Abstract
The nervous system exerts finely tuned control over all aspects of the life of an organism, including pain, sensation, growth, and development. Recent developments in tissue regeneration research have increasingly turned to small molecule peptides to tailor and augment the biological response following tissue loss or injury. In the present study, we have introduced the small molecule peptide galanin (GAL) as a novel scaffold-coating agent for the healing and regeneration of craniofacial tissues. Using immunohistochemistry, we detected GAL and GAL receptors in healthy periodontal tissues and in the proximity of blood vessels, while exposure to our periodontal disease regimen resulted in a downregulation of GAL. In a 3-dimensional bioreactor culture, GAL coating of collagen scaffolds promoted cell proliferation and matrix synthesis. Following subcutaneous implantation, GAL-coated scaffolds were associated with mineralized bone-like tissue deposits, which reacted positively for alizarin red and von Kossa, and demonstrated increased expression and protein levels of RUNX2, OCN, OSX, and iBSP. In contrast, the GAL receptor antagonist galantide blocked the effect of GAL on Runx2 expression and inhibited mineralization in our subcutaneous implantation model. Moreover, GAL coating promoted periodontal regeneration and a rescue of the periodontal defect generated in our periodontitis model mice. Together, these data demonstrate the efficacy of the neuropeptide GAL as a coating material for tissue regeneration. They are also suggestive of a novel role for neurogenic signaling pathways in craniofacial and periodontal regeneration.
Keywords: scaffold coating, neuroskeletogenesis, mineralization, neuropeptide, remodeling, galantide
Introduction
The nervous system affects and regulates all functions of the human body, including the physiology and metabolism of mineralized tissues such as bone and cartilage. The neurogenic control of skeletal homeostasis occurs through sympathetic and sensory neurotransmitters since osteoblasts, osteoclasts, or osteocytes lack classic synapses (Jones et al. 2004; Elefteriou 2005; Grässel 2014). Neural transmission ligands are frequently detected in close spatial association with bone cells, and neuropeptides such as neuropeptide Y, vasointestinal peptide, substance P, and calcitonin gene–related peptide exhibit potent effects on chondro-osteogenic differentiation, bone formation, or osteoclastogenesis and bone resorption (Lundy and Linden 2004; Sousa et al. 2009; Franquinho et al. 2010; Nunes et al. 2010; Grässel 2014). These studies suggest that neuropeptides are essential modulators of the effects of the nervous system on skeletal metabolism and mineral homeostasis.
One of the peptides involved in neuroskeletal homeostasis is the neuropeptide galanin (GAL), a highly conserved 29– to 30–amino acid peptide (Tatemoto et al. 1983; Schmidt et al. 1991). GAL signals via 3 G protein–coupled receptors, GALR1, GALR2, and GALR3 (Habert-Ortoli et al. 1994; Branchek et al. 2000), through which it affects its target organs. In previous studies, elevated concentrations of GAL have been detected in the fracture callus of fractured bones (McDonald et al. 2003) and demonstrated potential to facilitate bone formation associated with injury by inhibiting excess TNF-α and IL-1β production (McDonald et al. 2007). GAL is also necessary for the leptin-independent bone mass–inducing effects of the transcription factor ΔFosB on bone homeostasis via hypothalamic neurons (Idelevich et al. 2018; Idelevich et al. 2019). Thus, GAL is a prototypical neurogenic effector molecule involved in skeletal homeostasis.
The periodontal region is a mineralized tissue–connective tissue interface tightly regulated by homeostatic control of new bone formation and bone resorption. This narrow interface between adjacent soft and hard tissues is richly innervated by trigeminal nerve fibers that contain several neuropeptides, including substance P, calcitonin gene–related peptide, vasoactive intestinal peptide, neuropeptide Y, and GAL (Luthman et al. 1988; Deguchi et al. 2003; Lundy and Linden 2004). Previous studies have suggested that all 5 of these nerve-derived signal molecules may play individually distinct roles in the regulation of bone remodeling (Juarranz et al. 2005; McDonald et al. 2007; Franquinho et al. 2010; Grässel 2014). The presence of these neuroskeletal peptides in the periodontal region prompted us to speculate that neuropeptides are involved in the mineral homeostasis essential for the maintenance of the periodontal alveolus and healthy attachment.
In the present study, we have employed surface coatings as a means to harness the instructive capacity of neuropeptides for periodontal health and to test the ability of neurogenic signals to control periodontal mineral homeostasis. Surface modifications through coatings and other material design strategies are powerful tools to tune the biomimetic behavior of regenerative scaffolds (Richbourg et al. 2019). Here we have employed the neuropeptide GAL as a collagen (Col) sponge–coating material for mineralized tissue engineering and periodontal regeneration. Our data shed light on the potent instructive capacity of the GAL as a modulator and inducer of new mineralized tissue formation and periodontal homeostasis.
Materials and Methods
GAL Peptide Coating of Col Scaffold
Col sponge (Advanced BioMatrix) was cut to uniform size with a 5-mm biopsy punch (5-mm diameter × 1-mm thickness) and coated with 30 µL of phosphate buffered saline (PBS; control), GAL (Sigma), galantide (GT; a GAL receptor antagonist [Sigma]), or GAL + GT at a concentration of 10−8 M and incubated at 4 °C overnight. The coated Col sponges were freeze-dried.
Characterization of GAL-Coated Col Scaffolds
Scanning Electron Microscopy
For surface analysis, scaffolds were dried, sputter coated with gold/palladium, and then visualized with a scanning electron microscope (JSC-6010LA; JEOL).
Cell Attachment and Viability Analyses
Human periodontal ligament (hPDL) progenitor cells were isolated and cultured as described (Dangaria et al. 2010). hPDL cells (106) were seeded onto Col-control or Col-GAL scaffolds and cultured in 96-well plates in triplicate for 24 to 48 h. The cells were stained with DAPI for cell attachment counting. Live and dead cells were identified with a LIVE/DEAD Cell Imaging Kit (Invitrogen). The cells were stained with a calcein and BoBo-3 iodide for 15 min at 37 °C. Images were captured by confocal laser scanning microscopy.
GAL Release from a Coated Col Scaffold
Col-control and Col-GAL scaffolds (5-mm diameter × 1-mm thickness) were immersed in 1 mL of PBS at 37 °C for 1, 3, 5, 7, 9, 11, and 13 d. The solution was refreshed and collected at each time point. The concentration of GAL peptide in PBS was analyzed with a GAL ELISA kit (Abcam) and measured by a Spectro Max 250 (Molecular Devices).
3-Dimensional Culture of hPDL Cells Seeded onto Col Scaffolds in a 3-Dimensional Bioreactor
hPDL cells (106 per scaffold) were seeded onto Col-control or Col-GAL scaffolds (n = 5) and incubated at 37 °C for 2 h. The cell-seeded scaffolds were then transferred into 10-mL bioreactor vessels (Synthecon) and cultured for 7 d for BrdU incorporation and 14 d for hematoxylin and eosin and Masson’s staining. Three hours prior to culture termination, 10µM BrdU (bromodeoxyuridine/5-bromo-2′-deoxyuridine; Sigma) was added to the culture medium. The cultured scaffolds were harvested and processed for sections or RNA or protein extraction.
Periodontitis Mouse Model
All experimental procedures on rodents were approved by and conducted according to the guidelines of the Institutional Animal Care and Use Committee of Texas A&M University College of Dentistry. A completed copy of the ARRIVE checklist has been provided as a supplementary file. Wild-type C57BL/6 mice aged 8 wk were obtained from Charles River Laboratory. To induce periodontitis, sutures (5-0 silk suture) were placed around the left maxillary second molars for 4 wk as described earlier (Qian et al. 2019).
Subcutaneous Implantation
After anesthetization, the back skin of mice was sheared and sterilized, and 2 incisions were made on the upper and lower back areas. Four groups (n = 5 per group) of coated Col scaffolds (Col-control, Col-GAL, Col-GT, and Col-GAL/GT) were implanted into each side of the 2 incisions below the subcutis as described (Pan et al. 2013). The experiments were terminated 4 wk after implantation.
Periodontal Implantation
Periodontitis-treated mice were divided into 2 groups (n = 5): the treatment group (periodontitis/Col-GAL) and the defect control (periodontitis/Col-control). To readily access periodontitis-related bone defects, mice were subjected to gingival flap surgery. Briefly, incisions were placed from the mesial surface of the first molar to the distal surface of the third molar on the left maxilla. Full-thickness flaps were elevated, and Col-control or Col-GAL scaffolds were applied to the surface of the exposed alveolar bone and sutured in place (Francis et al. 2020). Experiments were terminated 6 wk after implantation.
Micro–Computed Tomography
Mouse left-side maxillae 6 wk after implantation were collected and fixed. Micro–computed tomography images from the prepared left maxillae were captured and reconstructed with a Scanco 40 µCT (Scanco Medical). Bone volume and mineral density were analyzed with a TeraRecon software package as described previously (Lu et al. 2016).
Tissue Processing and Histology
Maxillae and Col implants were dissected, fixed, embedded in paraffin, and then sectioned. For hematoxylin and eosin staining, sections were dipped in hematoxylin and then in eosin; for alizarin red staining, sections were stained with 1% alizarin red solution; for von Kossa staining, sections were stained with 5% silver nitrate; and Masson’s staining was performed with a Trichrome Staining Kit (Sigma). The stained sections were dehydrated, cleared in xylene, and covered.
Immunohistochemistry
A HistoMouse-Plus Kit (ThermoFisher) was used to detect the expression of GAL and its receptors (antibodies against GAL GTX88303, GALR1 GTX108207, GALR2 GTX100320, GALR3 GTX1081637; GenTex) as well as BRDU incorporation (ab152095; Abcam). In brief, sections were first incubated with primary antibodies overnight at 4 °C and then with biotinylated secondary antibodies and chromogen following the manufacturer’s instruction. Negative controls were processed without primary antibodies.
RNA Extraction and Quantitative Real-Time Polymerase Chain Reaction Analysis
Total RNA was extracted from mouse dental tissues or hPDL cells with the RNeasy Plus Mini Kit (Qiagen) according to the manufacturer’s instructions. One microgram of RNA was used for cDNA generation with SuperScript III (ThermoFisher Scientific). Polymerase chain reaction (PCR) primers of tested genes are listed in the Appendix Table. Transcript levels were quantified by SYBR Green (Applied Biosystems) based on quantitative real-time PCR (qRT-PCR) analysis on an ABI 7000 Sequence Detection System (Applied Biosystems). Expression levels were normalized to GAPDH levels. Relative expression levels were calculated with the 2-DDCt method, and values were represented as mean ± SD.
Protein Isolation and Western Blot Analysis
Subcutaneous implants were ground under liquid nitrogen, lysed in RIPA buffer (Sigma) with a proteinase inhibitor cocktail (ThermoFisher), and centrifuged. The protein concentration was evaluated by a Pierce BCA Protein Assay Kit (ThermoFisher). For blotting, 25 μg of protein was separated on a 20% precast polyacrylamide gel (Bio-Rad) and transferred to a PVDF membrane, which was incubated with primary antibodies anti-β-actin (ab8227; Abcam), RunX2 (ab75956), and iBSP (AM02057PU-S; Origene) at 4 °C overnight and then with the secondary anti-rabbit IgG antibody (ab6721). An enhanced chemiluminescence reagent (PerkinElmer) was used to detect the immunoreactive bands.
Statistical Analysis
Each experiment was repeated at least 3 times, and comparison of the results in each experimental group was performed by an unpaired Student’s t test with Welch’s correction for unequal variances. All calculations were carried out with Prism 6 (GraphPad).
Results
GAL and Its Receptors Are Expressed in Periodontal Tissues
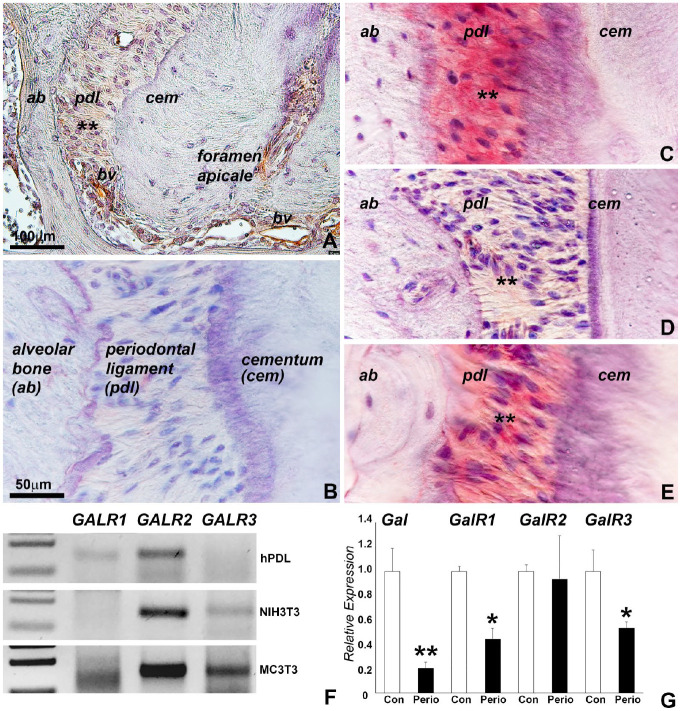
In prior studies, we identified GAL as a neurogenic small molecule involved in hPDL cell osteogenic differentiation (unpublished data). To explore GAL function in periodontal tissues, GAL was localized in the periodontal ligament via immunohistochemistry, and markedly increased GAL levels were detected in the periphery blood vessels (Fig. 1A). GAL was absent from the root cementum. To account for receptor-mediated GAL action, expression of the 3 known GAL receptors, GALR1, GALR2, and GALR3, was mapped to the periodontal ligament as demonstrated via immunohistochemistry (Fig. 1B–E) and qRT-PCR (Fig. 1F). Among these, GALR1 and GALR2 expression was confirmed in cultured hPDL cells, while GALR2 and GALR3 was detected in mouse NIH3T3 and MC3T3 cells. The expression levels of GAL, GALR1, GALR2, and GALR3 were altered in dental tissues from mice subjected to our periodontitis disease regimen, resulting in a downregulation of GAL, GALR1, and GALR3 under inflammatory condition (Fig. 1G). These data indicate that the expression of GAL and its receptors is affected by periodontal inflammation.
Figure 1.
Expression of galanin (GAL) and its receptors in periodontal ligament tissues. (A–E) Immunohistochemical localization of GAL, GALR1, GALR2, and GALR3 in the mouse periodontal ligament. GAL and its receptors were detected in the periodontal ligament (asterisks). High levels of GAL were associated with blood vessel walls (bv). Panel B is a negative control. (F) Quantitative real-time polymerase chain reaction (qRT-PCR) expression analysis of the GAL receptors GALR1, GALR2, and GALR3 in human periodontal ligament and mouse NIH3T3 and MC3T3 cells. qRT-PCR products were run in a 2% agarose gel. (G) qRT-PCR revealed changes in the expression of Gal and Gal receptors in dental tissues from mice subjected to a periodontitis regimen. Values are presented as mean ± SD. *P < 0.05. **P < 0.01.
GAL Coating Did Not Alter Col Scaffold Properties
Col scaffolds were coated with GAL peptide to deliver GAL for in vitro 3-dimensional cell culture and in vivo implantation. Scanning electron micrographs demonstrated protein precipitation on the surface of Col fibers and in the micropores of GAL-coated scaffolds (Fig. 2A, B). ELISA analysis of the coated scaffold revealed a burst of 14.6% of GAL peptide release within the first day and an accumulation of 30.4% within 3 d. During a period of 11 d, 62.5% of the GAL peptide was released from the scaffold (Fig. 2G). GAL coating on the surface of Col sponge did not affect cell adhesion properties (Fig. 2C, D). In contrast, GAL coating slightly increased cell survival rates as compared with the control group, demonstrated by a reduced number in red fluorescence–labeled cells in the live/death assay (Fig. 2E, F).
Figure 2.
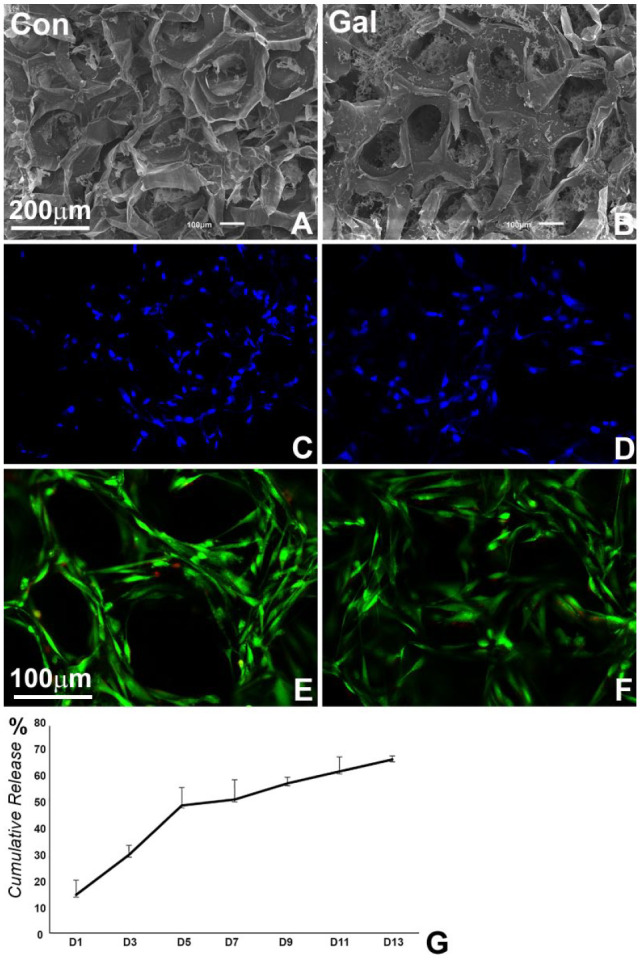
Characterization of the effect of galanin (GAL) coating on collagen scaffolds. (A, B) Scanning electron micrographs of phosphate buffered saline–coated control and GAL-coated collagen sponges. GAL was precipitated onto the surface and into the micropores of the collagen sponge (panel B vs. A). (C, D) Fluorescence staining (DAPI) of human periodontal ligament cell nuclei in GAL-coated and control collagen sponges 24 h after cell seeding. (E, F) Live/death analysis of human periodontal ligament cells in control and GAL-coated collagen sponges after 48 h of culture. Note that GAL coating did not alter cell attachment and survival in collagen scaffolds. (G) Controlled release of GAL from GAL-coated collagen sponges over a 13-d period. Values are presented as mean ± SD. Con, control.
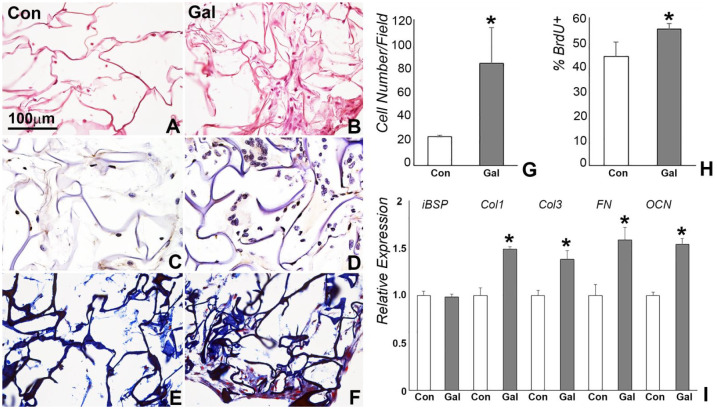
GAL Treatment Enhanced Cell Proliferation and ECM Formation of hPDL Cells in 3-Dimensional Culture
hPDL cells were seeded onto the control and GAL-coated scaffolds at a concentration of 106 per scaffold and cultured in a 3-dimensional bioreactor for 7 and 14 d. Hematoxylin and eosin staining demonstrated higher cell number and increased new matrix formation in GAL-treated scaffolds when compared with control scaffolds (Fig. 3B vs. 3A). The number of cells per field was 24 ± 1.4 on the control scaffold and 86 ± 29.7 on the GAL-coated scaffold (Fig. 3A, B, G). Of the cells on the GAL-coated scaffold, 57.3% ± 2.3% were proliferating cells, as compared with 45.9% ± 5.9% on the control scaffolds, as indicated by our BrdU incorporation assay (Fig. 3C, D, H). Masson’s staining suggested that the newly formed matrix in control and GAL-coated scaffolds contained Col fibers (Fig. 3E, F). In addition, qRT-PCR analysis showed significant upregulation of COL1, COL3, FN, and OCN genes but not iBSP (Fig. 3I).
Figure 3.
Human periodontal ligament proliferation and matrix formation in control and galanin (GAL)–coated collagen scaffolds in a 3-dimensional bioreactor. (A, B) Hematoxylin and eosin staining of collagen scaffolds 7 d after culture. Cell number and ECM formation were increased in the GAL-coated scaffold (panel B vs. A, G). (C, D, H) BrdU incorporation comparison of cell proliferation revealing a significant increase in the number of BrdU-positive cells (brown) in the GAL-coated scaffolds. (E, F) Masson staining of ECM connective tissue fibers in control and GAL-coated collagen scaffolds. (I) Quantitative real-time polymerase chain reaction analysis demonstrated significantly elevated levels of collagen I, collagen III, fibronectin, and osteocalcin gene expression. Values are presented as mean ± SD. Con, control. *P < 0.05.
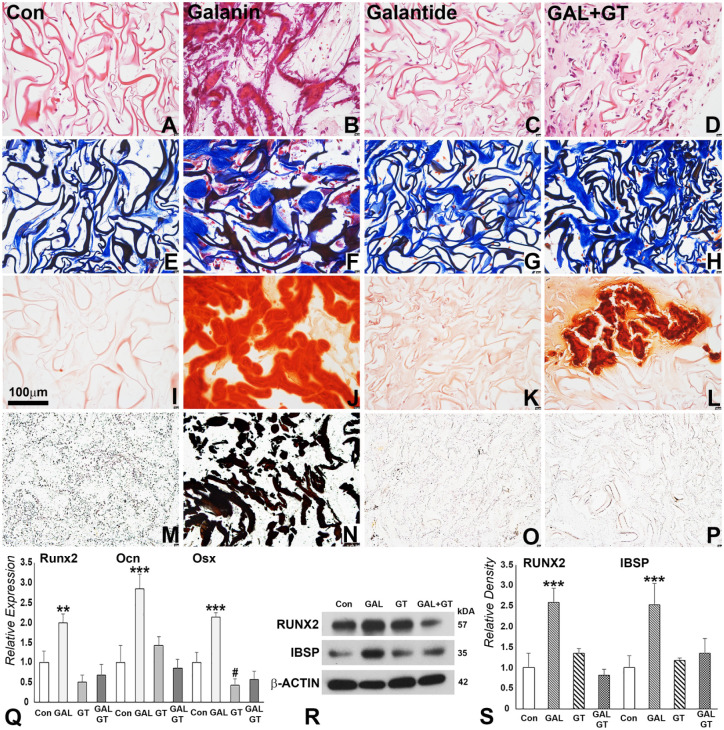
GAL Treatment Promoted Endogenous Stem Cell Homing and Mineralization in Subcutaneous Implants
To confirm the effects of GAL treatment on cell proliferation and differentiation, we introduced the GAL antagonist GT as part of our experimental strategy. PBS, GAL only, GT only, or GAL + GT were used to coat Col sponges, and the scaffolds were subcutaneously implanted for 4 wk. After 4 wk of implantation, deposition of osteoid-like tissue was observed in GAL-coated scaffolds as visualized with hematoxylin and eosin staining (Fig. 4B vs. A, C, D). These newly formed tissues contained connective tissue matrix (Fig. 4F) and were mineralized as revealed by alizarin red and von Kossa staining (Fig. 4J, N). In contrast, GT coating did not cause any significant changes in cell number and tissue structure in the scaffold when compared with the control scaffold and did not induce mineralized tissue formation (Fig. 4C, G, K, O). However, when GAL and GT were used to coat the scaffold, GT partially blocked GAL function, revealing decreased levels of matrix formation as compared with the control and GAL-coated scaffolds. The new matrix in GAL-coated scaffolds was enriched in calcium as illustrated by alizarin red staining (Fig. 4J) and contained inorganic phosphates according to the von Kossa stain applied to verify complete mineralization (Fig. 4N). qRT-PCR and Western blot analyses confirmed that the mineralized tissue marker gene products RUNX2, OCN, OSX, and iBSP were significantly upregulated at the mRNA and protein levels in the GAL-treated group (Fig. 4Q–S). However, the effect of GAL on bone-related gene expression was inhibited when GT was applied in combination with GAL, demonstrating the specificity of GAL as a mineralization-inducing agent.
Figure 4.
Mineralization parameter analysis of subcutaneously implanted control and galanin (GAL)–coated collagen scaffolds. For this study, collagen sponges were coated with phosphate buffered saline (control), GAL only, GAL antagonist galantide only (GT), or both (GAL + GT) and subcutaneously implanted for 4 wk. (A–D) Hematoxylin and eosin revealed trabecular structures in GAL- and GAL + GT–coated scaffolds. (E–H) Newly formed connective tissues as revealed by Masson’s trichrome staining. (F) Note the dense connective tissue blocks and red coloration indicative of osteoid. (I–L) Alizarin red staining and (M–P) von Kossa staining illustrate key mineralized tissue components (J, L) calcium and (N) phosphorus indicative of mineralized tissue formation in GAL- and GAL + GT–coated scaffolds. (Q) Quantitative real-time polymerase chain reaction analysis of bone-related protein expression in controls, GAL, GT, and GAL + GT treatment groups at the mRNA level. (R, S) Western blotting comparison of bone-related proteins subjected to GAL, GT, and GAL + GT treatment and corresponding densitometry analysis. #P < 0.05 downregulation. *P < 0.01. ***P < 0.001. Con, control.
GAL Treatment Restored Periodontal Tissue Integrity in a Mouse Periodontitis Model
Given the substantial effect of GAL on new connective tissue formation and mineralization, we decided to explore the applicability of GAL treatment in periodontal regeneration. For this study, GAL-coated and control scaffolds were implanted into alveolar bone defects of mice subjected to our periodontitis regimen for a period of 6 wk. Micro–computed tomographic analysis demonstrated a 24.8% reduction in the distance between cementum-enamel junction and alveolar bone crest in GAL-treated samples, as well as an increase in bone volume:tissue volume ratio, trabecular number and thickness, and a decrease in trabecular space (Fig. 5A–D, G–I). The enhanced bone density as a result of GAL treatment was confirmed by Masson’s staining (Fig. 5G, H). Mineral density in the GAL-coated scaffold was similar to the mineral density of the control scaffold (Fig. 5I). However, GAL greatly improved Sharpey’s fiber attachment, smoothened alveolar bone surface contours, reduced fibrotic tissue in the periodontal ligament, and improved the acellular cementum–periodontal fiber interface responsible for connective tissue attachment (Fig. 5F vs. E).
Figure 5.
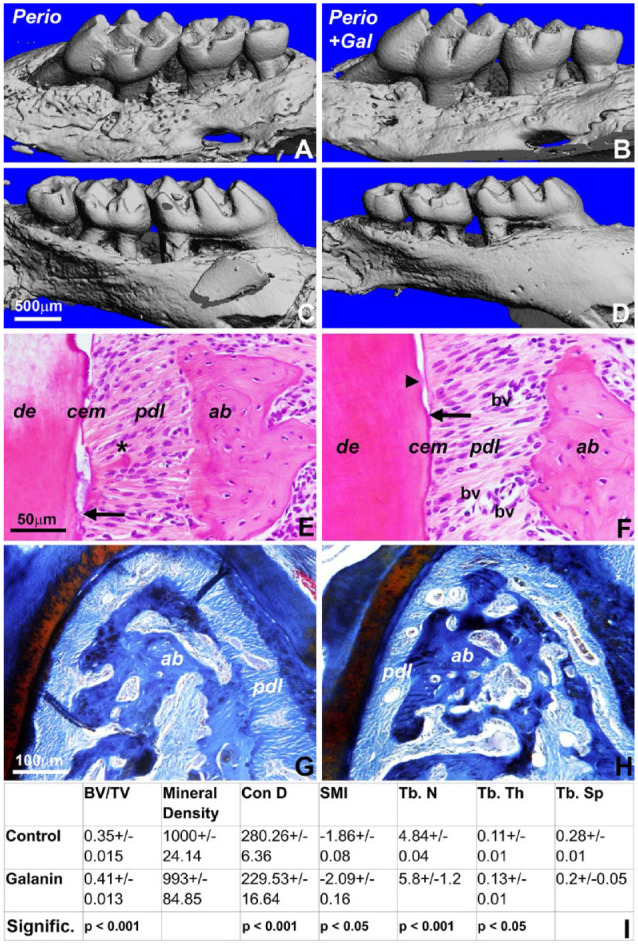
Restoration of periodontal tissue integrity in periodontitis mice by galanin (GAL) treatment. Three-dimensional reconstruction of micro–computed tomography images: (A, B) lingual view and (C, D) buccal view. (A, C) Periodontitis treatment group and (B, D) GAL treatment of periodontitis-exposed mice 6 wks after implantation. Note the smoothened bone contours in the GAL-treated group. Micrographs of hematoxylin and eosin–stained sections through the periodontia of mice (E) with periodontitis and (F) after GAL treatment. Arrows indicate the coronal-most position of Sharpey’s fibers (periodontal ligament [pdl]) attached to root cementum (cem). Note an island of reattaching Sharpey’s fibers in the GAL treatment group (arrowhead). The periodontal ligament of the inflamed periodontia revealed fibrotic tissue (asterisk), while the GAL-treated periodontal ligament featured healthy Sharpey’s fibers and increased blood vessel cross sections (panel F vs. E). Several blood vessels (bv) have been highlighted in the periodontal ligament of the GAL-treated group versus the control. ab, alveolar bone; de, dentin. (G, H) Masson’s staining of alveolar bone at the furcation between first molar roots. Note the increase in bone density following GAL treatment (panel H vs. G). (I) Alveolar bone parameters from the periodontitis control and GAL-treated mice.
Discussion
The present study was designed to examine the efficacy of the neuropeptide GAL as a coating material for periodontal tissue regeneration and a tool for mineralized tissue restoration. Our data confirmed GAL expression in periodontal tissues and verified the applicability of GAL as a Col scaffold–coating material to promote new mineralized tissue and connective tissue formation and to facilitate periodontal regeneration through bone remodeling and periodontal attachment restoration. Together, our studies are supportive of a novel role for neurogenic peptide signaling in craniofacial and periodontal regeneration.
According to our immunohistochemical analysis, GAL-like immunoreactivity was detected in periodontal ligament fibroblasts and periodontal extracellular matrix, with intense reactivity in the periphery of blood vessels. In addition, expression of all 3 GAL receptors was reported in the PDL. GALR1 and GALR2 were expressed in primary hPDL cells, and immunoreactivity for all 3 GAL receptors was detected in the periodontal ligament. Discrepancies in the level of immunodetection and qRT-PCR amplification signal between GALR2 and GALR3 are likely due to changes in the expression phenotype of cultured cells. The strong expression of a neuroendocrine peptide and its receptors in periodontal tissues may come as a surprise, as GAL was first isolated from porcine intestine (Tatemoto et al. 1983) and has been associated with the central and peripheral nervous system (Bedecs et al. 1995). However, nonneural expression of GAL-like immunoreactivity has been reported in several other tissues and cells: fibroblast cell lines (Yamamoto et al. 2014); primary epidermal keratinocytes (Lee et al. 2017); macrophage-like cells of the synovial membrane (Wu et al. 2004); fibroblast-like cells in skin during the formation of granulation tissue (Yamamoto et al. 2011); osteoprogenitor cells, osteoblasts, and chondrocytes of the hypertrophic zone during rib fracture repair (McDonald et al. 2003); and blood vessels, hair follicles, and sweat glands (Kofler et al. 2004; Lang et al. 2007). Moreover, previous studies suggested that GAL is involved in the regulation of inflammatory processes (Koller et al. 2017; Koller et al. 2019; Brzozowska and Calka 2021; Martinelli et al. 2021). Earlier studies have speculated about an application of GAL in tissue regeneration (McDonald et al. 2003; Yamamoto et al. 2011; Yamamoto et al. 2014), and one study applied GAL injections to promote calvarial bone formation via an injection strategy (McDonald et al. 2007). Together, these studies are supportive of our concept of using GAL as a scaffold-coating material for tissue engineering to regenerate tissues damaged or lost because of inflammation.
In the present study, local delivery of GAL induced ectopic bone formation in subcutaneous implants and restored periodontal tissue integrity in mice with periodontal disease. The effect of GAL on ectopic mineralization superseded the effect of its inhibitor GT, suggesting that GAL acts earlier or more efficient than its inhibitor. Our local delivery approach via Col scaffold coating was prompted by an earlier study revealing a significant increase of endogenous GAL-like immunoreactivity expression in a callus of fractured rat ribs during bone healing (McDonald et al. 2003). GAL local injection also rescued calvarial bone defects in a dose-dependent manner (McDonald et al. 2007), lending support to the concept of local GAL delivery as a means for bone tissue engineering. Subcutaneous implantation studies revealed evidence of mineralization in the GAL + GT group, likely due to an initial effect of GAL on osteogenic differentiation in the subcutaneous scaffold environment and a delayed action of the inhibitor.
Our study demonstrated that GAL treatment resulted in partial restoration of healthy alveolar bone height as indicated by a 24.8% reduction in the cementum-enamel junction–bone margin distance. In addition, GAL treatment led to a recontouring and smoothening of alveolar bone surfaces. From a periodontal perspective, increased bone height, increased attachment, and smoothening of bone surfaces are important goals of clinical periodontal therapy, suggesting a potential clinical application of GAL in the restoration of periodontal health following periodontal disease. Positive effects of GAL on bone formation and remodeling have also been reported in the mouse calvarial defect model mentioned earlier (McDonald et al. 2007). While the mechanism by which GAL regulates tissue regeneration is currently unknown, it has been shown that GAL affects cardiomyoblasts through the FoxO1 pathway (Martinelli et al. 2021), a possible candidate pathway as FoxO family members have been implicated in the regulation of osteoprogenitors, osteoblasts, and osteoclasts (Alharbi et al. 2018; Ma et al. 2020). In addition, anti-inflammatory effects of GAL have been indicated in animal models with chronic inflammatory diseases (Talero et al. 2006; Talero et al. 2007; Brzozowska and Calka 2021). These data suggest that the anti-inflammatory and regenerative effects of GAL will restore physiologic periodontal tissue homeostasis.
Author Contributions
W. Ma, contributed to design and data acquisition, drafted and critically revised the manuscript; H. Lyu, contributed to design and data analysis, drafted and critically revised the manuscript; M. Pandya, contributed to conception, data acquisition, analysis, and interpretation, drafted and critically revised the manuscript; G. Gopinathan, contributed to conception and data acquisition, drafted and critically revised the manuscript; X. Luan, contributed to conception, design, data acquisition, analysis, and interpretation, drafted and critically revised the manuscript; T.G.H. Diekwisch, contributed to conception, design, and data analysis, drafted and critically revised the manuscript. All authors gave final approval and agree to be accountable for all aspects of the work.
Supplemental Material
Supplemental material, sj-docx-1-jdr-10.1177_00220345211028852 for Successful Application of a Galanin-Coated Scaffold for Periodontal Regeneration by W. Ma, H. Lyu, M. Pandya, G. Gopinathan, X. Luan and T.G.H. Diekwisch in Journal of Dental Research
Footnotes
A supplemental appendix to this article is available online.
Declaration of Conflicting Interests: The authors declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The authors disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: Generous support for these studies was provided by National Institute of Dental and Craniofacial Research (grants R01 DE027930 to T.G.H. Diekwisch and DE019463 to X. Luan).
ORCID iD: T.G.H. Diekwisch  https://orcid.org/0000-0003-3356-9677
https://orcid.org/0000-0003-3356-9677
References
- Alharbi MA, Zhang C, Lu C, Milovanova TN, Yi L, Ryu JD, Jiao H, Dong G, O’Connor JP, Graves DT. 2018. FOXO1 deletion reverses the effect of diabetic-induced impaired fracture healing. Diabetes. 67(12):2682–2694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bedecs K, Berthold M, Bartfai T. 1995. Galanin—10 years with a neuroendocrine peptide. Int J Biochem Cell Biol. 27(4):337–349. [DOI] [PubMed] [Google Scholar]
- Branchek TA, Smith KE, Gerald C, Walker MW. 2000. Galanin receptor subtypes. Trends Pharmacol Sci. 21(3):109–117. [DOI] [PubMed] [Google Scholar]
- Brzozowska M, Całka J. 2021. Review: occurrence and distribution of galanin in the physiological and inflammatory states in the mammalian gastrointestinal tract. Front Immunol. 11:602070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dangaria SJ, Ito Y, Luan X, Diekwisch TG. 2010. Differentiation of neural-crest-derived intermediate pluripotent progenitors into committed periodontal populations involves unique molecular signature changes, cohort shifts, and epigenetic modifications. Stem Cells Dev. 20(1):39–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deguchi T, Takeshita N, Balam TA, Fujiyoshi Y, Takano-Yamamoto T. 2003. Galanin-immunoreactive nerve fibers in the periodontal ligament during experimental tooth movement. J Dent Res. 82(9):677–681. [DOI] [PubMed] [Google Scholar]
- Elefteriou F, Ahn JD, Takeda S, Starbuck M, Yang X, Liu X, Kondo H, Richards WG, Bannon TW, Noda M, et al. 2005. Leptin regulation of bone resorption by the sympathetic nervous system and CART. Nature. 434(7032):514–520. [DOI] [PubMed] [Google Scholar]
- Francis M, Gopinathan G, Salapatas A, Nares S, Gonzalez M, Diekwisch TGH, Luan X. 2020. SETD1 and NF-κB regulate periodontal inflammation through H3K4 trimethylation. J Dent Res. 99(13):1486–1493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Franquinho F, Liz MA, Nunes AF, Neto E, Lamghari M, Sousa MM. 2010. Neuropeptide Y and osteoblast differentiation—the balance between the neuro-osteogenic network and local control. FEBS J. 277(18):3664–3674. [DOI] [PubMed] [Google Scholar]
- Grässel SG. 2014. The role of peripheral nerve fibers and their neurotransmitters in cartilage and bone physiology and pathophysiology. Arthritis Res Ther. 16(6):485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Habert-Ortoli E, Amiranoff B, Loquet I, Laburthe M, Mayaux JF. 1994. Molecular cloning of a functional human galanin receptor. Proc Natl Acad Sci U S A. 91(21):9780–9783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Idelevich A, Sato K, Nagano K, Rowe G, Gori F, Baron R. 2018. Neuronal hypothalamic regulation of body metabolism and bone density is galanin dependent. J Clin Invest. 128(6):2626–2641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Idelevich A, Sato K, Nagano K, Rowe G, Gori F, Baron R. 2019. ΔFosB requires galanin, but not leptin, to increase bone mass via the hypothalamus, but both are needed to increase energy expenditure. J Bone Miner Res. 34(9):1707–1720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones KB, Mollano AV, Morcuende JA, Cooper RR, Saltzman CL. 2004. Bone and brain: a review of neural, hormonal, and musculoskeletal connections. Iowa Orthop J. 24:123–132. [PMC free article] [PubMed] [Google Scholar]
- Juarranz Y, Abad C, Martinez C, Arranz A, Gutierrez-Cañas I, Rosignoli F, Gomariz RP, Leceta J. 2005. Protective effect of vasoactive intestinal peptide on bone destruction in the collagen-induced arthritis model of rheumatoid arthritis. Arthritis Res Ther. 7(5):R1034–R1045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kofler B, Berger A, Santic R, Moritz K, Almer D, Tuechler C, Lang R, Emberger M, Klausegger A, Sperl W, et al. 2004. Expression of neuropeptide galanin and galanin receptors in human skin. J Invest Dermatol. 122(4):1050–1053. [DOI] [PubMed] [Google Scholar]
- Koller A, Bianchini R, Schlager S, Münz C, Kofler B, Wiesmayr S. 2017. The neuropeptide galanin modulates natural killer cell function. Neuropeptides. 64:109–115. [DOI] [PubMed] [Google Scholar]
- Koller A, Brunner SM, Bianchini R, Ramspacher A, Emberger M, Locker F, Schlager S, Kofler B. 2019. Galanin is a potent modulator of cytokine and chemokine expression in human macrophages. Sci Rep. 9(1):7237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lang R, Gundlach AL, Kofler B. 2007. The galanin peptide family: receptor pharmacology, pleiotropic biological actions, and implications in health and disease. Pharmacol Ther. 115(2):177–207. [DOI] [PubMed] [Google Scholar]
- Lee MJ, Oh JH, Park CH, Kim KH, Lee DH, Chung JH. 2017. Galanin contributes to ultraviolet irradiation-induced inflammation in human skin. Exp Dermatol. 26(8):744–747. [DOI] [PubMed] [Google Scholar]
- Lu X, Fukumoto S, Yamada Y, Evans CA, Diekwisch TG, Luan X. 2016. Ameloblastin, an extracellular matrix protein, affects long bone growth and mineralization. J Bone Miner Res. 31(6):1235–1246. [DOI] [PubMed] [Google Scholar]
- Lundy FT, Linden GJ. 2004. Neuropeptides and neurogenic mechanisms in oral and periodontal inflammation. Crit Rev Oral Biol Med. 15(2):82–98. [DOI] [PubMed] [Google Scholar]
- Luthman J, Johansson O, Ahlström U, Kvint S. 1988. Immunohistochemical studies of the neurochemical markers, CGRP, enkephalin, galanin, gamma-MSH, NPY, PHI, proctolin, PTH, somatostatin, SP, VIP, tyrosine hydroxylase and neurofilament in nerves and cells of the human attached gingiva. Arch Oral Biol. 33(3):149–158. [DOI] [PubMed] [Google Scholar]
- Ma X, Su P, Yin C, Lin X, Wang X, Gao Y, Patil S, War AR, Qadir A, Tian Y, et al. 2020. The roles of FoxO transcription factors in regulation of bone cells function. Int J Mol Sci. 21(3):692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martinelli I, Timotin A, Moreno-Corchado P, Marsal D, Kramar S, Loy H, Joffre C, Boal F, Tronchere H, Kunduzova O. 2021. Galanin promotes autophagy and alleviates apoptosis in the hypertrophied heart through FoxO1 pathway. Redox Biol. 40:101866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McDonald AC, Schuijers JA, Gundlach AL, Grills BL. 2007. Galanin treatment offsets the inhibition of bone formation and downregulates the increase in mouse calvarial expression of TNFα and GalR2 mRNA induced by chronic daily injections of an injurious vehicle. Bone. 40(4):895–903. [DOI] [PubMed] [Google Scholar]
- McDonald AC, Schuijers JA, Shen PJ, Gundlach AL, Grills BL. 2003. Expression of galanin and galanin receptor-1 in normal bone and during fracture repair in the rat. Bone. 33(5):788–797. [DOI] [PubMed] [Google Scholar]
- Nunes AF, Liz MA, Franquinho F, Teixeira L, Sousa V, Chenu C, Lamghari M, Sousa MM. 2010. Neuropeptide Y expression and function during osteoblast differentiation—insights from transthyretin knockout mice. FEBS J. 277(1):263–275. [DOI] [PubMed] [Google Scholar]
- Pan S, Dangaria S, Gopinathan G, Yan X, Lu X, Kolokythas A, Niu Y, Luan X. 2013. SCF promotes dental pulp progenitor migration, neovascularization, and collagen remodeling—potential applications as a homing factor in dental pulp regeneration. Stem Cell Rev Rep. 9(5):655–667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qian Y, Zhou X, Zhang F, Diekwisch THG, Luan X, Yang J. 2019. Triple PLGA/PCL scaffold modification including silver impregnation, collagen coating, and electrospinning significantly improve biocompatibility, antimicrobial, and osteogenic properties for orofacial tissue regeneration. ACS Appl Mater Interfaces. 11(41):37381–37396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richbourg NR, Peppas NA, Sikavitsas VI. 2019. Tuning the biomimetic behavior of scaffolds for regenerative medicine through surface modifications. J Tissue Eng Regen Med. 13(8):1275–1293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmidt WE, Kratzin H, Eckart K, Drevs D, Mundkowski G, Clemens A, Katsoulis S, Schäfer H, Gallwitz B, Creutzfeldt W. 1991. Isolation and primary structure of pituitary human galanin, a 30-residue nonamidated neuropeptide. Proc Natl Acad Sci U S A. 88(24):11435–11439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sousa DM, Herzog H, Lamghari M. 2009. NPY signalling pathway in bone homeostasis: Y1 receptor as a potential drug target. Curr Drug Targets. 10(1):9–19. [DOI] [PubMed] [Google Scholar]
- Talero E, Sánchez-Fidalgo S, Calvo JR, Motilva V. 2006. Galanin in the trinitrobenzene sulfonic acid rat model of experimental colitis. Int Immunopharmacol. 6(9):1404–1412. [DOI] [PubMed] [Google Scholar]
- Talero E, Sánchez-Fidalgo S, Calvo JR, Motilva V. 2007. Chronic administration of galanin attenuates the TNBS-induced colitis in rats. Regul Pept. 141(1–3):96–104. [DOI] [PubMed] [Google Scholar]
- Tatemoto K, Rökaeus A, Jörnvall H, McDonald TJ, Mutt V. 1983. Galanin—a novel biologically active peptide from porcine intestine. FEBS Lett. 164(1):124–128. [DOI] [PubMed] [Google Scholar]
- Wu Q, Hultenby K, Adlan E, Lindgren JU. 2004. Galanin in adjuvant arthritis in the rat. J Rheumatol. 31(2):302–307. [PubMed] [Google Scholar]
- Yamamoto H, Arai T, Ben S, Iguchi K, Hoshino M. 2011. Expression of galanin and galanin receptor mRNA in skin during the formation of granulation tissue. Endocrine. 40(3):400–407. [DOI] [PubMed] [Google Scholar]
- Yamamoto H, Iguchi K, Unno K, Kaji K, Hoshino M. 2014. Expression and release of progalanin in fibroblasts. Regul Pept. 194–195:55–62. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental material, sj-docx-1-jdr-10.1177_00220345211028852 for Successful Application of a Galanin-Coated Scaffold for Periodontal Regeneration by W. Ma, H. Lyu, M. Pandya, G. Gopinathan, X. Luan and T.G.H. Diekwisch in Journal of Dental Research