Abstract
Purpose of review
The field of multiple myeloma treatment has entered a new era with antibody-based approaches in clinical practice. In this review, we focus on the clinical approaches of utilizing antibody-based modality, specifically monoclonal antibodies, antibody-drug-conjugates, bispecific T-cell antibodies in the treatment of multiple myeloma.
Recent findings
Three monoclonal antibodies (daratumumab, isatuximab, elotuzumab) and one anti-BCMA antibody-drug-conjugate (belantamab mafodotin) have been approved by the FDA in the last 5 years for the treatment of multiple myeloma in both the frontline and relapsed settings. There are many ongoing clinical trials using novel targets and constructs, including bispecific antibodies against BCMA, GPRC5D and FCRH5. In addition to exploring efficacy, there are ongoing efforts to overcome the resistance to therapy.
Summary
Antibody-based therapy has improved the outcomes of patients with multiple myeloma and has been incorporated in the standard of care. We expect to see novel targets and constructs that can achieve deeper and more durable response while minimizing toxicity, as well as better strategies for toxicity management for existing agents. We also expect that antibody-based strategies will be used in earlier lines of therapy in the future.
Keywords: multiple myeloma, monoclonal antibody, antibody-drug conjugate, bispecific T cell antibodies, CD38, BCMA
Introduction:
Multiple myeloma (MM) is a cancer of plasma cells, accounting for 1.8% of all cancers, and the second most common hematological malignancies after non-Hodgkin lymphoma. The estimated number of new cases in 2020 was 32,270 [1]. The past two decades have seen a significant improvement in survival rates due to an introduction of proteasome inhibitors (PIs, such as bortezomib, carfilzomib and ixazomib) and immunomodulatory agents (IMiDs, such as thalidomide, lenolidomide and pomalidomide), as well as an improvement of hematopoietic stem cell transplantation. Despite these developments however, relapse is inevitable in almost all patients, and the 5-year relative survival rate is still 54% [1]. Furthermore, the prognosis of patients with genetically or clinically defined high-risk MM remains dismal. Hence, the development of agents with different modality to target MM cells or to potentiate the currently available therapies is crucial.
Antibody-based therapies and other immune-mediated approaches have emerged as an attractive approach for patients with MM to achieve a durable and deep response. Currently, monoclonal antibodies (mAbs), antibody-drug conjugates (ADCs), bispecific antibodies (BsAbs) that recruit T-cells, and chimeric antigen receptor (CAR) T cells are used in clinical practice, or are under clinical investigation. This review focuses on the current use of mAbs, ADCs, BsAbs for the treatment of MM, and those under clinical investigation. Figure 1 illustrates the different classes of monoclonal antibody-based therapies, available or under clinical investigation for the treatment of MM and their mechanisms of action. While CAR-T cell therapy has shown exciting results in MM, we will refer this topic to another source for review.
Figure 1:
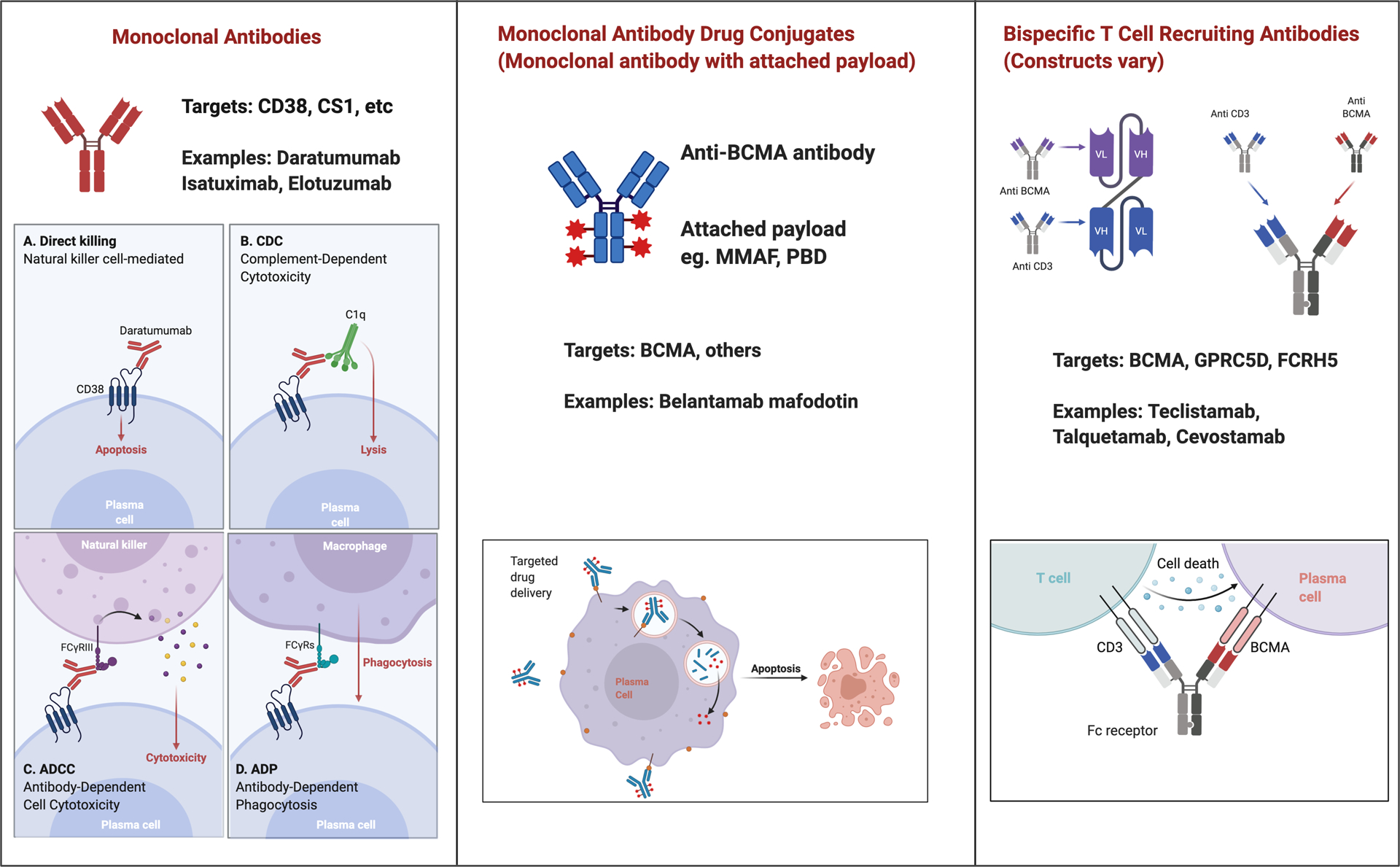
Different Antibody-based Therapies for Treatment of Multiple Myeloma: Monoclonal Antibodies, Antibody Drug Conjugates and Bispecific Antibodies
Monoclonal antibodies
Monoclonal antibodies have emerged as an effective therapeutic modality in many solid and hematological malignancies. The mechanisms of anti-tumor effect of mAbs vary by agents including (i) direct tumor cell killing such as induction of apoptosis or signaling inhibition, (ii) immune-mediated tumor cell killing such as antibody-dependent cell-mediated cytotoxicity (ADCC), complement–dependent cytotoxicity (CDC), induction of phagocytosis or T cell activation, (iii) stromal ablation such as inhibition of tumor blood vessel growth (Figure 1). The identification of antigens that are highly expressed on MM cells has led to the development of mAbs as a novel therapeutic platform.
Anti-CD38 antibodies
CD38 is a transmembrane pleiotropic glycoprotein that is highly expressed in plasma cells and MM cells, and at a lower level in other lymphoid and myeloid cells, red blood cells, platelets, and some solid tissues [2]. CD38 functions as a receptor of CD31, or as an ectoenzyme playing a role in homeostasis of nicotinamide dinucleotide (NAD+) and extracellular nucleotide as well as intracellular calcium [3]. Several anti-CD38 monoclonal antibodies have been developed (daratumumab, isatuximab, MOR-202, TAK-079 etc.) and these antibodies have multiple mechanisms of action including ADCC, CDC, antibody-dependent phagocytosis, and immune cell depletion or inhibition of immunosuppressive cells [2, 4]. Additionally, daratumumab has shown to reduce CD38-positive immune suppressor cells such as regulatory T cells (Tregs), regulatory B cells (Bregs), and myeloid-derived suppressor cells (MDSCs), thereby potentially enhancing anti-tumor immune response [5].
Daratumumab
Role in Relapsed/Refractory Myeloma:
Daratumumab was approved by the US Food and Drug Administration (FDA) in 2015 based on two open-label studies [6, 7]. It was initially approved as a monotherapy for relapsed or refractory (RR) MM patients who have received at least three prior lines of therapy. Pooled analysis of these studies reported an overall response rate (ORR) of 31.1%, median progression-free survival (PFS) of 4 months and median overall survival (OS) of 20.1 months in patients who received a median of five prior lines of therapy. The safety profile showed that infusion reactions were common with initial dosing (48%), though there was a low incidence of grade ≥3 infusion-related reactions (2.7%). The most common treatment-emergent adverse effects (TEAEs) included fatigue, nausea, anemia, thrombocytopenia, neutropenia and back pain, which occurred in more than 20% of the patients [8].
Combining daratumumab with lenalidomide showed an increase in NK cell-mediated ADCC in preclinical studies, which was translated into clinical studies that evaluated lenalidomide-dexamethasone (Rd) ± daratumumab. A phase III trial (POLLUX) that recruited 569 RRMM patients who were not lenalidomide-refractory demonstrated significantly higher ORR in the daratumumab triplet arm (93% vs 76%) as well as superior PFS at 12 months (83.2% vs 60.1%) and minimal residual disease (MRD) negativity rate (26% vs 6%) [9].
Similarly, daratumumab was combined with bortezomib-dexamethasone (Vd) regimen in a phase III trial (CASTOR). The triplet arm showed significantly higher ORR (83% vs 63%) as well as MRD negativity rate (12% vs 2%) in comparison to the Vd doublet [10].
Another study (CANDOR) evaluated daratumumab in combination with carfilzomib and dexamethasone (Kd) and showed significantly improved ORR (84% vs 75%). Hazard ratio for progression or death was 0.63. MRD negativity at 12 months was 13% in KdD arm versus 1% in Kd arm [11].
Combining daratumumab with other IMiDs is an important option as many patients eventually become refractory to lenalidomide. A phase I study [12] led to the FDA approval of daratumumab in combination of pomalidomide and dexamethasone (Pd). A phase III study (APOLLO) that compared subcutaneous daratumumab-Pd to Pd in patients who received more than 1 line of prior therapy showed longer PFS (9.9 vs 6.5 months) without unexpected safety concerns other than known safety profile of subcutaneous daratumumab and Pd [13].
Role in Frontline Treatment
Transplant-ineligible Patients:
Daratumumab in the frontline setting was first investigated in transplant-ineligible patients as a combination with bortezomib, melphalan and prednisone (ALCYONE) [14]. It was then explored in a combined regimen with lenalidomide and dexamethasone (MAIA) where an improved PFS (hazard ratio 0.56), depth of response [complete response (CR) rate 47.6% vs 24.9%] and MRD negativity rate (24.2% vs 7.3%) was shown in daratumumab arm compared to Rd-only arm [15].
Transplant-eligible Patients:
In CASSIOPEA trial, adding daratumumab to bortezomib, thalidomide and dexamethasone (D-VTd) was shown to significantly improve depth of response [stringent complete response (sCR) rate after day 100 post-transplant was 29% in D-VTd group vs 20% in VTd group], MRD negativity rate and PFS (hazard ratio 0.47) in transplant-eligible patients [16]. Daratumumab in combination with lenalidomide, bortezomib and dexamethasone (D-RVd), which is a more commonly used regimen in the United States, was also evaluated in newly diagnosed transplant-eligible patients (GRIFFIN). D-RVd group had improved sCR rate at the end of post-ASCT consolidation (42% vs 32%), which deepened with follow up (median of 22 months; 62% vs 45%) as well as higher MRD negativity rate (51% vs 20%) [17]. Stem cell harvest was not impacted by adding daratumumab, although plerixafor was more frequently used in D-RVd group. The engraftment, post-transplant was comparable.
Subcutaneous formulation and rapid intravenous dosing
When first introduced, intravenous daratumumab was typically administered slowly to minimize infusion reactions, requiring several hours to complete the infusion. The first or second infusion require 8 hours or more and subsequent infusions are 3–4 hours, which was a drawback with initial approval of daratumumab. Studies of rapid daratumumab intravenous infusion have shown that 90 minutes infusion from the third dose onwards is safe [18, 19], thereby greatly reducing the time needed to complete daratumumab infusion. Subcutaneous formulation of daratumumab is an attractive option given less treatment burden as well as easier administration. Daratumumab with human recombinant hyaluronidase showed comparable efficacy and safety profile as intravenous formulation, with less infusion-related reactions (12.7% vs 34.5%) and much shorter administration time (5 minutes in the subcutaneous group vs 7 hours for the first infusion, 4.3 hours for the second and 3.4 hours thereafter in the intravenous group [20]. The dosing of the subcutaneous form is fixed at1800 mg.
Potential laboratory interference by daratumumab
There are several caveats in laboratory measurements in patients treated with daratumumab. As it binds to CD38 on red blood cells and results in positive indirect Coombs test, daratumumab is known to interfere with blood group serological testing [21] and hence, transfusion laboratories need to be made aware so they can use alternate techniques to eliminate interference [22, 23]. Secondly, due to its nature as IgGk antibody, it may interfere with quantification of monoclonal immunoglobulin (M-protein) in patients with IgGk MM [24]. In this case, a mass spectrometry assay or daratumumab interference reflex assay (DIRA) can differentiate M-protein with daratumumab [24, 25], although these tests are not widely available. Thirdly, if anti-CD38 antibody was used for the measurement of MRD by flow cytometry, having daratumumab bound to CD38-positive cells may preclude the detection of residual disease [26].
Isatuximab
Isatuximab is another anti-CD38 antibody with similar mechanism of action to daratumumab, except that isatuximab can induce direct apoptosis without cross-linking [27]. Whether this difference leads to better clinical outcomes or overcome resistance of other anti-CD38 antibodies is unknown to date.
A phase III trial (ICARIA) showed that RRMM patients who had received at least two previous lines of therapy including lenalidomide and a PI, but not anti-CD38 antibody, had a significantly longer PFS when they were randomized to isatuximab-Pd group compared to Pd group (11.5 months vs 6.5 months) [28]. The infusion schedule is simpler than daratumumab and the infusion time is shorter (median infusion time for isatuximab: 3.3 hours for the first dose, 2.9 hours for subsequent doses [29]). This difference will become less relevant now that subcutaneous formulation of daratumumab is approved. Subcutaneous version of isatuximab is currently being evaluated in clinical trials (NCT04045795).
Isatuximab is also being evaluated in combination with Kd (IKEMA). An interim analysis showed Isa-Kd arm had a significant higher rate of ≥VGPR (73% vs 56% in Kd arm) as well as higher MRD negativity rate (30% vs 13%) [30].
Anti-SLAMF7 antibodies
Elotuzumab
Elotuzumab was approved by FDA for treatment of relapsed myeloma in combination with lenalidomide shortly after daratumumab. It targets CS1 (cell-surface glycoprotein CD2 subset 1), also known as SLAMF7 (signaling lymphocyte activation molecule F7), which is expressed in over 90% across all MM patients irrespective of cytogenetic abnormalities and disease course [31, 32]. It is also expressed on NK cells. Elotuzumab works by induction of NK cell-mediated ADCC, direct activation of NK cells, and interruption of the SLAMF7-mediated adhesion of MM cells to bone marrow stromal cells [33]. It demonstrated no efficacy as a single agent in phase I trial [34]. However, as a combination with Rd, it showed a significantly better PFS (19.4 months vs 14.9 months) as well as ORR (79% vs 66%) [35]. The benefit of elotuzumab was also seen when combined with Pd [36]. When combined with PI, elotuzumab demonstrated an improved outcome in a phase II trial [37], although this combination has not obtained FDA approval at the time of writing this article. A phase III trial (ELOQUENT-1) that investigated elotuzumab combined with Rd as a frontline setting did not show a significant improvement in PFS [38]. Also, phase II trial that compared RVd with and without elotuzumab in high-risk patients (SWOG-1211) showed that the addition of elotuzumab did not improve patient outcomes [39]. Currently, most clinicians use elotuzumab in relapsed disease in combination with an IMiD in patients who are experiencing a gradual progression.
Other monoclonal antibodies under clinical investigation
There are many other monoclonal antibodies evaluated in clinical trials. Examples are summarized in Table 1.
Table 1:
Selected Monoclonal Antibodies Investigated in Clinical Trials in Multiple Myeloma
| Agent | Target | Clinical Trial | Phase | Single vs combination |
|---|---|---|---|---|
| CJM112 | IL-17 | NCT03111992 | I | Single/combination (Anti-PD1 or SMAC-mimetic) |
| BHQ880 | DKK-1 | NCT01302886, NCT01337752 | II | Single/combination (bor and dex) |
| Lirilumab | KIR | NCT02252263 | I | With elotuzumab |
| MOR202/TJ202 | CD38 | |||
| NCT03952091 | III | With len and dex | ||
| TAK-079 | CD38 | NCT03439280 | I/II | Single/combination (pom/dex) |
| TAK-573 | CD38 | NCT03215030 | I/II | Single (phase I) With dex (phase II) |
| SEA-BCMA | BCMA | NCT03582033 | I | With dex |
Bor: bortezomib, len: lenalidomide, pom: pomalidomide, dex: dexamethasone
Antibody-drug conjugates
ADCs are a promising entity, enabling incorporation of the specificity of antibody to target tumor cells and cell killing of conjugated cytotoxins (Figure 1). Ideally, ADCs remain intact in systemic circulation and disassemble when internalized into the target cell, releasing the cytotoxic payload. ADCs have been successful in the treatment of lymphoma, acute leukemia and breast cancer thus far and many more are under clinical development [40]. In the myeloma field, belantamab mafodotin was approved in the United States in August 2020, and other ADCs are under clinical investigation.
Belantamab mafodotin
Belantamab mafodotin is a first-in-class anti-BCMA immunoconjugate, consisting of anti-BCMA monoclonal antibody conjugated to tubulin polymerization inhibitor monomethyl auristatin F (MMAF). BCMA, B-cell maturation antigen, belongs to the tumor necrosis factor receptor superfamily [41]. It is expressed exclusively on antibody-producing B-cells, playing a central role in B-cell maturation and differentiation into plasma cells [42]. BCMA is more highly expressed on myeloma cells than normal plasma cells [43–45], making it a promising antigen for targeted immunotherapy.
In phase I study, single-agent belantamab mafodotin induced ORR of 60% in patients with heavily pre-treated RRMM [46, 47]. In a phase II study, 196 patients with RRMM were randomly assigned to receive 2.5 mg/kg belantamab mafodotin or 3.4 mg/kg [48]. The median previous lines of therapy was 7 and 6, respectively and ≥80% of the patients had received >4 lines of therapy including proteasome inhibitor, IMiDs and anti-CD38 monoclonal antibody. The ORR was 31% in 2.5 mg/kg cohort and 34% in 3.4 mg/kg cohort. The most common grade ≥3 adverse events were keratopathy (27% in 2.5 mg/kg cohort and 21% in 3.4 mg/kg cohort), thrombocytopenia (20% and 33%), and anemia (20% and 40%). Infusion-related reactions of any grade occurred 21% and 16% of the patients with the 2.5 mg/kg and 3.4 mg/kg doses, respectively. The ocular toxicity presented as blurry vision and dry eye. Ophthalmic examination observed changes in corneal epithelium and patients had a dose reduction and/or delays. The mechanism of corneal injury is likely related to non-specific uptake of the MMAF by actively dividing corneal epithelial cells. Notably, other ADCs containing MMAF were also reported to have corneal toxicity [49]. Corticosteroid eye drops were ineffective for prophylaxis and the median time to resolution was 71 days in 2.5 mg/kg group and 96 days in 3.4 mg/kg group. Permanent vision loss was not reported. Based on these data, the FDA-recommended dose is 2.5 mg/kg every 3 weeks. Because of the potential ocular toxicity, patients who receive belantamab mafodotin require ophthalmic examinations at baseline, prior to each dose, and promptly for worsening symptoms.
MEDI 2228
MEDI 2228 is another ADC comprised of anti-BCMA antibody that preferentially binds to membrane-bound BCMA versus soluble BCMA, and pyrrrolobenzodiazepine (PBD) dimer, which cross-links DNA and leads to cell death once it is internalized and cleaved. In a phase I study (NCT03489525), among a cohort that received maximum tolerated dose (MTD) of 0.14 mg/kg (n=41), ORR was 66% with median duration of response of 6 months [50]. A further study with an expansion cohort is ongoing.
Other antibody-drug conjugates under clinical investigation
Other examples of antibody-drug conjugates that are currently investigated in clinical settings are listed in Table 2.
Table 2:
Selected Antibody-drug Conjugates Investigated in Clinical Trials in Multiple Myeloma
| Agent | Target | Clinical Trial | Phase | Single vs combination |
|---|---|---|---|---|
| CC99712 | BCMA | NCT04036461 | I | Single |
| AMG 224 | BCMA | NCT02561962 | I | Single |
| STRO-001 | CD74 | NCT03424603 | I | Single |
| FOR46 | CD46 | NCT03650491 | I | Single |
| TAK-169 | CD38 | NCT04017130 | I | Single |
Bispecific T-cell Recruiting Antibodies
Redirecting T cell activity to target tumor cells holds much promise for the treatment of hematological malignancies. Two main approaches of T cell redirection involve engineering T cells to express CARs or constructing recombinant protein that can physically link T cells and tumor cells. Bispecific antibodies can recognize two specific antigens or epitopes. They typically target a tumor specific antigen and the second receptor targets T cells (often the CD3 subunit of the T cell receptor) [51, 52]. By allowing them to bind to two unique antigens simultaneously, BsAbs can facilitate cell-cell interaction thereby activate T cells to exert cytotoxicity (Figure 1). Notably, the T cell responses are not restricted by T cell receptor specificity, presence of MHC class or additional T cell co-stimuli [53]. Unlike CAR-T cells, BsAbs are off-the-shelf products and do not require manufacturing process for individual patients. BsAbs targeting MM is an evolving field, and several constructs are under clinical investigation.
Anti-BCMA Bispecific Antibodies
As discussed in the previous section, BCMA is a promising target of MM. Anti-BCMA/CD3 BsAb construct, AMG 420, was studied in a phase I study [54]. AMG 420 was given 4 weeks continuously in 6-week cycles up to 10 cycles in 42 RRMM patients, who had ≥ 2 lines of prior therapy and no extramedullary disease. The ORR of all cohort was 31%. In a subgroup that received MTD of 400 ug/d (n=19), the ORR was 50%, with 50% MRD-negative rate. Among those who received at or below MTD dose (n=39), 15 developed grade 1 or 2 cytokine release syndrome (CRS). Due to the requirement of continuous infusion, the manufacturer discontinued further development of AMG 420 and is pursuing the next-generation AMG 701, which has an extended half-life allowing intermittent (weekly) dosing. A phase I study (NCT03287908) is demonstrating promising results, with ORR of 36% at doses of 3–18 mg (n=55); 83% at 9 mg doses with earlier escalation (n=6). CRS was observed in 65% among total of 85 patients, with grade 3 in 8 patients [55].
Teclistamab is a humanized anti-BCMA/CD3 BsAb that is being evaluated in a phase I study (NCT03145181). According to the data presented in December 2020, 149 patients with median prior lines of therapy of 6 (2–14) received either intravenous (IV; n=84) or subcutaneous (SC; n=65) teclistamab. CRS was observed in 55% of the patients, all grade 1–2. Neurotoxicity was observed in 7 patients (5%), with 2 patients with grade ≥3 (both IV dosing). Recommended phase 2 dose (RP2D) was determined as 1500 μg/kg SC and among those who received this dosing (n=22), the ORR was 73% with ≥VGPR was 55% [56]. A phase 2 study is currently recruiting (NCT04557098).
TNB-383B is another humanized anti-BCMA/CD3 BsAb armed with a unique anti-CD3 moiety and two anti-BCMA domains on a silenced IgG4 backbone with aims of reducing cytokine release, targeting cell-surface BCMA preferentially versus soluble BCMA, and preventing nonspecific T-cell activation, with a longer half-life [57]. This allows every 3 weeks dosing as opposed to weekly dosing in the other products with a short hospitalization for the first dose only. A phase I study showed ORR of 80% at doses ≥40 mg, with 73% ≥VGPR. CRS was observed in 45% of the 58 enrolled patients, all grade 1–2 [58].
REGN5458 is also an anti-BCMA/CD3 BsAb that is evaluated in phase I trial (NCT03761108) [59]. The summary of the current data is shown on Table 3.
Table 3:
Selected recent data from anti-BCMA/CD3 bispecific antibodies in clinical trials
| AMG-701 | Teclistamab | TNB-383B | REGN5458 | CC-93269 [60] | PF-3135 [61] | |
|---|---|---|---|---|---|---|
| Schedule | Weekly IV | Weekly IV or SC | IV q3w | Weekly IV | Weekly IV | Weekly SC |
| Patients | n=85 | n=149 (84 IV, 65 SC) | n=58 | n=49 | n=30 | n=30 |
| Median prior lines | 6 | 6 | 6 | 5 | 5 | 8 |
| ORR at therapeutic dose | 36% (3–18 mg; n=55) |
73% (1500 μg/kg; n=22) |
80% (40–60 mg; n=15) |
62.5 % (96 mg; n=8) |
89% (10 mg; n=9) |
80% (≥215 μg/kg) |
| Duration of response | NR | NR | NR | 6 months | NR | NR |
| CRS [All (Gr 3+)] | 65% (9%) | 55% (0%) | 45% (0%) | 39% (0%) | 77% (3%) | 73% (0%) |
| Neurotoxicity [All (Gr 3+)] | 6 patients* | 5% (1%; in IV cohort) | NR | 12% (0%) | NR | 20% (0%) |
NR: Not reported
6 patients reported in abstract in n=75, data not available from oral presentation with n=85
Anti-GPRC5D Bispecific Antibody
GPRC5D (G-protein coupled receptor family C group 5 member D) is a novel antigen that is expressed on MM cells, but with very limited expression on healthy tissues (hair follicles) [62, 63]. Talquetamab is a first-in-class anti-GPRC5D/CD3 BsAb that redirects T cells to GPRC5D-expressing myeloma cells. A phase I study (NCT03399799) demonstrated RP2D of 405 μg/kg SC with 69% ORR in this cohort (n=13). CRS was observed in 54% overall (n=157) with no grade ≥ 3 with SC dosing. Neurotoxicity was observed in 6% overall. Injection-site reactions occurred in 18% of the patients, all events were in grade 1–2 [64]. Two patients experienced a grade 3 or higher maculopapular rash, which was considered a dose-limiting toxicity. One patient experienced elevated lipase which was also considered a dose-limiting toxicity. Nail disorders were noted in 18% patients overall and 21% patients at the recommended phase 2 dose.
Anti-FcRH5 Bispecific Antibody
FcRH5 (Fc receptor-homolog 5) is expressed exclusively in the B cell lineage and retained in plasma cells [65]. One study demonstrated that all the samples from myeloma patients (8555 samples from 544 donors) had an expression of FcRH5 on MM cells [66], making it an attractive target. Cevostamab is a humanized anti-FcRH5/CD3 BsAb that is being evaluated in a phase I study (NCT03275103). In patients who received active dosing (≥3.6 mg step-up dose with ≥20 mg target dose; n=34), ORR was 53% with ≥VGPR of 32%. CRS was observed in 76% of all enrolled patients (n=53), with grade 3 in 1 patient (2%) [67]. A dose-escalation and expansion phase is ongoing.
Conclusions
The treatment of multiple myeloma has entered a new era with the emergence of antibody-based therapy. CD38-targeted mAbs, together with backbone regimen including PI and IMiDs have made responses more durable and improved survival outcomes in both frontline and relapsed/refractory settings. Arming naked antibodies with cytotoxins has shown a great effect, most exemplified by anti-BCMA ADCs, which induced a deep response as a single agent in heavily pretreated patients, including those who had progressed through anti-CD38 mAb, PI and IMiDs. Anti-BCMA BsAbs are an exciting new modality, with promising early data in clinical trials. Long-term effects of these new agents are yet to be determined, however based on the current data, we expect that they will be increasingly evaluated in earlier lines of therapy and/or in combination with other anti-myeloma agents (i.e. PI, IMiDs or novel agents under development).
Despite their promising effect, antibody-based therapy also has its caveats and limitations. First, on- or off-target adverse effects should be monitored closely. This was most illustrated in ocular toxicity by belantamab mafodotin. We expect that the identification of the mechanisms of toxicity and modification of the payload and/or linker would decrease unwanted effects in the future. We should also keep in mind that it is crucial to cautiously review the toxicity profile when we add other anti-MM drugs and choose drugs with non-overlapping toxicity.
Second, the expression of antigens by MM cells may affect the response to antibody-based therapy. A prior study showed that the pretreatment expression of CD38 correlated with patients’ response to daratumumab [68]. Interestingly, the frequency of pretreatment CD38-positive Tregs also correlated with durable response to daratumumab [69], indicating that antigen expression not only on tumor cells but also in tumor microenvironment may be an important factor in determining the response.
Finally, acquired resistance to antibody-based therapy may occur. For example, resistance to daratumumab may develop by downregulation of the expression of CD38, emergence of resistance to cell-killing mechanism, or alteration of immune system [70]. For ADCs, in addition to downregulation of antigen expression, defects of internalization and trafficking pathways, development of payload efflux pumps, impairment of lysosomal function and apoptotic dysregulation may also play a role in the development of resistance [71]. Additionally, MM is characterized by substantial intraclonal heterogeneity, and outgrowth of a subclone with low expression of antigen or acquired resistance mechanism may also result in disease progression. A better understanding of the primary and secondary resistance would facilitate the improvements in drug design and provide an insight to more effective combination strategies. For instance, all-trans retinoic acid increased CD38 expression and decreased complement-inhibitory proteins (i.e. CD55 and CD59) on MM cells in patients who developed daratumumab resistance, resulting in enhancement of daratumumab-mediated complement-dependent cytotoxicity [68]. Also, a combination of belantamab mafodotin with a gamma-secretase inhibitor resulted in blocking of BCMA shedding, thereby increasing the cell surface level of BCMA in a preclinical study [72]. This is currently being evaluated in a clinical trial (NCT04126200). Identification of novel targets is also important in addressing antigen escape.
At present, belantamab mafodotin is the only FDA approved anti-BCMA therapy. We expect that various BCMA-targeted agents in different platforms (ADC, BiTE, CAR-T) will be approved in the near future. Efficacy of using these agents in sequence and if so, the optimal sequencing order remains to be determined. Loss of BCMA expression or mutation in BCMA gene located at 16p locus is a potential mechanism of tumor escape [73]. In patients experiencing downregulation of functional BCMA, sequential therapy may not be feasible. It is not known whether BCMA expression would recover after a certain time interval following anti-BCMA therapy. In patients with ongoing BCMA expression, sequential therapy may be considered, though efficacy remains unknown. The optimal timing of such therapies in the course of myeloma treatment and integration of these agents into current therapeutics for MM also remains to be determined. Ongoing clinical trials are investigating the combination of these agents with currently approved therapies, concurrently or in sequence. Randomized trials are also comparing these therapies, particularly CAR-T cell therapies against standard triplet regimens in earlier lines of relapse. GPRC5D and FcRH5 are attractive targets in patients relapsing after anti-BCMA therapies and more mature data are awaited.
In summary, the field of antibody-based therapy in MM is rapidly growing and holds promise for MM patients. We expect to see novel targets, better strategies for toxicity management, and improvement in design to enhance the potency of the drug while minimizing the toxicity. We also expect that antibody-based strategies will be used in earlier lines of therapy in the future.
ACKNOWLEDGEMENTS:
Figure 1 was created with BioRender.com
Surbhi Sidana: Consultancy and Research Funding: Janssen; Research Funding: Magenta Therapeutics, Allogene.
FUNDING:
SS was supported by: KL2TR003143, KL2 Mentored Career Development Program, Stanford Clinical Translational Science Award Program
Footnotes
CONFLICTS OF INTEREST:
Hitomi Hosoya; No conflicts of interest
REFERENCES:
- 1.Surveillance, E., and End Results (SEER) Program, https://seer.cancer.gov/statfacts/html/mulmy.html. Accessed on October 15, 2020.
- 2.van de Donk NWCJ, Richardson PG, and Malavasi F, CD38 antibodies in multiple myeloma: back to the future. Blood, 2018. 131(1): p. 13–29. [DOI] [PubMed] [Google Scholar]
- 3.Hogan KA, Chini CCS, and Chini EN, The Multi-faceted Ecto-enzyme CD38: Roles in Immunomodulation, Cancer, Aging, and Metabolic Diseases. Front Immunol, 2019. 10: p. 1187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.van de Donk NW, et al. , Monoclonal antibodies targeting CD38 in hematological malignancies and beyond. Immunol Rev, 2016. 270(1): p. 95–112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Krejcik J, et al. , Daratumumab depletes CD38+ immune regulatory cells, promotes T-cell expansion, and skews T-cell repertoire in multiple myeloma. Blood, 2016. 128(3): p. 384–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lokhorst HM, et al. , Targeting CD38 with Daratumumab Monotherapy in Multiple Myeloma. N Engl J Med, 2015. 373(13): p. 1207–19. [DOI] [PubMed] [Google Scholar]
- 7.Lonial S, et al. , Daratumumab monotherapy in patients with treatment-refractory multiple myeloma (SIRIUS): an open-label, randomised, phase 2 trial. Lancet, 2016. 387(10027): p. 1551–1560. [DOI] [PubMed] [Google Scholar]
- 8.Usmani SZ, et al. , Clinical efficacy of daratumumab monotherapy in patients with heavily pretreated relapsed or refractory multiple myeloma. Blood, 2016. 128(1): p. 37–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.**.Dimopoulos MA, et al. , Daratumumab, Lenalidomide, and Dexamethasone for Multiple Myeloma. N Engl J Med, 2016. 375(14): p. 1319–1331. [DOI] [PubMed] [Google Scholar]; This study was the first trial to incorporate daratumumab into IMiD-based therapy and showed efficacy.
- 10.Palumbo A, et al. , Daratumumab, Bortezomib, and Dexamethasone for Multiple Myeloma. N Engl J Med, 2016. 375(8): p. 754–66. [DOI] [PubMed] [Google Scholar]
- 11.Dimopoulos M, et al. , Carfilzomib, dexamethasone, and daratumumab versus carfilzomib and dexamethasone for patients with relapsed or refractory multiple myeloma (CANDOR): results from a randomised, multicentre, open-label, phase 3 study. Lancet, 2020. 396(10245): p. 186–197. [DOI] [PubMed] [Google Scholar]
- 12.Chari A, et al. , Daratumumab plus pomalidomide and dexamethasone in relapsed and/or refractory multiple myeloma. Blood, 2017. 130(8): p. 974–981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Dimopoulos MA, et al. , Apollo: Phase 3 Randomized Study of Subcutaneous Daratumumab Plus Pomalidomide and Dexamethasone (D-Pd) Versus Pomalidomide and Dexamethasone (Pd) Alone in Patients (Pts) with Relapsed/Refractory Multiple Myeloma (RRMM). Blood, 2020. 136(Supplement 1): p. 5–6. [Google Scholar]
- 14.Mateos MV, et al. , Daratumumab plus Bortezomib, Melphalan, and Prednisone for Untreated Myeloma. N Engl J Med, 2018. 378(6): p. 518–528. [DOI] [PubMed] [Google Scholar]
- 15.**.Facon T, et al. , Daratumumab plus Lenalidomide and Dexamethasone for Untreated Myeloma. N Engl J Med, 2019. 380(22): p. 2104–2115. [DOI] [PMC free article] [PubMed] [Google Scholar]; This trial demonstrated addition of daratumumab to Rd improved PFS in upfront setting in transplant-ineligible patients.
- 16.**.Moreau P, et al. , Bortezomib, thalidomide, and dexamethasone with or without daratumumab before and after autologous stem-cell transplantation for newly diagnosed multiple myeloma (CASSIOPEIA): a randomised, open-label, phase 3 study. Lancet, 2019. 394(10192): p. 29–38. [DOI] [PubMed] [Google Scholar]; This trial showed efficacy of incorporating daratumumab to VTd regimen in newly diagnosed transplant-eligible patients.
- 17.**.Voorhees PM, et al. , Daratumumab, lenalidomide, bortezomib, and dexamethasone for transplant-eligible newly diagnosed multiple myeloma: the GRIFFIN trial. Blood, 2020. 136(8): p. 936–945. [DOI] [PMC free article] [PubMed] [Google Scholar]; This study added daratumumab to RVd regimen in newly diagnosed transplant-eligible patients and showed improved sCR rate as well as MRD negativity rate.
- 18.Barr H, et al. , Ninety-minute daratumumab infusion is safe in multiple myeloma. Leukemia, 2018. 32(11): p. 2495–2518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lombardi J, et al. , Safety of ninety-minute daratumumab infusion. J Oncol Pharm Pract, 2020: p. 1078155220951231. [DOI] [PubMed] [Google Scholar]
- 20.Mateos MV and Usmani SZ, Subcutaneous versus intravenous daratumumab in multiple myeloma - Authors’ reply. Lancet Haematol, 2020. 7(8): p. e559. [DOI] [PubMed] [Google Scholar]
- 21.United States Food and Drug Administration. Darzalex: label information. 2018. https://www.accessdata.fda.gov/drugsatfda_docs/label/2018/761036s013lbl.pdf Accessed on Oct 13, 2020.
- 22.Murphy MF, et al. , Interference of New Drugs with Compatibility Testing for Blood Transfusion. N Engl J Med, 2016. 375(3): p. 295–6. [DOI] [PubMed] [Google Scholar]
- 23.Chapuy CI, et al. , International validation of a dithiothreitol (DTT)-based method to resolve the daratumumab interference with blood compatibility testing. Transfusion, 2016. 56(12): p. 2964–2972. [DOI] [PubMed] [Google Scholar]
- 24.van de Donk NW, et al. , Interference of daratumumab in monitoring multiple myeloma patients using serum immunofixation electrophoresis can be abrogated using the daratumumab IFE reflex assay (DIRA). Clin Chem Lab Med, 2016. 54(6): p. 1105–9. [DOI] [PubMed] [Google Scholar]
- 25.Mills JR, et al. , A universal solution for eliminating false positives in myeloma due to therapeutic monoclonal antibody interference. Blood, 2018. 132(6): p. 670–672. [DOI] [PubMed] [Google Scholar]
- 26.Flores-Montero J, et al. , Next Generation Flow for highly sensitive and standardized detection of minimal residual disease in multiple myeloma. Leukemia, 2017. 31(10): p. 2094–2103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Moreno L, et al. , The Mechanism of Action of the Anti-CD38 Monoclonal Antibody Isatuximab in Multiple Myeloma. Clin Cancer Res, 2019. 25(10): p. 3176–3187. [DOI] [PubMed] [Google Scholar]
- 28.Attal M, et al. , Isatuximab plus pomalidomide and low-dose dexamethasone versus pomalidomide and low-dose dexamethasone in patients with relapsed and refractory multiple myeloma (ICARIA-MM): a randomised, multicentre, open-label, phase 3 study. Lancet, 2019. 394(10214): p. 2096–2107. [DOI] [PubMed] [Google Scholar]
- 29.Mikhael J, et al. , A phase 1b study of isatuximab plus pomalidomide/dexamethasone in relapsed/refractory multiple myeloma. Blood, 2019. 134(2): p. 123–133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Martin T, et al. , Depth of Response and Response Kinetics of Isatuximab Plus Carfilzomib and Dexamethasone in Relapsed Multiple Myeloma: Ikema Interim Analysis. Blood, 2020. 136(Supplement 1): p. 7–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Hsi ED, et al. , CS1, a potential new therapeutic antibody target for the treatment of multiple myeloma. Clin Cancer Res, 2008. 14(9): p. 2775–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Tai YT, et al. , Anti-CS1 humanized monoclonal antibody HuLuc63 inhibits myeloma cell adhesion and induces antibody-dependent cellular cytotoxicity in the bone marrow milieu. Blood, 2008. 112(4): p. 1329–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Veillette A and Guo H, CS1, a SLAM family receptor involved in immune regulation, is a therapeutic target in multiple myeloma. Crit Rev Oncol Hematol, 2013. 88(1): p. 168–77. [DOI] [PubMed] [Google Scholar]
- 34.Zonder JA, et al. , A phase 1, multicenter, open-label, dose escalation study of elotuzumab in patients with advanced multiple myeloma. Blood, 2012. 120(3): p. 552–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Lonial S, et al. , Elotuzumab Therapy for Relapsed or Refractory Multiple Myeloma. N Engl J Med, 2015. 373(7): p. 621–31. [DOI] [PubMed] [Google Scholar]
- 36.Dimopoulos MA, et al. , Elotuzumab plus Pomalidomide and Dexamethasone for Multiple Myeloma. N Engl J Med, 2018. 379(19): p. 1811–1822. [DOI] [PubMed] [Google Scholar]
- 37.Jakubowiak A, et al. , Randomized phase 2 study: elotuzumab plus bortezomib/dexamethasone vs bortezomib/dexamethasone for relapsed/refractory MM. Blood, 2016. 127(23): p. 2833–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Bristol Myers Squibb Reports Primary Results of ELOQUENT-1 Study Evaluating Empliciti (elotuzumab) Plus Revlimid (lenalidomide) and Dexamethasone in Patients with Newly Diagnosed, Untreated Multiple Myeloma (https://bit.ly/3cJ17P9) Accessed on October 15, 2020.
- 39.Usmani SZ, et al. , Bortezomib, lenalidomide, and dexamethasone with or without elotuzumab in patients with untreated, high-risk multiple myeloma (SWOG-1211): primary analysis of a randomised, phase 2 trial. Lancet Haematology, 2021. 8(1): p. e45–e54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Birrer MJ, et al. , Antibody-Drug Conjugate-Based Therapeutics: State of the Science. J Natl Cancer Inst, 2019. 111(6): p. 538–549. [DOI] [PubMed] [Google Scholar]
- 41.Madry C, et al. , The characterization of murine BCMA gene defines it as a new member of the tumor necrosis factor receptor superfamily. Int Immunol, 1998. 10(11): p. 1693–702. [DOI] [PubMed] [Google Scholar]
- 42.Tai YT and Anderson KC, Targeting B-cell maturation antigen in multiple myeloma. Immunotherapy, 2015. 7(11): p. 1187–99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Claudio JO, et al. , A molecular compendium of genes expressed in multiple myeloma. Blood, 2002. 100(6): p. 2175–86. [DOI] [PubMed] [Google Scholar]
- 44.Tarte K, et al. , Gene expression profiling of plasma cells and plasmablasts: toward a better understanding of the late stages of B-cell differentiation. Blood, 2003. 102(2): p. 592–600. [DOI] [PubMed] [Google Scholar]
- 45.Novak AJ, et al. , Expression of BCMA, TACI, and BAFF-R in multiple myeloma: a mechanism for growth and survival. Blood, 2004. 103(2): p. 689–94. [DOI] [PubMed] [Google Scholar]
- 46.Trudel S, et al. , Targeting B-cell maturation antigen with GSK2857916 antibody-drug conjugate in relapsed or refractory multiple myeloma (BMA117159): a dose escalation and expansion phase 1 trial. Lancet Oncol, 2018. 19(12): p. 1641–1653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Trudel S, et al. , Antibody-drug conjugate, GSK2857916, in relapsed/refractory multiple myeloma: an update on safety and efficacy from dose expansion phase I study. Blood Cancer J, 2019. 9(4): p. 37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.**.Lonial S, et al. , Belantamab mafodotin for relapsed or refractory multiple myeloma (DREAMM-2): a two-arm, randomised, open-label, phase 2 study. Lancet Oncol, 2020. 21(2): p. 207–221. [DOI] [PubMed] [Google Scholar]; This trial evaluated belantamab mafodotin in relapsed/refractory setting and showed efficacy, leading to the FDA approval of the drug.
- 49.Eaton JS, et al. , Ocular Adverse Events Associated with Antibody-Drug Conjugates in Human Clinical Trials. J Ocul Pharmacol Ther, 2015. 31(10): p. 589–604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Kumar SK, et al. , Phase 1, First-in-Human Study of MEDI2228, a BCMA-Targeted ADC in Patients with Relapsed/Refractory Multiple Myeloma. Blood, 2020. 136(Supplement 1): p. 26–27. [Google Scholar]
- 51.Huehls AM, Coupet TA, and Sentman CL, Bispecific T-cell engagers for cancer immunotherapy. Immunol Cell Biol, 2015. 93(3): p. 290–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Lejeune M, et al. , Bispecific, T-Cell-Recruiting Antibodies in B-Cell Malignancies. Front Immunol, 2020. 11: p. 762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Baeuerle PA and Reinhardt C, Bispecific T-cell engaging antibodies for cancer therapy. Cancer Res, 2009. 69(12): p. 4941–4. [DOI] [PubMed] [Google Scholar]
- 54.Topp MS, et al. , Anti-B-Cell Maturation Antigen BiTE Molecule AMG 420 Induces Responses in Multiple Myeloma. J Clin Oncol, 2020. 38(8): p. 775–783. [DOI] [PubMed] [Google Scholar]
- 55.Harrison SJ, et al. , A Phase 1 First in Human (FIH) Study of AMG 701, an Anti-B-Cell Maturation Antigen (BCMA) Half-Life Extended (HLE) BiTE® (bispecific T-cell engager) Molecule, in Relapsed/Refractory (RR) Multiple Myeloma (MM). Blood, 2020. 136(Supplement 1): p. 28–29. [Google Scholar]
- 56.**.Garfall AL, et al. , Updated Phase 1 Results of Teclistamab, a B-Cell Maturation Antigen (BCMA) × CD3 Bispecific Antibody, in Relapsed and/or Refractory Multiple Myeloma (RRMM). Blood, 2020. 136(Supplement 1): p. 27–27. [Google Scholar]; Teclistamab, anti-BCMA/CD3 bispecific antibody, was evaluated in this phase I study. We expect that bispecific antibodies will be incorporated in the treatment of multiple myeloma in the near future.
- 57.Buelow B, et al. , Development of a fully human t-cell engaging bispecific antibody for the treatment of multiple myeloma. Journal of Clinical Oncology, 2018. 36(5_suppl): p. 60–60. [Google Scholar]
- 58.Rodriguez C, et al. , Initial Results of a Phase I Study of TNB-383B, a BCMA × CD3 Bispecific T-Cell Redirecting Antibody, in Relapsed/Refractory Multiple Myeloma. Blood, 2020. 136(Supplement 1): p. 43–44. [Google Scholar]
- 59.Madduri D, et al. , REGN5458, a BCMA × CD3 Bispecific Monoclonal Antibody, Induces Deep and Durable Responses in Patients with Relapsed/Refractory Multiple Myeloma (RRMM). Blood, 2020. 136(Supplement 1): p. 41–42. [Google Scholar]
- 60.Costa LJ, et al. , First Clinical Study of the B-Cell Maturation Antigen (BCMA) 2+1 T Cell Engager (TCE) CC-93269 in Patients (Pts) with Relapsed/Refractory Multiple Myeloma (RRMM): Interim Results of a Phase 1 Multicenter Trial. Blood, 2019. 134(Supplement_1): p. 143–143. [Google Scholar]
- 61.Lesokhin AM, et al. , Preliminary Safety, Efficacy, Pharmacokinetics, and Pharmacodynamics of Subcutaneously (SC) Administered PF-06863135, a B-Cell Maturation Antigen (BCMA)-CD3 Bispecific Antibody, in Patients with Relapsed/Refractory Multiple Myeloma (RRMM). Blood, 2020. 136(Supplement 1): p. 8–9.32614959 [Google Scholar]
- 62.Smith EL, et al. , GPRC5D is a target for the immunotherapy of multiple myeloma with rationally designed CAR T cells. Sci Transl Med, 2019. 11(485). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Pillarisetti K, et al. , A T-cell-redirecting bispecific G-protein-coupled receptor class 5 member D × CD3 antibody to treat multiple myeloma. Blood, 2020. 135(15): p. 1232–1243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Chari A, et al. , A Phase 1, First-in-Human Study of Talquetamab, a G Protein-Coupled Receptor Family C Group 5 Member D (GPRC5D) × CD3 Bispecific Antibody, in Patients with Relapsed and/or Refractory Multiple Myeloma (RRMM). Blood, 2020. 136(Supplement 1): p. 40–41. [Google Scholar]
- 65.Polson AG, et al. , Expression pattern of the human FcRH/IRTA receptors in normal tissue and in B-chronic lymphocytic leukemia. Int Immunol, 2006. 18(9): p. 1363–73. [DOI] [PubMed] [Google Scholar]
- 66.Li J, et al. , Membrane-Proximal Epitope Facilitates Efficient T Cell Synapse Formation by Anti-FcRH5/CD3 and Is a Requirement for Myeloma Cell Killing. Cancer Cell, 2017. 31(3): p. 383–395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Cohen AD, et al. , Initial Clinical Activity and Safety of BFCR4350A, a FcRH5/CD3 T-Cell-Engaging Bispecific Antibody, in Relapsed/Refractory Multiple Myeloma. Blood, 2020. 136(Supplement 1): p. 42–43. [Google Scholar]
- 68.Nijhof IS, et al. , CD38 expression and complement inhibitors affect response and resistance to daratumumab therapy in myeloma. Blood, 2016. 128(7): p. 959–70. [DOI] [PubMed] [Google Scholar]
- 69.Kitadate A, et al. , Pre-treatment CD38-positive regulatory T cells affect the durable response to daratumumab in relapsed/refractory multiple myeloma patients. Haematologica, 2020. 105(1): p. e37–e40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Saltarella I, et al. , Mechanisms of Resistance to Anti-CD38 Daratumumab in Multiple Myeloma. Cells, 2020. 9(1). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.García-Alonso S, Ocaña A, and Pandiella A, Resistance to Antibody-Drug Conjugates. Cancer Res, 2018. 78(9): p. 2159–2165. [DOI] [PubMed] [Google Scholar]
- 72.Eastman S, et al. , Synergistic Activity of Belantamab Mafodotin (anti-BCMA immuno-conjugate) with PF-03084014 (gamma-secretase inhibitor) in Bcma-Expressing Cancer Cell Lines. 2019. p. 4401. [Google Scholar]
- 73.Samur MK, et al. , Biallelic loss of BCMA as a resistance mechanism to CAR T cell therapy in a patient with multiple myeloma. Nat Commun, 2021. 12(1): p. 868. [DOI] [PMC free article] [PubMed] [Google Scholar]


