Abstract
Hereditary hemochromatosis (HH) is a common genetic metabolic disorder characterized by excessive iron absorption and elevated serum iron levels, which accumulate in various organs, such as the heart, pancreas, gonads, and damage these organs. There are only a few articles and clinical studies describing the characteristics of cardiac involvement in HH along with the significance of early diagnosis and management in preventing complications. In this review article, we have reviewed multiple pieces of literature and gathered available information regarding the subject. We compiled the data to investigate the importance of early detection of symptoms, regular monitoring, and prompt management with strict adherence to reverse or prevent complications. This article has reviewed different aspects of cardiac hemochromatosis, such as pathogenesis, clinical presentation, diagnosis, and management. Recognition of early symptoms, diagnosis of cardiac involvement with various modalities, and implementation of early treatment are essentially the foundation of better outcomes in HH.
Keywords: cardiac arrhythmia, primary iron overload, cardiac hemochromatosis, iron overload cardiomyopathy, hereditary hemochromatosis
Introduction and background
HH is an inherited, multi-systemic, progressive metabolic disease characterized by systemic iron overload and iron deposition in various visceral organs [1]. It is one of the most common inherited metabolic diseases worldwide and predominantly found among the Caucasian population with a frequency of the mutated gene C282Y found in one to two individuals in every 500 and less frequently found among Hispanics, Asians, and African Americans [2].
Historically, the first case of HH was described by French physician Armand Trousseau in the mid-19th century [3]. In 1935, Joseph Sheldon, a British geriatrician, described 311 case records in his monologue and concluded that hemochromatosis is an inherited disorder and resulted from the accumulation of iron in multiple organs [4]. Hemochromatosis was first linked to the HFE gene in 1996 by Feder et al.; 83% of patients were homozygous for missense mutation of HFE in the study [5]. Later on, multiple other genes were identified responsible for a minority of cases of HH such as hemojuvelin (HJV), transferrin receptor 2 (TfR2), and ferroportin [6-8]. The genetic mutations that contribute to the pathogenesis of HH are multiple and have an expected outcome of excessive iron concentration in plasma.
The pathogenesis of HH involves the failure of the downregulation of iron metabolism. HFE-related hemochromatosis results from abnormally low levels of hepcidin, a key regulator of iron absorption from the duodenum, and the release of iron from the macrophage, secreted by the hepatocytes in response to decreasing plasma levels of iron [9]. The disorder can present with various symptoms, the most common being fatigue, malaise, and arthralgia [10]. In any individual with suspicion of HH, measurement of serum iron, ferritin, and transferrin saturation (TS) is recommended [11]. Management primarily involves the reduction of serum iron as well as total body iron by phlebotomy, as it has shown to slow the course of the disease, reverse organ damage, and prevent long-term complications [11-12]. Iron chelators are alternative therapy required in patients with anemia requiring chronic blood transfusion, unstable hemodynamic status, and a few refractory cases [13-14]. Heart failure and hepatic carcinoma are leading causes of mortality in HH, and approximately one-third of patients succumb to death as a result of cardiac causes [15-16]. The cardiac involvement in HH can have an insidious progression that eventually, over the course of the disease, leads to irreversible myocyte damage as the cells are overwhelmed by the deposition of iron, making the management of such cases challenging [17].
For reasons mentioned in the preceding paragraph, it is of paramount importance to detect cardiac involvement in patients of HH at the earliest, with prompt initiation of treatment before the advent of complications.
In this article, we discuss the following aims and objectives: 1. Explore the early manifestations of cardiac hemochromatosis; 2. Emphasize the importance of early diagnosis of cardiac involvement; 3. Outline various modalities of management of cardiac hemochromatosis.
Review
Iron metabolism and pathogenesis of cardiac hemochromatosis
Iron has a substantial role in various biochemical reactions, and it is also the main component of biomolecules, such as hemoglobin and myoglobin, and multiple enzymes [18]. Furthermore, iron catalyzes oxidative reactions, which generate reactive oxygen species (ROS) [19-20]. Hence, excessive amounts of iron will lead to grave effects by generating excessive amounts of ROS, which mandates strict control of its level in the body. There is no specific pathway of iron excretion from the body except the losses that occur through the shedding of intestinal mucosal cells and menstruation in females [21]. Therefore, the most effective way to control the iron level in the body is through restricted absorption and not excretion.
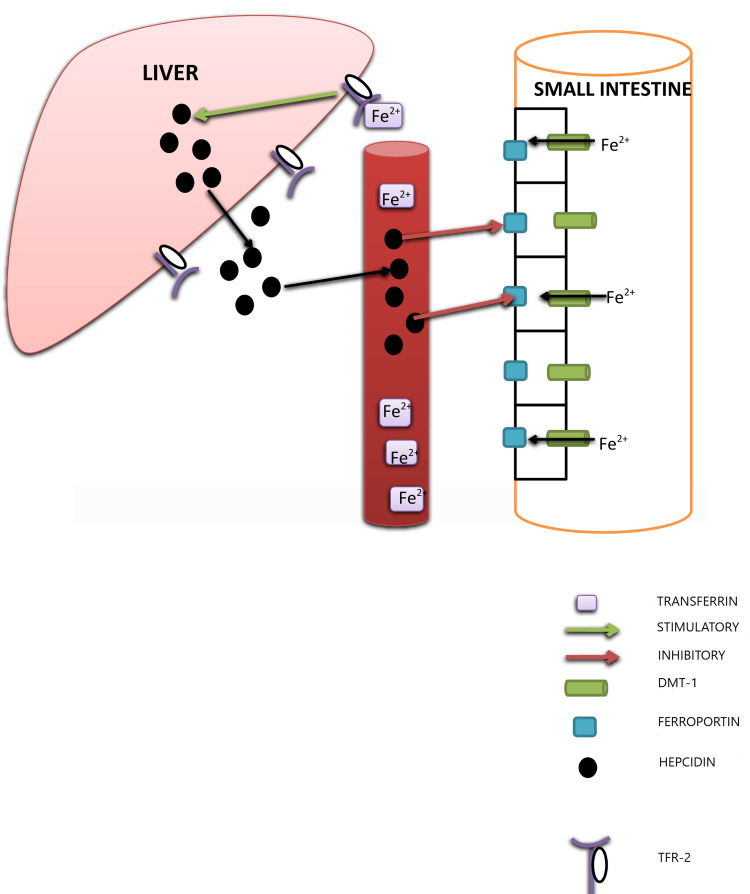
Hepcidin controls the activity of ferroportin, an iron exporter present on the basolateral surface of enterocytes, macrophages, and hepatocytes (Figure 1) [22]. Although mechanisms and mediators responsible are different, all types of HH, in common, have abnormally low hepcidin levels. Reduced hepcidin level manifests as uninhibited activity of ferroportin and resultant excessive iron absorption, especially in classical HH [23]. More importantly, in classical HH, the macrophage plays a significant role than the duodenal enterocytes [23]. In patients of HH, macrophages release more iron than an average individual [24]. Irrespective of the clinical subtype, HH invariably involves dysfunctional iron metabolism. Usually, the body absorbs 1 to 2 mg iron daily; in HH, however, this reaches up to 8 to 10 mg per day [25]. Initially, transferrin saturation increases, which is the earliest biochemical marker of iron overload [26]. Gradually, as transferrin saturation reaches 75%, further iron remains in free form in the plasma, which has been termed non-transferrin-bound-iron (NTBI) and is central to the pathogenesis of HH, mainly through the generation of reactive oxygen species [19].
Figure 1. Schematic representation of iron metabolism and regulation.
DMT-1: divalent metal transporter-1; TFR-2: transferrin receptor-2
HH can be divided into various subtypes (Table 1) [27].
Table 1. Clinical subtypes of hereditary hemochromatosis.
HAMP: hepcidin anti-microbial peptide; HJV: hemojuvelin; tfr-2: transferrin receptor-2
| SUBTYPE | INHERITANCE | MUTATED GENE | SEVERITY | AGE OF ONSET | AFFECTED POPULATION | PHENOTYPE |
| Type 1 | Autosomal Recessive | HFE | Mild | 4-5 decade | Middle-aged white male | Liver disease, Arthralgia, Fatigue |
| Type 2 | Autosomal Recessive | 2a: HJV | Severe | 1-2 decade | Young, white/non-white, Male/female | Cirrhosis, Arrhythmia, Heart failure |
| 2B: HAMP | ||||||
| Type 3 | Autosomal Recessive | tfr-2 | Mild to severe | 3-4 decade | Young, white/non-white, Male/female | Endocrinopathy, Cardiomyopathy, Liver failure |
| Type 4 | Autosomal Dominant | Ferroportin | Mild | 4-5 decade | White/non-white, Male/female of all ages | Liver abnormalities, Marginal anemia, Hyperferritinemia |
Cardiac hemochromatosis is a common occurrence in patients of HH. It involves a multitude of presentations, which include but are not limited to cardiomyopathy, arrhythmia, and sudden cardiac death [28]. Gradually, in HH, as iron concentration builds up, the free iron eventually starts to accumulate in the heart. The free iron enters cardiomyocytes through the L-type calcium channel (LTCC) [29]. The free iron generates ROS through the Fenton reaction. It dramatically accelerates the production of hydroxyl ions, which damage the organelle membrane, cell membrane, and lysosomes, eventually leading to cell death in the heart [30]. Since cardiac myocytes have a high number of mitochondria and fewer antioxidants, they are particularly susceptible to damage from ROS [31]. All the subtypes have similar syndromic presentations because of identical pathogenesis and organ involvement. The only difference is the severity of the disease, with the juvenile form of HH involving the heart at an early stage with rapid deterioration [28]. Excess iron deposition begins from the epicardium and progresses towards the endocardium, which explains the preservation of ventricular systolic function till late in the disease [32]. In contrast to the previously held opinion that free iron gets deposited in the interstitial space, recent histological observations have shown that the iron is indeed deposited in the sarcoplasm [33]. Therefore, iron deposition in the heart is a form of storage rather than an infiltrative process, and potentially, it can be reversed with the initiation of appropriate therapy.
Cardiac hemochromatosis: clinical presentation
About 15% of HH patients have cardiac symptoms as a chief complaint. Symptoms appear insidiously and progress gradually, consisting of non-specific symptoms such as malaise, fatigue, and lethargy that worsen over time [28]. These symptoms are mainly the result of cardiomyopathy that can be either restrictive or dilated and has a progressive course that can result in death due to congestive heart failure if not treated promptly. Palpitations and sudden cardiac death occur due to iron deposition in the cardiac conduction system [34]. Udani et al. conducted a retrospective cohort study on 63,846,188 hospitalized patients to determine the prevalence and risk of CV manifestations in patients with hemochromatosis and discovered that among 64,590 patients with hemochromatosis, 27.8% had one or more cardiovascular manifestations [35]. Among CV manifestations, arrhythmias, congestive heart failure (CHF), and pulmonary hypertension were the most common [35].
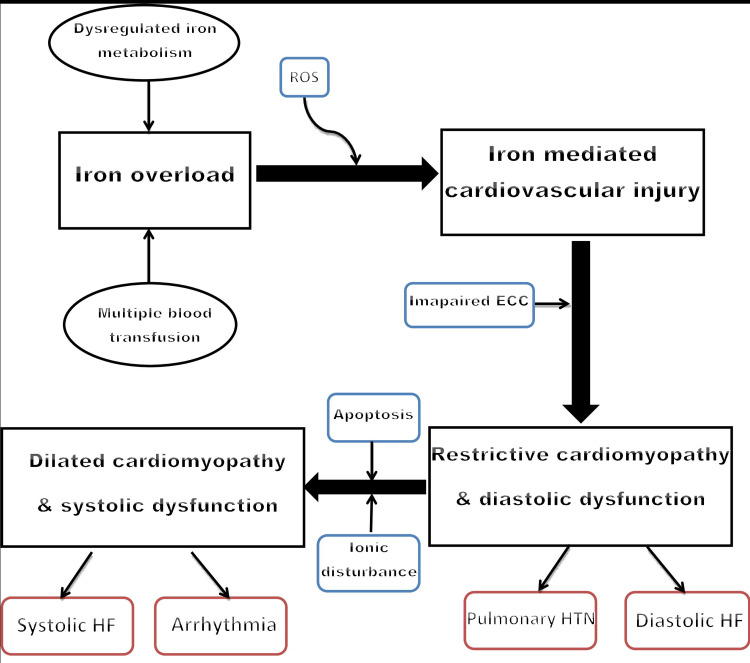
Iron overload cardiomyopathy (IOC) can be either systolic or diastolic cardiac dysfunction originating from iron accumulation in cardiomyocytes, independent of other concomitant abnormalities such as ischemic cardiomyopathy, atherosclerosis, or valvular diseases [36]. IOC is a cardinal cause of mortality, especially in the second and third decades of life. In the long term, it has been called one of the most significant predictors of mortality in patients with iron overload [37]. IOC has common characteristics and progresses in a predictable way irrespective of the type of hemochromatosis, characterized by early restrictive cardiomyopathy (RCM) with diastolic dysfunction, which, if not treated in the early stage, invariably progresses to late dilated cardiomyopathy (DCM) with impaired systolic function (Figure 2) [38]. Oxidative stress in cardiomyocytes due to excessive free iron accumulation leads to impaired excitation-contraction coupling (ECC) and impaired function of sarcoendoplasmic reticulum calcium ATPase 2 (SERCA2) and sodium-calcium exchanger (NCX) channel leading to high intracellular calcium and sodium concentration, respectively [39-40]. These ionic changes within cardiomyocytes collectively lead to reduced peak systolic and high diastolic calcium levels, resulting in impaired systolic and diastolic function, respectively [41]. After entering into cardiomyocytes through L-type calcium channels, Fe+2 leads to the slowing of the Ca+2 current resulting in high intracellular Ca+2, which explains the early diastolic dysfunction [29]. Later on, with persistently elevated levels of Fe+2, transport of Ca+2 across the membrane is impaired because Fe+2 competes for the same iron transporter, which manifests as late systolic dysfunction seen in IOC [29]. Initially, patients have no overt symptoms but eventually, as diastolic dysfunction develops, non-specific symptoms such as mild to moderate exertional fatigue and exercise intolerance, appear [42]. Shizukuda et al. carried out a study on 43 asymptomatic HH patients with echocardiographic monitoring to evaluate the relationship between left ventricular diastolic function and oxidative stress and demonstrated a significant association between oxidative stress and left ventricular diastolic dysfunction in patients with HH in the early stage, with no overt symptoms and more than 55% ejection fraction (EF) [43]. Early in the disease, restrictive cardiomyopathy presents as diastolic heart failure with symptoms such as exertional dyspnea, exercise intolerance, orthopnea, peripheral swelling, and paroxysmal nocturnal dyspnea [44]. Later, untreated cases progress to irreversible dilated cardiomyopathy due to apoptosis of cardiomyocytes because of long-term iron deposition and subsequent damage by ROS. Dilated cardiomyopathy presents as systolic dysfunction and is often biventricular.
Figure 2. The spectrum of iron overload cardiomyopathy.
ROS: reactive oxygen species; ECC: excitation-contraction coupling; HF: heart failure; HTN: hypertension
Rhythm abnormalities in cardiac hemochromatosis are common. Its etiopathogenesis involves iron deposition in the sinoatrial (SA) node, atrioventricular (AV) node, and cardiac conduction system [45]. In a clinical study undertaken by Shizukuda et al., 42 asymptomatic patients with homozygosity of C282Y showed no significant incidence of arrhythmia. Over six months, reducing iron levels in asymptomatic HH patients did not statistically reduce the incidence of arrhythmias [46]. They concluded that oxidative stress, rather than systemic iron-overload, is the main culprit behind arrhythmia in HH. After treatment with multiple phlebotomies, subjects have constantly elevated oxidative stress, which explains the persistence of arrhythmia in patients with near-normal serum iron levels [46]. These abnormalities include atrial tachyarrhythmias, premature ventricular beats, nodal block, and ventricular tachycardia, among which supraventricular arrhythmia is the most common cardiac rhythm disorder [16,47]. The iron itself is arrhythmogenic, as it can interfere with the cardiac conduction system [48]. Kenneth et al. conducted a study on the experimental animal model of IOC to determine the molecular and cellular mechanisms responsible for arrhythmia in subjects with iron overload. They hypothesized and demonstrated that iron-induced reduction in the sodium current is central to the pathogenesis of arrhythmia [49]; apart from this, they discovered fixed regions of conduction block that contribute to arrhythmia by establishing a re-entrant circuit [49]. Thus, action potential shortening and abnormal electrical impulse conduction due to heterogeneous iron deposition are essentially responsible for unidirectional block and arrhythmia in hemochromatosis [50]. Irregular rhythm may be due to atrial fibrillation, premature atrial contraction, or premature ventricular contractions. On the other hand, regular rhythm with rapid heart rate is due to atrial flutter, paroxysmal supraventricular tachycardia, or sustained ventricular tachycardia [50]. Symptoms may include palpitation, fatigue, chest discomfort, and frank syncope resulting from bradyarrhythmias [51].
There is little evidence to support the exact pathophysiological mechanism accounting for pulmonary hypertension in hemochromatosis. It is theorized that iron-induced damage of endothelium results in vasoconstriction in addition to smooth muscle proliferation of the media [52]. Sudden cardiac death that occurs in hemochromatosis is due to end-stage irreversible cardiomyopathy culminating in cardiogenic shock or fatal arrhythmia [35].
Diagnosis
With the advancement in diagnostic techniques, numerous patients with cardiac involvement in HH are diagnosed early. Still, a large number of patients remain undiagnosed or underdiagnosed. It is imperative to detect cardiac disease in the early asymptomatic stage before it progresses to irreversible cardiomyopathy and subsequent heart failure. Early diagnosis though challenging, is essential, and various modalities, such as biochemical, genetic, radiological, and histological analysis, should be incorporated [53]. Physical examination and thorough history evaluation are similarly essential. A significant degree of cardiac dysfunction has already occurred before symptoms of cardiac hemochromatosis appear. Hence, early detection using sensitive markers is vital.
Serum transferrin saturation more than 55% and ferritin levels greater than 200 in pre-menopausal women and 300 in men and post-menopausal women is called iron overload [54]. Both markers, however, have low sensitivity and specificity. Their levels are also elevated in inflammatory and infectious conditions. Conversely, high cardiac iron accumulation can also occur in the setting of low levels of serum ferritin, suggesting no significant correlation between serum ferritin levels and severity of myocardial involvement [55]. These markers are chiefly helpful for screening purposes. Genetic screening for C282Y mutation is widely available. It helps to detect family members who are homozygous for the mutation.
Transthoracic echocardiography (TTE) is an inexpensive, non-invasive, and widely available technique to screen patients for cardiac hemochromatosis. It is challenging to diagnose early diastolic dysfunction by using M-mode echocardiography. Changes observed are increased left ventricle (LV) early diastolic filling and left atrial (LA) dimensions with preserved systolic function [56]. Enhanced left atrial contractile function that occurs even before LV diastolic dysfunction may be the earliest marker of cardiac iron overload on echocardiography [57]. Tissue Doppler imaging (TDI) is a modern technique to diagnose early involvement of the heart in HH. Palka et al. tested a hypothesis in their study on 18 HH patients that LV diastolic filling indexes are helpful in early detection of cardiac involvement in HH and used TDI as a diagnostic imaging technique. They observed a reduction in peak systolic and early diastolic filling using TDI and concluded that TDI is a valuable tool for early detection of cardiac hemochromatosis [58].
Magnetic resonance imaging (MRI) is the best non-invasive method to detect iron-overload quantitatively in the heart. Iron in tissues acts as a paramagnetic force and disrupts the magnetic signals received by the detector, which results in a heterogeneous image of the tissue displayed as early darkening on the MRI [59]. Cardiac MRI using late gadolinium enhancement (LGE) is a useful technique to identify the extent of cardiac tissue fibrosis that transcends non-contrast MRI for comprehensive cardiac assessment. The sensor in MRI refocuses on the radio waves derived from the tissue, which is called echo time. In tissues with iron overload, as the echo time increases, the darkening of images occurs earlier. The time constant for gradient echo is called T2*. T2* is a sensitive and highly specific method that can quantify and track myocardial iron overload [60]. Mid-ventricular iron overload is representative of global cardiac iron overload and can quantitate cardiac iron deposition [60]. Furthermore, MRI can also be used to determine ejection fraction, cardiac tissue mass, and viability.
Management
Therapeutic phlebotomy and iron-chelators are the mainstays of therapy. Early initiation of treatment can reverse LV dysfunction, and as the iron deposition is not interstitial, cardiac function can be restored entirely [33].
Phlebotomy is the least toxic and most effective method to control serum iron levels. It entails the removal of 400 to 500 milliliters of blood, which roughly correlates to 200 to 250 mg of iron at each session once or twice, initially. Around 1 to 2 gm iron can be removed per month [61]. This treatment helps to mobilize iron from tissues such as the heart, liver, and pancreas. The target of the therapy is to achieve serum ferritin levels below 30 ng/ml and transferrin saturation less than 30% [62]. After the target level is reached, maintenance treatment every two to three months is required with strict monitoring of hematocrit and serum iron levels. This frequency varies among patients depending on the iron reaccumulation rates [63]. A study on 22 patients with HH carried out by Candell-Riera et al. to determine the efficacy of phlebotomy for management of HH demonstrated significant improvement in LV function after a long-term treatment [56]. Echocardiographic findings showed a reduction in diameter and mass of LV in patients with abnormal LV parameters initially [56].
Iron-chelators are used in refractory cases and patients with CHF. They increase the rate of iron excretion by binding iron in plasma and tissue [64]. Deferoxamine, deferiprone, and deferasirox have been approved as iron-chelators. Deferoxamine is given subcutaneously or intravenously while the other two are delivered through the oral route. Intensive combination therapy with deferoxamine and deferiprone rapidly improves cardiac function to the normal state and reduces serum iron levels [65]. A new therapeutic approach includes calcium channel blockers (CCB). CCBs block iron uptake of cardiomyocytes through LTCC and prevent deposition of iron in the heart. An experimental study on mice demonstrated that amlodipine reduces iron uptake in the heart and reduces the amount of ROS generation [66]. It is imperative to initiate guideline-directed medical treatment (GDMT) for CHF, such as angiotensin-converting enzyme inhibitors (ACEI), angiotensin receptor blockers (ARB), diuretics, beta-blockers, and inotropes, in patients who develop heart failure in the late stage of the disease. ACEI and ARB prevent remodeling of the heart and worsening of CHF. Beta-blockers decrease myocardial oxygen consumption and reduce mortality in the long term.
Limitations
This article solely focuses on cardiac abnormalities arising due to iron deposition in the heart and ignores other co-existing factors that can contribute to the modification of cardiac functions. Practically, many factors can influence cardiac functions, including environment, comorbidities, and genetic predisposition. The article has not considered disparities in the genetic structure of individuals that can result in certain people developing more severe and rapid cardiac dysfunction.
Conclusions
It is evident from the review article that, relatively, there are few studies regarding the emphasis on the early recognition and treatment of cardiac hemochromatosis. To summarize, HH can present as iron overload cardiomyopathy, arrhythmias, pulmonary hypertension, and CHF. IOC evolves predictably, beginning as RCM with diastolic dysfunction and, if left untreated, progresses to DCM with systolic dysfunction. CHF ensues as IOC progresses. Iron deposition in the heart generates ROS, damaging cardiomyocytes and triggering a cascade leading to impaired cardiac function. Diagnostic approaches to detect IOC are echocardiography, Doppler imaging, and cardiac MRI. Family members of patients should be screened for HH by genetic techniques. Cardiac function should be regularly monitored in asymptomatic patients and patients with symptoms other than cardiac. Cardiac hemochromatosis is best managed by therapeutic phlebotomy, and refractory cases are treated with iron chelators. The clinical implication of this article is to give a glance at various aspects of cardiac hemochromatosis. This article can serve as a shared resource to obtain knowledge, gain deep insight, and guide physicians to acquire additional information regarding the topic. Lastly, we feel that active investigation through clinical studies and systemic analysis is required to form a rigid construct and manage the condition more efficiently.
The content published in Cureus is the result of clinical experience and/or research by independent individuals or organizations. Cureus is not responsible for the scientific accuracy or reliability of data or conclusions published herein. All content published within Cureus is intended only for educational, research and reference purposes. Additionally, articles published within Cureus should not be deemed a suitable substitute for the advice of a qualified health care professional. Do not disregard or avoid professional medical advice due to content published within Cureus.
Footnotes
The authors have declared that no competing interests exist.
References
- 1.Haemochromatosis. Brissot P, Pietrangelo A, Adams PC, de Graaff B, McLaren CE, Loréal O. Nat Rev Dis Primers. 2018;4:18016. doi: 10.1038/nrdp.2018.16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Hereditary hemochromatosis: the most frequent inherited human disease [Article in Polish] Sikorska K, Bielawski KP, Romanowski T, Stalke P. https://pubmed.ncbi.nlm.nih.gov/17245317/ Postepy Hig Med Dosw (Online) 2006;60:667–676. [PubMed] [Google Scholar]
- 3.Hereditary haemochromatosis through 150 years. Ulvik RJ. Tidsskr Nor Laegeforen. 2016;136:2017–2021. doi: 10.4045/tidsskr.15.1003. [DOI] [PubMed] [Google Scholar]
- 4.Haemochromatosis. Sheldon JH. Lancet. 1934;224:1031–1036. [Google Scholar]
- 5.A novel MHC class I-like gene is mutated in patients with hereditary haemochromatosis. Feder JN, Gnirke A, Thomas W, et al. Nat Genet. 1996;13:399–408. doi: 10.1038/ng0896-399. [DOI] [PubMed] [Google Scholar]
- 6.Mutations in HFE2 cause iron overload in chromosome 1q-linked juvenile hemochromatosis. Papanikolaou G, Samuels ME, Ludwig EH, et al. Nat Genet. 2004;36:77–82. doi: 10.1038/ng1274. [DOI] [PubMed] [Google Scholar]
- 7.The gene TFR2 is mutated in a new type of haemochromatosis mapping to 7q22. Camaschella C, Roetto A, Calì A, et al. Nat Genet. 2000;25:14–15. doi: 10.1038/75534. [DOI] [PubMed] [Google Scholar]
- 8.Autosomal-dominant hemochromatosis is associated with a mutation in the ferroportin (SLC11A3) gene. Montosi G, Donovan A, Totaro A, et al. J Clin Invest. 2001;108:619–623. doi: 10.1172/JCI13468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hepcidin, a urinary antimicrobial peptide synthesized in the liver. Park CH, Valore EV, Waring AJ, Ganz T. J Biol Chem. 2001;276:7806–7810. doi: 10.1074/jbc.M008922200. [DOI] [PubMed] [Google Scholar]
- 10.A survey of 2,851 patients with hemochromatosis. Symptoms and response to treatment. McDonnell SM, Preston BL, Jewell SA, et al. Am J Med. 1999;106:619–624. doi: 10.1016/s0002-9343(99)00120-5. [DOI] [PubMed] [Google Scholar]
- 11.Serum ferritin is a biomarker for liver mortality in the hemochromatosis and iron overload screening study. Adams PC, Barton JC, Guo H, Alter D, Speechley M. Ann Hepatol. 2015;14:348–353. [PubMed] [Google Scholar]
- 12.Long-term survival in patients with hereditary hemochromatosis. Niederau C, Fischer R, Pürschel A, Stremmel W, Häussinger D, Strohmeyer G. Gastroenterology. 1996;110:1107–1119. doi: 10.1053/gast.1996.v110.pm8613000. [DOI] [PubMed] [Google Scholar]
- 13.Therapeutic recommendations in HFE hemochromatosis for p.Cys282Tyr (C282Y/C282Y) homozygous genotype. Adams P, Altes A, Brissot P, et al. Hepatol Int. 2018;12:83–86. doi: 10.1007/s12072-018-9855-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Iron mobilization using chelation and phlebotomy. Flaten TP, Aaseth J, Andersen O, Kontoghiorghes GJ. J Trace Elem Med Biol. 2012;26:127–130. doi: 10.1016/j.jtemb.2012.03.009. [DOI] [PubMed] [Google Scholar]
- 15.Survival and causes of death in hemochromatosis. Observations in 163 patients. Strohmeyer G, Niederau C, Stremmel W. Ann N Y Acad Sci. 1988;526:245–257. doi: 10.1111/j.1749-6632.1988.tb55510.x. [DOI] [PubMed] [Google Scholar]
- 16.Iron in the heart. Etiology and clinical significance. Buja LM, Roberts WC. Am J Med. 1971;51:209–221. doi: 10.1016/0002-9343(71)90240-3. [DOI] [PubMed] [Google Scholar]
- 17.Idiopathic hemochromatosis, an iron storage disease. A. Iron metabolism in hemochromatosis. Finch SC, Finch CA. Medicine (Baltimore) 1955;34:381–430. doi: 10.1097/00005792-195512000-00001. [DOI] [PubMed] [Google Scholar]
- 18.Hemoglobin and myoglobin as reducing agents in biological systems. Redox reactions of globins with copper and iron salts and complexes. Postnikova GB, Shekhovtsova EA. Biochemistry (Mosc) 2016;81:1735–1753. doi: 10.1134/S0006297916130101. [DOI] [PubMed] [Google Scholar]
- 19.Iron overload: effects on cellular biochemistry. Sousa L, Oliveira MM, Pessôa MT, Barbosa LA. Clin Chim Acta. 2020;504:180–189. doi: 10.1016/j.cca.2019.11.029. [DOI] [PubMed] [Google Scholar]
- 20. Chemistry and biology of eukaryotic iron metabolism. Aisen P, Enns C, Wessling-Resnick M. Int J Biochem Cell Biol. 2001;33:940–959. doi: 10.1016/s1357-2725(01)00063-2. [DOI] [PubMed] [Google Scholar]
- 21.Disorders of iron metabolism. Andrews NC. N Engl J Med. 1999;341:1986–1995. doi: 10.1056/NEJM199912233412607. [DOI] [PubMed] [Google Scholar]
- 22.Hepcidin regulates cellular iron efflux by binding to ferroportin and inducing its internalization. Nemeth E, Tuttle MS, Powelson J, et al. Science. 2004;306:2090–2093. doi: 10.1126/science.1104742. [DOI] [PubMed] [Google Scholar]
- 23.Hepcidin, a key regulator of iron metabolism and mediator of anemia of inflammation. Ganz T. Blood. 2003;102:783–788. doi: 10.1182/blood-2003-03-0672. [DOI] [PubMed] [Google Scholar]
- 24.Model of reticuloendothelial iron metabolism in humans: abnormal behavior in idiopathic hemochromatosis and in inflammation. Fillet G, Beguin Y, Baldelli L. Blood. 1989;74:844–851. [PubMed] [Google Scholar]
- 25.Idiopathic hemochromatosis. a study of three families. Balcerzak SP, Westerman MP, Lee RE, Doyle AP. Am J Med. 1966;40:857–873. doi: 10.1016/0002-9343(66)90201-4. [DOI] [PubMed] [Google Scholar]
- 26.Iron kinetics with emphasis on iron overload. Cook JD, Barry WE, Hershko C, Fillet G, Finch CA. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC1903996/ Am J Pathol. 1973;72:337–343. [PMC free article] [PubMed] [Google Scholar]
- 27.Online Mendelian inheritance in man (OMIM) Hamosh A, Scott AF, Amberger J, Valle D, McKusick VA. Hum Mutat. 2000;15:57–61. doi: 10.1002/(SICI)1098-1004(200001)15:1<57::AID-HUMU12>3.0.CO;2-G. [DOI] [PubMed] [Google Scholar]
- 28.Cardiac involvement in hemochromatosis. Gulati V, Harikrishnan P, Palaniswamy C, Aronow WS, Jain D, Frishman WH. Cardiol Rev. 2014;22:56–68. doi: 10.1097/CRD.0b013e3182a67805. [DOI] [PubMed] [Google Scholar]
- 29.Modulation of iron uptake in heart by L-type Ca2+ channel modifiers: possible implications in iron overload. Tsushima RG, Wickenden AD, Bouchard RA, Oudit GY, Liu PP, Backx PH. Circ Res. 1999;84:1302–1309. doi: 10.1161/01.res.84.11.1302. [DOI] [PubMed] [Google Scholar]
- 30.Iron loading of cultured cardiac myocytes modifies sarcolemmal structure and increases lysosomal fragility. Link G, Pinson A, Hershko C. https://www.translationalres.com/article/0022-2143(93)90233-O/fulltext. J Lab Clin Med. 1993;121:127–134. [PubMed] [Google Scholar]
- 31.Mitochondrial dysfunction in cardiac disease: ischemia-reperfusion, aging, and heart failure. Lesnefsky EJ, Moghaddas S, Tandler B, Kerner J, Hoppel CL. J Mol Cell Cardiol. 2001;33:1065–1089. doi: 10.1006/jmcc.2001.1378. [DOI] [PubMed] [Google Scholar]
- 32.Iron overload cardiomyopathies: new insights into an old disease. Liu P, Olivieri N. Cardiovasc Drugs Ther. 1994;8:101–110. doi: 10.1007/BF00877096. [DOI] [PubMed] [Google Scholar]
- 33.Endomyocardial biopsy in hemochromatosis: clinicopathologic correlates in six cases. Olson LJ, Edwards WD, Holmes DR Jr, Miller FA Jr, Nordstrom LA, Baldus WP. J Am Coll Cardiol. 1989;13:116–120. doi: 10.1016/0735-1097(89)90558-5. [DOI] [PubMed] [Google Scholar]
- 34.Heart failure and malignant ventricular tachyarrhythmias due to hereditary hemochromatosis with iron overload cardiomyopathy. Demant AW, Schmiedel A, Büttner R, Lewalter T, Reichel C. Clin Res Cardiol. 2007;96:900–903. doi: 10.1007/s00392-007-0568-y. [DOI] [PubMed] [Google Scholar]
- 35.Cardiovascular manifestations in hospitalized patients with hemochromatosis in the United States. Udani K, Chris-Olaiya A, Ohadugha C, Malik A, Sansbury J, Paari D. Int J Cardiol. 2021;342:117–124. doi: 10.1016/j.ijcard.2021.07.060. [DOI] [PubMed] [Google Scholar]
- 36.Iron-overload cardiomyopathy: pathophysiology, diagnosis, and treatment. Murphy CJ, Oudit GY. J Card Fail. 2010;16:888–900. doi: 10.1016/j.cardfail.2010.05.009. [DOI] [PubMed] [Google Scholar]
- 37.Survival and causes of death in cirrhotic and in noncirrhotic patients with primary hemochromatosis. Niederau C, Fischer R, Sonnenberg A, Stremmel W, Trampisch HJ, Strohmeyer G. N Engl J Med. 1985;313:1256–1262. doi: 10.1056/NEJM198511143132004. [DOI] [PubMed] [Google Scholar]
- 38.Iron overload cardiomyopathy in clinical practice. Kremastinos DT, Farmakis D. Circulation. 2011;124:2253–2263. doi: 10.1161/CIRCULATIONAHA.111.050773. [DOI] [PubMed] [Google Scholar]
- 39.Hemochromatosis protein (HFE) knockout mice as a novel model of hemochromatosis: implications for study and management of iron-overload cardiomyopathy. Zhabyeyev P, Oudit GY. Can J Cardiol. 2017;33:835–837. doi: 10.1016/j.cjca.2017.04.013. [DOI] [PubMed] [Google Scholar]
- 40.Decreased sodium and increased transient outward potassium currents in iron-loaded cardiac myocytes. Implications for the arrhythmogenesis of human siderotic heart disease. Kuryshev YA, Brittenham GM, Fujioka H, Kannan P, Shieh CC, Cohen SA, Brown AM. Circulation. 1999;100:675–683. doi: 10.1161/01.cir.100.6.675. [DOI] [PubMed] [Google Scholar]
- 41.Role of L-type Ca2+ channels in iron transport and iron-overload cardiomyopathy. Oudit GY, Trivieri MG, Khaper N, Liu PP, Backx PH. J Mol Med (Berl) 2006;84:349–364. doi: 10.1007/s00109-005-0029-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Cardiac involvement in hemochromatosis. Lewis HP. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC2249101/ Trans Am Clin Climatol Assoc. 1954;65:49–76. [PMC free article] [PubMed] [Google Scholar]
- 43.Does oxidative stress modulate left ventricular diastolic function in asymptomatic subjects with hereditary hemochromatosis? Shizukuda Y, Bolan CD, Tripodi DJ, et al. Echocardiography. 2009;26:1153–1158. doi: 10.1111/j.1540-8175.2009.00956.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Muhlestein JB. Hemochromatosis: Genetics, Pathophysiology, Diagnosis and Treatment. Cambridge, United Kingdom: Cambridge University Press; 2000. Cardiac abnormalities in hemochromatosis. hemochromatosis: genetics, pathophysiology, diagnosis, and treatment; pp. 297–310. [Google Scholar]
- 45.Earliest cardiac toxicity induced by iron overload selectively inhibits electrical conduction. Schwartz KA, Li Z, Schwartz DE, Cooper TG, Braselton WE. J Appl Physiol (1985) 2002;93:746–751. doi: 10.1152/japplphysiol.01144.2001. [DOI] [PubMed] [Google Scholar]
- 46.Incidence of cardiac arrhythmias in asymptomatic hereditary hemochromatosis subjects with C282Y homozygosity. Shizukuda Y, Tripodi DJ, Zalos G, et al. Am J Cardiol. 2012;109:856–860. doi: 10.1016/j.amjcard.2011.11.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Cardiac abnormalities in primary hemochromatosis. Dabestani A, Child JS, Perloff JK, Figueroa WG, Schelbert HR, Engel TR. Ann N Y Acad Sci. 1988;526:234–244. doi: 10.1111/j.1749-6632.1988.tb55509.x. [DOI] [PubMed] [Google Scholar]
- 48.Physiology and pathophysiology of iron cardiomyopathy in thalassemia. Wood JC, Enriquez C, Ghugre N, et al. Ann N Y Acad Sci. 2005;1054:386–395. doi: 10.1196/annals.1345.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Optical mapping reveals conduction slowing and impulse block in iron-overload cardiomyopathy. Laurita KR, Chuck ET, Yang T, et al. J Lab Clin Med. 2003;142:83–89. doi: 10.1016/S0022-2143(03)00060-X. [DOI] [PubMed] [Google Scholar]
- 50.The effect of iron stores on corrected qt dispersion in patients undergoing peritoneal dialysis. Wu VC, Huang JW, Wu MS, et al. Am J Kidney Dis. 2004;44:720–728. [PubMed] [Google Scholar]
- 51.Incidence of symptomatic arrhythmias and utilization of arrhythmia testing in 10-year follow up of cardiac asymptomatic hereditary hemochromatosis subjects. Shizukuda Y, Lingamaneni P, Rosing DR. Am J Cardiol. 2021;157:153–154. doi: 10.1016/j.amjcard.2021.07.031. [DOI] [PubMed] [Google Scholar]
- 52.Determinants of pulmonary hypertension in patients with beta-thalassemia major and normal ventricular function. Vlahos AP, Koutsouka FP, Papamichael ND, et al. Acta Haematol. 2012;128:124–129. doi: 10.1159/000338825. [DOI] [PubMed] [Google Scholar]
- 53.Iron overload cardiomyopathy: better understanding of an increasing disorder. Gujja P, Rosing DR, Tripodi DJ, Shizukuda Y. J Am Coll Cardiol. 2010;56:1001–1012. doi: 10.1016/j.jacc.2010.03.083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Screening for hereditary hemochromatosis: a clinical practice guideline from the American College of Physicians. Qaseem A, Aronson M, Fitterman N, Snow V, Weiss KB, Owens DK. Ann Intern Med. 2005;143:517–521. doi: 10.7326/0003-4819-143-7-200510040-00010. [DOI] [PubMed] [Google Scholar]
- 55.Serum ferritin concentration in sickle cell crisis. Brownell A, Lowson S, Brozović M. J Clin Pathol. 1986;39:253–255. doi: 10.1136/jcp.39.3.253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Cardiac hemochromatosis: beneficial effects of iron removal therapy. An echocardiographic study. Candell-Riera J, Lu L, Serés L, et al. Am J Cardiol. 1983;52:824–829. doi: 10.1016/0002-9149(83)90422-8. [DOI] [PubMed] [Google Scholar]
- 57.Significance of left atrial contractile function in asymptomatic subjects with hereditary hemochromatosis. Shizukuda Y, Bolan CD, Tripodi DJ, et al. Am J Cardiol. 2006;98:954–959. doi: 10.1016/j.amjcard.2006.04.040. [DOI] [PubMed] [Google Scholar]
- 58.The role of Doppler left ventricular filling indexes and Doppler tissue echocardiography in the assessment of cardiac involvement in hereditary hemochromatosis. Palka P, Macdonald G, Lange A, Burstow DJ. J Am Soc Echocardiogr. 2002;15:884–890. doi: 10.1067/mje.2002.118032. [DOI] [PubMed] [Google Scholar]
- 59.Mimicking liver iron overload using liposomal ferritin preparations. Wood JC, Fassler JD, Meade T. Magn Reson Med. 2004;51:607–611. doi: 10.1002/mrm.10735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Multislice multiecho T2* cardiovascular magnetic resonance for detection of the heterogeneous distribution of myocardial iron overload. Pepe A, Positano V, Santarelli MF, et al. J Magn Reson Imaging. 2006;23:662–668. doi: 10.1002/jmri.20566. [DOI] [PubMed] [Google Scholar]
- 61.Practical management of iron overload. Porter JB. Br J Haematol. 2001;115:239–252. doi: 10.1046/j.1365-2141.2001.03195.x. [DOI] [PubMed] [Google Scholar]
- 62.How I treat hemochromatosis. Adams PC, Barton JC. Blood. 2010;116:317–325. doi: 10.1182/blood-2010-01-261875. [DOI] [PubMed] [Google Scholar]
- 63.Rate of iron reaccumulation following iron depletion in hereditary hemochromatosis. Implications for venesection therapy. Adams PC, Kertesz AE, Valberg LS. J Clin Gastroenterol. 1993;16:207–210. doi: 10.1097/00004836-199304000-00008. [DOI] [PubMed] [Google Scholar]
- 64.Action of chelators in iron-loaded cardiac cells: accessibility to intracellular labile iron and functional consequences. Glickstein H, El RB, Link G, et al. Blood. 2006;108:3195–3203. doi: 10.1182/blood-2006-05-020867. [DOI] [PubMed] [Google Scholar]
- 65.Normalisation of total body iron load with very intensive combined chelation reverses cardiac and endocrine complications of thalassaemia major. Farmaki K, Tzoumari I, Pappa C, Chouliaras G, Berdoukas V. Br J Haematol. 2010;148:466–475. doi: 10.1111/j.1365-2141.2009.07970.x. [DOI] [PubMed] [Google Scholar]
- 66.Amlodipine decreases iron uptake and oxygen free radical production in the heart of chronically iron overloaded mice. Crowe S, Bartfay WJ. Biol Res Nurs. 2002;3:189–197. doi: 10.1177/109980040200300404. [DOI] [PubMed] [Google Scholar]