Abstract
Porcine islet xenotransplantation is a viable strategy to treat diabetes. Its translation has been limited by the pre-clinical development of a clinically available immunosuppressive regimen. We tested two clinically relevant induction agents in a non-human primate (NHP) islet xenotransplantation model to compare depletional versus nondepletional induction immunosuppression. Neonatal porcine islets were isolated from GKO or hCD46/GKO transgenic piglets and transplanted via portal vein infusion in diabetic rhesus macaques. Induction therapy consisted of either basiliximab (n=6) or rhesus specific anti-thymocyte globulin (rhATG, n=6), combined with a maintenance regimen using B7 costimulation blockade, tacrolimus with delayed transition to sirolimus, and mycophenolate mofetil. Xenografts were monitored by blood glucose levels and porcine C-peptide measurements. Of the six receiving basiliximab induction, engraftment was achieved in 4 with median graft survival of 14 days. All six receiving rhATG induction engrafted with significantly longer xenograft survival at 40.5 days (p=0.03). These data suggest that depletional induction provides superior xenograft survival to nondepletional induction, in the setting of a costimulation blockade-based maintenance regimen.
Keywords: Islet Transplantation, Xenotransplantation, Rhesus anti-thymocyte globulin, belatacept
Introduction
There are over 1.5 million people currently living with type one diabetes mellitus (T1DM) in the United States.1 Definitive therapy requires endocrine function replacement in the form of allogeneic pancreas or islet transplantation. Compared to conventional insulin therapy, β-cell replacement restores glucose homeostasis and provides additional benefits among those experiencing hypoglycemia unawareness and severe glycemic lability.2,3 However, the lack of pancreata for transplantation remains a major barrier. A near limitless source of islet tissue could be attained through porcine islet xenotransplantation.
Continued advances in genetic engineering and novel immunosuppressive therapeutics continue to bring islet xenotransplantation closer to clinical reality.4 Multiple groups have independently achieved prolonged xenograft survival, up to 950 days, in pre-clinical non-human primate (NHP) models.5–11 However, these results have relied heavily on the use of investigational immunosuppressive agents that are not clinically available.12,13 CD154 blockade seems essential to ensuring successful transplant outcomes in most preclinical islet xenotransplantation studies,14 however, no CD154-specific agent is available for clinical use, particularly for development in xenotransplantation.15,16
To ultimately facilitate a clinical trial of porcine islet xenotransplantation, a relevant immunosuppressive strategy must be shown to be effective. To date, few studies have achieved prolonged graft survival using solely FDA-approved agents.17 One challenge is the lack of appropriate depletional agents that mimic the effect of those used clinically. While rabbit anti-thymocyte globulin (ATG) effectively depletes lymphocytes in humans, its effect is inconsistent and extremely high doses with inevitable toxicities are required to attain similar effects in preclinical NHP models.18 Similarly, the use of alemtuzumab in NHPs has been hindered by the expression of CD52 on red blood cells of most old world monkeys, again causing off target effects.19 Many groups have utilized rhesus-specific anti-CD4 and anti-CD8 monoclonal antibodies;20,21 however, those agents do not recapitulate the nondepleting biological effects associated with thymoglobulin. We have recently reported on the development of a novel rhesus-specific anti-thymocyte globulin (rhATG), a polyclonal IgG preparation purified from rabbits immunized against thymus recovered from rhesus macaques which serves to emulate the effect of ATG in humans. 22
Although non-depletional induction using basiliximab or daclizumab has been used in early clinical islet transplant studies,23 most contemporary protocols prefer depletional induction using thymoglobulin, and to a lesser extend alemtuzumab, in combination with a TNF-ɑ inhibitor.24–28 A pilot clinical xenotransplantation study conducted in China suggests depletional induction using OKT-3 may be associated with improved neonatal porcine islet (NPI) survival.29 In this study, we compared the efficacy of depletional induction using rhATG and non-depletional induction using basiliximab (anti-IL2R), both in conjunction with clinically available, costimulation blockade-based maintenance immunosuppression. We demonstrate that in the context of costimulation blockade, depletional induction improves engraftment and survival of NPI xenografts in NHPs.
Materials and Methods
Neonatal porcine islet procurement, isolation, and culture
Neonatal piglets of α1,3-galactosyl transferase knockout background, with or without knock-in of human CD46 (GKO and GKO/hCD46), were obtained from Revivicor Inc. (Blacksburg, VA, USA). The genotype of the piglets was confirmed via direct sequencing and expression of hCD46 on porcine peripheral blood mononuclear cells (PBMCs) was verified through flow cytometry at our facility. Piglets between 1 to 7 days of age were underwent terminal pancreatectomy under a protocol approved by the Duke Institutional Animal Care and Use Committee (IACUC). A previously described modified Korbutt technique30 was used to isolate porcine islet cells from the recovered neonatal pancreatic tissue. NPIs were maintained in culture using NPI culture media (Corning, Corning, NY, USA) supplemented with penicillin/streptomycin (Corning) for 6–8 days. On the day of transplantation, NPIs were quantified in islet equivalents (IEQs) using dithizone (Sigma-Aldrich, St. Louis, MO, USA). NPI viability was evaluated using fluorescein diacetate (Sigma-Aldrich, St. Louis, MO, USA) and propidium iodide (Sigma-Aldrich, St. Louis, MO, USA) live-dead counterstaining. All NPI preparations underwent endotoxin testing to exclude bacterial contamination prior to transplantation.
Diabetes induction and islet transplantation
Rhesus macaques were rendered diabetic chemically through IV administration of streptozocin (STZ, 1250 mg/m2 IV; Sigma, St Louis, MO, USA) 3–5 weeks prior to the planned transplantation and diabetes was confirmed by hyperglycemia with concomitant reduction and poor responsiveness of rhesus C-peptide levels (C-peptide ELISA assay; Abcam, Cambridge, MA, USA) to dextrose bolus. On day of transplantation, NPI preparations were washed and suspended in 20–30 ml of CMRL 1066 solution (Corning, Corning, NY, USA) supplemented with 200 units of heparin and etanercept at 3mg/kg (Enbrel; Amgen, Philadelphia, PA, USA). All rhesus macaque recipients underwent mini-laparotomy while under general anesthesia. At least 50,000 IEQs/kg of NPIs were infused through the portal vein via gravity through a 22-gauge catheter. As most piglets were born to parents of heterozygous human CD46 knocked in background, we received a variable number of GKO/heterologous hCD46 piglets each time. Thus, although we prefer to use islets of a single genotype, five out of twelve animals received an islet preparation of mixed genotypes to make up a minimal 50,000 IEQs/kg NPI dosage. All the above procedures were approved by the Duke IACUC.
Experimental groups and immunosuppressive regimens
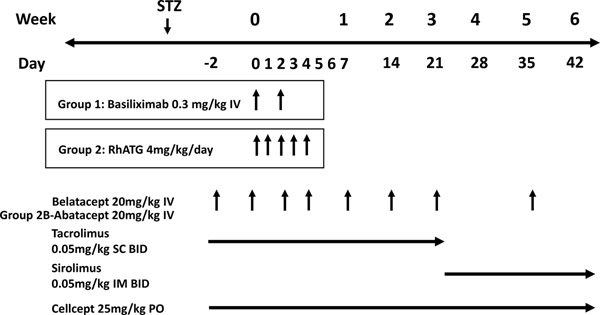
The immunosuppression and prophylaxis regimens applied in this study are depicted in Figure 1. Six animals (Group 1) received the IL-2R antagonist basiliximab (Simulect, Novartis, E. Hanover, NJ) as induction at 0.3mg/kg IV on day of transplant and postoperative day (POD) 2. The rest (Group 2A and 2B) received depletional induction with rhATG (NHP Reagent Resource, Worcester, MA, USA) at 4mg/kg IV daily for 5 doses from day of transplant to POD 4. Maintenance immunosuppression consisted of 20mg/kg belatacept (Nulojix, Bristol Meyers Squibb, Princeton, NJ, USA) administered IV on POD −2, 0, 4, 7, 14, 21 and biweekly afterwards, tacrolimus (Prograf, Astellas Pharma US, Inc. Northbrook, IL, USA) at 0.05mg/kg subcutaneously twice daily from days −2 to 21, sirolimus (LC laboratory, Woburn, MA, USA) at 0.05mg/kg intramuscularly twice daily from day 22 onwards, and mycophenolate mofetil (CellCept, Genentech, San Francisco, CA, USA) in oral suspension at 25mg/kg daily. Tacrolimus and sirolimus dosages were adjusted as needed to achieve a trough level of 8–12ng/ml. Three animals (Group 2B) received abatacept (Orencia, Bristol Meyers Squibb, Princeton, NJ, USA) in place of belatacept at 20mg/kg, due to a temporary manufacturing-related constraint. Rhesus CMV prophylaxis was initiated on POD −2 and consisted of oral valganciclovir at 60mg twice daily among those receiving basiliximab induction (Group 1). Animals receiving rhATG induction (Group 2A and 2B) received intramuscularly administered ganciclovir at 6mg/kg twice daily from POD 0 to 30 and valganciclovir thereafter.
Figure 1. Immunosuppressive regimens.
This outlines the demographics of recipients, study groups, and islet preparation characteristics. “Mixed” refers to a combination of NPIs from neonatal piglets with GKO and GKO/hCD46 background from mixed litters.
Recipient care and xenograft monitoring
Post-transplantation monitoring consisted of daily clinical assessment by the veterinary staff, as well as weekly laboratory studies including complete blood count and complete metabolic panel. Rhesus CMV was monitored using quantitative polymerase chain reaction from whole blood weekly and when clinically indicated. Intramuscular ganciclovir at 6mg/kg was given as treatment if recipient serum CMV exceeded 10,000 copies/ml.
Following streptozocin induction, animal morning fasting and afternoon postprandial blood glucose levels were monitored daily by either ear- or tail-stick. Based on blood glucose readings, animals received NPH insulin twice daily and long-acting lantus once in the evening per sliding scale to maintain a target fasting blood glucose (FBG) <200 mg/dL. Intravenous glucose tolerance tests (IVGTTs) with a bolus of dextrose (500 mg/kg) were performed pre-diabetes, pre-transplant, and then every two weeks during the post-transplantation period. IVGTT glucose levels were measured 5 minutes prior to dextrose bolus, immediately following dextrose bolus, and then at 10-, 30-, 60- and 90-min intervals. Porcine c-peptide was measured from sera obtained at each IVGTT timepoint and from serial samples obtained throughout the study period using the Mercodia Porcine C-peptide ELISA (Mercodia, Uppsala, Sweden).
Monitoring of peripheral blood mononuclear cell (PBMC) population
Flow cytometric analysis of PBMCs was performed pre-transplant, weekly thereafter and at terminal endpoint on all recipients. In addition, cells were isolated from lymph nodes and spleen tissue recovered at euthanasia and subjected to similar flow analysis. Cells were stained with the following fluorophore-conjugated antibodies: CD19-PE (Beckman Coulter, Indianapolis, IN, USA), CD28-PE-Cy7, CD10-PerCP-Cy5.5, CD95-eFlour450, CD27-PE-Cy7 (Biolegend, San Diego, CA, USA), CD25-PE (Miltinyi Biotech, Bergisch Gladbach, Germany), IgD-FITC (Southern Biotech, Birmingham, AL, USA), CD2-PE, CD3-APC-Cy7(-Pacific Blue, or PerCP), CD4-PerCP-Cy5.5, CD8-BV510, CD20-APC-Cy7, CD45-PE-Cy7, CD69-FITC, IgM-BV510, and Ki67-FITC (BD Bioscience, San Jose, CA, USA). Secondary analysis of data was performed using FlowJo software (Tree Star, San Carlos, CA, USA).
Experimental endpoints and histologic evaluation
Graft survival was defined as the last day porcine c-peptide levels were detected in animal serum samples (plasma samples were used instead whenever no available serum samples exited). Failure of engraftment was defined as serum porcine c-peptide below the lower range of detection at two weeks post-transplantation. Animals judged to be severely ill, as determined by veterinary staff assessment of acuity and irreversibility of illness, animal distress, or weight loss (loss of 25% of body weight from pre-transplant baseline) were euthanized in accordance with IACUC guidelines.
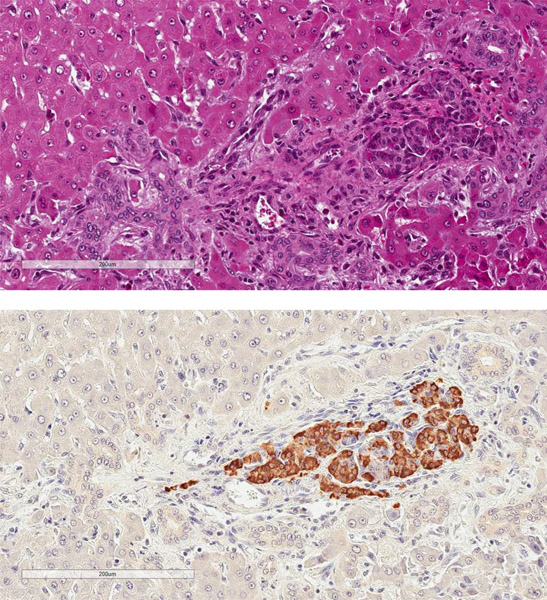
At the time of recipient endpoint, lymph nodes, spleen, and the liver were collected in 10% formalin for histologic evaluation. These samples were embedded in paraffin before subsequent sectioning and hematoxylin and eosin staining. Additionally, slides were stained for hCD46 and insulin to help identify viable NPIs at tissue recovery. Images were digitally scanned by using Aperio Scanscope AT Turbo (Leica Biosystems, Buffalo Grove, IL).
Statistical Analysis
Statistical analysis was performed using GraphPad Prism v9.1 (GraphPad, San Diego, CA, USA). Kaplan-Meier survival analysis was performed for experimental endpoint of graft rejection. Flow data was compared using two-way ANOVA of longitudinal cell frequencies and absolute counts.
Results
Depletional induction promotes engraftment and xenograft survival compared to nondepletional induction
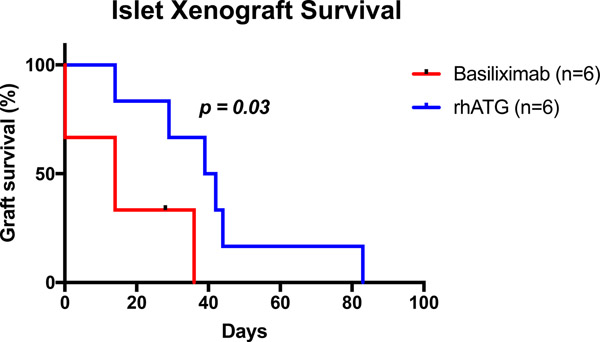
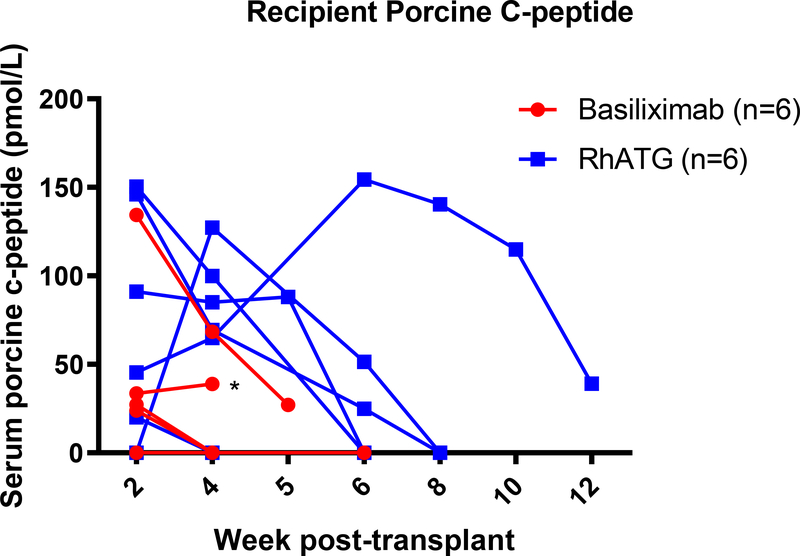
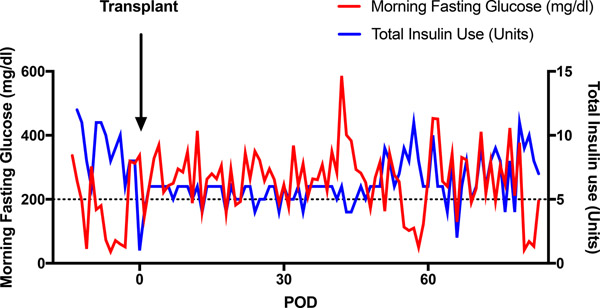
A total of twelve diabetic rhesus macaques, ranging from 3.2kg to 6.8kg, successfully underwent islet transplantation (Table 1). All animals received B7 blockade (in the form of belatacept or abatacept), tacrolimus with transition to sirolimus and mycophenolate mofetil (MMF). Group 1 (n=6) was given anti-IL2R induction and belatacept; group 2A (n=3) was given rhATG induction and belatacept while group 2B (n=3) was given rhATG induction and abatacept. Groups 2A and 2B were combined (Group 2) for analysis. Group 1 received an average NPI dosage of 58,636±5,837 (SD) IEQs/kg compared to 70,239±10,552 IEQs/kg among group 2 animals (p=0.36). In group 1, one animal received GKO NPIs, four received GKO/hCD46 NPIs, and one received a mixture of genotypes. Two animals in group 2 received GKO/hCD46 NPIs, while the rest received mixed GKO and GKO/hCD46 NPIs. Of the six receiving anti-IL2R induction, engraftment was achieved in 4 and the median graft survival was 14 days. Of the six receiving rhATG induction, all engrafted and the median graft survival was 40.5 days, significantly longer compared with the anti-IL2R induction group (p=0.03, Figure 2). Of note, one recipient in group 1 was euthanized early after established engraftment due to distress secondary to a biliary stricture. This was likely due to an intraoperative thermal injury or manipulation of the bile duct during dissection and cannulation of the portal vein, which are anatomically adjacent to one another. Although most recipients demonstrated successful engraftment, none achieved insulin independence following transplant. Even among those with detectable porcine c-peptide (Figure 3A), fasting blood glucose levels below 200mg/dl were not consistently achieved (Figure 3B).
Table 1.
Summary of study groups and graft outcomes.
| Recipient ID | Recipient weight (kg) | Immunosuppression regimens | Total NPIs transplanted (IEQs) | NPI dosage (IEQs/kg) | Genotypes of piglets | Graft survival |
|---|---|---|---|---|---|---|
|
| ||||||
| DF3F | 5.1 | 1 | 200,000 | 39,216 | GKO/CD46 | 0 |
| DF9Z | 3.2 | 1 | 253,000 | 79,063 | GKO | 0 |
| DFT0 | 3.9 | 1 | 210,000 | 53,846 | GKO/CD46 | 28 |
| DF17 | 4.2 | 1 | 300,000 | 71,429 | GKO/CD46 | 28 |
| H722 | 5.6 | 1 | 300,000 | 53,571 | GKO/CD46 | 14 |
| G06D | 3.2 | 1 | 175,000 | 54,688 | Mixed | 14 |
| H720 | 6.2 | 2A | 350,000 | 56,452 | Mixed | 14 |
| H878 | 6.8 | 2A | 400,000 | 58,824 | Mixed | 83 |
| H11R | 6.2 | 2A | 300,000 | 48,387 | Mixed | 44 |
| H59H | 4.5 | 2B | 518,000 | 115,111 | Mixed | 29 |
| H70N | 5 | 2B | 437,770 | 87,554 | GKO/CD46 | 39 |
| H69F | 4.7 | 2B | 259,000 | 55,106 | GKO/CD46 | 42 |
Figure 2. Islet Xenograft Survival.
Recipients receiving rhATG demonstrated a higher rate of primary engraftment (100% versus 66%) and longer graft survival based on the presence of porcine C-peptide during dextrose bolus stimulation (p=0.03).
Figure 3. Islet xenograft function.
(a) Weekly assessment of porcine C-peptide (without dextrose bolus stimulation) illustrates function specific to NPIs after transplantation. Asterisk indicates endpoint due to complication. (b). Representative morning fasting glucose and insulin requirement for one recipient throughout the study period. Despite presence of serum porcine c-peptide, insulin independence was not achieved.
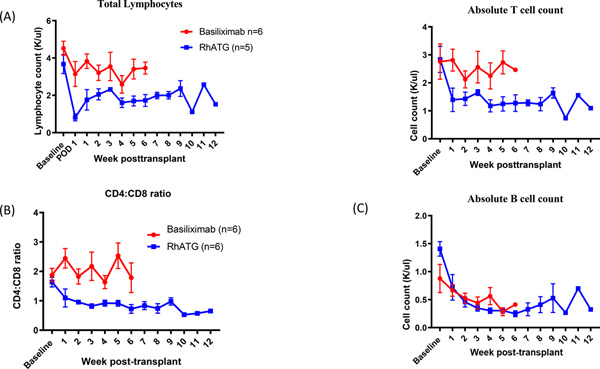
RhATG efficiently depletes lymphocytes with inversion of CD4:CD8 ratio
To account for the observed differences in graft survival, we compared the effects of induction agent on lymphocyte populations. We observed a 78.4 ± 4.1% (n=6) reduction of absolute lymphocyte count following the first dose of rhATG on POD1. Some level of lymphocyte reconstitution took place soon thereafter, but overall the depletional effect was sustained up to study endpoints (p<0.01, Figure 4). This degree of lymphocyte depletion was not demonstrated among animals receiving anti-IL2R induction. Suppression of CD4 T cells accounted for the observed differences in lymphocyte counts between the two treatment groups. In contrast, CD8 T cells rapidly repopulate following depletion and by 1-week post-transplantation, the CD8 T cell count was comparable between the two groups. As a result, CD4:CD8 ratio significantly decreased in rhATG induction group, compared to anti-IL2R group (p<0.01, Figure 4b). Absolute B cell count was reduced in both groups in a parallel fashion (p=ns, Figure 4c).
Figure 4. RhATG effectively depletes T lymphocytes.
(a) RhATG treated animals demonstrated reduction in total lymphocyte (p<0.01) and T cell absolute counts (p<0.01) when compared to basiliximab controls. B cell counts were similarly decreased in both study groups (p=ns). (b) RhATG treated recipients underwent inversion CD4:CD8 ratio following induction which remained throughout the study period (p<0.01).
Lymphocyte depletion with rhATG increases FoxP3+ Treg frequency
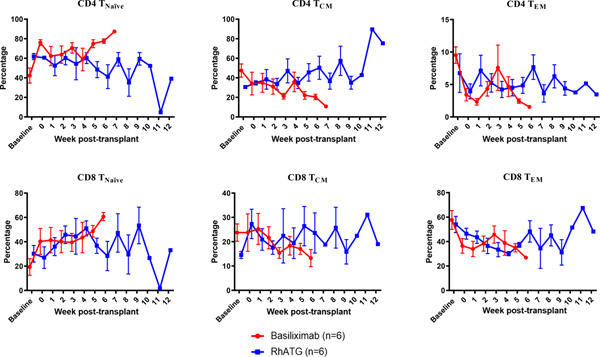
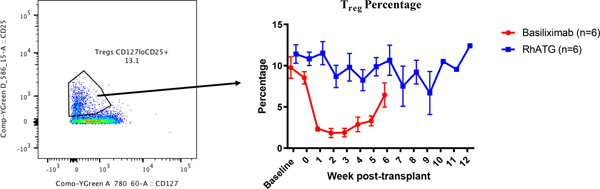
Among CD4 and CD8 T cells, we observed similar trends in both groups—a relative increase of naïve subsets and relative decrease of both central memory and effector memory cells, in both (Figure 5a). Regulatory T cells (Treg) were defined as CD4+CD127lowCD25+ T cells and we observed equal reduction of absolute Treg counts in both groups. While the relative frequency of Tregs in peripheral blood was maintained in animals receiving rhATG induction, a reduction of the Treg frequency was observed among animals receiving anti-IL2R (p<0.01, Figure 5b). B cell subsets were interrogated based on surface expression of CD27 and IgD. The percentage of naïve B cells tended to increase following transplantation; however, no differences were detected between the two groups (data not shown).
Figure 5. T cell subtype analysis.
(a) Similar T cell subtype frequencies between induction groups(p=ns in all comparisons). (b) Preservation of Treg frequency in rhATG treated recipients (p<0.01).
Adequate CMV suppression with antiviral prophylaxis with rhATG
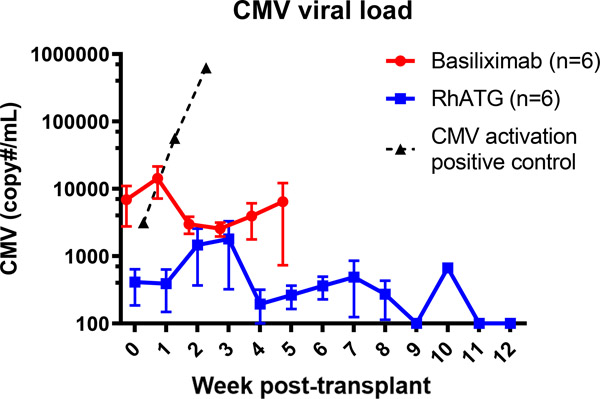
All animals received oral valganciclovir while on immunosuppression. In anticipation of viral reactivation associated with depletional induction, animals in group 2 received intramuscular ganciclovir for the first month in place of oral valganciclovir post-transplantation with transition to oral valganciclovir at POD 30. Throughout the study period, no recipient developed clinical manifestations of CMV infection. Three animals in group 1 developed >10,000 copies/ml transiently with reduction of viremia following a short course of intramuscular ganciclovir. None of the animals receiving rhATG developed >10,000 copies/ml during the study period (Figure 6).
Figure 6. Control of rhCMV.
Viral titers of rhCMV were adequately controlled with oral valganciclovir or IM ganciclovir in the post-op period. The dotted line is a positive control from a non-transplant animal which received depletional induction using anti-CD4 and anti-CD8 demonstrating rapid viral reactivation with lymphocyte depletion.
Cellular infiltration predominates islet xenograft rejection
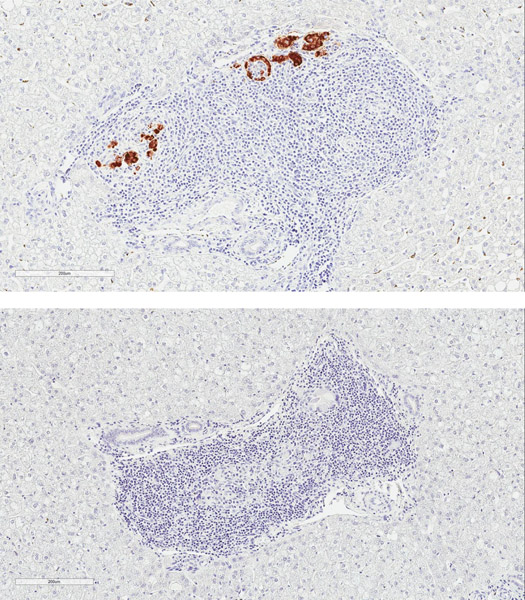
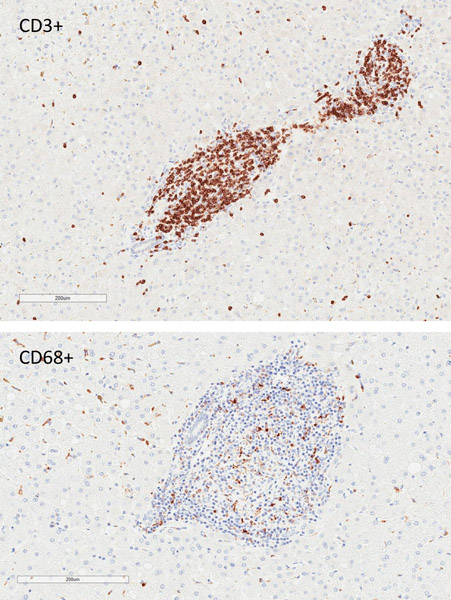
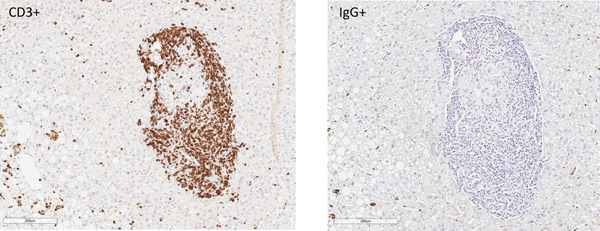
Rejection was confirmed on terminal histology, with appearance correlating with xenograft function at experimental endpoint (Figure 7). Two recipients in group 1 with primary engraftment failure had no identifiable intrahepatic islets on multiple sections of histology (data not shown). One animal (DF17) was euthanized for complications related to biliary stricture after demonstrating engraftment. Although serum porcine c-peptide was still positive at terminal endpoint in this animal, dense cellular infiltrate was found surrounding most intrahepatic NPIs on histology suggestive of an ongoing process (Figure 7a). The other recipients that had lost graft function had substantial cellular infiltration of intrahepatic islets with minimal staining of insulin (Figure 7b). This appeared to be a largely T cell response (Figure 7c and 7d).
Figure 7. Cell mediated rejection of xenoislets.
(a) H&E and insulin staining of islets in a recipient with detectable porcine C-peptide. (b) Representative images of islets at terminal endpoint with minimal presence of insulin. (c) Predominant cellular infiltrate at the time of graft rejection with CD3+ T cells and CD68+ macrophages. (d) Islet cluster with strong CD3 positivity and minimal staining of IgG.
Discussion
Here we report our experience using rhATG as a polyclonal depletion agent in a preclinical rhesus model of xenoislet transplantation. The observed depletional and immune-modulatory effects of rhATG recapitulated those of ATG in clinical use. RhATG was also well tolerated when used with appropriate viral prophylaxis. Our results demonstrate that depletional induction using rhATG promoted xenoislet engraftment and survival when compared to ILR2 targeted induction in the setting of costimulation blockade-based immunosuppression.
Several NHP xenotransplantation studies to date have used ATG as a surrogate for its use in the clinic, largely due to a lack of better options. Although effective, its dosing requirement and specificity in NHP models are intuitively unpredictable.31 We argue that rhATG, which is prepared in a parallel fashion to ATG, may serve as an accurate surrogate in the rhesus model.22 We show that rhATG reliably depletes T cells with the effect persisting throughout the study period. More importantly, we observed a sustained reversal of the peripheral CD4:CD8 ratio associated with a higher frequency of Tregs after induction, a phenomenon commonly observed in patients who have received ATG induction.32,33 These signatures have been described in clinical studies and associated with improved transplant outcomes.34,35 Our finding that depletional induction prolongs graft survival compared to anti-IL2R induction is also consistent with studies in clinical kidney and islet allotransplantation studies.26,36 The depletion arm may have additionally benefited from the inhibitory effects of leukocyte adhesion and trafficking molecules which are known effects of ATG.37 Indeed this may be of particular significance as inhibition of LFA-1 has been demonstrated in clinical islet and preclinical xenoislet models to be beneficial.38–40 Secondly, rhATG may possess some immune-modulatory effects as seen with ATG use clinically, especially when used together with costimulation blockade.41 These findings in this study support the overall immune-modulatory effect of rhATG in rhesus may parallel effects of ATG in humans.
Pre-clinical studies have repeatedly demonstrated porcine islet xenotransplantation as a feasible method to treat diabetes. However, many have relied on investigational agents and therefore preclude transition into clinical trial development.12 However, the present study was constructed using only clinically relevant drugs with current FDA approval. Additionally, we excluded steroids and minimized CNI-associated renal and beta cell toxicity with early conversion of tacrolimus to mTOR inhibition. Regardless of study arm and these modifications, we were unable to achieve insulin independence in all animals transplanted, with the longest xenograft survival being 83 days. It is unlikely this is related to inadequate dosing of NPIs, as our group had shown previously that a comparable NPI dose would have resulted in insulin independence for up to 249 days in the same model when investigational immunosuppressive drugs were utilized.9,42 In our study, fasting serum porcine c-peptide levels post-transplantation were uniformly below 0.5 ng/ml for all NPI recipients. Based on reports from other groups, a sustained serum fasting porcine c-peptide level above 0.5 ng/ml is generally required for insulin independence.6,8,42 The fact that recipients failed to maintain normoglycemia with short term engraftment suggests islet loss at two key stages of the host immune response—an early nonspecific loss of NPI mass related to an instant blood mediated inflammatory reaction (IBMIR) and graft rejection mediated by adaptive responses.
IBMIR is a well described phenomenon which is directly responsible for the acute loss of islet mass upon portal vein infusion.43–46 We included heparin and etanercept to our NPI preparations, however several studies have employed more sophisticated perioperative immunosuppressive regimens to curtail the IBMIR. Van der Windt et al. used a combination of aspirin, prostacyclin and continuous LMW-DS infusion to suppress endothelial cell activation and platelet aggregation.8 Herring et al. included pentoxifylline in their clinical islet transplant protocol.25 Many other clinical available agents such as nicotinamide, melagatran and gabexate mesilate have been shown to prevent IBMIR in vitro or in rodent models.47–49 Cobra venom factor (CVF) has historically been used in many preclinical studies to deplete complement factors, but it would be more clinically relevant to evaluate available complement inhibitors in future islet xenotransplantation studies. Besides TNF-⍺, a panel of other cytokines are released during the initial islet damage and therefore the addition of anakinra (anti-IL1R) and/or tocilizumab (anti-IL6R) may further avoid early islet loss.50,51 These interventions certainly warrant further study, however for the purposes of this study focused on the role of depletional induction we chose a simplified and commonly used protocol. Many of the NPIs in our study included hCD46 on a GKO background which has shown to have some potential benefits when compared to GKO alone in IBMIR.52 The glucose homeostasis functions of porcine islets can also be modified to improve their efficacy, which may further reduce the quantity needing to survive for successful functional engraftment.53,54 Indeed, this presents a clear opportunity for further targeted genetic modification which may ultimately be critical for early porcine xenoislet engraftment.55–57
Costimulation based immunosuppression has been tested in small clinical islet allotransplant studies with promising results.38,39 However, our study utilizing a clinically available agents were only able to support xenograft for a maximum of 83 days. Our results suggest B7 inhibition alone may be insufficient for xenograft maintenance without the inhibition of CD40-CD154 interactions.14 Fc silent anti-CD154 mAb and CD28 Dab are two agents that have shown promise in preclinical studies,58,59 with an anti-CD40mAb actively in clinical trial.60 Novel strategies with off-label use of approved immunosuppressive agents add more options for future clinical trial consideration.61 Cell-based therapies, in the form of regulatory T cells or mesenchymal stem cells, have shown promises in promoting a tolerogenic host environment, although they have largely failed to induce tolerance in islet xenotransplantation studies.6 One trial in China is presently testing the efficacy of recipient derived regulatory T cells in combination with a belatacept, tacrolimus and MMF based regimen in islet xenotransplantation (NCT03162237). Ultimately, the design of any porcine islet xenotransplant clinical trial will require approved immunosuppression or cell-based therapy with a well-documented favorable risk profile.
Our study has several important limitations. First, there was an inconsistent phenotype among the islets used: not all islets harbored the hCD46 knock-in and therefore the direct effect of this modification on islet survival is difficult to measure. Additionally, islet preparations were inconsistent in dosing across animals, with group 2 on average receiving more islets. This however did not appear to correlate with longer engraftment. The animals in the two groups purposefully received different CMV prophylaxis in anticipation of a differential effect of our immunosuppressive regimens. However, due to this we saw greater reactivation of CMV in the basiliximab arm than the rhATG arm. The expansion of anti-CMV CD8 T cells with heterologous specificity for xenograft islets cannot be excluded as a factor contributing to the shorter survival in this group. Due to a worldwide supply chain disruption, we were unable to utilize belatacept in all animals in Group 2 and instead adopted abatacept. Though we anticipate that the two drugs would have similar effects, our study groups are not adequately powered for such an assessment.
These data demonstrated the benefit of depletional induction in prolonging islet xenograft survival and endorses the use of rhATG as an excellent parallel to ATG in the NHP preclinical model. Islet xenograft loss was not prevented acutely due to IBMIR and long-term engraftment was poor despite suppression of B7 costimulation in combination with conventional agents. Though islet xenotransplantation has certainly cleared the conceptual barrier, progress is currently impeded the use of experimental immunosuppressive agents. The next step towards clinical trials will depend on the convergence of purposeful genetic modification and clinically available therapeutics proven by rigorous preclinical study.
Acknowledgments
This study was funded by National Institutes of Health (NIH) grant U01-AI090956 (A.D.K.). We would like to thank the Duke University Substrate Core for their assistance in serum rhesus CMV level monitoring. Anti–rhesus thymocyte globulin used in this study was produced by the NIH Nonhuman Primate Reagent Resource (National Institute of Allergy and Infectious Diseases contract HHSN 2722001300031C).
Abbreviations:
- T1DM
Type one diabetes mellitus
- NHPs
Non-human primates
- rhATG
Anti–rhesus anti-thymocyte globulin
- GKO
α1,3-galactosyltransferase knockout
- hCD46
human CD46
- STZ
Streptozocin
- NPIs
Neonatal porcine islets
- IACUC
Institutional Animal Care and Use Committee
- IEQs
Islet equivalents
- IVGTT
Intravenous glucose tolerance test
- ATG
Rabbit anti-thymocyte globulin
- POD
postoperative day
- PBMC
peripheral blood mononuclear cell
- MMF
mycophenolate mofetil
- Treg
T regulatory cells
- IBMIR
instant blood mediated inflammatory reaction
- DAMPs
damage associated molecular patterns
- CNIs
calcineurin inhibitors
Footnotes
Disclosure
The authors of this manuscript have no conflicts of interest to disclose as described by Xenotransplantation.
References
- 1.Centers for Disease Control and Prevention. National Diabetes Statistics Report, 2017. Atlanta, GA: Centers for Disease Control and Prevention, US Department of Health and Human Services;2017. [Google Scholar]
- 2.Hering BJ, Clarke WR, Bridges ND, et al. Phase 3 Trial of Transplantation of Human Islets in Type 1 Diabetes Complicated by Severe Hypoglycemia. Diabetes Care. 2016;39(7):1230–1240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lablanche S, Vantyghem MC, Kessler L, et al. Islet transplantation versus insulin therapy in patients with type 1 diabetes with severe hypoglycaemia or poorly controlled glycaemia after kidney transplantation (TRIMECO): a multicentre, randomised controlled trial. Lancet Diabetes Endocrinol. 2018;6(7):527–537. [DOI] [PubMed] [Google Scholar]
- 4.Cowan PJ, Tector AJ. The Resurgence of Xenotransplantation. Am J Transplant. 2017;17(10):2531–2536. [DOI] [PubMed] [Google Scholar]
- 5.Shin JS, Kim JM, Kim JS, et al. Long-term control of diabetes in immunosuppressed nonhuman primates (NHP) by the transplantation of adult porcine islets. Am J Transplant. 2015;15(11):2837–2850. [DOI] [PubMed] [Google Scholar]
- 6.Shin JS, Min BH, Kim JM, et al. Failure of transplantation tolerance induction by autologous regulatory T cells in the pig-to-non-human primate islet xenotransplantation model. Xenotransplantation. 2016;23(4):300–309. [DOI] [PubMed] [Google Scholar]
- 7.Bottino R, Wijkstrom M, van der Windt DJ, et al. Pig-to-monkey islet xenotransplantation using multi-transgenic pigs. Am J Transplant. 2014;14(10):2275–2287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.van der Windt DJ, Bottino R, Casu A, et al. Long-term controlled normoglycemia in diabetic non-human primates after transplantation with hCD46 transgenic porcine islets. Am J Transplant. 2009;9(12):2716–2726. [DOI] [PubMed] [Google Scholar]
- 9.Thompson P, Badell IR, Lowe M, et al. Islet xenotransplantation using gal-deficient neonatal donors improves engraftment and function. Am J Transplant. 2011;11(12):2593–2602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Cardona K, Korbutt GS, Milas Z, et al. Long-term survival of neonatal porcine islets in nonhuman primates by targeting costimulation pathways. Nat Med. 2006;12(3):304–306. [DOI] [PubMed] [Google Scholar]
- 11.Hering BJ, Wijkstrom M, Graham ML, et al. Prolonged diabetes reversal after intraportal xenotransplantation of wild-type porcine islets in immunosuppressed nonhuman primates. Nature medicine. 2006;12(3):301–303. [DOI] [PubMed] [Google Scholar]
- 12.Samy KP, Martin BM, Turgeon NA, Kirk AD. Islet cell xenotransplantation: a serious look toward the clinic. Xenotransplantation. 2014;21(3):221–229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Liu Z, Hu W, He T, et al. Pig-to-Primate Islet Xenotransplantation: Past, Present, and Future. Cell Transplant. 2017;26(6):925–947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Samy KP, Butler JR, Li P, Cooper DKC, Ekser B. The Role of Costimulation Blockade in Solid Organ and Islet Xenotransplantation. J Immunol Res. 2017;2017:8415205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kawai T, Andrews D, Colvin RB, Sachs DH, Cosimi AB. Thromboembolic complications after treatment with monoclonal antibody against CD40 ligand. Nat Med. 2000;6(2):114. [DOI] [PubMed] [Google Scholar]
- 16.Boumpas DT, Furie R, Manzi S, et al. A short course of BG9588 (anti-CD40 ligand antibody) improves serologic activity and decreases hematuria in patients with proliferative lupus glomerulonephritis. Arthritis Rheum. 2003;48(3):719–727. [DOI] [PubMed] [Google Scholar]
- 17.Chen Y, Stewart JM, Gunthart M, et al. Xenoantibody response to porcine islet cell transplantation using GTKO, CD55, CD59, and fucosyltransferase multiple transgenic donors. Xenotransplantation. 2014;21(3):244–253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Preville X, Flacher M, LeMauff B, et al. Mechanisms involved in antithymocyte globulin immunosuppressive activity in a nonhuman primate model. Transplantation. 2001;71(3):460–468. [DOI] [PubMed] [Google Scholar]
- 19.van der Windt DJ, Smetanka C, Macedo C, et al. Investigation of lymphocyte depletion and repopulation using alemtuzumab (Campath-1H) in cynomolgus monkeys. American journal of transplantation : official journal of the American Society of Transplantation and the American Society of Transplant Surgeons. 2010;10(4):773–783. [DOI] [PubMed] [Google Scholar]
- 20.Adams AB, Kim SC, Martens GR, et al. Xenoantigen Deletion and Chemical Immunosuppression Can Prolong Renal Xenograft Survival. Ann Surg. 2018;268(4):564–573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Burghuber CK, Kwun J, Page EJ, et al. Antibody-Mediated Rejection in Sensitized Nonhuman Primates: Modeling Human Biology. American journal of transplantation : official journal of the American Society of Transplantation and the American Society of Transplant Surgeons. 2016;16(6):1726–1738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Shaw BI, Schmitz R, Flores WJ, et al. A comparative study of human- and rhesus-specific anti-thymocyte globulins in Rhesus macaques. Clinical transplantation. 2021:e14369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Shapiro AM, Lakey JR, Ryan EA, et al. Islet transplantation in seven patients with type 1 diabetes mellitus using a glucocorticoid-free immunosuppressive regimen. N Engl J Med. 2000;343(4):230–238. [DOI] [PubMed] [Google Scholar]
- 24.Rickels MR, Robertson RP. Pancreatic Islet Transplantation in Humans: Recent Progress and Future Directions. Endocr Rev. 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hering BJ, Kandaswamy R, Ansite JD, et al. Single-donor, marginal-dose islet transplantation in patients with type 1 diabetes. JAMA. 2005;293(7):830–835. [DOI] [PubMed] [Google Scholar]
- 26.Bellin MD, Barton FB, Heitman A, et al. Potent induction immunotherapy promotes long-term insulin independence after islet transplantation in type 1 diabetes. American journal of transplantation : official journal of the American Society of Transplantation and the American Society of Transplant Surgeons. 2012;12(6):1576–1583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Barton FB, Rickels MR, Alejandro R, et al. Improvement in outcomes of clinical islet transplantation: 1999–2010. Diabetes Care. 2012;35(7):1436–1445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Brooks AM, Walker N, Aldibbiat A, et al. Attainment of metabolic goals in the integrated UK islet transplant program with locally isolated and transported preparations. Am J Transplant. 2013;13(12):3236–3243. [DOI] [PubMed] [Google Scholar]
- 29.Wang W, Mo Z, Ye B, Hu P, Liu S, Yi S. A clinical trial of xenotransplantation of neonatal pig islets for diabetic patients. Zhong nan da xue xue bao Yi xue ban = Journal of Central South University Medical sciences. 2011;36(12):1134–1140. [DOI] [PubMed] [Google Scholar]
- 30.Korbutt GS, Elliott JF, Ao Z, Smith DK, Warnock GL, Rajotte RV. Large scale isolation, growth, and function of porcine neonatal islet cells. J Clin Invest. 1996;97(9):2119–2129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Anderson DJ, Kirk AD. Primate models in organ transplantation. Cold Spring Harb Perspect Med. 2013;3(9):a015503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Rosenblum JM, Kirk AD. Recollective homeostasis and the immune consequences of peritransplant depletional induction therapy. Immunol Rev. 2014;258(1):167–182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Grafals M, Smith B, Murakami N, et al. Immunophenotyping and efficacy of low dose ATG in non-sensitized kidney recipients undergoing early steroid withdrawal: a randomized pilot study. PLoS One. 2014;9(8):e104408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Bas J, Mestre M, Grinyo JM, et al. Peripheral blood lymphoid subsets and long-term clinical course of kidney recipients: a longitudinal study. Cytometry. 1998;34(2):103–112. [DOI] [PubMed] [Google Scholar]
- 35.Tang Q, Leung J, Melli K, et al. Altered balance between effector T cells and FOXP3+ HELIOS+ regulatory T cells after thymoglobulin induction in kidney transplant recipients. Transpl Int. 2012;25(12):1257–1267. [DOI] [PubMed] [Google Scholar]
- 36.Brennan DC, Daller JA, Lake KD, Cibrik D, Del Castillo D, Thymoglobulin Induction Study G. Rabbit antithymocyte globulin versus basiliximab in renal transplantation. N Engl J Med. 2006;355(19):1967–1977. [DOI] [PubMed] [Google Scholar]
- 37.Mohty M Mechanisms of action of antithymocyte globulin: T-cell depletion and beyond. Leukemia. 2007;21(7):1387–1394. [DOI] [PubMed] [Google Scholar]
- 38.Turgeon NA, Avila JG, Cano JA, et al. Experience with a novel efalizumab-based immunosuppressive regimen to facilitate single donor islet cell transplantation. American journal of transplantation : official journal of the American Society of Transplantation and the American Society of Transplant Surgeons. 2010;10(9):2082–2091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Posselt AM, Szot GL, Frassetto LA, et al. Islet transplantation in type 1 diabetic patients using calcineurin inhibitor-free immunosuppressive protocols based on T-cell adhesion or costimulation blockade. Transplantation. 2010;90(12):1595–1601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Thompson P, Badell IR, Lowe M, et al. Alternative immunomodulatory strategies for xenotransplantation: CD40/154 pathway-sparing regimens promote xenograft survival. American journal of transplantation : official journal of the American Society of Transplantation and the American Society of Transplant Surgeons. 2012;12(7):1765–1775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Furukawa A, Wisel SA, Tang Q. Impact of Immune-Modulatory Drugs on Regulatory T Cell. Transplantation. 2016;100(11):2288–2300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Thompson P, Cardona K, Russell M, et al. CD40-specific costimulation blockade enhances neonatal porcine islet survival in nonhuman primates. Am J Transplant. 2011;11(5):947–957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.van der Windt DJ, Marigliano M, He J, et al. Early islet damage after direct exposure of pig islets to blood: has humoral immunity been underestimated? Cell transplantation. 2012;21(8):1791–1802. [DOI] [PubMed] [Google Scholar]
- 44.Nilsson B, Ekdahl KN, Korsgren O. Control of instant blood-mediated inflammatory reaction to improve islets of Langerhans engraftment. Current opinion in organ transplantation. 2011;16(6):620–626. [DOI] [PubMed] [Google Scholar]
- 45.Naziruddin B, Iwahashi S, Kanak MA, Takita M, Itoh T, Levy MF. Evidence for instant blood-mediated inflammatory reaction in clinical autologous islet transplantation. American journal of transplantation : official journal of the American Society of Transplantation and the American Society of Transplant Surgeons. 2014;14(2):428–437. [DOI] [PubMed] [Google Scholar]
- 46.Goto M, Tjernberg J, Dufrane D, et al. Dissecting the instant blood-mediated inflammatory reaction in islet xenotransplantation. Xenotransplantation. 2008;15(4):225–234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Ozmen L, Ekdahl KN, Elgue G, Larsson R, Korsgren O, Nilsson B. Inhibition of thrombin abrogates the instant blood-mediated inflammatory reaction triggered by isolated human islets: possible application of the thrombin inhibitor melagatran in clinical islet transplantation. Diabetes. 2002;51(6):1779–1784. [DOI] [PubMed] [Google Scholar]
- 48.Jung DY, Park JB, Joo SY, et al. Effect of nicotinamide on early graft failure following intraportal islet transplantation. Exp Mol Med. 2009;41(11):782–792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Tokodai K, Goto M, Inagaki A, et al. C5a-inhibitory peptide combined with gabexate mesilate prevents the instant blood-mediated inflammatory reaction in a rat model of islet transplantation. Transplantation proceedings. 2010;42(6):2102–2103. [DOI] [PubMed] [Google Scholar]
- 50.McCall M, Pawlick R, Kin T, Shapiro AM. Anakinra potentiates the protective effects of etanercept in transplantation of marginal mass human islets in immunodeficient mice. American journal of transplantation : official journal of the American Society of Transplantation and the American Society of Transplant Surgeons. 2012;12(2):322–329. [DOI] [PubMed] [Google Scholar]
- 51.Sahraoui A, Kloster-Jensen K, Ueland T, Korsgren O, Foss A, Scholz H. Anakinra and tocilizumab enhance survival and function of human islets during culture: implications for clinical islet transplantation. Cell transplantation. 2014;23(10):1199–1211. [DOI] [PubMed] [Google Scholar]
- 52.Samy KP, Gao Q, Davis RP, et al. The role of human CD46 in early xenoislet engraftment in a dual transplant model. Xenotransplantation. 2019;26(6):e12540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Mourad NI, Perota A, Xhema D, Galli C, Gianello P. Transgenic Expression of Glucagon-Like Peptide-1 (GLP-1) and Activated Muscarinic Receptor (M3R) Significantly Improves Pig Islet Secretory Function. Cell transplantation. 2017;26(5):901–911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Mourad NI, Gianello P. Gene Editing, Gene Therapy, and Cell Xenotransplantation: Cell Transplantation Across Species. Current transplantation reports. 2017;4(3):193–200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Ryczek N, Hryhorowicz M, Lipiński D, Zeyland J, Słomski R. Evaluation of the CRISPR/Cas9 Genetic Constructs in Efficient Disruption of Porcine Genes for Xenotransplantation Purposes Along with an Assessment of the Off-Target Mutation Formation. Genes (Basel). 2020;11(6). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Kemter E, Denner J, Wolf E. Will Genetic Engineering Carry Xenotransplantation of Pig Islets to the Clinic? Current diabetes reports. 2018;18(11):103. [DOI] [PubMed] [Google Scholar]
- 57.Hryhorowicz M, Lipiński D, Hryhorowicz S, Nowak-Terpiłowska A, Ryczek N, Zeyland J. Application of Genetically Engineered Pigs in Biomedical Research. Genes (Basel). 2020;11(6). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Kim S, Higginbotham L, Mathews DV, et al. CD28 directed immunotherapy is more potent than belatacept in a pig-to-nonhuman primate renal xenotransplant model. Xenotransplantation. 2017;24(5). [Google Scholar]
- 59.Kim SC, Wakwe W, Higginbotham LB, et al. Fc-Silent Anti-CD154 Domain Antibody Effectively Prevents Nonhuman Primate Renal Allograft Rejection. Am J Transplant. 2017;17(5):1182–1192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Nashan B, Tedesco H, van den Hoogen M, et al. CFZ533, a New Anti-CD40 mAB Demonstrates Comparable Efficacy and Better Renal Function versus Tacrolimus in De-Novo CNI-Free Kidney Transplantation. American Journal of Transplantation. 2018;18:400–400. [Google Scholar]
- 61.Kim JM, Hong SH, Chung H, et al. Long-term porcine islet graft survival in diabetic non-human primates treated with clinically available immunosuppressants. Xenotransplantation. 2021;28(2):e12659. [DOI] [PubMed] [Google Scholar]