Abstract
Backgrounds and aims:
Abdominal aortic calcification (AAC) is associated with weaker grip strength, an established risk factor for fall-related hospitalizations. However, its association with long-term fall-related hospitalisations remains unknown. This study investigated the association between AAC and long-term fall-related hospitalizations in community-dwelling older women.
Methods:
Fall-related hospitalizations were obtained from linked data over 14.5-years in a prospective cohort of 1053 older women (mean age 75.0 ± 2.6 years). At baseline (1998/99), AAC was assessed from lateral spine images obtained using dual-energy X-ray absorptiometry, and scored using a semi-quantitative method (AAC24, range 0–24). The presence of any AAC was defined by AAC24 ≥ 1.
Results:
Over 14.5-years, 413 (39.2%) women experienced a fall-related hospitalization. In the multivariable-adjusted model, each unit increase in baseline AAC24 was associated with a 3% increase in relative hazards for a fall-related hospitalization (HR 1.03 95%CI, 1.01 to 1.07). Compared to women with no AAC, women with any AAC had a 40% (HR 1.40 95%CI, 1.11 to 1.76) and 39% (HR 1.39 95%CI, 1.10 to 1.76) greater risk for fall-related hospitalizations in the minimal and multivariable-adjusted models, respectively. This relationship was not attenuated by including measures of muscle function such as grip strength and timed-up-and-go.
Conclusions:
The presence of AAC is associated with long-term fall-related hospitalizations risk, independent of muscle function, in community-dwelling older women. Concurrent assessment of AAC may be a simple and cost-effective way to identify older women at higher risk of falling as part of routine osteoporosis screening.
Keywords: Abdominal aortic calcification, Vascular calcification, Muscle function, Falls, Ageing
1. Introduction
Falls are a major cause of concern for older populations and are the leading cause of injury-related hospitalizations [1]. Approximately 42% of older adults aged 75 years and above, fall annually [2]. The causes of falls are multifactorial in nature, often including acute mediating physiological and/or environmental factors [3]. As a result of the injuries suffered from falling, physical mobility and quality of life may be compromised [1]. Furthermore, mobility impairment may limit the ability to undertake regular physical activity. This can lead to additional burden of chronic disease, which in-turn exacerbates falling propensity, culminating in a vicious cycle [4,5].
Transient cardiovascular events (e.g. syncope, and hypotension), long-standing cardiovascular disease (CVD) (e.g. hypertension and atrial fibrillation) and blood pressure lowering medications have been associated with higher falls risk [4,6]. For example, more than half of the individuals hospitalized with CVD have a higher falls risk, thereby exacerbating injury and mortality risk [7]. Recently, we reported a positive association between severe-abdominal aortic calcification (AAC24 ≥ 6) and 5-year decline in muscle strength in older women [8], with the latter known to increase falls propensity [9,10].
Abdominal aortic calcification (AAC), a complex and multifactorial process of mineral deposition in arterial wall [11–13], is a stable marker of atherosclerotic vascular disease. AAC has been shown to predict future CVD events, poor prognosis [14–16] and all-cause mortality [17]. AAC can be assessed relatively quickly and cost-effectively from lateral spine imaging (LSI), initially developed to identify vertebral fractures using a low radiation bone densitometer [18,19]. Severe AAC has also been shown to be associated with low bone mineral density (BMD) and an increased risk of fractures [20,21]. Part of this observed increase in fracture with greater AAC might also be related to a potential contribution to the risk of falling.
While AAC may simply be a marker of falls risk factors, such as advanced age and chronic kidney disease, there are several putative mechanisms whereby AAC may influence risk of falls [15,22,23]. Firstly, AAC may be a marker of risk of future CVD events such as stroke. Furthermore, severe AAC may result in aggravation of ischemic heart disease leading to arrythmias and loss of consciousness, which may directly cause falls or negatively impact falls risk factors such as dizziness, and subsequent muscle weakness or paralysis [24]. Secondly, signalling pathways regulating arterial calcification may be involved in muscle function and falls risk [25]. Finally, advanced atherosclerosis with AAC may also be a marker of macro and microvascular disease impairing blood and nutrient supply to the muscles leading to impaired muscle strength and/or greater decline in its trajectories [8,26]. Given such associations, it is plausible that AAC may also be associated with a higher risk of falling due to vascular causes. This is especially important for vulnerable populations such as older women who typically experience a higher proportion of fall-related injuries compared to their male counterparts [27]. To our knowledge this has not yet been evaluated. Therefore, the aim of this study was to examine the association between AAC and injurious falls that resulted in hospitalizations in a longitudinal study of community-dwelling older women.
2. Materials and methods
2.1. Participants
Community-dwelling Western Australian older women in the Perth Longitudinal Study of Aging Women (PLSAW) were included in this study. Briefly, PLSAW was an extension of the Calcium Intake Fracture Outcome Study (CAIFOS) in which 1460 women aged 70 years or over were enrolled in a five-year, double-blind, randomized controlled trial of daily calcium supplementation to prevent fracture as described previously [28]. Baseline (1998/1999) AAC was measured in 1053 women. A diagrammatic representation of the study design according to CONSORT guidelines is presented in Supplementary Fig. 1. Ethics approval was granted by the Human Ethics Committee of the University of Western Australia. Both studies were retrospectively registered on the Australian New Zealand Clinical Trials Registry (CAIFOS trial registration number #ACTRN12615000750583 and PLSAW trial registration number #ACTRN12617000640303) and complied with the Declaration of Helsinki. Human ethics approval for the use of linked data was provided by the Human Research Ethics Committee of the Western Australian Department of Health (project number #2009/24). All participants provided written informed consent that included consent to future access to Western Australian Health Department data on coded and dated hospital discharges.
2.2. Assessment of abdominal aortic calcification (AAC)
Measurements of AAC were collected over 1 year, during 1998 (baseline) and 1999 (year 1). AAC was scored from 0 to 24 derived from digitally enhanced lateral single-energy images of the thoraco-lumbar spine using a Hologic 4500A bone densitometer (Hologic, Bedford, MA, USA). A single experienced investigator (JTS) read all images using a validated semi-quantitative scoring system, as detailed previously [29]. The AAC24 scoring system scores AAC relative to each vertebral height (L1–L4) and is scored as; 0 (no calcification), 1 (≤1/3 of the aortic wall), 2 (>1/3 to ≤2/3 of the aortic wall) or 3 (>2/3 of the aortic wall) for both the anterior and posterior aortic walls giving a maximum possible score of up to 24. Both intra and inter-rater agreements have been reported as very good [29,30]. The presence of any AAC was defined by an AAC24 score ≥1.
2.3. Falls outcome assessment
Injurious fall-related hospitalizations over 14.5 years were tracked through the Western Australian Data Linkage System (Department of Health Western Australia, East Perth, Australia) and retrieved from the Western Australia Hospital Morbidity Data Collection (HMDC). Records were obtained for each of the study participants from 1998 until 2013 using the International Classification of External Causes of Injury codes and the International Classification of Diseases (ICD) coded diagnosis data pertaining to all public and private inpatient hospitalizations in Western Australia. This allows ascertainment of hospitalizations independent of self-report and avoids the problems of patient self-reporting and loss to follow-up. Falls from standing height or less, not resulting from external force were included (ICD-10 codes: W01, W05–W08, W10, W18, and W19). A fall was considered injurious if it required hospitalizations.
2.4. Baseline assessment
Prevalent atherosclerotic vascular disease (ASVD) was obtained from primary discharge diagnoses from hospital records (1980–1998) as described previously [31]. Cardiovascular hospitalizations and deaths (1998–2013) [32] and any history of knee replacement procedures were identified using the linked data provided by the Western Australian Data Linkage System. Participants were asked about participation in sport, recreation, and/or regular physical activities undertaken three months prior to their baseline visit [33]. The level of activity, expressed in kilojoules per day, was then calculated using a validated method applying the type of activity, time engaged in the activity, and the participant’s body weight [34]. Smoking history was coded as non-smoker or smoked ever (if they had consumed >1 cigarette per day for more than three months at any time in their life or is a current smoker). Body weight was measured using digital scales to the nearest 0.1 kg and height was assessed using a wall-mounted stadiometer to the nearest 0.1 cm, whilst participants were wearing light clothes and without socks and shoes. Body mass index (BMI) (kg/m2) was then calculated. Treatment (placebo or calcium) over the five years of the CAIFOS trial was included as a covariate. Current medication use at baseline was used to assess prevalent diabetes mellitus. Medications were verified by participants’ general practitioner where possible and were coded (T89001–T90009) using the International Classification of Primary Care-Plus (ICPC-Plus) method which allows aggregation of different terms for similar pathologic entities as defined by the ICD-10 coding system [35]. Socioeconomic status was calculated using the Socioeconomic Indexes for Areas developed by the Australian Bureau of Statistics as described previously [36]. Self-reported dizziness was categorized as frequent (experienced more than once within a week) and infrequent (experienced less than 4 per month or never encountered). Total hip bone mineral density was determined by DXA using the same Hologic Acclaim 4500A fan beam densitometer (Hologic Corp, Waltham, MA, USA). Physical balance was defined as poor (unable to maintain a tandem stance for 10 s or more) and normal (maintain a tandem stance for greater than 10 s) from the sharpened Romberg test as described previously [37]. Details pertaining to the assessment of grip strength and TUG in this cohort have been previously described [38]. Participants self-reported their history of previous falls in the three months prior to baseline clinical visit.
2.5. Statistical analysis
The primary outcome of the study was a fall-related hospitalization while the presence of any AAC was the primary exposure variable. Follow up was available for each of the participants starting from their baseline visit until the first injurious fall, death or the end of the study follow-up period (14.5-years). The relationship between AAC24 score and fall-related hospitalizations was explored using restricted cubic splines within Cox-proportional hazards models, the hazard ratios of fall-related hospitalizations were plotted against the exposure variable (AAC24). This allowed us to examine whether the association between baseline AAC and subsequent fall-related hospitalizations was non-linear. Based on this, subsequent analyses were undertaken with AAC dichotomized into two group, absence (AAC24 = 0) and presence of AAC (AAC24 ≥ 1).
Kaplan-Meier survival curves and the log-rank test were used to assess univariate associations between AAC24 categories (absence/presence AAC) and fall-related hospitalizations. Two models of adjustment were adopted; Minimally-adjusted: age, BMI and treatment code (calcium or placebo), and Multivariable-adjusted: Minimally-adjusted plus, prevalent ASVD, smoked ever, prevalent diabetes, statin use, anti-hypertensive medication use, socioeconomic status, physical activity and self-reported prevalent falls three months prior the baseline visit. Cox proportional hazards assumptions were tested on the basis of Schoenfeld residuals, computed using the ‘estat phtest’ command in Stata. p-values of >0.05 were recorded for all analyses including covariates indicating that proportional hazards assumptions were not violated. For the primary analysis, we treated deaths as censored. Cox-proportional hazard modelling was used to determine the relative hazard for a fall-related hospitalization within the follow up period assuming an individual remains alive. Hence, it assumes the risk of a fall-related hospitalization would have remained the same during the remainder of the follow-up period in those who died as in those who did not. Statistical analyses were performed using IBM SPSS Statistics for Windows, version 24.0 (IBM Corp., Armonk, NY, USA), and R software (version 3.4.2, R Foundation for Statistical Computing, Vienna, Austria) [39]. Statistical significance was set at a 2-sided Type 1 error rate of p < 0.05 for all tests.
2.6. Additional analysis
Given the shape of the spline observed in Fig. 1, the relationship between AAC24 and fall-related hospitalizations was also further explored by separating into AAC24 categories based on severity of AAC; No AAC (AAC score 0); Low to Moderate AAC (AAC24 score 1 to 5) and Severe AAC (AAC24 ≥ 6). We also performed further analyses after excluding women with prevalent ASVD at baseline and re-examined the relationship between AAC and fall-related hospitalizations as AAC is an advanced marker of ASVD which is associated with high risk of falls. In addition, we have explored the association between AAC and fall-related hospitalization after excluding women with any CVD hospitalization (n = 443) and any knee replacement procedures (n = 115) over 14.5-years to assess if the relationship between AAC and falls is independent of any cardiovascular events and knee surgeries, respectively. We also examined the impact of including muscle strength (grip strength) and physical function tests (timed-up-and-go; TUG) on the relationship between AAC24 and fall-related hospitalizations as muscle function is a key contributor to risk of a fall-related hospitalization [38]. Specifically, we included baseline grip strength and TUG (separately and combined) in the multivariable-adjusted model. Further, we also examined the association after including baseline hip BMD in the multivariable model. Finally, we performed interaction tests between AAC24 and other falls risk factors (smoking, physical activity, prevalent ASVD, mean systolic blood pressure, antihypertensive medications use, statin use, self-reported dizziness, lipid profiles, hand grip strength, TUG and fear of falling) as potential modifiers of the association between AAC24 and fall-related hospitalizations in age and treatment (placebo/calcium)-adjusted models with a significance level of p for interaction <0.1.
Fig. 1.
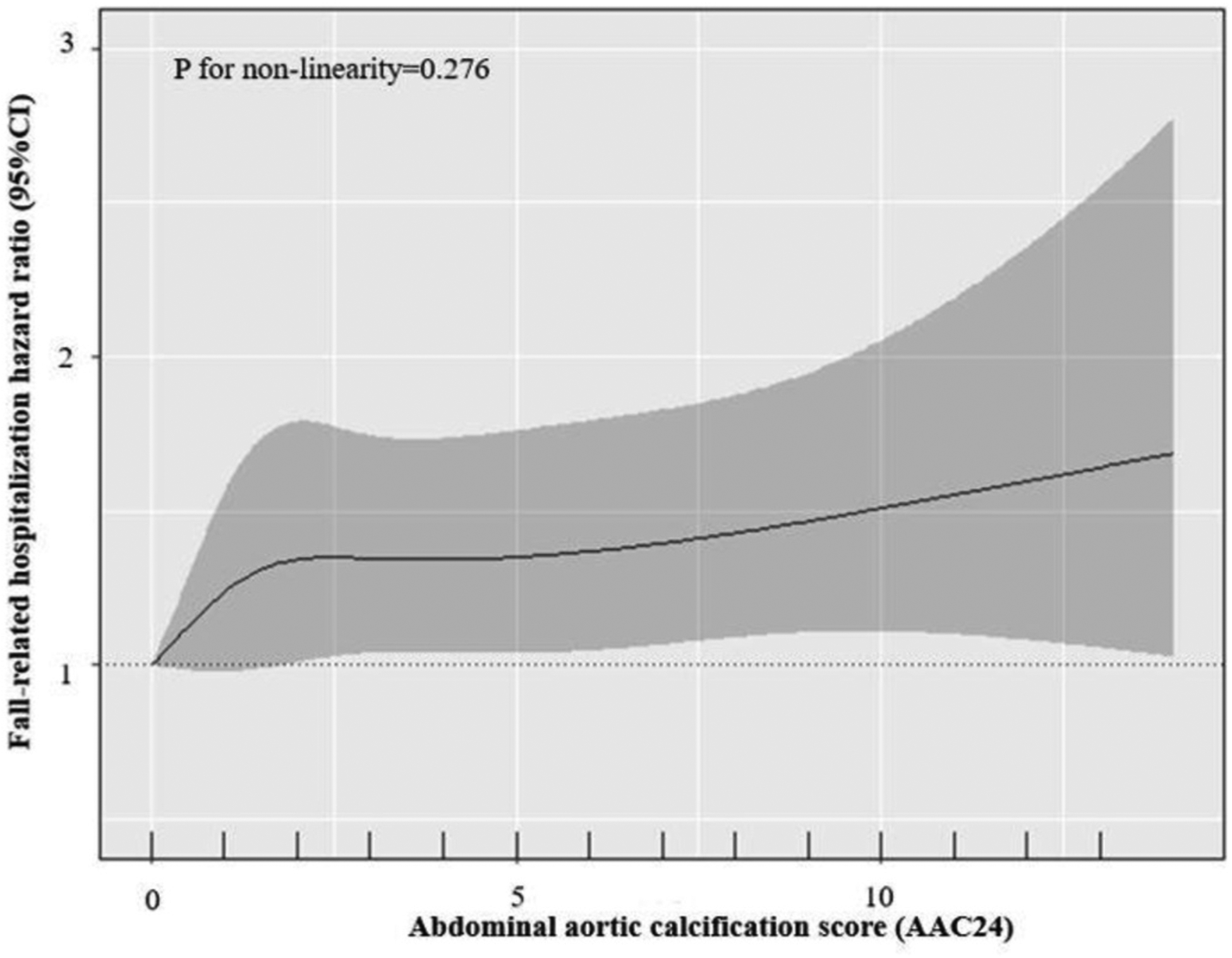
Cubic spline regression analysis of the hazard ratio and 95%CI for fall-related hospitalizations over 14.5 years and abdominal aortic calcification 24 score (AAC24).
The reference value is set at AAC24 = 0.
3. Results
Baseline characteristics of participants according to the presence of AAC are presented in Table 1. Mean age of the participants was 75.0 ± 2.6 y and the median (IQR) AAC24 was 2 (0–4). Women presenting with AAC were more likely to have a history of smoking and to be using lipid-lowering medications in comparison to women with no AAC.
Table 1.
Baseline characteristics according to the presence or absence of AACa.
| All participants | Abdominal aortic calcificationb | ||
|---|---|---|---|
| Absent | Present | ||
| Number | 1053 | 287 (27.3) | 766 (72.7) |
| Demographics | |||
| Age, years | 75.0 ± 2.6 | 74.9 ± 2.6 | 75 ± 2.6 |
| Calcium treatment group, yes % | 514 (48.8) | 146 (50.9) | 368 (48.0) |
| Body mass index (BMI)c, kg/m2 | 27.1 ± 4.5 | 27.3 ± 4.7 | 27.1 ± 4.4 |
| Prevalent atherosclerotic vascular disease, yes % | 118 (11.2) | 24 (8.4) | 94 (12.3) |
| Smoked everd, yes % | 377 (35.9) | 72 (25.2) | 305 (39.9) |
| Prevalent diabetes mellitus, yes % | 63 (6.0) | 14 (4.9) | 49 (6.4) |
| Lipid lowering medications, yes % | 198 (18.8) | 41 (14.3) | 157 (20.4) |
| Blood pressure lowering medication, yes % | 452 (42.9) | 114 (39.7) | 338 (44.1) |
| Infrequent dizziness (≤4 per month)e, yes % | 997 (95.1) | 269 (94.1) | 728 (95.5) |
| Frequent dizziness (≥4 per month), yes % | 51 (4.9) | 17 (5.9) | 34 (4.5) |
| Fear of fallinge, yes % | 261 (24.9) | 71 (24.8) | 190 (24.9) |
| Socioeconomic statusf, yes % | |||
| Top 10% most highly disadvantaged | 44 (4.2) | 11 (3.9) | 33 (4.3) |
| Highly disadvantaged | 123 (11.8) | 38 (13.3) | 85 (11.2) |
| Moderate-highly disadvantaged | 167 (16.0) | 39 (13.7) | 128 (16.9) |
| Low-moderately disadvantaged | 155 (14.8) | 55 (19.3) | 100 (13.2) |
| Low disadvantaged | 222 (21.3) | 57 (20.0) | 165 (21.7) |
| Top 10% least disadvantaged | 333 (31.9) | 85 (29.8) | 248 (32.7) |
| Physical function | |||
| Physical activityc, kJ/day | 496 (188–876) | 474 (193–954) | 505 (189–860) |
| Poor balance, eye closedg, yes % | 870 (83.4) | 245 (85.7) | 625 (82.6) |
| Normal balance, eye closed, yes % | 173 (16.6) | 41 (14.3) | 132 (17.4) |
| Grip strengthf, kg | 20.7 ± 4.6 | 20.7 ± 4.4 | 20.8 ± 4.7 |
| Timed-up-and-goh, sec | 9.3 (8.1–10.8) | 9.1 (8.1–11.0) | 9.3 (8.1–10.8) |
| Prevalent fallsi, yes % | 111 (10.7) | 30 (10.7) | 81 (10.7) |
Data presented as mean ± SD, median (IQR) or number n and (%).
AAC were categorized based on AAC24 score. Absent, AAC24 = 0; Present, AAC24 ≥ 1.
n = 1052.
n = 1050.
n = 1048.
n = 1044.
n = 1043.
n = 1051,
n = 1037.
Bolded numbers indicate p < 0.05 and are a comparison between groups using t-test, Mann–Whitney U test, Chi-square test where appropriate.
3.1. Abdominal aortic calcification and fall-related hospitalizations
Over 14.5-years (11,838 person years) of follow-up (mean ± SD; 11.2 ± 3.9 y), 39.2% (413/1053) of women experienced a fall-related hospitalization. The relationship between AAC24 and fall-related hospitalizations is presented in Fig. 1 (p for non-linearity = 0.276). Despite a ‘linear relationship’ being recorded, the gradient risk of falls appeared greatest between no AAC (AAC24 = 0) to mild AAC (AAC24 1–2), and gradually increased in a linear manner from there on. Specifically, each unit increase in baseline AAC24 was associated with 3% higher relative hazard for a fall-related hospitalization (Table 2). Kaplan-Meier survival curves indicated that women with any AAC had higher risk of a fall-related hospitalization compared to women with no AAC (log-rank test: p = 0.006) (Fig. 2). Specifically, compared to no AAC, the presence of any AAC was associated with 40% and 39% higher relative hazards for a fall-related hospitalization in minimally and multivariable-adjusted models, respectively (Table 2).
Table 2.
Hazard ratios (HR) for injurious fall-related hospitalizations by abdominal aortic calcification score (AAC24)a.
| Injurious falls | HR per unit increase in AAC score | Abdominal aortic calcificationb | ||
|---|---|---|---|---|
| Absent | Present | |||
| AAC Score | Number | 1053 | 287 | 766 |
| Events, n (%) | 413 (39.2) | 96 (33.4) | 317 (41.4) | |
| Minimally-adjusted | 1.03 (1.00–1.07) | 1.00 | 1.40 (1.11–1.76) | |
| Multivariable-adjusted* | 1.03 (1.01–1.07) | 1.00 | 1.39 (1.10–1.76) | |
Hazard ratios (95% CI) for injurious falls by AAC score (continuous and discrete) analyzed using Cox-proportional hazard models. Minimally-adjusted = age, treatment code and BMI; multivariable-adjusted = age, treatment code, BMI, prevalent atherosclerotic vascular disease, smoked ever, prevalent diabetes mellitus, statin use, blood pressure lowering medication use, socioeconomic status, physical activity and self-reported prevalent falls.
AAC were categorized based on AAC24 score. Absent, AAC24 = 0; present, AAC24 ≥ 1.
Multivariable analysis in 1024 women. Bolded numbers indicate p < 0.05.
Fig. 2.
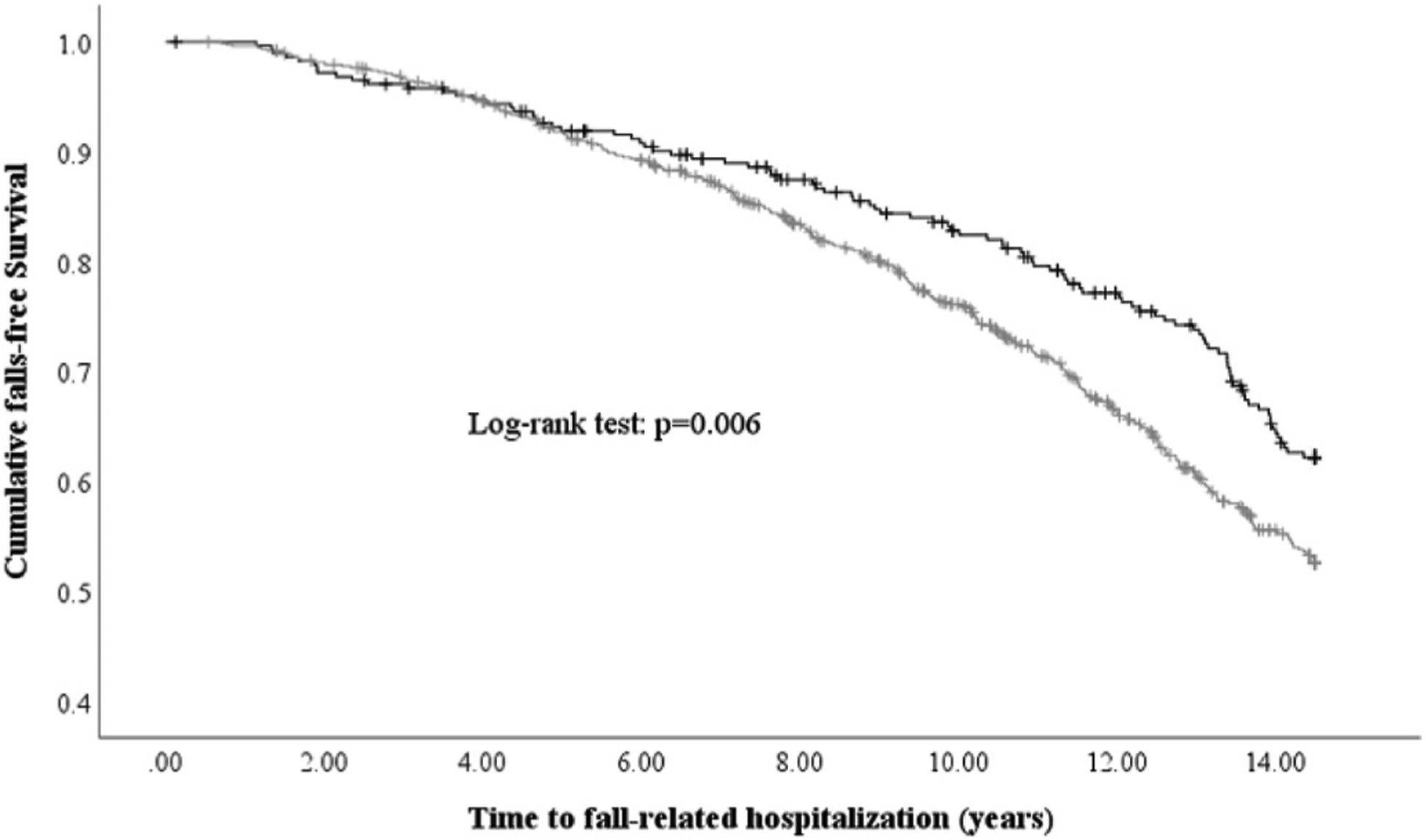
Kaplan-Meier survival curve for abdominal aortic calcification (AAC) score categories for fall-related-hospitalizations.
No-AAC (AAC24 = 0), any AAC (AAC24 ≥ 1) categories are represented by black and light grey lines, respectively.
3.2. Additional analyses
The presence of low to moderate AAC24 (AAC24 1–5) and severe AAC24 (AAC24 > 5) was associated with 39% (HR 1.39 95%CI, 1.09 to 1.78, p = 0.008) and 40% (HR 1.40 95%CI, 1.03 to1.90, p = 0.032) greater relative hazards for an injurious falls respectively compared to no AAC in the multivariable-adjusted model. We performed additional analyses excluding women with prevalent or incident events that may have been related to falls. When excluding individuals with ASVD (n = 118), women presenting with any AAC had greater risk for a fall-related hospitalization compared to women with no AAC in both the minimal (HR 1.40 95%CI, 1.10 to 1.78) and multivariable (HR 1.41 95%CI, 1.10 to 1.81) adjusted models. The association between AAC and falls did not reach significance although the point estimate remained similar with the original finding [multivariable-adjusted (HR 1.39 95%CI, 0.99 to 1.94) vs (HR 1.39 95%CI, 1.10 to 1.76)] after excluding women with any CVD hospitalizations and deaths (n = 443) over 14.5-years. The risk of fall-related hospitalizations remained similar in older women with any AAC after excluding those with any knee joint replacement (n = 115) in both minimal (HR 1.39 95%CI, 1.08 to 1.78) and multivariable (HR 1.38 95%CI, 1.07 to 1.79) adjusted models. Grip strength, (per kg increase; HR 0.97 95%CI, 0.94 to 0.99, p < 0.01) and TUG, (per sec increase; HR 1.05 95%CI, 1.02 to 1.08, p < 0.01 were significantly associated with fall-related hospitalizations. However, including grip strength and TUG (separately and combined) in the multivariable-adjusted model did not alter the relationship between AAC24 and fall-related hospitalizations (Supplementary Table 1). Model parameters that remained significantly associated with fall-related hospitalizations for each of the aforementioned analyses are listed in Supplementary Table 1. Similarly, including baseline hip BMD into the multivariable-adjusted model did not attenuate the association for AAC and fall-related hospitalizations (per unit increase in AAC24 score, HR 1.03 95%CI, 1.00 to 1.06; presence of any AAC, HR 1.37 95%CI, 1.08 to 1.74). None of the risk factors examined for interaction were found to modify the relationship between AAC24 and fall-related hospitalizations (p for interaction >0.1).
4. Discussion
Considering the growing social and economic burden of injurious falling, strategies to identify risk factors are crucial for the development of primordial or primary prevention strategies. In this study, each point increase in AAC24 was associated with 3% greater risk for future fall-related hospitalizations. Furthermore, the presence of AAC that was seen in more than 7 out of 10 women was associated with 39% higher risk for a fall-related hospitalization compared to women with no AAC. To our knowledge, this is the first study to identify AAC as a risk factor for the most serious type of falls requiring hospitalizations in community-dwelling older women. These findings remained significant when adjusting or excluding women with clinical ASVD at baseline, suggesting that the presence of AAC provides complimentary prognostic information to clinical disease [15]. The findings also remained unchanged after excluding women with incident CVD events although it didn’t reach statistical significance, which may be attributable to reduction in the statistical power of the study. The association was not attenuated after excluding women with any knee joint replacement and including hip BMD into the model. Such findings do not highlight specific underlying mechanisms, making it even more important to unravel why the association was observed.
The findings also remained comparable when adjusted for measures of muscle strength and function, and hip BMD suggesting that AAC provides further prognostic information from functional measures. Importantly, the Kaplan-Meier curves demonstrated the risk of falls diverged after about 5 years in the women with AAC. Perhaps, such findings may be explained by the presence of AAC serving as predictor for fall risk factors such as CVD and its sequelae or declines in muscle strength. Thus, the health benefits of lifestyle interventions including a healthy diet and exercise known to improve cardiovascular and musculoskeletal health should continue to be promoted, especially in those with AAC. Considering over two-thirds of women presented with AAC, the observed 8% absolute risk difference and 39% relative risk difference for a fall-related hospitalization in women with AAC compared to those without AAC over 14.5-years is notable.
Due to the complex multifactorial causes of falling, identifying the singular most important risk factor may not be possible. For example, the influence of fall propensity risk factors such as neuromuscular function, vision or cognitive state may be different between fallers and the circumstances surrounding a single fall event [40]. Nonetheless, ASVDs, arterial stiffness and/or carotid-intima media thickness (CIMT), have been associated with fall propensity risk factors such as lower muscle strength [41], poor balance and impaired gait [42]. Higher levels of arterial plaque have also been linked with impaired mobility [43]. In our cohort, severe AAC (AAC24 score ≥6) was associated with a greater decline in muscle (grip) strength but not with a measure of physical function (TUG) over five years. It was suggested that AAC may be more closely related to discrete measurements of muscle function (e.g. grip strength) requiring fewer muscle groups and therefore less neuromuscular coordination [8]. Given the presence of AAC was associated with fall-related hospitalizations risk independent of measures of muscle strength and function, it suggests AAC may identify women at a higher risk of developing of falls by an as yet unknown mechanism.
It is known that parameters of vascular disease are related to impaired muscle function. For example in the Multi-Ethnic Study of Atherosclerosis of 6490 Americans free of CVD (aged 45–84 years, 53% female, ~9.2 years follow-up), greater CIMT and coronary artery calcification was related to faster decline in self-reported walking pace and walking speed, respectively [43]. Such results suggest an inverse relationship between vascular disease and physical functioning, thereby contributing to increased falls propensity. In our additional analysis, we report that grip strength, TUG and AAC24 were all independently associated with injurious falls in the multivariable-adjusted model. This suggests that AAC may simply reflect higher risk of CVD that contribute towards falls propensity independent of muscle strength and physical function. Nonetheless, it is possible that the aforementioned parameters might still be interrelated, especially since vascular factors have been suggested to play an important and under-recognized role in motor function [42]. It is hypothesized that vascular disease limits the capacity of an individual to undertake physical activity or low physical activity increases an individual’s propensity to develop vascular disease [9,44]. Collectively, such events have the potential to exacerbate other falls propensity risk factors and cardiometabolic diseases. It is noteworthy that the association between AAC and fall-related hospitalizations was not attenuated after excluding women with prevalent ASVD, as well as including muscle function measures into the multivariable model. Such findings suggest that AAC may independently predict falls risk regardless of clinical vascular disease and poor musculoskeletal function. However, caution must be applied to interpreting these results given the potential for large changes in muscle and vascular parameters over the course of the follow up.
In regards to the links between falls propensity and vascular disease, numerous falls propensity risk factors such as dizziness, use of multiple medications, poor vision and impaired cognition may also manifest as part of CVD and its sequelae [4,9]. Risk factors for serious injury from falls were studied in 1103 community-dwelling Americans (mean age 76.9 years, 73% female) over a median of ~2.5 years. Cognitive impairment, the presence of at least two chronic conditions and poor gait and balance were associated with a higher relative risk for serious falls-related injury by 120%, 100% and 80%, respectively [9]. Furthermore, medications commonly prescribed as part of managing metabolic syndrome may also increase falls risk [44,45]. For example, cardiovascular medications such as digoxin, class I anti-arrhythmic drugs and diuretics have been implicated in higher falls risk [4]. Type 2 diabetes mellitus, which often presents in conjunction with vascular disease [46,47] has also been identified as a risk factor for falls in older women. Consequently, the cause of injurious falls is likely to be multifactorial in nature. Although not all data and potential confounders were available in our cohort, our multivariable-adjusted model were based on established risk factors, while also including CVD medications (e.g. anti-hypertensive, statins), prevalent disease and lifestyle factors (e.g. physical activity). This is an important consideration to determine if AAC is an independent to other known risk factors for injurious falls.
The study has several strengths including a long-term prospective follow-up (14.5-years) in a large number of older Australian women. LSI using DXA machines were used to quantify AAC, which were blindly scored by a single highly experienced investigator (JTS). LSI represents a cost-effective method, which can be incorporated as part of the routine diagnosis of osteoporosis, to assess AAC (to reduce fall-related hospitalizations risk). Future studies investigating the prognostic value of AAC, captured at the time of routine bone density testing, on falls risk and other non-vascular outcomes is warranted. Limitations of the study include its observational nature which does not permit causal links to be established and increases the possibility of bias due to residual confounding. AAC was assessed semi-quantitatively using AAC24 point scoring system which is operator dependent. However, good inter-operator agreement between our highly experienced investigator (JTS) and another experienced investigator with high intraclass correlation coefficients (ICC 0.89 95%CI 0.80–0.94) for AAC scores have been reported [30]. Unlike computed tomography, lateral spine imaging using either standard radiographs or DXA has lower image resolution that may impact the identification of smaller AACs. Nevertheless, with recent improvements to image resolution, AAC assessment via DXA represents a safe, reliable and low-cost approach that is already undertaken clinically as part of osteoporosis screening to identify people with vertebral fractures [48]. Given the study was undertaken in older community-dwelling women, the findings of this study may not be generalisable to other populations such as older men. Finally, as AAC usually presents in conjunction with other vascular disease, any un-measured confounders cannot be ruled out when considering the observed relationship between AAC and falls.
4.1. Conclusion
In this study, we demonstrated that the AAC is independently associated with increased risk for fall-related hospitalizations in community-dwelling older Australian women. Although the mechanisms underpinning this relationship remain unclear, the presence of AAC24 may be considered a long-term risk factor for injurious falls in older women. Considering the large proportion of older women undertaking routine bone densitometry scans, including AAC24 assessment may present a simple and cost-effective method to identify individuals with higher falls risk, thus enabling early inclusion into falls prevention programs to facilitate healthy ageing.
Supplementary Material
Acknowledgements
We thank the staff at the Data Linkage Branch, Hospital Morbidity Data Collection and Registry of Births, Deaths and Marriages for their work on providing data for this study.
Financial support
The study was funded by Healthway the Western Australian Health Promotion Foundation, National Health and Medical Research Council (NHMRC) of Australia project grants (254627, 303169 and 572604) and a Rebecca L. Cooper Medical Research Foundation grant. The salary of M.S is supported by a Royal Perth Hospital Research Foundation Career Advancement Fellowship. The salary of J.M.H is supported by a NHMRC of Australia Senior Research Fellowship. The salary of J.R.L is supported by a National Heart Foundation of Australia Future Leader Fellowship (ID: 102817). N.P.B is funded by a NHMRC of Australia Early Career Fellowship (ID: 1159914). The salary of L.C.B is supported by an NHMRC of Australia Emerging Leadership Investigator Grant (ID: 1172987) and a National Heart Foundation of Australia Post-Doctoral Research Fellowship (ID: 102498). D.P.K’s time was supported by a grant from the National Institute of Arthritis, Musculoskeletal and Skin Diseases (R01 AR 041398). None of these funding agencies had any input into any aspect of the design and management of this study.
Footnotes
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Appendix A. Supplementary data
Supplementary data to this article can be found online at https://doi.org/10.1016/j.atherosclerosis.2021.05.003.
References
- [1].Ballestas T, Xiao J, McEvoy S, Somerford P, The epidemiology of injury in Western Australia, 2000–2008, Department of Health WA, Perth, 2011. [Google Scholar]
- [2].Downton JH, Andrews K, Prevalence, characteristics and factors associated with falls among the elderly living at home, Aging (Milano) 3 (1991) 219–228. [DOI] [PubMed] [Google Scholar]
- [3].Tinetti ME, Speechley M, Ginter SF, Risk factors for falls among elderly persons living in the community, N. Engl. J. Med 319 (1988) 1701–1707. [DOI] [PubMed] [Google Scholar]
- [4].Ambrose AF, Paul G, Hausdorff JM, Risk factors for falls among older adults: a review of the literature, Maturitas 75 (2013) 51–61. [DOI] [PubMed] [Google Scholar]
- [5].Schwartz AV, Hillier TA, Sellmeyer DE, Resnick HE, Gregg E, et al. , Older women with diabetes have a higher risk of falls: a prospective study, Diabetes Care 25 (2002) 1749–1754. [DOI] [PubMed] [Google Scholar]
- [6].Rubenstein LZ, Josephson KR, The epidemiology of falls and syncope, Clin. Geriatr. Med 18 (2002) 141–158. [DOI] [PubMed] [Google Scholar]
- [7].Manemann SM, Chamberlain AM, Boyd CM, Miller DM, Poe KL, et al. , Fall risk and outcomes among patients hospitalized with cardiovascular disease in the community, Circ Cardiovasc Qual Outcomes 11 (2018) e004199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Rodríguez AJ, Lewis JR, Scott DS, Kiel DP, Schousboe JT, et al. , Aortic calcification is associated with five-year decline in handgrip strength in older women, Calcif. Tissue Int 103 (2018) 589–598. [DOI] [PubMed] [Google Scholar]
- [9].Tinetti ME, Doucette J, Claus E, Marottoli R, Risk factors for serious injury during falls by older persons in the community, J. Am. Geriatr. Soc 43 (1995) 1214–1221. [DOI] [PubMed] [Google Scholar]
- [10].Sim M, Prince RL, Scott D, Daly RM, Duque G, et al. , Utility of four sarcopenia criteria for the prediction of falls-related hospitalization in older Australian women, Osteoporos. Int 30 (2019) 167–176. [DOI] [PubMed] [Google Scholar]
- [11].Gambardella J, Wang X, Mone P, Khondkar W, Santulli G, Genetics of adrenergic signaling drives coronary artery calcification, Atherosclerosis 310 (2020) 88–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Lahousse L, Bos D, Wijnant SRA, Kavousi M, Stricker BH, et al. , Atherosclerotic calcification in major vessel beds in chronic obstructive pulmonary disease: the Rotterdam Study, Atherosclerosis 291 (2019) 107–113. [DOI] [PubMed] [Google Scholar]
- [13].Hanley C, Shields KJ, Matthews KA, Brooks MM, Janssen I, et al. , Associations of cardiovascular fat radiodensity and vascular calcification in midlife women: the SWAN cardiovascular fat ancillary study, Atherosclerosis 279 (2018) 114–121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Lewis JR, Schousboe JT, Lim WH, Wong G, Wilson KE, et al. , Long-term atherosclerotic vascular disease risk and prognosis in elderly women with abdominal aortic calcification on lateral spine images captured during bone density testing: a prospective study, J. Bone Miner. Res 33 (2018) 1001–1010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Leow K, Szulc P, Schousboe JT, Kiel DP, Teixeira-Pinto A, et al. , Prognostic value of abdominal aortic calcification: a systematic review and meta-analysis of observational studies, J Am Heart Assoc 10 (2021) e017205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Bolland MJ, Wang TK, van Pelt NC, Horne AM, Mason BH, et al. , Abdominal aortic calcification on vertebral morphometry images predicts incident myocardial infarction, J. Bone Miner. Res 25 (2010) 505–512. [DOI] [PubMed] [Google Scholar]
- [17].Bellasi A, Di Lullo L, Russo D, Ciarcia R, Magnocavallo M, et al. , Predictive value of measures of vascular calcification burden and progression for risk of death in incident to dialysis patients, J. Clin. Med 10 (3) (2021) 376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Lewis JR, Schousboe JT, Lim WH, Wong G, Zhu K, et al. , Abdominal aortic calcification identified on lateral spine images from bone densitometers are a marker of generalized atherosclerosis in elderly women, Arterioscler. Thromb. Vasc. Biol 36 (2016) 166–173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Schousboe JT, Lewis JR, Kiel DP, Abdominal aortic calcification on dual-energy X-ray absorptiometry: methods of assessment and clinical significance, Bone 104 (2017) 91–100. [DOI] [PubMed] [Google Scholar]
- [20].Schulz E, Arfai K, Liu X, Sayre J, Gilsanz V, Aortic calcification and the risk of osteoporosis and fractures, J. Clin. Endocrinol. Metab 89 (2004) 4246–4253. [DOI] [PubMed] [Google Scholar]
- [21].Lewis JR, Eggermont CJ, Schousboe JT, Lim WH, Wong G, et al. , Association between abdominal aortic calcification, bone mineral density, and fracture in older women, J. Bone Miner. Res 34 (2019) 2052–2060. [DOI] [PubMed] [Google Scholar]
- [22].Yang SW, Yang HF, Chen YY, Chen WL, Unraveling the link between metabolic syndrome and abdominal aortic calcification, Nutr. Metabol. Cardiovasc. Dis 31 (2021) 464–471. [DOI] [PubMed] [Google Scholar]
- [23].Akbay E, Çoner A, Akinci S, Adar A, Çakan F, et al. , Aortic Arch Calcification: a Novel Parameter for Prediction of Masked Hypertension, Blood Press Monit, 2021. [DOI] [PubMed] [Google Scholar]
- [24].Iribarren C, Sidney S, Sternfeld B, Browner WS, Calcification of the aortic arch: risk factors and association with coronary heart disease, stroke, and peripheral vascular disease, JAMA 283 (2000) 2810–2815. [DOI] [PubMed] [Google Scholar]
- [25].Ali H, Zmuda JM, Cvejkus RK, Kershaw EE, Kuipers AL, et al. , Wnt pathway inhibitor DKK1: a potential novel biomarker for adiposity, J Endocr Soc 3 (2019) 488–495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Ramírez-Vélez R, García-Hermoso A, Correa-Rodríguez M, Lobelo F, Gonźalez-Ruiz K, et al. , Abdominal aortic calcification is associated with decline in handgrip strength in the US adult population≥ 40 years of age, Nutr. Metabol. Cardiovasc. Dis 31 (2021) 1035–1043. [DOI] [PubMed] [Google Scholar]
- [27].Stevens JA, Sogolow ED, Gender differences for non-fatal unintentional fall related injuries among older adults, Inj. Prev 11 (2005) 115–119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Prince RL, Devine A, Dhaliwal SS, Dick IM, Effects of calcium supplementation on clinical fracture and bone structure: results of a 5-year, double-blind, placebo-controlled trial in elderly women, Arch. Intern. Med 166 (2006) 869–875. [DOI] [PubMed] [Google Scholar]
- [29].Kauppila LI, Polak JF, Cupples LA, Hannan MT, Kiel DP, et al. , New indices to classify location, severity and progression of calcific lesions in the abdominal aorta: a 25-year follow-up study, Atherosclerosis 132 (1997) 245–250. [DOI] [PubMed] [Google Scholar]
- [30].Schousboe JT, Wilson KE, Kiel DP, Detection of abdominal aortic calcification with lateral spine imaging using DXA, J. Clin. Densitom 9 (2006) 302–308. [DOI] [PubMed] [Google Scholar]
- [31].Lewis JR, Lim W, Dhaliwal SS, Zhu K, Lim EM, et al. , Estimated glomerular filtration rate as an independent predictor of atherosclerotic vascular disease in older women, BMC Nephrol. 13 (2012) 58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Lewis JR, Lim WH, Wong G, Abbs S, Zhu K, et al. , Association between high-sensitivity cardiac troponin I and cardiac events in elderly women 6, J Am Heart Assoc, 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].Bruce DG, Devine A, Prince RL, Recreational physical activity levels in healthy older women: the importance of fear of falling, J. Am. Geriatr. Soc 50 (2002) 84–89. [DOI] [PubMed] [Google Scholar]
- [34].McArdle WD, Katch FI, Katch VL, Exercise Physiology: Nutrition, Energy, and Human Performance, Lippincott Williams & Wilkins, 2010. [Google Scholar]
- [35].Britt H, Scahill S, Miller G, ICPC PLUS© for community health? A feasibility study, Health Inf. Manag. J 27 (1997) 171–175. [DOI] [PubMed] [Google Scholar]
- [36].Sim M, Blekkenhorst LC, Lewis JR, Bondonno CP, Devine A, et al. , Vegetable and fruit intake and injurious falls risk in older women: a prospective cohort study, Br. J. Nutr 120 (2018) 925–934. [DOI] [PubMed] [Google Scholar]
- [37].Austin N, Devine A, Dick I, Prince R, Bruce D, Fear of falling in older women: a longitudinal study of incidence, persistence, and predictors, J. Am. Geriatr. Soc 55 (2007) 1598–1603. [DOI] [PubMed] [Google Scholar]
- [38].Sim M, Lewis JR, Blekkenhorst LC, Bondonno CP, Devine A, et al. , Higher dietary nitrate intake is associated with better muscle function in older women, J. Cachexia Sarcopenia Muscle (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [39].R Core Team, R: a Language and Environment for Statistical Computing, R Foundation for Statistical Computing, Vienna, Austra, 2019. http://www.R-project.org/. [Google Scholar]
- [40].Sanders KM, Lim K, Stuart AL, Macleod A, Scott D, et al. , Diversity in fall characteristics hampers effective prevention: the precipitants, the environment, the fall and the injury, Osteoporos. Int 28 (2017) 3005–3015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [41].Park J, Park H, Muscle strength and carotid artery flow velocity is associated with increased risk of atherosclerosis in adults, Cardiol. J 24 (2017) 385–392. [DOI] [PubMed] [Google Scholar]
- [42].Elbaz A, Ripert M, Tavernier B, Février B, Zureik M, et al. , Common carotid artery intima-media thickness, carotid plaques, and walking speed, Stroke 36 (2005) 2198–2202. [DOI] [PubMed] [Google Scholar]
- [43].Everson-Rose SA, Mendes de Leon CF, Roetker NS, Lutsey PL, Alonso A, Subclinical cardiovascular disease and changes in self-reported mobility: multi-ethnic study of atherosclerosis, J Gerontol A Biol Sci Med Sci 73 (2018) 218–224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [44].Huang AR, Mallet L, Rochefort CM, Eguale T, Buckeridge DL, et al. , Medication-related falls in the elderly, Drugs Aging 29 (2012) 359–376. [DOI] [PubMed] [Google Scholar]
- [45].Leipzig RM, Cumming RG, Tinetti ME, Drugs and falls in older people: a systematic review and meta-analysis: II. Cardiac and analgesic drugs, J. Am. Geriatr. Soc 47 (1999) 40–50. [DOI] [PubMed] [Google Scholar]
- [46].Vinik AI, Vinik EJ, Colberg SR, Morrison S, Falls risk in older adults with type 2 diabetes, Clin. Geriatr. Med 31 (2015) 89–99. [DOI] [PubMed] [Google Scholar]
- [47].Sanchis P, Rivera R, Fortuny R, Río C, Mas-Gelabert M, et al. , Role of advanced glycation end products on aortic calcification in patients with type 2 diabetes mellitus, J. Clin. Med 9 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [48].Setiawati R, Di Chio F, Rahardjo P, Nasuto M, Dimpudus FJ, et al. , Quantitative assessment of abdominal aortic calcifications using lateral lumbar radiograph, dual-energy X-ray absorptiometry, and quantitative computed tomography of the spine, J. Clin. Densitom 19 (2016) 242–249. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


