Abstract
Objective:
To study the association between nicotine or cannabis metabolite presence in maternal urine and child neurodevelopmental outcomes.
Methods:
We conducted a secondary analysis of two parallel multi-center randomized controlled trials of treatment for hypothyroxinemia or subclinical hypothyroidism among pregnant individuals enrolled at 8–20 weeks gestation. All maternal-infant dyads with a maternal urine sample at enrollment and child neurodevelopmental testing were included (n= 1197). Exposure was urine samples positive for nicotine (cotinine) or cannabis (11-nor-9-carboxy-delta-9-tetrahydrocannabinol, THC-COOH) or both metabolites. Primary outcome was child intelligence quotient (IQ) at 60 months. Secondary outcomes included cognitive, motor and language, attention, behavioral and social competency, and differential skills assessments at 12, 24, 36 and 48 months. Quantile regression analysis was performed with confounder adjustment.
Results:
Of 1197 pregnant individuals, 99 (8.3%) had positive cotinine and 47 (3.9%) had positive THC-COOH samples; 33 (2.8%) were positive for both. Groups differed in self-reported race and ethnicity, education, marital status, insurance and thyroid status. Median IQ was similar between cotinine-exposed and unexposed (90 versus 95, adjusted difference in medians (ADM) −2.47 (95% confidence interval (CI) −6.22 to 1.29)) and THC-COOH-exposed and unexposed (89 versus 95, ADM −1.35 (95% CI −7.76 to 5.05)) children. In secondary outcome analysis, children with THC-COOH exposure compared with those unexposed had higher attention scores at 48 months of age (57 versus 49, ADM 6.0 (95% CI 1.11 to 10.89)).
Conclusions:
Neither prenatal nicotine nor cannabis exposure was associated with a difference in IQ. Cannabis exposure was associated with worse attention scores in early childhood. Longitudinal studies assessing associations between child neurodevelopmental outcomes and prenatal nicotine and cannabis exposure with a focus on timing, and quantity of exposure are needed.
Clinical Trial Registration:
Précis
Neither nicotine nor cannabis exposure in the early prenatal period is associated with difference in child intelligence quotient at 5 years of age.
Introduction
Nicotine product use steadily decreased among US adults over the past two decades,1–3 while tetrahydrocannabinol (THC)-containing cannabis use increased,4, 5 including among pregnant individuals.6–8 Despite a decrease in cigarette smoking prevalence from 14.9% in 2005 to 10.7% in 2014 among pregnant individuals,6 7–25% of pregnant individuals report use of nicotine products. 9–11 From 2001 to 2013, self-reported cannabis use increased among pregnant individuals from 2.4% in 2002 to 3.9% in 2014 nationwide.12 Similarly from 2009–2016, prenatal cannabis use increased from 4.2% to 7.1% in a single health system that utilized universal urine biochemical testing during prenatal care.7
While prenatal use of nicotine and cannabis products is strongly discouraged due to concerns for maternal and neonatal risks,13–16 the effect of prenatal nicotine17–19 or cannabis20–24 exposure on child neurodevelopmental outcomes remains unclear. Four longitudinal human studies demonstrated an association between prenatal cannabis exposure and long-term adverse child neurodevelopment.25 A major limitation in the methodology for many studies related to prenatal nicotine and cannabis exposure and child neurodevelopmental outcomes is reliance on self-reported use which may underestimate the true association.26, 27 To address these knowledge gaps, we examined the association between the presence of nicotine or cannabis metabolites in maternal urine during early pregnancy and child neurodevelopmental outcomes at 1 to 5 years of age. We hypothesized that children with exposure to either nicotine or cannabis would have worse neurodevelopmental outcomes compared to unexposed children.
Methods
This is a secondary analysis of the Eunice Kennedy Shriver National Institute of Child Health and Human Development Maternal-Fetal Medicine Units Network two parallel randomized controlled trials of treatment for hypothyroxinemia or subclinical hypothyroidism among pregnant individuals enrolled at 8–20 weeks gestation. Study enrollment occurred from 2006–2009 and maternal-infant dyad follow-up continued until 2015. The Institutional Review Board (IRB) at each of 15 centers approved the parent trials.28 The local IRB deemed this study exempt as it is a secondary analysis of deidentified data. This study follows STROBE guidelines for reporting of observational studies.
The details of the parent trials were described previously. Briefly, participants with a singleton gestation at 8–20 weeks gestation who were diagnosed with either subclinical hypothyroidism (TSH ≥ 4.0mU/L and a normal free thyroxine (T4) level 0.86 to 1.90 ng/dL) (N=677) or hypothyroxinemia (free T4 <0.86ng/dl and normal TSH 0.08 to 3.99 Mu/L) (N=526) were randomized either to levothyroxine or placebo from 2006–2009 to examine the effect of treatment with levothyroxine on child neurodevelopmental outcomes. Children underwent annual developmental and behavioral testing for five years and follow-up concluded in 2014 with a 96% longitudinal follow-up rate. In both trials, there were no significant differences by treatment group for maternal or pregnancy outcomes, or child neurodevelopmental outcomes at 12, 24, 36, 48 or 60 months of age. For the parent trials, pregnant individuals with known “illicit drug or alcohol abuse during current pregnancy” were excluded. For this secondary analysis, we included all participants with maternal urine samples at study enrollment and available child neurodevelopmental testing results.
Urine samples were obtained at the time of randomization as part of the original trial protocol. None of the samples underwent freeze/thaw cycles prior to this analysis. The most stable and prominent metabolites for nicotine (cotinine) and cannabis (11-nor-9-carboxy-delta-9-tetrahydrocannabinol, THC-COOH) were selected as the primary biomarkers of exposure. Urine was refrigerated, shipped to the central laboratory, and frozen at −80 degrees Celsius until processing.
Urine samples were assessed qualitatively using immunoassay and samples with positive results were reflexed to confirmation by liquid chromatography tandem mass spectrometry using clinically validated tests at ARUP Laboratories (Salt Lake City, Utah). The limit of detection for qualitative urine cotinine screening was 100 ng/mL and confirmatory limit of detection was 5 ng/mL. The limit of detection for screening and confirmation tests differ because immunoassays tests are generally sensitive but often not precise while confirmatory tests are highly specific with low false positive and false negative rates. For participants with self-reported tobacco use, the screening test was presumed to be positive, and urine samples were assessed using confirmatory testing only. If participants reported tobacco use but confirmatory testing was negative, they were included in the non-exposed group. Cotinine detection time is approximately 7 days with active nicotine use. Qualitative urine THC-COOH screening limit of detection was 20 ng/mL and a confirmatory limit of detection was 15 ng/mL. THC-COOH detection time is approximately 3 days for a single use, 5–7 days for moderate use (4 times per week), 10 days for heavy use (daily use) and 30 days for chronic heavy (daily use for multiple months).29, 30 Quantitative values were reported for liquid chromatography tandem mass spectrometry confirmatory testing for both nicotine and THC-COOH in ng/mL.
The primary outcome was full-scale IQ assessed with the Wechsler Preschool and Primary Scale of Intelligence III (WPPSI-III) at 5 years of age. Results are expressed as age standardized scores, with an expected population mean of 100 and a standard deviation of 15. Secondary outcomes in infants and children included the same neurodevelopmental outcomes as the parent randomized trials: (1) cognitive, motor, and language scores on the Bayley Scales of Infant Development, Third Edition (Bayley-III), at 12 months and 24 months of corrected age; (2) DAS overall scores at 36 months of age; (3) specific scores on the DAS (subtests regarding recall of digits forward and recognition of pictures) (4) Conners’ Rating Scales–Revised at 48 months of age for assessment of attention; and (5) scores on the Child Behavior Checklist at 36 months and 60 months of age for assessment of behavioral and social competency. Notably, the Conners Rating Scales is validated for use at 48 months of age.31, 32
We compared individual-level covariates associated with THC-containing cannabis and nicotine product use and child neurodevelopmental outcomes including maternal age, body mass index, gestational age at delivery and social determinants of health including maternal education, marital status, insurance status, self-reported race and ethnicity.27, 33, 34 We included race and ethnicity as a co-variate as pediatric neurodevelopmental tests are subject to racial and cultural bias35 and access to early childhood education is not universal36, and therefore may influence our outcomes of interest. We categorized race and ethnicity as White, Black, Hispanic and Other, which included Asian, American Indian and a participant selected option of “Other.” Because of small sample size, we combined these groups to protect participant confidentiality. We also assessed differences between groups for study-related baseline characteristics including gestational age at urine sample collection, thyroid status (subclinical hypothyroidism or subclinical hypothyroxinemia) and randomized trial treatment group. Maternal baseline, delivery and study characteristics were compared between exposed and unexposed groups (cotinine positive compared to negative; THC-COOH positive compared to negative) using the chi-square test, Fisher’s exact test and Wilcoxon rank sum test as appropriate. Due to insufficient numbers of women with dual exposure (cotinine and THC-COOH positive), a separate comparison of dual exposed could not be performed, and this group was included as exposed in both cotinine and THC-COOH models.
We estimated that with our fixed sample size there would be 80% power to show a difference of at least 6 IQ points based on a two-sided Mann-Whitney-Wilcoxon test, assuming an alpha level of 0.05, and that 60 participants would be tobacco users (5% of available cohort) and 1096 tobacco non-users. For marijuana use, we estimated that there would be 60 marijuana users (5% of available cohort) and 1096 non-marijuana users.
Quantile regression models were used for both the primary and secondary outcomes defined on a continuous scale with adjustment for potential confounders. Results are reported as adjusted median scores. Initial regression models were adjusted for thyroid status (hypothyroxinemia or the subclinical hypothyroidism) and treatment group in the parent trial and demographic variables that included education, race and ethnicity, insurance type, marital status and child age at exam. Final parsimonious models were adjusted for differences in child age at exam, insurance type, race and ethnicity and maternal education. We chose to include race and ethnicity and maternal education as important social determinants of health that are associated with child neurodevelopmental exam performance.37, 38 For outcomes that were associated with either nicotine or THC-COOH exposure, we performed exploratory analyses to evaluate the correlation between quantitative values of the substance of interest and continuous scores for the neurodevelopmental outcomes of interest. Statistical significance was defined as p <0.05. No corrections were made for multiple comparisons as this was an unplanned secondary (hypothesis-generating) analysis using all available neurodevelopmental outcomes in the parent trials. All statistical analysis was completed using SAS, version 9.4 (Cary, NC: SAS Institute Inc.).
Results
Of 1203 maternal-child dyads included in the parent trials, 1197 (99.5% of the overall cohort) met inclusion criteria for this study (Figure 1). Of these, 99 (8.3%) were positive for cotinine and 47 (3.9%) were positive for THC-COOH; 33 (2.8%) were positive for both (Figure 1). Of the 99 participants who were cotinine positive, 82 self-reported tobacco use and were positive for cotinine (median 626, range 5–3248 ng/mL). In addition, 17 individuals who did not self-report tobacco use were positive for cotinine (median 283, range 37–1949 ng/mL). There were 14 individuals who self-reported tobacco use in pregnancy but had negative confirmatory urine testing for cotinine; they were included in the cotinine negative group. Overall positive THC-COOH results ranged from16–501 ng/mL. One individual was THC-COOH screen positive with insufficient urine for confirmatory testing and was included in the THC-COOH negative group. Five-year follow-up outcome data was assessed for 92% of the offspring.
Figure 1:
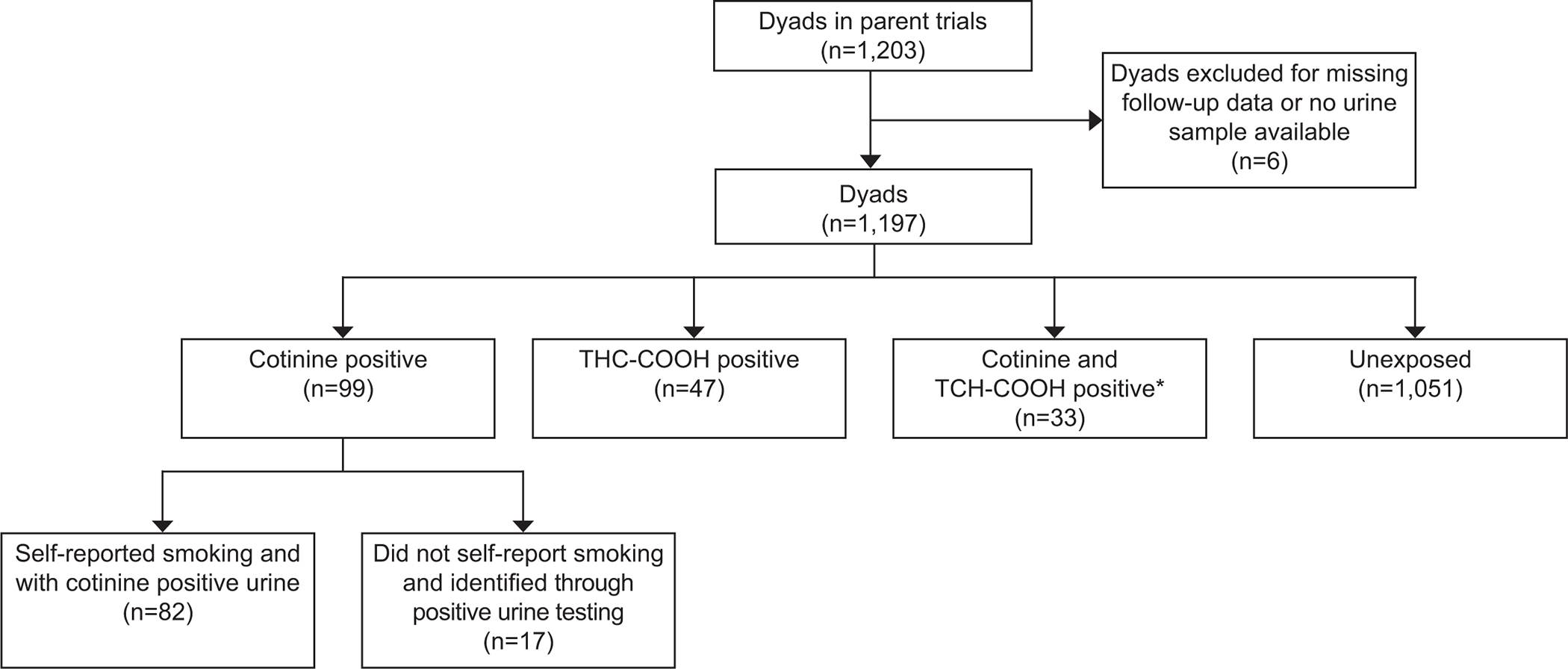
Population cohort. *Not mutually exclusive with THC and cotinine positive groups. THC-COOH, 11-nor-9-carboxy-delta-9-tetrahydrocannabinol.
When comparing cotinine positive and negative groups, there were significant differences in completed education level, marital status, insurance, race and ethnicity and baseline thyroid status (Table 1). In the analysis comparing THC-COOH positive and negative groups, the baseline characteristic variables that differed between groups included the ones in the cotinine analysis, with additional difference in maternal age. There were no differences in median gestational age at urine sample collection or gestational age at delivery for either analysis.
Table 1.
Maternal and study characteristics of exposure by cotinine and THC-COOH
| Cotinine | THC-COOH | |||||
|---|---|---|---|---|---|---|
| Positive | Negative | P-value | Positive | Negative | P-value | |
| N=99 | N=1098 | N=47 | N=1150 | |||
| Maternal age (years) | 26.6 ±5.9 | 27.8 ±5.7 | 0.05 | 26.1 ±6.1 | 27.8 ±5.7 | 0.04 |
| Maternal BMI (kg/m 2 ) | 30.0 ±8.0 | 29.0 ±6.5 | 0.47 | 29.3 ±8.1 | 29.0 ±6.6 | 0.65 |
| Education | <0.001 | 0.003 | ||||
| Less than high school |
33 (33) | 498 (45) | 20 (43) | 511 (44) | ||
| High school | 61 (62) | 354 (32) | 25 (53) | 390 (34) | ||
| College | 5 (5) | 246 (22) | 2 (4) | 249 (22) | ||
| Married/partner | 41 (41) | 838 (76) | <0.001 | 21 (45) | 858 (75) | <0.001 |
| Private insurance | 13 (13) | 325 (30) | <0.001 | 3 (6) | 335 (29) | <0.001 |
| Race or ethnicity | <0.001 | <0.001 | ||||
| Black | 41 (41) | 136 (12) | 21 (45) | 156 (14) | ||
| Hispanic | 11 (11) | 621 (57) | 5 (11) | 627 (55) | ||
| Other* | 2 (2) | 23 (2) | 2 (4) | 23 (2) | ||
| White | 45 (46) | 318 (29) | 19 (40) | 344 (30) | ||
| Gestational age at randomization & urine sample (weeks) | 17.4 ± 3.0 | 17.1 ±3.0 | 0.21 | 17.5 ±2.9 | 17.1 ±3.0 | 0.34 |
| Gestational age at delivery (weeks) | 38.1 ±4.7 | 39.0 ±2.6 | 0.42 | 38.5 ±4.4 | 39.0 ±2.7 | 0.30 |
| Levothyroxine Treatment group | 47 (48) | 554 (51) | 0.57 | 25 (53) | 576 (50) | 0.68 |
| Thyroid status | <0.001 | <0.001 | ||||
| Subclinical hypothyroxinemia | 27 (27) | 647 (59) | 9 (19) | 665 (58) | ||
| Subclinical hypothyroidism | 72 (73) | 451 (41) | 38 (81) | 485 (42) | ||
Includes Asian, American Indian and participant-selected “other” category for race.
Tables 2 and 3 include the main findings. In unadjusted analyses for cotinine, there were differences in IQ at 60 months, the Bayley Cognitive and Motor scores at 12 months, Cognitive and Behavioral Checklist scores at 36 months and DAS Digits Forward and Picture Recognition at 48 months. However, after adjustment for confounders, there were no differences by cotinine exposure group in the adjusted medians for either primary or secondary outcomes (Table 2). In unadjusted analyses for THC-COOH, there were differences in IQ at 60 months, Cognitive and Behavioral Checklist scores at 36 months, Conners’ Attention Scale scores, DAS Digits Forward and Picture Recognition at 48 months (Table 3). However, after adjustment for confounders, the only finding that remained significant was that children exposed to THC-COOH compared with unexposed children had higher adjusted medians for the Conners’ Attention Scale score at 48 months of age. In an exploratory analysis, there was no significant correlation between quantitative urine THC-COOH levels and attention scores (Spearman’s correlation coefficient −0.029, p = 0.86).
Table 2:
Cotinine exposure and primary and secondary child neurodevelopmental outcomes at 12–60 months of age
| Outcome | Cotinine | Unadjusted Difference in Medians (95% CI) | Adjusted Difference in Medians (95% CI) | |
|---|---|---|---|---|
| Positive | Negative | |||
| Primary outcome | ||||
| N=85 | N=1022 | |||
| WPPSI III IQ score at age 60 months* | 90 [81–100] | 95 [85–104] | −5.0 (−8.82 to −1.18) | −2.47 (−6.22 to 1.29) |
| Secondary outcomes | ||||
| 12 months | ||||
| Bayley – Cognitive* | 105 [95–110] | 100 [90–110] | 5 (2.15 to 7.85) | 0 (−4.95 to 4.95) |
| Bayley – Motor* | 97 [94–110] | 97 [91–103] | 0 (−3.75 to 3.75) | 0 (−4.17 to 4.17) |
| Bayley – Language | 97 [86–109] | 94 [86–103] | 3 (−1.33 to 7.33) | 0.75 (−4.46 to 5.96) |
| 24 months | ||||
| Bayley – Cognitive | 90 [85–100] | 90 [85–95] | 0 (0 to 0) | 0 (−2.82 to 2.82) |
| Bayley – Motor | 97 [91–103] | 97 [91–103] | 0 (−2.55 to 2.55) | 0 (−3.94 to 3.94) |
| Bayley – Language | 94 [84.5–100] | 89 [79–97] | 5 (1.71 to 8.29) | 0 (−3.31 to 3.31) |
| 36 months | ||||
| DAS II General Conceptual Ability Score | 91.5 [81–100] | 90 [81–100] | 1.0 (−3.49 to 5.49) | −0.20 (−4.19 to 3.79) |
| CBCL T score* | 52[43–58] | 46 [40–54] | 6.0 (3.54 to 8.45) | 2.42 (−1.78 to 6.62) |
| 48 months | ||||
| Conners | 52[44–58] | 49 [44–56.5] | 3.0 (0.38 to 5.62) | 0 (−2.54 to 2.54) |
| DAS II subtest Digits Forward* | 91 [76–113] | 84 [53–106] |
7.0 (−0.63 to 14.63) |
−0.22 (−10.81 to 10.36) |
| DAS II subtest Picture Recognition* | 74 (46–94] | 74 [65–94] | 0 (−5.83 to 5.83) | −3.45 (−10.97 to 4.06) |
| 60 months | ||||
| CBCL T score | 46 [40–55] | 44 [37–53] | 2.00 (−0.25 to 4.25) | 1.67 (−2.54 to 5.88) |
Data displayed as median and interquartile range
P-value < 0.05 in univariate analysis based on the Wilcoxon Rank Sum Test.
WPPS III, Wechsler Preschool and Primary Scale of Intelligence-III; DAS II, Differential Ability Scales-II; Bayley, Bayley Scales of Infant Development-III; Conners, Conners’ Rating Scales-Revised for assessment of attention; CBCL, Child Behavior Checklist for behavioral and social competency
Quantile regression model adjusters included insurance type, education, race and ethnicity, and child age at exam.
Number of participants in each outcome: WPPS III: 1107; 12 month Bayley cognitive: 1106; 12 month Bayley motor: 1103; 12 month Bayley language: 1099; 24 month Bayley cognitive: 1076; 24 month Bayley motor: 1065; 24 month Bayley language: 1053; DAS II: 1088; 36 month CBCL: 1092; 48 month Conners: 1068; 48 month DAS II Digits Forward: 1054; 48 month DAS II Picture Recognition: 1057; 60 month CBCL: 1110.
Table 3:
THC exposure and primary and secondary child neurodevelopmental outcomes at 12–60 months of age
| Outcome | THC-COOH | Unadjusted Difference in Medians (95% CI) | Adjusted Difference in Medians (95% CI) | |
|---|---|---|---|---|
| Positive | Negative | |||
| N=41 | N=1066 | |||
| Primary outcome | ||||
| WPPSI III IQ score age 60 months* | 89 [81–99] | 95 [85–104] | −6.0 (−14.33 to 2.33) | −1.35 (−7.76 to 5.05) |
| Secondary outcomes | ||||
| 12 months | ||||
| Bayley – Cognitive | 100 [95–112.5] | 100 [90–110] | 0 (−5.47 to 5.47) | 0 (−6.54 to 6.54) |
| Bayley – Motor | 97 [92.5–110] | 97 [92.5–110] | 0 (−4.02 to 4.02) | 0 (−4.69 to 4.69) |
| Bayley – Language | 95.5 [87.5–109] | 94 [86–103] | 3.0 (−2.37 to 8.37) | 3.0 (−3.23 to 9.23) |
| 24 months | ||||
| Bayley – Cognitive | 90 [85–95] | 90 [85–100] | 0 (−2.36 to 2.36) | 0 (−3.88 to 3.88) |
| Bayley – Motor | 97 [94–100] | 97 [91–103] | 0 (−3.17 to 3.17) | 0 (−4.36 to 4.36) |
| Bayley – Language | 94 [84.5–97] | 89 [79–97] | 5.0 (1.74 to 8.26) | −0.67 (−4.37 to 3.03) |
| 36 months | ||||
| DAS II General Conceptual Ability Score | 89 [81–99] | 90 [81–100] | 0 (−6.60 to 6.60) | −2.40 (−8.61 to 3.81) |
| CBCL T score* | 54 [43–62] | 46 [40–54] | 8.0 (4.12 to 11.88) | 4.42 (−1.20 to 10.05) |
| 48 months | ||||
| Conners* | 57 [48–62] | 49 [44–56] | 8.0 (4.12 to 11.88) | 6.0 (1.11 to 10.89) |
| DAS II subtest Digits Forward* | 91 [76–113] | 84 [53–106] | 7.0 (−4.03 to 18.03) | 4.89 (−10.31 to 20.09) |
| DAS II subtests Picture recognition* | 74 [46–7] | 74 [65–94] | 0 (−7.77 to 7.77) | −4.73 (−13.33 to 3.87) |
| 60 months | ||||
| CBCL T score | 45 [39–55] | 44 [37–53] | 1.0 (−3.64 to 5.64) | −2.0 (−6.91 to 2.91) |
Data displayed as median and interquartile range
P-value < 0.05 in univariate analysis based on the Wilcoxon Rank Sum Test. Bold denotes p-value <0.05 in adjusted analysis.
WPPS III, Wechsler Preschool and Primary Scale of Intelligence-III; DAS II, Differential Ability Scales-II; Bayley, Bayley Scales of Infant Development-III; Conners, Conners’ Rating Scales-Revised, for assessment of attention; CBCL, Child Behavior Checklist, for behavioral and social competency
Quantile regression model adjusters included insurance type, education, race and ethnicity, and child age at exam.
Number of participants in each outcome: WPPS III: 1107; 12 month Bayley cognitive: 1106; 12 month Bayley motor: 1103; 12 month Bayley language: 1099; 24 month Bayley cognitive: 1076; 24 month Bayley motor: 1065; 24 month Bayley language: 1053; 36 month DAS II: 1088; 36 month CBCL: 1092; 48 month Conners: 1068; 48 month DAS II Digits Forward: 1054; 48 month DAS II Picture Recognition: 1057; 60 month CBCL: 1110.
Discussion
In this secondary analysis of two parallel RCTs, we examined the association between cotinine and THC exposure and early childhood neurodevelopmental outcomes. In this study with over 99% child follow up for the primary outcome, we found no difference between exposed and unexposed in child IQ at 60 months of age. Cannabis exposure between 8 and 20 weeks gestation was associated with higher Conners attention scores at 48 months of age. These results should be interpreted with caution. While the difference between exposed and unexposed children was statistically significant, both groups’ median T score was within the average range 40–59 (16–83 percentile) which is associated with typical levels of attention concern for the child’s age and sex.39 In addition, we did not adjust for multiple comparisons as this analysis was intended to be hypothesis-generating in order to guide future work in this area.
Our results build on previous research demonstrating an association between prenatal THC exposure and adverse neurodevelopmental outcomes among young children, particularly attention.40 The endocannabinoid system is active in fetal brain development. The endocannabinoid receptor, CB1, plays a major role in fetal brain development by regulating neural progenitor differentiation into neurons and glia and guiding axonal migration and synaptogenesis. Therefore, dysregulation of this process through exposure to exogenous cannabis resulting in abnormal neurodevelopment is biologically plausible.
Evidence related to neurodevelopmental outcomes with cannabis exposure in humans comes predominantly from four longitudinal studies: Adolescent Brain Cognitive Development (ABCD) Study (data release 2.0.1), Ottawa Prenatal Prospective Study (OPPS), Maternal Health Practices and Child Development (MHPCD), and Generation R, a population based prospective cohort in the Netherlands starting in 2002. Long-term follow-up is complete for ABCD, OPPS and MHPCD studies, while Generation R is ongoing. For all of these studies, cannabis use was ascertained by maternal self-report.
Recent data from the Adolescent Brain Cognitive Development (ABCD) study, a cross-sectional study of 11489 children with 655 with prenatal cannabis exposure demonstrated that exposure was associated with worse attention and hyperactivity on the Child Behavior Checklist (all |β| > 0.047; all false discovery rate –corrected P < .001). In the MHPCD study (N=564), at 6 years of age, prenatal cannabis exposure was associated with a significant increase in impulsivity (more errors of commission) but a positive effect on attention (fewer errors of omission).41 Similar findings in impairment of short-term memory and in verbal and abstract or visual reasoning were found in the MHPCD cohort at 3 years of age.42, 43 At 6 years of age, the OPPS study (N=698) found prenatal cannabis exposure was associated with decreased attention and increased impulsivity and hyperactivity.44 As the cohorts were followed over the next 9 to 12 years, executive function and difficulty organizing and integrating specific cognitive and output processes were observed.45–47 Data from the Generation R cohort demonstrate that prenatal self-reported cannabis use in early pregnancy correlated with worse attention using the Child Behavior Checklist among girls, but not boys, at age 18 months.48 Thus, our findings are consistent with those of prior studies demonstrating an association between prenatal cannabis exposure and worse attention in childhood.
Limitations of these prior studies include the small sample of prenatal cannabis–exposed offspring; potential maternal underreporting of use during pregnancy; imprecise data on timing and amount, frequency, and potency of cannabis exposure; and lack of data on some potential confounders. Nevertheless, based on findings of these studies, the United States Surgeon General,14 American Academy of Pediatrics and American College of Obstetricians and Gynecologists4 note concerns regarding the potential for maternal cannabis use to adversely affect fetal neurodevelopment.
Our study addresses two of the major limitations of these other studies. First, we ascertained exposure through urine assays for nicotine and cannabis metabolites as opposed to self-report, which is important as self-report underestimates use by as much as ten-fold.26 Second, we had an extraordinarily high child follow-up rate in this cohort resulting in an available sample size exceeding that of most existing studies of neurodevelopment and maternal cannabis and nicotine product use.
Additional strengths include the generalizability of the cohort, which was assembled through recruitment of pregnant participants from 15 centers across the United States resulting in a racially and socioeconomically diverse cohort. Prior studies are focused on subsets of the population in a single location. In addition, all study data were collected prospectively by experienced perinatal research staff. All neurodevelopmental testing was performed using standardized instruments following centralized training and certification. Finally, all laboratory analyses were performed by a CLIA-certified national reference laboratory for drug testing.
Limitations of this study are primarily related to the design as an unplanned secondary analysis and the number of children exposed to THC-COOH is low. Because this secondary analysis is exploratory, we did not adjust for multiple comparisons which may have resulted in alpha error. We are not powered to detect modest differences in the primary and secondary outcomes based on our exposures of interest. However, the finding of worse attention scores with cannabis exposure is consistent with existing literature.40–48 We only had a study enrollment urine specimen, which did not allow for investigation of quantity and duration of perinatal substance use. The parent RCT excluded individuals with “known illicit drug or alcohol abuse during current pregnancy.” Therefore, pregnant individuals with cannabis use disorder who may have the highest levels of exposure were excluded, potentially biasing the results towards the null. Nonetheless, nearly 4% of the study population were THC-COOH positive, which is consistent with the estimated prevalence of prenatal marijuana use in the literature at the time of study enrollment. Additionally, other substance use including opioid, methamphetamine, cocaine and polysubstance use, which may significantly influence child neurodevelopmental outcomes was not evaluated with urine drug testing for this analysis due to small volumes of available urine. 49–57 In addition, small numbers of nicotine and cannabis dual exposure precluded meaningful analysis related to potential additive effects of dual exposure. Finally, while more than 99% of all children had data on the primary outcome available, 8–10% of children had missing data on the secondary outcomes including attention at 48 months (n=1068, 89.2%).
In terms of the urine testing, we assessed only the most stable metabolite of cannabis and nicotine. There are hundreds of active substances in cannabis and nicotine-containing products and these metabolites may not be the most predictive of adverse child neurodevelopmental outcomes. Additionally, urine toxicology has variable detection windows depending on quantity and timing of use. The presence or absence of metabolites in one sample indicates substance exposure in a limited window of detection, which likely biases these results towards the null. We are also unable to distinguish between the timing or type of maternal exposure (e.g. smoking, vaping) based on urine metabolite testing. Additionally, we are unable to distinguish between active versus passive use of cannabis and nicotine-containing products.
Results on neurodevelopmental outcomes after prenatal cannabis exposure are inconsistent across studies and may be due to unmeasured confounding, including difference in postnatal environment and caregiver characteristics, including maternal mental health disorders.58 While maternal depression treated with tricyclic anti-depressants and selective serotonin reuptake inhibitors was an exclusion criteria for this trial, pregnant individuals undergoing non-pharmacological treatment, untreated mental health disorder or other mental health conditions were not excluded. Only 242 participants (20.2% of cohort) completed depression assessments (Center for Epidemiological Studies - Depression); therefore, adjustment for baseline maternal mental health was not possible. Other unmeasured confounders may include paternal or other household member cannabis or nicotine product use, children’s social and school environment,59 maternal stress levels,60 exposure to systemic violence61 and discrimination,62 which all influence child neurodevelopmental outcomes. Other significant risk factors for attention disorders include environmental exposures such as high levels of lead,63 mercury64 and polychlorinated biphenyls (PCBs)65, fetal alcohol exposure,66 adverse childhood events (ACE)67–69 and gene susceptibility70 which were not systematically collected as part of the parent study.
Neither prenatal cannabis nor cotinine exposure was associated with differences in child IQ at age 60 months. However, prenatal THC exposure was associated with higher (worse) attention scores at 48 months. Results of this study suggest the need for high quality studies that aim to examine the child neurodevelopmental effects of prenatal exposure to cannabis and nicotine. Future studies should include those with prospective longitudinal design assessing timing, quantity and co-exposure to nicotine, cannabis and other substances over the course of pregnancy, as well as assessment of critical confounding factors in order to better elucidate the relationship between maternal nicotine and cannabis use and child neurodevelopmental outcomes.
Supplementary Material
Acknowledgments
Supported by Eunice Kennedy Shriver National Institute of Child Health and Human Development (NICHD) and the National Institute of Neurological Disorders and Stroke (NINDS) [HD34116, HD40512, HD27917, HD34208, HD40485, HD40560, HD53097, HD27869, HD40500, HD40545, HD27915, HD40544, HD53118, HD21410, and U10 HD36801.] and UL1TR003096, TL1TR003106, KL2TR003097 grants. In addition, Marcela C. Smid was supported by Women’s Reproductive Health Research (WRHR K12, 1K12 HD085816) Career Development Program and Dr. Metz by R01DA049832 during the completion of this project. Comments and views of the authors do not necessarily represent views of the NIH.
Financial Disclosure
This study was funded in part by the University of Utah School of Medicine H.A. and Edna Benning Presidential Endowment. Dr. Smid serves as a medical consultant for Gilead Science Inc for hepatitis C treatment in pregnancy. Dr. Metz reports receiving UptoDate royalties for two topics on VBAC, and serving as a medical consultant to Pfizer for design of a SARS-CoV-2 vaccination trial in pregnancy. Funds for Dr. Metz were paid to her institution from Pfizer for being a site PI for an RSV vaccine trial. Funds were paid to her institution from Gestvision (site PI for preeclampsia POC test). Dr. Metz and Smid also report money was paid to her institution from NIDA. Dr. Tita reports support from NIH/NCATS and money was paid to his institution from Pfizer for a vaccine study and the CDC. reports money was paid to his institution from Pfizer for a vaccine study and the CDC. Dr. Miller reports money was paid to her institution from Pfizer as a site PI for a study on COVID vaccine in pregnancy. The authors did not report any potential conflicts of interest.
Footnotes
Presented at the Society for Maternal-Fetal Medicine’s 41st Annual Pregnancy Meeting (virtual), January 25–30, 2021.
Contributor Information
Marcela C. Smid, Departments of Obstetrics and Gynecology of the University of Utah Health, Salt Lake City, UT.
Torri D. Metz, Departments of Obstetrics and Gynecology of the University of Utah Health, Salt Lake City, UT.
Gwen A. McMillin, Department of Pathology, University of Utah Health and ARUP Laboratories, Salt Lake City UT.
Lisa Mele, George Washington University Biostatistics Center, Washington, DC.
Brian M. Casey, University of Texas - Southwestern, Dallas, TX.
Uma M. Reddy, Eunice Kennedy Shriver National Institute of Child Health and Human Development, Bethesda, MD.
Ronald J. Wapner, Columbia University, New York, NY.
John M. Thorp, Jr., University of North Carolina, Chapel Hill, NC.
George R. Saade, University of Texas Medical Branch, Galveston, TX.
Alan T.N. Tita, University of Alabama at Birmingham, Birmingham, AL.
Emily S. Miller, Northwestern University, Chicago IL.
Dwight J. Rouse, Brown University, Providence, RI.
Baha Sibai, University of Texas – Houston, Houston, TX.
Maged M. Costantine, The Ohio State University, Columbus, OH.
Brian M. Mercer, Case Western Reserve University, Cleveland, OH.
Steve N. Caritis, University of Pittsburgh, Pittsburgh, PA.
References
- 1.Jamal A, Phillips E, Gentzke AS, et al. Current Cigarette Smoking Among Adults - United States, 2016. MMWR Morb Mortal Wkly Rep. January 19 2018;67(2):53–59. doi: 10.15585/mmwr.mm6702a1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Wang TW, Asman K, Gentzke AS, et al. Tobacco Product Use Among Adults - United States, 2017. MMWR Morb Mortal Wkly Rep. November 9 2018;67(44):1225–1232. doi: 10.15585/mmwr.mm6744a2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Creamer MR, Wang TW, Babb S, et al. Tobacco Product Use and Cessation Indicators Among Adults - United States, 2018. MMWR Morb Mortal Wkly Rep. November 15 2019;68(45):1013–1019. doi: 10.15585/mmwr.mm6845a2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kerr WC, Lui C, Ye Y. Trends and age, period and cohort effects for marijuana use prevalence in the 1984–2015 US National Alcohol Surveys. Addiction. March 2018;113(3):473–481. doi: 10.1111/add.14031 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Steigerwald S, Wong PO, Cohen BE, et al. Smoking, Vaping, and Use of Edibles and Other Forms of Marijuana Among U.S. Adults. Ann Intern Med. December 18 2018;169(12):890–892. doi: 10.7326/m18-1681 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Goodwin RD, Cheslack-Postava K, Nelson DB, et al. Smoking during pregnancy in the United States, 2005–2014: The role of depression. Drug Alcohol Depend. October 1 2017;179:159–166. doi: 10.1016/j.drugalcdep.2017.06.021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Young-Wolff KC, Tucker LY, Alexeeff S, et al. Trends in Self-reported and Biochemically Tested Marijuana Use Among Pregnant Females in California From 2009–2016. JAMA. December 26 2017;318(24):2490–2491. doi: 10.1001/jama.2017.17225 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Skelton KR, Hecht AA, Benjamin-Neelon SE. Association of Recreational Cannabis Legalization With Maternal Cannabis Use in the Preconception, Prenatal, and Postpartum Periods. JAMA Netw Open. February 1 2021;4(2):e210138. doi: 10.1001/jamanetworkopen.2021.0138 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Curtin SC, Matthews TJ. Smoking Prevalence and Cessation Before and During Pregnancy: Data From the Birth Certificate, 2014. Natl Vital Stat Rep. February 10 2016;65(1):1–14. [PubMed] [Google Scholar]
- 10.Drake P, Driscoll AK, Mathews TJ. Cigarette Smoking During Pregnancy: United States, 2016. NCHS Data Brief. February 2018;(305):1–8. [PubMed] [Google Scholar]
- 11.Centers for Disease C, Prevention. Smoking during pregnancy--United States, 1990–2002. MMWR Morb Mortal Wkly Rep. October 8 2004;53(39):911–5. [PubMed] [Google Scholar]
- 12.Brown QL, Sarvet AL, Shmulewitz D, Martins SS, Wall MM, Hasin DS. Trends in Marijuana Use Among Pregnant and Nonpregnant Reproductive-Aged Women, 2002–2014. Jama. January 10 2017;317(2):207–209. doi: 10.1001/jama.2016.17383 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.American College of Obstetricians and Gynecologists. Committee Opinion, Number 807: Tobacco and Nicotine Cessation During Pregnancy Obstet Gynecol. 2020;135(5):e221–e229. doi: 10.1097/AOG.0000000000003822 [DOI] [PubMed] [Google Scholar]
- 14.Office of the Surgeon General. US Surgeon General’s Advisory: Marijuana Use and the Developing Brain; 2019. Retrieved from https://www.hhs.gov/surgeongeneral/reports-and-publications/addiction-and-substance-misuse/advisory-on-marijuana-use-and-developing-brain/index.html. Accessed August 17, 2021.
- 15.American College of Obstetricians and Gynecologists. Committee Opinion No. 722: Marijuana Use During Pregnancy and Lactation. Obstet Gynecol. October 2017;130(4):e205–e209. doi: 10.1097/aog.0000000000002354 [DOI] [PubMed] [Google Scholar]
- 16.Adams JM. Smoking Cessation-Progress, Barriers, and New Opportunities: The Surgeon General’s Report on Smoking Cessation. JAMA. June 23 2020;323(24):2470–2471. doi: 10.1001/jama.2020.6647 [DOI] [PubMed] [Google Scholar]
- 17.Yang Q, Pan L, Shen C, et al. Mothers’ prenatal tobacco smoke exposure is positively associated with the occurrence of developmental coordination disorder among children aged 3–6 years: A cross-sectional study in a rural area of Shanghai, China. Tob Induc Dis. 2020;18:25. doi: 10.18332/tid/119115 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Minatoya M, Araki A, Itoh S, et al. Prenatal tobacco exposure and ADHD symptoms at pre-school age: the Hokkaido Study on Environment and Children’s Health. Environ Health Prev Med. December 7 2019;24(1):74. doi: 10.1186/s12199-019-0834-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Polanska K, Krol A, Merecz-Kot D, et al. Environmental Tobacco Smoke Exposure during Pregnancy and Child Neurodevelopment. Int J Environ Res Public Health July 17 2017;14(7)doi: 10.3390/ijerph14070796 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Metz TD, Stickrath EH. Marijuana use in pregnancy and lactation: a review of the evidence. Am J Obstet Gynecol. December 2015;213(6):761–78. doi: 10.1016/j.ajog.2015.05.025 [DOI] [PubMed] [Google Scholar]
- 21.Hurd YL. Cannabis and the developing brain challenge risk perception. J Clin Invest. August 3 2020;130(8):3947–3949. doi: 10.1172/JCI139051 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Grant KS, Petroff R, Isoherranen N, Stella N, Burbacher TM. Cannabis use during pregnancy: Pharmacokinetics and effects on child development. Pharmacol Ther. February 2018;182:133–151. doi: 10.1016/j.pharmthera.2017.08.014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.El Marroun H, Brown QL, Lund IO, et al. An epidemiological, developmental and clinical overview of cannabis use during pregnancy. Prev Med. November 2018;116:1–5. doi: 10.1016/j.ypmed.2018.08.036 [DOI] [PubMed] [Google Scholar]
- 24.Corsi DJ, Donelle J, Sucha E, et al. Maternal cannabis use in pregnancy and child neurodevelopmental outcomes. Nat Med. October 2020;26(10):1536–1540. doi: 10.1038/s41591-020-1002-5 [DOI] [PubMed] [Google Scholar]
- 25.Metz TD, Borgelt LM. Marijuana Use in Pregnancy and While Breastfeeding. Obstet Gynecol. November 2018;132(5):1198–1210. doi: 10.1097/aog.0000000000002878 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Metz TD, Silver RM, McMillin GA, et al. Prenatal Marijuana Use by Self-Report and Umbilical Cord Sampling in a State With Marijuana Legalization. Obstet Gynecol. January 2019;133(1):98–104. doi: 10.1097/aog.0000000000003028 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Rodriguez CE, Sheeder J, Allshouse AA, et al. Marijuana use in young mothers and adverse pregnancy outcomes: a retrospective cohort study. Bjog. November 2019;126(12):1491–1497. doi: 10.1111/1471-0528.15885 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Casey BM, Thom EA, Peaceman AM, et al. Treatment of Subclinical Hypothyroidism or Hypothyroxinemia in Pregnancy. N Engl J Med. March 2 2017;376(9):815–825. doi: 10.1056/NEJMoa1606205 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Verstraete AG. Detection times of drugs of abuse in blood, urine, and oral fluid. Ther Drug Monit. April 2004;26(2):200–5. doi: 10.1097/00007691-200404000-00020 [DOI] [PubMed] [Google Scholar]
- 30.Vandevenne M, Vandenbussche H, Verstraete A. Detection time of drugs of abuse in urine. Acta Clin Belg. Nov-Dec 2000;55(6):323–33. doi: 10.1080/17843286.2000.11754319 [DOI] [PubMed] [Google Scholar]
- 31.DuPaul GJ, Power TJ, McGoey KE, Ikeda MJ, Anastopoulos AD. Reliability and validity of parent and teacher ratings of attention-deficit hyperactivity disorder symptoms. Journal of Psychoeducational Assessment. March 1998;16(1):55–68. doi:Doi 10.1177/073428299801600104 [DOI] [Google Scholar]
- 32.Conners CK, Sitarenios G, Parker JD, Epstein JN. The revised Conners’ Parent Rating Scale (CPRS-R): factor structure, reliability, and criterion validity. J Abnorm Child Psychol. August 1998;26(4):257–68. doi: 10.1023/a:1022602400621 [DOI] [PubMed] [Google Scholar]
- 33.Dotters-Katz SK, Smid MC, Manuck TA, Metz TD. Risk of neonatal and childhood morbidity among preterm infants exposed to marijuana. J Matern Fetal Neonatal Med. December 2017;30(24):2933–2939. doi: 10.1080/14767058.2016.1269165 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Metz TD, Allshouse AA, Hogue CJ, et al. Maternal marijuana use, adverse pregnancy outcomes, and neonatal morbidity. Am J Obstet Gynecol. October 2017;217(4):478.e1–478.e8. doi: 10.1016/j.ajog.2017.05.050 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Olson K, Jacobson K. Cross-Cultural Considerations in Pediatric Neuropsychology: A Review and Call to Attention. Appl Neuropsychol Child. 2015;4(3):166–77. doi: 10.1080/21622965.2013.830258 [DOI] [PubMed] [Google Scholar]
- 36.Greenberg JP, Kahn JM. Early childhood education and care use: Differences by race/ethnicity and age. Journal of Children and Poverty. 2012;18(1):23–54. [Google Scholar]
- 37.Linsell L, Malouf R, Morris J, Kurinczuk JJ, Marlow N. Prognostic Factors for Poor Cognitive Development in Children Born Very Preterm or With Very Low Birth Weight: A Systematic Review. JAMA Pediatr. December 2015;169(12):1162–72. doi: 10.1001/jamapediatrics.2015.2175 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Duncan AF, Watterberg KL, Nolen TL, et al. Effect of ethnicity and race on cognitive and language testing at age 18–22 months in extremely preterm infants. J Pediatr. June 2012;160(6):966–71.e2. doi: 10.1016/j.jpeds.2011.12.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Conners Comprehensive Behavior Rating Scales. Conners Comprehensive Behavior Rating Update. April, 2009. Accessed September 24th 2021. https://www.acer.org/files/CBRS-Supplement.pdf
- 40.Paul SE, Hatoum AS, Fine JD, et al. Associations Between Prenatal Cannabis Exposure and Childhood Outcomes: Results From the ABCD Study. JAMA Psychiatry. January 1 2021;78(1):64–76. doi: 10.1001/jamapsychiatry.2020.2902 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Leech SL, Richardson GA, Goldschmidt L, Day NL. Prenatal substance exposure: effects on attention and impulsivity of 6-year-olds. Neurotoxicol Teratol. Mar-Apr 1999;21(2):109–18. doi: 10.1016/s0892-0362(98)00042-7 [DOI] [PubMed] [Google Scholar]
- 42.Fried PA, Watkinson B. 36- and 48-month neurobehavioral follow-up of children prenatally exposed to marijuana, cigarettes, and alcohol. Journal of developmental and behavioral pediatrics : JDBP. April 1990;11(2):49–58. [PubMed] [Google Scholar]
- 43.Day N, Sambamoorthi U, Taylor P, et al. Prenatal marijuana use and neonatal outcome. Neurotoxicol Teratol. May-Jun 1991;13(3):329–34. doi: 10.1016/0892-0362(91)90079-c [DOI] [PubMed] [Google Scholar]
- 44.Fried PA, Watkinson B, Gray R. A follow-up study of attentional behavior in 6-year-old children exposed prenatally to marihuana, cigarettes, and alcohol. Neurotoxicol Teratol. Sep-Oct 1992;14(5):299–311. doi: 10.1016/0892-0362(92)90036-a [DOI] [PubMed] [Google Scholar]
- 45.Fried PA, Watkinson B, Gray R. Differential effects on cognitive functioning in 9- to 12-year olds prenatally exposed to cigarettes and marihuana. Neurotoxicol Teratol. May-Jun 1998;20(3):293–306. doi: 10.1016/s0892-0362(97)00091-3 [DOI] [PubMed] [Google Scholar]
- 46.Richardson G, Day N. A comparison of the effects of prenatal marijuana, alcohol, and cocaine use on 10-year child outcome. Neurotoxicology and Teratology. 1997;3(19):257. [Google Scholar]
- 47.Huizink AC, Mulder EJ. Maternal smoking, drinking or cannabis use during pregnancy and neurobehavioral and cognitive functioning in human offspring. Neurosci Biobehav Rev. 2006;30(1):24–41. doi: 10.1016/j.neubiorev.2005.04.005 [DOI] [PubMed] [Google Scholar]
- 48.El Marroun H, Hudziak JJ, Tiemeier H, et al. Intrauterine cannabis exposure leads to more aggressive behavior and attention problems in 18-month-old girls. Drug Alcohol Depend. November 1 2011;118(2–3):470–4. doi: 10.1016/j.drugalcdep.2011.03.004 [DOI] [PubMed] [Google Scholar]
- 49.Smith LM, Paz MS, LaGasse LL, et al. Maternal depression and prenatal exposure to methamphetamine: neurodevelopmental findings from the infant development, environment, and lifestyle (ideal) study. Depress Anxiety. June 2012;29(6):515–22. doi: 10.1002/da.21956 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Arria AM, Derauf C, Lagasse LL, et al. Methamphetamine and other substance use during pregnancy: preliminary estimates from the Infant Development, Environment, and Lifestyle (IDEAL) study. Matern Child Health J. May 2006;10(3):293–302. doi: 10.1007/s10995-005-0052-0 [DOI] [PubMed] [Google Scholar]
- 51.Derauf C, LaGasse LL, Smith LM, et al. Demographic and psychosocial characteristics of mothers using methamphetamine during pregnancy: preliminary results of the infant development, environment, and lifestyle study (IDEAL). Am J Drug Alcohol Abuse. 2007;33(2):281–9. doi: 10.1080/00952990601175029 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.McGlone L, Mactier H. Infants of opioid-dependent mothers: neurodevelopment at six months. Early Hum Dev. January 2015;91(1):19–21. doi: 10.1016/j.earlhumdev.2014.10.006 [DOI] [PubMed] [Google Scholar]
- 53.Conradt E, Crowell SE, Lester BM. Early life stress and environmental influences on the neurodevelopment of children with prenatal opioid exposure. Neurobiol Stress. November 2018;9:48–54. doi: 10.1016/j.ynstr.2018.08.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Conradt E, Flannery T, Aschner JL, et al. Prenatal Opioid Exposure: Neurodevelopmental Consequences and Future Research Priorities. Pediatrics. September 2019;144(3)doi: 10.1542/peds.2019-0128 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Bandstra ES, Morrow CE, Vogel AL, et al. Longitudinal influence of prenatal cocaine exposure on child language functioning. Neurotoxicol Teratol. May-Jun 2002;24(3):297–308. doi: 10.1016/s0892-0362(02)00192-7 [DOI] [PubMed] [Google Scholar]
- 56.Lester BM, Tronick EZ, LaGasse L, et al. The maternal lifestyle study: effects of substance exposure during pregnancy on neurodevelopmental outcome in 1-month-old infants. Pediatrics. December 2002;110(6):1182–92. doi: 10.1542/peds.110.6.1182 [DOI] [PubMed] [Google Scholar]
- 57.Andrews K, Francis DJ, Riese ML. Prenatal cocaine exposure and prematurity: neurodevelopmental growth. Journal of developmental and behavioral pediatrics : JDBP. August 2000;21(4):262–70. doi: 10.1097/00004703-200008000-00002 [DOI] [PubMed] [Google Scholar]
- 58.National Academies of Sciences E, Medicine, Health, et al. The National Academies Collection: Reports funded by National Institutes of Health. The Health Effects of Cannabis and Cannabinoids: The Current State of Evidence and Recommendations for Research. National Academies Press (US) Copyright 2017 by the National Academy of Sciences. All rights reserved.; 2017. [PubMed] [Google Scholar]
- 59.Miguel PM, Pereira LO, Silveira PP, Meaney MJ. Early environmental influences on the development of children’s brain structure and function. Dev Med Child Neurol. October 2019;61(10):1127–1133. doi: 10.1111/dmcn.14182 [DOI] [PubMed] [Google Scholar]
- 60.O’Sullivan A, Monk C. Maternal and Environmental Influences on Perinatal and Infant Development. Future of Children. 2020;30(2) [Google Scholar]
- 61.Toso K, de Cock P, Leavey G. Maternal exposure to violence and offspring neurodevelopment: A systematic review. Paediatr Perinat Epidemiol. March 2020;34(2):190–203. doi: 10.1111/ppe.12651 [DOI] [PubMed] [Google Scholar]
- 62.Webb E Discrimination against children. Arch Dis Child. September 2004;89(9):804–8. doi: 10.1136/adc.2003.046300 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Needleman HL. Lead and impaired abilities. Dev Med Child Neurol. April 1982;24(2):196–8. doi: 10.1111/j.1469-8749.1982.tb08802.x [DOI] [PubMed] [Google Scholar]
- 64.Yoshimasu K, Kiyohara C, Takemura S, Nakai K. A meta-analysis of the evidence on the impact of prenatal and early infancy exposures to mercury on autism and attention deficit/hyperactivity disorder in the childhood. Neurotoxicology. September 2014;44:121–31. doi: 10.1016/j.neuro.2014.06.007 [DOI] [PubMed] [Google Scholar]
- 65.Lackmann GM, Schaller KH, Angerer J. Organochlorine compounds in breast-fed vs. bottle-fed infants: preliminary results at six weeks of age. Sci Total Environ. August 15 2004;329(1–3):289–93. doi: 10.1016/j.scitotenv.2004.03.014 [DOI] [PubMed] [Google Scholar]
- 66.Streissguth AP, Barr HM, Olson HC, Sampson PD, Bookstein FL, Burgess DM. Drinking during pregnancy decreases word attack and arithmetic scores on standardized tests: adolescent data from a population-based prospective study. Alcoholism, clinical and experimental research. April 1994;18(2):248–54. doi: 10.1111/j.1530-0277.1994.tb00009.x [DOI] [PubMed] [Google Scholar]
- 67.Mehari K, Iyengar SS, Berg KL, Gonzales JM, Bennett AE. Adverse Childhood Experiences and Obesity Among Young Children with Neurodevelopmental Delays. Matern Child Health J. August 2020;24(8):1057–1064. doi: 10.1007/s10995-020-02940-4 [DOI] [PubMed] [Google Scholar]
- 68.Kambeitz C, Klug MG, Greenmyer J, Popova S, Burd L. Association of adverse childhood experiences and neurodevelopmental disorders in people with fetal alcohol spectrum disorders (FASD) and non-FASD controls. BMC Pediatr. December 16 2019;19(1):498. doi: 10.1186/s12887-019-1878-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Weinstein D, Staffelbach D, Biaggio M. Attention-deficit hyperactivity disorder and posttraumatic stress disorder: differential diagnosis in childhood sexual abuse. Clin Psychol Rev. April 2000;20(3):359–78. doi: 10.1016/s0272-7358(98)00107-x [DOI] [PubMed] [Google Scholar]
- 70.Faraone SV, Perlis RH, Doyle AE, et al. Molecular genetics of attention-deficit/hyperactivity disorder. Biol Psychiatry. June 1 2005;57(11):1313–23. doi: 10.1016/j.biopsych.2004.11.024 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


