Objective:
This study sought to clarify cancer risk in fighter aviators.
Methods:
US Air Force officers who served between 1970 and 2004 were followed through 2018 for incidence and mortality of 10 cancers: colon and rectum; pancreas; melanoma skin; prostate; testis; urinary bladder; kidney and renal pelvis; brain and other nervous system; thyroid; and non-Hodgkin lymphoma. Fighter aviators were compared with other officers and the general US population.
Results:
Compared with other officers, male fighter aviators had greater adjusted odds of developing testis, melanoma skin, and prostate cancers; mortality odds were similar for all cancers. When compared with the US population, male fighter aviators were more likely to develop and die from melanoma skin cancer, prostate cancer, and non-Hodgkin lymphoma.
Conclusions:
Military fighter aviation may be associated with slightly increased risk of certain cancers.
Keywords: military personnel, neoplasms, pilots
The association between military aviation and cancer risk is unclear. Investigations in Bulgaria,1 Sweden,2 and the United States3 have reported similar cancer rates between military aviators and their respective civilian populations, except a higher rate of melanoma skin cancer in US aviators3 and urinary bladder cancer in US and Bulgarian aviators.1,3 When compared to the narrower population of their military peers, US aviators were found to have higher rates of testis and urinary bladder cancer in one retrospective cohort study3 and greater odds of testis cancer in one case–control study,4 but these findings were not replicated in more recent US cohort studies.5–7 Another case–control study found an elevated crude odds of brain cancer in aviators, but adjustment for military rank negated statistical significance.8
Several concerns limit the generalizability of prior military aviation studies. First, each featured a relatively small number of cases. The largest cancer-specific sample size was 73 cases of prostate cancer7; most cancer counts were in the single digits. Second, they had variable inclusion criteria regarding airframe type and seat position, such as combining fixed-wing and rotary-wing airframes or restricting to pilots proper, thus complicating generalizability. Third, none incorporated cancer mortality, which may be a more relevant outcome.
In light of these concerns, the US Congress directed the Secretary of Defense to investigate cancer outcomes in military aviators.9 This study was designed to determine the incidence of and mortality from 10 high-interest cancers among US Air Force fighter aviators, to include backseat aircrew, and how this risk compares to other US Air Force officers and to the general US population. The objective was to provide conclusive findings to health care policymakers who develop force health protection and preventive strategies.
MATERIALS AND METHODS
Study Design and Population
This is a retrospective cohort study comparing US Air Force fighter aviators to other US Air Force officers (an “internal” comparison group) and to the general US population (an “external” comparison group). The term “fighter aviator” is used to designate both pilots and backseat aircrew. Fighter aviators and other officers were included if they served on Air Force active duty at any time between June 1970 and December 2004; they were followed for outcomes through 2018. Air Force personnel data are unavailable prior to June 1970. Demographic information was received from the Air Force Personnel Center. The personnel analyst coded officers as fighter aviators if they had at least 100 hours in any seat of any fighter airframe (n = 34,679) or a Rated Distribution and Training Management code or a Major Weapon System code consistent with fighter aviation (n = 308).
Demographic and Military Variables
The received personnel dataset included name, social security number, birth date, sex, race, ethnicity (ie, Hispanic or non-Hispanic), and military entrance and exit dates, when available. Some missing birth dates were included by utilizing other available databases. If the sex variable was missing, it was manually assigned according to first name. Race and ethnicity variables were merged and grouped as American Indian/Alaskan Native (AI/AN), Asian/Pacific Islander (PI), black, white, Hispanic, and other. Officers of Hispanic ethnicity were assigned to the Hispanic group, regardless of race, and those with multiple races (n = 7821) were assigned using the rarest group method: AI/AN, then Asian/PI, then black. Those missing both race and ethnicity (n = 27) were assigned to the other category. Merged race/ethnicity categories are hereafter shortened to “race.” The military start date was established as the entered active duty date, or, for those missing this date (n = 77), the total active federal commissioned service date. The military end date was defined as the last “as of” date, defined as either the date of separation, retirement, or last snapshot at which the person had a record.
Demographics of fighter aviators and other officers were compared with Student's t tests (for age) and chi-square tests (for sex and race), with statistical significance established at an alpha of 0.05. Since 99.4% of fighter aviators were male, and since two of the cancers exclusively afflict males, all results were stratified by sex.
Cancer Types
Based on the findings of previously published studies and of a voluntary member survey10 conducted by the Red River Valley Fighter Pilots Association, 10 cancers were deemed high-interest: colon and rectum; pancreas; melanoma skin; prostate; testis; urinary bladder; kidney and renal pelvis; brain and other nervous system; thyroid; and non-Hodgkin lymphoma.
Cancer Incidence: Definitions and Case Capture
The final personnel dataset was merged with diagnostic data in the Automated Central Tumor Registry (ACTUR), the Veterans Affairs Central Cancer Registry (VACCR), and the Defense Medical Surveillance System (DMSS). ACTUR, which is managed by the Joint Pathology Center, is the central cancer registry for the US Department of Defense and contains some but not all cancers diagnosed in service members since 1998. VACCR, which includes cases since 1995, is the equivalent registry for the US Veterans Health Administration. DMSS contains diagnostic codes received in TRICARE, the global health care program for US service members, retirees, and their families. Inpatient visit codes begin in 1990 and outpatient encounter codes begin in 1996.11 Cancer incidence was defined according to the Ninth and Tenth Revisions of the International Classification of Diseases (ICD-9 and ICD-10), using taxonomy from the Surveillance, Epidemiology, and End Results (SEER) Program.12 All cases identified in ACTUR and VACCR were included. Cases identified only in DMSS had to meet the oncological case definition established by the US Defense Health Agency.13 Based on a previous study that confirmed cases by chart review, the case definition has high positive predictive values for each of the studied cancers, from a high of 99.6% for testis cancer to a low of 78.1% for non-Hodgkin lymphoma.14 The incidence date was defined differently by source: the registry-assigned date for ACTUR and VACCR and the first date with a case-defining code for DMSS. An individual was counted once per cancer type.
Cancer Incidence: Fighter Aviators and Other Officers
To account for demographic differences, fighter aviators and other officers were assigned to categories, each of which reflected a distinct combination of sex, age at active duty entrance (in 5-year age groups), and age at incidence censoring (in 5-year age groups). Incidence censoring was based on either the incidence date, or, for those without cancer, the date of the last medical encounter in DMSS; for those without any encounters in DMSS, the last military record (“as of”) date was used. Other officers were included in the analysis if they “matched” to a demographic bucket with at least one fighter aviator. Crude and adjusted odds ratios (OR) with 95% confidence intervals (CI) were calculated for each cancer using logistic regression, with the significance level set at an alpha of 0.05. ORs were adjusted for race and exact age at incidence censoring. Mean age at cancer diagnosis (with 95% CIs), stratified by sex and adjusted for race, was calculated for fighter aviators and other officers and compared with Student's t test.
Cancer Incidence: Fighter Aviators and the General Population
Fighter aviators were assigned to one of 192 combinations of sex, race, and age at incidence censoring. Expected diagnosis counts of each cancer site were based on the experience of the general US population, indirectly adjusted for sex, race, and age. Expected counts were retrieved from the Cancer Query System, the online portal to the National Cancer Institute's DevCan program, which provides cancer risk statistics from the SEER 21 Registries Incidence and Mortality database in 3-year intervals for the inclusive years of 2000 to 2017.15 Risks from the six intervals were weighted equally. Per SEER methodology, ages were categorized in 5-year categories based on age at incidence censoring, beginning at age 20. The starting age was established at 20 years, rather than birth, because fighter aviators by definition would be at least 20-years old at the start of their military aviation careers. The ending age was established at both the beginning and end of each 5-year interval, with the expected diagnoses calculated as the mean of the two (eg, for Hispanic males aged 60.00 to 64.99 years, the number of expected cases was defined as the mean of expected cases at ages 60 and 65). Race was stratified as AI/AN, Asian/PI, black, white, Hispanic, and other, with expected cases in the lattermost category based on overall risk. Expected counts were calculated for each sex–age–race category, with probability rounded to five decimal places. Standardized incidence ratios (SIR) with 95% CIs were calculated using exact Poisson regression.16
Cancer Mortality: Definitions and Case Capture
The final personnel dataset was merged with death certificate data archived in the National Death Index (NDI) Plus, the central repository for deaths occurring in the United States since January 1979. Mortality data were available through December 2018. NDI Plus was accessed through the Joint Department of Veterans Affairs and Department of Defense Suicide Data Repository. Conditions on death certificates are coded in NDI Plus according to ICD-9 (before 1999) and ICD-10 (since 1999). Consistent with SEER methodology,17 the underlying cause of death recorded on the death certificate was used to define mortality.
Cancer Mortality: Fighter Aviators and Other Officers
As with the incidence analysis, fighter aviators and other officers were assigned to categories based on sex, age group at active duty entrance, and age group at mortality censoring, with other officers included if they matched to a demographic bucket with at least one fighter aviator. Mortality censoring was based on either the date of death (from any cause) or the last date of the outcome surveillance period (December 31, 2018). Crude and adjusted ORs with 95% CIs were calculated for each cancer using logistic regression, with the significance level set at an alpha of 0.05. ORs were adjusted for race and exact age at mortality censoring. Race-adjusted mean age at cancer death (with 95% CIs) was calculated for fighter aviators and other officers and compared with Student's t test.
Cancer Mortality: Fighter Aviators and the General Population
As with the incidence analysis, fighter aviators were assigned to one of 192 sex–race–age categories. Expected deaths from each cancer were based on the experience of the general US population, as described above. Standardized mortality ratios (SMR) with 95% CIs were calculated using exact Poisson regression.16
Statistical Analysis and Regulatory Approval
Data were analyzed using SAS version 9.4 (SAS Institute Inc., Cary, NC). For adjusted ORs that were statistically significant, E-values based on the point estimate were computed using an online calculator (https://www.evalue-calculator.com/), with the outcome type defined as a low-prevalence (<15%) OR. E-values quantify the potential impact of unmeasured confounding in observational studies.18 Attributable risks were also calculated for statistically significant adjusted ORs. This project was approved as exempt research by the Air Force Research Laboratory Institutional Review Board (#FWR20200049E).
RESULTS
Population
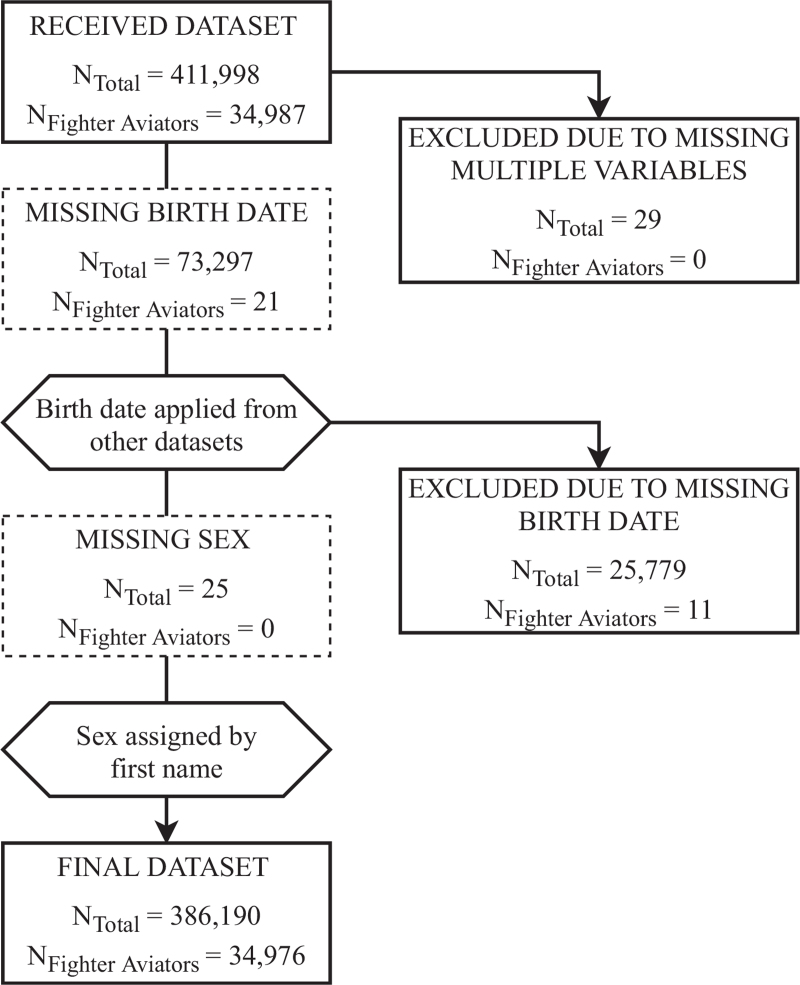
The received personnel dataset had 411,998 officers who served on active duty during the 35-year exposure period. Twenty-nine (0.007%) officers were excluded due to multiple missing variables. Birth dates were missing for 73,297 (17.8%) officers, but 47,518 (64.8%) were recovered by utilizing other available databases. The remaining 25,779 (6.3%) officers with missing birth dates were excluded from the final dataset. Missing sex was manually assigned according to first name for 25 officers. The final dataset had 386,190 officers, of whom 34,976 (9.1%) were fighter aviators (Fig. 1).
FIGURE 1.
Flow diagram of study participants, stratified by total US Air Force officers and fighter aviators.
Fighter aviators were more likely than other officers to be male (99.4% vs 83.7%) and white (80.6% vs 74.1%), and they had a larger outcome surveillance window: fighter aviators were approximately 24 months younger at active duty entrance, 93 months older at incidence censoring, and 51 months older at mortality censoring (P < 0.001 for all) (Table 1).
TABLE 1.
Demographic Characteristics, US Air Force Fighter Aviators, and Other Officers, 1970–2004 (N = 386,190)
| Fighter Aviators (n = 34,976) | Other Officers (n = 351,214) | P | |
| Age, mean (SD) | |||
| At active duty entrance | 23.1 (3.4) | 25.1 (4.5) | <0.001 |
| At incidence censoring∗ | 57.4 (17.7) | 49.7 (19.7) | <0.001 |
| At mortality censoring† | 64.1 (14.4) | 59.8 (16.0) | <0.001 |
| Sex, no. (%) | |||
| Male | 34,760 (99.4) | 293,840 (83.7) | <0.001 |
| Female | 216 (0.6) | 57,374 (16.3) | |
| Race, no. (%) | |||
| White | 28,196 (80.6) | 260,330 (74.1) | <0.001 |
| Black | 5960 (17.0) | 64,199 (18.3) | |
| Hispanic | 193 (0.6) | 6483 (1.8) | |
| AI/AN | 189 (0.5) | 6591 (1.9) | |
| Asian/PI | 80 (0.2) | 4562 (1.3) | |
| Other/missing | 358 (1.0) | 9049 (2.6) | |
AI, American Indian; AN, Alaskan Native; PI, Pacific Islander; SD, standard deviation.
Age at first cancer diagnosis, or age at the last medical encounter in the Defense Medical Surveillance System, or age at the last “as of” date in the personnel record.
Age at death or on the last day of the surveillance period (December 31, 2018).
Cancer Incidence
The most frequent cancer diagnosis among male fighter aviators was prostate (n = 2124), followed by melanoma skin (n = 416) and colon and rectum (n = 387). After matching, 274,451 (93.4%) other male officers and 41,811 (72.9%) other female officers were available for analysis. Compared to their officer peers and adjusted for race and age at incidence censoring, male fighter aviators had greater odds of being diagnosed with cancers of the testis (by 29%), melanoma skin (by 24%), and prostate (by 23%); the remaining cancers had similar adjusted odds (Table 2). Compared to males in the general US population, standardized for race and age group, male fighter aviators were more likely to be diagnosed with melanoma skin cancer (by 25%), prostate cancer (by 19%), and non-Hodgkin lymphoma (by 13%), and less likely to be diagnosed with cancers of the kidney and renal pelvis (by 69%), testis (by 62%), colon and rectum (by 29%), thyroid (by 29%), and urinary bladder (by 15%) (Table 3). Three female fighter aviators were diagnosed with a cancer; their adjusted odds of each cancer were similar to other female officers (Table 2), and their standardized incidence was similar to females in the general US population (Tables 3).
TABLE 2.
Cancer Incidence, US Air Force Fighter Aviators (n = 34,976), and Other Officers (n = 316,262), Exposure Period of 1970–2004, Followed Through 2018
| Fighter Aviators | Other Officers∗ | |||||
| No. | % | No. | % | Crude OR (95% CI) | Adjusted OR (95% CI)† | |
| Males | n = 34,760 | n = 274,451 | ||||
| Colon and rectum | 387 | 1.11 | 2946 | 1.07 | 1.04 (0.93–1.15) | 1.00 (0.89–1.12) |
| Pancreas | 169 | 0.49 | 1316 | 0.47 | 1.01 (0.86–1.19) | 0.94 (0.76–1.11) |
| Melanoma skin | 416 | 1.20 | 2375 | 0.87 | 1.39 (1.25–1.54) | 1.24 (1.11–1.38) |
| Prostate | 2124 | 6.11 | 12,637 | 4.60 | 1.35 (1.29–1.41) | 1.23 (1.17–1.30) |
| Testis | 43 | 0.12 | 244 | 0.09 | 1.39 (1.01–1.93) | 1.29 (1.15–2.12) |
| Urinary bladder | 305 | 0.88 | 2204 | 0.80 | 1.09 (0.97–1.23) | 1.04 (0.92–1.18) |
| Kidney and renal pelvis | 78 | 0.22 | 657 | 0.24 | 0.94 (0.74–1.19) | 0.87 (0.68–1.12) |
| Brain and nervous system | 104 | 0.30 | 861 | 0.31 | 0.95 (0.78–1.17) | 0.88 (0.71–1.10) |
| Thyroid | 72 | 0.21 | 463 | 0.17 | 1.23 (0.96–1.58) | 1.12 (0.86–1.44) |
| Non-Hodgkin lymphoma | 309 | 0.89 | 1963 | 0.72 | 1.25 (1.10–1.40) | 1.15 (0.99–1.21) |
| Females | n = 216 | n = 41,811 | ||||
| Melanoma skin | 1 | 0.46 | 121 | 0.29 | 1.60 (0.08–8.10) | 1.58 (0.35–18.2) |
| Thyroid | 1 | 0.46 | 194 | 0.46 | 1.00 (0.05–5.01) | 1.18 (0.29–10.5) |
| Non-Hodgkin lymphoma | 1 | 0.46 | 105 | 0.25 | 1.85 (0.09–9.36) | 1.62 (0.55–37.2) |
CI, confidence interval; OR, odds ratio.
Matched on sex, age group at active duty entrance, and age group at incidence censoring.
Adjusted for race and exact age at incidence censoring.
TABLE 3.
Cancer Incidence, US Air Force Fighter Aviators (n = 34,976), and the General US Population, Exposure Period of 1970–2004, Followed Through 2018
| Fighter Aviators (Observed) | General US Population (Expected) | SIR (95% CI)∗ | |
| Males (n = 34,760) | |||
| Colon and rectum | 387 | 542.4 | 0.71 (0.64–0.79) |
| Pancreas | 169 | 149.5 | 1.13 (0.97–1.31) |
| Melanoma skin | 416 | 332.2 | 1.25 (1.13–1.38) |
| Prostate | 2124 | 1779 | 1.19 (1.14–1.25) |
| Testis | 43 | 114.0 | 0.38 (0.27–0.51) |
| Urinary bladder | 305 | 357.7 | 0.85 (0.76–0.95) |
| Kidney and renal pelvis | 78 | 254.6 | 0.31 (0.24–0.38) |
| Brain and nervous system | 104 | 98.0 | 1.06 (0.87–1.29) |
| Thyroid | 72 | 101.1 | 0.71 (0.56–0.90) |
| Non-Hodgkin lymphoma | 309 | 273.1 | 1.13 (1.01–1.27) |
| Females (n = 216) | |||
| Melanoma skin | 1 | 0.66 | 1.51 (0.04–8.43) |
| Thyroid | 1 | 0.97 | 1.04 (0.03–5.77) |
| Non-Hodgkin lymphoma | 1 | 0.22 | 4.53 (0.11–25.2) |
CI, confidence interval; SIR, standardized incidence ratio; US, United States.
Standardized for sex, age group, and race.
Age at Cancer Diagnosis
Male fighter aviators were diagnosed with six cancers at later ages than other male officers, adjusted for race: for colon and rectum cancer, by a mean of 31 months (P < 0.001); for pancreas cancer, by 74 months (P < 0.001); for melanoma skin cancer, by 33 months (P = 0.012); for kidney and pelvis cancer, by 93 months (P < 0.001); for brain and nervous system cancer, by 59 months (P = 0.005), and for non-Hodgkin lymphoma, by 41 months (P < 0.001). No statistical differences were noted for female fighter aviators and other female officers (Supplementary Table 1).
Cancer Mortality
The most frequent causes of death among male fighter aviators were cancers of the prostate (n = 197), colon and rectum (n = 168), and pancreas (n = 166). After matching, 293,667 (99.9%) other male officers were available for analysis. Compared to other officers, male fighter aviators had similar race-adjusted mortality odds for all cancers (Table 4). Compared to males in the general US population, standardized for race and age group, male fighter aviators were less likely to die from colon and rectum cancer (by 24%), and more likely to die from melanoma skin cancer (by 64%), non-Hodgkin lymphoma (by 32%), and prostate cancer (by 23%) (Table 5). No female fighter aviators died from any of the studied cancers.
TABLE 4.
Cancer Mortality, Male US Air Force Fighter Aviators (n = 34,760), and Other Officers (n = 293,667), Exposure Period of 1970–2004, Followed Through 2018
| Fighter Aviators | Other Officers∗ | |||||
| No. | % | No. | % | Crude OR (95% CI) | Adjusted OR (95% CI)† | |
| Colon and rectum | 168 | 0.48 | 1480 | 0.50 | 0.96 (0.82–1.13) | 0.92 (0.78–1.08) |
| Pancreas | 166 | 0.48 | 1311 | 0.45 | 1.07 (0.91–1.26) | 0.97 (0.82–1.14) |
| Melanoma skin | 88 | 0.25 | 601 | 0.20 | 1.24 (0.99–1.55) | 1.14 (0.91–1.44) |
| Prostate | 197 | 0.57 | 1750 | 0.60 | 0.95 (0.82–1.10) | 0.89 (0.76–1.03) |
| Testis | 3 | 0.01 | 21 | 0.01 | 1.21 (0.29–3.68) | 1.58 (0.46–5.49) |
| Urinary bladder | 62 | 0.18 | 521 | 0.18 | 1.01 (0.77–1.31) | 0.91 (0.69–1.19) |
| Kidney and renal pelvis | 68 | 0.20 | 562 | 0.19 | 1.02 (0.79–1.32) | 0.92 (0.71–1.18) |
| Brain and nervous system | 92 | 0.26 | 812 | 0.28 | 0.96 (0.77–1.19) | 0.87 (0.70–1.09) |
| Thyroid | 11 | 0.03 | 51 | 0.01 | 1.82 (0.91–3.41) | 1.54 (0.79–2.99) |
| Non-Hodgkin lymphoma | 119 | 0.34 | 863 | 0.29 | 1.17 (0.96–1.41) | 1.08 (0.88–1.31) |
CI, confidence interval; OR, odds ratio.
Matched on sex, age group at active duty entrance, and age group at mortality censoring.
Adjusted for race and exact age at mortality censoring.
TABLE 5.
Cancer Mortality, Male US Air Force Fighter Aviators (n = 34,760), and the General US Population, Exposure Period of 1970–2004, Followed Through 2018
| Fighter Aviators (Observed) | General US Population (Expected) | SMR (95% CI)∗ | |
| Colon and rectum | 168 | 222.1 | 0.76 (0.65–0.88) |
| Pancreas | 166 | 157.2 | 1.06 (0.90–1.23) |
| Melanoma skin | 88 | 53.5 | 1.64 (1.32–2.03) |
| Prostate | 197 | 160.5 | 1.23 (1.06–1.41) |
| Testis | 3 | 4.7 | 0.63 (0.13–1.85) |
| Urinary bladder | 62 | 68.7 | 0.90 (0.69–1.16) |
| Kidney and renal pelvis | 68 | 69.3 | 0.98 (0.76–1.24) |
| Brain and nervous system | 92 | 81.7 | 1.13 (0.91–1.38) |
| Thyroid | 11 | 6.0 | 1.83 (0.91–3.27) |
| Non-Hodgkin lymphoma | 119 | 90.3 | 1.32 (1.09–1.58) |
CI, confidence interval; SMR, standardized mortality ratio; US, United States.
Standardized for age group and race.
Age at Cancer Death
The race-adjusted mean age at death was similar between male fighter aviators and other officers for all cancers except colon and rectum. Of males who died from colon and rectum cancer, mean age at death was 27 months earlier in fighter aviators than their officer peers (P = 0.022) (Supplementary Table 2).
E-Values
Of all comparisons between fighter aviators and other officers, statistically significant adjusted ORs were found for diagnoses of melanoma skin, prostate, and testis cancers. The respective E-values for the point estimates were 1.90, 1.79, and 1.76.
Attributable Risks
In absolute terms, for every 10,000 male fighter aviators, compared to other male officers, there were 150 additional lifetime cases of prostate cancer, 33 additional lifetime cases of melanoma skin cancer, and three additional lifetime cases of testis cancer.
DISCUSSION
This is the largest study of cancer incidence in military aviators and the first to incorporate mortality data. For all cancers except testis, the counts in this study vastly exceed those from previous studies. The number of aviators with prostate cancer (n = 2124) and colon and rectum cancer (n = 387), for example, surpass the previously largest studies by 29 times8 and 19 times.3 Enhanced statistical power adds credibility to the finding that US Air Force fighter aviators who served between 1970 and 2004 had similar cancer incidence and mortality as their fellow officers, with the exception of greater incidence of melanoma skin and prostate cancers, and a suggestive association with non-Hodgkin lymphoma. These are the same three cancers—the only three cancers—for which the standardized incidence and mortality ratios were statistically elevated. Conversely, fighter aviators were less likely than the general population to be diagnosed with colon and rectum, testis, urinary bladder, kidney and renal pelvis, and thyroid cancers, and less likely to die from colon and rectum cancer. This juxtaposition, in which an apparent healthy worker effect extends to most but not all cancers, suggests that fighter aviators were more susceptible to melanoma skin cancer, prostate cancer, and potentially non-Hodgkin lymphoma.
Several explanations should be considered. While melanoma skin and prostate cancers are screen-detectable, the constellation of findings does not reflect common biases associated with screening. Differential screening uptake is unlikely because the pattern does not extend to colon and rectum cancer, which is also detectable by screening. Lead time and length time biases—that is, detection of less advanced and less aggressive cancers—would not explain concomitant elevations of standardized incidence and mortality ratios. Screening bias would also fail to explain why fighter aviators were more likely than fellow officers to be diagnosed with melanoma skin and prostate cancers, despite equivalent mortality. This could reflect insufficient observation time or post-military differences in access to specialty care, such as dermatologists and urologists.
Occupational explanations are also elusive, as these cancers have dissimilar risk factors. Ionizing radiation may be associated with non-Hodgkin lymphoma, but it does not constitute an important risk factor for melanoma skin or prostate cancers.19 Background natural radiation from galactic cosmic radiation (GCR),20 solar particle events,21 and terrestrial gamma flashes from lightning strikes22 can affect anyone, but the risk increases with altitude. Nonetheless, epidemiologic studies have not established a definitive link between these exposures and cancer outcomes among aviators. A study of male European pilots found no association between GCR and cancer mortality,23 while a study of German cockpit crew members found that those with the highest cumulative effective dose of GCR (≥25 mSv) had significantly lower cancer mortality than their peers in the general population.24
Ultraviolet radiation is an established risk factor for melanoma skin cancer,25 although it is difficult in observational studies to distinguish occupational from recreational exposures.26 A study of 322 commercial flights in Europe found that pilots’ monthly intra-cockpit exposure was significantly less than office workers’ weekend recreational exposure.27 One study reported a direct relationship between ultraviolet radiation and the risk of some non-Hodgkin lymphoma subtypes,28 but others have documented an inverse relationship between ultraviolet radiation and non-Hodgkin lymphoma, prostate cancer, and other cancers—a relationship that may be mediated by endogenous synthesis of vitamin D3 following cutaneous exposure to ultraviolet radiation.29
Radium-painted instruments were once used in some retired US fighter jets to facilitate nighttime operations. Radium paint was manufactured by mixing radium salt, luminescent material, and a binding glue. Workers who applied these paints suffered from well-documented health effects, leading to its phase out in the 1960s.30 Fighter aviators in our cohort who flew the F-4A (n = 10,634) and F-100 (n = 2285) were likely exposed to radium-painted instruments. Based on our review of publicly available photographs of these cockpits, each of these aircraft had 25 to 30 instruments that were luminesced with radium paint. A recent measurement at the Smithsonian National Air and Space Museum, just beyond the acrylic surface of a display case containing 60 legacy radium instruments with the highest radioactivity, found a radiation dose rate below 0.02 mSv/hour.30 Given the half-life of radium (1600 years), these instruments are 97% to 98% as radioactive as they were when operational in the 1960s. Even if aircrew were exposed to a full 0.02 mSv/hour, they would require 10,000 hours in an unventilated cockpit to receive a cumulative dose of 200 mSv, the lowest dose found to increase cancer risk.31 To our knowledge, no studies have demonstrated detrimental effects from radium paint on aviators.
Based on studies in more vulnerable military occupations, electromagnetic frequency and aromatic hydrocarbons are unlikely carcinogens for fighter aviators. French Navy personnel working near radar systems had similar all-cause and cancer-specific mortality as their unexposed peers,32 and a meta-analysis concluded that occupational exposure to radar conveys no significant cancer risk if proper preventive measures are followed.33 US Air Force ground crew are routinely exposed to ambient benzene,34 an established leukemogen that may increase the risk of non-Hodgkin lymphoma.35 However, among the most highly exposed group of fuel maintenance workers, pre-work breath concentrations in smokers exceeded post-work concentrations in non-smokers,34 suggesting that recreational exposure to tobacco combustion may confound the relationship between occupational exposure to jet fuel combustion and cancer outcomes. To our knowledge, no intra-cockpit air sampling studies or aviator breath sampling studies have been conducted.
A final and important explanation is non-occupational risk factors. Incidence of and mortality from all-site cancer is directly related to family history and unhealthy lifestyles36; mortality is indirectly related to socioeconomic status.37 In terms of their genetic, behavioral, and socioeconomic profiles, we suspect that fighter aviators are quite dissimilar than their peers in the general population and largely similar to their peers in the US Air Force officer population. However, unmeasured confounding in the associations between fighter aviators and other officers could still threaten internal validity, as indicated by the modest E-values of 1.90 and 1.79 for the respective diagnoses of melanoma skin and prostate cancers. In other words, one or more unmeasured confounders would need to be associated with both fighter aviation and melanoma skin cancer incidence by ORs of at least 1.90, above and beyond the measured demographic confounders, to explain away the observed effect. This E-value of less than 2, in the context of a large observational study, raises concern of unmeasured confounding.38
Two additional findings merit consideration. First, fighter aviators were less likely than the standardized general population to be diagnosed with or die from colon and rectum cancer, by 29% and 24%, although they had equivalent incidence and mortality as their officer peers. This may reflect differences in health behaviors. Physical inactivity, overweight and obesity, and inadequate fruit and vegetable consumption account for 15% of colon and rectum cancer mortality in high-income countries.39 We suspect that US Air Force officers, including fighter aviators, are healthier than the broader US population. In other words, the data suggest a healthy worker effect, not a healthy aviator effect. Second, testicular cancer diagnosis was greater in fighter aviators than fellow officers. A hospital-based case–control study of US Air Force officers also found an increased odds of testicular cancer among aviators. Unable to establish a biologically plausible mechanism, the study investigators encouraged testicular self-examination.4 Despite our similar finding, we cannot endorse this recommendation because testicular cancer screening has well-described harms,40 and the similar cause-specific mortality odds between fighter aviators and other officers suggests that enhanced detection would not necessarily reduce mortality. Further research on this topic is warranted.
Limitations
In addition to its lack of data on potentially confounding variables, this study had six limitations. First, it likely did not capture all cancer diagnoses and deaths among fighter aviators and other officers. Under-capture of outcomes would likely be non-differential by exposure status, thus biasing results to the null. Under-capture of outcomes in fighter aviators would falsely underestimate standardized ratios, making fighter aviators appear healthier than the general population. The impact of this limitation is likely minimal: for deaths, because we used death certificate data from NDI Plus, a compendious archive that outperforms even the Death Master File maintained by the Social Security Administration41; and for diagnoses, because all 8946 cancer deaths in NDI Plus were also in the cancer registries or diagnostic data, underscoring the robustness of these data sources.
Second, we could not capture outcomes that have not yet occurred. The latency period between carcinogenic exposure and cancer diagnosis, and between carcinogenic exposure and cancer death, can span decades. The minimum follow-up period in our study, for someone who entered active duty on the last day of the exposure period, is 14 years. Relative to other military aviation studies, this is a substantial interval between the closure of the exposure and outcome windows. However, an even longer follow-up period may unveil differences in cancer outcomes among fighter aviators. It may be beneficial to reassess this cohort after several more years have elapsed.
Third, we assumed correct classification of fighter aviators and non-fighter aviator officers. To verify, we cross-matched the personnel assignments with those from a previous study, in which we assigned fighter pilot status based on Air Force Specialty Codes.5 For the overlapping period of 1986 through 2004 (n = 88,260), the overall concordance was 97.9%. Of the 4949 officers we had classified as fighter pilots in the previous study, only 47 (0.9%) were not classified as fighter aviators in the received personnel dataset. Of the 83,311 we had previously classified as other officers, the personnel dataset classified 1844 (2.2%) as fighter aviators—likely reflecting the addition of backseat aircrew, who were not included in the prior study.
Fourth, by retaining non-fighter aviators (eg, bomber pilots) in the comparison group, the effects of potential carcinogenic exposures related to flight may have been biased toward the null. We made this decision during study design development to isolate the exposure of fighter aviation, rather than aviation writ large. Future studies should analyze cancer outcomes in other airframe types.
Fifth, we could not account for residual confounding by year and by age. US incidence of some cancers (eg, colon and rectum) has decreased since 2000, while that of others (eg, pancreas) has increased. Colon and rectum and prostate cancer mortality have declined precipitously.42 Because of these non-monotonic trends and the outcome observation period of this study, we calculated SIRs and SMRs based on an average of US population data from 2000 through 2017. This accounts for some but not all of the period effect associated with cancer data vacillation, leaving residual confounding by year. Because US population cancer statistics are arranged in 5-year intervals, we calculated interval-specific probability of diagnosis and death as the mean of the interval boundaries. For example, a former fighter aviator who just turned 70-years old at incidence or mortality censoring would be assigned the same outcome probability as someone who is one day shy of 75 years—despite the latter's higher probability de facto. This results in residual confounding by age. Both types of residual confounding could bias results either toward or away from the null.
Finally, although we mitigated the threat of false discovery by a priori selection of 10 high-interest cancers, we nonetheless performed nearly 70 unique tests of association. We chose not to adjust the alpha level for multiple comparisons to minimize missed discovery for these important cancers. Whereas false positive results might reveal fallacious associations and spur further research, false negative results might conceal genuine associations and impede such research.
CONCLUSIONS
Male US Air Force fighter aviators who served on active duty between 1970 and 2004 were more likely than their fellow officers to be diagnosed with melanoma skin and prostate cancers (by adjusted odds of 24% and 23%), and they experienced greater standardized incidence ratios (by 25% and 19%) and mortality ratios (by 64% and 23%) when compared to the general US population. For non-Hodgkin lymphoma, the SIR and SMR were elevated by 13% and 32%. Although these effect sizes are statistically significant, none is especially salient—each would be considered epidemiologically “small,43” and the E-values associated with the ORs highlight the threat from unmeasured confounding. Nonetheless, the uniformity of these findings suggests that military fighter aviation may be associated with these three cancers.
Fighter aviators in this cohort should be offered enhanced strategies for combatting melanoma skin cancer, prostate cancer, and non-Hodgkin lymphoma. Because primary and secondary prevention strategies may have unintended harms (eg, sun avoidance leading to hypovitaminosis D or prostate specific antigen screens leading to unnecessary biopsies), we discourage universal recommendations. Rather, we support individualized discussions between fighter aviators and their health care providers, in which risks and benefits are evaluated in the context of shared clinical decision-making, akin to a grade C recommendation from the US Preventive Services Task Force.44 This study should prompt additional research of decommissioned US Air Force aircraft that contained radium-painted instruments. The findings of this study may or may not apply to fighter aviators in other US or international military services, or to fighter aviators who began serving after 2004. At this point, lacking a clear exposure-outcome pathway, generalization to other military and civilian aircrew is inadvisable.
Supplementary Material
Supplementary Material
Acknowledgments
The authors thank Anthony Robbins, MD, PhD, for assisting with interpretation of results.
Footnotes
This project was supported in part by an appointment to the Research Participation Program for the US Air Force School of Aerospace Medicine, administered by the Oak Ridge Institute for Science and Education through an agreement between the US Department of Energy and US Air Force School of Aerospace Medicine, Public Health and Preventive Medicine Department. The work was partially funded by a Studies and Analysis (Defense Health Program) grant from the 711th Human Performance Wing (#2020-002).
This project was approved as exempt research by the Air Force Research Laboratory Institutional Review Board (#FWR20200049E). The views expressed in this manuscript are those of the authors and do not necessarily reflect the official policy or position of the Air Force, the Department of Defense, or the US Government.
The authors report no conflicts of interest.
Clinical significance: Within the context of shared clinical decision-making, health care providers may wish to discuss primary and secondary preventive strategies for melanoma skin cancer, prostate cancer, and non-Hodgkin lymphoma with current and former fighter pilots.
Supplemental digital contents are available for this article.
REFERENCES
- 1.Milanov L, Dimitrov D, Danon S. Cancer incidence in Republic of Bulgaria aircrew, 1964–1994. Aviat Space Environ Med 1999; 70:681–685. [PubMed] [Google Scholar]
- 2.Hammar N, Linnersjö A, Alfredsson L, Dammström BG, Johansson M, Eliasch H. Cancer incidence in airline and military pilots in Sweden 1961–1996. Aviat Space Environ Med 2002; 73:2–7. [PubMed] [Google Scholar]
- 3.Grayson JK, Lyons TJ. Cancer incidence in United States Air Force aircrew, 1975–89. Aviat Space Environ Med 1996; 67:101–104. [PubMed] [Google Scholar]
- 4.Yamane GK, Johnson R. Testicular carcinoma in US Air Force aviators: a case–control study. Aviat Space Environ Med 2003; 74:846–850. [PubMed] [Google Scholar]
- 5.Robbins AS, Pathak SR, Webber BJ, et al. Malignancy in US Air Force fighter pilots and other officers, 1986–2017: a retrospective cohort study. PLoS One 2020; 15:e0239437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Walker C. Incidence of Testicular Cancer in US Air Force Officer Aviators: 1998–2008. United States Air Force School of Aerospace Medicine, June 2011, https://apps.dtic.mil/sti/pdfs/ADA554672.pdf. AFRL-SA-WP-SR-2012-0001. Accessed April 1, 2021. [Google Scholar]
- 7.Rogers D, Boyd DD, Fox EE, et al. Prostate cancer incidence in Air Force aviators compared with non-aviators. Aviat Space Environ Med 2011; 82:1067–1070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Grayson JK, Lyons TJ. Brain cancer, flying, and socioeconomic status: a nested case–control study of USAF aircrew. Aviat Space Environ Med 1996; 67:1152–1154. [PubMed] [Google Scholar]
- 9.National Defense Authorization Act for Fiscal Year 2021, H.R. 6395, 116th Cong. (2020). https://www.congress.gov/bill/116th-congress/house-bill/6395/text/enr. Accessed April 1, 2021. [Google Scholar]
- 10.Aviator Medical Issues. https://www.river-rats.org/page/Medical. Accessed April 1, 2021. [Google Scholar]
- 11.Rubertone MV, Brundage JF. The Defense Medical Surveillance System and the Department of Defense serum repository: glimpses of the future of public health surveillance. Am J Public Health 2002; 92:1900–1904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.National Cancer Institute. Appendix B: Comparison of ICD-O-3, ICD-10-CM, ICD-10 and ICD-9-CM. https://training.seer.cancer.gov/icd10cm/appendix-b/. Accessed April 1, 2021. [Google Scholar]
- 13.Defense Health Agency. Surveillance Case Definitions: Oncology. https://www.health.mil/Military-Health-Topics/Combat-Support/Armed-Forces-Health-Surveillance-Branch/Epidemiology-and-Analysis/Surveillance-Case-Definitions. Accessed April 1, 2021. [Google Scholar]
- 14.Webber BJ, Rogers AE, Pathak SR, Robbins AS. Positive predictive value of an algorithm used for cancer surveillance in the U.S. Armed Forces. MSMR 2019; 26:18–22. [PubMed] [Google Scholar]
- 15.National Cancer Institute. Cancer Query System: Probability of Developing or Dying of Cancer. https://surveillance.cancer.gov/devcan/canques.html. Accessed April 1, 2021. [Google Scholar]
- 16.Liddell FD. Simple exact analysis of the standardised mortality ratio. J Epidemiol Community Health 1984; 38:85–88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Weir HK, Thun MJ, Hankey BF, et al. Annual report to the nation on the status of cancer, 1975–2000, featuring the uses of surveillance data for cancer prevention and control. J Natl Cancer Inst 2003; 95:1276–1299. [DOI] [PubMed] [Google Scholar]
- 18.Mathur MB, Ding P, Riddell CA, VanderWeele TJ. Website and R package for computing E-values. Epidemiology 2018; 29:e45–e47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.National Research Council. Health Risks from Exposure to Low Levels of Ionizing Radiation: BEIR VII Phase 2. 2006; Washington, DC: The National Academies Press, 141-258. [PubMed] [Google Scholar]
- 20.Bramlitt ET, Shonka JJ. Radiation exposure of aviation crewmembers and cancer. Health Phys 2015; 108:76–86. [DOI] [PubMed] [Google Scholar]
- 21.Anderson CM, Burns DM, Dodd KW, Feuer EJ. Chapter 2: birth-cohort-specific estimates of smoking behaviors for the U.S. population. Risk Anal 2012; 32: (Suppl 1): S14–S24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Dwyer JR, Smith DM, Cummer SA. High-energy atmospheric physics: terrestrial gamma-ray flashes and related phenomena. Space Sci Rev 2012; 173:133–196. [Google Scholar]
- 23.Langner I, Blettner M, Gundestrup M, et al. Cosmic radiation and cancer mortality among airline pilots: results from a European cohort study (ESCAPE). Radiat Environ Biophys 2004; 42:247–256. [DOI] [PubMed] [Google Scholar]
- 24.Hammer GP, Blettner M, Langner I, Zeeb H. Cosmic radiation and mortality from cancer among male German airline pilots: extended cohort follow-up. Eur J Epidemiol 2012; 27:419–429. [DOI] [PubMed] [Google Scholar]
- 25.Narayanan DL, Saladi RN, Fox JL. Ultraviolet radiation and skin cancer. Int J Dermatol 2010; 49:978–986. [DOI] [PubMed] [Google Scholar]
- 26.Nicholas JS, Swearingen CJ, Kilmer JB. Predictors of skin cancer in commercial airline pilots. Occup Med (Lond) 2009; 59:434–436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Baczynska KA, Brown S, Chorley AC, et al. In-flight UV-A exposure of commercial airline pilots. Aerosp Med Hum Perform 2020; 91:501–510. [DOI] [PubMed] [Google Scholar]
- 28.Zhang Y, Holford TR, Leaderer B, et al. Ultraviolet radiation exposure and risk of non-Hodgkin's lymphoma. Am J Epidemiol 2007; 165:1255–1264. [DOI] [PubMed] [Google Scholar]
- 29.Holick MF. Biological effects of sunlight, ultraviolet radiation, visible light, infrared radiation and vitamin D for health. Anticancer Res 2016; 36:1345–1356. [PubMed] [Google Scholar]
- 30.Norquest S, Kile A, Peters D. Working with a collection of radioactive aircraft instruments. Objects Specialty Group Postprints 2015; 22:169–180. [Google Scholar]
- 31.Muirhead CR, O’Hagan JA, Haylock RG, et al. Mortality and cancer incidence following occupational radiation exposure: third analysis of the National Registry for Radiation Workers. Br J Cancer 2009; 100:206–212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Dabouis V, Arvers P, Debouzy JC, Sebbah C, Crouzier D, Perrin A. First epidemiological study on occupational radar exposure in the French Navy: a 26-year cohort study. Int J Environ Health Res 2016; 26:131–144. [DOI] [PubMed] [Google Scholar]
- 33.Safari Variani A, Saboori S, Shahsavari S, Yari S, Zaroushani V. Effect of occupational exposure to radar radiation on cancer risk: a systematic review and meta-analysis. Asian Pac J Cancer Prev 2019; 20:3211–3219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Pleil JD, Smith LB, Zelnick SD. Personal exposure to JP-8 jet fuel vapors and exhaust at air force bases. Environ Health Perspect 2000; 108:183–192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Guo H, Ahn S, Zhang L. Benzene-associated immunosuppression and chronic inflammation in humans: a systematic review. Occup Environ Med 2021; 78:377–384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Drake I, Dias JA, Teleka S, Stocks T, Orho-Melander M. Lifestyle and cancer incidence and mortality risk depending on family history of cancer in two prospective cohorts. Int J Cancer 2020; 146:1198–1207. [DOI] [PubMed] [Google Scholar]
- 37.Ward E, Jemal A, Cokkinides V, et al. Cancer disparities by race/ethnicity and socioeconomic status. CA Cancer J Clin 2004; 54:78–93. [DOI] [PubMed] [Google Scholar]
- 38.VanderWeele TJ, Ding P. Sensitivity analysis in observational research: introducing the E-value. Ann Intern Med 2017; 167:268–274. [DOI] [PubMed] [Google Scholar]
- 39.Danaei G, Vander Hoorn S, Lopez AD, Murray CJ, Ezzati M. Comparative Risk Assessment collaborating group (Cancers). Causes of cancer in the world: comparative risk assessment of nine behavioural and environmental risk factors. Lancet 2005; 366:1784–1793. [DOI] [PubMed] [Google Scholar]
- 40.Lin K, Sharangpani R. Screening for testicular cancer: an evidence review for the U.S. Preventive Services Task Force. Ann Intern Med 2010; 153:396–399. [DOI] [PubMed] [Google Scholar]
- 41.Lash TL, Silliman RA. A comparison of the National Death Index and Social Security Administration databases to ascertain vital status. Epidemiology 2001; 12:259–261. [DOI] [PubMed] [Google Scholar]
- 42.Siegel RL, Miller KD, Fuchs HE, Jemal A. Cancer statistics. CA Cancer J Clin 2021; 71:7–33. [DOI] [PubMed] [Google Scholar]
- 43.Chen H, Cohen P, Chen S. How big is a big odds ratio? Interpreting the magnitudes of odds ratios in epidemiological studies. Commun Stat—Simul Comput 2010; 39:860–864. [Google Scholar]
- 44.Harris RP, Helfand M, Woolf SH, et al. Methods Work Group, Third US Preventive Services Task Force. Current methods of the US Preventive Services Task Force: a review of the process. Am J Prev Med 2001; 20:S21–S35. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.