Abstract
Thyroid hormone is known to participate in the control of intestine maturation at weaning. Its action is mediated by the thyroid hormone nuclear receptors, encoded by the TRα and TRβ genes. Since previous studies have shown that TRβ plays a minor role in the gut, we focused here our analysis on the TRα gene. The TRα locus generates the TRα1 receptor together with the splicing variant TRα2 and the truncated products TRΔα1 and TRΔα2, which all lack an intact ligand binding domain. The TRΔα isoforms are transcribed from an internal promoter located in intron 7, and their distribution is restricted to a few tissues including those of the intestine. In order to define the functions of the different isoforms encoded by the TRα locus in the intestinal mucosa, we produced mice either lacking all known TRα products or harboring a mutation which inactivates the intronic promoter. We performed a detailed analysis of the intestinal phenotypes in these mice and compared it to that of the previously described TRα−/− mice, in which TRα isoforms are abolished but the TRΔα isoforms remain. This comparative analysis leads us to the following conclusions: (i) the TRα1 receptor mediates the T3-dependent functions in the intestine at weaning time and (ii) the TRΔα products negatively control the responsiveness of the epithelial cells to T3. Moreover, we show that TRΔα proteins can interfere with the transcription of the intestine-specific homeobox genes cdx1 and cdx2 and that their activity is regulated by TRα1. Altogether these data demonstrate that cooperation of TRα and TRΔα products is essential to ensure the normal postnatal development of the intestine and that mutations in the TRα locus can generate different phenotypes caused by the disruption of the equilibrium between these products.
The intestinal mucosa is composed of smooth muscle and connective tissues, two mesodermal derivatives, and of epithelial tissue derived from the endoderm (18). The epithelium is characterized by continuous cell renewal from multipotent stem cells within the crypts. The epithelial cells acquire differentiated phenotypes (enterocyte, goblet, or enteroendocrine) during a vertical migration toward the villus tip, and finally they die and are exfoliated into the lumen. The Paneth cells, the other cytotype composing the intestinal epithelium, migrate to the crypt base and represent the only resident differentiated cell type of this compartment (10). Small intestine mucosal thickness depends on the rate of crypt cell division and migration as well as the lifespan of the villus cells. All these parameters change markedly during normal development and in response to various environmental, dietary, and hormonal factors (19). The enterocytes represent the most abundant cell type composing the small intestine epithelium (95% [10]), and their function in nutrient uptake and metabolism takes part in ensuring the organism's homeostasis. Their differentiation is characterized by functional polarization, i.e., the expression of digestive enzymes integrated in the apical brush border membranes (18). At weaning, the passage from milk to solid diet is characterized in rodents by a switch in the expression of lactase to various α-glucosidases such as sucrase (13) and increased expression of other brush border enzymes such as alkaline phosphatase (14). Thyroid hormones have been shown to take part in the developmental processes responsible for intestinal postnatal maturation, such as the increase in the number of crypts and proliferating cells as well as the onset of the adult-type digestive enzyme expression (3, 15; reviewed in references 12 and 13). The molecular mechanisms as well as the direct regulation of these processes by the thyroid hormone remain under discussion. It has been shown that cdx1 and cdx2 homeobox genes control intestinal epithelial cell proliferation as well as the expression of enterocyte differentiated markers (reviewed in reference 8). This and a previous work (25) suggest that the thyroid hormone signaling pathway could involve these major regulators of intestinal homeostasis.
The action of T3, the active form of thyroid hormones, is mediated by its binding to thyroid hormone receptors (TR), which belong to the nuclear hormone receptor family of transcription factors (22). The activity of TRs is modulated by the T3 binding that leads to the activation or the repression of target genes (22). The TRs are encoded by two genes, TRα and TRβ (N1RA1 and N1RA2 according to the Nuclear Receptors Nomenclature Committee [1999]) (26, 31). The TRβ locus encodes the TRβ1, TRβ2, and TRβ3 receptors and the TRΔβ3 truncated isoform, generated by different promoter usage and alternative splicing (31, 32). The TRα gene has been shown to encode the T3 receptor TRα1 and several other isoforms unable to bind T3 (5, 20, 21). The TRα2 isoform, produced by alternative splicing of the primary transcript, retains DNA recognition capacity and can act as an antagonist of the T3 receptors, TRα1, TRβ1, and TRβ2 in vitro (20). Recently, we have demonstrated that the TRα gene also produces transcripts from an internal promoter located in intron 7, TRΔα1 and TRΔα2 (5), whose expression is restricted to a few organs including the lung and the small intestine (7). The sequences of these isoforms are identical to the C-terminal sequences of TRα1 and TRα2, respectively. They lack the DNA binding domain and part of the ligand binding domain, leading to their inability to bind DNA and T3. In vitro experiments showed that they can repress the transactivation activity of TR and retinoic acid receptors (5), suggesting that these truncated isoforms could act as modulators of nuclear hormone receptor activities in the tissues in which the different proteins are coexpressed. However, the molecular basis of their action as well as their physiological role is still unknown. Several in vivo studies aimed at clarifying the functions of the different isoforms encoded by the TRα gene have been performed in different laboratories. By using the homologous recombination technology, we and others have generated strains of mice lacking the expression of either TRα1 or both TRα1 and TRα2, as well as the respective double mutants combining the deletion in TRα and TRβ genes (reviewed in reference 6). The TRα−/− animals, which lack both the TRα1 and TRα2 isoforms but still express the TRΔα isoforms, display growth retardation, become progressively hypothyroid by weaning time, and die thereafter. In addition, they show bone and small intestine developmental alterations (7). In contrast, TRβ−/− mice do not display intestinal development retardation, suggesting that TRα is the only locus involved in postnatal small intestine development (25). However, as the TRα−/− mice retain the expression of the TRΔα isoforms we cannot exclude that in these animals the unbalanced expression of the TRα/TRΔα products is one of the causes of the strong phenotype observed in these animals. To investigate this possibility we have generated new TRα mutant mice. The TRα0 mutation was designed to abolish the expression of all transcripts from the TRα locus (9a.). The TRα7 mutation described here was aimed at selectively suppressing the TRΔα transcripts while retaining the normal expression of the TRα transcripts. As all TRα products are expressed in the small intestine, our study focused on the morphofunctional comparative analysis between mice harboring different mutations in the TRα locus. We demonstrate that (i) removing only the TRα1 and TRα2 products in TRα−/− mice results in a severe alteration of intestinal development and function, (ii) further abolishing of the expression of the TRΔα isoforms in TRα0/0 mice generates a milder phenotype, and (iii) specifically deleting the intronic promoter to prevent the expression of the TRΔα isoforms in TRα7/7 mice results in an enhanced response of the epithelial cells to T3. In conclusion, these data demonstrate that the balance between the TRα and TRΔα isoforms is critical to ensure normal postnatal intestinal development.
MATERIALS AND METHODS
Construction of TRα-targeting vector.
To construct the TRα7 targeting vector, the 5′ and 3′ homology fragments were generated by PCR, using VIS/PIAS and Pr3S/RevS as primer pairs, respectively, and Expand High Fi reagent, and were successively inserted into pBSKII (Stratagene). The loxP PGKNeor PGKTK loxP cassette was inserted between the 5′ and 3′ arms in sense orientation.
Targeted disruption of TRα gene.
ENS embryonic stem (ES) cells were electroporated with 40 μg of linearized targeting vector and then selected with 250 μg of G418 (Gibco-BRL) per ml and 0.2 μM ganciclovir. The TRα7 allele was obtained in two steps. Upon electroporation with the recombination vector and selection with G418, the cells containing the targeted [loxP NeoTK loxP] cassette were identified by PCR-based screening using 6S1 and pT102AS as oligonucleotide primers. Positive clones were subsequently transfected with a Cre-expressing vector. To identify clones having excised the loxP flanked selection cassette among the ganciclovir-resistant colonies, DNA was cut by PstI and submitted to Southern blot analysis, using a probe hybridizing to the 3′ end of intron 7 and to exon 8. To determine the promoter activities of the −270 to +237, −114 to +237, and −51 to +237 portions of intron 7, the corresponding fragments were inserted upstream of a chloramphenicol acetyltransferase (CAT) reporter gene into a BAS-CAT vector (Promega). Cells were cotransfected with the CAT-expressing vector and a plasmid containing the beta-galactosidase gene under the control of the PGK promoter.
Animals and tissues.
The small intestine was dissected from the ligament of Treitz to the rectum. We collected the first (proximal jejunum) and the last (distal ileum) fourths of the small intestine as well as the proximal part of the colon. The intestinal segments were immediately fixed for morphological analysis or frozen in liquid nitrogen and stored at −80°C until they were used for enzymatic analysis or RNA extraction.
The epithelium and connective and smooth muscle tissues were isolated from proximal jejunums, distal ilea, and proximal colons of three 12-day-old wild-type mice, as described in reference 25.
Experimental hypothyroidism and hyperthyroidism.
TH deficiency was induced in wild-type, TRα0/0, and TRα7/7 mice by a low-iodine diet supplemented with 0.15% propylthiouracil (PTU) purchased from Harlan/Teklad. The animals were treated from birth until they were sacrificed. Hyperthyroidism was induced in one-half of PTU-treated animals by daily injection of 0.25 μg of L-T3 per mouse for 2 days. For each experimental condition at least three animals per genotype were used. The levels of free T4 and T3 in serum were measured using a VIDAS enzyme-linked immunosorbent assay kit (Biomérieux).
Purification of RNAs, RNase protection assay, and RT-PCR analysis.
Total RNA from intestine was isolated by the improved acid-guanidine-phenol-chloroform method. The RNase protection assay was performed as described in reference 9a.
Reverse transcription (RT) was performed as described in reference 7. cDNA (0.05 μg) was used for each PCR with Eurobio Taq. To perform the detection of TRΔα1 and TRΔα2 we used oligonucleotide pairs α7S/α1A and α7S/α2A, respectively. The primers used for Cdx1 and Cdx2 mRNA detection and semiquantitative RT-PCR conditions are described in reference 25.
Sequences of oligonucleotides used.
All the primers are from MWG (Ebersberg, Germany) and have the following sequences: VIS, GGAGATGATTCGCTCACTGCAG; PIAS, GTCATGCTGCCTGCAGATAG; Pr3S, GGTGTGGAGAGGATGCACTGAAG; RevS, GGAGGTGGTAGAGTTTGCCAAAC; α7S, GTGTGGAGAGGATGCACTGAAGT; 6S1, GGTTCTAGATGATTCGAAGCGG; α1A, CGACTTTCATGTGGAGGAAG; α2A, CCTGAACAACATGCATTCCGA; α15A, CAGCCTGCAGCAGAGCCACTTCCGT; pT102AS, CCTCGAGCGGCCATAACTTCG.
Morphological staining and immunohistochemistry.
Three-week-old mice were killed by cervical dislocation, and their small intestines were immediately removed. Proximal jejunums and distal ilea were collected separately and fixed in 4% paraformaldehyde overnight at 4°C. They were then embedded in paraffin, and 5-μm sections were applied to polylysine-coated slides. For morphological observations, after dewaxing and rehydration, the slides were stained with Schiff's reagent or hematoxylin and eosin. Immunohistochemistry experiments were performed with a monoclonal antibody (Novocastra Laboratories) for Ki67 detection in proliferating cells (27), a polyclonal PA1-211 (Affinity Bioreagents, Inc.) for TRα1 and TRΔα1 protein localization, and a polyclonal antibody recognizing the N-terminal part of both TRα1 and TRα2 proteins (generous gift of D. Baas [1]). These antibodies were used in combination with secondary biotinylated antibody and a streptavidin-peroxidase detection system (Histomouse; Zymed). The tissue was counterstained lightly with hematoxylin. Before incubation with the primary antibody, the slides were immersed in 0.01 M citrate buffer, pH 6, and microwaved for 15 min.
Cells, plasmids, and transfections.
The Caco2-TC7 cell line (kindly gift of A. Zweibaum, Paris, France [4]) corresponds to a highly differentiated subclone of the human colonic adenocarcinoma Caco2 cell line that exhibits spontaneous enterocyte-like differentiation in culture (11). The cells were maintained in Dulbecco's modified Eagle medium (Biomedia) supplemented with 10% of heat inactivated fetal calf serum (Biomedia). The reporter pCdx1-4Luc and pCdx2-1Luc plasmids containing promoter fragments of the murine Cdx1 and Cdx2 genes have already been described (23). The plasmids containing the cDNA coding for human TRα1 (pSG5hTRα1), for mouse TRΔα1 (pSG5mTRΔα1), and for mouse TRΔα2 (pSG5mTRΔα2) have already been reported (5). Caco2TC7 cells have been transfected as described (23) using the Exgen transfection reagent (Euromedex). Luciferase activity was measured 48 h after transfection using the luciferase assay system (Promega).
Enzymatic activities.
Sucrase and lactase enzymatic activities were measured in the purified brush border membrane as previously described (28). The samples were incubated with the appropriate substrate in 0.1-mol/liter maleate buffer (0.056 mol of sucrose per liter, pH 6.25; 0.056 mol of lactose per liter, pH 5.8), and liberated glucose was measured. Enzyme activities were expressed as milliunits per milligram of brush border protein. One unit hydrolyzes 1 mmol of substrate per min at 37°C.
Statistics.
Numerical results are presented as means ± standard deviations (SD). Groups were compared using the Student t test, with P values of <0.05 considered significant.
RESULTS
Generation of the TRα7/7 mutant mice.
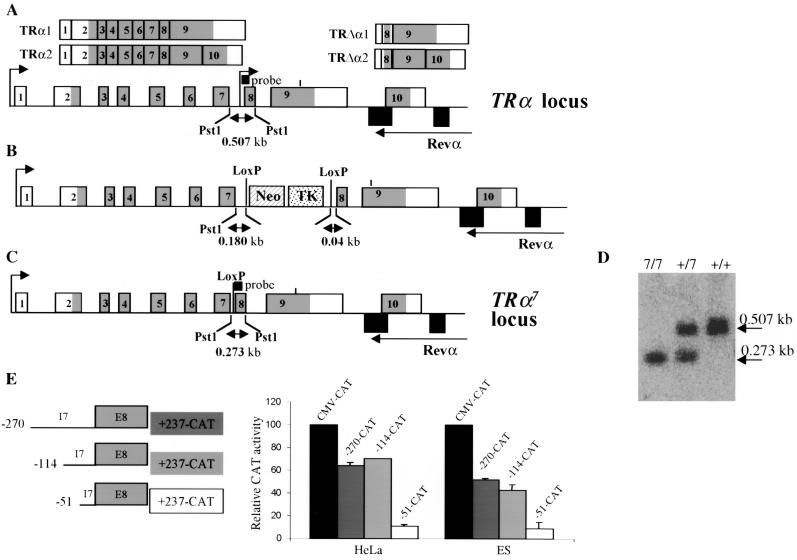
To introduce the TRα7 mutation in the TRα locus (Fig. 1A), we deleted a limited portion of intron 7 in order to abolish the activity of the internal promoter. The extent of this deletion was determined after assaying the transcriptional activities of different truncated fragments of intron 7 following transfection in HeLa and ES cells (Fig. 1E). We thereby identified a 234-bp fragment whose deletion abolished 80% of the transcriptional activity in this assay and designed a vector in which this fragment was replaced by a Neor-TK cassette flanked by loxP sites. ES cell clones screened for homologous recombination of this construct (Fig. 1B) were transfected with a vector expressing the Cre recombinase fused to a nuclear localization signal under the control of a PGK promoter. ES cells which had excised the loxP-NeoR-TK-loxP fragment (Fig. 1C) were selected by ganciclovir resistance and identified by Southern blot screening (Fig. 1D). Mice homozygous for the TRα7 mutation were named TRα7/7.
FIG. 1.
Targeted mutagenesis of the TRα gene by homologous recombination. (A) Structure of the TRα gene. The upper arrows indicate the two transcription start sites. The differential splice site in exon 9 is indicated by a vertical bar. Exons are numbered starting downstream to the distal promoter. Grey-shaded areas represent the coding regions. Structures of the transcripts are shown at the top. (B) TRα mutated locus in which the loxP Neor TK loxP cassette replaces a 257-bp fragment of intron 7. Cells expressing this construct have then been transfected with a plasmid encoding the Cre recombinase to obtain the TRα7 locus. (C) Structure of the targeted allele TRα7 containing a 257-bp deletion of intron 7, corresponding to the active portion of the internal promoter. The probe used for Southern blot analysis and the size of the fragment detected after digestion with PstI are indicated. (D) Southern blot analysis showing wild-type (+/+), heterozygous (+/7), and homozygous (7/7) littermates. (E) Promoter activities of the −270 to +237, −114 to +237 and −51 to +237 fragments of intron 7 have been tested in HeLa and ES cells. Results are mean values ± SD of two independent experiments conducted in duplicate.
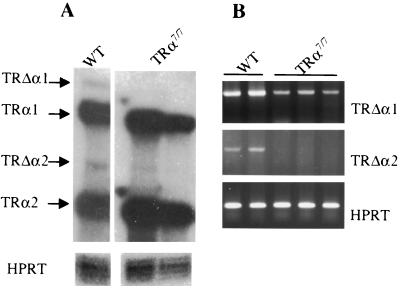
To further characterize the new mutant mice, we compared the expressions of the different RNA products of the TRα locus in the distal ilea of TRα7/7 mice to those of wild-type mice by RNase protection and RT-PCR analysis. Using specific probes in an RNase protection assay, we detected similar amounts of TRα1 and TRα2 mRNAs in wild-type and TRα7/7 mice (Fig. 2A), showing that the mutation introduced in intron 7 did not affect the production of these transcripts. The same experiment also showed that the mutation reduced the mRNA expression of both TRΔα1 and TRΔα2. The reduced expression of the TRΔα isoforms was also confirmed by semiquantitative RT-PCR (Fig. 2B). Using a sense primer within intron 7, which has been shown to contain the transcription initiation site of TRΔα mRNAs (5), and either an α1-specific or an α2-specific oligonucleotide as antisense primer, we detected the TRΔα1 and TRΔα2 transcripts in wild-type mice. Under the PCR conditions used, we were unable to detect a TRΔα2 transcript in TRα7/7 animals, while the TRΔα1 mRNA was strongly reduced compared to that of wild-type mice. These analyses indicate that TRα7/7 animals display normal levels of TRα1 and TRα2 transcripts and decreased amounts of TRΔα transcripts. The decreased level of expression of the TRΔα isoforms in TRα7/7 animals is consistent with the reduced promoter activity of the deleted intron 7.
FIG. 2.
Expression of the different transcripts encoded by the TRα locus in the distal ilea of wild-type and TRα7/7 mutant mice. (A) An RNase protection assay was performed using RNA isolated from the distal ilea of animals carrying the indicated genotypes. The protected fragments are indicated on the left. (B) Semiquantitative RT-PCR analysis to selectively detect TRΔα1 and TRΔα2 mRNAs in the distal ilea of wild-type (WT) and TRα7/7 mice. For the semiquantitative RT-PCR, a preliminary assay was conducted to define the appropriate range of cycles consistent with an exponential increase of the amount of the PCR products in each experimental condition. For both panels, HPRT was used as internal control.
Analysis of the TRΔα1 and TRΔα2 expression patterns in the intestinal mucosa. (i) mRNA expression.
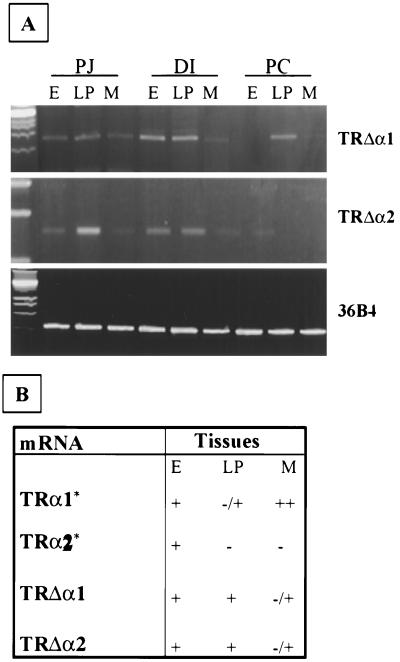
We performed RT-PCR analysis to evaluate the longitudinal expression of the TRΔα truncated isoforms on separated epithelia, laminae propriae, and muscle layers of 12-day-old wild-type mice (Fig. 3A and B). TRΔα1 mRNA is expressed in almost all tissues in each region analyzed, with a higher expression in the epithelium and lamina propria of the distal ileum and a very faint expression in the muscular layers of the distal ileum and proximal colon. These data are in agreement with the data concerning the protein immunostaining using specific antibody (see below). TRΔα2 mRNA shows a distribution similar to that of TRΔα1 except that the maximal expression is in the lamina propria of the proximal jejunum.
FIG. 3.
Expression of TRΔα1 and TRΔα2 in the gut. (A) Semiquantitative RT-PCR analysis was performed to study the longitudinal expression of the two transcripts in the different tissue compartments of 12-day-old wild-type mice. 36-B4 was used as internal control. Standard molecular masses for DNA size are in the first lane. (B) Summary of the expression pattern of the four mRNAs encoded by the TRα gene in the different tissues composing the distal ileum mucosa. E, epithelium; LP, lamina propria; M, muscle; PJ, proximal jejunum; DI, distal ileum; PC, proximal colon: ∗, described in reference 25.
(ii) Protein expression.
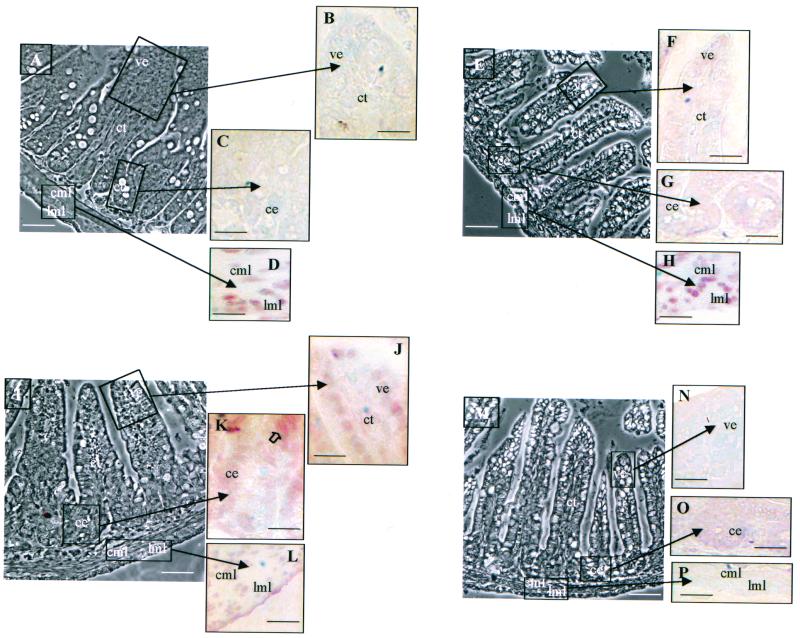
We used specific antisera to analyze the expression pattern of the TRα locus products in mice carrying different TRα mutations. Using the anti-Nα antiserum, which recognizes the N-terminal region common to TRα1 and TRα2 proteins, we observed a similar nuclear labeling in muscle layers of TRα7/7 and wild-type distal ileum (Fig. 4D and H), consistent with the unmodified mRNA expression of these two isoforms in TRα7/7 animals. In contrast, when using the anti-Cα1 antibody, which recognizes the C-terminal part common to TRα1 and TRΔα1 proteins, the signals in these two mouse strains were very different. While a strong nuclear signal in the differentiated epithelial cells of the villi and a fainter signal in the laminae propriae and muscle layers were detected in wild-type mice (Fig. 4J to L), no labeling was observed in TRα7/7 mice (Fig. 4N to P). The loss of staining in TRα7/7 mice definitely shows that the protein labeled by the anti-Cα1 reagent in wild-type mice is TRΔα1. These data also demonstrate that even though a small amount of TRΔα1 mRNA can be revealed by RNase protection assay in the distal ilea of TRα7/7 mice, the amount of TRΔα1 protein is strongly reduced to below the detection limit. Using specific antibodies against TRα2 and TRΔα2 proteins, we could not reveal any staining (not shown). Table 1 summarizes the data concerning the expression of the proteins produced by the TRα locus in the intestinal mucosae of mice with different mutations in the TRα gene.
FIG. 4.
Immunolocalization of TRα1/TRα2 and TRΔα1 proteins. We analyzed the expression patterns of TRα and TRΔα1 proteins on distal ileum paraffin-embedded sections (5 μm) of wild-type (A through D and I through L) and TRα7/7 (E through H and M through P) intestines from 3-week-old mice. Two different antibodies were used, one (anti-Nα) recognizing the N-terminal part common to TRα1 and TRα2 on wild-type (A through D) and TRα7/7 (E through H) mouse tissues and the other (anti-Cα1) recognizing the C-terminal part common to TRα1 and TRΔα1 on wild-type (I through L) and TRα7/7 (M through P) mouse tissues. Technical controls included the use of phosphate-buffered saline or the antibody preincubated with an excess of the recognized peptide. Phase contrast low magnification (A, E, I, M) bar, 30 μm; bright field high magnification (B to D, F to H, J to L, N to P) bar, 70 μm; ce, crypt epithelium; ve, villus epithelium; ct, connective tissue; cml, circular muscle layer; 1ml, longitudinal muscle layer. The open arrow in K indicates the stronger nuclear staining of epithelial cells above the crypt-villus junction.
TABLE 1.
Expression of the TRα isoforms in the different tissues composing the distal small intestine mucosa
| Immunolabeling (isoform) | Expressiona in indicated tissuesb of TRα isoforms for mice with genotype:
|
|||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Wild type
|
TRα−/−
|
TRα0/0
|
TRα7/7
|
|||||||||||||
| Ce | Ve | LP | M | Ce | Ve | LP | M | Ce | Ve | LP | M | Ce | Ve | LP | M | |
| N-TRαc (TRα1/α2) | − | − | − | + | − | − | − | − | − | − | − | − | − | − | − | + |
| C-TRαc (TRα1/Δα1) | −/+ | ++ | −/+ | −/+ | ++ | ++ | −/+ | −/+ | − | − | − | − | − | − | − | − |
The symbols − to ++ correspond to the relative intensities of the signals.
Ce, crypt epithelium; Ve, villus epithelium; LP, lamina propria or connective tissue; M, muscle layers.
See also reference 9a.
Morphological and functional parameters of the small intestine are more affected in TRα−/− mice than in TRα0/0 and TRα7/7 mice.
It has previously been shown that the morphology of the small intestine, the proliferation of crypt epithelial cells, and their differentiation were altered in mice lacking TRα1 and TRα2 expression (TRα−/−) and that this impairment was more severe in the distal part of the small intestine (25). Interestingly, mice lacking the expression of all the products of the TRα locus (TRα0/0) display a milder alteration of the intestinal mucosa than the TRα−/− mice (9a). In order to determine the molecular basis of these differences, we examined morphofunctional parameters in the distal small intestines of TRα0/0 and TRα7/7 mice and compared them to those described for wild-type and TRα−/− mice.
(i) Morphology.
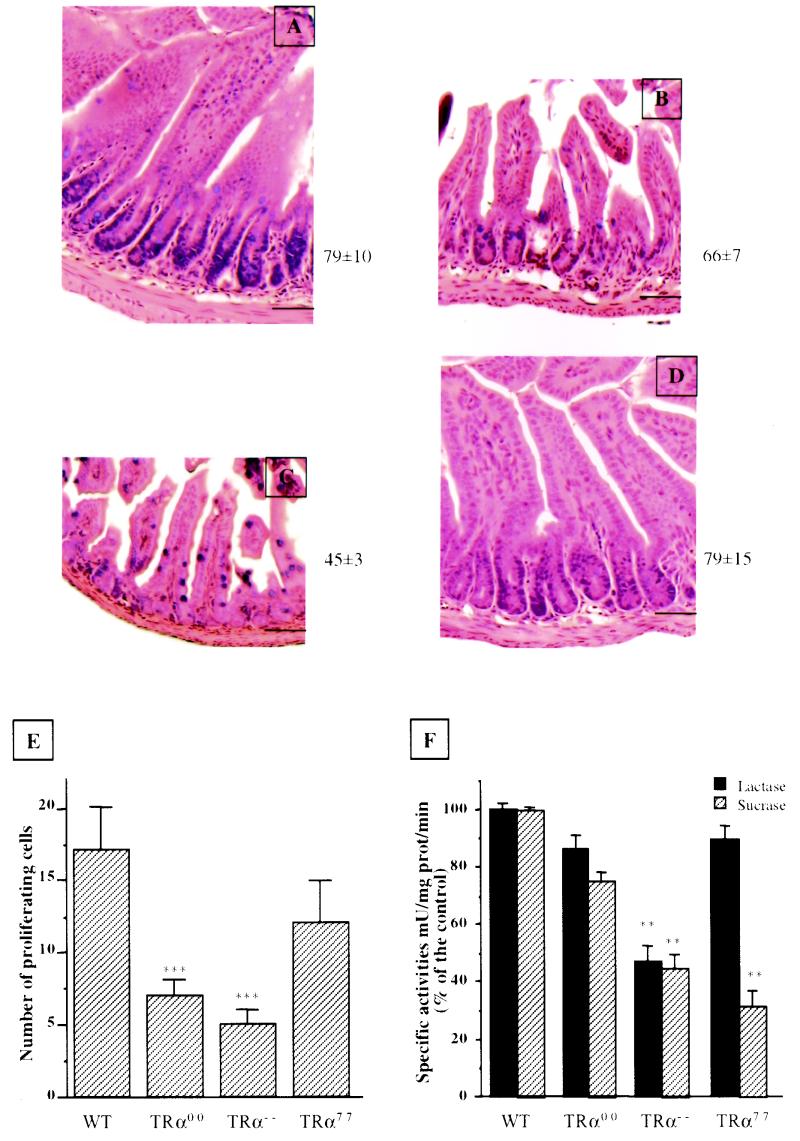
As shown in Fig. 5, the thickness of the mucosa in TRα0/0 mice was decreased compared to that of wild-type mice (Fig. 5B versus A), with a reduction of the villus height, resulting from a reduction of the number of epithelial cells per crypt-villus axis. This phenotype was milder than but comparable to the one observed in the TRα−/− ileum (Fig. 5C). No obvious morphological alteration was observed in the TRα7/7 distal ileum (Fig. 5D) compared to that of the wild type. A previous study described a decreased number of goblet cells in the distal ileum of 3-week-old TRα−/− mice compared to the wild type (25). In order to investigate whether the other TRα mutants also show this alteration, we determined the number of mucus-producing goblet cells stained by Schiff's reagent as previously described (25). The TRα−/− and TRα0/0 mice show similar significant decreases in the number of goblet cells compared to that of the wild type (TRα−/−, 4 ± 1 [mean ± SD]; TRα0/0, 6 ± 1; wild type, 14 ± 2); in contrast, the TRα7/7 mice displayed an increased amount (20 ± 3) statistically significantly different from that of the wild type (P < 0.05). The Paneth cells, a differentiated epithelial cytotype located in the intestinal crypts, displayed no altered proportions as demonstrated by specific staining or morphological criteria (data not shown).
FIG. 5.
(A through D) Morphological appearance of distal small intestine in 3-week-old animals. Five-micrometer histological sections of wild-type (A), TRα0/0 (B), TRα−/− (C), and TRα7/7 (D) mice were stained with hematoxylin and eosin. Bar, 30 μm. The numbers at the right of each picture indicate the number of epithelial cells per crypt-villus axis. (E) Analysis of proliferation of epithelial cells. The number of proliferating cells per crypt was evaluated after immunolabeling of cells expressing Ki67 antigen on distal ileum paraffin-embedded sections (5 μm). The Student t test indicated that in TRα0/0 and TRα−/− mice the number of Ki67-positive cells is significantly decreased compared to the number in wild-type animals. For each pair a P value of <0.0001 (∗∗∗) was obtained (n = 15). (F) Functional analysis of the differentiation markers of small intestine epithelial cells. Lactase and sucrase brush border activities were analyzed at the proximal jejunum level in 3-week-old wild-type and mutant animals. Enzymatic activities are represented as percentages of the respective wild-type levels. Statistical analysis was performed using the Student t test by comparing each group of mutant animals and the control. ∗, P < 0.05; ∗∗, P < 0.001 (n = 4).
(ii) Epithelial proliferation.
We then checked the effects of the TRα mutations on cell proliferation by determining the number of Ki67-positive cells per crypt (Fig. 5E). In TRα0/0 mice the number of Ki67-positive cells was significantly reduced, consistent with the reduction in the number of cells per axis described above. This decrease was more important in TRα−/− mice. The proliferation in TRα7/7 mice was also slightly decreased, although this difference did not reach statistical significance compared to wild-type mice. This is consistent with the absence of obvious intestinal morphological alteration in TRα7/7 mice.
(iii) Epithelial differentiation.
In order to analyze whether the functional polarization typical of differentiated enterocytes and the ontogenetic rise in sucrase expression occurring in mature enterocytes at weaning were affected by the different mutations of the TRα gene, we measured the enzymatic activities of lactase and sucrase in 3-week-old mutant mice (Fig. 5F). This study was conducted on brush border preparations from the proximal jejunal epithelium, as these enzymes are mainly expressed in this region of the small intestine (13). In TRα0/0 mice, both enzymatic activities were moderately decreased. In TRα−/− mice, the reduction of both activities was more dramatic. Interestingly, in TRα7/7 mice the lactase activity was not affected but the sucrase activity was considerably decreased, to less than 35% of that of the wild type.
In summary, in TRα−/− mice the epithelial proliferation was decreased and morphological (25) and biochemical parameters characteristic of enterocyte differentiation were impaired; in TRα0/0 mice, the proliferation was decreased but the differentiation was almost normal; in TRα7/7 mice, the functional polarization was normal but the expression of some markers specific for the mature differentiated epithelial cells was impaired. In addition, these data show that the TRα locus controls the fate of both enterocytes and goblet cells.
cdx1 and cdx2 gene expression is controlled by the TRΔα isoforms.
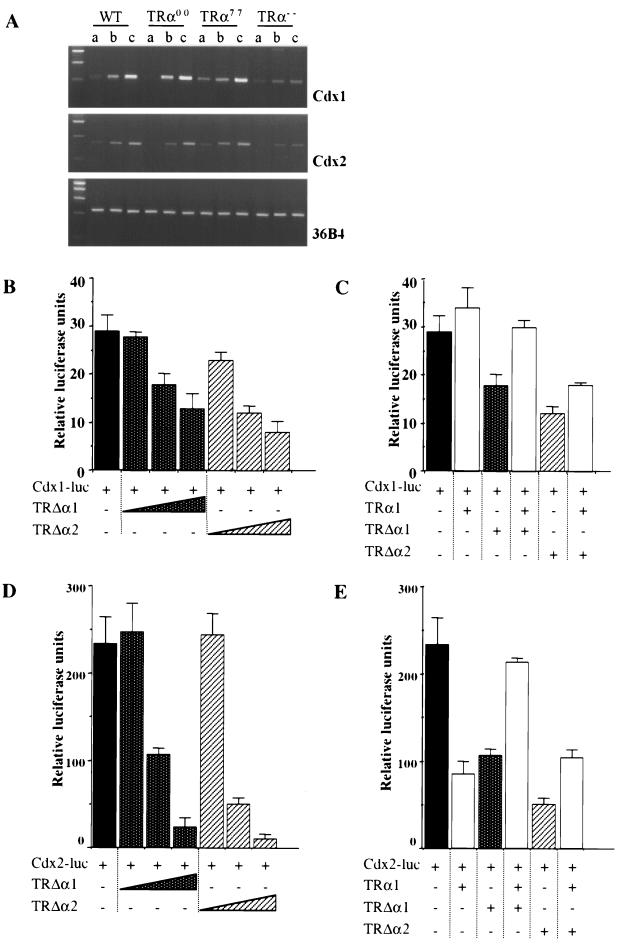
In an attempt to identify the molecular mechanisms responsible for the impaired proliferation and differentiation of the epithelial cells in TRα mutant mice, we examined the levels of expression of the Cdx1 and Cdx2 transcripts, which encode key homeoproteins involved in the control of intestinal epithelium homeostasis (reviewed in reference 8). The amounts of Cdx1 and Cdx2 mRNAs were estimated by semiquantitative RT-PCR analysis along the proximodistal intestinal axis. Figure 6A and a previous report (25) show that the increasing gradient of expression of these genes along the proximodistal axis is maintained in mice of each genotype. Moreover, the amounts of both transcripts were specifically decreased in each region of TRα−/− mice but unchanged in TRα0/0 and TRα7/7 mice compared to the amounts in wild-type mice. These data suggest that the TRΔα products, which are still expressed in TRα−/− mice, could have a negative regulatory role on the transcription of the cdx genes in the absence of TRα1. To challenge this assumption, we investigated the effects of TRΔα1 and TRΔα2 products on the activities of the cdx1 and cdx2 promoters by transient transfection assays in the human colonic cell line Caco2-TC7, using 5′ regulatory regions previously described (23). Figure 6 shows that both TRΔα1 and TRΔα2 repressed the activities of the cdx1 (Fig. 6B) and cdx2 (Fig. 6D) promoters in a dose-dependent manner. TRΔα1 did not affect the activity of a mouse β-actin promoter in Caco2-TC7 cells (data not shown) and has been previously shown to have no effect on various promoters in HeLa cells (5). TRΔα2 repressed the β-actin promoter activity by 50% when 1 μg of vector was transfected. TRΔα2 has previously been shown to exert transcriptional inhibition towards several promoters (5). Therefore, we assume that while the specificity of the TRΔα1 isoform appears tight, TRΔα2 can inhibit a wider spectrum of promoters. However, since TRΔα1 but not TRΔα2 was detected in the epithelium of distal ilea of wild-type and TRα−/− mice, this protein is likely to be responsible for the decreased levels of cdx transcripts observed in mutant mice. In vivo, the amount of cdx transcripts is reduced in TRα−/− mice, which lack the TRα1 and TRα2 isoforms. We have shown that TRα1 accelerated the degradation of the TRΔα1 protein in transfected HeLa cells (9a). We examined whether the expression of TRα1 in Caco2-TC7 cells was able to counteract the activity of the TRΔα isoforms. In the absence of T3, TRα1 did not affect the activity of the cdx1 promoter but inhibited the cdx2 promoter by 60% (Fig. 6C and E). The addition of T3 resulted in a slightly increased activity of the cdx1 promoter and did not affect the cdx2 promoter activity (data not shown). Remarkably, TRα1 in the absence (Fig. 6C) or in the presence (data not shown) of T3 partly alleviated the repression exerted by TRΔα1 and TRΔα2 on the activity of the cdx1 promoter. The coexpression of TRα1 and TRΔα1 or TRΔα2 resulted in the alleviation of the inhibitory activity of each product taken separately and led to at least partial restoration of full transcriptional activity of the cdx2 promoter (Fig. 6E). The TRα2 cotransfection did not modify the inhibitory activity of the TRΔα isoforms on the cdx promoters (data not shown). The modulation of the activity of the TRΔα products by TRα1 in transient transfection assays is consistent with the decreased levels of Cdx transcripts observed in vivo in the absence of TRs in TRα−/− mice (Fig. 6A). We conclude that transfection experiments and in vivo observations support the existence of reciprocal interactions between TRΔα products and the TRα1 protein. We propose that TRΔα products can alter the transcription of specific genes when they are not “buffered” by TRα1.
FIG. 6.
Expression and transcriptional regulation of cdx1 and cdx2 homeobox genes. (A) Representative results of RT-PCR analysis of cdx1 and cdx2 gene expression along the intestinal proximo-distal axis of knockout and wild-type (WT) animals. 36-B4 was used as an internal control. (B) Relative levels of luciferase activity triggered by the cdx1 promoter (1 μg of the pCdx1-4luc plasmid) in Caco2-TC7 cells cotransfected with increasing amounts of the expression vector pSG5mTRΔα1 or pSG5mTRΔα2 (10 ng, 100 ng, and 1 μg). (C) Relative levels of luciferase activity triggered by the cdx1 promoter as in panel B in Caco2-TC7 cells cotransfected or not with 900 ng of the expression vector pSG5hTRα1 and 100 ng of either the pSG5mTRΔα1 or the pSG5mTRΔα2 plasmid. (D) Relative levels of luciferase activity triggered by the cdx2 promoter (1 μg of the pCdx2-1luc plasmid) in Caco2-TC7 cells cotransfected with increasing amounts of the expression vector pSG5mTRΔα1 or pSG5mTRΔα2 (10 ng, 100 ng, and 1 μg). (E) Relative levels of luciferase activity triggered by the cdx2 promoter as in panel D in Caco2-TC7 cells cotransfected or not with 900 ng of the expression vector pSG5hTRα1 and 100 ng of either the pSG5mTRΔα1 or the pSG5mTRΔα2 plasmid. The cells were transiently transfected with a total of 2 μg of DNA/well; when necessary, the empty vector pSG5 was added. Cells were lysed 48 h after transfection. Results are mean values ± SD of three independent experiments conducted in triplicate.
TRΔα products control responsiveness to T3.
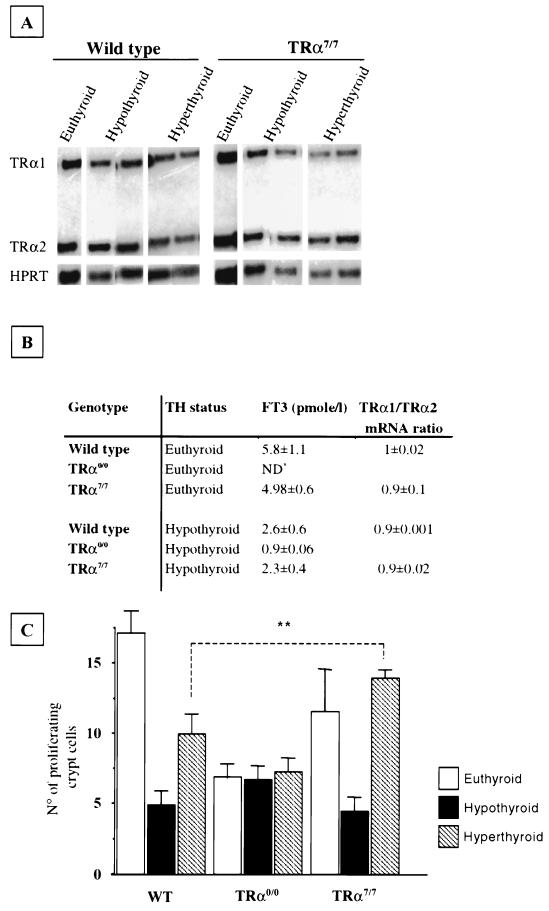
We have shown that the TRα0/0 animals displayed a decreased number of proliferating crypt cells and that TRα7/7 mice had a lower sucrase activity than did the wild type. To check whether altered T3 responsiveness could account for these phenotypes, we analyzed several morphological and functional parameters in wild-type, TRα0/0, and TRα7/7 mice in which experimental hypothyroidism or hyperthyroidism had been induced. Wild-type and TRα7/7 animals following a low-iodine diet supplemented with PTU (hypothyroid) displayed a reduction of the mucosa thickness related to a reduction of the crypt-villus height. This correlates with a decrease in the number of the epithelial cells per crypt-villus axis compared to the respective euthyroid 3-week-old mice (data not shown). It is noteworthy that hypothyroid TRα0/0 animals displayed the same morphological appearance as euthyroid animals of the same age. Indeed, the number of epithelial cells was not significantly decreased in TRα0/0 hypothyroid animals compared to that in euthyroid animals (data not shown). Upon administration of T3, which induced a hyperthyroid status, the number of epithelial cells did not change significantly in any of the strains analyzed. This is not surprising as the complete renewal of the epithelium is accomplished in 3 to 4 days (10), whereas T3 was administered only 48 h before the animals were sacrificed. Instead, we measured the number of proliferating crypt cells in the same experimental conditions (Fig. 7C). It is worth noting that hypothyroidism strongly reduced the numbers of proliferating cells in wild-type and TRα7/7 mice but was ineffective in TRα0/0 mice compared to euthyroid animals of the corresponding genotypes (Fig. 7C). Administration of T3 stimulated the proliferation in wild-type but not in TRα0/0 mice despite higher levels of serum T3 (Fig. 7B), demonstrating that the proliferative response to T3 is mediated by the TRα1 receptor. Surprisingly, T3-induced stimulation of proliferation was much larger in TRα7/7 mice (threefold increase) than in wild-type animals (twofold increase), although similar blood concentrations of T3 were measured in both sets of mice (Fig. 7B). In order to check whether the hypothyroid or hyperthyroid status modified TRα1 and TRα2 mRNA expression in wild-type and TRα7/7 animals, we quantified the relative abundance of the two mRNAs by RNase protection assay and densitometric analysis in both strains (Fig. 7A and B). The results clearly indicate that the relative amounts of TRα1 and TRα2 were not dependent on the thyroid hormone status.
FIG. 7.
Effects of hypothyroidism and hyperthyroidism induction on the small intestine. (A) Analysis of TRα1 and TRα2 expression by RNase protection assay on wild-type and TRα7/7 hypothyroid and hyperthyroid animals. The protected fragments are indicated on the left; HPRT was used as internal control. (B) Free T3 (FT3) blood concentrations (in picomoles per liter) in hypothyroid, euthyroid, and hyperthyroid animals of the indicated genotypes and densitometric analysis of the protected bands in the RNase protection assay. The study was performed to quantify the relative amounts of TRα1 and TRα2 in animals of different thyroid hormone statuses. Results are mean values ± SD; n = 3 or 4. ND∗, not determined. The total T3 concentration has been shown not to be different in TRα0/0 and wild-type mice (24). (C) Analysis of epithelial proliferation. The numbers of proliferating cells per crypt in hypothyroid and hyperthyroid animals were evaluated after immunolabeling of cells expressing Ki67 antigen on distal ileum paraffin-embedded sections (5 μm). The Student t test indicated that in TRα7/7 T3-treated animals the number of proliferating cells was significantly increased compared to that in wild-type T3-treated animals. ∗∗, P < 0.001.
This study indicates that in vivo, as in vitro (5), the TRΔα isoforms repress the responsiveness to T3 mediated by TRα1. In addition it enables us to definitively conclude that the TRα1 receptor is the only mediator of the T3-stimulated proliferation in the intestinal epithelium.
DISCUSSION
Thyroid hormone has long been suggested to be a regulator of the intestine developmental changes occurring at weaning (reviewed in reference 13). Indeed, the thyroid hormone-dependent intestinal remodeling during amphibian metamorphosis is well characterized (17, 29). The actions of the thyroid hormones are mediated by the TRs acting as transcription factors. Previous work and our more recent findings have led to the identification of four transcripts generated by the TRα locus. As all the isoforms produced by the TRα locus are expressed in the intestine, we analyzed morphofunctional parameters of this organ in mice with different TRα gene mutations. This enabled us to assign specific functions to the different TRα products. We demonstrated that the TRα1 receptor mediates the T3-dependent functions in the small intestine. We provide genetic evidence for the functions exercised by the TRΔα products in the postnatal intestinal development as well as in the control of T3 responsiveness.
The unbalanced expression of TRα and TRΔα products accounts for the strong intestinal phenotype of TRα−/− mutants.
It has been shown that in TRα−/− mice the expression of the TRΔα transcripts was maintained in the absence of the TRα transcripts and that severe alterations occurred, particularly in the distal small intestine (25). The intestinal phenotype is characterized by impairment of cell proliferation and differentiation, associated with the downregulation of cdx1 and cdx2 homeobox gene expression. In contrast, in the TRα0/0 mice, which lack all TRα and TRΔα isoforms, only the proliferation of the epithelial cells is affected. These data establish that the TRα− and the TRα0 mutations generate very different phenotypes. Theoretically, the severity of the phenotype in TRα−/− mice could be attributed either to the low concentration of thyroid hormones associated with this mutation (7) or to the unbalanced expression of the TRΔα and TRα products. The former hypothesis can be definitely ruled out since intestinal impairment is already observed in 2-week-old TRα−/− mice which retain normal TH concentrations at this preweaning age (25). Ruling out the difference in the thyroid hormone status as the factor accounting for the discrepancies between the phenotypes observed in TRα−/− and TRα0/0 mice implies that the cause must be the altered balance between the TRα and TRΔα products of the TRα locus.
TRα1 mediates T3-dependent functions in intestinal epithelial cells.
The data reported in the present study clearly show that the proliferation of epithelial cells in the crypts of the small intestine, which was shown to be enhanced at weaning (30, 33), is modulated by T3 through a TRα1-dependent pathway. The further deletion of the TRβ genes does not worsen this phenotype (9a), demonstrating that it is exclusively mediated by the TRα1 receptor. Moreover, the experiment of induced hypothyroidism and hyperthyroidism in TRα0/0 mice confirms that TRα1 is the only receptor which mediates the T3-dependent control of crypt cell proliferation, at least during the weaning period. We also showed that the numbers of mucus-producing goblet cells are decreased in similar proportions in TRα−/− and TRα0/0 animals, indicating that the TRα1 receptor is indeed involved in the previously described role of T3 on goblet cell maturation (2). Finally, we show that in TRα0/0 mice the epithelial differentiation is slightly but not significantly impaired, suggesting that the T3-TRα1 pathway is not a major regulator of intestinal epithelial differentiation.
Functional interference between TRα1 and TRΔα isoforms.
In TRα7/7 mice, the levels of expression of the TRα1 and TRα2 transcripts are unchanged, but the production levels of TRΔα1 and TRΔα2 transcripts and of the TRΔα1 protein are reduced. Despite the persistence of small amounts of TRΔα transcripts, a clear alteration in the intestinal epithelium is observed in these mice. This emphasizes the physiological importance of these proteins in wild-type animals. The enhancement of T3-stimulated proliferation as well as the increase in the number of goblet cells in untreated TRα7/7 mice compared to wild-type mice suggests that TRΔα products down modulate the activity of T3. Since we have shown that the proliferation of crypt epithelial cells and the goblet cell maturation depend on T3-TRα1, this implies that TRΔα products regulate the activity of TRα1, in agreement with our previous observations in transient transfection experiments (5). Another striking feature of TRα7/7 mice is the low specific activity of sucrase. In contrast to what is observed with TRα−/− mice, this is not the consequence of an impaired differentiation since lactase activity (this paper) and the number of sucrase-expressing cells, as assessed by immunohistochemical staining using antisucrase antisera (our unpublished data), are not affected in TRα7/7 mice. Instead, it may reflect a delay in the maturation process at weaning. The colocalization of the TRΔα1 and sucrase proteins in the epithelial cells (13) of the villi in wild-type mice is consistent with a potential regulatory effect of TRΔα isoforms on sucrase gene expression. Since no significant reduction of sucrase activity is observed in the small intestines of TRα0/0 mice, lacking not only TRΔα but also TRα products, the down-regulation of sucrase expression observed in TRα7/7 mice is not due to an autonomous activity of TRΔα isoforms but might be the result of interference with the other TRα gene products, although the mechanisms involved are unknown. Altogether, our data support the assumption that TRΔα products modulate the activity of TRα proteins in vivo and that this modulation is essential to ensure the normal maturation of the small intestine.
Genetic and biochemical evidence for TRΔα functions.
TRα0/0 mice, which have lost both the TRα and TRΔα genes, do not exhibit the very severe phenotype shown by TRα−/− mice. We conclude that this phenotype is the consequence of the unbalanced expression of the TRΔα versus the TRα gene in TRα−/− mutants. We have demonstrated that the stability of the TRΔα1 protein is negatively controlled by the α1 receptor (9a), and that the inhibitory activity of TRΔα1 over the transcription of the cdx1 and cdx2 promoters was alleviated by the expression of TRα1. This suggests that, when TRα1 is present, the amount and hence the activity of TRΔα1 are blunted. In contrast, in the absence of the α1 receptor (i.e., in TRα−/− mice), the stability and thus the amount of TRΔα1 protein are increased, leading to deleterious effects. Careful examination of the expression pattern of the TRΔα1 protein in the intestinal epithelium supports this assumption. In wild-type mice, this protein is detected as a faint signal in the crypts compared to the stronger staining in the villi. In TRα−/− mice, the staining reaches the same intensity in both compartments, revealing increased amounts of the TRΔα1 protein in the crypts of these animals compared to amounts in the wild type. In striking correlation with this altered expression pattern, the cell proliferation in crypts and their further differentiation are obviated in TRα−/− mice. Interestingly, in vitro and in vivo in the absence of TRα1, the products of the TRΔα genes can repress the transcription of cdx1 and cdx2, two genes which control intestinal cell proliferation and differentiation (8). The molecular mechanisms of such repression are not yet clear. The TRΔα products may act on the cdx promoters or may interfere in vivo with other nuclear receptors such as retinoic acid receptors, as suggested by transfection experiments (5; our unpublished observations) and by the direct activation of the cdx1 promoter by retinoic acid (16). They may, however, also affect other signaling pathways.
In conclusion, we demonstrate here the physiological importance of TRΔα products. We show that these isoforms, in the absence of the TRα proteins, alter the transcription of specific genes that regulate intestinal homeostasis, impair cell proliferation and differentiation in the small intestine epithelial cells, and generate a severe, lethal phenotype. We show that a reduced expression of the TRΔα gene increases T3-mediated functions and leads to a decreased sucrase activity in enterocytes. Therefore, the TRΔα isoforms should interfere with T3-dependent and T3-independent pathways. We conclude that the TRα locus generates in the intestine TRΔα and TRα isoforms, which negatively control each other and whose balanced expression is critical for the correct development of the small intestine at weaning.
ACKNOWLEDGMENTS
We warmly thank Claude Legrand and Aude Conscience for their expert technical help and Denise Aubert, who manages the transgenic facility at ENS Lyon. We thank D. Belgarbi and C. Morin for animal breeding. We thank Thomas Lamonerie for providing the plasmid containing the loxP flanked selection cassette and the Cre expression vector. We also thank F. Flamant and M. Kedinger for critical reading of the manuscript.
This work was supported by grants from the Association pour la Recherche contre le Cancer and Région Rhône-Alpes (grant no. 700006058) and by a grant from Human Frontier Scientific Program (fellowship RG0347/1999.M). C.D.D. was a recipient of a fellowship from la Fondation Ipsen.
REFERENCES
- 1.Baas D, Fressinaud C, Ittel M E, Reeber A, Dalençon D, Puymirat J, Sarlievre L L. Expression of thyroid hormone receptor isoforms in rat oligodendrocyte cultures. Effect of 3,5,3′-triiodo-l-thyronine. Neurosci Lett. 1994;176:47–51. doi: 10.1016/0304-3940(94)90868-0. [DOI] [PubMed] [Google Scholar]
- 2.Black B L, Moog F. Goblet cells in embryonic intestine: accelerated differentiation in culture. Science. 1977;197:368–370. doi: 10.1126/science.560059. [DOI] [PubMed] [Google Scholar]
- 3.Castillo R O, Gregory F, Glasscock F, Noren K M, Reisenauer A M. Pituitary regulation of postnatal small intestinal ontogeny in the rat: differential regulation of digestive hydrolase maturation by thyroxine and growth hormone. Endocrinology. 1991;129:1417–1423. doi: 10.1210/endo-129-3-1417. [DOI] [PubMed] [Google Scholar]
- 4.Chantret L, Rodolosse A, Barbat A, Dussaulx E, Brot-Laroche E, Zweibaum A, Rousset M. Differential expression of sucrase-isomaltase in clones isolated from early and late passages of the cell line Caco-2: evidence for glucose-dependent negative regulation. J Cell Sci. 1994;107:213–225. doi: 10.1242/jcs.107.1.213. [DOI] [PubMed] [Google Scholar]
- 5.Chassande O, Fraichard A, Gauthier K, Flamand F, Legrand C, Savatier P, Laudet V, Samarut J. Identification of transcripts initiated from an internal promoter in the c-erbAα locus that encode inhibitors of retinoic acid receptor-α and triiodothyronine receptor activities. Mol Endocrinol. 1997;11:1278–1290. doi: 10.1210/mend.11.9.9972. [DOI] [PubMed] [Google Scholar]
- 6.Forrest D, Vennstrom B. Functions of thyroid hormone receptors in mice. Thyroid. 2000;10:41–52. doi: 10.1089/thy.2000.10.41. [DOI] [PubMed] [Google Scholar]
- 7.Fraichard A, Chassande O, Plateroti M, Roux J P, Trouillas J, Dehay C, Legrand C, Gauthier K, Kedinger M, Malaval L, Rousset B, Samarut J. The TRα gene encoding a thyroid hormone receptor is essential for post-natal development and thyroid hormone production. EMBO J. 1997;16:4412–4420. doi: 10.1093/emboj/16.14.4412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Freund J-N, Domon-Dell C, Kedinger M, Duluc I. The Cdx1 and Cdx2 homeobox genes in the intestine. Biochem Cell Biol. 1998;76:1–12. doi: 10.1139/o99-001. [DOI] [PubMed] [Google Scholar]
- 9.Gauthier K, Chassande O, Plateroti M, Roux J P, Legrand C, Pain B, Rousset B, Weiss R, Trouillas J, Samarut J. Different functions for the thyroid hormone receptors TRα and TRβ in the control of thyroid hormone production and post-natal development. EMBO J. 1999;18:623–631. doi: 10.1093/emboj/18.3.623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9a.Gauthier K, Plateroti M, Harvey C B, Williams G R, Weiss R E, Refetoff S, Willott J F, Sundin V, Roux J-P, Malaval L, Hara M, Samarut J, Chassande O. Genetic analysis reveals different functions for the products of the thyroid hormone receptor α locus. Mol Cell Biol. 2001;21:4748–4760. doi: 10.1128/MCB.21.14.4748-4760.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Gordon J I. Intestinal epithelial cell differentiation: new insights from chimeric and transgenic mice. J Cell Biol. 1989;108:1187–1194. doi: 10.1083/jcb.108.4.1187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Grasset E, Pinto M, Dussaulx E, Zweibaum A, Desjeux J F. Epithelial properties of human colonic carcinoma cell line Caco-2: electrical parameters. Am J Physiol. 1984;247:C260–C267. doi: 10.1152/ajpcell.1984.247.3.C260. [DOI] [PubMed] [Google Scholar]
- 12.Henning S J. Functional development of the gastrointestinal tract. In: Johnson L R, et al., editors. Physiology of the gastrointestinal tract. 2nd ed. New York, N.Y: Raven; 1987. pp. 285–300. [Google Scholar]
- 13.Henning S J, Rubin D C, Shulman J. Ontogeny of the intestinal mucosa. In: Johnson L R, et al., editors. Physiology of the gastrointestinal tract. 3rd ed. New York, N.Y: Raven; 1994. pp. 571–601. [Google Scholar]
- 14.Hodin R A, Sherman M, Chamberlain M, Meng S. Pattern of rat intestinal brush border enzyme gene expression changes with epithelial growth state. Am J Physiol. 1995;269:C385–C391. doi: 10.1152/ajpcell.1995.269.2.C385. [DOI] [PubMed] [Google Scholar]
- 15.Hodin R A, Shei A, Morin M, Meng S. Thyroid hormone and the gut: selective transcriptional activation of a villus-enterocyte marker. Surgery. 1996;120:138–143. doi: 10.1016/s0039-6060(96)80280-7. [DOI] [PubMed] [Google Scholar]
- 16.Houle M, Prinos P, Iulianella A, Buchard N, Lohnes D. Retinoic acid regulation of Cdx1: an indirect mechanism for retinoids and vertebral specification. Mol Cell Biol. 2000;20:6579–6586. doi: 10.1128/mcb.20.17.6579-6586.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kaltenbach J C. Endocrinology of amphibian metamorphosis. In: Gilbert L I, Tate J B, Atkinson B G, editors. Metamorphosis. New York, N.Y: Academic Press; 1996. pp. 403–431. [Google Scholar]
- 18.Kedinger M. Growth and development of intestinal mucosa. In: Campbell F C, editor. Small bowel enterocyte culture and transplantation. Georgetown, Tex: Landes; 1994. pp. 1–31. [Google Scholar]
- 19.Klein R M. Small intestinal proliferation during development. In: Lebenthal E, editor. Human gastrointestinal development. New York, N.Y: Raven; 1989. pp. 367–392. [Google Scholar]
- 20.Koenig R J, Lazar A A, Hodin R A, Brent G A, Larsen P R, Chin W W, Moore D D. Inhibition of thyroid hormone action by a non-hormone binding c-erbA protein generated by alternative mRNA splicing. Nature. 1989;337:659–661. doi: 10.1038/337659a0. [DOI] [PubMed] [Google Scholar]
- 21.Lazar M A, Hodin R A, Chin W W. Human carboxyl-terminal variant of α-type c-erbA inhibit trans-activation by thyroid hormone receptors without binding thyroid hormone. Proc Natl Acad Sci USA. 1989;86:7771–7774. doi: 10.1073/pnas.86.20.7771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lazar M A. Thyroid hormone receptors: multiple forms, multiple possibilities. Endocr Rev. 1993;14:184–193. doi: 10.1210/edrv-14-2-184. [DOI] [PubMed] [Google Scholar]
- 23.Lorentz O, Cadoret A, Duluc I, Capeau J, Gespach C, Cherqui J, Freund J N. Down regulation of the colon tumor suppressor homeobox gene cdx2 by oncogenic Ras. Oncogene. 1999;18:87–92. doi: 10.1038/sj.onc.1202280. [DOI] [PubMed] [Google Scholar]
- 24.Macchia P E, Takeuchi Y, Kawai T, Cua K, Gauthier K, Chassande O, Seo H, Hayashi Y, Samarut J, Murata Y, Weiss R E, Refetoff S. Increased sensitivity to thyroid hormone in mice with complete deficiency of thyroid hormone receptor α. Proc Natl Acad Sci USA. 2001;98:349–354. doi: 10.1073/pnas.011306998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Plateroti M, Chassande O, Fraichard A, Gauthier K, Freund J N, Samarut J, Kedinger M. Involvement of T3Rα- and β-receptor subtypes in mediation of T3 functions during post-natal murine intestinal development. Gastroenterology. 1999;116:1367–1378. doi: 10.1016/s0016-5085(99)70501-9. [DOI] [PubMed] [Google Scholar]
- 26.Sap J, Munoz A, Damm K, Goldberg Y, Ghysdael J, Leutz A, Beug H, Vennstrom B. The c-erbA protein is a high affinity receptor for thyroid hormone. Nature. 1986;324:635–640. doi: 10.1038/324635a0. [DOI] [PubMed] [Google Scholar]
- 27.Schlüter C, Duchrow M, Wohlenberg C, Becker M H, Key G, Flad H D, Gerdes J. The cell proliferation-associated antigen of antibody Ki67: a very large, ubiquitous nuclear protein with numerous repeated elements, representing a new kind of cell cycle-maintaining proteins. J Cell Biol. 1993;123:513–522. doi: 10.1083/jcb.123.3.513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Simon-Assmann P, Kedinger M, Grenier J F, Haffen K. Control of brush border enzymes by dexamethasone in the foetal rat intestine cultured in vitro. J Pediatr Gastroenterol Nutr. 1982;1:257–265. doi: 10.1097/00005176-198201020-00017. [DOI] [PubMed] [Google Scholar]
- 29.Tata J R. Gene expression during metamorphosis: an ideal model for post-embryonic development. Bioessays. 1993;15:239–248. doi: 10.1002/bies.950150404. [DOI] [PubMed] [Google Scholar]
- 30.Wall A J, Middleton W R J, Pearse A G E, Booth C C. Intestinal mucosa hyperplasia following induced hyperthyroidism in the rat. Virchows Arch. 1970;6:79–87. doi: 10.1007/BF02899113. [DOI] [PubMed] [Google Scholar]
- 31.Weinberger C, Thompson C C, Ong E S, Lebo R, Gruol D J, Evans R M. The c-erbA gene encodes a thyroid hormone receptor. Nature. 1986;324:641–646. doi: 10.1038/324641a0. [DOI] [PubMed] [Google Scholar]
- 32.Williams, G. R. Cloning and characterization of two novel thyroid hormone receptor b isoforms. Mol. Cell. Biol. 20:8329–8342. [DOI] [PMC free article] [PubMed]
- 33.Yeh K Y, Moog F. Development of the small intestine in the hypophysectomized rat. II. Influence of cortisone growth hormone and prolactin. Dev Biol. 1975;49:173–184. doi: 10.1016/0012-1606(75)90271-7. [DOI] [PubMed] [Google Scholar]