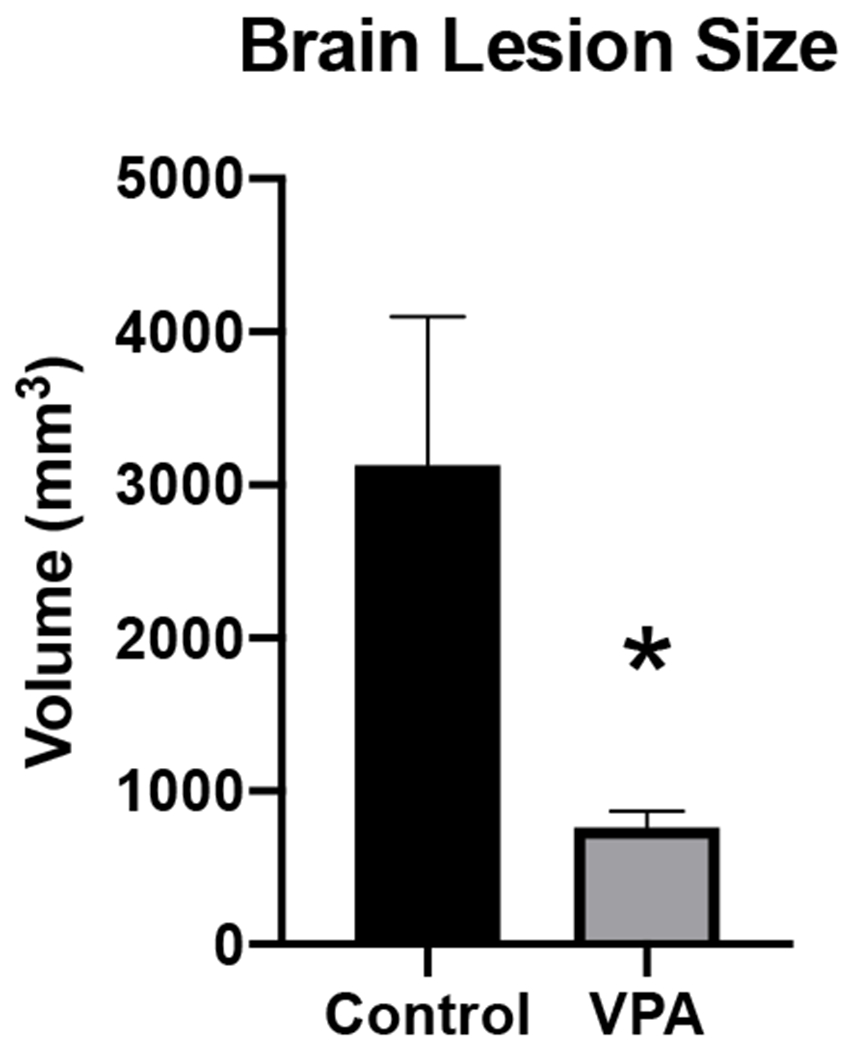
– Brain Lesion size. Brain lesions at 3 days after injury (PID 3), animals treated with VPA had significantly smaller brain lesion sizes compared to the control group.
Pharmacokinetic and Pharmacodynamic Analysis
The mean ± SD total VPA Cmax was 382.8 μg/mL ± 91.1 and the mean ± SD free VPA Cmax was 266.2 μg/mL ± 67.9 in the VPA group. The median [min, max] percent free concentration was 73.6% [37.2%, 98.3%]. A similar exponential decline relationship was observed between free VPA (R2= 0.73) concentrations compared to total VPA concentrations (R2= 0.73) and lesion size. Figure 4 illustrates this relationship of free VPA concentrations and this measure of neuroprotection.
Figure 4.
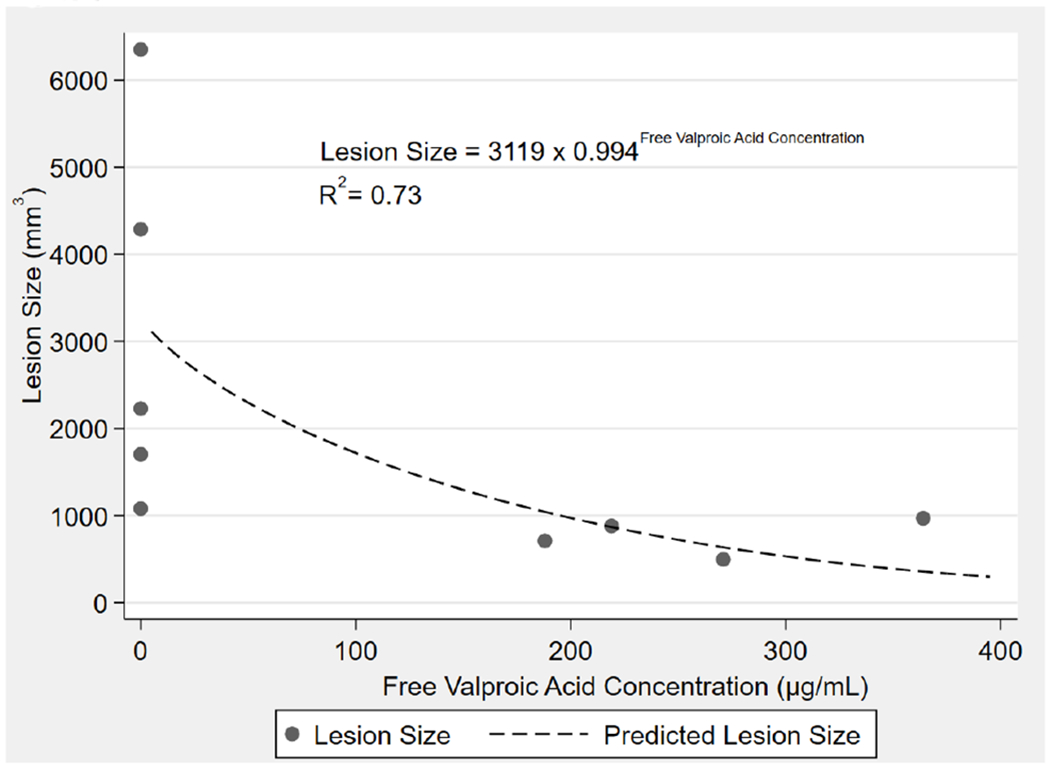
Concentration of free VPA plotted against brain lesion size. The graph demonstrates there is a strong correlation with the concentration of unbound VPA at the end of infusion and brain lesion size. fVPA = free VPA.
Discussion
This is the first study to examine the effect of VPA administration on neurologic outcomes in a clinically realistic model of isolated nonlethal TBI. Our group has previously demonstrated that a early administration of a single dose of VPA (150 mg/kg) improves neurologic outcomes in polytrauma TBI models that include hemorrhagic shock. However, it was previously unknown if this dose would have any benefits in the setting of isolated TBI. Isolated TBI is the most common form of head injury in the civilian setting. Current treatments primarily focus on providing supportive care and avoiding secondary insults, as no specific neuroprotective agents are available. Thus, a feasible pharmacologic intervention that improves recovery could have a meaningful impact. This work demonstrating neuroprotective benefits of VPA in isolated nonlethal TBI has relevance for a much larger patient population.
In addition to the neuroprotection benefits demonstrated in this study, the pharmacokinetic knowledge gained in this study is valuable. Previous models involved significant hemorrhage (at least 40% of estimated blood volume) and significant resuscitation (animals in prior studies typically received large volume crystalloid resuscitation) that likely have a clinically meaningful impact on the of pharmacokinetic properties such as free drug concentration and volume of distribution. It was unclear how VPA levels would be impacted in this study that omitted hemorrhage and resuscitation, and this relationship is important to delineate in order to answer practical questions regarding drug dosing in different patient populations. Prior work with those more severe models that established the neuroprotective benefits of VPA, showed a dose of VPA of 150 mg/kg administered over 1 hour resulted in a mean total VPA concentration of 477 μg/mL ± 39.8 and mean free VPA concentration of 214 μg/mL ± 121.8.7 Those levels are very similar to the total and free VPA concentrations demonstrated in this study of 382.8 μg/mL ± 91.1 and 266.2 μg/mL ± 67.9, respectively. This shows that hemorrhage and resuscitation has minimal effect on VPA concentration levels. The similar VPA concentration levels between the very different models likely explains the similar results of decreased Neurologic Severity Score and reduced brain lesion size. Furthermore, prior work showed that injured animals had relatively high levels of free VPA concentration (54.3%) compared to the expected 5-15% in healthy subjects.13,14 We had previously theorized the hemorrhagic shock and resuscitation increased free VPA levels by creating a state of hypoalbumenia that decreased plasma protein binding.14–16 The current study also demonstrates a high free-fraction of VPA, albeit with wide variability, observed in the free-fraction between animals. However, the relationship of lesion-size reduction to VPA concentration were predicted equally well by total and free values suggesting that free values may not necessarily be more informative in this model. This would suggest there may not be a need to adjust dosing of VPA based on severity of TBI or presence of other injuries. This will have implications for dose optimization in future clinical trials.
There are limitations to this study. There is an increased chance of a Type I error given the small sample size due to ethical and fiscal considerations. Another limitation is that clinical evaluation may not be sensitive enough to detect subtle neurologic deficits in swine. However, to avoid observer error, each animal was evaluated by two separate examiners. Furthermore, the more objective brain lesion measurement was in concordance with the NSS findings.
In summary, this study demonstrates that VPA treatment is safe, reduces neurologic injury, and decreases brain lesion size compared to control in a swine model on nonlethal, mild-moderate, isolated TBI. This is an important practical consideration to inform future clinical trials. Given the substantial burden of isolated TBI on the population these findings could be an important step in developing a therapeutic option for millions who are currently only managed with supportive care.
Supplementary Material
Acknowledgement:
This work was supported by the US Army Medical Research and Materiel Command under Contract No. W81XWH-17-C-0246 to Dr. Alam. The views, opinions and/or findings contained in this report are those of the author(s) and should not be construed as an official Department of the Army position, policy or decision unless so designated by other documentation. In addition, Dr. Biesterveld was supported by the National Institute of General Medical Sciences of the National Institutes of Health under Award Number F32GM130010. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Footnotes
These data were presented at the 51st Annual meeting of the Western Trauma Association, February 2021.
The authors do not report any conflicts of interest related to this study.
Level of Evidence: Therapeutic, V
References
- 1.Peterson AB, Daugherty J, Breiding MJ. Surveillance Report of Traumatic Brain Injury-related Emergency Department Visits, Hospitalizations, and Deaths—United States, 2014. Centers for Disease Control and Prevention, U.S. Department of Health and Human Services. TBI Surveillance Report. 2019. [Google Scholar]
- 2.Dewan MC, Rattani A, Gupta S, Baticulon RE, Hung Y, Punchak M, Agrawal A, Adekeye AO, Shrime MG, Rubiano AM et al. Estimating the global incidence of traumatic brain injury. J Neurosurg. 2018;130(4) 1039–1408. [DOI] [PubMed] [Google Scholar]
- 3.Ma VY, Chan L, & Carruthers KJ Incidence, prevalence, costs, and impact on disability of common conditions requiring rehabilitation in the United States: stroke, spinal cord injury, traumatic brain injury, multiple sclerosis, osteoarthritis, rheumatoid arthritis, limb loss, and back pain. Arch Phys Med Rehabil. 2014;95(5):986–995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Biesterveld, Ben E. MD; Williams Aaron M. MD; Pai Manjunath P. PharmD; Dennahy Isabel S. MD; Graham Nathan J. BS; Chtraklin Kiril DVM; Siddiqui Ali Z. BS; O'Connell Rachel L. BS; Bhatti Umar F. MD; Liu Baoling MD, et al. Dose optimization of valproic acid in a lethal model of traumatic brain injury, hemorrhage, and multiple trauma in swine, J Trauma Acute Care Surg. 2019;87(5):1133–1139. [DOI] [PubMed] [Google Scholar]
- 5.Nikolian VC, Georgoff PE, Pai MP, Dennahy IS, Chtraklin K, Eidy H, Ghandour MH, Han Y, Srinivasan A, Li Y, et al. Valproic acid decreases brain lesion size and improves neurologic recovery in swine subjected to traumatic brain injury, hemorrhagic shock, and polytrauma. J Trauma Acute Care Surg. 2017;83(6):1066–1073. [DOI] [PubMed] [Google Scholar]
- 6.Halaweish I, Bambakidis T, Chang Z, Wei H, Liu B, Li Y, Bonthrone T, Srinivasan A, Bonham T, Chtraklin K, et al. Addition of low-dose valproic acid to saline resuscitation provides neuroprotection and improves longterm outcomes in a large animal model of combined traumatic brain injury and hemorrhagic shock. J Trauma Acute Care Surg. 2015;79(6):911–919. [DOI] [PubMed] [Google Scholar]
- 7.Wakam GK, Biesterveld BE, Pai MP, Kemp MT, O'Connell RL, Williams AM, Srinivasan A, Chtraklin K, Siddiqui AZ, Bhatti UF, et al. Administration of Valproic Acid in Clinically Approved Dose Improves Neurologic Recovery and Decreases Brain Lesion Size in Swine Subjected to Hemorrhagic Shock and Traumatic Brain Injury. J Trauma Acute Care Surg. 2020; Nov 20. doi: 10.1097/TA.0000000000003036. [DOI] [PubMed] [Google Scholar]
- 8.Sugerman DE, Xu L, Pearson WS, Faul M. Patients with severe traumatic brain injury transferred to a Level I or II trauma center: United States, 2007 to 2009. J Trauma Acute Care Surg 2012;73(6):1491–1499. [DOI] [PubMed] [Google Scholar]
- 9.Rhee P, Talon E, Eifert S, Anderson D, Stanton K, Koustova E, Ling G, Burris D, Kaufmann C, Mongan P, et al. Induced hypothermia during emergency department thoracotomy: an animal model J Trauma Acute Care Surg. 2000;48(3):439–47. [DOI] [PubMed] [Google Scholar]
- 10.Jin G, Duggan M, Imam A, Demoya MA, Sillesen M, Hwabejire J, Jepsen CH, Liu B, Mejaddam AY, Lu J, et al. Pharmacologic resuscitation for hemorrhagic shock combined with traumatic brain injury. J Trauma Acute Care Surg. 2012;73(6):1461–70. [DOI] [PubMed] [Google Scholar]
- 11.Imam AM, Jin G, Duggan M, Sillesen M, Hwabejire JO, Jepsen CH, DePeralta D, Liu B, Lu J, deMoya MA, et al. Synergistic effects of fresh frozen plasma and valproic acid treatment in a combined model of traumatic brain injury and hemorrhagic shock. Surgery. 2013;154(2):388–396 [DOI] [PubMed] [Google Scholar]
- 12.Halaweish I, Bambakidis T, He W, Linzel D, Chang Z, Srinivasan A, Dekker SE, Liu B, Li Y, Alam HB. Early resuscitation with fresh frozen plasma for traumatic brain injury combined with hemorrhagic shock improves neurologic recovery. J Am Coll Surg. 2015;220(5):809–819. [DOI] [PubMed] [Google Scholar]
- 13.Cornford EM, Diep CP, Pardridge WM . Blood-brain barrier transport of valproic acid. J Neurochem. 1985;44(5):1541–50. [DOI] [PubMed] [Google Scholar]
- 14.VandenBerg A, Broadway J, Lalich C, Kennedy R, Williams K. Valproate serum concentrations in patients with hypoalbuminemia and medical complications. Ment Health Clin. 2018;7(1):13–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Li Z, Gao W, Liu G, & Chen W Prediction of Serum-Free and Cerebrospinal Fluid Valproic Acid Levels in Patients With Hypoalbuminemia After Craniotomy. Ther Drug Monit. 2020;42(4):610–616. [DOI] [PubMed] [Google Scholar]
- 16.Drisaldi A, Weeda E, Neyens R, Orvin N, Bonilha L, Campbell Z, Bohm N. Accuracy of Valproic Acid Concentration Correction Based on Serum Albumin. Neurocrit Care. 2019;30(2):301–306. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


